Abstract
The small GTPase Ras is mutated to remain in the active oncogenic state in one-third of human cancers, thereby promoting tumorigenesis. It has recently come to light that one consequence of oncogenic Ras signaling is secretion of cytokines vascular endothelial growth factor (VEGF), interleukin 6 (IL6), hCXCL1 (Gro-α) and hCXCL8 (IL8). As the latter two belong to the ELR+ Cys-X-Cys (CXC) chemokine family, we investigated whether the entire family of ELR+ CXC chemokines plays a role in oncogenic Ras-mediated tumorigenesis. We now demonstrate that oncogenic Ras induced the expression and secretion of the ELR+ CXC chemokine family in different tumorigenic human cells and that these chemokines are elevated in tumor specimens. Moreover, genetic ablation of the common receptor for these chemokines, mCXCR2, reduced oncogenic Ras-driven tumorigenesis in mice. Taken together, we suggest that oncogenic Ras induces the secretion of the ELR+ CXC chemokine family to promote tumorigenesis. This chemokine signature may identify the presence of Ras activation in cancer and perhaps even serve as targets for oncogenic Ras-driven tumor cells.
Introduction
One of three Ras small GTPases genes HRAS, NRAS or KRAS acquire a mutation rendering the encoded protein constitutively guanosine triphosphate (GTP) bound and thereby in the active and oncogenic state in one-third of human cancers and as high as 90% of pancreatic cancers (1). Constitutively active GTP-bound Ras binds to and activates a number of proteins that transmit a potent oncogenic signal fostering many tumorigenic phenotypes (2). Mounting evidence supports the notion that oncogenic Ras signaling also induces the secretion of cytokines, which in turn promote tumor growth. Specifically, activated Ras has been shown to upregulate VEGF, which acts on endothelial cells to promote vasculature formation (3,4). Moreover, Ras induces expression of hCXCL8 in HeLa cells infected with activated HRas as assayed by enzyme-linked immunosorbent assay (ELISA) (5). hCXCL8 is an ELR+ chemokine most well known for its ability to recruit neutrophils to sites of inflammation (6). Injection of CXCL8 neutralizing antibodies in a xenograft model led to a decrease in Ras-induced tumorigenesis (5), presumably in a paracrine fashion since the tumor cells lacked the hCXCR1 and hCXCR2 receptors for hCXCL8 (5). Inhibition of hCXCL8 also led to an increase in necrosis consistent with a defect in tumor vasculature and paracrine mechanism of action. It should also be noted that hCXCL8 is upregulated in pancreatic (7), lung (8), melanoma (9), breast (10,11), prostate (12,13) and ovarian (10,11) cancers. Studies in our laboratory have demonstrated a role for interleukin (IL6) in Ras-induced tumorigenesis (14). IL6 acts in a pleiotropic manner eliciting cell survival, angiogenic, as well as metastatic effects on tumors (15). It has been shown by ELISA and reverse transcription–polymerase chain reaction (RT–PCR) that Ras induces expression of IL6 and that knockdown of IL6 with IL6 short hairpin RNA (shRNA) reduces tumorigenic growth of both genetically defined cells as well as in pancreatic cancer cell lines. Furthermore, IL6 null mice show a decrease in dimethylbenzanthracene (DMBA)/12-O-tetradecanoylphorbol 13-acetate (TPA) induced sporadic tumors as compared with wild-type controls (14). As knockdown of IL6 in cells lacking the IL6 receptor reduced tumorigenesis, IL6 presumably acts in a paracrine fashion. Indeed, knockdown of IL6 also resulted in decreased tumor vasculature. As with hCXCL8, IL6 is upregulated in a variety of human cancers, including pancreatic, lung, colorectal, melanoma, breast, prostate and ovarian (16). Studies have also demonstrated that hCXCL1 is upregulated by activated Ras and that this activation is required for proper tumorigenesis (17,18). As with hCXCL8, hCXCL1 is best known as a neutrophil chemoattractant (19). HRas- and KRas- expressing immortalized ovarian cells express elevated levels of hCXCL1 as compared with non-Ras expressing controls (17). Moreover, shRNA-mediated inhibition of hCXCL1 led to a decrease in Ras tumorigenesis in a xenograft model (18). It appears that hCXCL1 also acts in a paracrine manner, although through a different mechanism compared to hCXCL8 or IL6. Increased expression of hCXCL1 led to an increase in senescent fibroblasts in the tumor area and addition of hCXCL1 to the media of cultured fibroblasts induced senescence (18). Lastly, co-injecting such senescent fibroblasts with non-tumorigenic cells into mice actually promoted tumorigenesis.
Of these four chemokines, hCXCL1 and hCXCL8 belong to the ELR+ CXC family of chemokines, comprised of CXCL1 (GRO-α), 2 (GRO-β), 3 (GRO-γ), 5 (ENA-78), 6 (GCP-2), 7 (NAP-2) and 8 (IL-8) in humans or mCXCL1, 2, 3, 5 and 6 in mice. These chemokines are characterized by a canonical Cys-X-Cys (CXC) motif preceded by a Glu-Leu-Arg (ELR) sequence, which promotes an interaction and subsequent activation of primarily the CXCR2 receptor, although some of these chemokines can bind additional receptors (20,21). Activation of receptors by ELR+ CXC chemokines in immune cells can lead to a chemotactic response, recruiting activated cells to the site of chemokine secretion and eliciting a localized immune response, which could facilitate angiogenesis (21,22). Furthermore, CXCR1 and CXCR2 receptors are found on endothelial cells and activation of these receptors has been shown to inhibit apoptosis, induce migration and tube formation in endothelial cells, processes linked to angiogenesis (23).
The ability of ELR+ CXC chemokines and their activated receptors to promote angiogenesis, and at least in the case of hCXCL1 and hCXCL8, to foster oncogenic Ras-driven tumorigenesis (5,18) prompted us to investigate the involvement of the entire family of ELR+ CXC chemokines in oncogenic Ras-driven tumorigenesis.
Materials and methods
Vectors
pBabePuro, pBabeBleo and pBabePuro-HRasG12V, pCMVNeo-TET, pSUPER-RETRO-PURO-TETO-KRasG12D shRNA, pBabeHygro-TERT, pBabeNeo-SV40 T-Ag and pBabeZeo-cMycT58A were described previously (24–26). pBabeBleo-FLAG-NRasG12V was generated by subcloning PCR-amplified NRas complementary DNA (cDNA) (using primers 5′-CGGGATCCGCCACCATGGACTACAAAGACGATGACGACAAGACTGAGTACAAACTG-3′ and 5′-GAATTCCTTACATCACCACACATGG-3′) into the BamHI and EcoRI sites of pBabeNeo. pBabeNeo-FLAG-KRasG12V was generated by subcloning PCR-amplified KRasG12V cDNA (using primers 5′-CGGGATCCGCCACCATGGACTACAAAGACGATGACGACAAGACTGAATATAAACTTGTG-3′ and 5′-ACGCGTCGACTTACATAATTACACACTTTGTCTT-3′) into the BamHI and SalI sites of pBabeNeo. pSUPER-RETRO-PURO-hCXCL1 shRNA1 and shRNA2 were engineered to encode the shRNA sequence 5′-GCATCGCTTAGGAGGAAGTCTT-3′ and 5′-GCACACTGTCCTATTATA-3′, respectively. pSUPER-RETRO-PURO encoding the shRNA sequence 5′-GACAGGTTTCTGTAGAAGA-3′ was used as a scramble control.
Cell lines
HEK-HT, HEK-HT HRasG12V, BJ-HT, BJ-HT HRasG12V, HSMM-HT and HSMM-HT HRasG12V cell lines were described previously (24,27–29). HEK-HT cells were stably infected with a retrovirus derived from pBabebleo or pBabebleo-NRasG12V using a protocol described previously (30) or transiently transfected with pBabeneo or pBabeneo-KRasG12V, generating the cells HEK-HT-vector or HEK-HT-NRasG12V and HEK-HT-vector or HEK-HT-KRasG12V, respectively. Primary HEK cells were stably infected with retroviruses derived from plasmids pBabeHygro-hTERT and pBabeNeo-SV40 T-Ag. Resultant HEK hTERT/T-Ag cells or HeLa (American Type Culture Collection, Manasass, VA) cells were then stably infected with retroviruses derived from plasmids pBabeZeocMycT58A or pBabePuro-FLAG-KRasG12V using a protocol described previously (30). SW1990 (American Type Culture Collection) cells were stably transduced with pCMVNeo-TET and pSUPER-RETRO-PURO-TETO-KRasG12D shRNA similar to approaches described previously (24) to create the cell line SW1990 DOX KRasG12D shRNA. HRasG12V HEK-HT and AsPC1, Panc1, SW1990 and Capan1 cells (American Type Culture Collection) were stably infected with retroviruses derived from pSUPER-RETRO-PURO scramble or hCXCL1 shRNA-1 using a protocol described previously (30).
RT–PCR
Total RNA isolated from HeLa, HEK-HT, BJ-HT, HSMM-HT and SW1990-DOX-KRasG12D shRNA (in the absence and presence of 64 μg/ml doxycyclin) cells was reverse transcribed using an oligo-dT primer and amplified with the gene specific primer pairs 5′-ACCTCCTCGCCAGCTCTT-3′ and 5′-CTTCAGGAACAGCCACCAG-3′ to detect hCXCL1; 5′-AGCTCTCCTCCTCGACA-3′ and 5′-CTTCAGGAACAGCCACCAA-3′to detect hCXCL2; 5′-AAGTGTGAATGTAAGGTCCCC 3′ and 5′-CTTTCCAGCTGTCCCTAGAA-3′ to detect hCXCL3; 5′-GAGAGCTGCGTTGCGTTTG-3′ and 5′-TTTCCTTGTTTCCACCGTCCA-3′ to detect hCXCL5; 5′-CGCTGGTCCTGTCTCTGCT-3′and 5′-GTTTTTCTTGTTTCCACTGTCC-3′ to detect hCXCL6; 5′-ACCATGAGCCTCAGACTTGATACC-3′ and 5′-TTAATCAGCAGATTCATCACCTGCC-3′ to detect hCXCL7; 5′-TTGCCAAGGAGTGCTAAAGAAC-3′ and 5′-GTCACTTCTACGGTCATTTG-3′ to detect hCXCL8; 5′-GAGAGACCCTCACTGCTG-3′ and 5′-GATGGTACATGACAAGGTGC-3′ to detect GAPDH; 5′-ACGAGCACAAGCTCACC-3′ and 5′-TTTCCACACCTGGTTGC-3′ to detect cMyc and 5′-TGTGGTAGTTGAGCTGGTG-3′ and 5′-TGACCTGCTGTGTCGAGAAT-3′to detect ectopic KRas messenger RNA (mRNA). Total RNA isolated from DMBA/TPA-induced tumors, normal B6 mouse skin or frozen human tissues reverse transcribed with oligo-dT and subsequently amplified with the following gene-specific primer pairs. 5′-GCTGGGATTCACCTCAAGAA-3′ and 5′-TCTCCGTTACTTGGGGACAC-3′ to detect mCXCL1; 5′-AAGTTTGCCTTGACCCTGAA-3′ and 5′-AGGCACATCAGGTACGATCC-3′ to detect mCXCL2; 5′-ATCCAGAGCTTGACGGTGAC-3′ and 5′-GGATGGATCGCTTTTCTCTG-3′ to detect mCXCL3; 5′-TGCCCCTTCCTCAGTCATAG-3′ and 5′-GTGCATTCCGCTTAGCTTTC-3′ to detect mCXCL5; 5′-GCGCTGCAGATGTACGAATA-3′ and 5′-AGGAAAATGGTTTGGCACAG 3′ to detect mCXCL7 and 5′-TGGTCTTTCTGGTGCTTGTCTCAC-3′ and 5′-ATTTCAATGTGAGGCGGGTGGAAC-3′ to detect B2M mRNA. Number of PCR cycles (94°C for 30 s, 55°C for 60 s, 72°C for 90 s) varied from 20 to 35 depending on cell line and transcript.
Immunoblot
Flag-HRasG12V, β-actin and Flag-NRasG12V were detected by immunoblot with the antibodies α-Pan-Ras (Oncogene Research Products, La Jolla, CA), α-actin C-2 (Santa Cruz Biotechnology, Santa Cruz, CA) and α-Flag (Sigma, St. Louis, MI) antibodies, respectively.
Chemokine detection
Cells were seeded at 80% confluency in the absence of serum, and 48 h later, isolated media was centrifuged to remove debris and diluted 1:1 (HEK cells), 1:3 (BJ fibroblasts) or not diluted (human skeletal muscle myoblasts) in sample diluent (Raybiotech, Norcross, GA). Human serum samples (Bioreclamation, Liverpool, NY) were undiluted. The levels of the indicated ELR+ CXC chemokines in samples were quantitated using a Custom Quantibody Array as per manufacture's protocol (Raybiotech). Results were normalized per milligram of protein extracted with RIPA buffer (1x phosphate buffered saline (PBS), 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS) from total cell lysate of the same cells which media was tested in ELISA. Pancreatic cancer cell lines (AsPC1, Panc1, SW1990, Capan1 and SW1990-DOX-KRasG12D shRNA in the absence and presence of 64 μg/ml doxycyclin) were seeded at 80% confluency on day 1. Cells were then serum starved on day 2 for 48 h and media was collected. Conditioned media were diluted 1:4 in sample diluent. hCXCL1-ELISA was performed using manufacturer's instructions (R & D systems, Minneapolis, MN). Results are normalized to total protein.
DMBA/TPA skin carcinogenesis
The backs of 24 (12 male, 12 female) wild-type Balb/c mice and 14 (7 male, 7 female) homozygous mCXCR2 knockout (Il8rbtm1Mwm/Il8rbtm1Mwm) Balb/c mice (Jackson Laboratory, Bar Harbor, ME) were topically treated with 150μl of 125μg/ml DMBA (Sigma) in dimethyl sulfoxide. One week later, the same region was topically treated twice a week with 150μl of 1 × 10−4M TPA (Sigma) in dimethyl sulfoxide for 20 weeks. Tumor number and size were recorded weekly. All procedures were done using a protocol approved by the DUMC Institutional Animal Care and Use Committee.
Xenografts
Ten million cells in PBS were injected into the flanks of SCID–Beige mice following published protocols (30). Following removal from the mice, tumors were fixed in formalin, paraffin embedded, sectioned and stained with Ki-67, CD-31 and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay using standard protocols.
Results
Induction of ELR+ CXC chemokines by oncogenic HRas
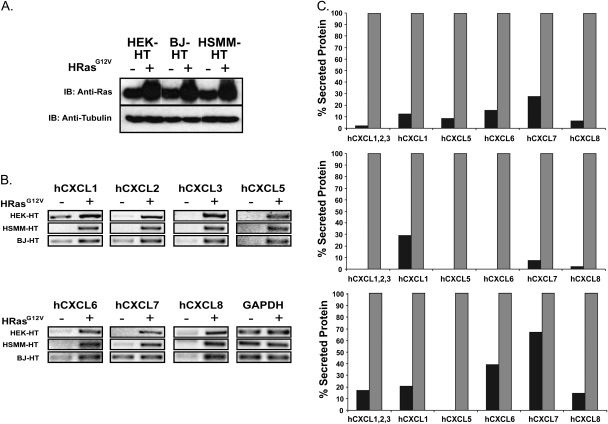
HEK cells expressing the early region of SV40 encoding T-Ag and t-Ag, and hTERT, the catalytic subunit of telomerase (HEK-HT cells), are driven to a tumorigenic state upon expression of oncogenic HRas (HRasG12V). Because of an absolute requirement for oncogenic Ras for tumorigenesis and the defined genetic background of these cells (28,29), we tested whether the mRNA and secreted protein levels of the human ELR+ CXC chemokine family (hCXCL1, 2, 3, 5, 6, 7 and 8) were elevated in HEK-HT cells stably infected with a retrovirus encoding HRasG12V or no transgene. Appropriate HRasG12V expression was validated by immunoblot (Figure 1A), and chemokine mRNA and secreted protein levels assessed by RT–PCR and antibody array, respectively. Consistent with previous observations (5,31), HRasG12V increased the mRNA and secreted protein levels of hCXCL1 and hCXCL8, and as shown here, also the remaining five chemokines (Figure 1B and C and supplementary Table I is available at Carcinogenesis Online). We conclude that oncogenic HRas induces the expression and secretion of the entire ELR+ CXC family of chemokines in HEK-HT cells.
Fig. 1.
HRasG12V increases expression and secretion of ELR+ CXC chemokines. (A) Immunoblot (IB) using a pan-Ras demonstrating ectopic HRasG12V expression, and (B) RT–PCR of ELR+ CXC chemokines demonstrating elevated chemokines levels upon expression of HRasG12V in HEK-HT, BJ-HT and HSMM-HT cells expressing HRasG12V (+) compared with a control vector (−). Tubulin and GAPDH serves as loading controls. (C) Antibody array demonstrating increased secretion of ELR+ CXC chemokines in HEK-HT, BJ-HT and HSMM-HT cells expressing HRasG12V (grey bar) compared with a vector control (black bar). For ease of comparison between different chemokines and cell lines, the level of the each chemokine in the media collected from the indicated cell lines was normalized to the total protein in the media and depicted as a percentage of the level of the corresponding chemokine in the Ras-expressing cells, which was set at 100%.
Induction of ELR+ CXC chemokines by oncogenic HRas in different cell types
To test whether the concerted expression of the ELR+ CXC chemokine family by oncogenic HRas was independent of cell type, the mRNA and secreted protein levels of ELR+ CXC chemokines were measured in human skeletal muscle myoblasts (HSMM-HT) and human fibroblasts (BJ-HT) similarly expressing SV40 early region and hTERT in the absence or presence of HRasG12V (Figure 1A). Again, both these cell types are dependent upon oncogenic HRas for tumor growth (24,27–29), providing a means to compare chemokine level with Ras-mediated tumor potential. RT–PCR and antibody array analysis revealed that, with the possible exception of hCXCL7 in BJ-HT cells, all seven chemokines were elevated upon expression of HRasG12V in both cell types (Figure 1B and C and supplementary Table I is available at Carcinogenesis Online). Thus, oncogenic HRas induces secretion of the ELR+ CXC family of chemokines independent of cell type.
Induction of ELR+ CXC chemokines by all three Ras family members
Oncogenic mutations to HRAS are typically detected in 10% of the relatively rare tumors of the bladder. NRAS is more commonly mutated and found in ∼30% of AML cancers. However, by far most tumors are associated with oncogenic KRAS mutations (1). Given this prevalence of mutations in specific RAS genes in different cancers and the unknown effect of Ras isoform on secretion of these cytokines, we tested whether oncogenic NRas and KRas would similarly induce expression of the ELR+ CXC chemokine family. To this end, HEK-HT cells were stably retrovirally infected or transfected with a vector encoding NRasG12V, KRasG12V or as a control, no transgene, after which appropriate expression of ectopic Ras was verified (supplementary Figure 1A and B is available at Carcinogenesis Online) and chemokine levels assessed by RT–PCT. KRasG12V induced expression of all seven ELR+ CXC chemokines, whereas NRasG12V induced all but hCXCL5 and hCXCL6 (supplementary Figure 1C is available at Carcinogenesis Online). Thus, oncogenic versions of any of the three Ras family members increases the levels of ELR+ CXC chemokines.
ELR+ CXC chemokines are upregulated by oncogenic Ras but not oncogenic Myc
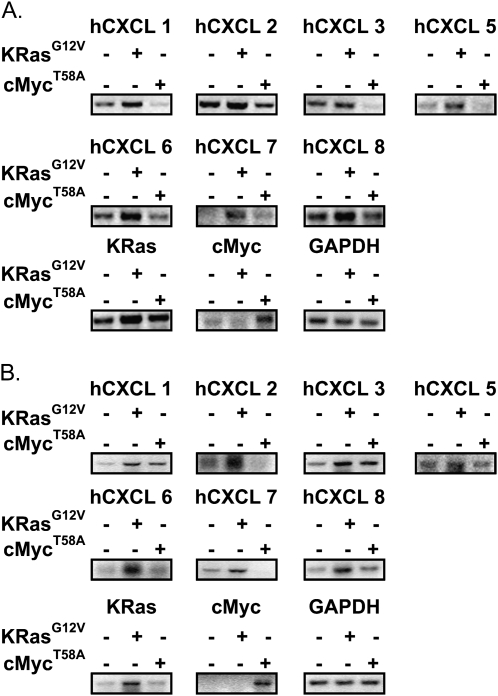
To assess whether the upregulation of ELR+ CXC chemokines was specific to Ras and not other oncogenes, the mRNA level of hCXCL1, 2, 3, 5, 6, 7 and 8 was measured in HEK cells engineered to express hTERT, SV40 T-Ag and a control vector or one encoding either oncogenic KRasG12V or oncogenic cMycT58A (26). Small t-Ag was not expressed in these cells since it can activate cMyc (26,32). Consistent with the above experiments, expression of oncogenic KRasG12V in the resultant HEK-hTERT/T-Ag cells induced the expression, as assessed by RT–PCR, of all seven of these chemokines compared with vector control cells. In contrast, expression of cMycT58A, if anything, reduced the level of these chemokines (Figure 2A). To validate these results in an independent setting, we measured the mRNA levels of these chemokines by RT–PCR in HeLa cells engineered to stably express no transgene, KRasG12V or cMycT58A. In agreement with the previous experiment, only oncogenic KRasG12V induced expression of ELR+ CXC chemokines (Figure 2B). Thus, oncogenic Ras, but not oncogenic cMyc, induces ELR+ CXC chemokines.
Fig. 2.
Oncogenic cMycT58A does not uniformly induce expression of ELR+ CXC chemokines in HEK-hTERT/T-Ag or HeLa cells. Detection of ELR+ CXC chemokines by RT–PCR in KRasG12V and cMycT58A expressing (A) HEK-hTERT/T-Ag and (B) HeLa cells. GAPDH serves as a loading control.
Knockdown of oncogenic KRas in pancreatic cancer cells reduces ELR+ CXC chemokines levels
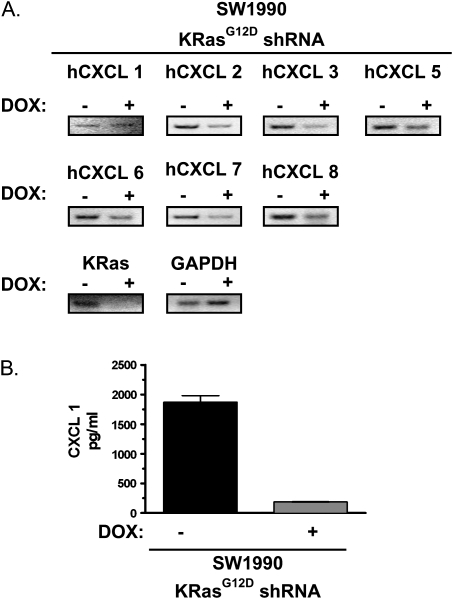
To independently validate that oncogenic Ras induces the expression of ELR+ CXC chemokines, we tested whether knockdown of endogenous oncogenic KRas in pancreatic cancer cells suppressed expression of these chemokines. Specifically, SW1990 human pancreatic cancer cells were engineered to express doxycycline-inducible shRNA targeting the oncogenic KRasG12D transcript, such that the level of hCXCL1, 2, 3, 5, 6, 7 and 8 could be measured in the same cells, except in the absence or presence of endogenous KRasG12D. hCXCL2 to hCXCL8 mRNA levels were reduced, as assessed by RT–PCR (Figure 3A), and CXCL1 secreted protein levels were reduced (Figure 3B) in SW1990 cells upon the addition of doxycycline, which was shown to suppress KRasG12D expression (Figure 3A). Thus, the endogenous mutant KRasG12D protein increases the level of ELR+ CXC chemokines.
Fig. 3.
ELR+ CXC chemokine expression and hCXCL 1 levels are regulated by (A) Detection of ELR+ CXC chemokines and KRas by RT–PCR in SW1990 cells expressing doxycyclin (dox)-inducible KRasG12D shRNA treated with dox for 48 h compared with untreated cells. GAPDH serves as loading control. (B) hCXCL 1 levels in conditioned media from serum starved SW1990 cells expressing doxycycline (dox-inducible KRasG12D shRNA treated with dox for 48 h compared was assayed by ELISA.
ELR+ CXC chemokines are upregulated in tumors
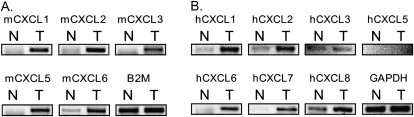
Given the limitations of studying cell lines as a model of cancer (33), we next determined whether ELR+ CXC expression was elevated during carcinogen-induced tumorigenesis in mice, given that such a model reflects the spontaneous nature of cancer in an in vivo environment. Specifically, premalignant papillomas characterized by oncogenic HRAS mutations (34) occur spontaneously in mice treated topically with 7,12 DMBA followed by repeated application of TPA. Thus, the mRNA levels assessed by RT–PCR of mCXCL1, 2, 3, 5 and 6 (corresponding to the seven aforementioned human ELR+ CXC chemokines) were compared between the skin of an untreated mouse and the carcinogen-induced tumor of a mouse treated with DMBA/TPA. We found that the level of each of these ELR+ CXC chemokines was elevated in the DMBA/TPA-induced skin tumor as compared with normal mouse skin (Figure 4A). We next validated these results by comparing the levels of the ELR+ CXC chemokines in a matched pair of normal versus tumor tissue from a patient diagnosed with pancreatic adenocarcinoma, given that this is the cancer most commonly associated with oncogenic Ras mutations (1). RT–PCR analysis revealed that of these chemokines, hCXCL1, 2, 6, 7 and 8 mRNA levels were elevated in the pancreatic adenocarcinoma tumor sample (Figure 4B). Thus, independent models of Ras tumorigenesis demonstrate an increase in the level of ELR+ CXC chemokines.
Fig. 4.
Upregulation of ELR+ CXC chemokines in oncogenic Ras-driven mouse and human tumors. Detection by RT–PCR of the indicated ELR+ CXC chemokines from a (A) DMBA/TPA-induced tumor (T) versus normal mouse skin (N) or (B) matched pair of tumor (T) and normal tissue (N) removed from the pancreas of a patient diagnosed with pancreatic cancer. B2M or GAPDH serves as a loading control.
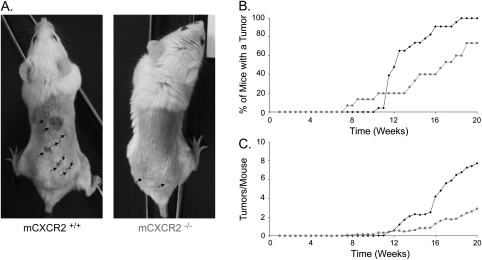
mCXCR2−/− mice are resistant to oncogenic Ras-driven tumors
The one common feature among all ELR+ CXC chemokines is that they bind to and activate the CXCR2 receptor (20). As such, we reasoned that disruption of this receptor would be a means to test whether the concerted increase in ELR+ CXC chemokines by oncogenic Ras was necessary for oncogenic Ras-driven tumorigenesis. mCXCR2−/− mice (35) were thus tested for resistance to DMBA/TPA-induced tumors, again because this model recapitulates the spontaneous nature of cancer, and tumors arising in this system typically harbor oncogenic RAS mutations (34). mCXCR2+/+ and mCXCR2−/− mice were treated topically with DMBA, followed by TPA and tumor number and size were monitored over time. We found that there was a delay in the onset of tumors, as the point when 50% of mice exhibited tumors occurred at week 12 in control mice, but at week 16.5 in the mCXCR2−/− mice (Figure 5A and B). There was also a decrease in the number of mice with tumors; specifically, control mice all formed tumors by week 18.5, whereas at the termination of the experiment almost a quarter of the mCXCR2−/− mice still were tumor-free (Figure 5A and B). Lastly, control mCXCR2+/+ mice formed more tumors per mouse, having an average of 7.7 tumors/mouse compared with 2.9 for the mCXCR2−/− mice at the termination of the experiment (Figure 5A and C). In agreement, a mCXCR2 neutralizing antibody has also been shown to decrease the size of KRasG12D-driven murine lung tumors (36). Thus, the common receptor for ELR+ CXC chemokines, mCXCR2, is integrally involved in mouse models of Ras-driven tumorigenesis.
Fig. 5.
mCXCR2 −/− mice are resistant to DMBA/TPA-induced carcinogenesis. (A) Representative mice at the termination of experiment (arrow: tumors), (B) number of mice with tumors or (C) number of tumors per mouse versus time in weeks of 24 mCXCR2+/+ (black diamond) and 14 mCXCR 2−/− (grey square) mice treated topically with DMBA followed by TPA.
The ELR+ CXC chemokine hCXCL1 is required for tumorigenesis
Given that loss of the common ELR+ CXC receptor mCXCR2 reduced oncogenic Ras-mediated tumorigenesis, we next tested whether tumor-derived ELR+ CXC chemokines promote tumorigenesis. To this end, we focused on hCXCL1 since it was uniformly elevated in cells expressing oncogenic Ras, oncogenic Ras-driven tumors (Figures 1, 2 and 4 and supplementary Figure 1 is available at Carcinogenesis Online) and the serum of pancreatic cancer patients (Figure 6).
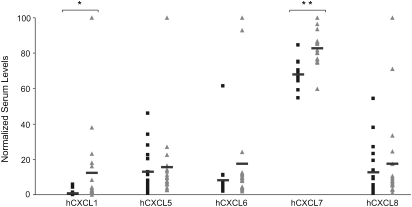
Fig. 6.
hCXCL1 and hCXCL7 are upregulated in pancreatic adenocarcinoma serum. Serum concentrations of the indicated ELR+ CXC chemokines as assessed by antibody array. Samples from age- and sex-matched normal donors (black square) and patients diagnosed with pancreatic adenocarcinoma (grey triangle). Bar indicates mean; *P < 0.05, **P < 1 × 10−5.
We first tested whether knockdown of hCXCL1 reduced the tumorigenic growth of oncogenic Ras-transformed cells. Specifically, HRasG12V HEK-HT cells stably infected with a retrovirus encoding a scramble control sequence or hCXCL1 shRNA-1 that reduced the secreted level of hCXCL1 (supplementary Figure 2A is available at Carcinogenesis Online) were injected into the flanks of four mice each. At the termination of the experiment, tumors derived from cells in which hCXCL1 expression was reduced were a third the size of scramble control tumors (supplementary Figure 2B is available at Carcinogenesis Online). We validated these results with a second shRNA, again demonstrating that the same cells engineered to express an independent hCXCL1 shRNA-2 (supplementary Figure 2A is available at Carcinogenesis Online) exhibited reduced tumorigenic growth when injected into immunocompromised mice (supplementary Figure 2B is available at Carcinogenesis Online).
In order to determine how hCXCL1 shRNA inhibited tumor growth, tumors were extracted and stained for Ki-67, TUNEL and CD-31 to assess cell proliferation, apoptosis and tumor vasculature, respectively. It was shown by Ki-67 staining that scramble control cells proliferate at a significantly higher rate than hCXCL1 shRNA cells. Furthermore, TUNEL staining indicated a higher level of apoptosis in hCXCL1 shRNA cells. Interestingly, CD-31 staining was higher in the hCXCL1 shRNA tumors than in the scramble control tumors (supplementary Figure 3A and B is available at Carcinogenesis Online). Thus, oncogenic Ras-induced secretion of hCXCL1 appears to promote a number of tumor phenotypes.
To address whether hCXCL1 was also required for the tumorigenic growth of human pancreatic cancer cells, we stably infected the human pancreatic cancer cell lines AsPC1, Panc1, SW1990 and Capan1 with a retrovirus encoding a scramble control sequence or hCXCL1 shRNA-1, which was validated to reduce the levels of hCXCL1-secreted protein (supplementary Figure 4A is available at Carcinogenesis Online). These eight cell lines were then injected into the flanks of four immunocompromised mice each and when the first control tumor reached maximum volume (<1.5 cm3), tumors were excised, photographed and weighed. Knockdown of hCXCL1 reduced tumor weight by approximately half in all but Capan1 cells (supplementary Figure 4B and C is available at Carcinogenesis Online), cells which incidentally exhibited the poorest knockdown and highest endogenous secreted levels of hCXCL1 (supplementary Figure 4A is available at Carcinogenesis Online and data not shown). These results agree with the observation that hCXCL1 is upregulated by Ras and is required for Ras-driven tumorigenesis (18). Thus, knockdown of an ELR+ CXC chemokine reduces oncogenic Ras-driven tumor growth.
In order to determine how hCXCL1 shRNA inhibited tumor growth, Panc-1 tumors were extracted and stained for Ki-67, TUNEL and CD-31 to assess cell proliferation, apoptosis and tumor vasculature, respectively. It was shown by Ki-67 staining that scramble control cells proliferate at a significantly higher rate that do hCXCL1 shRNA cells. Furthermore, TUNEL staining indicated a higher level of apoptosis in hCXCL1 shRNA cells. Additionally, CD-31 staining was found to show lower levels of vasculature in the hCXCL1 shRNA tumors than in the scramble control tumors (supplementary Figure 5A and B is available at Carcinogenesis Online). Thus, oncogenic Ras-induced secretion of hCXCL1 appears to promote a number of tumor phenotypes.
Elevated serum levels of hCXCL1 and hCXCL7 pancreatic cancer patients
Lastly, we tested whether the elevation of ELR+ CXC chemokines observed in tumorigenic cells of multiple models of oncogenic Ras tumorigenesis could be detected in a more clinically available tissue, namely serum. We again chose pancreatic cancer for this analysis, since KRAS is mutated in >90% of all pancreatic adenocarcinoma (1). Serum isolated from 20 patients diagnosed with pancreatic adenocarcinoma versus 19 age- and sex-matched normal donors was assayed by antibody array for the presence of ELR+ CXC chemokines hCXCL1, 5, 6, 7 and 8. Although only hCXCL1 and hCXCL7 levels were statistically higher in pancreatic cancer patients, the highest levels of chemokines were detected exclusively in cancer patients, suggesting the possibility of a more broad increase in ELR+ CXC chemokines in a subset of pancreatic cancer patients (Figure 6).
Discussion
We demonstrate that expression of any of the three oncogenic Ras proteins, but not an unrelated oncogene cMyc, and in different tumorigenic cells and tumor tissues, there is an increase of ELR+ CXC chemokines and that genetic ablation of the common mCXCR2 receptor for these chemokines in mice or knockdown of a representative ELR+ CXC chemokine (hCXCL1) in pancreatic cancer cells reduces oncogenic Ras-driven tumorigenesis. We thus suggest that oncogenic Ras induces the secretion of the ELR+ CXC chemokine family to promote tumorigenesis. This could have two impacts. First, such a secreted protein signature induced by oncogenic Ras may serve as a non-invasive means of detecting oncogenic Ras-driven malignancies, such as pancreatic cancer. Indeed, we (Figure 6) and others (7,37) detect elevated levels of hCXCL1, hCXCL7 and hCXCL8 in serum of pancreatic cancer patients. Second, although RAS is one of the most widely activated proto-oncogenes in human cancer (1), and inhibition of oncogenic Ras expression is well known to cause tumor regression (24,38,39), Ras has proven refractory to pharmacological inhibition as a means to treat oncogenic Ras-driven cancers (40). However, chemokines and their receptors that are required for Ras oncogenesis, such as ELR+ CXC chemokines and CXCR2, are druggable proteins, being both bioavailable and susceptible to inhibition by neutralizing antibodies (41). Indeed, neutralizing antibodies to CXCL8 and CXCR2 have been demonstrated to reduce oncogenic Ras-driven tumor growth (5,36). As such, the induction of ELR+ CXC chemokines by oncogenic Ras may have diagnostic, prognostic and perhaps even therapeutic value.
Supplementary material
Supplementary Table 1 and Figures 1–5 can be found at http://carcin.oxfordjournals.org/
Funding
National Institutes of Health grant CA123031 to C.M.C.
Supplementary Material
Acknowledgments
We would like to thank Jay Stringer, Brooke Ancrile and Kian-Huat Lim for technical assistance.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- CXC
Cys-X-Cys
- DMBA
dimethylbenzanthracene
- ELISA
enzyme-linked immunosorbent assay
- GTP
guanosine triphosphate
- HEK
human embryonic kidney
- IL
interleukin
- mRNA
messenger RNA
- RT–PCR
reverse transcription–polymerase chain reaction
- shRNA
short hairpin RNA
- TPA
12-O-tetradecanoylphorbol 13-acetate
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
- VEGF
vascular endothelial growth factor
References
- 1.Bos JL. ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–4689. [PubMed] [Google Scholar]
- 2.Downward J. Targeting RAS signalling pathways in cancer therapy. Nat. Rev. Cancer. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 3.Okada F, et al. Impact of oncogenes in tumor angiogenesis: mutant K-ras up-regulation of vascular endothelial growth factor/vascular permeability factor is necessary, but not sufficient for tumorigenicity of human colorectal carcinoma cells. Proc. Natl. Acad. Sci. USA. 1998;95:3609–3614. doi: 10.1073/pnas.95.7.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rak J, et al. Oncogenes and tumor angiogenesis: differential modes of vascular endothelial growth factor up-regulation in ras-transformed epithelial cells and fibroblasts. Cancer Res. 2000;60:490–498. [PubMed] [Google Scholar]
- 5.Sparmann A, et al. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell. 2004;6:447–458. doi: 10.1016/j.ccr.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 6.Ahuja SK, et al. The CXC chemokines growth-regulated oncogene (GRO) alpha, GRObeta, GROgamma, neutrophil-activating peptide-2, and epithelial cell-derived neutrophil-activating peptide-78 are potent agonists for the type B, but not the type A, human interleukin-8 receptor. J. Biol. Chem. 1996;271:20545–20550. doi: 10.1074/jbc.271.34.20545. [DOI] [PubMed] [Google Scholar]
- 7.Wigmore SJ, et al. Cytokine regulation of constitutive production of interleukin-8 and -6 by human pancreatic cancer cell lines and serum cytokine concentrations in patients with pancreatic cancer. Int. J. Oncol. 2002;21:881–886. doi: 10.3892/ijo.21.4.881. [DOI] [PubMed] [Google Scholar]
- 8.Tas F, et al. Serum vascular endothelial growth factor (VEGF) and interleukin-8 (IL-8) levels in small cell lung cancer. Cancer Invest. 2006;24:492–496. doi: 10.1080/07357900600814771. [DOI] [PubMed] [Google Scholar]
- 9.Brennecke S, et al. Decline in angiogenic factors, such as interleukin-8, indicates response to chemotherapy of metastatic melanoma. Melanoma Res. 2005;15:515–522. doi: 10.1097/00008390-200512000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Lambeck AJ, et al. Serum cytokine profiling as a diagnostic and prognostic tool in ovarian cancer: a potential role for interleukin 7. Clin. Cancer Res. 2007;13:2385–2391. doi: 10.1158/1078-0432.CCR-06-1828. [DOI] [PubMed] [Google Scholar]
- 11.Lokshin AE, et al. Circulating IL-8 and anti-IL-8 autoantibody in patients with ovarian cancer. Gynecol. Oncol. 2006;102:244–251. doi: 10.1016/j.ygyno.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Lehrer S, et al. Serum interleukin-8 is elevated in men with prostate cancer and bone metastases. Technol. Cancer Res. Treat. 2004;3:411. doi: 10.1177/153303460400300501. [DOI] [PubMed] [Google Scholar]
- 13.Pfitzenmaier J, et al. Elevation of cytokine levels in cachectic patients with prostate carcinoma. Cancer. 2003;97:1211–1216. doi: 10.1002/cncr.11178. [DOI] [PubMed] [Google Scholar]
- 14.Ancrile B, et al. Oncogenic Ras-induced secretion of IL6 is required for tumorigenesis. Genes Dev. 2007;21:1714–1719. doi: 10.1101/gad.1549407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ancrile BB, et al. Oncogenic ras-induced expression of cytokines: a new target of anti-cancer therapeutics. Mol. Interv. 2008;8:22–27. doi: 10.1124/mi.8.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trikha M, et al. Targeted anti-interleukin-6 monoclonal antibody therapy for cancer: a review of the rationale and clinical evidence. Clin. Cancer Res. 2003;9:4653–4665. [PMC free article] [PubMed] [Google Scholar]
- 17.Liu J, et al. A genetically defined model for human ovarian cancer. Cancer Res. 2004;64:1655–1663. doi: 10.1158/0008-5472.can-03-3380. [DOI] [PubMed] [Google Scholar]
- 18.Yang G, et al. The chemokine growth-regulated oncogene 1 (Gro-1) links RAS signaling to the senescence of stromal fibroblasts and ovarian tumorigenesis. Proc. Natl. Acad. Sci. USA. 2006;103:16472–16477. doi: 10.1073/pnas.0605752103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahuja SK, et al. CXC chemokines bind to unique sets of selectivity determinants that can function independently and are broadly distributed on multiple domains of human interleukin-8 receptor B. Determinants of high affinity binding and receptor activation are distinct. J. Biol. Chem. 1996;271:225–232. doi: 10.1074/jbc.271.1.225. [DOI] [PubMed] [Google Scholar]
- 20.Allen SJ, et al. Chemokine: receptor structure, interactions, and antagonism. Annu. Rev. Immunol. 2007;25:787–820. doi: 10.1146/annurev.immunol.24.021605.090529. [DOI] [PubMed] [Google Scholar]
- 21.Rossi D, et al. The biology of chemokines and their receptors. Annu. Rev. Immunol. 2000;18:217–242. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 22.Coussens LM, et al. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li A, et al. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J. Immunol. 2003;170:3369–3376. doi: 10.4049/jimmunol.170.6.3369. [DOI] [PubMed] [Google Scholar]
- 24.Lim KH, et al. Reduction in the requirement of oncogenic Ras signaling to activation of PI3K/AKT pathway during tumor maintenance. Cancer Cell. 2005;8:381–392. doi: 10.1016/j.ccr.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 25.Kendall SD, et al. A network of genetic events sufficient to convert normal human cells to a tumorigenic state. Cancer Res. 2005;65:9824–9828. doi: 10.1158/0008-5472.CAN-05-1543. [DOI] [PubMed] [Google Scholar]
- 26.Yeh E, et al. A signalling pathway controlling c-Myc degradation that impacts oncogenic transformation of human cells. Nat. Cell Biol. 2004;6:308–318. doi: 10.1038/ncb1110. [DOI] [PubMed] [Google Scholar]
- 27.Linardic CM, et al. Genetic modeling of human rhabdomyosarcoma. Cancer Res. 2005;65:4490–4495. doi: 10.1158/0008-5472.CAN-04-3194. [DOI] [PubMed] [Google Scholar]
- 28.Hamad NM, et al. Distinct requirements for Ras oncogenesis in human versus mouse cells. Genes Dev. 2002;16:2045–2057. doi: 10.1101/gad.993902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hahn WC, et al. Creation of human tumour cells with defined genetic elements. Nature. 1999;400:464–468. doi: 10.1038/22780. [DOI] [PubMed] [Google Scholar]
- 30.O’Hayer K, et al. A genetically defined normal human somatic cell system to study ras oncogenesis in vivo and in vitro. Methods Enzymol. 2006;407:637–647. doi: 10.1016/S0076-6879(05)07050-3. [DOI] [PubMed] [Google Scholar]
- 31.Yang G, et al. The chemokine growth-regulated oncogene 1 (Gro-1) links RAS signaling to the senescence of stromal fibroblasts and ovarian tumorigenesis. Proc. Natl. Acad. Sci. USA. 2006;103:16472–16477. doi: 10.1073/pnas.0605752103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janssens V, et al. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem. J. 2001;353:417–439. doi: 10.1042/0264-6021:3530417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharpless NE, et al. The mighty mouse: genetically engineered mouse models in cancer drug development. Nat. Rev. Drug Discov. 2006;5:741–754. doi: 10.1038/nrd2110. [DOI] [PubMed] [Google Scholar]
- 34.Quintanilla M, et al. Carcinogen-specific mutation and amplification of Ha-ras during mouse skin carcinogenesis. Nature. 1986;322:78–80. doi: 10.1038/322078a0. [DOI] [PubMed] [Google Scholar]
- 35.Cacalano G, et al. Neutrophil and B cell expansion in mice that lack the murine IL-8 receptor homolog. 1994 doi: 10.1126/science.8036519. Science, 265, 682–684. [DOI] [PubMed] [Google Scholar]
- 36.Wislez M, et al. High expression of ligands for chemokine receptor CXCR2 in alveolar epithelial neoplasia induced by oncogenic kras. Cancer Res. 2006;66:4198–4207. doi: 10.1158/0008-5472.CAN-05-3842. [DOI] [PubMed] [Google Scholar]
- 37.Ebrahimi B, et al. Cytokines in pancreatic carcinoma: correlation with phenotypic characteristics and prognosis. Cancer. 2004;101:2727–2736. doi: 10.1002/cncr.20672. [DOI] [PubMed] [Google Scholar]
- 38.Chin L, et al. Essential role for oncogenic Ras in tumour maintenance. Nature. 1999;400:468–472. doi: 10.1038/22788. [DOI] [PubMed] [Google Scholar]
- 39.Felsher DW. Reversibility of oncogene-induced cancer. Curr. Opin. Genet. Dev. 2004;14:37–42. doi: 10.1016/j.gde.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 40.Friday BB, et al. K-ras as a target for cancer therapy. Biochim. Biophys. Acta. 2005;1756:127–144. doi: 10.1016/j.bbcan.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 41.Adams GP, et al. Monoclonal antibody therapy of cancer. Nat. Biotechnol. 2005;23:1147–1157. doi: 10.1038/nbt1137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.