Abstract
OBJECTIVE
To compare the effect of a high-fat, high-carbohydrate meal (HFHC) with that of a high-fiber and fruit meal on the concentrations of endotoxin (lipopolysaccharide [LPS]), LPS-binding protein (LBP), the expression of toll-like receptors (TLRs), and the suppressor of cytokine signaling-3 (SOCS-3) in mononuclear cells.
RESEARCH DESIGN AND METHODS
Healthy lean subjects were given 910 calories of either an HFHC meal (n = 10) or an American Heart Association (AHA)-recommended meal rich in fiber and fruit (n = 10) after an overnight fast. Blood was collected before and at 1, 2, and 3 h after the meal. Cellular indexes of oxidative and inflammatory stress; the expression of SOCS-3, TLR2, and TLR4 in mononuclear cells; and plasma concentrations of LPS and LBP were measured.
RESULTS
HFHC meal intake induced an increase in plasma LPS concentration and the expression of SOCS-3, TLR2, and TLR4 protein, reactive oxygen species generation, and nuclear factor-κB binding activity (P < 0.05 for all). These increases were totally absent after the AHA meal rich in fiber and fruit.
CONCLUSIONS
The novel changes described after the HFHC meal elucidate further the mechanisms underlying postprandial inflammation and also provide the first evidence explaining the pathogenesis of insulin and leptin resistance mediated by SOCS-3 after such meals. In contrast, an AHA meal does not induce these effects.
We have previously shown that macronutrients including glucose and cream and high-fat, high-carbohydrate (HFHC) meals induce inflammation and oxidative stress, as reflected in increased reactive oxygen species (ROS) generation, increased expression of p47phox, an NADPH oxidase subunit, and nuclear factor (NF)-κB binding and other proinflammatory mediators in mononuclear cells (MNCs) and plasma in normal subjects (1). In contrast, ROS generation by MNCs and other indexes of oxidative damage in the body fall with short-term caloric restriction (2) and weight loss in human obesity over a period of 4 weeks (3). We have also shown that a 48-h fast in normal subjects reduces ROS generation by MNCs, p47phox expression, and oxidative damage of phenylalanine (3). Caloric restriction and weight loss over longer periods of time also result in a reduction in the concentrations of proinflammatory cytokines and C-reactive protein (CRP) (3).
Toll-like receptors (TLRs) are a variety of pathogen pattern recognition receptors that recognize pathogen-associated molecular patterns from bacterial and viral products and other pathogens (4). TLR4 recognizes endotoxin (lipopolysaccharide [LPS]). On the basis of its interaction with LPS, TLR4 may be a mediator and a modulator of endotoxin-induced inflammation and shock. TLR4 has also been shown to play an important role in the pathogenesis of atherosclerosis (5), diet-induced obesity, and related insulin resistance (6). TLR2, in a heterodimeric association with TLR1 or TLR6, recognizes certain lipopeptides, peptidoglycans, and other lipid moieties derived from gram-positive bacteria (7). Both TLR4 and TLR2 are expressed in atherosclerotic plaques (8).
Recent work has also demonstrated that the expression of TLR2 and TLR4 is increased in patients with type 1 and type 2 diabetes (9,10). In addition, it has also been shown that the plasma concentration of LPS is significantly higher in these patients and that its concentration is related to plasma insulin concentration and insulin resistance (9). High-fat diet–induced insulin resistance and obesity are known to be TLR4 dependent, such that TLR4 deletion protects mice from NF-κB–mediated inflammation and the development of insulin resistance (6). We have recently shown that a low-dose insulin infusion in type 2 diabetic subjects caused a significant suppression of TLR1, -2, -4, -7, and -9 mRNA expression and the suppression of PU.1, a major transcription factor that regulates the expression of many TLRs in addition to exerting its known anti-inflammatory action (11). However, the possibility that food intake may either increase TLR expression or increase plasma endotoxin concentration has not been investigated in humans.
Suppressor of cytokine signaling-3 (SOCS-3) has been shown to interfere with insulin and leptin signal transduction (12,13) in experimental animals. Proinflammatory cytokines, tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6, induce an increase in the expression of SOCS-3 in animal models (13). Our recent work has shown that SOCS-3 expression in the circulating MNCs of the obese humans is markedly increased in parallel with other indexes of inflammation and insulin resistance when compared with that in normal subjects (14,15).
Because macronutrient intake causes inflammation and oxidative stress, we have now hypothesized that the intake of an HFHC meal induces SOCS-3, TLR2, and TLR4 in parallel with the induction of cellular oxidative stress and inflammation and that this is associated with an increase in plasma endotoxin (LPS) and LPS-binding protein (LBP) concentrations. In addition, we hypothesized that a meal based on fiber and fruit, as recommended by the American Heart Association (AHA), does not induce inflammation or an increase in the expression of TLRs and SOCS-3 and the plasma concentrations of LPS or LBP.
RESEARCH DESIGN AND METHODS
Two groups (n = 10 each) of normal healthy lean (BMI <25 kg/m2) subjects 20–50 years old were included in the study. Subjects from both groups had comparable mean age, BMI, and sex distribution. After an overnight fast, a baseline blood sample was collected. Subjects from the first group (five males, BMI 23.1 ± 0.6 kg/m2, mean age 32.4 ± 1.3 years) were asked to ingest a 910-calorie HFHC meal (egg muffin and sausage muffin sandwiches and two hash browns, which contain 88 g carbohydrates, 51 g fat [33% saturated] and 34 g protein [carbohydrates 41%, protein 17%, and fat 42%]), while subjects from the second group (six males, BMI 22.8 ± 0.6 kg/m2, mean age 31.2 ± 1.1 years) were given an isocaloric meal rich in fruit and fiber consisting of oatmeal, milk, orange juice, raisins, peanut butter, and English muffin (carbohydrates 58%, protein 15%, and fat 27%) as recommended by the AHA. This meal has been termed the “AHA meal” in the subsequent text of this article. Blood samples were collected at 0, 1, 2, and 3 h. None of the subjects were smokers, and those who drank alcohol had <30 ml/day. The study was approved by the human research committee of the State University of New York at Buffalo. Each informed participant signed a consent form.
MNC isolation
Blood samples were collected in Na-EDTA as an anticoagulant. An anticoagulated blood sample (3.5 ml) was carefully layered over 3.5 ml polymorphonuclear leukocyte (PMN) medium (Cedarlane Laboratories, Hornby, Ontario, Canada). Samples were centrifuged, and at the end of the centrifugation, two bands separate out at the top of the red blood cell pellet. The MNC band was harvested and washed twice with Hank's balanced salt solution. This method yields >95% pure PMN and MNC suspensions.
ROS generation assay
A total of 500 μl PMN or MNC (2 × 105 cells) were delivered into a Chrono-log Lumi-Aggregometer plastic flat-bottom cuvette. A total of 15 μl of 10 mmol/l luminol was then added, followed by 1.0 μl of 10 mmol/l formylmethionyl leucinyl phenylalanine (fMLP). Chemiluminescence was recorded for 15 min. Our method, developed independently, is similar to that published by Tosi and Hamedani (16) and correlated with that measured by the ferricytochrome C method (16). Intra- and interassay variations in ROS generation are <10% for readings obtained 1–2 weeks apart in normal healthy subjects.
Western blotting and NF-κB–binding activity
Total cell lysates and nuclear extracts were prepared from freshly isolated MNCs. Consensus sequence for electromobility shift assay for NF-κB–binding activity was used (Santa Cruz Biotechnology, Santa Cruz, CA). Monoclonal antibodies against p47phox (BD Biosciences), TLR2, TLR4, CD14, and SOCS-3 (Abcam, Cambridge, MA) and a polyclonal antibody against actin (Santa Cruz Biotechnology) were used, and the membranes were developed using super signal west-femto, chemiluminescence reagent (Pierce Chemical, Rockford, IL). Densitometry was performed using molecular analyst software (Bio-Rad, Hercules, CA), and all values were corrected for loading with actin.
LPS, LBP, and proinflammatory mediator measurements in plasma
Plasma LPS concentration was measured by a commercially available kit (Cambrex Limulus Amebocyte Lysate [LAL] kit; Lonza, Walkersville, MD). This assay has a sensitivity range of 0.1–1.0 endotoxin unit (EU)/ml. Normal values from lean subjects measured in our laboratory ranged from 0.15 to 0.35 EU/ml. Inter- and intraassay variations for this test were <10%. Plasma samples used for LPS determination were stored in LPS-free glass tubes to prevent loss of endotoxin to plastic tube walls. All materials used for the assay were rendered LPS-free. Plasma was diluted 10-fold and heated to 75°C for 5 min before LPS measurement. LBP was measured using an immunoassay kit from Cell Sciences (Canton, MA) with an intraassay variation of <5% calculated from our results. Plasma matrix metalloproteinase-9 (MMP-9) and TNF-α ELISA kits were purchased from R&D Systems (Minneapolis, MN) with intra- and interassay variations of <5% and <10%, respectively. CRP measurement was done using immunoassay kits from Alpha Diagnostic International (San Antonio, TX) with coefficient of variation for intraassay precision of <5% and interassay precision of <7%.
Determination of LPS content in meals
Ingredients of HFHC and AHA meals were mixed, homogenized, and filtered. Dilutions from meal homogenates were prepared in plasma or LPS-free water to determine the LPS content of each. Plasma was used as a diluent to maintain similar testing medium, as in the post-challenge LPS measurements. LPS concentrations were then measured as described using LAL assay, and change in LPS was calculated (n = 3 each).
Measurement of plasma insulin and free fatty acid concentrations
Insulin was measured from plasma samples using an enzyme-linked immunosorbent assay kit (Diagnostics Systems Laboratories, Webster, TX). Free fatty acid (FFA) concentrations were measured using the Half-Micro calorimetric kit from Roche Diagnostics (Indianapolis, IN). Inter- and intraassay variations for the FFA test were <10%.
Statistical analysis
Statistical analysis was carried out using SigmaStat software (Systat Software, San Jose, CA). Data are presented as means ± SE. The percent change is calculated from the means of the groups. Percent change from baseline was calculated, and statistical analysis was carried out using Holm-Sidak one-way repeated-measures ANOVA (RMANOVA). Dunnett two-factor RMANOVA method was used for comparisons between the two groups. Correlation analysis was performed using Spearman rank-order correlation between changes in TLRs and SOCS-3 protein and markers of oxidative stress and inflammation.
RESULTS
Effect of HFHC and AHA meals on glucose, insulin, and lipid concentrations
The intake of an HFHC meal induced a significant increase in glucose concentration at 1, 2, and 3 h. However, the AHA meal did not induce an increase at these times. In fact, there was a tendency for the glucose concentration to fall. However, there was a similar increase in insulin concentrations after both meals (Table 1). Both the HFHC and AHA meals increased triglyceride concentrations. The increase after the HFHC meal was significantly greater. FFA concentrations fell significantly after both meals.
Table 1.
Change in metabolic profile after a 910-calorie AHA or HFHC meal in normal subjects
| Mealtime | 0 h | 1 h | 2 h | 3 h | |
|---|---|---|---|---|---|
| Glucose (mg/dl) | AHA | 79 ± 2 | 75 ± 3 | 73 ± 4 | 73 ± 3 |
| HFHC | 82 ± 3 | 92 ± 6*† | 88 ± 5† | 89 ± 3 | |
| Insulin (μIU/ml) | AHA | 7.7 ± 1.2 | 44.8 ± 8.1* | 34.9 ± 6.4* | 21.0 ± 5.8* |
| HFHC | 6.8 ± 0.9 | 49.9 ± 10.5* | 31.3 ± 8.1* | 24.1 ± 5.6* | |
| Cholesterol (mg/dl) | AHA | 149 ± 13 | 153 ± 14 | 146 ± 13 | 150 ± 14 |
| HFHC | 165 ± 9 | 162 ± 8 | 161 ± 9 | 161 ± 10 | |
| Triglycerides (mg/dl) | AHA | 95 ± 12 | 104 ± 11 | 138 ± 14* | 158 ± 16* |
| HFHC | 107 ± 12 | 127 ± 14* | 160 ± 11* | 191 ± 8*† | |
| FFA (mmol/l) | AHA | 0.36 ± 0.04 | 0.18 ± 0.03* | 0.20 ± 0.04* | 0.24 ± 0.04* |
| HFHC | 0.35 ± 0.05 | 0.19 ± 0.03* | 0.24 ± 0.04* | 0.29 ± 0.04 | |
| MNC ROS generation (mV) | AHA | 169 ± 26 | 201 ± 30 | 198 ± 31 | 182 ± 27 |
| HFHC | 224 ± 39 | 264 ± 39 | 408 ± 108*† | 323 ± 63*† | |
| PMN ROS generation (mV) | AHA | 182 ± 32 | 204 ± 45 | 196 ± 49 | 131 ± 22 |
| HFHC | 179 ± 30 | 214 ± 28 | 302 ± 57*† | 222 ± 44† | |
| TBARS (nmol/l) | AHA | 0.98 ± 0.11 | 1.10 ± 0.12 | 0.92 ± 0.09 | 0.96 ± 0.12 |
| HFHC | 1.19 ± 0.10 | 1.44 ± 0.12* | 1.53 ± 0.11*† | 1.66 ± 0.09*† | |
| P47phox protein (%) | AHA | 100 | 88 ± 7* | 94 ± 10 | 94 ± 7 |
| HFHC | 100 | 131 ± 12*† | 131 ± 13*† | 134 ± 7*† | |
| NF-κB binding (%) | AHA | 100 | 113 ± 7 | 120 ± 9 | 120 ± 11 |
| HFHC | 100 | 132 ± 11 | 177 ± 18*† | 157 ± 14*† | |
| MMP-9 (ng/ml) | AHA | 259 ± 18 | 284 ± 28 | 279 ± 18 | 282 ± 26 |
| HFHC | 269 ± 28 | 312 ± 30 | 323 ± 31* | 349 ± 31*† | |
| TNF-α (pg/ml) | AHA | 1.87 ± 027 | 1.79 ± 0.25 | 1.82 ± 0.32 | 1.80 ± 0.31 |
| HFHC | 2.09 ± 0.32 | 1.933 ± 0.27 | 2.00 ± 0.30 | 1.96 ± 0.27 | |
| CRP (μg/ml) | AHA | 1.40 ± 0.3 | 1.45 ± 0.2 | 1.47 ± 0.3 | 1.50 ± 0.3 |
| HFHC | 1.43 ± 0.2 | 1.43 ± 0.2 | 1.52 ± 0.2 | 1.54 ± 0.2 |
Data are means ± SE. n = 10 each.
*P < 0.05 vs. baseline with one-way RMANOVA;
†P < 0.05 between groups with two-way RMANOVA.
Effect of HFHC and AHA meals on oxidative stress
ROS generation by MNC and PMN increased significantly by a peak of 78 ± 20% and 65 ± 23% over the baseline, respectively, 2 h after the HFHC meal (Table 1). NADPH oxidase subunit, p47phox, also increased significantly at 1 h by 32 ± 18% over the baseline (P < 0.05) after the HFHC meal and continued thereafter (Table 1). Thiobarbituric acid reactive substance (TBARS) concentrations increased gradually and significantly after the HFHC meal and reached 43 ± 17% above the baseline at 3 h (Table 1). AHA meal intake was not associated with any significant change in ROS generation, p47phox expression, or TBARS.
Effect of HFHC and AHA meals on NF-κB DNA binding in the MNC and plasma MMP-9, CRP, and IL-6 concentrations
DNA binding by NF-κB increased significantly by 72 ± 24% over the baseline (P < 0.05, Table 1) at 2 h after HFHC meal intake, whereas AHA did not cause any significant change in NF-κB (Table 1). HFHC meal induced an increase in MMP-9 concentration, while there was no significant increase in MMP-9 after the AHA meal. In addition, there was no change in plasma CRP and TNF-α concentrations after either meal.
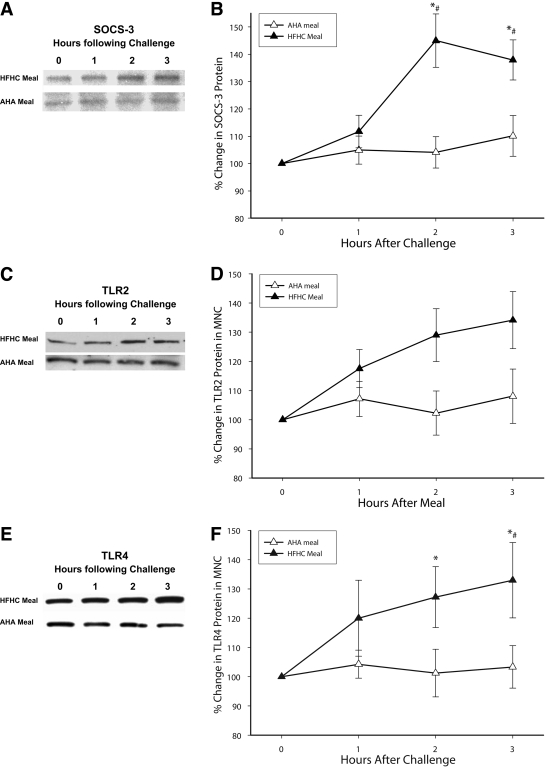
Effect of HFHC and AHA meal intake on SOCS-3, TLR2, and TLR4
SOCS-3, TLR2, and TLR4 expression increased significantly at 2 and 3 h after an HFHC meal (Fig. 1). There was no significant change in these proteins after AHA meal intake. CD14 expression and PU.1 binding did not change after either meal.
Figure 1.
SOCS-3 (A and B), TLR2 (C and D), and TLR4 (E and F) protein levels by Western blotting after an HFHC or AHA meal challenge in normal healthy subjects (n = 10 each). A: Representative SOCS-3 immunoblot in total cell lysate from MNCs. B: SOCS-3 protein densitometry (n = 10 each). C: Representative TLR2 immunoblot in total cell lysate from MNCs. D: TLR2 protein densitometry (n = 10 each). E: Representative TLR4 immunoblot in total cell lysate from MNCs. F: TLR4 protein densitometry (n = 10 each). *P < 0.05 by RMANOVA compared with baseline; #P < 0.05 by two-way RMANOVA comparing groups.
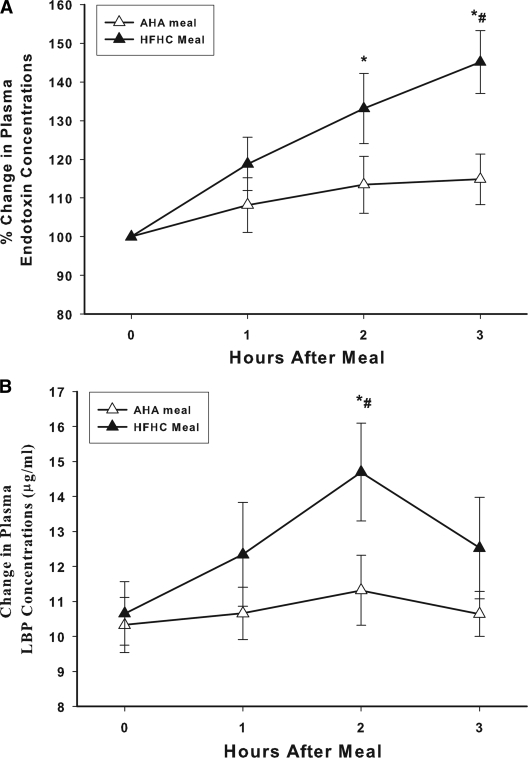
Effect of the HFHC and AHA meals on plasma LPS and LBP concentrations
Plasma endotoxin (LPS) concentrations increased significantly after the intake of the HFHC meal from 0.39 ± 0.07 to 0.58 ± 0.10 EU/ml at 3 h (47 ± 14% over the baseline, P < 0.05) (Fig. 2A). This was associated with an increase in plasma levels of LBP by 34 ± 14% over the baseline at 2 h (from 10.5 ± 1.5 to 14.7 ± 2.2 μg/ml, P < 0.05) (Fig. 2B). The intake of the AHA meal did not induce any significant change in either LPS or LBP plasma concentrations. The total LPS content of the HFHC and AHA meals measured from meal homogenates was 4,200 and 5,700 EU, respectively.
Figure 2.
Plasma endotoxin (A) and LBP (B) concentrations after HFHC or AHA meal challenges in normal healthy subjects (n = 10 each). Plasma endotoxin levels were measured by LAL assay. Basal endotoxin concentrations in the HFHC group were 0.39 ± 0.07 EU/ml and in AHA 0.29 ± 0.07 EU/ml. *P < 0.05 RMANOVA compared with baseline; #P < 0.05 by two-way RMANOVA comparing groups.
Relationships between SOCS-3 and TLR expression and indexes of oxidative stress and inflammation
The maximum percent increase in TLR2 protein (at 3 h) was significantly related to maximal changes in NF-κB and ROS generation in the MNCs at 2 h (r = 0.414, P < 0.01, and r = 0.488, P < 0.01, respectively). There was a trend toward a significant relationship between TLR4 protein and both ROS generation and NF-κB (r = 0.274, P = 0.10, and r = 0.321, P = 0.10, respectively). There was no relationship between SOCS-3 protein levels at 3 h and either NF-κB or ROS generation. The expression of SOCS-3 was significantly related to that of TLR4 (r = 0.612, P = 0.003).
CONCLUSIONS
Our data show clearly for the first time that HFHC meal intake acutely induces an increase in SOCS-3, TLR4, and TLR2 protein expression in parallel with the increase in ROS generation, p47phox expression, NF-κB–binding activity, and plasma MMP-9 concentrations. We have also shown for the first time that the intake of such a meal induces an increase in plasma LPS and LBP concentrations. On the other hand, the intake of the AHA meal, rich in fruit and fiber, did not cause any significant increase in any of these proinflammatory mediators, including the expression of SOCS-3, TLR2, and TLR4. These observations are relevant to the pathogenesis of postprandial oxidative and inflammatory stress, insulin resistance, and atherosclerosis since the cumulative effects of such meals may manifest not only in chronic oxidative and inflammatory stress, but also potentially insulin resistance and atherosclerosis. Whereas chronic intake of high-fat diets has been shown to lead to insulin resistance through an increase in TLR2 and TLR4 expression in experimental animals, there are no data on the effect of a single meal on TLR2, TLR4, or SOCS-3 expression in either humans or experimental animals.
It is relevant that insulin, the hormone secreted in response to macronutrient intake, exerts the opposite effect: it suppresses the expression of TLR4 and TLR2 and exerts a general anti-inflammatory effect and ROS-suppressive effect (11,17). Clearly, the magnitude of insulin increase after an HFHC meal is not able to neutralize the proinflammatory effect of this meal, including the induction of SOCS-3, TLR4, and TLR2. In contrast, a similar insulin response after the fruit and fiber AHA meal is associated with a total absence of postprandial inflammation.
There was also a significantly greater increase in glucose and triglyceride concentrations after the HFHC meal than after the AHA meal. Glucose concentrations after the HFHC meal were still elevated at 1 and 2 h, whereas those after the AHA meal were not significantly different from the baseline. This occurred in spite of the greater amount of carbohydrates in the AHA meal. This phenomenon is intriguing, but we have previously observed it when comparing the effect of glucose with orange juice (18). Insulin increase after the AHA meal was similar to that after an HFHC meal in spite of the fact that the excursion of glucose was significantly greater after the latter meal. Both meals resulted in similar and rapid reductions in FFA concentrations, probably due to insulin release.
It is of interest that PU.1, the major transcription factor regulating TLR gene expression, did not alter after HFHC intake in spite of the increase in the expression of TLR. It is thus likely that the increased protein expression of TLRs 4 and 2 after the HFHC meal is mediated by other transcription factors that need further exploration. Nevertheless, it is relevant that the suppression of TLRs by insulin is associated with a reduction in PU.1 activity (11).
Because the onset of oxidative stress and inflammation occur early after the intake of the HFHC meal, as observed in our data, it is unlikely that the induction of TLRs contributes to the early inflammatory response after their intake. However, it is possible that TLRs may modulate the latter part of the response or modify the response to a subsequent proinflammatory challenge. It would be of interest to determine the duration of the increase in TLR expression in response to macronutrient challenge, including repeated challenge, and also determine the possible increased expression of TLRs in human obesity, the metabolic syndrome, and type 2 diabetes. We have previously shown that the magnitude of oxidative and inflammatory stress after an HFHC meal is greater and more prolonged in the obese when compared with normal subjects (19).
We also observed an increase in the plasma concentration of LPS and LBP after the HFHC meal intake but not after the AHA meal. Whereas the LPS content of the meal probably contributes substantially to the increase in plasma concentrations of LPS, it is possible that some contribution also comes from LPS in the gastrointestinal tract since LPS is fat soluble. In addition, it was recently shown that fat intake leads to increased intestinal permeability for LPS (20). However, it was remarkable that the AHA meal did not alter plasma LPS concentrations in spite of having LPS content similar to that of the HFHC meal.
LBP serves the function of binding and carrying LPS in plasma to CD14, which initially binds to LPS and then presents it to TLR4, which triggers the LPS-induced signal transduction cascade. This results in NF-κB activation and the transcription of proinflammatory genes. The concomitant increase in plasma LPS, LBP, and TLR4 expression is the perfect combination to aggravate inflammation induced by gram-negative organisms. The increase in LBP concentrations after an HFHC meal is of interest since its concentrations have previously been shown to be inversely related to insulin sensitivity (21).
Our observations are relevant to the pathogenesis of diet-induced obesity and insulin resistance since the deletion of TLR4 and TLR2 in mice leads to the prevention of high-fat diet–induced insulin resistance (6,22). Thus, the acute induction of increased TLR2 and TLR4 expression by a single HFHC meal is intriguing and may contribute to insulin resistance after chronic and repetitive intake of such a meal. Our data are consistent with those of Dasu et al. (23), who demonstrated that high concentrations of glucose induce an increase in TLR expression in vitro.
Our data show clearly for the first time that the intake of an HFHC fast-food meal results in a significant increase in the expression of SOCS-3 protein in the circulating MNCs. In contrast, there was no change in SOCS-3 expression after the AHA meal. The induction of SOCS-3 after an HFHC meal, in combination with the fact that its expression is increased in the obese, suggests the possibility that repeated and chronic excessive fat and carbohydrate intake may result in a chronic increase in its expression. We have recently shown that SOCS-3 expression is increased in the obese and is inversely related to the phosphorylation of the insulin receptor and that it is directly related to insulin resistance (homeostasis model assessment–insulin resistance), BMI, and other indexes of inflammation (24).
Our observations are to some extent limited by the fact that we do not have detailed data on the subjects' dietary and exercise habits before their participation in this study. Just as the state of obesity can affect the oxidative and inflammatory stress responses to macronutrient challenge, eating habits and exercise may also do so. However, this fact should not affect the validity of our data since the responses with the HFHC meal and the AHA meal were consistently observed in each participant and the two groups tested were similar.
In conclusion, the intake of an HFHC meal results in an increase in SOCS-3, TLR4, and TLR2 mRNA and protein expression in parallel with an increase in LPS and LBP concentrations and the induction of a comprehensive oxidative and inflammatory stress response characterized by an increase in ROS generation and NF-κB binding in MNCs. The intake of the HFHC meal, therefore, potentially results in the induction of molecules that interfere with insulin and leptin signal transduction, a characteristic of human obesity. In contrast, an equicaloric fruit and fiber meal does not induce these changes. These observations have important implications for the understanding of the pathogenic mechanisms involved in postprandial inflammation, diet-induced obesity, insulin resistance, type 2 diabetes, and atherosclerosis.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Dandona P, Aljada A, Chaudhuri A, Mohanty P, Garg R: Metabolic syndrome: a comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation 2005; 111: 1448– 1454 [DOI] [PubMed] [Google Scholar]
- 2. Raitakari OT, Lai N, Griffiths K, McCredie R, Sullivan D, Celermajer DS: Enhanced peripheral vasodilation in humans after a fatty meal. J Am Coll Cardiol 2000; 36: 417– 422 [DOI] [PubMed] [Google Scholar]
- 3. Dandona P, Aljada A, Bandyopadhyay A: Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol 2004; 25: 4– 7 [DOI] [PubMed] [Google Scholar]
- 4. Janssens S, Beyaert R: Role of toll-like receptors in pathogen recognition. Clin Microbiol Rev 2003; 16: 637– 646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xu XH, Shah PK, Faure E, Equils O, Thomas L, Fishbein MC, Luthringer D, Xu XP, Rajavashisth TB, Yano J, Kaul S, Arditi M: Toll-like receptor-4 is expressed by macrophages in murine and human lipid-rich atherosclerotic plaques and upregulated by oxidized LDL. Circulation 2001; 104: 3103– 3108 [DOI] [PubMed] [Google Scholar]
- 6. Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS: TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 2006; 116: 3015– 3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hallman M, Ramet M, Ezekowitz RA: Toll-like receptors as sensors of pathogens. Pediatr Res 2001; 50: 315– 321 [DOI] [PubMed] [Google Scholar]
- 8. Edfeldt K, Swedenborg J, Hansson GK, Yan ZQ: Expression of toll-like receptors in human atherosclerotic lesions: a possible pathway for plaque activation. Circulation 2002; 105: 1158– 1161 [PubMed] [Google Scholar]
- 9. Creely SJ, McTernan PG, Kusminski CM, Fisher M, Da Silva NF, Khanolkar M, Evans M, Harte AL, Kumar S: Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am J Physiol Endocrinol Metab 2007; 292: E740– E747 [DOI] [PubMed] [Google Scholar]
- 10. Devaraj S, Dasu MR, Rockwood J, Winter W, Griffen SC, Jialal I: Increased toll-like receptor (TLR) 2 and TLR4 expression in monocytes from patients with type 1 diabetes: further evidence of a proinflammatory state. J Clin Endocrinol Metab 2008; 93: 578– 583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ghanim H, Mohanty P, Deopurkar R, Sia CL, Korzeniewski K, Abuaysheh S, Chaudhuri A, Dandona P: Acute modulation of toll-like receptors by insulin. Diabetes Care 2008; 31: 1827– 1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bjorbaek C, El-Haschimi K, Frantz JD, Flier JS: The role of SOCS-3 in leptin signaling and leptin resistance. J Biol Chem 1999; 274: 30059– 30065 [DOI] [PubMed] [Google Scholar]
- 13. Ueki K, Kondo T, Tseng YH, Kahn CR: Central role of suppressors of cytokine signaling proteins in hepatic steatosis, insulin resistance, and the metabolic syndrome in the mouse. Proc Natl Acad Sci U S A 2004; 101: 10422– 10427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ghanim H, Aljada A, Hofmeyer D, Syed T, Mohanty P, Dandona P: Circulating mononuclear cells in the obese are in a proinflammatory state. Circulation 2004; 110: 1564– 1571 [DOI] [PubMed] [Google Scholar]
- 15. Bistrian BR, Khaodhiar L: Chronic systemic inflammation in overweight and obese adults. JAMA 2000; 283: 2235. [PubMed] [Google Scholar]
- 16. Tosi MF, Hamedani A: A rapid, specific assay for superoxide release from phagocytes in small volumes of whole blood. Am J Clin Pathol 1992; 97: 566– 573 [DOI] [PubMed] [Google Scholar]
- 17. Dandona P, Aljada A, Mohanty P, Ghanim H, Hamouda W, Assian E, Ahmad S: Insulin inhibits intranuclear nuclear factor κB and stimulates IκB in mononuclear cells in obese subjects: evidence for an anti-inflammatory effect? J Clin Endocrinol Metab 2001; 86: 3257– 3265 [DOI] [PubMed] [Google Scholar]
- 18. Ghanim H, Mohanty P, Pathak R, Chaudhuri A, Sia CL, Dandona P: Orange juice or fructose intake does not induce oxidative and inflammatory response. Diabetes Care 2007; 30: 1406– 1411 [DOI] [PubMed] [Google Scholar]
- 19. Patel C, Ghanim H, Ravishankar S, Sia CL, Viswanathan P, Mohanty P, Dandona P: Prolonged reactive oxygen species generation and nuclear factor-κB activation after a high-fat, high-carbohydrate meal in the obese. J Clin Endocrinol Metab 2007; 92: 4476– 4479 [DOI] [PubMed] [Google Scholar]
- 20. Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R: Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008; 57: 1470– 1481 [DOI] [PubMed] [Google Scholar]
- 21. Gubern C, Lopez-Bermejo A, Biarnes J, Vendrell J, Ricart W, Fernandez-Real JM: Natural antibiotics and insulin sensitivity: the role of bactericidal/permeability-increasing protein. Diabetes 2006; 55: 216– 224 [PubMed] [Google Scholar]
- 22. Caricilli AM, Nascimento PH, Pauli JR, Tsukumo DM, Velloso LA, Carvalheira JB, Saad MJ: Inhibition of toll-like receptor 2 expression improves insulin sensitivity and signaling in muscle and white adipose tissue of mice fed a high-fat diet. J Endocrinol 2008; 199: 399– 406 [DOI] [PubMed] [Google Scholar]
- 23. Dasu MR, Devaraj S, Zhao L, Hwang DH, Jialal I: High glucose induces toll-like receptor expression in human monocytes: mechanism of activation. Diabetes 2008; 57: 3090– 3098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ghanim H, Aljada A, Daoud N, Deopurkar R, Chaudhuri A, Dandona P: Role of inflammatory mediators in the suppression of insulin receptor phosphorylation in circulating mononuclear cells of obese subjects. Diabetologia 2007; 50: 278– 285 [DOI] [PubMed] [Google Scholar]