Abstract
Treatment of human head and neck squamous cell carcinoma (HNSCC) cell lines with guggulsterone, a widely available, well-tolerated nutraceutical, demonstrated dose-dependent decreases in cell viability with EC50s ranging from 5 to 8 μM. Guggulsterone induced apoptosis and cell cycle arrest, inhibited invasion and enhanced the efficacy of erlotinib, cetuximab and cisplatin in HNSCC cell lines. Guggulsterone induced decreased expression of both phosphotyrosine and total signal transducer and activator of transcription (STAT)-3, which contributed to guggulsterone's growth inhibitory effect. Hypoxia-inducible factor (HIF)-1α was also decreased in response to guggulsterone treatment. In a xenograft model of HNSCC, guggulsterone treatment resulted in increased apoptosis and decreased expression of STAT3. In vivo treatment with a guggulsterone-containing natural product, Guggulipid, resulted in decreased rates of tumor growth and enhancement of cetuximab's activity. Our results suggest that guggulsterone-mediated inhibition of STAT3 and HIF-1α provide a biologic rationale for further clinical investigation of this compound in the treatment of HNSCC.
Introduction
Head and neck squamous cell carcinoma (HNSCC), the sixth most common cancer in the United States (1), and the single most common cancer in many developing countries, is associated with a mortality rate of over 50% (2). Therapies include surgical intervention, radiation, conventional chemotherapy and strategies to inhibit the epidermal growth factor receptor (EGFR). Patients who survive their initial treatment often suffer severe morbidity resulting from damage to the upper aerodigestive tract secondary to treatment or from tumor invasion. Second primary tumors occur at a rate of 4–7% per year, among the highest for any neoplasm (3). This is thought to result from “field cancerization,” molecular changes that arise throughout the upper aerodigestive tract in response to carcinogen exposure (4). A great need exists, therefore, for new therapies, including complementary and preventive approaches to treating HNSCC.
The extract of the plant Commiphora mukul is used in Indian ayurvedic medicine to treat numerous ailments. Guggulsterone, a compound contained in this plant resin, is widely available as a dietary supplement and has been used in many clinical trials that have focused on its cholesterol-lowering potential. Guggulsterone's anticancer activity was first demonstrated by Shishodia et al. (5) who, in various cell lines, including HNSCC, demonstrated guggulsterone-induced inhibition of nuclear factor kappa B (NFκB), a transcription factor that plays a role in inflammation and cancer. Subsequent studies demonstrated guggulsterone's anticancer activity in a variety of cancer cell lines and in animal models of prostate and skin cancer (6–8).
Previous reports have demonstrated interactions between NFκB and another oncogenic transcription factor, signal transducer and activator of transcription (STAT)-3 (9), both of which are known to play important roles in HNSCC. STAT3 enhances cellular transformation (10) and mediates survival and growth in HNSCC cell lines and tumor xenografts (11–13). Ahn et al. (14) recently demonstrated guggulsterone-induced decreases in levels of phosphotyrosine STAT3, seen in both multiple myeloma and HNSCC cell lines.
Hypoxia-inducible factor (HIF)-1α, which is regulated by both NFκB (15) and STAT3 (16), is known to regulate genes involved in tumor growth, tumor angiogenesis, invasion, metastasis and drug resistance in various cancers, including HNSCC (17). HIF-1α expression is a predictor of poor prognosis and resistance to radiotherapy in human HNSCC (17).
In the current study, we investigated guggulsterone's effects in HNSCC pre-clinical models and the role of STAT3 signaling in mediating these effects. Our results demonstrate that guggulsterone induces apoptosis and cell cycle arrest, decreases invasiveness and enhances the effects of currently available HNSCC therapies. Furthermore, the expression of HIF-1α was found to decrease dramatically with guggulsterone treatment. Guggulsterone's growth inhibitory effects were mediated, at least in part, by modulation of STAT3 signaling, notably characterized by decreases in both total STAT3 as well as phosphotyrosine STAT3 expression. Guggulsterone inhibited HNSCC tumor growth in vivo, thereby supporting the future clinical development of this compound in HNSCC.
Materials and methods
Cell lines and reagents
Human HNSCC cell lines included PCI-37a, created at the University of Pittsburgh (18), UM-22b, provided by Dr Thomas Carey (University of Michigan, Ann Arbor, MI) (19), and 1483 provided by Dr Gary Clayman (MD Anderson Cancer Center, Houston, TX) (20). Human SV40-immortalized esophageal epithelial cells, Het-1a, were purchased from American Type Culture Collection (Manassas, VA). E and Z guggulsterone (Steraloids, Newport, RI) were dissolved in 100% dimethyl sulfoxide for cell treatments and suspended in saline for animal treatments. Guggulipid (Sabinsa, Piscataway, NJ) was suspended in 5% ethanol/corn oil for animal treatments. Cisplatin (Bedford Laboratories, Bedford, OH) and cetuximab (ImClone, New York, NY) were diluted in saline, and erlotinib (Chemietek, Indianapolis, IN) was dissolved in dimethyl sulfoxide.
Trypan blue dye exclusion assays
Cells were suspended in trypan blue dye (Invitrogen, Carlsbad, CA) and counted under an inverted microscope. EC50s were calculated using GraphPad Prism software.
Apoptosis assay
Histone-associated DNA fragments were detected using a Cell Death Detection ELISA (Roche Diagnostics, Indianapolis, IN) according to the manufacturer's instructions. Enrichment factor = (average blanked optical density of guggulsterone-treated cells)/(average blanked optical density of vehicle-treated cells). Enrichment factor > 1 indicates an increase in apoptosis.
Cell cycle analysis
Cells were fixed in 70% ethanol/phosphate-buffered saline, stained with propidium iodide/RNase A (BD Biosciences, San Diego, CA) and subject to flow cytometric analysis with a BD FACSCalibur flow cytometer. BD CellQuest and Modfit software were used for data acquisition and analysis.
Matrigel invasion assays
Cells were plated, in serum-free Dulbecco’s modified Eagle’s medium in matrigel-coated modified Boyden inserts (8 μm pore) (BD Biosciences), the lower well containing Dulbecco’s modified Eagle’s medium/10% fetal bovine serum. Both the insert and outer well contained drug solutions and epidermal growth factor (10 ng/ml). Matrigel inserts were fixed, stained and counted (at least four fields per insert) as described previously (21).
Immunoblotting for STAT3, phosphotyrosine STAT3, HIF-1α and β-actin
As described previously (21), whole-cell lysates were subject to sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes, which were blocked in 5% non-fat dry milk in 0.2% Tween 20/phosphate-buffered saline and probed with antibodies for phosphotyrosine STAT3, total STAT3 (Cell Signaling Technology, Beverly, MA) or HIF-1α (BD Biosciences). Membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (Bio-Rad Laboratories, Hercules, CA), developed using luminol reagents (Santa Cruz Biotechnology, Santa Cruz, CA) and visualized by autoradiography. Densitometry was performed using DigiDoc1000 software. Relative units = densitometric value for protein of interest/densitometric value for corresponding β-actin band.
STAT3 small interfering RNA transfection
The 1483 and UM-22b cells were transfected with siGenome Duplex STAT3 or GFP duplex 1 (Dharmacon, Lafayette, CO) plus Lipofectamine 2000 (Invitrogen), in OPTIMEM 1 medium (Invitrogen) for 4 h. Dulbecco’s modified Eagle’s medium/10% fetal bovine serum was then replaced.
In vivo tumor xenograft studies
Guggulsterone study, pre-treatment model.
Female nude mice (6–8 weeks old; eight in each of two groups) were treated daily, by oral gavage, with 2 mg guggulsterone (Steraloids) or with saline. After 2 weeks, 1 × 106 1483 and UM-22b cells were injected into each animal's left and right flank, respectively. Daily treatments continued for another 3 weeks and mice were euthanized 1 week after the last treatment. Tumors were fixed, sectioned onto glass slides and stained with terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling or, by immunohistochemistry, for STAT3 (Cell Signaling Technology). Slides were scored by a pathologist (Dr. Raja Seethala) who was blinded to treatment groups. Immunohistochemical score = (% tumor section scored as 1+) × 1 + (% tumor section scored as 2+) × 2+ (% tumor section scored as 3+) × 3.
Guggulipid and cetuximab study, therapy model.
In total, 40 female nude mice (6–8 weeks old) were inoculated with 2 × 106 UM-22b cells into each animal's right flank. After tumor outgrowth, 10 days later, mice were randomized to four groups, based on tumor volumes, and treated daily by oral gavage, with 25.9 mg guggulipid (7.73% guggulsterone; Sabinsa), re-suspended in corn oil/5% ethanol, twice per week with 0.8 mg cetuximab (ImClone), by intraperitoneal injection, with both drugs or with the corresponding vehicles. Blinded tumor measurements were taken three times per week.
Statistical analyses
Statistical analyses of in vitro results and tissue stains were done using the non-parametric Mann–Whitney test (two-tailed and exact). Xenograft growth curves were analyzed with a general linear model that assumed animals were random effects. Data were examined for the interaction between treatment group and day of observation, testing whether the slopes of the growth curves (volume versus day of observation) were significantly different for the control and treatment groups.
Results
Guggulsterone inhibits the growth of HNSCC cell lines
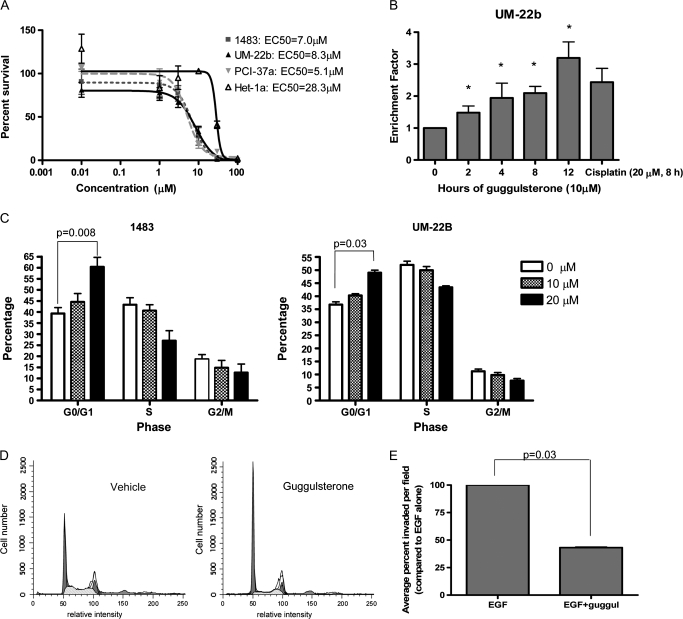
After 72 h of treatment of 1483, UM-22b or PCI-37a cells with an even mixture of guggulsterone's stereoisomers, inhibition of HNSCC cell growth occurred with EC50s of 7.0 μM for 1483, 8.3 μM for UM-22b and 5.1 μM for PCI-37a (Figure 1A). Dose-dependent growth inhibition was seen as early as 24 h after treatment, with similar results for the even mixture of stereoisomers versus either stereoisomer alone (data not shown). The EC50 concentrations are comparable with those observed in guggulsterone treatment of other cancer cell lines (5,22,23). Furthermore, the concentrations required to inhibit the growth of HNSCC cell lines are physiologically attainable in vivo. Though pharmacokinetic parameters for guggulsterone have not been determined in humans, the maximal plasma concentration of guggulsterone (Cmax) in rats was ∼3.3 μM following oral gavage with 50 mg guggulsterone/kg body wt (24). In an immortalized esophageal cell line, Het-1a, guggulsterone was found to have an EC50 of 28.3 μM, suggesting that, at physiologically attainable concentrations, guggulsterone has a lesser effect on non-neoplastic cells. The plant extract, sold as a dietary supplement, contains both stereoisomers of guggulsterone in nearly equal amounts (data not shown). Since the plant extract is more likely than the synthetic compound to be used as a clinical formulation and because, based on our observations, there is no apparent advantage to using one stereoisomer over the other, subsequent in vitro studies were performed using an even mixture of the two stereoisomers.
Fig. 1.
Guggulsterone treatment decreases viability, induces apoptosis, arrests the cell cycle and inhibits invasion in HNSCC cell lines. (A) 1483, UM-22b, PCI-37a and Het-1a cells were treated with guggulsterone (0.01–100 μM) for 72 h, stained with trypan blue dye and counted. Experiments were done with triplicate samples and performed twice with similar results. (B) UM-22b cells were treated with guggulsterone (10 μM) or cisplatin (20 μM), as a positive control. Histone-associated DNA fragments were detected, by enzyme-linked immunosorbent assay, in cell lysates. Enrichment factor = (average blanked optical density of guggulsterone-treated cells)/(average blanked optical density of vehicle-treated cells). Enrichment factor > 1 represents an increase in apoptosis. The experiment was performed four times, each with duplicate wells, with similar results (*P = 0.03 for all time points). (C) 1483 and UM-22b were treated with guggulsterone (10 and 20 μM) for 24 h, fixed, stained with propidium iodide and analyzed by flow cytometry. The y axis represents the percentage of the entire cell population within each sample currently in each phase of the cell cycle. Each experiment was performed at least four times, each with triplicate samples, with similar results (P = 0.008 for 1483 and P = 0.03 for UM-22b). (D) Representative cell cycle analysis histograms of 1483 cells treated with vehicle or guggulsterone (20 μM). (E) UM-22b cells were plated, in serum-free Dulbecco’s modified Eagle’s medium, in matrigel inserts within wells containing Dulbecco’s modified Eagle’s medium/10% fetal bovine serum. Both inserts and outer wells contained either guggulsterone (8.3 μM) or dimethyl sulfoxide, with epidermal growth factor (10 ng/ml). After 24 h, matrigel inserts were fixed, stained and numbers of invading cells counted. The experiment was performed four times, using duplicate samples and counting at least four fields per well (P = 0.03).
Guggulsterone induces apoptosis and cell cycle arrest and inhibits invasion in HNSCC cell lines
Guggulsterone has been reported to induce apoptosis in prostate carcinoma (23), monocytic leukemia (22) and multiple myeloma (14) cells. In HNSCC cells, we examined guggulsterone's effect on DNA degradation through detection of histone-associated DNA fragments in cell lysates, a marker of apoptosis. Guggulsterone (10 μM) induced a time-dependent increase in histone-associated DNA fragments in UM-22b cells; 12 h of treatment resulted in a 3.2-fold increase in histone-associated DNA fragments (P = 0.03) (Figure 1B); 72 h guggulsterone treatment of 1483 and UM-22b cells results in relatively modest, but statistically significant by densitometric analysis, decreases in pro-caspase 3, an enzyme mediator of apoptosis that is inactive in its pro-form and cleaved upon activation (P = 0.03; Figure S1). Consistent increases in the caspase 3 cleavage product were not observed, however, indicating that guggulsterone's induction of caspase 3-mediated apoptosis is, at most, modest.
We next examined the effects of guggulsterone on the cell cycle, which is often aberrantly regulated in cancer cells. Flow cytometric analysis of propidium iodide stained 1483 and UM-22b cells that had been treated with guggulsterone for 24 h revealed a dose-dependent increase in the proportion of cells in the G0/G1 phases of the cell cycle as compared with vehicle control. This population increased by 13.3 and 53.6% at 10 and 20 μM, respectively, in 1483 cells and by 9.5 and 33.2%, respectively, in UM-22b cells (Figure 1C and D). These results were statistically significant at 20 μM guggulsterone (P = 0.008 for 1483 and P = 0.03 for UM-22b). Similar results have been reported previously in human monocytic leukemia and multiple myeloma cells (14,22). In UM-22b cells, expression levels of proteins that promote the transition from the G1 to the S phase of the cell cycle, including CDK4, CDK6, CDK2, phosphorylated Rb, cyclin E and cyclin D1, which is a target gene for both STAT3 and NFκB (25,26), showed modest decreases following 16 h of guggulsterone (10 μM) treatment (Figure S2).
The invasive properties of HNSCC cells contribute to the morbidity and mortality associated with this neoplasm. An in vitro matrigel invasion assay was used to determine the effect of guggulsterone treatment on the invasive potential of UM-22b, a cell line derived from a cervical lymph node HNSCC metastasis. Epidermal growth factor was used to stimulate invasion. Cells were allowed to invade while treated for 24 h with 8.3 μM guggulsterone, the 72-h EC50, which results in only small decreases in viability after 24 h. Guggulsterone treatment resulted in a 56.8% decrease in average numbers of invasive cells (Figure 1E).
Guggulsterone enhances the growth inhibitory and anti-invasion activities of HNSCC therapies
Guggulsterone has recently been shown to inhibit drug efflux transporters involved in multi-drug resistance to cancer therapies (27). We examined the effect of combining guggulsterone with two different HNSCC therapies, the widely used chemotherapeutic drug, cisplatin, and the small molecule EGFR tyrosine kinase inhibitor, erlotinib (Tarceva), which is in advanced stages of clinical testing (Figure 2A). Another EGFR-inhibiting therapy, cetuximab (C225), has been approved for treatment of HNSCC but is known to only inhibit growth in vivo, and not in vitro, perhaps, because its efficacy relies on the presence of extracellular factors. Combining the ∼72-h EC50 concentration of each drug (1.5 μM for cisplatin and 10 μM for erlotinib) with guggulsterone at its EC50 (7.0 μM in 1483 cells) significantly enhanced each drug's growth inhibitory effect (P = 0.03 for both drugs; Figure 2A). The inhibitory effect that both erlotinib (5 μM) and cetuximab (4 μg/ml) have on HNSCC invasion was also enhanced with the addition of guggulsterone at its 72-h EC50 (8.3 μM in UM-22b cells) (P = 0.03 for both drugs) (Figure 2B).
Fig. 2.
Guggulsterone enhances the activity of cisplatin, erlotinib and cetuximab in HNSCC cells. (A) 1483 cells were treated with guggulsterone (7 μM) and/or erlotinib (10 μM) or cisplatin (1.5 μM). Trypan blue dye exclusion assays were performed after 72 h. Each experiment was performed with triplicate samples a total of four times with similar results (P = 0.03). (B) UM-22b cells were plated in serum-free Dulbecco’s modified Eagle’s medium, in matrigel inserts within wells containing Dulbecco’s modified Eagle’s medium/10% fetal bovine serum. Both inserts and outer wells contained EGF (10 ng/ml) and either guggulsterone (8.3 μM) or dimethyl sulfoxide, with or without erlotinib (5 μm) or cetuximab (4 μg/ml). After 24 h, matrigel inserts were fixed and stained and numbers of invading cells counted. The experiment was performed four times, using duplicate samples and counting at least four fields per well (P = 0.03 for both drugs).
Guggulsterone decreases total and phosphotyrosine STAT3 expression levels in HNSCC cell lines and enhances STAT3 inhibition in combination with EGFR-targeting therapy
After 72 h, guggulsterone-treated 1483, UM-22b and PCI-37a cells demonstrated dose-dependent decreases in both phosphotyrosine STAT3 and total STAT3 that were statistically significant, upon densitometric analysis (P = 0.03) (Figure 3). This effect, though less pronounced, was seen as early as 24 h after treatment (data not shown). Guggulsterone treatment did not decrease levels of phosphorylated or total p42/p44 mitogen-activated protein kinase, which is stimulated by some of the same upstream mediators as STAT3. Nor did guggulsterone decrease levels of STAT1, which is thought to behave as a tumor suppressor in HNSCC and which has an analogous protein sequence and structure to STAT3 (data not shown). Phosphoserine STAT3, whose role in HNSCC is less clear than that of phosphotyrosine STAT3, was also found to decrease with guggulsterone treatment, perhaps reflecting the observed decrease in total STAT3 (Figure S3). In contrast to results reported by Ahn et al. (14), who demonstrated decreases in phosphotyrosine STAT3, but not total STAT3, upon treatment of HNSCC cells with the Z but not the E isomer, in our study, decreases in both phosphotyrosine and total STAT3 were seen with each stereoisomer of guggulsterone alone (data not shown). Using Real-time polymerase chain reaction of mRNA from 1483 and UM-22b cells treated with guggulsterone for up to 24 h, we were, however, unable to detect changes in mRNA levels of STAT3 with guggulsterone treatment (data not shown), indicating that guggulsterone may affect either translation or degradation of the STAT3 protein. Another recent study has demonstrated inhibition of total STAT3 protein expression in colorectal cancer cells treated with Z-guggulsterone, albeit at much higher concentrations (28).
Fig. 3.
Guggulsterone decreases total and phosphotyrosine STAT3 expression levels in HNSCC cell lines and enhances STAT3 inhibition in combination with EGFR-targeting therapy. (A) 1483, UM-22b and PCI-37a cells were treated with guggulsterone (5 or 10 μM) in Dulbecco’s modified Eagle’s medium/10% fetal bovine serum for 72 h. Whole-cell lysates were probed for total and phosphotyrosine STAT3. Densitometric analyses of bands, normalized to β-actin, appear below each blot. Each experiment was performed four times with similar results (P = 0.03 for all three cell lines). (B) UM-22b cells were treated with erlotinib (10 μM), guggulsterone (10 μM) or both drugs. Cells were treated with drug-containing serum-free Dulbecco’s modified Eagle’s medium, which was changed daily, for 72 h, and then with Dulbecco’s modified Eagle’s medium containing epidermal growth factor (10 μg/ml) for 10 min before harvesting lysates. Lysates were probed for phosphotyrosine STAT3, total STAT3 and β-actin. Densitometric analyses of bands, normalized to β-actin, appear beside the blot. The experiment was repeated four times with similar results (P = 0.03).
Guggulsterone was found to enhance STAT3 inhibition resulting from treatment of UM-22b cells with the small molecule EGFR inhibitor, erlotinib (Figure 3B; P = 0.03). UM-22b cells were treated with serum-free medium containing erlotinib alone compared with the combination of erlotinib with guggulsterone, for 72 h, and then stimulated, for 10 min, with epidermal growth factor. Similar results were observed in combining guggulsterone with the EGFR-targeting antibody, cetuximab (data not shown). These data provide a possible mechanism for guggulsterone's ability to enhance the growth inhibitory and anti-invasion activities of EGFR inhibitors and further support the potential therapeutic use of guggulsterone in combination with EGFR-targeting therapies in HNSCC.
STAT3 contributes to the growth inhibitory effects of guggulsterone in HNSCC cell lines
To determine whether or not the observed decrease in STAT3 contributes to guggulsterone's anticancer activity in HNSCC cell lines, STAT3-specific small interfering RNA (siRNA) was used to knock down STAT3 and green fluorescent protein (GFP)-specific siRNA used as a control. STAT3 knockdown was optimal 48 h after transient transfection (Figure 4A). Therefore, guggulsterone (10 μM) treatments of 1483 and PCI-37a cells were performed after a 48-h transient transfection with STAT3 or GFP siRNA, until 96 h of transient transfection, when STAT3 expression resumes, resulting in 48 h of guggulsterone treatment. At this time point, STAT3 siRNA transfection, compared with transfection with GFP siRNA, does not result in a noticeable or significant effect on viability of vehicle-treated UM-22b or PCI-37a cells (Figure S4). Despite this, to account for potential differences in cell numbers, the percent survival of each guggulsterone-treated sample was calculated as a comparison with the vehicle control sample transfected with the corresponding siRNA; 1483 cells transfected with GFP siRNA showed a 32.5% decrease in the number of viable cells after 48 h of guggulsterone treatment, but only a 9.7% decrease in cell viability in STAT3 siRNA-transfected cells treated with guggulsterone. PCI-37a cells transfected with GFP siRNA demonstrated a 43.0% decrease in cell viability with guggulsterone treatment compared with a 22.0% decrease in cell viability in the same cells transfected with STAT3 siRNA (Figure 4B; P = 0.015 for 1483, P = 0.03 for PCI-37a). The effects of guggulsterone treatment, therefore, were abrogated by siRNA-mediated knockdown of STAT3. Thus, effects on STAT3 signaling are required, at least in part, for the growth inhibitory effect of guggulsterone in HNSCC cells.
Fig. 4.
Knockdown of STAT3 inhibits guggulsterone's effect on viability of HNSCC cells. (A) 1483 and PCI-37a cells were transfected with STAT3 siRNA or GFP siRNA for 48 h. Whole-cell lysates were probed for total STAT3 and NFκB, a negative control for protein knockdown by siRNA. (B) 1483 and PCI-37a cells were transfected with STAT3 siRNA or GFP siRNA for 48 h, treated with guggulsterone (10 μM) for an additional 48 h and viability measured through trypan blue dye exclusion assay. The experiments shown in (B) were performed at least four times, each with triplicate samples, with similar results (P = 0.015 for 1483 and P = 0.03 for PCI-37a).
As STAT3 knockdown only partly abrogates guggulsterone's growth inhibitory effect, it is probable that other molecular factors involved in HSNCC cell growth and survival are affected by guggulsterone. The role of NFκB has been investigated at high concentrations of guggulsterone (5). Using a luciferase promoter assay, we have found that, at physiologically relevant concentrations up to 20 μM, guggulsterone does not significantly inhibit NFκB's transcriptional activity in HNSCC cells (data not shown).
Guggulsterone inhibits the expression of HIF-1α
Expression of HIF-1α is correlated with invasiveness of HNSCC cell lines, including UM-22b (29), resistance of HNSCC to radiotherapy and poor prognosis in HNSCC patients (17). HIF-1α over-expression has been found to be an early change in preneoplastic tissue and is thought to be important in carcinogenesis (30). HIF-1α is known to be regulated by both NFκB (15) and c-Jun-N-terminal kinase (31), which has also been implicated in guggulsterone's anticancer activity (22,32). Upon 48 h of treatment of UM-22b and 1483 cells with guggulsterone (10 μM), protein levels of HIF-1α decreased dramatically (Figure 5). The experiments shown here were performed under normoxia, though a similar trend was also observed under hypoxia (data not shown).
Fig. 5.
Guggulsterone inhibits expression of HIF-1α in HNSCC cells. UM-22b and 1483 cells were treated with guggulsterone (10 μM) for 48 h. Whole-cell lysates were probed for HIF-1α. The experiment was performed at least twice, for each cell line, with similar results.
Knockdown of STAT3 with siRNA or inhibition with a STAT3-targeted decoy oligonucleotide results in almost complete abrogation of HIF-1α expression. Furthermore, HIF-1α is not expressed in embryonic fibroblasts derived from STAT3 knockout mice (data not shown). HIF-1α expression has already been found to rely on STAT3 activity in myeloid cells (16). These data suggest that guggulsterone, a STAT3 inhibitor, primarily affects HIF-1α expression at the transcriptional level.
Guggulsterone's and guggulipid's in vivo effects on HNSCC tumor xenografts
A xenograft model of HNSCC was used to investigate the in vivo effects of guggulsterone in this neoplasm, specifically on STAT3. In order to determine the effects of guggulsterone on tumor outgrowth, eight athymic nude mice per group were treated with guggulsterone (2 mg), or with saline, by daily oral gavage, for 2 weeks prior to tumor inoculation. Oral administration was selected as it resembles the most likely eventual usage of guggulsterone for cancer therapy. After 2 weeks of treatment, mice were inoculated subcutaneously with 1483 or UM-22b cells in either flank. Daily guggulsterone treatments continued for an additional 3 weeks. One week after completing treatment, the animals were killed and tumors stained with terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling for detection of apoptosis and, by immunohistochemistry, for total STAT3. Percentages of terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling-positive cells increased 2.2-fold in 1483 xenografts and 4-fold in UM-22b xenografts from mice treated with guggulsterone versus vehicle, indicating that guggulsterone induced apoptosis in these xenografts (Figure 6A). We also observed a decrease in STAT3 expression of 87.3% in UM-22b-derived tumors and of 44.3% in 1483-derived tumors (Figure 6B).
Fig. 6.
Guggulsterone's in vivo effects in HNSCC xenografts. (A) and (B) Nude mice (eight per group) were treated orally with 2 mg/mouse/day of guggulsterone or with saline. After 2 weeks, mice were inoculated with 1 × 106 UM-22b and 1483 cells, in the right and left flank, respectively. Treatments continued for 3 weeks following inoculation. One week after discontinuation of treatment, mice were killed and tumors harvested, sectioned and stained with (A) terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling and (B) by immunohistochemistry, for STAT3 (20× photomicrographs). Internal controls for STAT3 include vascular endothelial cells, inflammatory cells (positive) and nerves (negative). Immunohistochemical score = (% tumor section scored as 1+) × 1 + (% tumor section scored as 2+) × 2+ (% tumor section scored as 3+) × 3. (C) Nude mice (N = 40) were inoculated with 2 × 106 UM-22b cells and, after tumor outgrowth 10 days later, randomized into 4 groups of 10. Mice were then treated daily, by oral gavage, with 26 mg Guggulipid (Sabinsa), the equivalent of 2 mg/mouse/day of guggulsterone, with 0.8 mg cetuximab, twice weekly by intraperitoneal injection, with a combination of both drugs or with the corresponding vehicles. Tumors were measured three times per week. Average rates of tumor growth were found to be significantly decreased in the Guggulipid-treated group compared with vehicle (P = 0.0007) and in the cetuximab/Guggulipid combination group compared with cetuximab alone (P = 0.0017).
Guggulipid (Sabinsa) is a dietary supplement, composed of a standardized extract of C. mukul, and sold by General Nutrition Centers. Because guggulipid is more likely to be administered clinically than the synthetic guggulsterone used in our other studies, we chose to test the in vivo effects of guggulipid, alone and in combination with cetuximab. Correcting for the amount of guggulsterone present in guggulipid, this extract inhibited the growth of HNSCC cell lines in vitro with EC50’s slightly lower than those observed for synthetic guggulsterone and decreased expression levels of phosphotyrosine STAT3 and total STAT3 at the same concentrations and to a comparable degree as synthetic guggulsterone (data not shown). Mice were treated either orally with 25.9 mg/mouse of guggulipid, the equivalent of 2 mg/mouse of guggulsterone (7.73% of guggulipid), daily, twice weekly with 0.8 mg cetuximab by intraperitoneal injection, with both drugs or with corresponding vehicles. Blinded measurements of tumors were taken three times per week (Figure 6C). The average rate of tumor growth was 24.3 mm3/day for the guggulipid-treated group, 30.7% lower than that for the vehicle-treated group, 35.0 mm3/day, and 18.9 mm3/day for the cetuximab/guggulipid combination group, 27.0% lower than that for the group treated with cetuximab alone, 24.3 mm3/day. Using a general linear model analysis, rates of tumor growth were found to be significantly different for the vehicle and guggulipid groups (P = 0.0007) and between the cetuximab and cetuximab/guggulipid combination groups (P = 0.0017).
Discussion
Guggulsterone's safety (8) makes it a good candidate for complementary and preventive therapy. In the present study, guggulsterone treatment of HNSCC cell lines was found to induce growth inhibition associated with apoptosis, cell cycle arrest, decreased invasiveness and enhancement of the effects of current therapies including the chemotherapy agent cisplatin and EGFR inhibitors cetuximab and erlotinib. The growth inhibitory effect of guggulsterone is mediated, at least in part, through inhibition of STAT3 signaling. Guggulsterone also abrogated expression of HIF-1α, a therapeutic target downstream of STAT3. In vivo, when given in the form of guggulipid, guggulsterone treatments resulted in increased apoptosis and decreased expression of STAT3, as well as growth inhibition and enhancement of the efficacy of the EGFR-targeting antibody, cetuximab.
The in vivo anticancer activity of synthetic guggulsterone, as opposed to a C. mukul extract like guggulipid, has been shown in a xenograft model of prostate cancer (7) and a chemical carcinogenesis model of skin cancer (6). We chose to test the guggulsterone-containing nutraceutical, guggulipid, whose anticancer activity has not been demonstrated previously in vivo because pure, synthetic guggulsterone is expensive and currently unavailable as a clinical formulation. Guggulipid inhibited HNSCC growth and enhanced cetuximab's activity in vivo.
Our findings suggest that guggulsterone inhibits the growth of HNSCC, at least in part, via decreases in STAT3. Ahn et al. (14) have reported decreases in phosphotyrosine but not total STAT3 induced by the Z but not the E stereoisomer of guggulsterone. This is in contrast to our observations that either stereoisomer alone as well as the even mixture decrease levels of both total STAT3, which was seen in vitro and in vivo, as well as phosphotyrosine STAT3. The mechanism of guggulsterone's inhibition of STAT3 reported by Ahn et al. was induction of the protein tyrosine phosphatase SHP-1, which explains changes in phosphotyrosine STAT3 but does not account for the decreases in total STAT3. Guggulsterone's effect on total STAT3 levels is of interest, particularly in light of evidence that STAT3’s translocation to the nucleus may occur independently of tyrosine phosphorylation and that STAT3 activity may be regulated by other post-translational modifications, aside from tyrosine phosphorylation (33–35). The cellular mechanisms of regulating STAT3 levels through transcription, translation and degradation are incompletely understood. Using real-time polymerase chain reaction of mRNA from 1483 and UM-22b cells treated, for up to 24 hours, with guggulsterone, we were unable to detect changes in mRNA levels of STAT3 with guggulsterone treatment, suggesting that either STAT3 translation or degradation may be altered by guggulsterone.
Several cancer therapies currently under investigation in pre-clinical studies, including various natural compounds, inhibit STAT3 tyrosine phosphorylation (36,37). With many of these natural compounds, inhibition of STAT3 has been shown to be mediated through effects on signaling mediators upstream of STAT3 (36). To our knowledge, these natural compounds have not been shown to induce a decrease in total STAT3 levels as we have observed with guggulsterone treatment of HNSCC.
Mechanisms of guggulsterone's anticancer activity upstream of STAT3 and its direct inhibitor, SHP-1, have not been studied in HNSCC. Guggulsterone antagonizes the farnesoid X receptor (FXR), a nuclear receptor, which regulates lipid metabolism but also plays a role in breast cancer cell invasiveness (38) and survival of Barrett's esophagus cells (39). We observed FXR expression in all four HNSCC cell lines tested (data not shown). Guggulsterone is also an agonist of the pregnane X receptor (40), which regulates expression of CYP3a, an enzyme involved in carcinogen metabolism and whose expression is decreased in HNSCC tumors compared with adjacent normal tissue (41). Agonists of the retinoid X receptor, which dimerizes with both the FXR and pregnane X receptor, have been found to inhibit STAT3 in HNSCC cell lines (42) and have shown promise as chemopreventive therapies for HNSCC, albeit with unacceptable toxicity. Investigation into guggulsterone's effects on the FXR, pregnane X receptor and retinoid X receptor in HNSCC cell lines may help to elucidate the mechanisms of guggulsterone's anticancer activity in HNSCC.
In addition to directly regulating the expression of oncogenes, both NFκB and STAT3 have been shown to promote tumorigenesis via their role in inflammation (43,44), which plays an important role in HNSCC (44). The involvement of reactive oxygen species in HNSCC carcinogenesis has also been found to be associated with the actions of inflammatory mediators, including NFκB (45). Guggulsterone has been shown to function as an antioxidant (46,47) as well as induce increases in reactive oxygen species in prostate carcinoma cells (32). Guggulsterone may have complex effects on levels of reactive oxygen species that depend on the individual system and have implications for guggulsterone's therapeutic potential.
In a human myeloid cell line, STAT3 has been found to be required for HIF-1α expression (16). In our study, dramatic abrogation of HIF-1α expression was seen in treatment of UM-22b cells with guggulsterone. To our knowledge, this is the first evidence of guggulsterone's effect on HIF-1α. Various pre-clinical studies have been devoted to targeting HIF-1α with specific inhibitors and natural products (48).
Further investigation is required to determine the mechanism of guggulsterone-induced decreases in total STAT3. The data presented here, demonstrating guggulsterone's ability to target STAT3 and HIF-1α and to enhance the efficacies of HNSCC therapies, are suggestive of the clinical utility of guggulsterone, a safe and inexpensive nutraceutical, as a potential complementary therapy for the treatment of HNSCC.
Supplementary material
Supplementary Figures S1–S4 can be found at http://carcin.oxfordjournals.org/.
Funding
National Institutes of Health (1F30 ES015669 01 for R.J.L., CA101753 for S.V.S., P50CA097190-01 and R01 CA101840 for J.R.G.).
Supplementary Material
Acknowledgments
We would like to acknowledge William E. Gooding (Department of Biostatistics, University of Pittsburgh) who was helpful in reviewing our statistical analyses.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- EGFR
epidermal growth factor receptor
- FXR
farnesoid X receptor
- HNSCC
head and neck squamous cell carcinoma
- HIF
hypoxia-inducible factor
- NFκB
nuclear factor kappa B
- siRNA
small interfering RNA
- STAT
signal transducer and activator of transcription
References
- 1.Jemal A, et al. Cancer statistics, 2006. CA Cancer J. Clin. 2006;2:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.Sturgis EM. A review of social and behavioral efforts at oral cancer preventions in India. Head Neck. 2004;26:937–944. doi: 10.1002/hed.20075. [DOI] [PubMed] [Google Scholar]
- 3.Do KA, et al. Second primary tumors in patients with upper aerodigestive tract cancers: joint effects of smoking and alcohol (United States) Cancer Causes Control. 2003;14:131–138. doi: 10.1023/a:1023060315781. [DOI] [PubMed] [Google Scholar]
- 4.Slaughter DP, et al. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;5:963–968. doi: 10.1002/1097-0142(195309)6:5<963::aid-cncr2820060515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 5.Shishodia S, et al. Guggulsterone inhibits NF-kappaB and ikappaBalpha kinase activation, suppresses expression of anti-apoptotic gene products, and enhances apoptosis. J. Biol. Chem. 2004;45:47148–47158. doi: 10.1074/jbc.M408093200. [DOI] [PubMed] [Google Scholar]
- 6.Sarfaraz S, et al. Guggulsterone modulates MAPK and NF-kappaB pathways and inhibits skin tumorigenesis in SENCAR mice. Carcinogenesis. 2008;10:2011–2018. doi: 10.1093/carcin/bgn180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiao D, et al. Z-guggulsterone, a constituent of ayurvedic medicinal plant commiphora mukul, inhibits angiogenesis in vitro and in vivo. Mol. Cancer Ther. 2008;1:171–180. doi: 10.1158/1535-7163.MCT-07-0491. [DOI] [PubMed] [Google Scholar]
- 8.Urizar NL, et al. Gugulipid: a natural cholesterol-lowering agent. Annu. Rev. Nutr. 2003:303–313. doi: 10.1146/annurev.nutr.23.011702.073102. [DOI] [PubMed] [Google Scholar]
- 9.Squarize CH, et al. Molecular cross-talk between the NFkappaB and STAT3 signaling pathways in head and neck squamous cell carcinoma. Neoplasia. 2006;9:733–746. doi: 10.1593/neo.06274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bromberg JF, et al. Stat3 activation is required for cellular transformation by v-src. Mol. Cell. Biol. 1998;5:2553–2558. doi: 10.1128/mcb.18.5.2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grandis JR, et al. Requirement of Stat3 but not Stat1 activation for epidermal growth factor receptor-mediated cell growth in vitro. J. Clin. Invest. 1998;7:1385–1392. doi: 10.1172/JCI3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kijima T, et al. Stat3 activation abrogates growth factor dependence and contributes to head and neck squamous cell carcinoma tumor growth in vivo. Cell Growth Differ. 2002;8:355–362. [PubMed] [Google Scholar]
- 13.Rubin Grandis J, et al. Epidermal growth factor receptor–mediated stat3 signaling blocks apoptosis in head and neck cancer. Laryngoscope. 2000;5:868–874. doi: 10.1097/00005537-200005000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Ahn KS, et al. Guggulsterone, a farnesoid x receptor antagonist, inhibits constitutive and inducible stat3 activation through induction of a protein tyrosine phosphatase shp-1. Cancer Res. 2008;11:4406–4415. doi: 10.1158/0008-5472.CAN-07-6696. [DOI] [PubMed] [Google Scholar]
- 15.Rius J, et al. Nf-kappaB links innate immunity to the hypoxic response through transcriptional regulation of hif-1alpha. Nature. 2008;7196:807–811. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niu G, et al. Signal transducer and activator of transcription 3 is required for hypoxia-inducible factor-1alpha rna expression in both tumor cells and tumor-associated myeloid cells. Mol. Cancer Res. 2008;7:1099–1105. doi: 10.1158/1541-7786.MCR-07-2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brennan PA, et al. Hypoxia-inducible factor 1alpha in oral cancer. J. Oral Pathol. Med. 2005;7:385–389. doi: 10.1111/j.1600-0714.2005.00335.x. [DOI] [PubMed] [Google Scholar]
- 18.Heo DS, et al. Biology, cytogenetics, and sensitivity to immunological effector cells of new head and neck squamous cell carcinoma lines. Cancer Res. 1989;18:5167–5175. [PubMed] [Google Scholar]
- 19.Sacks PG, et al. Establishment and characterization of two new squamous cell carcinoma cell lines derived from tumors of the head and neck. Cancer Res. 1988;10:2858–2866. [PubMed] [Google Scholar]
- 20.Riser BL, et al. Monocyte killing of human squamous epithelial cells: role for thrombospondin. Cancer Res. 1989;21:6123–6129. [PubMed] [Google Scholar]
- 21.Koppikar P, et al. Combined inhibition of c-src and epidermal growth factor receptor abrogates growth and invasion of head and neck squamous cell carcinoma. Clin. Cancer Res. 2008;13:4284–4291. doi: 10.1158/1078-0432.CCR-07-5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shishodia S, et al. Guggulsterone inhibits tumor cell proliferation, induces S-phase arrest, and promotes apoptosis through activation of c-jun N-terminal kinase, suppression of Akt pathway, and downregulation of antiapoptotic gene products. Biochem. Pharmacol. 2007;1:118–130. doi: 10.1016/j.bcp.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh SV, et al. Caspase-dependent apoptosis induction by guggulsterone, a constituent of ayurvedic medicinal plant commiphora mukul, in pc-3 human prostate cancer cells is mediated by Bax and Bak. Mol. Cancer Ther. 2005;11:1747–1754. doi: 10.1158/1535-7163.MCT-05-0223. [DOI] [PubMed] [Google Scholar]
- 24.Verma N, et al. Pharmacokinetics of guggulsterone after intravenous and oral administration in rats. Pharmacy Pharmacol. Commun. 1999;5:349–354. [Google Scholar]
- 25.Joyce D, et al. NF-kappaB and cell-cycle regulation: the cyclin connection. Cytokine Growth Factor Rev. 2001;1:73–90. doi: 10.1016/s1359-6101(00)00018-6. [DOI] [PubMed] [Google Scholar]
- 26.Sinibaldi D, et al. Induction of p21WAF1/CIP1 and cyclin d1 expression by the Src oncoprotein in mouse fibroblasts: role of activated STAT3 signaling. Oncogene. 2000;48:5419–5427. doi: 10.1038/sj.onc.1203947. [DOI] [PubMed] [Google Scholar]
- 27.Nabekura T, et al. Effects of plant sterols on human multidrug transporters abcb1 and abcc1. Biochem. Biophys. Res. Commun. 2008;2:363–368. doi: 10.1016/j.bbrc.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 28.Kim ES, et al. Guggulsterone inhibits angiogenesis by blocking STAT3 and VEGF expression in colon cancer cells. Oncol. Rep. 2008;6:1321–1327. [PubMed] [Google Scholar]
- 29.Cohen NA, et al. Dysregulation of hypoxia inducible factor-1alpha in head and neck squamous cell carcinoma cell lines correlates with invasive potential. Laryngoscope. 2004;3:418–423. doi: 10.1097/00005537-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Zhong H, et al. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999;22:5830–5835. [PubMed] [Google Scholar]
- 31.Shemirani B, et al. Hypoxic induction of HIF-1alpha and VEGF expression in head and neck squamous cell carcinoma lines is mediated by stress activated protein kinases. Oral Oncol. 2002;3:251–257. doi: 10.1016/s1368-8375(01)00052-5. [DOI] [PubMed] [Google Scholar]
- 32.Singh SV, et al. Guggulsterone-induced apoptosis in human prostate cancer cells is caused by reactive oxygen intermediate dependent activation of c-Jun NH2-terminal kinase. Cancer Res. 2007;15:7439–7449. doi: 10.1158/0008-5472.CAN-07-0120. [DOI] [PubMed] [Google Scholar]
- 33.Liu L, et al. STAT3 nuclear import is independent of tyrosine phosphorylation and mediated by importin-alpha3. Proc. Natl. Acad. Sci. USA. 2005;23:8150–8155. doi: 10.1073/pnas.0501643102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Komyod W, et al. Are stats arginine-methylated? J. Biol. Chem. 2005;23:21700–21705. doi: 10.1074/jbc.C400606200. [DOI] [PubMed] [Google Scholar]
- 35.Yuan ZL, et al. STAT3 dimerization regulated by reversible acetylation of a single lysine residue. Science. 2005;5707:269–273. doi: 10.1126/science.1105166. [DOI] [PubMed] [Google Scholar]
- 36.Aggarwal BB, et al. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem. Pharmacol. 2006;10:1397–1421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 37.Leeman RJ, et al. STAT3 as a therapeutic target in head and neck cancer. Expert Opin. Biol. Ther. 2006;3:231–241. doi: 10.1517/14712598.6.3.231. [DOI] [PubMed] [Google Scholar]
- 38.Silva J, et al. Lipids isolated from bone induce the migration of human breast cancer cells. J. Lipid Res. 2006;4:724–733. doi: 10.1194/jlr.M500473-JLR200. [DOI] [PubMed] [Google Scholar]
- 39.De Gottardi A, et al. Expression of the bile acid receptor fxr in barrett's esophagus and enhancement of apoptosis by guggulsterone in vitro. Mol. Cancer. 2006;5:48. doi: 10.1186/1476-4598-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burris TP, et al. The hypolipidemic natural product guggulsterone is a promiscuous steroid receptor ligand. Mol. Pharmacol. 2005;3:948–954. doi: 10.1124/mol.104.007054. [DOI] [PubMed] [Google Scholar]
- 41.Ali S, et al. Cytochrome p450 and glutathione transferase expression in squamous cell cancer. Clin. Cancer Res. 2004;13:4412–4416. doi: 10.1158/1078-0432.CCR-04-0145. [DOI] [PubMed] [Google Scholar]
- 42.Song JI, et al. Abrogation of transforming growth factor-alpha/epidermal growth factor receptor autocrine signaling by an RXR-selective retinoid (lgd1069, targretin) in head and neck cancer cell lines. Cancer Res. 2001;15:5919–5925. [PubMed] [Google Scholar]
- 43.Li Y, et al. Activation of the signal transducers and activators of the transcription 3 pathway in alveolar epithelial cells induces inflammation and adenocarcinomas in mouse lung. Cancer Res. 2007;18:8494–8503. doi: 10.1158/0008-5472.CAN-07-0647. [DOI] [PubMed] [Google Scholar]
- 44.Allen CT, et al. Role of activated nuclear factor-kappaB in the pathogenesis and therapy of squamous cell carcinoma of the head and neck. Head Neck. 2007;10:959–971. doi: 10.1002/hed.20615. [DOI] [PubMed] [Google Scholar]
- 45.Bradburn JE, et al. The effects of reactive species on the tumorigenic phenotype of human head and neck squamous cell carcinoma (HNSCC) cells. Anticancer Res. 2007;6B:3819–3827. [PMC free article] [PubMed] [Google Scholar]
- 46.Wang X, et al. The hypolipidemic natural product commiphora mukul and its component guggulsterone inhibit oxidative modification of LDL. Atherosclerosis. 2004;2:239–246. doi: 10.1016/j.atherosclerosis.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 47.Saxena G, et al. Gugulipid, an extract of commiphora whighitii with lipid-lowering properties, has protective effects against streptozotocin-induced memory deficits in mice. Pharmacol. Biochem. Behav. 2007;4:797–805. doi: 10.1016/j.pbb.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 48.Semenza GL. Targeting hif-1 for cancer therapy. Nat. Rev. Cancer. 2003;10:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.