Abstract
We have previously reported that sulindac, a non-steroidal anti-inflammatory drug, inhibited tumor formation in the small intestine but increased tumors in the colon of ApcMin/+ mice, a model of human familial adenomatous polyposis. To further explore intestinal regional responses, we studied effects of sulindac on additional gene-targeted mouse models of human intestinal tumorigenesis; these were (i) Apc1638N/+ mouse (chain termination mutation in exon 15 of the Apc gene); (ii) Mlh1+/− mouse (DNA mismatch repair deficiency, a mouse model of human hereditary non-polyposis colorectal cancer) and (iii) double-heterozygous Mlh1+/−Apc1638N/+ mutant mouse. Mice were fed AIN-76A control diet with or without 0.02% sulindac for 6 months. Intestinal regional tumor incidence, multiplicity, volume and degree of inflammation were used as end points. The results showed the following: (i) sulindac inhibited tumor development in the small intestine of Apc1638N/+ mice; (ii) in contrast, sulindac increased tumors in the small intestine of Mlh1 mutant mice, a neoplastic effect which persisted in heterozygous compound Mlh1+/−Apc1638N/+ mutant mice; (iii) sulindac increased tumors in the cecum of all mice regardless of genetic background; (iv) sulindac decreased inflammation in the small intestine of Apc1638N/+ mice, but it increased inflammation in the small intestine of Mlh1+/− mice and Mlh1+/−Apc1638N/+ mice and (v) sulindac enhanced inflammation in the cecum of all mutant mice. Findings indicate that the effects of sulindac in the intestine of these mutant mouse models are probably related to genetic background and appear to be associated with its inflammatory-inducing response.
Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) have been shown to possess chemopreventive properties against gastrointestinal neoplasia. For example, sulindac decreased the number and size of colorectal adenomas in familial adenomatous polyposis (FAP) patients (1–8) and in non-FAP cohorts at increased risk of adenoma recurrence (9). Nonetheless, colorectal cancer appears to recur in FAP patients after prolonged sulindac treatment (6,10–13). Sulindac also caused regression of small intestinal tumors in the ApcMin/+ mouse, a mouse model of FAP (14–16), the small intestine being the major site of tumor development in mouse models. We have previously observed (17) regional effects of sulindac on intestinal tumorigenesis in the ApcMin/+ mouse, wherein these mice when given sulindac for 9 weeks showed decreased number and volume of tumors in the small intestine but increased tumor formation in the cecal portion of the colon. A similar regional effect of piroxicam on intestinal neoplasia was reported in double-mutant Mlh1+/−/Apc1638N/+ mice (18). It is noteworthy that sulindac administration induced tumors in the cecum of wild-type C57BL/6J mice, a mouse strain with very low susceptibility to intestinal cancer (19).
Here, we further explored the regional responses of the intestine to dietary sulindac, using additional mouse genetic models that simulate increased sensitivity to human intestinal tumorigenesis, including (i) Apc1638N/+ mice (chain termination mutation in exon 15 of the Apc gene) (20); (ii) Mlh1+/− mice (DNA mismatch repair deficiency, a mouse model of human hereditary non-polyposis colorectal cancer) and (iii) double-heterozygous Mlh1+/−Apc1638N/+ mutant mice (21). The results indicate that the regional effects of sulindac on tumor development in these mice are associated with chronic inflammation and appears to be related to their genetic background.
Materials and methods
Mice and diets
Genetically modified mice at 5–6 weeks of age were provided by Dr Winfried Edelmann, Department of Cell Biology, Albert Einstein College of Medicine, Bronx, NY. These included Apc1638N/+ (n = 31), Mlh1+/− (n = 38) and double-heterozygous Mlh1+/−Apc1638N/+ mice (n = 22). Mice were fed AIN-76A diet and maintained on a 12 h light–dark cycle in a temperature and humidity-controlled room. After 1 week of diet acclimatization, mice of different genotypes were randomized to two dietary groups and either maintained on AIN-76A diet or AIN-76A diet containing sulindac (200 p.p.m.) (Research Diets, Brunswick, NJ). The average consumption of sulindac was ∼0.6 mg/day per mouse, equivalent to the dose that inhibited tumor growth reported in ApcMin/+ mice (14) and to the dose used in our previous study with ApcMin/+ mice (17). Mice were weighed weekly and tested for fecal bleeding to monitor both intestinal inflammation and tumor formation. All animals were euthanized after 6 months of feeding.
Evaluation of tumor and inflammation development
The gastrointestinal tract was removed, opened longitudinally, and the contents were washed in cold phosphate-buffered saline (pH 7.4). Each specimen was examined under a dissecting microscope for tumors after fixation in 10% neutral-buffered formalin. Tumor multiplicity (number of mice with tumors), incidence (number of tumors per mouse), location and volume (mm3) were recorded. All tumors found in the gastrointestinal tract were excised for processing; tissue section slides were prepared and stained with hematoxylin and eosin for histological examination. The tumor diagnosis was based on the Histological Typing of Intestinal Tumours and The Pathology of Mouse Models of Intestinal Cancer (22) with modifications. Other organs and lymph nodes were also examined. Two segments of flat mucosa were taken from duodenum, cecum, proximal and distal colon and fixed in 10% neutral-buffered formalin, and tissue sections were stained with hematoxylin and eosin for histological analysis and evaluation of inflammation.
The degree of inflammation was assessed using the following semiquantitative scoring system (23): (i) no inflammatory cells; (ii) few plasma and lymphoid cells; (iii) clusters of plasma and lymphoid cells; (iv) granulomatous tissue formed with acute and chronic inflammatory cells, mononuclear cells, fiber and fibroblastic cells and (v) micro-abscess formation. The depth of inflammatory infiltration was evaluated as follows: (i) confined to the mucosa propria; (ii) extended to the submucosa; (iii) spreading to the muscularis and (iv) spreading to the serosa or beyond. Inflammation severity was calculated as the degree of inflammatory cells × depth of inflammatory infiltration.
Results
Tumor development
Small intestine.
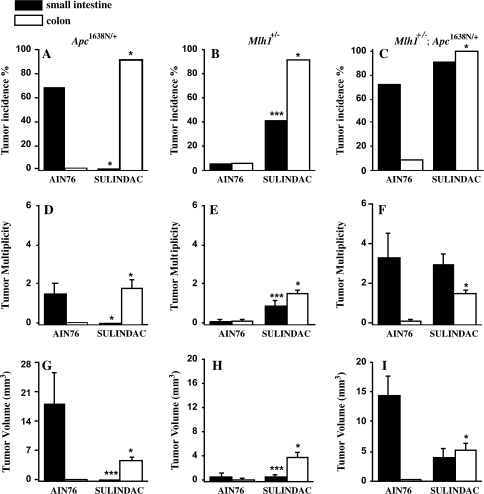
Spontaneous tumor incidence in the small intestine of Apc1638N/+ mice, Mlh1+/− mice and double-mutant Mlh1+/−Apc1638N/+ mice fed AIN-76A for 6 months was 68, 6 and 73%, respectively (Figure 1). Sulindac treatment for the same period resulted in marked changes in tumor development in each of the genetic groups (Figure 1 and Table I). Sulindac completely eliminated tumor formation, as measured by tumor incidence (0 versus 68%, P < 0.001), multiplicity (0.0 versus 1.5, P < 0.001) and volume (0.0 versus 18.0 mm3, P < 0.05) in the small intestine of Apc1638N/+ mice, compared with mice fed unmodified AIN-76A control diet (Figure 1A, D and G). In contrast, dietary sulindac increased tumor incidence 5.8-fold (41 versus 6%, P < 0.05) and tumor number 8-fold (0.9 versus 0.1, P < 0.05) in the small intestine of Mlh1+/− mice (Figure 1B, E and H). Sulindac was ineffective in suppressing tumor incidence (91 versus 73% control) or multiplicity (3.0 versus 3.3) in the small intestine of compound Mlh1+/−; Apc1638N/+ mice, but there appeared to be a decrease in tumor size (4.1 versus 14.5 mm3), which was not statistically significant (Figure 1C, F and I). Importantly, the increased susceptibility to spontaneous tumors in the small intestine appears to be related to alterations in the Apc gene in both the Apc1638N/+ and the compound Mlh1+/−; Apc1638N/+ mice.
Fig. 1.
Tumor development in the intestine of mice with Apc1638N/+ and/or Mlh1 mutations. Tumor incidence, multiplicity and volume (mm3) in small intestine (filled bars) and colon (open bars) of Apc1638N/+ (A, D and G), Mlh1+/− (B, E and H) and Mlh1+/−; Apc1638N/+ mice (C, F and I). Histograms are expressed as mean ± SEM. Comparisons between sulindac and control groups (AIN76A) of each mouse strain using Fisher’s exact probability (two-tailed) test for tumor incidence; Mann–Whitney or exact binomial calculation for tumor multiplicity and volume. *P < 0.001, **P < 0.05, ***P < 0.01 versus AIN-76A.
Table I.
Summary of intestinal tumor development in mutant mice after feeding sulindac
|
Apc1638N/+ |
Mlh1+/− |
Mlh1+/−/Apc1638N/+ |
|
| Sulindac added to AIN-76A diet | Sulindac added to AIN-76A diet | Sulindac added to AIN-76A diet | |
| Tumor incidence | |||
| Small intestine | − | + | − |
| Colon | + | + | + |
| Tumor multiplicity | |||
| Small intestine | − | + | − |
| Colon | + | + | + |
| Tumor volume | |||
| Small intestine | − | + | − |
| Colon | + | + | + |
Decreased (−) or increased (+) tumor incidence, multiplicity or volume compared with mice fed unmodified control AIN-76A diet.
Cecum.
Tumors were mainly located in the cecum and upper part of the colon near the junction with the ileum. Tumor incidence was 0, 6 and 9%, respectively, in single-mutant Apc1638N/+ mice, Mlh1+/− mice and double-mutant mice fed AIN-76A diet, respectively (Figure 1). In mice treated with sulindac, tumor incidence, multiplicity and volume were greatly increased regardless of genetic status. Thus, in Apc1638N/+ mice, tumor incidence increased to 92%, multiplicity to 1.8 tumors per mouse and volume to 4.7 mm3 per mouse, with all increases significant at P < 0.001 (Figure 1A, D and G, Table I). In Mlh1+/− mice, dietary sulindac increased tumor incidence in cecum and proximal colon 14-fold (91 versus 6%), tumor multiplicity (1.5 versus 0.1 tumors per mouse) and increased tumor volume by 17.5-fold (3.7 versus 0.2 mm3), all increases significant at P < 0.001 (Figure 1B, E and H). In double-heterozygous Mlh1+/−Apc1638N/+ mice, dietary sulindac led to a 10-fold increase of tumor incidence (100 versus 9%), 14-fold increase of tumor multiplicity (1.5 versus 0.1) and increased tumor volume 50-fold (5.1 versus 0.1 mm3), all values significant at the P < 0.001 (Figure 1C, F and I).
Most of these tumors in control group mice fed AIN-76A were adenomas. After sulindac treatment, however, the number of invasive adenocarcinomas in Apc1638N/+ and Mlh1+/−Apc1638N/+ mice significantly increased in the colon: 57 and 32%, respectively. These findings demonstrated that sulindac not only markedly increased tumor formation but also accelerated tumor progression from benign to malignant in the cecum and proximal colon of mice, regardless of their genetic etiology.
Inflammation in small intestine and colon.
In order to determine if there was a mechanistic relationship between tumor development and inflammation in the absence and presence of sulindac, the extent of inflammation was evaluated in different intestinal segments along the cephalo-caudal axis, from samples of duodenum, cecum, proximal and distal colon of the Apc1638N/+ and Mlh1+/− mice. In Apc1638N/+ mice fed AIN-76A control diet, inflammation was more severe in duodenum than in cecum and was absent in proximal and distal colon (Table II). Significantly, after sulindac treatment, the inflammatory severity score decreased in small intestine (0.00 versus 2.68, P < 0.001) and greatly increased in cecum (6.00 versus 0.29, P < 0.001) compared with mice fed unmodified AIN-76A control diet; no inflammation was detected in the remainder of the colon after sulindac treatment (Table II).
Table II.
Inflammation measurements in the intestine of mice with Apc1638 and Mlh1 mutations
| Inflammation scoresa |
|||
| Degree | Depth | Severity | |
| Apc1638N/+ mice | |||
| AIN-76A | |||
| Duodenum | 1.32 ± 0.27 | 1.21 ± 0.26 | 2.68 ± 0.78 |
| Cecum | 0.29 ± 0.16 | 0.21 ± 0.11 | 0.29 ± 0.16 |
| Proximal colon | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Distal colon | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Sulindac | |||
| Duodenum | 0.00 ± 0.001 | 0.00 ± 0.001 | 0.00 ± 0.001 |
| Cecum | 2.42 ± 0.291 | 2.25 ± 0.281 | 6.00 ± 0.911 |
| Proximal colon | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Distal colon | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Mlh1+/− mice | |||
| AIN-76A | |||
| Duodenum | 0.00 ± 0.00* | 0.00 ± 0.00* | 0.00 ± 0.00* |
| Cecum | 0.33 ± 0.17 | 0.33 ± 0.17 | 0.33 ± 0.17 |
| Proximal colon | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Distal colon | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Sulindac | |||
| Duodenum | 1.18 ± 0.321* | 1.23 ± 0.341* | 3.64 ± 1.261* |
| Cecum | 2.18 ± 0.241 | 1.82 ± 0.181 | 4.73 ± 0.601 |
| Proximal colon | 0.09 ± 0.09 | 0.09 ± 0.09 | 0.18 ± 0.18 |
| Distal colon | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
Mean ± SEM.
Inflammatory scores included degree of inflammatory cells, depth of inflammatory cell infiltration and inflammatory severity (degree × depth). By Mann–Whitney test and binomial exact calculation compared with corresponding AIN-76A: 1P < 0.001; compared with corresponding diet group of Apc1638N/+ mice: *P < 0.001.
Slight inflammation occurring spontaneously was observed in the cecum of Mlh1 mutant mice maintained on AIN-76A control diet. However, following sulindac treatment, the inflammatory severity score in these mice increased significantly in both small intestine (3.64 versus 0.00, P < 0.001) and cecum (4.73 versus 0.33, P < 0.001) compared with mice fed AIN-76A control diet (Table II).
In all instances, inflammatory cells after sulindac administration were located mainly in and around the area of tumor formation and included neutrophils, lymphoid cells, plasma cells and mononuclear cells extending from the upper part of intestinal wall to the muscularis and serosa. Increased inflammation was evident in the proximal colon after sulindac treatment, although not statistically significant.
Discussion
It is mandatory that the effects of sulindac as a potential chemopreventive agent are taken in context. Present results using mice with different genetic backgrounds, all with an increased propensity to develop colorectal cancer, provide further insight on the potential effects of the drug. Sulindac inhibited tumorigenesis in small intestine of Apc1638N/+ mice but significantly increased the incidence, multiplicity and volume of tumors in the cecum of these mice. These results are in agreement with previous findings in ApcMin/+ mice (17). We extended these studies to Mlh1+/− and Mlh1+/−Apc1638N/+ mice wherein sulindac treatment resulted in a marked increase in tumor incidence, multiplicity and volume in both small intestine and cecum. Thus, in contrast to the single-mutant Apc1638N/+ mice in which sulindac protected against tumor formation in the small intestine, we saw no such protection by sulindac in the small intestine of Mlh1+/− or Mlh1+/−Apc1638N/+ mice which could be due to loss of wild type Apc allele. These genetic alterations would presumably affect the histopathology of the intestine in these mice, accounting for their increased propensity to develop intestinal cancer and in turn differentially alter their response to sulindac in the small intestine and cecum.
In the present study, we have examined these findings as related to the inflammatory process and its potential association with the development of intestinal tumorigenesis in Apc1638N/+ and Mlh1+/− mice fed either unmodified AIN-76A diet or AIN-76A supplemented with sulindac. Surprisingly, moderate to severe inflammation was present in the duodenum of Apc1638N/+ mice fed AIN-76A but was absent in mice with an Mlh1+/− mutation maintained on the same diet. In Apc1638N/+ mice, inflammation was decreased after sulindac. Whether the presence or absence of inflammation in the same region of the intestine reflects the different genetic lesions in mice or an interaction of diet with a specific genotype remains to be determined. It is also noteworthy that sulindac-enhanced inflammation developed predominantly in the cecum of these mice where most tumors were also found.
Using quantitative computer-assisted image analysis, we previously showed (17) that intestinal regional response of ApcMin/+ mice to sulindac may be accounted for, at least in part, by the increased expression of Bax, a pro-apoptotic protein, and decreased expression of Bcl-xl, an anti-apoptotic protein of the Bcl-2 family. An opposite Bax/Bcl-xl pattern was observed in cecal mucosa and in tumors therein; these changes were also associated with widespread ulceration and limited perforation in cecal mucosal surface and tumors after sulindac treatment, which are histopathologic signs of severe inflammation.
These observations are consistent with reports showing that sustained use of NSAIDs, including sulindac, induces damage not only to the upper gastrointestinal tract but also to the small and large intestine, resulting in the activation of an inflammatory cascade leading to mucosal ulceration (24–26). While this proinflammatory process has been attributed to NSAIDs’ induction of changes in mucosal permeability and the consequent influx of toxic luminal factors into the mucosa (24,26,27), an important compounding effect might be the intrinsic ability of NSAIDs, including sulindac, to generate reactive oxygen species (28–31).
While sulindac-induced reactive oxygen species was shown to enhance apoptosis in cancer cells (29), we surmise that sustained stimulation by sulindac of reactive oxygen species formation would ultimately lead to oxidative stress, a key stimulus of the inflammatory response and carcinogenesis (32,33). Thus, sulindac-induced oxidative stress was found to induce oncogenic COX-2 overexpression in various cell lines (31) and to activate p38 (29,34), a mitogen-activated protein kinase that participates in the generation of key proinflammatory cytokines and chemokines and in cancer development (35–38).
There is a large body of evidence showing that chronic inflammatory conditions increase the risk of cancer: the causative link between inflammation in inflammatory bowel disease and colon cancer is a salient case in point (39–41). One may therefore propose that sulindac-induced inflammation contributes to the regional effects of sulindac on tumor formation in the small intestine and cecum of mice. This view is supported by the observation that chemically induced inflammation accelerated colonic adenoma formation in ApcMin/+ mice (42,43) and enhanced colon carcinogenesis in Mlh1+/− mice (44).
Funding
National Cancer Institute; National Institutes of Health (NO1-CN-15116, NO1-CN-43308, RO1 CA 87559).
Acknowledgments
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- FAP
familial adenomatous polyposis
- NSAID
non-steroidal anti-inflammatory drug
References
- 1.Labayle D, et al. Sulindac causes regression of rectal polyps in familial adenomatous polyposis. Gastroenterology. 1991;101:635–639. doi: 10.1016/0016-5085(91)90519-q. [DOI] [PubMed] [Google Scholar]
- 2.Nugent KP, et al. Randomized controlled trial of the effect of sulindac on duodenal and rectal polyposis and cell proliferation in patients with familial adenomatous polyposis. Br. J. Surg. 1993;80:1618–1619. doi: 10.1002/bjs.1800801244. [DOI] [PubMed] [Google Scholar]
- 3.Giardiello FM, et al. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N. Engl. J. Med. 1993;328:1313–1316. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- 4.Giardiello FM, et al. Sulindac induced regression of colorectal adenomas in familial adenomatous polyposis: evaluation of predictive factors. Gut. 1996;38:578–581. doi: 10.1136/gut.38.4.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winde G, et al. Complete reversion and prevention of rectal adenomas in colectomized patients with familial adenomatous polyposis by rectal low-dose sulindac maintenance treatment: Advantages of a low-dose non steroidal anti-inflammatory drug regimen in reversing adenomas exceeding 33 months. Dis. Colon Rectum. 1995;38:813–830. doi: 10.1007/BF02049838. [DOI] [PubMed] [Google Scholar]
- 6.Cruz-Correa M, et al. Long-term treatment with sulindac in familial adenomatous polyposis: a prospective cohort study. Gastroenterology. 2002;122:641–645. doi: 10.1053/gast.2002.31890. [DOI] [PubMed] [Google Scholar]
- 7.Keller JJ, et al. Rectal epithelial apoptosis in familial adenomatous polyposis with sulindac. Gut. 1999;45:795–796. doi: 10.1136/gut.45.6.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giardiello FM, et al. Primary chemoprevention of familial adenomatous polyposis with sulindac. N. Engl. J. Med. 2002;346:1054–1059. doi: 10.1056/NEJMoa012015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyskens FL, et al. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev. Res. 2008;1:32–38. doi: 10.1158/1940-6207.CAPR-08-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niv Y, et al. Adenocarcinoma in the rectal segment in familial polyposis coli is not prevented by sulindac therapy. Gastroenterology. 1994;107:854–857. doi: 10.1016/0016-5085(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 11.Lynch HT, et al. Rectal cancer after prolonged sulindac chemoprevention. Cancer. 1995;75:936–938. doi: 10.1002/1097-0142(19950215)75:4<936::aid-cncr2820750407>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 12.Matsubashi N, et al. Rectal cancer after sulindac therapy for a sporadic adenomatous colonic polyp. Am. J. Gastroenterol. 1998;93:2261–2266. doi: 10.1111/j.1572-0241.1998.00630.x. [DOI] [PubMed] [Google Scholar]
- 13.Tonelli F, et al. Long term treatment with sulindac in familial adenomatosis polyposis: is there an actual efficacy in prevention of rectal cancer? J. Surg. Oncol. 2000;74:15–20. doi: 10.1002/1096-9098(200005)74:1<15::aid-jso4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 14.Boolbol SK, et al. Cyclooxygenase-2 overexpression and tumor formation are blocked by sulindac in a murine model of familial adenomatous polyposis. Cancer Res. 1996;56:2556–2560. [PubMed] [Google Scholar]
- 15.Beazer-Barclay Y, et al. Sulindac suppresses tumorigenesis in the Min mouse. Carcinogenesis. 1996;17:1757–1760. doi: 10.1093/carcin/17.8.1757. [DOI] [PubMed] [Google Scholar]
- 16.Chiu CH, et al. Sulindac causes rapid regression of preexisting tumors in Min/+ mice independent of prostaglandin biosysnthesis. Cancer Res. 1997;57:4267–4273. [PubMed] [Google Scholar]
- 17.Yang K, et al. Regional response leading to tumorigenesis after sulindac in small and large intestine of mice with Apc mutations. Carcinogenesis. 2003;24:605–611. doi: 10.1093/carcin/24.3.605. [DOI] [PubMed] [Google Scholar]
- 18.Palmerini E, et al. Piroxicam increases colon tumorigenesis and promotes apoptosis in Mlh1+/-Apc1638N/+ mice. Anticancer Res. 2007;27:3807–3812. [PubMed] [Google Scholar]
- 19.Yang K, et al. Sulindac induces benign and malignant tumors with increased COX-2 expression in the proximal colon of normal C57Bl/6 mice. Proc. Am. Assoc. Cancer Res. 2005;46:1427. [Google Scholar]
- 20.Fodde R, et al. A targeted chain-termination mutation in the mouse Apc gene results in multiple intestinal tumors. Proc. Natl Acad. Sci. USA. 1994;91:8969–8973. doi: 10.1073/pnas.91.19.8969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edelmann W, et al. Tumorigenesis in Mlh1 and Mlh1/Apc1638N mutant mice. Cancer Res. 1999;59:1301–1307. [PubMed] [Google Scholar]
- 22.Boivin GP, et al. Pathology of mouse models of intestinal cancer: consensus report and recommendations. Gastroenterology. 2003;124:762–777. doi: 10.1053/gast.2003.50094. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki K, et al. Sulindac rapidly induces inflammation and neoplasms in the intestine of A/J mice. Proc. Am. Assoc. Cancer Res. 2007;48:2007. [Google Scholar]
- 24.Bjarnason I, et al. Side effects of nonsteroidal anti-inflammatory drugs on the small and large intestine in humans. Gastroenterology. 1993;104:1832–1847. doi: 10.1016/0016-5085(93)90667-2. [DOI] [PubMed] [Google Scholar]
- 25.Higuchi K, et al. Present status and strategy of NSAIDs-induced small bowel injury. J. Gastroenterol. 2009 doi: 10.1007/s00535-009-0102-2. (epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 26.Lanas A, et al. Microbial flora in NSAID-induced intestinal damage: a role for antibiotics? Digestion. 2006;73(suppl. 1):136–150. doi: 10.1159/000089789. [DOI] [PubMed] [Google Scholar]
- 27.Bjarnason I, et al. Intestinal permeability in the pathogenesis of NSAID-induced enteropathy. J. Gastroenterol. 2009;44(suppl. XIX):23–29. doi: 10.1007/s00535-008-2266-6. [DOI] [PubMed] [Google Scholar]
- 28.Minami T, et al. Sulindac enhances the proteasome inhibitor bortezomib-mediated oxidative stress and anticancer activity. Clin. Cancer Res. 2005;11:5248–5256. doi: 10.1158/1078-0432.CCR-05-0085. [DOI] [PubMed] [Google Scholar]
- 29.Seo S-K, et al. Sulindac-derived reactive oxygen species induce apoptosis of human multiple myeloma cells via p38 mitogen activated protein kinase-induced mitochondrial dysfunction. Apoptosis. 2007;12:195–209. doi: 10.1007/s10495-006-0527-5. [DOI] [PubMed] [Google Scholar]
- 30.Adachi M, et al. Nonsteroidal anti-inflammatory drugs and oxidative stress in cancer cells. Histol. Histopathol. 2007;22:437–442. doi: 10.14670/HH-22.437. [DOI] [PubMed] [Google Scholar]
- 31.Sun Y, et al. Chemopreventive agents induce oxidative stress in cancer cells leading to COX-2 overexpression and COX-2 independent cell death. Carcinogenesis. 2009;30:93–100. doi: 10.1093/carcin/bgn242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hussain SP, et al. Radical causes of cancer. Nat. Rev. Cancer. 2003;3:276–285. doi: 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
- 33.Chang CL, et al. Oxidative stress inactivates the human DNA mismatch repair system. Am. J. Physiol. Cell Physiol. 2002;283:148–154. doi: 10.1152/ajpcell.00422.2001. [DOI] [PubMed] [Google Scholar]
- 34.Sun Y, et al. Selective inhibitors of MEK1/ERK44/42 and p38 mitogen-activated protein kinases potentiate apoptosis induction by sulindac sulfide in human colon carcinoma cells. Mol. Cancer Ther. 2005;4:51–59. [PubMed] [Google Scholar]
- 35.Kumar S, et al. p38 MAP kinases: key signaling molecules are therapeutic targets for inflammatory diseases. Nat. Rev. Drug Discov. 2003;2:717–726. doi: 10.1038/nrd1177. [DOI] [PubMed] [Google Scholar]
- 36.Saklatvala J. The p38 MAP kinase pathway as a therapeutic target in inflammatory disease. Curr. Opin. Pharmacol. 2004;4:372–377. doi: 10.1016/j.coph.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 37.Cuenda A, et al. P38 MAP-kinases pathway regulation, function and role in human diseases. Biochim. Biophys. Acta. 2007;1773:1358–1375. doi: 10.1016/j.bbamcr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 38.Cohen P. Targeting protein kinases for the development of anti-inflammatory drugs. Curr. Opin. Cell Biol. 2009;21:317–324. doi: 10.1016/j.ceb.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 39.Mantovani A, et al. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 40.Yang GY, et al. Inflammatory bowel disease: a model of chronic inflammation-induced cancer. Methods Mol. Biol. 2009;511:193–233. doi: 10.1007/978-1-59745-447-6_9. [DOI] [PubMed] [Google Scholar]
- 41.Colotta F, et al. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1061–1073. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 42.Cooper HS, et al. The role of mutant Apc in the development of dysplasia and cancer in the mouse model of dextran sulfate sodium-induced colitis. Gastroenterology. 2001;121:1407–1416. doi: 10.1053/gast.2001.29609. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka T, et al. Dextran sodium sulfate strongly promotes colorectal carcinogenesis in Apc (Min/+): mice: inflammatory stimuli by dextran sodium sulfate results in the development of multiple colonic neoplasms. Int. J. Cancer. 2006;118:25–34. doi: 10.1002/ijc.21282. [DOI] [PubMed] [Google Scholar]
- 44.Huang EH, et al. Induction of inflammatory bowel disease accelerates adenoma formation in Min+/- mice. Surgery. 2006;139:782–788. doi: 10.1016/j.surg.2005.11.005. [DOI] [PubMed] [Google Scholar]