Abstract
OBJECTIVE
Given evidence of both indirect and direct signaling, we tested the hypothesis that increased β-cell–mediated signaling of α-cells negates direct α-cell signaling in the regulation of glucagon secretion in humans.
RESEARCH DESIGN AND METHODS
We measured plasma glucagon concentrations before and after ingestion of a formula mixed meal and, on a separate occasion, ingestion of the sulfonylurea glimepiride in 24 basal insulin-infused, demonstrably β-cell–deficient patients with type 1 diabetes and 20 nondiabetic, demonstrably β-cell–sufficient individuals; the latter were infused with glucose to prevent hypoglycemia after glimepiride.
RESULTS
After the mixed meal, plasma glucagon concentrations increased from 22 ± 1 pmol/l (78 ± 4 pg/ml) to 30 ± 2 pmol/l (103 ± 7 pg/ml) in the patients with type 1 diabetes but were unchanged from 27 ± 1 pmol/l (93 ± 3 pg/ml) to 26 ± 1 pmol/l (89 ± 3 pg/ml) in the nondiabetic individuals (P < 0.0001). After glimepiride, plasma glucagon concentrations increased from 24 ± 1 pmol/l (83 ± 4 pg/ml) to 26 ± 1 pmol/l (91 ± 4 pg/ml) in the patients with type 1 diabetes and decreased from 28 ± 1 pmol/l (97 ± 5 pg/ml) to 24 ± 1 pmol/l (82 ± 4 pg/ml) in the nondiabetic individuals (P < 0.0001). Thus, in the presence of both β-cell and α-cell secretory stimuli (increased amino acid and glucose levels, a sulfonylurea) glucagon secretion was prevented when β-cell secretion was sufficient but not when β-cell secretion was deficient.
CONCLUSIONS
These data indicate that, among the array of signals, indirect reciprocal β-cell–mediated signaling predominates over direct α-cell signaling in the regulation of glucagon secretion in humans.
The regulation of pancreatic islet α-cell glucagon secretion is complex (1–10). It involves direct signaling of α-cells (1) and indirect signaling of α-cells by β-cell (2–6) and δ-cell (7) secretory products, the autonomic nervous system (8,9), and gut incretins (10).
Appropriate glucagon secretory responses occur from the perfused pancreas (3,5) and perifused islets (2). Low plasma glucose concentrations stimulate glucagon secretion from the transplanted (i.e., denervated) human pancreas (11) and the denervated dog pancreas (12). Therefore, we have focused on the intraislet regulation of glucagon secretion. Furthermore, because selective destruction of β-cells results in loss of the glucagon response to hypoglycemia in type 1 diabetes (13), and partial reduction of the β-cell mass in minipigs results in impaired postprandial suppression of glucagon secretion (14), we have focused on the role of β-cell–mediated signaling in the regulation of glucagon secretion.
Findings from studies of the perfused rat (3,4) and human (5) pancreas, rats in vivo (6), rat islets (2), isolated rat α-cells (2), and humans (15–18) have been interpreted to indicate that a β-cell secretory product or products tonically restrains basal α-cell glucagon secretion during euglycemia and that a decrease in β-cell secretion, coupled with low glucose concentrations at the α-cells, signals an increase in glucagon secretion in response to hypoglycemia. Parenthetically, the relative roles of the candidate β-cell secretory products (insulin, zinc, γ-aminobutyric acid, and amylin, among others) (2) that normally restrain α-cell glucagon secretion remain to be determined. However, that interpretation rests, in part, on results of studies in isolated rat α-cells (2), which are debated (1), and on the evidence that the islet microcirculation flows from β-cells to α-cells to δ-cells (4), which is also debated (19). Furthermore, it does not address the plausible possibility that a decrease in intraislet δ-cell somatostatin secretion might also signal an increase in α-cell glucagon secretion during hypoglycemia (7).
Given that interpretation, it follows that an increase in β-cell secretion would signal a decrease in glucagon secretion in the postprandial state (14). The concept is an interplay of indirect reciprocal β-cell–mediated signaling of α-cells and of direct α-cell signaling in the regulation of glucagon secretion.
There is, in our view, compelling evidence that, among other mechanisms, both indirect reciprocal β-cell–mediated signaling of α-cells (2–6) and direct α-cell signaling (1) are involved in the regulation of glucagon secretion by nutrients, hormones, neurotransmitters, and drugs. Given that premise, we posed the question: Which of these predominates in humans? Accordingly, we tested the hypothesis that increased β-cell–mediated signaling of α-cells negates direct α-cell signaling in the regulation of glucagon secretion in humans. To do so, we measured plasma glucagon responses to ingestion of a mixed meal and, on a separate occasion, to ingestion of the sulfonylurea glimepiride in patients with type 1 diabetes and in nondiabetic individuals. We conceptualized patients with type 1 diabetes as a model of α-cells isolated from β-cells because their β-cells had been destroyed but they have functioning α-cells. (Their α-cells are not, of course, isolated from other islet cells, including δ-cells.) Increased plasma amino acid and glucose levels after a mixed meal and sulfonylureas normally stimulate β-cell secretion; increased plasma amino acid and perhaps glucose (2) levels after a mixed meal and sulfonylureas (1) stimulate α-cell secretion. Our hypothesis predicts that such factors that normally stimulate both β-cells and α-cells would stimulate glucagon secretion in patients with type 1 diabetes but not in nondiabetic individuals, i.e., in the virtual absence and the presence of β-cell function, respectively. Indeed, a mixed meal (20,21) and the secretagogues tolbutamide (22), glyburide (23), and repaglinide (23) have been reported to raise plasma glucagon concentrations in patients with type 1 diabetes, but all of those studies lacked nondiabetic control subjects.
RESEARCH DESIGN AND METHODS
Twenty-four patients with type 1 diabetes (9 women) and 20 nondiabetic individuals (10 women) gave their written consent to participate in this study, which was approved by the Washington University Human Research Protection Office and conducted at the university's Clinical Research Unit (CRU). Their mean ± SD ages were 25.9 ± 9.5 and 27.0 ± 3.2 years, BMIs were 26.2 ± 3.8 and 24.6 ± 5.0 kg/m2, and A1C levels were 7.5 ± 1.1 and 5.3 ± 0.3%, respectively. The duration of type 1 diabetes was 11.8 ± 8.2 years. Twelve of the patients were using a continuous subcutaneous insulin infusion regimen with a rapid-acting insulin analog, and 12 were using a multiple daily injection insulin regimen with glargine as the basal insulin in 10 and NPH as the basal insulin in 2 with rapid-acting analogs as the prandial insulin.
The patients with type 1 diabetes took their last dose of long-acting insulin (if used) 24 h before the study. They used a rapid-acting insulin to control their diabetes until they were admitted to the CRU the evening before study. Regular human insulin (Novolin R; Novo Nordisk, Princeton, NJ) was infused intravenously to maintain near-euglycemia (∼5.6 mmol/l [100 mg/dl]) overnight. After an overnight fast, an arterialized venous sampling line was inserted into a hand vein (with that hand kept in an ∼55°C Plexiglas box), and the insulin infusion was adjusted to maintain stable euglycemia; that insulin infusion dose was then continued through the end of the study. Nondiabetic individuals presented to the CRU, after an overnight fast, on the day of study.
Both study groups ingested a formula mixed meal (Ensure Plus; 355 kcal, 50 g carbohydrate, 13 g protein, and 11 g fat) on one study occasion and 4.0 mg glimepiride (Amaryl; sanofi-aventis U.S., Bridgewater, NJ) on the other study occasion. Glucose was infused intravenously to maintain euglycemia after glimepiride ingestion in the nondiabetic individuals. Blood samples were drawn every 15 minutes. Heart rates and blood pressures (Propaq Encore; Protocol Systems, Beaverton, OR) were measured throughout.
Analytical methods
Plasma glucose was measured with a glucose oxidase method (YSI Glucose Analyzer; YSI, Yellow Springs, OH). Plasma insulin, C-peptide, cortisol, and growth hormone were measured with solid-phase, two-site chemiluminescent immunometric assays (Immulite 1000; Siemens, Los Angeles, CA); plasma glucagon and pancreatic polypeptide were measured with Linco radioimmunoassays (Millipore, Temecula, CA). Plasma epinephrine and norepinephrine were measured with a single isotope derivative (radioenzymatic) method, and blood lactate and serum nonesterified fatty acids were measured with enzymatic techniques.
Statistical methods
Time and condition-related variables were analyzed by repeated-measures mixed-model ANOVA. Contrasts of interest were compared with Student's t test. P < 0.05 was considered to indicate significant differences.
RESULTS
Fasting plasma glucose and glucagon concentrations
Fasting plasma glucagon concentrations were similar in the two groups when they were sampled, after an overnight fast, at the screening visits but were lower (P = 0.0007 and 0.0278) in the patients with type 1 diabetes after intravenous insulin infusion to maintain near-euglycemia overnight (Table 1).
Table 1.
Fasting plasma glucose and glucagon concentrations in patients with type 1 diabetes and nondiabetic individuals sampled at the screening visit and at 0 min in the mixed meal and glimepiride studies after intravenous insulin infusion to maintain near-euglycemia overnight in the patients with type 1 diabetes
| Type 1 diabetes | Nondiabetic | |
|---|---|---|
| n | 24 | 20 |
| Plasma glucose (mmol/l) | ||
| Screening | 7.7 ± 0.7 | 4.7 ± 0.1 |
| Mixed-meal study | 5.6 ± 0.2 | 4.9 ± 0.1 |
| Glimepiride study | 6.0 ± 0.2 | 4.9 ± 0.1 |
| Plasma glucagon (pmol/l) | ||
| Screening | 27 ± 1 | 27 ± 1 |
| Mixed-meal study | 22 ± 1* | 27 ± 1 |
| Glimepiride study | 24 ± 1† | 28 ± 1 |
Data are means ± SEM. To convert glucose to mg/dl divide by 0.05551, glucagon to pg/ml divide by 0.2871.
*P = 0.0007.
†P = 0.0278.
Plasma glucose, C-peptide, insulin, and epinephrine
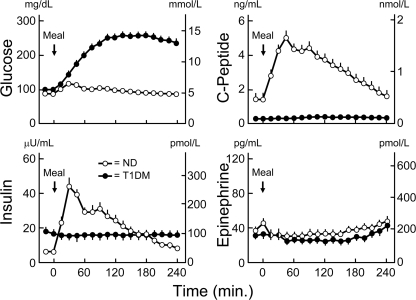
In the patients with type 1 diabetes, plasma C-peptide concentrations remained low, plasma insulin concentrations were constant, and plasma glucose concentrations increased from 5.6 ± 0.2 mmol/l (101 ± 3 mg/dl) to a peak of 14.3 ± 0.6 mmol/l (257 ± 11 mg/dl) after ingestion of the mixed meal (Fig. 1). In the nondiabetic individuals, plasma glucose concentrations increased from 4.9 ± 0.0 mmol/l (88 ± 1 mg/dl) to a peak of 6.6 ± 0.2 mmol/l (118 ± 3 mg/dl) at 30 min, plasma C-peptide concentrations increased from 0.5 ± 0.9 nmol/l (1.4 ± 0.1 ng/ml) to a peak of 1.6 ± 0.1 nmol/l (5.0 ± 0.4 ng/ml) at 45 min, and plasma insulin concentrations increased from 36 ± 6 pmol/l (6 ± 1 μU/ml) to a peak of 264 ± 30 pmol/l (44 ± 5 μU/ml) at 30 min (Fig. 1). The plasma glucose, C-peptide, and insulin levels differed between the patients and control subjects (all P < 0.0001). Plasma epinephrine concentrations were low and similar (Fig. 1).
Figure 1.
Means ± SEM plasma glucose, C-peptide, insulin, and epinephrine concentrations before and after ingestion of a mixed meal in basal insulin–infused patients with type 1 diabetes (T1DM) (●) and nondiabetic (ND) individuals (○). Differences in glucose, C-peptide, and insulin levels, P < 0.0001.
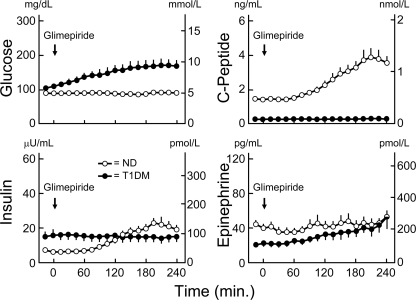
In the patients with type 1 diabetes, plasma C-peptide concentrations remained low, plasma insulin concentrations were constant, and plasma glucose concentrations increased gradually from 6.0 ± 0.2 mmol/l (108 ± 3 mg/dl) to a peak of 9.4 ± 0.9 mmol/l (169 ± 17 mg/dl) at 225 min after ingestion of glimepiride (Fig. 2). In the nondiabetic individuals, glucose was infused to prevent hypoglycemia (the lowest mean plasma glucose concentration was 4.6 ± 0.9 mmol/l [84 ± 1 mg/dl] at 165 min), plasma C-peptide concentrations increased from 0.5 ± 0.9 nmol/l (1.4 ± 0.1 ng/ml) to a peak of 1.3 ± 0.2 nmol/l (3.8 ± 0.6 ng/ml) at 210 min, and plasma insulin concentrations increased from 36 ± 6 pmol/l (6 ± 1 μU/ml) to a peak of 138 ± 24 pmol/l (23 ± 4 μU/ml) at 195 min (Fig. 2). The plasma glucose, C-peptide, and insulin levels differed between the patients and the control subjects (all P < 0.0001). Plasma epinephrine concentrations were low and similar (Fig. 2).
Figure 2.
Means ± SEM plasma glucose, C-peptide, insulin, and epinephrine concentrations before and after ingestion of the sulfonylurea glimepiride in basal insulin–infused patients with type 1 diabetes (T1DM) (●) and nondiabetic (ND) individuals (○), the latter infused with glucose intravenously to prevent hypoglycemia. Differences in insulin, C-peptide, and insulin levels, P < 0.0001.
Plasma glucagon
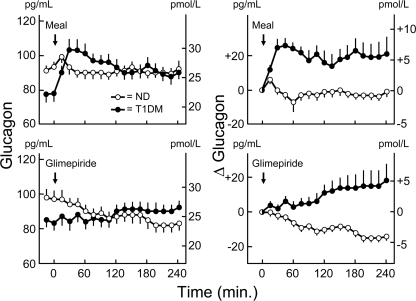
In the patients with type 1 diabetes, plasma glucagon concentrations increased from 22 ± 1 pmol/l (78 ± 4 pg/ml) to a peak of 30 ± 2 pmol/l (103 ± 7 pg/ml) at 30 min after ingestion of the mixed meal and from 24 ± 1 pmol/l (83 ± 4 pg/ml) to a peak of 26 ± 1 pmol/l (91 ± 4 pg/ml) at 135 min after ingestion of glimepiride (Fig. 3). In the nondiabetic individuals, plasma glucagon concentrations tended to decline from 27 ± 1 pmol/l (93 ± 3 pg/ml) to 26 ± 1 pmol/l (89 ± 3 pg/ml) at 105 min after ingestion of the mixed meal and decreased from 28 ± 1 pmol/l (97 ± 5 pg/ml) to a nadir of 24 ± 1 pmol/l (82 ± 4 pg/ml) at 195 min after ingestion of glimepiride (Fig. 3). The baseline-adjusted plasma glucagon levels differed between the patients and control subjects (both P < 0.0001).
Figure 3.
Means ± SEM plasma glucagon concentrations (left panels) and change in plasma glucagon from zero time (right panels) before and after ingestion of a mixed meal (top panels) and of the sulfonylurea glimepiride (bottom panels) in basal insulin–infused patients with type 1 diabetes (T1DM) (●) and nondiabetic (ND) individuals (○). Differences in baseline-adjusted glucagon levels, P < 0.0001.
Other neuroendocrine and metabolic parameters
Plasma norepinephrine concentrations were unchanged after the mixed meal and glimepiride and did not differ between the groups (data not shown). After the mixed meal, plasma pancreatic polypeptide concentrations increased from 25 ± 4 pmol/l (105 ± 17 pg/ml) to a peak of 59 ± 9 pmol/l (246 ± 39 pg/ml) at 30 min in the patients with type 1 diabetes and from 29 ± 3 pmol/l (123 ± 14 pg/ml) to a peak of 67 ± 9 pmol/l (279 ± 36 pg/ml) at 15 min in the nondiabetic individuals; they were unchanged after glimepiride. Pancreatic polypeptide levels did not differ between the patients and control subjects. Plasma cortisol and growth hormone concentrations also did not differ between the patients and control subjects under either condition (data not shown).
After ingestion of the mixed meal, blood lactate concentrations increased from 784 ± 93 to 1,071 ± 160 μmol/l at 120 min in the patients with type 1 diabetes and from 660 ± 56 to 1,590 ± 127 μmol/l at 75 min in the nondiabetic individuals (P = 0.0221). Lactate levels were unchanged and similar in both groups after ingestion of glimepiride. Serum nonesterified fatty acid concentrations were unaltered in the patients after the mixed meal and glimepiride but were suppressed from 646 ± 64 to 210 ± 23 μmol/l at 75 min and from 786 ± 92 to 310 μmol/l at 195 min, respectively, under the two conditions in the control subjects (both P < 0.0001).
CONCLUSIONS
We tested the hypothesis that increased β-cell–mediated signaling of α-cells negates direct α-cell signaling in the regulation of glucagon secretion in humans. To do so, we contrasted the plasma glucagon responses to the ingestion of a mixed meal and sulfonylurea glimepiride, conditions that normally stimulate both β-cells and α-cells, in nondiabetic individuals and patients with type 1 diabetes, i.e., in individuals with normal and virtually absent β-cell function, respectively. The data support our hypothesis. Plasma glucagon concentrations were unchanged or decreased in the nondiabetic individuals with intact β-cell function but were increased in the patients with β-cell failure after ingestion of both the mixed meal and glimepiride.
We attribute these dichotomous plasma glucagon concentration responses to the presence and virtual absence of β-cell function in the nondiabetic individuals and the patients with type 1 diabetes, respectively. It is likely that that was not the only difference between the two groups. For example, appropriate provocative testing would most likely have demonstrated reduced autonomic (adrenomedullary, sympathetic, and parasympathetic) responses in the patients with type 1 diabetes (24). However, reduced autonomic responses, which are all normally stimulatory (8), could not explain the observed increased glucagon responses (8,9). In the present study, plasma epinephrine and norepinephrine concentrations (measures of sympathoadrenal activity) and plasma pancreatic polypeptide concentrations (a measure of parasympathetic activity) were comparable in both groups under both conditions. Therefore, differences in autonomic signaling could not explain the observed differences in the glucagon responses. Among the gut incretins, glucagon-like peptide-1 (GLP-1), but not glucose-dependent insulinotropic polypeptide (GIP), normally suppresses glucagon secretion (10). Thus, reduced GLP-1, but not GIP, actions in patients with diabetes could explain less suppression of glucagon secretion after a mixed meal. However, that suppression would not explain the observed increased glucagon responses to a mixed meal or those to glimepiride in the patients. Thus, we conclude that our data are consistent with the interpretation that indirect reciprocal β-cell–mediated signaling predominates over direct α-cell signaling in the regulation of glucagon secretion in humans. That conclusion does not exclude an additional role for δ-cell somatostatin.
The relative contributions of the potential β-cell secretory products that normally restrain α-cell glucagon secretion (2) remain to be clarified. That issue is of considerable biological interest. However, the identity of the relevant β-cell secretory products is not critical to the interpretation of our evidence that indirect reciprocal β-cell–mediated signaling, whatever the signaling molecules, predominates over direct α-cell signaling in the regulation of glucagon secretion.
The significantly lower baseline plasma glucagon concentrations in the patients with type 1 diabetes on both the mixed-meal and the glimepiride study occasions were most likely the result of the intravenous insulin infusions used to maintain near-euglycemia overnight. Fasting plasma glucagon concentrations were similar in the patients with type 1 diabetes and in the nondiabetic individuals sampled after an overnight fast in the absence of overnight insulin infusion in the patients. These findings document the fact that replacement doses of exogenous insulin or one of the components of the insulin preparation suppress glucagon secretion. Notably, however, the lower baseline plasma glucagon concentrations did not preclude the finding of an enhanced glucagon response to both stimuli.
The α-cell actions of sulfonylureas are complex. For example, low concentrations of tolbutamide stimulate glucagon release from mouse islets, whereas high concentrations are inhibitory (1). In humans, intravenous tolbutamide, which stimulated β-cell secretion, neither raised nor lowered basal plasma glucagon concentrations (15,17) but prevented the glucagon response to hypoglycemia (15). In the present study, the sulfonylurea glimepiride raised plasma glucagon concentrations in the virtual absence of β-cell function, implying a direct α-cell stimulatory effect.
This study was not designed to assess the impact of glucagon on glycemia in type 1 diabetes. Plasma glucose concentrations increased substantially after ingestion of the mixed meal in the patients with type 1 diabetes. That result is plausibly attributable to the absence of an increase in insulin secretion; an additional effect of the documented increase in glucagon secretion cannot be excluded. Plasma glucose concentrations rose after ingestion of glimepiride in the patients with type 1 diabetes despite ongoing insulin infusion. Although it is conceivable that the documented increase in glucagon secretion contributed to that increase in plasma glucose, the temporal pattern suggests that an additional, unidentified factor was involved. The possibility of imperfect insulin replacement remains.
The finding of predominant β-cell regulation of α-cell glucagon secretion has clinical implications. Given that a decrease in an intraislet β-cell secretory product, in concert with low α-cell glucose concentrations, normally signals an increase in α-cell glucagon secretion during hypoglycemia (15–18), it follows that, because it stems fundamentally from β-cell failure, the pathophysiology of glucose counterregulation is the same in type 1 diabetes and advanced type 2 diabetes, albeit with different time courses (24): in the setting of a period of therapeutic hyperinsulinemia and the resulting falling plasma glucose concentrations, β-cell failure causes neither a decrease in insulin secretion nor an increase in glucagon secretion leading to an episode of hypoglycemia; the latter causes an attenuated sympathoadrenal response to subsequent hypoglycemia and, thus, the clinical syndromes of defective glucose counterregulation and hypoglycemia unawareness, the components of hypoglycemia-associated autonomic failure in diabetes (24).
Predominant β-cell regulation of α-cell glucagon secretion may also be relevant to the pathogenesis of postprandial as well as postabsorptive hyperglycemia in type 1 diabetes and in type 2 diabetes (14) if failure of postprandial suppression of glucagon plays an important role (25). Oral glucose tolerance tests in individuals with normal glucose tolerance, impaired glucose tolerance, and type 2 diabetes documented an inverse relationship between the suppression of plasma glucagon and plasma glucose concentrations (25). Given the present findings, it is conceivable that diminished early β-cell secretion accounted for both progressively increased plasma glucose concentrations and progressively decreased suppression of plasma glucagon concentrations with progressive deterioration of glucose tolerance.
In summary, these data indicate that, among the array of signals, indirect reciprocal β-cell–mediated signaling of α-cells predominates over direct α-cell signaling in the regulation of glucagon secretion in humans.
Acknowledgments
This study was supported in part by National Institutes of Health Grants R37-DK-27085 and UL1-RR-24992 and by a fellowship award from the American Diabetes Association.
P.E.C. has served as a consultant to MannKind Corp., Marcadia Biotech, and Merck & Co. No other potential conflicts of interest relevant to this article were reported.
Parts of this study were presented in abstract form at the 68th Scientific Sessions of the American Diabetes Association, San Francisco, California, 6–10 June 2008.
We acknowledge the assistance of the staff of the Washington University Clinical Research Unit in the performance of this study; the technical assistance of Krishan Jethi, Michael Morris, Zina Lubovich, Laura Karsteter, Nalima Parikh, Tanya Eden, and Melissa McKenna; and the assistance of Janet Dedeke in the preparation of this article.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. MacDonald PE, De Marinis YZ, Ramracheya R, Salehi A, Ma X, Johnson PR, Cox R, Eliasson L, Rorsman P: A KATP channel-dependent pathway within α-cells regulates glucagon release from both rodent and human islets of Langerhans. PLoS Biol 2007; 5: 1236– 1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gromada J, Franklin I, Wollheim CB: α-Cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr Rev 2007; 28: 84– 116 [DOI] [PubMed] [Google Scholar]
- 3. Maruyama H, Hisatomi A, Orci L, Grodsky GM, Unger RH: Insulin within islets is a physiologic glucagon release inhibitor. J Clin Invest 1984; 74: 2296– 2299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Samols E, Stagner JI, Ewart RBL, Marks V: The order of islet microvascular cellular perfusion is B→A→D in the perfused rat pancreas. J Clin Invest 1988; 82: 350– 353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brunicardi FC, Kleinman R, Moldovan S, Nguyen TH, Watt PC, Walsh J, Gingerich R: Immunoneutralization of somatostatin, insulin and glucagon causes alterations in islet cell secretion in the isolated perfused human pancreas. Pancreas 2001; 23: 302– 308 [DOI] [PubMed] [Google Scholar]
- 6. Zhou H, Tran PO, Yang S, Zhang T, LeRoy E, Oseid E, Robertson RP: Regulation of α-cell function by the β-cell during hypoglycemia in Wistar rats: the “switch-off hypothesis.” Diabetes 2004; 53: 1482– 1487 [DOI] [PubMed] [Google Scholar]
- 7. Hauge-Evans AC, King AJ, Carmignac D, Richardson CC, Robinson IC, Low MJ, Christie MR, Persaud SJ, Jones PM: Somatostatin secreted by islet δ-cells fulfills multiple roles as a paracrine regulator of islet function. Diabetes 2009; 58: 403– 411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Taborsky GJ, Jr, Ahrén B, Havel PJ: Autonomic mediation of glucagon secretion during hypoglycemia. Diabetes 1998; 47: 995– 1005 [DOI] [PubMed] [Google Scholar]
- 9. Marty N, Dallaporta M, Thorens B: Brain glucose sensing, counterregulation, and energy homeostasis. Physiology (Bethesda) 2007; 22: 241– 251 [DOI] [PubMed] [Google Scholar]
- 10. Holst JJ: The physiology of glucagon-like peptide 1. Physiol Rev 2007; 87: 1409– 1439 [DOI] [PubMed] [Google Scholar]
- 11. Diem P, Redmon JB, Abid M, Moran A, Sutherland DE, Halter JB, Robertson RP: Glucagon, catecholamine and pancreatic polypeptide secretion in type 1 diabetic recipients of pancreas allografts. J Clin Invest 1990; 86: 2008– 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sherck SM, Shiota M, Saccomando J, Cardin S, Allen EJ, Hastings JR, Neal DW, Williams PE, Cherrington AD: Pancreatic response to mild non-insulin-induced hypoglycemia does not involve extrinsic neural input. Diabetes 2001; 50: 2487– 2496 [DOI] [PubMed] [Google Scholar]
- 13. Gerich JE, Langlois M, Noacco C, Karam JH, Forsham PH: Lack of glucagon response to hypoglycemia in diabetes: evidence for an intrinsic pancreatic α cell defect. Science 1973; 182: 171– 173 [DOI] [PubMed] [Google Scholar]
- 14. Meier JJ, Kjems LL, Veldhuis JD, Lefèbvre P, Butler PC: Postprandial suppression of glucagon secretion depends on intact pulsatile insulin secretion: further evidence for the intraislet insulin hypothesis. Diabetes 2006; 55: 1051– 1056 [DOI] [PubMed] [Google Scholar]
- 15. Banarer S, McGregor VP, Cryer PE: Intraislet hyperinsulinemia prevents the glucagon response to hypoglycemia despite an intact autonomic response. Diabetes 2002; 51: 958– 965 [DOI] [PubMed] [Google Scholar]
- 16. Gosmanov NR, Szoke E, Israelian Z, Smith T, Cryer PE, Gerich JE, Meyer C: Role of the decrement in intraislet insulin for the glucagon response to hypoglycemia in humans. Diabetes Care 2005; 28: 1124– 1131 [DOI] [PubMed] [Google Scholar]
- 17. Israelian Z, Gosmanov NR, Szoke E, Schorr M, Bokhari S, Cryer PE, Gerich JE, Meyer C: Increasing the decrement in insulin secretion improves glucagon responses to hypoglycemia in advanced type 2 diabetes. Diabetes Care 2005; 28: 2691– 2696 [DOI] [PubMed] [Google Scholar]
- 18. Raju B, Cryer PE: Loss of the decrement in intraislet insulin plausibly explains loss of the glucagon response to hypoglycemia in insulin-deficient diabetes. Diabetes 2005; 54: 757– 764 [DOI] [PubMed] [Google Scholar]
- 19. Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren P-O, Caicedo A: The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci U S A 2006; 103: 2334– 2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brown RJ, Sinaii N, Rother KI: Too much glucagon, too little insulin. Diabetes Care 2008; 31: 1403– 1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pörksen S, Nielsen LB, Kaas A, Kocova M, Chiarelli F, Orskov C, Holst JJ, Ploug KB, Hougaard P, Hansen L, Mortensen HB. Hvidøre Study Group on Childhood Diabetes. Meal-stimulated glucagon release is associated with blood glucose level and does not interfere with glycemic control in children and adolescents with new-onset type 1 diabetes. J Clin Endocrinol Metab 2007; 92: 2910– 2916 [DOI] [PubMed] [Google Scholar]
- 22. Bohannon NV, Lorenzi M, Grodsky GM, Karam JH: Stimulatory effects of tolbutamide infusion on plasma glucagon in insulin-dependent diabetic subjects. J Clin Endorinol Metab 1982; 54: 459– 462 [DOI] [PubMed] [Google Scholar]
- 23. Østergård T, Degn KB, Gall M-A, Carr RD, Veldhuis JD, Thomsen MK, Rizza RA, Schmitz O: The insulin secretagogues glibenclamide and repaglinide do not influence growth hormone secretion in humans but stimulate glucagon secretion during profound insulin deficiency. J Clin Endocrinol Metab 2004; 89: 297– 302 [DOI] [PubMed] [Google Scholar]
- 24. Cryer PE: The barrier of hypoglycemia in diabetes. Diabetes 2008; 57: 3169– 3176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Abdul-Ghani M, DeFronzo RA: Fasting hyperglycemia impairs glucose-but not insulin-mediated suppression of glucagon secretion. J Clin Endocrinol Metab 2007; 92: 1778– 1784 [DOI] [PubMed] [Google Scholar]