Abstract
OBJECTIVE
There is limited information from large-scale prospective studies regarding the prediction of type 1 diabetes by specific types of pancreatic islet autoantibodies, either alone or in combination. Thus, we studied the extent to which specific autoantibodies are predictive of type 1 diabetes.
RESEARCH DESIGN AND METHODS
Two cohorts were derived from the first screening for islet cell autoantibodies (ICAs) in the Diabetes Prevention Trial–Type 1 (DPT-1). Autoantibodies to GAD 65 (GAD65), insulinoma-associated antigen-2 (ICA512), and insulin (micro-IAA [mIAA]) were also measured. Participants were followed for the occurrence of type 1 diabetes. One cohort (Questionnaire) included those who did not enter the DPT-1 trials, but responded to questionnaires (n = 28,507, 2.4% ICA+). The other cohort (Trials) included DPT-1 participants (n = 528, 83.3% ICA+).
RESULTS
In both cohorts autoantibody number was highly predictive of type 1 diabetes (P < 0.001). The Questionnaire cohort was used to assess prediction according to the type of autoantibody. As single autoantibodies, ICA (3.9%), GAD65 (4.4%), and ICA512 (4.6%) were similarly predictive of type 1 diabetes in proportional hazards models (P < 0.001 for all). However, no subjects with mIAA as single autoantibodies developed type 1 diabetes. As second autoantibodies, all except mIAA added significantly (P < 0.001) to the prediction of type 1 diabetes. Within the positive range, GAD65 and ICA autoantibody titers were predictive of type 1 diabetes.
CONCLUSIONS
The data indicate that the number of autoantibodies is predictive of type 1 diabetes. However, mIAA is less predictive of type 1 diabetes than other autoantibodies. Autoantibody number, type of autoantibody, and autoantibody titer must be carefully considered in planning prevention trials for type 1 diabetes.
Autoantibodies to islet cell antigens are known predictors of type 1 diabetes and are commonly present at its diagnosis (1–12). Islet cell autoantibodies (ICAs), the first identified (1,2), actually represent autoimmunity to several different antigens. More recently, autoantibodies specific to single tissue antigens, termed biochemical autoantibodies, have been identified (4,7,8,11–13). These include antibodies to GAD 65 (GAD65), the antibody to an insulinoma-associated antigen-2 (ICA512), and antibodies to insulin (IAA).
Type 1 diabetes prevention trials have used autoantibodies to screen for individuals at increased risk who might be candidates for participation (14–16). The Diabetes Prevention Trial–Type 1 (DPT-1) assessed parenteral and oral insulin as potential prevention modalities. First- and second-degree relatives of type 1 diabetic patients were screened for the presence of ICA, which was required for eligibility. Although not relevant to the trials, biochemical autoantibodies were subsequently measured from screening samples to learn more about their prediction of type 1 diabetes. The prevalence of autoantibodies according to various subgroups has been reported for DPT-1 (17).
We used two DPT-1 cohorts to examine the prediction of type 1 diabetes by ICA and biochemical autoantibodies, as few large-scale studies have examined the prediction of type 1 diabetes by a variety of single autoantibodies in large numbers of individuals of whom many ultimately developed type 1 diabetes. One cohort includes DPT-1 participants who participated in the trials (the Trials cohort), and the other cohort includes participants who did not participate in either trial but responded to questionnaires (the Questionnaire cohort) used to ascertain information regarding the diagnosis of type 1 diabetes. The differing perspectives of these two cohorts and the large number of individuals studied, almost 30,000, provide a unique opportunity for studying the prediction of type 1 diabetes by autoantibodies.
RESEARCH DESIGN AND METHODS
All participants were relatives of patients with type 1 diabetes. There were 97,273 serum samples collected and tested for ICA at the initial screening. Informed consent was obtained from all subjects. As described elsewhere (14,15), eligibility for the trials was further assessed on the basis of metabolic abnormalities (parenteral insulin trial) and the presence of IAA (oral insulin trial). There were 711 individuals who participated in the DPT-1 trials. Of the screening samples, 84% were later tested for the presence of GAD65, ICA512, and IAA measured by the micro method (mIAA).
Questionnaires were mailed to 79,292 individuals who did not enter the trials. Those who were ICA+ did not meet the criteria for trial entry or chose not to enter the trials. Responses were received from 37,017 subjects. Those who had all autoantibody determinations and sufficiently complete data were included in the analyses (n = 29,035).
Procedures
Questionnaire cohort.
Participants were asked whether they were informed by a physician that they had developed type 1 diabetes. If participants answered affirmatively, they were asked when they received the diagnosis. The follow-up interval was the time between the date of the response to the questionnaire and the date of the initial screen for autoantibodies (those who did not develop type 1 diabetes) or between the date of diagnosis as indicated on the questionnaire and the date of the initial screen (those who developed type 1 diabetes). The mean ± SD age of the individuals in the Questionnaire cohort (n = 28,507) was 17.9 ± 13.0 years (55% female). The duration of follow-up was 4.2 ± 2.4 years.
Trials cohort.
The procedures for the DPT-1 trials have been described elsewhere (14,15). In both the parenteral and oral insulin trials, oral glucose tolerance tests were scheduled for 6-month intervals. Blood samples were obtained for plasma glucose and C-peptide measurements in the fasting state and at 30, 60, 90, and 120 min. Those with glucose values in the diabetic range (fasting glucose ≥126 mg/dl and/or 2-h glucose ≥200 mg/dl) were asked to return for confirmation at a follow-up visit. The follow-up interval was the time between the date of last contact and the date of the first screen (those who did not develop type 1 diabetes) or the time between the date of diagnosis and the date of the first screen (those who developed type 1 diabetes). In 61% of those with type 1 diabetes in the DPT-1 trials, diabetes was diagnosed at a routine visit. In others, diabetes was diagnosed clinically. There was no overall effect of the intervention in either trial (14,15). The mean ± SD age of those in the Trials cohort (n = 528) was 12.2 ± 9.3 years (43% female). The Trials cohort was significantly younger and had a lower proportion of female participants than the Questionnaire cohort (P < 0.001 for both). The duration of follow-up was 4.4 ± 2.2 years.
Laboratory measures
ICA.
ICA values were determined by an immunofluorescence assay on frozen sections of blood type O human pancreas in the DPT-1 ICA Core Laboratory (Gainesville, FL, February 1994–September 1997 and January 1999–October 2003; New Orleans, LA, September 1997–January 1999). ICA values of ≥10 Juvenile Diabetes Foundation (JDF) units were considered positive. In the 1995 Immunology of Diabetes Society workshop (18), this ICA assay had a specificity of 100% and a sensitivity of 74.4% for patients with new-onset type 1 diabetes who were aged <30 years. Based on a receiver operating characteristic curve, in this dataset with a positive JDF value of 10, the assay sensitivity was 75.0% with a 95.7% specificity (no age influence on the values).
GAD65, ICA512, and mIAA.
Autoantibodies against GAD65 and ICA512bdc (ICA512) were determined at the Barbara Davis Center (Denver, CO). IAA (using the micro-volume requiring assay) values were determined at the Barbara Davis Center or the Joslin Diabetes Center (Boston, MA). As described previously, a combined GAD65 and ICA512 radioassay was performed (19). Labeled recombinant GAD65 and ICA512 autoantibodies were produced by in vitro transcription/translation with differential labeling ([3H]GAD65 and [35S]ICA512) (8,13). The levels of both autoantibodies were expressed as an index. The upper limits of normal (0.032 for GAD65 and 0.049 for ICA512) were established as the 99th percentile for GAD65 and for ICA512 from receiver operating characteristic curves in 198 healthy control subjects and 50 patients with new-onset diabetes. In this dataset, a GAD65 index of 0.032 (used for the analysis) provided a 41.8% sensitivity and a 98.3% specificity (no difference by age-group), and for ICA512 an index of 0.049 (used for the analysis) resulted in a sensitivity of 57.5% and specificity of 98.5% (no difference by age-group). In the 2000, 2002, and the 2003 Diabetes Antibody Standardization Program (DASP) for proficiency testing, the sensitivity/specificity results for the GAD65 assay were 84%/96%, 90%/93%, and 84%/98% and for the ICA512 assay were 52%/100%, 62%/99%, and 58%/100%, respectively. The interassay coefficients of variation for ICA512 and GAD65 were 8 and 10%, respectively.
The mIAA assay (20) was performed as described and expressed as an index with the upper limits of 0.02 and 0.01 (Boston and Denver laboratories, respectively) based on the 99th percentile of healthy control values. In the combined dataset, a positive index value had 36.8% sensitivity and 92.7% specificity. Determination of mIAA on samples began later than for GAD65 and ICA512 (assay development needed). In the 2003 DASP proficiency testing, the sensitivity for the mIAA assay was 74 and 56% and the specificity was 90 and 98%, respectively, for the Denver and Boston laboratories. The correlation coefficient between both laboratories was r = 0.90 (P < 0.0001). The interassay coefficient of variation for mIAA was 16%.
Data analysis
Student t tests and χ2 tests were used to compare groups. The log-rank test was used to compare the distributions of event times between groups. Cox proportional hazards regression models were used to examine effects on type 1 diabetes risk over time. The Kaplan-Meier estimate of the survival function was used to obtain estimates of type 1 diabetes occurrence. Spearman correlations were performed to assess associations. Log transformations were performed for certain analyses.
SAS (version 9.1.3) was used for the analyses. All P values are two-sided. The level of significance was P < 0.05.
RESULTS
The prevalence of each autoantibody at the initial screening is shown in Table 1 for both cohorts. Autoantibody prevalence was much higher in the Trials cohort, which is attributable to the selection for ICA positivity and for the additional trial entry criteria. Although some of those in the Trials cohort were ICA− at the initial screening, all eventually became ICA+ before randomization.
Table 1.
Prevalence of positive autoantibodies at initial screening
| Questionnaire cohort | Trials cohort | |
|---|---|---|
| n | 28,507 | 528 |
| ICA | 674 (2.4) | 440 (83.3) |
| GAD65 | 907 (3.2) | 363 (68.8) |
| ICA512 | 315 (1.1) | 258 (48.9) |
| mIAA | 525 (1.8) | 136 (25.8) |
Data are n or n (%).
In the Questionnaire cohort, ICA512 positivity was most commonly associated with one or more autoantibodies (65%), whereas mIAA positivity was least commonly associated with other autoantibodies (22%). The percentages of GAD65 and ICA positivity with associated autoantibodies were 37 and 39%, respectively.
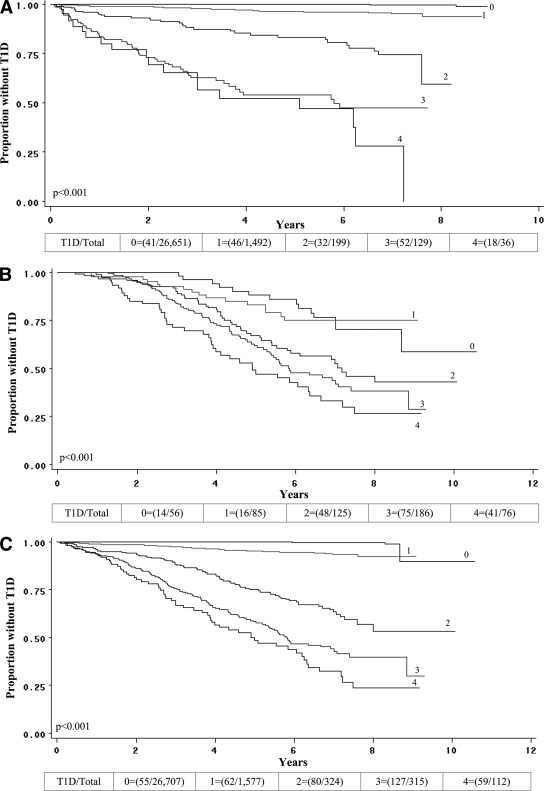
The occurrence of type 1 diabetes according to the number of biochemical autoantibodies is shown separately for the Questionnaire and Trials cohorts and in the aggregate in Fig. 1. In each cohort, there tended to be an increasing occurrence of type 1 diabetes as the number of autoantibodies increased (P < 0.001 for both).
Figure 1.
Curves indicate the occurrence of type 1 diabetes (T1D) over follow-up according to the number of autoantibodies present at the initial screening in the Questionnaire cohort (A), in the Trials cohort (B), and in the cohorts combined (C). In all three panels, there were significant trends among the groups of an increasing occurrence of type 1 diabetes with increasing autoantibody number. The numbers (1–4) indicate the number of autoantibodies. (The fraction in parentheses indicates the number who developed type 1 diabetes among the number in the group at baseline.)
The occurrence of type 1 diabetes among those with a single autoantibody is shown in Table 2 for the Questionnaire cohort. None of the 407 individuals with the presence of only mIAA developed type 1 diabetes. The occurrence of type 1 diabetes was similar for those with GAD65 alone (4.4%), ICA512 alone (4.6%), and ICA alone (3.9%). In proportional hazards models, there were significant associations between the occurrence of type 1 diabetes and each of those autoantibodies occurring singly (P < 0.001 for all). When age was added as a covariate, the associations remained highly significant (P < 0.001).
Table 2.
Associations of type 1 diabetes occurrence with the presence of single autoantibodies in the Questionnaire cohort at initial screening
| Type 1 diabetes/total (%) | HR (95% CI) | |
|---|---|---|
| GAD65 | 25/568 (4.4) | 27.6 (16.8–45.4)* |
| ICA512 | 5/110 (4.6) | 29.5 (11.6–74.7)* |
| mIAA | 0/407 (0) | — |
| ICA | 16/407 (3.9) | 27.5 (15.4–49.0)* |
Reference group: negative for all autoantibodies (type 1 diabetes/total = 41 of 26,651).
*P < 0.001.
Supplemental Table A1 (available at http://care.diabetesjournals.org/cgi/content/full/dc09-0934/DC1) shows the occurrence of type 1 diabetes among those who were single autoantibody–positive in the combined Questionnaire and Trials cohorts. The occurrence of type 1 diabetes for those with ICA alone was somewhat higher than the occurrence for the other autoantibodies alone.
The distribution of mIAA values was examined to determine whether the lack of occurrence of type 1 diabetes in those with mIAA alone could be the result of a preponderance of low titers. Because mIAAs were measured in two laboratories (positive results: n = 123 for Boston and n = 284 for Denver) and the threshold was higher for an abnormal value in Boston than in Denver (0.02 vs. 0.01), the distributions were examined for each laboratory. Among those with abnormal values, the median values for Boston and Denver were 0.108 and 0.024, respectively. The values for the 75th percentiles were 0.182 and 0.051, respectively. Thus, an appreciable proportion of the titers was clearly elevated.
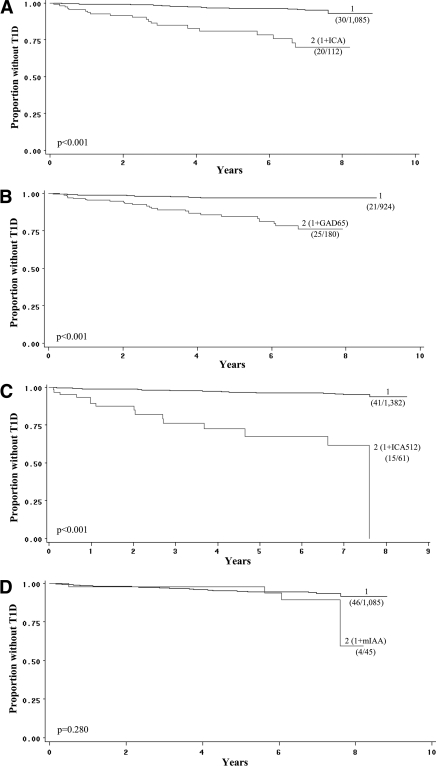
Figure 2 shows the effect of adding each autoantibody as a second autoantibody to the presence of a single autoantibody (any of the other three). When ICA, ICA512, or GAD65 was each included as a second autoantibody, the occurrence of type 1 diabetes was significantly greater (P < 0.001 for each) than when there was single autoantibody positivity. However, with mIAA as a second autoantibody there was no significant difference from the presence of one autoantibody. (There was little difference in risk increment between GAD65 and ICA when either was the first autoantibody and the other was added [data not shown].) When each of the autoantibodies was present additionally as a third autoantibody (supplemental Fig. A1, available in an online appendix), the risk increased appreciably with ICA and ICA512 (both P < 0.001) but did not increase significantly with GAD65 and mIAA.
Figure 2.
Curves indicate the occurrence of type 1 diabetes (T1D) in the Questionnaire cohort over follow-up for the presence of one autoantibody and for the additional presence of a second autoantibody at the initial screening. Thus, in each panel the groups that included a specific second positive autoantibody were compared with the group that had one positive autoantibody from any of the others. The panels show that when ICA (A), GAD65 (B), or ICA512 (C) each was present as a second autoantibody, there were significant and similar increases in the occurrence of type 1 diabetes; however, there was no increase when mIAA was present as a second autoantibody (D). The number shown for each curve (1, 2) indicates the number of autoantibodies. Proportions of those who developed type 1 diabetes are shown for each curve. (The fraction in parentheses indicates the number who developed type 1 diabetes among the number in the group at baseline.)
The Trials cohort was used to assess the influence of a second autoantibody besides ICA. There was an increase in the percentage of those developing type 1 diabetes when GAD65 and ICA512 each was present besides ICA (ICA alone: 13 of 65 [20%], ICA with GAD65: 30 of 87 [34%], and ICA with ICA512: 11 of 22 [50%]). However, when age was included as a covariate in proportional hazards models, neither the additional presence of GAD65 nor that of ICA512 was significant. The numbers for the additional presence of mIAA as a second autoantibody were too small for a meaningful analysis; however, 3 of 6 (50%) developed type 1 diabetes.
Associations of autoantibody titers were examined in the combined cohorts. Of those who did not develop type 1 diabetes (n = 28,652), ICA512 and GAD65 titers were much more strongly correlated (r = 0.31) than the titers of any other autoantibody pair (r ranged from 0.03 to 0.13). However, among those who developed type 1 diabetes (n = 383), although the ICA512-GAD65 correlation remained similar (r = 0.30), the correlations of other autoantibody pairs tended to increase, especially when the pair included an ICA titer (with ICA titer: r = 0.39 for GAD65 titer, r = 0.51 for ICA512 titer, and r = 0.34 for mIAA titer). In this large dataset, all correlations were significant (P < 0.001 for all).
The association between the development of type 1 diabetes and titer (log-transformed) was examined among those who were positive for single autoantibodies in the combined cohorts. Because of the lack of cases of type 1 diabetes for those with mIAA positivity alone, the analysis was not performed for that autoantibody. GAD65 titer (type 1 diabetes/total = 27 of 582) and ICA titer (29 of 472) were each predictive of type 1 diabetes (P < 0.01 for both, with and without age as a covariate). There was borderline significance (P = 0.04 and P = 0.07 with age added) for the ICA512 titer (6 of 113), but the number for that analysis was small.
CONCLUSIONS
The analyses presented above were designed specifically to discern the extent to which positivity for a single autoantibody predicts the occurrence of type 1 diabetes. They showed that among those in the Questionnaire cohort, ICA, ICA512, and GAD65, as single positive autoantibodies, were similarly predictive of type 1 diabetes. In addition, each of those autoantibodies appeared to add significant increments of risk when they were included as second autoantibodies in the Questionnaire cohort. However, type 1 diabetes was not associated with mIAA as a single autoantibody, and the inclusion of mIAA as a second or a third autoantibody appeared to have little effect. The addition of single biochemical autoantibodies to ICA in the Trials cohort did not significantly increase type 1 diabetes risk with age included as a covariate. This finding could be related to the selection criteria for that cohort and to the small numbers.
The findings from this study are better understood by considering them in the context of the characteristics of each cohort. The vast majority of those in the Questionnaire cohort were ICA− at the initial screening, whereas those in the Trials cohort were mostly ICA+ at the initial screening, and eventually all became positive before randomization. Moreover, the Trials cohort was selected for additional characteristics, including metabolic impairment and IAA positivity. These factors, together with glucose tolerance test surveillance in that cohort, could explain the relatively stronger association of type 1 diabetes with single positivity of ICA when the cohorts were combined.
The finding that autoantibody number predicts type 1 diabetes is consistent with other studies (9,15). The lack of a mIAA effect was similar to a previous finding of little effect when IAA was included as a third autoantibody (21). However, there have been other studies that showed a higher risk associated with IAA positivity (15,22,23). Other autoantibodies, either accounted for or unaccounted for, could have explained these associations. ICA+ individuals who were also IA-2βA+ have been observed to have a high risk for type 1 diabetes (11). The addition of ICA to other autoantibodies has been shown to substantially increase the risk of type 1 diabetes in first-degree relatives (24). An autoantibody to zinc transporter 8 has recently been found to be predictive of type 1 diabetes in children (12).
The percentages of common positivity with at least one other autoantibody ranged from 22% for mIAA to 65% for ICA512. In addition, the correlations of titers between autoantibody pairs differed, and the correlations varied according to the subsequent development of type 1 diabetes. It is possible that associations among autoantibodies according to positivity and titer are a function of the stage of progression to type 1 diabetes in the study population. If so, the serial follow-up of these associations could provide insight into both the prediction and pathogenesis of type 1 diabetes.
Among those with single positivity of GAD65 and ICA, type 1 diabetes was predicted by titer. Thus, titers can provide useful information, even within the positive range. In a previous report, among those with positive autoantibodies, the titers of IA-2 antigen and IAA were both predictive of type 1 diabetes (25).
IAA has been the most difficult to measure in multiple DASP workshops. Although a cutoff is set at the 99th percentile for each of the autoantibodies, the strength of signal for IAA of patients with new-onset type 1 diabetes is much closer to the range of signal for normal control subjects compared with that for either GAD65 or ICA512 autoantibodies. Thus, it is possible that a sizable proportion of mIAA+ values represents false-positive results or low-affinity IAA associated with lower risk (26). Because confirmation and persistence were not determined in the current study, these factors will be important to evaluate in future studies.
The findings in this study might not be fully generalizable because they were derived from relatives of patients with type 1 diabetes. As discussed above, the Trials cohort was selected on the basis of certain criteria. In addition, the composition of the Questionnaire cohort could have been influenced by the willingness to respond to the questionnaire and even to some extent by the absence of the qualifying criteria for entry into the trials. As discussed above, the increased occurrence of type 1 diabetes in the Trials cohort is probably attributable to selection factors for trial entry and to oral glucose tolerance test surveillance.
Our findings show that although autoantibody number is a predictor of type 1 diabetes, the particular type and titer of an autoantibody can influence prediction. Moreover, it is evident that the frequencies and the associations of autoantibodies with each other can vary to a great extent. These factors must be carefully considered in planning prevention trials for type 1 diabetes.
Supplementary Material
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Bottazzo GF, Florin-Christensen A, Doniach D: Islet cell antibodies in diabetes mellitus with autoimmune polyendocrine deficiencies. Lancet 1974; 2: 1279– 1282 [DOI] [PubMed] [Google Scholar]
- 2. Lendrum R, Walker G, Cudworth AG, Theophanides C, Pyke DA, Bloom A, Gamble DR: Islet-cell antibodies in diabetes mellitus. Lancet 1976; 2: 1273– 1276 [DOI] [PubMed] [Google Scholar]
- 3. Gorsuch AN, Spencer KM, Lister J, McNally JM, Dean BM, Bottazzo GF, Cudworth AG: Evidence for a long prediabetic period in type I (insulin dependent) diabetes mellitus. Lancet 1981; 2: 1363– 1365 [DOI] [PubMed] [Google Scholar]
- 4. Palmer JP, Asplin CM, Clemons P, Lyen K, Tatpati O, Raghu PK, Paquette TL: Insulin antibodies in insulin-dependent diabetics before insulin treatment. Science 1983; 222: 1337– 1339 [DOI] [PubMed] [Google Scholar]
- 5. Wilkin T, Hoskins PJ, Armitage M, Rodier M, Casey C, Diaz JL, Pyke DA, Leslie RD: Value of insulin autoantibodies as serum markers for insulin-dependent diabetes mellitus. Lancet 1985; 480– 481 [DOI] [PubMed] [Google Scholar]
- 6. Baekkeskov S, Landin M, Kristensen JK, Srikanta S, Bruining GJ, Mandrup-Poulsen T, de Beaufort C, Soeldner JS, Eisenbarth G, Lindgren F: Antibodies to a 64,000 Mr human islet cell antigen precede the clinical onset of insulin-dependent diabetes. J Clin Invest 1987; 79: 926– 934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Christie MR, Vohra G, Champagne P, Daneman D, Delovitch TL: Distinct antibody specificities to a 64-kD islet cell antigen in type 1 diabetes as revealed by trypsin treatment. J Exp Med 1990; 172: 789– 794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Payton MA, Hawkes CJ, Christie MR: Relationship of 37,000 and 40,000-Mr tryptic fragment of islet antigens in insulin dependent diabetes to the protein tyrosine phosphatase-like molecule IA-2 (ICA 512). J Clin Invest 1995; 96: 1506– 1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Verge CF, Gianani R, Kawasaki E, Yu L, Pietropaolo M, Chase HP, Eisenbarth GS: Number of autoantibodies (against insulin, GAD or ICA512/IA2) rather than particular autoantibody specificities determines risk of type I diabetes. J Autoimmun 1996; 9: 379– 383 [DOI] [PubMed] [Google Scholar]
- 10. Kulmala P, Savola K, Petersen JS, Vahasalo P, Karjalainen J, Lopponen T, Dyrberg T, Akerblom HK, Knip M: Prediction of insulin-dependent diabetes mellitus in siblings of children with diabetes: a population-based study. The Childhood Diabetes in Finland Study Group. J Clin Invest 1998; 101: 327– 336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Achenbach P, Bonifacio E, Williams AJK, Ziegler AG, Gale EAM, Bingley PJ. ENDIT Group. Autoantibodies to IA-2β improve diabetes risk assessment in high-risk relatives. Diabetologia 2008; 51: 488– 492 [DOI] [PubMed] [Google Scholar]
- 12. Achenbach P, Lampasona V, Landherr U, Koczwara K, Krause S, Grallert H, Winkler C, Pfluger M, Illig T, Bonifacio E, Ziegler A-G: Autoantibodies to zinc transporter 8 and SLC3018 genotype stratify type 1 diabetes risk. Diabetologia 2009; 52: 1881– 1888 [DOI] [PubMed] [Google Scholar]
- 13. Grubin CE, Daniels T, Toivola B, Landin-Olsson M, Hagopian WA, Li L, Karlsen AE, Boel E, Michelsen B, Lernmark A: A novel radioligand binding assay to determine diagnostic accuracy of isoform-specific glutamic acid decarboxylase antibodies in childhood IDDM. Diabetologia 1994; 37: 344– 350 [DOI] [PubMed] [Google Scholar]
- 14. Diabetes Prevention Trial–Type 1 Diabetes Study Group. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med 2002; 346: 1685– 1691 [DOI] [PubMed] [Google Scholar]
- 15. Skyler JS, Krischer JP, Wolfsdorf J, Cowie C, Palmer JP, Greenbaum C, Cuthbertson D, Rafkin-Mervis LE, Chase HP, Leschek E: Effects of oral insulin in relatives of patients with type 1 diabetes: the Diabetes Prevention Trial–Type 1. Diabetes Care 2005; 28: 1068– 1076 [DOI] [PubMed] [Google Scholar]
- 16. Bingley PJ, Gale EAM. European Nicotinamide Diabetes Intervention Trial (ENDIT) Group. Progression to type 1 diabetes in islet cell antibody-positive relatives in the European Nicotinamide Diabetes Intervention Trial: the role of additional immune, genetic and metabolic markers of risk. Diabetologia 2006; 49: 881– 890 [DOI] [PubMed] [Google Scholar]
- 17. Krischer JP, Cuthbertson DD, Liping Yu, Orban Tihamer, Maclaren N, Jackson R, Winter WE, Schatz DA, Palmer JP, Eisenbarth GS: Screening strategies for identification of multiple antibody-positive relatives of individuals with type 1 diabetes. J Clin Endocrinol Metab 2003; 88: 103– 108 [DOI] [PubMed] [Google Scholar]
- 18. Bottazzo GF, Gleichmann H: Immunology and Diabetes Workshops: report of the First International Workshop on Standardization of Cytoplasmic Islet Cell Antibodies. Diabetologia 1986; 29: 125– 126 [Google Scholar]
- 19. Yu L, Rewers M, Gianani R, Kawasaki E, Zhang Y, Verge C, Chase P, Klingensmith G, Erlich H, Norris J, Eisenbarth GS: Antiislet autoantibodies usually develop sequentially rather than simultaneously. J Clin Endocrinol Metab 1996; 81: 4264– 4267 [DOI] [PubMed] [Google Scholar]
- 20. Williams AJ, Bingley PJ, Bonifacio E, Palmer JP, Gale EA: A novel micro-assay for insulin autoantibodies. J Autoimmun 1997; 10: 473– 478 [DOI] [PubMed] [Google Scholar]
- 21. Greenbaum CJ, Sears KL, Kahn SE, Palmer JP: Relationship of β-cell function and autoantibodies to progression and nonprogression of subclinical type 1 diabetes: follow-up of the Seattle Family Study. Diabetes 1999; 48: 170– 175 [DOI] [PubMed] [Google Scholar]
- 22. Bingley PJ, Christie MR, Bonifacio E, Bonfanti R, Shattock M, Fonte MT, Bottazzo GF, Gale EA: Combined analysis of autoantibodies improves prediction of IDDM in islet cell antibody-positive relatives. Diabetes 1994; 43: 1304– 1310 [DOI] [PubMed] [Google Scholar]
- 23. Bingley PJ. for the ICARUS Group. Interactions of age, islet cell antibodies, insulin autoantibodies, and first-phase insulin response in predicting risk of progression to IDDM in ICA+ relatives. Diabetes 1996; 45: 1720– 1728 [DOI] [PubMed] [Google Scholar]
- 24. Pitropaolo M, Libman IM, Pietropaolo SL, Riley K, LaPorte RE, Drash AL, Mazumdar S, Trucco M, Becker DJ: Cytoplasmic islet cell antibodies remain valuable in defining risk of progression to type 1 diabetes in subjects with other islet autoantibodies. Pediatr Diabetes 2005; 6: 181– 183 [DOI] [PubMed] [Google Scholar]
- 25. Achenbach P, Warncke K, Reiter J, Naserke HE, Williams AJK, Bingley PJ, Bonifacio E, Ziegler A-G: Stratification of type 1 diabetes risk on the basis of islet autoantibody characteristics. Diabetes 2004; 53: 384– 392 [DOI] [PubMed] [Google Scholar]
- 26. Achenbach P, Koczwara K, Knopff A, Naserke H, Ziegler A-G, Bonifacio E: Mature high-affinity immune responses to (pro)insulin anticipate the autoimmune cascade that leads to type 1 diabetes. J Clin Invest 2004; 114: 589– 597 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.