Abstract
Recent studies have demonstrated that K-ras mutations in lung epithelial cells elicit inflammation that promotes carcinogenesis in mice (intrinsic inflammation). The finding that patients with chronic obstructive pulmonary disease (COPD), an inflammatory disease of the lung, have an increased risk of lung cancer after controlling for smoking suggests a further link between lung cancer and extrinsic inflammation. Besides exposure to cigarette smoke, it is thought that airway inflammation in COPD is caused by bacterial colonization, particularly with non-typeable Hemophilus influenzae (NTHi). Previously, we have shown that NTHi-induced COPD-like airway inflammation promotes lung cancer in an airway conditional K-ras-induced mouse model. To further test the role of inflammation in cancer promotion, we administered the natural anti-inflammatory agent, curcumin, 1% in diet before and during weekly NTHi exposure. This significantly reduced the number of visible lung tumors in the absence of NTHi exposure by 85% and in the presence of NTHi exposures by 53%. Mechanistically, curcumin markedly suppressed NTHi-induced increased levels of the neutrophil chemoattractant keratinocyte-derived chemokine by 80% and neutrophils by 87% in bronchoalveolar lavage fluid. In vitro studies of murine K-ras-induced lung adenocarcinoma cell lines (LKR-10 and LKR-13) indicated direct anti-tumoral effects of curcumin by reducing cell viability, colony formation and inducing apoptosis. We conclude that curcumin suppresses the progression of K-ras-induced lung cancer in mice by inhibiting intrinsic and extrinsic inflammation and by direct anti-tumoral effects. These findings suggest that curcumin could be used to protract the premalignant phase and inhibit lung cancer progression in high-risk COPD patients.
Introduction
Lung cancer is the major cause of cancer-related mortality in both men and women worldwide (1,2). Cigarette smoking causes 90% of all lung cancers and is thought to do so primarily by inducing DNA mutations (3). In addition, epidemiologic data indicate that chronic inflammation also plays a role in lung epithelial carcinogenesis (4). Tumor cells produce cytokines and chemokines that attract leukocytes, which provide an inflammatory microenvironment in favor of malignant conversion and tumor development (intrinsic inflammation) (5–7). Lung inflammatory diseases such as chronic obstructive pulmonary disease (COPD) are characterized by leukocyte infiltration of the airways that is regulated by a variety of mediators such as cytokines, chemokines and adhesion molecules (extrinsic inflammation). Multiple studies have found that smokers with COPD have a 1.3- to 4.9-fold increased risk of lung cancer compared with smokers without COPD (4,8,9). In view of the high incidence and mortality of lung cancer (10), the high prevalence and morbidity of COPD (11), and the lack of a recommended screening method for lung cancer, a therapeutic strategy that targets inflammation to prevent progression of COPD and cancer would be of great value.
Despite the fact that smoking causes most cases of COPD, only 25% of smokers develop COPD. This variable susceptibility to COPD most probably reflects genetic variations in the inflammatory response to inhaled smoke and to microorganisms colonizing the injured airways of smokers (12,13). The most common colonizing bacterium is non-typeable (i.e. unencapsulated) Haemophilus influenzae (NTHi) (14,15). This organism is found in the lower respiratory tract of ∼30% of individuals with COPD at any time, and the acquisition of new serotypes is associated with exacerbations of COPD (14,16–18). We have previously established a COPD-like model of airway inflammation induced by repetitive exposure to an aerosolized lysate of NTHi (19) and shown that this type of inflammation enhances lung carcinogenesis in a K-ras induced mouse model (20).
Curcumin (1,7-bis (4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3, 5-dione) is a naturally occurring polyphenolic phytochemical isolated from the rhizome of the medicinal plant Curcuma longa (turmeric) (21). It is commonly used as a spice, food additive or dietary pigment. It has been shown that curcumin has several pharmacologic effects including anti-inflammatory (22–26), antioxidant (27,28), anti-tumoral (29–34) and wound healing activities (35,36). There are only limited and contradictory data available on the effects of curcumin on lung inflammation and tumor promotion (37–39). In this study, we report that dietary administration of curcumin effectively suppresses NTHi-induced COPD-like airway inflammation and lung cancer progression in mice.
Materials and methods
Animals
Specific pathogen-free 5- to 6-week-old female C57BL/6 mice were purchased from Harlan (Indianapolis, IN). CCSPCre/LSL-K-rasG12D mice (CC-LR) were generated as described previously (20). Briefly, this is a mouse generated by crossing a mouse harboring the LSL-K-rasG12D allele with a mouse containing Cre recombinase inserted into the Clara cell secretory protein (CCSP) locus (20). All mice were housed in specific pathogen-free conditions and handled in accordance with the Institutional Animal Care and Use Committee of M. D. Anderson Cancer Center. Mice were monitored daily for evidence of disease or death.
Curcumin treatment
Female wild type (WT) C57BL/6 and CC-LR mice were fed a powdered diet (5053 Pico Lab Rodent Diet 20, Purina LabDiet, Richmond, IN) mixed with 0.2, 0.5, 1 and 2% wt/wt curcumin from 7 days before NTHi lysate exposure to the end of the study. Curcumin (curcumin 78.1%, demethoxycurcumin 17.7% and bisdemethoxycurcumin 4.2%) was purchased from Sigma–Aldrich (St Louis, MO). Curcumin administration did not alter the weight of mice, and consumption of the diet with curcumin was not noticeably different from the diet without curcumin.
NTHi lysate aerosol exposure
A lysate of NTHi strain 12 was prepared as described previously (19), the protein concentration was adjusted to 2.5 mg/ml in phosphate buffered saline (PBS), and the lysate was frozen in 10 ml aliquots at −80°C. To deliver the lysate to mice by aerosol, a thawed aliquot was placed in an AeroMist CA-209 nebulizer (CIS-US, Bedford, MA) driven by 10 l/min of room air supplemented with 5% CO2 for 20 min. WT mice were exposed to the lysate starting at 6 weeks of age for 1 week, and CC-LR and LSL-K-rasG12D mice were exposed starting at 6 weeks of age once a week for 8 weeks.
Histologic analysis
Tissues were taken from mice with the following genotypes: WT, CC-LR and LSL-K-rasG12D (control). Mice were anesthetized and killed by intraperitoneal injection of Avertin (Sigma–Aldrich, St Louis, MO) and then tracheotomized and cannulated with a luer stub adapter cannula. Lungs were inflated with 10% buffered formalin (Sigma–Aldrich), removed and placed in 10% buffered formalin for 18 h. Tissues then were transferred to 75% ethanol, embedded in paraffin, sectioned and stained with hematoxylin and eosin. Formalin-fixed, paraffin-embedded sections (5 μm) were labeled with anti-Ki-67 antibody at 1:100 (rat monoclonal clone TEC-3; Dako, Glostrup, Denmark). Slides were then incubated with secondary antibody, exposed to Alkaline Phosphatase Standard ABC Kit (Vector Laboratories, Burlingame, CA), developed with Alkaline Phosphatase Substrate Kit I (Vector Laboratories) and counterstained with 4′-6-diamidino-2-phenylindole (Molecular Probes, Eugene, OR).
Assessment of lung tumor burden and inflammation
On the first day, after the first NTHi exposure in WT mice and the first day after the eighth NTHi exposure in CC-LR and LSL-K-rasG12D mice, animals were euthanized by intraperitoneal injection of Avertin. In some mice, lung surface tumor numbers were counted and then lungs were prepared for histological analysis. Hematoxylin and eosin sections were prepared from 3–5 animals per group. Five randomly selected microscopic fields from peripheral and central regions of the lungs were photographed, and the percentage of the lung field occupied by tumors measured by overlaying of these images on a dotted grid. In other mice, bronchoalveolar lavage fluid (BALF) was obtained by sequentially instilling and collecting two aliquots of 1 ml PBS through a tracheostomy cannula, and lungs were removed and frozen for further experiments. BALF total leukocyte count was determined using a hemacytometer, and cell populations were determined by cytocentrifugation of 300 μl of BALF followed by Wright–Giemsa staining (19). The remaining BALF (∼1400 μl) was centrifuged at 1250g for 10 min, and supernatants were collected and stored at −70°C. Cytokine concentrations were measured in duplicate by multiplexed sandwich enzyme-linked immunosorbent assay using SearchLight Proteome Arrays (Pierce, Rockford, IL). Blood was also collected by cardiac puncture, and serum was separated for curcumin measurement.
Assessment of nuclear factor-kappaB activation by electrophoretic mobility shift assay
To assess nuclear factor-kappaB (NF-κB) activation, lungs were removed within 5 min of completion of NTHi aerosol exposure and flash frozen in liquid nitrogen and underwent electrophoretic mobility shift assay essentially as described previously (40). Briefly, nuclear protein extracts were prepared from lung tissues of NTHi challenged and non-challenged mice. To avoid proteolysis during the extraction process, the lung tissue was pulverized with a mortar under liquid nitrogen and immediately transferred into ice-cold extraction buffer. Protein concentration was determined with the bicinchoninic acid (BCA) protein assay (Pierce–KMF, Sankt-Augustin, Germany). Nuclear extracts were incubated with 32P-end-labeled 45mer double-stranded NF-κB oligonucleotide (4 μg of protein with 16 fmol of DNA) from the human immunodeficiency virus long terminal repeat (5′-TTGTTACAAGGGACTTTCCGCTGGGGACTTTCCAGGGAGGCGTGG-3′; boldface indicates NF-κB-binding sites) for 15 min at 37°C. The resulting DNA–protein complex was separated from free oligonucleotide on 6.6% native polyacrylamide gels. A double-stranded mutant oligonucleotide (5′-TTGTTACAACTCACTTTCCGCTGCTCACTTTCCAGGGAGGCGTGG-3′; boldface indicates consensus NF-κB-binding sequence) was used to examine the specificity of binding of NF-κB to the DNA. The dried gels were visualized, and radioactive bands were quantitated using a PhosphorImager (Molecular Dynamics, Sunnyvale, CA) with ImageQuant software.
Curcumin measurement in lung and serum
Aliquots (100 μl) of mouse sera were diluted with 800 μl of PBS containing Ca+2 and Mg+2 (Ca,Mg-PBS). An aliquot (100 μl) of a beta-glucuronidase/sulfatase enzyme solution in Ca,Mg-PBS (Sigma–Aldrich; 100 U/μl) was added to the samples, which were then incubated overnight at 37°C to cleave curcumin-glucuronide and curcumin-sulfate conjugates. Treated samples were then processed as described previously using solid phase extraction prior to analysis by liquid chromatography-mass spectrometry-tandem mass spectrometry (41).
Frozen lung tissue was pulverized using a liquid nitrogen-cooled mortar and pestle. Pulverized tissue was then suspended in 1 ml of Ca,Mg-PBS and homogenized using a Misonix 300 sonic homogenizer. An aliquot (500 μl) of the resulting tissue homogenate was diluted with 250 μl of Ca,Mg-PBS and then treated with 250 μl of beta-glucuronidase/sulfatase enzyme solution (100 U/μl) in Ca,Mg-PBS and incubated at 37°C overnight. Digested lung tissue homogenates were centrifuged to remove particulates and 200 μl aliquots of the supernatant were then processed by solid phase extraction as described previously (41).
Sample quantification was done using extracted control mouse plasma samples spiked with a curcumin reference standard containing the three curcuminoid compounds (Sigma–Aldrich). A calibration curve was generated using MassLynx 4.1 QuanLynx software for the nominal concentration and the peak area for each of the three curcuminoids. Quantification of the mouse samples was made using this calibration curve based on sample peak area (41). Reported data are expressed as micromolars curcuminoids in serum or as nanograms of curcuminoids per milligram of lung homogenate.
Colony formation assay
LKR-10 and LKR-13 cells, lung adenocarcinoma cell lines derived by serial passaging of minced lung adenocarcinoma tissues isolated from a K-rasLA1 mouse (42) were kindly provided by Dr Jonathan Kurie (M. D. Anderson Cancer Center). The cells were passaged in RPMI 1640 supplemented with 10% fetal bovine serum on standard Falcon plasticware (Becton Dickinson, Bedford, MA) at 37°C in an atmosphere containing 5% CO2 (43). Exponentially growing cells were seeded into six-well culture plates (0.75 × 104 cells per well) overnight before treatment with curcumin dissolved in 100% dimethyl sulfoxide. The medium was removed and replaced with fresh medium containing these agents every 3 days. After 7 days of incubation, the cells were fixed with methanol:acetic acid (3:1 vol/vol) and stained with crystal violet in methanol (0.5% vol/vol) to visualize the colonies.
Cell growth inhibition assay
LKR-10 and LKR-13 cells were seeded into 96-well culture plates (1 × 104 cells per well) in quadruplicates in keratinocyte-serum free media with 3% fetal bovine serum, allowed to adhere overnight at 37°C and followed by treatment with curcumin for 3 days as in the colony forming assay. An automated plate reader (model MR5000, Dynatech Laboratories, Chantilly, VA) was used to estimate cell numbers with the sulforhodamine B assay (44). The inhibition of cell growth was calculated as (1−At/Ac) × 100%, where At and Ac represent the average of absorbancies of treated and control cultures, respectively. Concentration response curves were plotted, and the median inhibition concentration of curcumin was calculated by interpolation after 3 days.
Apoptosis analysis by determination of poly (adenosine diphosphate-ribose) polymerase cleavage
Samples containing 30 μg of total cellular protein mixed in sample buffer [50 mM Tris–HCl (pH 6.8), 0.3% glycerol, 0.03% β-mercaptoethanol, 10% sodium dodecyl sulfate and 0.001% bromphenol blue] were electrophoretically separated through 12% sodium dodecyl sulfate–polyacrylamide slab gels and followed by transfer onto nitrocellulose membranes (Bio-Rad Laboratories, Hercules, CA). Briefly, cell monolayers were washed twice with ice-cold PBS and collected in lysis buffer containing 150 mM NaCl, 0.02% NaN3, 2% Igepal CA-630 (octylphenyl-polyethylene glycol, Sigma–Aldrich), 0.5% sodium deoxycholate, 0.2% sodium dodecyl sulfate and 50 mM Tris–HCl (pH 8.0) supplemented with the protease inhibitors leupeptin (1 μg/ml), aprotinin (1 μg/ml), pepstatin (0.5 μg/ml) and phenylmethylsulfonyl fluoride (100 μg/ml). Protein concentrations were measured using the Bradford protein assay (Bio-Rad Laboratories). After blocking with 3% nonfat dry milk solution in 0.1% (wt/vol) Tween 20 in PBS, the membranes were probed with primary antibody against poly (adenosine diphosphate-ribose) polymerase (1:1000, Cell Signaling Technology, Charlottesville, VA). Antibody binding was detected with horseradish peroxidase-linked secondary antibody and enhanced chemiluminescence (Amersham Biosciences Corp., Piscataway, NJ). Loading and transferring control was confirmed by probing the membranes with anti-β-actin antibody (Sigma–Aldrich).
Statistical methods
Summary statistics for cell counts in BALF and nuclear staining of Ki-67 in tumoral cells were computed within treatment groups, and analysis of variance with adjustment for multiple comparisons was performed to examine the differences between the mean cell counts of the control group and each of the NTHi/curcumin treatment groups. For tumor counts and curcumin levels in NTHi-treated/curcumin-treated mice, comparisons of groups were made using Student's t-test. Differences were considered significant for P <0.05.
Results
Effect of curcumin on NTHi-induced (extrinsic) and tumor-induced (intrinsic) airway inflammation
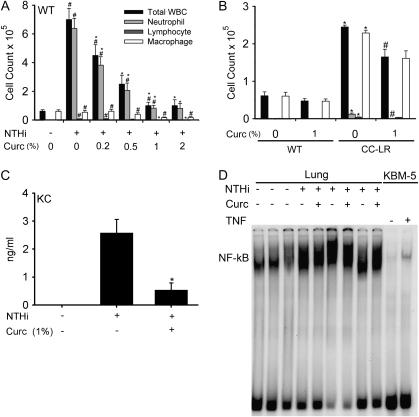
To explore the dose-response relationship between curcumin exposure and its anti-inflammatory effect on NTHi-induced (extrinsic) airway inflammation in WT mice, we fed several groups of 6-week-old female C57BL/6 mice a powder diet mixed with different concentrations of curcumin (0.2, 0.5, 1 and 2% by weight) for 8 days and exposed them to the aerosolized NTHi lysate once at day 7. All treatment groups and controls were killed on day 8 (1 day after NTHi exposure), and BALF was collected. In WT mice without curcumin treatment, NTHi exposure resulted in a rise in total leukocyte numbers dominated by neutrophils (from <1% of total BALF leukocytes before exposure to 81% after exposure). Curcumin treatment resulted in a marked reduction of total leukocyte and neutrophil counts, starting at 0.2% curcumin concentration and reaching a plateau at 1% (Figure 1A).
Fig. 1.
Effect of curcumin on inflammatory cell infiltration into BALF. (A) WT mice were treated with different concentrations of curcumin in the diet for 8 days and then exposed to an NTHi aerosol at day 7 of treatment. Total and lineage-specific leukocyte numbers in BALF 1 day after NTHi aerosol exposure are shown (mean ± SE, #P < 0.05 for NTHi exposure versus no exposure, *P < 0.05 for NTHi exposure versus NTHi exposure plus curcumin treatment). (B) CC-LR mice were treated with 1% curcumin in the diet for 8 days. Total and lineage-specific leukocyte numbers in BALF are shown (mean ± SE, *P < 0.05 for curcumin treatment versus without treatment, #P < 0.05 for 0.05 for CC-LR with curcumin treatment versus CC-LR without treatment). (C) Level of the neutrophil chemoattractant KC in BALF before and after treatment with 1% dietary curcumin in the presence of NTHi lysate exposure (mean ± SE, *P < 0.05 for curcumin treatment versus no treatment). (D) Electrophoretic mobility shift assay analysis of DNA-binding activity to the NF-κB consensus sequence in the lung after NTHi exposure (lanes 4, 6 and 8 from the left) and after dietary curcumin treatment (lanes 5, 7 and 9). Lanes 1, 2 and 3 show DNA-binding activity after PBS exposure (controls). Lanes 10 and 11 show KBM-5 cells (human leukemia cell line) treated or non-treated with 0.05 nM tumor necrosis factor (TNF).
To test the effect of curcumin on tumor-induced (intrinsic) inflammation, we fed a group of 10-week-old female CC-LR mice with a 1% curcumin diet for 8 days and studied their BALF. As we described before (20), non-curcumin-treated CC-LR mice showed elevated levels of macrophages and neutrophils even in the absence of exposure to NTHi (Figure 1B), similar to what has been reported in other models that induce expression of activated K-ras in the airways (45,46). Curcumin treatment of CC-LR mice resulted in a significant reduction of total leukocyte and neutrophil counts (12.1 × 103 without curcumin versus 0.2 × 103 with curcumin).
Leukocyte recruitment in response to NTHi lysate was accompanied by an increase in cytokines and chemokines in BALF, 4 h after NTHi exposure (data not shown). There were markedly increased levels of inflammatory cytokines [interleukin (IL)-6, tumor necrosis factor and IL-1β], the T helper 1 cytokine interferon-γ and the neutrophil chemokine keratinocyte-derived chemokine (KC) (Figure 1C), as we have shown previously (19). Curcumin treatment resulted in a significant reduction of the KC level (Figure 1C), though it did not have any effect on the high levels of inflammatory or T helper 1 cytokines (data not shown). Consistent with the changes in the levels of inflammatory cytokines after NTHi exposure, DNA-binding activity to the NF-κB consensus sequence was significantly increased in the lung shortly after NTHi exposure as detected by electrophoretic mobility shift assay (Figure 1D, lanes 4, 6 and 8 from the left). Surprisingly, dietary curcumin did not inhibit NTHi-induced NF-κB activation in the lung (Figure 1D, lanes 5, 7 and 9 from the left). Lanes 1, 2 and 3 show DNA-binding activity in the lung shortly after PBS exposure (control group). Lanes 10 and 11 show KBM-5 cells (human leukemia cell line) treated or non-treated with tumor necrosis factor (experimental positive control).
Curcumin measurement in serum and lung
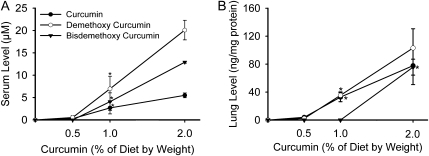
The levels of curcumin and curcuminoid compounds in mouse serum (Figure 2A) and lung homogenates (Figure 2B) were measured after 8 days of varying levels of dietary curcumin treatment (1 day after NTHi exposure). The curcuminoids showed dose-dependent serum and lung concentrations, with demethoxycurcumin the most abundant molecular species in both serum and lung. However, their concentrations were not measurable in the serum at the 0.5% curcumin dose despite the fact that curcumin showed a strong biological effect in suppressing NTHi-induced neutrophilic influx at this concentration.
Fig. 2.
Levels of curcumin and curcuminoid compounds in mouse serum and lung tissue. WT mice were treated with different concentrations of curcumin in the diet for 8 days and their blood and lung tissue were collected for curcuminoid measurement. (A) Dose-dependent serum levels and (B) dose-dependent levels in lung homogenates (mean ± SE, *P < 0.05 for curcumin versus no treatment).
Effect of curcumin on lung cancer progression
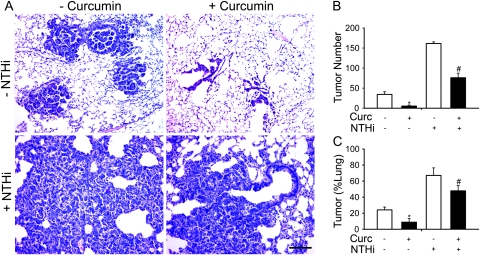
Since 1% curcumin in the diet was sufficient to effectively suppress NTHi-induced airway inflammation and did not show any toxic effect in WT and CC-LR mice, two groups of 6-week-old CC-LR mice were treated with 1% curcumin for 8 weeks, with one of these groups exposed to the NTHi lysate once weekly for 8 weeks. Two other groups of mice were not treated with curcumin, with one of these exposed weekly to the NTHi lysate for 8 weeks. All groups were killed one day after the eighth NTHi exposure while still under curcumin treatment, and the effect of curcumin on lung tumor progression was analyzed by determining the number of tumors visible on the pleural surface of the lungs and the tumor volume per lung. Curcumin significantly reduced the number of tumors on the lung surface of CC-LR mice not exposed to NTHi lysate by 85% (34 ± 6 without curcumin versus 5 ± 2 with curcumin) and after 8 weekly NTHi exposures by 53% (161 ± 4 without curcumin versus 76 ± 11 with curcumin) (Figure 3B). Curcumin also lowered tumor volume per lung in curcumin-treated mice not exposed to NTHi lysate by 64% (24.1 ± 3.5% versus 8.8 ± 4.6%) and after NTHi exposure by 39% (67.1 ± 9.4% versus 47.9 ± 6.6%) (Figure 3C) by both reducing the size of individual tumors and the total number of tumors (Figure 3A).
Fig. 3.
Effect of curcumin on tumor burden in the CC-LR mouse model of lung cancer. (A) Histopathological appearance of lung tissue after treatment with curcumin in NTHi-exposed or unexposed CC-LR mice. (B) Lung surface tumor number after curcumin treatment in NTHi exposed or unexposed CC-LR mice. (C) Percentage of lung tissue occupied by tumor after curcumin treatment in NTHi-exposed or unexposed CC-LR mice (mean ± SE, *P < 0.05 for CC-LR without NTHi exposure versus CC-LR without NTHi exposure plus curcumin treatment, #P < 0.05 for CC-LR with NTHi exposure versus CC-LR with NTHi exposure plus curcumin treatment).
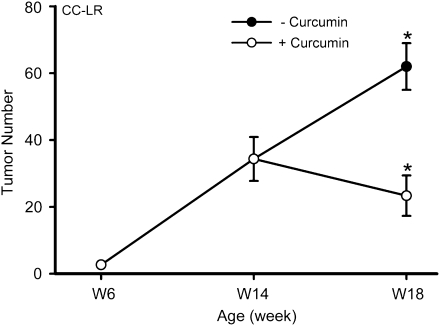
To further test the efficacy of curcumin in suppressing existing tumors, we started the curcumin treatment of CC-LR mice not treated with NTHi at 14 weeks of age, when the lung tumors are well developed. Four weeks of treatment with 1% dietary curcumin reduced the number of visible tumors on the lung surface of CC-LR mice at age 18 weeks by 63% (62 ± 7 without curcumin versus 23 ± 6 with curcumin) (Figure 4).
Fig. 4.
Effect of curcumin on late tumor progression in the CC-LR mouse model of lung cancer. Lung surface tumor number after curcumin treatment in CC-LR mice (mean ± SE, *P < 0.05 for 18-week-old CC-LR with or without curcumin treatment versus 14-week-old CC-LR without curcumin treatment).
Effect of curcumin on lung tumor cell proliferation in vivo
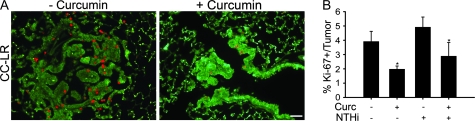
In order to evaluate the effect of curcumin on cell proliferation in CC-LR mice, the relative number of cells showing positive staining for the cell proliferation marker, Ki-67, was measured in tumor tissues from the four groups by immunohistochemistry (Figure 5). The numbers of labeled Ki-67 positive tumor cells were quantitated as a fraction of total tumor nuclei per high power field (×40) in five fields from three mice of each of the treatment groups. Results were expressed as percentage of Ki-67 positive cells ± SE.
Fig. 5.
Effect of curcumin on cell proliferation. Lung tissues from CC-LR mice were stained immunohistochemically for the Ki-67 proliferation marker. (A) Representative photomicroscopy of Ki-67 positive cells in lung tissue in CC-LR mice with or without curcumin treatment. (B) Quantitative analysis of Ki-67 positive staining in lung tissues from CC-LR mice with or without curcumin treatment in the presence or absence of NTHi exposure (mean ± SE, *P < 0.05 for 14-week-old CC-LR with or without NTHi exposure versus 14-week-old CC-LR with or without NTHi exposure plus curcumin treatment).
NTHi exposure increased the expression of Ki-67 in tumor tissue, and conversely, curcumin significantly decreased the expression of Ki-67 in tumor tissues compared with both NTHi exposed and unexposed control groups (P < 0.05 versus control).
Effect of curcumin on lung cancer cell growth, proliferation and apoptosis
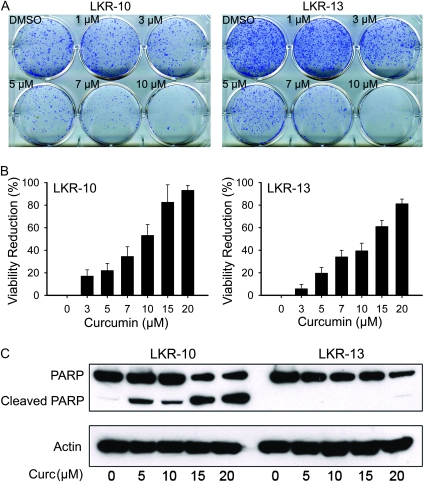
The effect of curcumin on cell growth of two ras-induced mouse lung adenocarcinoma cell lines (LKR-10 and LKR-13) was assessed. In general, these two cell lines behave similarly with slight differences in their in vivo growth and in their invasive ability when grown in 3D culture. Curcumin showed a strong dose-dependent effect in suppressing colony formation by both LKR-10 and LKR-13 cell lines (Figure 6A) at concentrations achieved in vivo (Figure 2A). The inhibitory effect of curcumin on total cell viability of the cell lines was also found to be dose-dependent in the sulforhodamine B assay (Figure 6B). Total and cleaved poly (adenosine diphosphate-ribose) polymerase levels were measured as an indicator of apoptosis, and curcumin similarly showed a dose-dependent effect (Figure 6C). Poly (adenosine diphosphate-ribose) polymerase levels in LKR-10 cells were higher than those in LKR-13, but cell viability differences were less striking, suggesting that curcumin is mainly growth inhibitory in LKR-13 cells, but both pro-apoptotic and growth inhibitory in LKR-10 cells (Figure 6C).
Fig. 6.
Effect of curcumin on lung cancer cell line clonogenicity and apoptosis in vitro. (A) The inhibitory effect of curcumin on colony formation of LKR-10 and LKR-13 cells. (B) The sulforhodamine B assay shows the inhibitory effect of curcumin on LKR-10 and LKR-13 cell viability (mean ± SE). (C) Western blot analysis of poly (adenosine diphosphate-ribose) polymerase (PARP) cleavage indicates increased apoptosis by curcumin mainly in LKR-10 cells. Upper and lower bands indicate intact and cleaved-PARP, respectively.
Discussion
The pooled global prevalence of COPD in adults ≥40 years is ∼10%, and it is a leading cause of morbidity and mortality in the USA (11). Smoking is the most important cause of inflammation in COPD (47). However, among smokers with COPD, even following withdrawal of cigarette smoke, inflammation persists and lung function continues to deteriorate (47), possibly because bacterial colonization of smoke-damaged airways perpetuates airway injury and inflammation (12,13). Thus, besides smoking cessation, additional strategies that stop the progression to advanced COPD are highly attractive. Worldwide, lung cancer is the leading cause of cancer mortality, with the highest rates currently observed in Europe and North America (10). There are limited numbers of effective therapeutic regimens available for lung cancer, and once diagnosed, the 5 year survival rate is only 8–12% (10). Therefore, any new modalities to supplement current treatments for lung cancer would be of interest. In both diseases (COPD and lung cancer), one such modality that has received attention recently is curcumin, which is used as a dietary ingredient and for its anti-inflammatory and wound healing properties. We have found that 1% curcumin in the diet is able to suppress NTHi-induced COPD-like airway inflammation and K-ras initiated lung cancer in mice.
Curcumin has previously been found to have significant anti-carcinogenic effects in mice, interfering not only with the initiation phase but also with the promotion/progression phase of chemical carcinogen-induced tumor formation in organs other than the lung (37,48). However, in the lungs, curcumin was inactive in the post-initiation stage of a carcinogen-induced lung cancer model, since feeding 2% curcumin in the diet had no effect on 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis in A/J mice (37,38). Further, in a recent study by Dance-Barnes et al. (39), treatment of a transgenic mouse model of lung cancer that expresses the human K-ras (G12C) allele in a doxycycline-inducible and lung-specific manner with dietary curcumin (4000 p.p.m.) actually increased tumor multiplicity, tumor progression and lung inflammation. In contrast, our data show that curcumin treatment prevents tumor development in a K-ras initiated lung cancer model (CC-LR mouse), consistent with the existing data showing its anti-tumoral effect in other organs (37,49). Our study and Dance-Barnes’s are similar in that carcinogenesis was initiated in both by an activated allele of K-ras (G12D in ours and G12C in Dance-Barnes’s), expressed in airway secretory cells under control of the CCSP promoter, and the concentrations of dietary curcumin were similar (1% in our study and 0.4% in Dance-Barnes’s). However, the studies differ markedly in their mechanisms of transgene expression. We used a hit-and-run strategy in which the Cre recombinase is expressed in airway secretory cells by the CCSP promoter prior to the 6th week of postnatal life to induce rearrangement and activation of a conditional K-ras allele, with permanent subsequent expression under control of the endogenous K-ras promoter in an inflammatory model (20). This strategy was adopted because we had found that the CCSP promoter is downregulated by inflammation, resulting in reduced carcinogenesis when the oncogene remains under control of the CCSP promoter (20). Dance-Barnes et al. (39) used a bitransgenic strategy with the reverse tetracycline transactivator under control of the CCSP promoter and an activated K-ras allele under control of a tetracycline-inducible promoter. Either or both of these promoters could be influenced by curcumin, resulting in upregulation of the expression of the K-ras transgene. Since tumor progression was mild in this model, occurring late in life and at a low grade, carcinogenesis should be sensitive to small changes in oncogene expression. While these differences in the genetic models could explain the discrepant findings between our studies, further experiments will be required to provide evidential support.
Curcumin is a potent immunomodulatory agent that can attenuate the activation of T cells, B cells, macrophages, neutrophils, natural killer cells and dendritic cells (50). Curcumin is also reported to downregulate the expression of pro-inflammatory cytokines including tumor necrosis factor, IL-1 and IL-6 (50). The inflammatory component of a developing neoplasm may include a diverse leukocyte population of neutrophils, dendritic cells, macrophages, eosinophils and mast cells, as well as lymphocytes, all of which are capable of producing an array of cytokines, reactive oxygen species, serine and cysteine proteases and matrix metalloproteinases (5,6). Sustained cell proliferation in this environment rich in inflammatory cells, growth factors, activated stroma and DNA-damage-promoting agents (intrinsic inflammation) potentiates neoplastic risk (5,7). Such pro-tumor inflammatory microenvironments promote not only malignant conversion and development of solid tumors but also the dissemination of neoplastic cells into the blood vasculature and lymphatics by driving the invasive capacity of malignant cells (51) and expansion of angiogenic vasculature (52). CC-LR mice show elevated levels of macrophages and neutrophils even in the absence of exposure to NTHi (Figure 1B), similar to what has been reported in other models that induce expression of activated K-ras in the airways (intrinsic inflammation) (45,46). Curcumin not only reverses the promoting effect of intrinsic (K-ras-driven) and extrinsic (NTHi-induced) airway inflammation on established lung cancer (Figure 3) but also inhibits and suppresses tumor progression in a late phase (Figure 4). One of the possible mechanisms for these findings could be the anti-inflammatory effect of curcumin described by us (Figure 1) and other groups (50).
As shown in Figure 1C, the suppressive effect of curcumin on neutrophil recruitment was associated with a markedly reduced level of the neutrophil chemokine KC in BALF. KC is a functional mouse homologue of human IL-8. It is a member of the CXC chemokine family that contains the ELR motif (ELR+) (53). This subtype of chemokines mediates neutrophil migration to sites of inflammation through CXCR2 (54,55). There is a marked upregulation of CXCR2 in airway epithelial cells in COPD and this correlated with the increased number of neutrophils in the airways (56–58). Also, several studies have shown roles for CXC chemokines (including KC) and their receptors in tumorigenesis, including angiogenesis, attraction of leukocytes to tumor sites and induction of tumor cell migration and homing in metastatic sites (59–61). Our findings support the CXC/CXCR2/neutrophil axis as a possible target for curcumin in suppressing the COPD-like inflammation, and a mechanistic pathway indirectly involved in the anti-tumoral effect of curcumin. Therefore, curcumin could simultaneously be a treatment for patients with COPD at high risk of lung cancer by suppressing the ongoing neutrophilic inflammation induced by bacterial colonization and other inflammatory stimuli.
Existing data show that the direct anti-tumoral activity of curcumin occurs through suppressing cell proliferation and activating cell apoptosis by downregulation of NF-κB-regulated gene products involved in cellular proliferation (e.g. cyclin D1) and anti-apoptosis (e.g. Bcl-2) (62). Of interest, NF-κB inhibition by curcumin also leads to the downregulation of various pro-inflammatory cytokines (50). Our results also indicate that curcumin suppresses the proliferation of lung cancer cell lines in vitro (Figure 6A and B) and tumor cells in vivo (Figure 5). Similar to finding of other, this was associated with cell-cycle arrest and increased apoptosis (Figure 6C). Surprisingly, curcumin did not inhibit NTHi-induced NF-κB activation (Figure 1D) and the subsequent increase in inflammatory cytokine production. Therefore, dissecting signaling pathways mediating these phenomena induced by curcumin in our model needs further investigation, which will provide a rational basis for testing selective anti-inflammatory/anti-tumoral agents.
In animal studies, curcumin shows poor systemic bioavailability after oral administration (63–65). For example, administration of curcumin 1.0 g/kg to mice by a single gavage resulted in plasma levels of 130–220 ng/ml 1 h later (64). In rats, 500 mg/kg curcumin by a single gavage results in a peak plasma concentration of 1.8 ng/ml of free curcumin (63). In humans, studies support the poor bioavailability of oral curcumin. For example, to achieve a detectable blood level, gram doses of 90–99% pure curcumin were required, and the highest dose tested (8 g) resulted in <1 μg/ml curcumin (66–68). The most recent human study showed that a single 10 or 12 g oral dose of curcumin generates mainly curcumin glucuronide and curcumin sulfate in blood (69). Like the other studies, a high rate of curcumin conjugation through glucuronidation and sulfation may explain low concentrations of free curcumin when administered orally (66,67). Curcumin is the most studied curcuminoid with respect to several biological activities. However, some experiments have demonstrated that two other curcuminoids, desmethoxycurcumin and bisdesmethoxycurcumin, also possess biological activities, but with different potency from curcumin (70). For example, desmethoxycurcumin and bisdesmethoxycurcumin show higher anti-metastasis potency than curcumin (71). We also found low serum concentrations of curcuminoids in our study when curcumin was administered at 1–2% of diet (Figure 2A). However, curcumin showed a strong biological effect in suppressing NTHi-induced COPD-like inflammation even when undetectable in serum after administration at 0.5% of diet (Figure 1A). This indicates that sufficient curcumin is absorbed to provide a useful effect even when present in modest concentration in the diet.
In summary, the results presented here support further the effectiveness of curcumin as a therapeutic agent, alone or in combination with current therapy against lung cancer. It could also be considered for use by current smokers or ex-smokers with or without COPD as a chemopreventive agent. Further understanding of the molecular mechanisms affected by curcumin treatment will provide a basis for rationally directed clinical testing of the efficacy of this agent and other selective anti-inflammatory/anti-tumoral agents in preventing COPD progression and lung carcinogenesis.
Funding
National Cancer Institute (UO1 CA105352).
Acknowledgments
We thank Linda Foot and Dafna Lotan for their assistance in the completion of this project.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- BALF
bronchoalveolar lavage fluid
- CC-LR
CCSPCre/LSL-K-rasG12Dmice
- CCSP
Clara cell secretory protein
- COPD
chronic obstructive pulmonary disease
- IL
interleukin
- KC
keratinocyte-derived chemokine
- NTHi
non-typeable Hemophilus influenzae
- PBS
phosphate buffered saline; WT, wild type
References
- 1.Parkin DM, et al. Global cancer statistics, 2002. CA Cancer J. Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Jett JR, et al. Screening for lung cancer: current status and future directions: Thomas A. Neff lecture. Chest. 2004;125:158S–162S. doi: 10.1378/chest.125.5_suppl.158s. [DOI] [PubMed] [Google Scholar]
- 3.Stellman SD, et al. Smoking and lung cancer risk in American and Japanese men: an international case-control study. Cancer Epidemiol. Biomarkers Prev. 2001;10:1193–1199. [PubMed] [Google Scholar]
- 4.Shacter E, et al. Chronic inflammation and cancer. Oncology (Williston Park) 2002;16:217–226. 229. [PubMed] [Google Scholar]
- 5.Coussens LM, et al. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin EY, et al. Role of infiltrated leucocytes in tumour growth and spread. Br. J. Cancer. 2004;90:2053–2058. doi: 10.1038/sj.bjc.6601705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balkwill F, et al. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–217. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Tockman MS, et al. Airways obstruction and the risk for lung cancer. Ann. Intern. Med. 1987;106:512–518. doi: 10.7326/0003-4819-106-4-512. [DOI] [PubMed] [Google Scholar]
- 9.Skillrud DM, et al. Higher risk of lung cancer in chronic obstructive pulmonary disease. A prospective, matched, controlled study. Ann. Intern. Med. 1986;105:503–507. doi: 10.7326/0003-4819-105-4-503. [DOI] [PubMed] [Google Scholar]
- 10.Guessous I, et al. Lung cancer screening: current situation and perspective. Swiss Med. Wkly. 2007;137:304–311. doi: 10.4414/smw.2007.11582. [DOI] [PubMed] [Google Scholar]
- 11.Halbert RJ, et al. Global burden of COPD: systematic review and meta-analysis. Eur. Respir. J. 2006;28:523–532. doi: 10.1183/09031936.06.00124605. [DOI] [PubMed] [Google Scholar]
- 12.Rennard SI. COPD: overview of definitions, epidemiology, and factors influencing its development. Chest. 1998;113:235S–241S. doi: 10.1378/chest.113.4_supplement.235s. [DOI] [PubMed] [Google Scholar]
- 13.Saetta M, et al. Cellular and structural bases of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2001;163:1304–1309. doi: 10.1164/ajrccm.163.6.2009116. [DOI] [PubMed] [Google Scholar]
- 14.King PT, et al. Adaptive immunity to nontypeable Haemophilus influenzae. Am. J. Respir. Crit. Care Med. 2003;167:587–592. doi: 10.1164/rccm.200207-728OC. [DOI] [PubMed] [Google Scholar]
- 15.Murphy TF. Haemophilus influenzae in chronic bronchitis. Semin. Respir. Infect. 2000;15:41–51. doi: 10.1053/srin.2000.0150041. [DOI] [PubMed] [Google Scholar]
- 16.Bandi V, et al. Nontypeable Haemophilus influenzae in the lower respiratory tract of patients with chronic bronchitis. Am. J. Respir. Crit. Care Med. 2001;164:2114–2119. doi: 10.1164/ajrccm.164.11.2104093. [DOI] [PubMed] [Google Scholar]
- 17.Soler N, et al. Airway inflammation and bronchial microbial patterns in patients with stable chronic obstructive pulmonary disease. Eur. Respir. J. 1999;14:1015–1022. doi: 10.1183/09031936.99.14510159. [DOI] [PubMed] [Google Scholar]
- 18.Monso E, et al. Bacterial infection in chronic obstructive pulmonary disease. A study of stable and exacerbated outpatients using the protected specimen brush. Am. J. Respir. Crit. Care Med. 1995;152:1316–1320. doi: 10.1164/ajrccm.152.4.7551388. [DOI] [PubMed] [Google Scholar]
- 19.Moghaddam SJ, et al. Haemophilus influenzae lysate induces aspects of the chronic obstructive pulmonary disease phenotype. Am. J. Respir. Cell Mol. Biol. 2008;38:629–638. doi: 10.1165/rcmb.2007-0366OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moghaddam SJ, et al. Promotion of lung carcinogenesis by chronic obstructive pulmonary disease-like airway inflammation in a K-ras-induced mouse model. Am. J. Respir. Cell Mol. Biol. 2009;40:443–453. doi: 10.1165/rcmb.2008-0198OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aggarwal BB, et al. Curcumin: the Indian solid gold. Adv. Exp. Med. Biol. 2007;595:1–75. doi: 10.1007/978-0-387-46401-5_1. [DOI] [PubMed] [Google Scholar]
- 22.Srimal RC, et al. Pharmacology of diferuloyl methane (curcumin), a non-steroidal anti-inflammatory agent. J. Pharm. Pharmacol. 1973;25:447–452. doi: 10.1111/j.2042-7158.1973.tb09131.x. [DOI] [PubMed] [Google Scholar]
- 23.Arora RB, et al. Anti-inflammatory studies on Curcuma longa (turmeric) Indian J. Med. Res. 1971;59:1289–1295. [PubMed] [Google Scholar]
- 24.Mukhopadhyay A, et al. Anti-inflammatory and irritant activities of curcumin analogues in rats. Agents Actions. 1982;12:508–515. doi: 10.1007/BF01965935. [DOI] [PubMed] [Google Scholar]
- 25.Srivastava R. Inhibition of neutrophil response by curcumin. Agents Actions. 1989;28:298–303. doi: 10.1007/BF01967418. [DOI] [PubMed] [Google Scholar]
- 26.Jobin C, et al. Curcumin blocks cytokine-mediated NF-kappa B activation and proinflammatory gene expression by inhibiting inhibitory factor I-kappa B kinase activity. J. Immunol. 1999;163:3474–3483. [PubMed] [Google Scholar]
- 27.Ramsewak RS, et al. Cytotoxicity, antioxidant and anti-inflammatory activities of curcumins I-III from Curcuma longa. Phytomedicine. 2000;7:303–308. doi: 10.1016/S0944-7113(00)80048-3. [DOI] [PubMed] [Google Scholar]
- 28.Ruby AJ, et al. Anti-tumour and antioxidant activity of natural curcuminoids. Cancer Lett. 1995;94:79–83. doi: 10.1016/0304-3835(95)03827-j. [DOI] [PubMed] [Google Scholar]
- 29.Huang MT, et al. Inhibitory effects of dietary curcumin on forestomach, duodenal, and colon carcinogenesis in mice. Cancer Res. 1994;54:5841–5847. [PubMed] [Google Scholar]
- 30.Huang MT, et al. Inhibitory effects of curcumin on tumor initiation by benzo[a]pyrene and 7,12-dimethylbenz[a]anthracene. Carcinogenesis. 1992;13:2183–2186. doi: 10.1093/carcin/13.11.2183. [DOI] [PubMed] [Google Scholar]
- 31.Rao CV, et al. Chemoprevention of colon carcinogenesis by dietary curcumin, a naturally occurring plant phenolic compound. Cancer Res. 1995;55:259–266. [PubMed] [Google Scholar]
- 32.Pereira MA, et al. Effects of the phytochemicals, curcumin and quercetin, upon azoxymethane-induced colon cancer and 7,12-dimethylbenz[a]anthracene-induced mammary cancer in rats. Carcinogenesis. 1996;17:1305–1311. doi: 10.1093/carcin/17.6.1305. [DOI] [PubMed] [Google Scholar]
- 33.Bhide SV, et al. Chemoprevention of mammary tumor virus-induced and chemical carcinogen-induced rodent mammary tumors by natural plant products. Breast Cancer Res. Treat. 1994;30:233–242. doi: 10.1007/BF00665965. [DOI] [PubMed] [Google Scholar]
- 34.Singletary K, et al. Inhibition of 7,12-dimethylbenz[a]anthracene (DMBA)-induced mammary tumorigenesis and DMBA-DNA adduct formation by curcumin. Cancer Lett. 1996;103:137–141. doi: 10.1016/0304-3835(96)04224-3. [DOI] [PubMed] [Google Scholar]
- 35.Sidhu GS, et al. Enhancement of wound healing by curcumin in animals. Wound Repair Regen. 1998;6:167–177. doi: 10.1046/j.1524-475x.1998.60211.x. [DOI] [PubMed] [Google Scholar]
- 36.Sidhu GS, et al. Curcumin enhances wound healing in streptozotocin induced diabetic rats and genetically diabetic mice. Wound Repair Regen. 1999;7:362–374. doi: 10.1046/j.1524-475x.1999.00362.x. [DOI] [PubMed] [Google Scholar]
- 37.Huang MT, et al. Inhibitory effects of curcumin on tumorigenesis in mice. J. Cell. Biochem. Suppl. 1997;27:26–34. [PubMed] [Google Scholar]
- 38.Hecht SS, et al. Evaluation of butylated hydroxyanisole, myo-inositol, curcumin, esculetin, resveratrol and lycopene as inhibitors of benzo[a]pyrene plus 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis in A/J mice. Cancer Lett. 1999;137:123–130. doi: 10.1016/s0304-3835(98)00326-7. [DOI] [PubMed] [Google Scholar]
- 39.Dance-Barnes ST, et al. Lung tumor promotion by curcumin. Carcinogenesis. 2009;30:1016–1023. doi: 10.1093/carcin/bgp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chaturvedi MM, et al. Assay for redox-sensitive transcription factor. Methods Enzymol. 2000;319:585–602. doi: 10.1016/s0076-6879(00)19055-x. [DOI] [PubMed] [Google Scholar]
- 41.Dhillon N, et al. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin. Cancer Res. 2008;14:4491–4499. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- 42.Johnson L, et al. Somatic activation of the K-ras oncogene causes early onset lung cancer in mice. Nature. 2001;410:1111–1116. doi: 10.1038/35074129. [DOI] [PubMed] [Google Scholar]
- 43.Wislez M, et al. High expression of ligands for chemokine receptor CXCR2 in alveolar epithelial neoplasia induced by oncogenic kras. Cancer Res. 2006;66:4198–4207. doi: 10.1158/0008-5472.CAN-05-3842. [DOI] [PubMed] [Google Scholar]
- 44.Skehan P, et al. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 45.Ji H, et al. K-ras activation generates an inflammatory response in lung tumors. Oncogene. 2006;25:2105–2112. doi: 10.1038/sj.onc.1209237. [DOI] [PubMed] [Google Scholar]
- 46.Wislez M, et al. Inhibition of mammalian target of rapamycin reverses alveolar epithelial neoplasia induced by oncogenic K-ras. Cancer Res. 2005;65:3226–3235. doi: 10.1158/0008-5472.CAN-04-4420. [DOI] [PubMed] [Google Scholar]
- 47.Shapiro SD. End-stage chronic obstructive pulmonary disease: the cigarette is burned out but inflammation rages on. Am. J. Respir. Crit. Care Med. 2001;164:339–340. doi: 10.1164/ajrccm.164.3.2105072c. [DOI] [PubMed] [Google Scholar]
- 48.Aggarwal BB, et al. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23:363–398. [PubMed] [Google Scholar]
- 49.Kuttan G, et al. Antitumor, anti-invasion, and antimetastatic effects of curcumin. Adv. Exp. Med. Biol. 2007;595:173–184. doi: 10.1007/978-0-387-46401-5_6. [DOI] [PubMed] [Google Scholar]
- 50.Jagetia GC, et al. “Spicing up” of the immune system by curcumin. J. Clin. Immunol. 2007;27:19–35. doi: 10.1007/s10875-006-9066-7. [DOI] [PubMed] [Google Scholar]
- 51.Goswami S, et al. Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer Res. 2005;65:5278–5283. doi: 10.1158/0008-5472.CAN-04-1853. [DOI] [PubMed] [Google Scholar]
- 52.Bergers G, et al. Tumorigenesis and the angiogenic switch. Nat. Rev. Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 53.Strieter RM, et al. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J. Biol. Chem. 1995;270:27348–27357. doi: 10.1074/jbc.270.45.27348. [DOI] [PubMed] [Google Scholar]
- 54.Murphy PM. Neutrophil receptors for interleukin-8 and related CXC chemokines. Semin. Hematol. 1997;34:311–318. [PubMed] [Google Scholar]
- 55.Waugh DJ, et al. The interleukin-8 pathway in cancer. Clin. Cancer Res. 2008;14:6735–6741. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 56.Barnes PJ. Cytokine-directed therapies for the treatment of chronic airway diseases. Cytokine Growth Factor Rev. 2003;14:511–522. doi: 10.1016/s1359-6101(03)00058-3. [DOI] [PubMed] [Google Scholar]
- 57.Donnelly LE, et al. Chemokine receptors as therapeutic targets in chronic obstructive pulmonary disease. Trends Pharmacol. Sci. 2006;27:546–553. doi: 10.1016/j.tips.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 58.Panina P, et al. Chemokine receptors in chronic obstructive pulmonary disease (COPD) Curr. Drug Targets. 2006;7:669–674. doi: 10.2174/138945006777435272. [DOI] [PubMed] [Google Scholar]
- 59.Strieter RM, et al. Cancer CXC chemokine networks and tumour angiogenesis. Eur. J. Cancer. 2006;42:768–778. doi: 10.1016/j.ejca.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 60.Raman D, et al. Role of chemokines in tumor growth. Cancer Lett. 2007;256:137–165. doi: 10.1016/j.canlet.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vandercappellen J, et al. The role of CXC chemokines and their receptors in cancer. Cancer Lett. 2008;267:226–244. doi: 10.1016/j.canlet.2008.04.050. [DOI] [PubMed] [Google Scholar]
- 62.Aggarwal S, et al. Curcumin (diferuloylmethane) down-regulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of IkappaBalpha kinase and Akt activation. Mol. Pharmacol. 2006;69:195–206. doi: 10.1124/mol.105.017400. [DOI] [PubMed] [Google Scholar]
- 63.Ireson C, et al. Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol ester-induced prostaglandin E2 production. Cancer Res. 2001;61:1058–1064. [PubMed] [Google Scholar]
- 64.Pan MH, et al. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab. Dispos. 1999;27:486–494. [PubMed] [Google Scholar]
- 65.Asai A, et al. Occurrence of orally administered curcuminoid as glucuronide and glucuronide/sulfate conjugates in rat plasma. Life Sci. 2000;67:2785–2793. doi: 10.1016/s0024-3205(00)00868-7. [DOI] [PubMed] [Google Scholar]
- 66.Cheng AL, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–2900. [PubMed] [Google Scholar]
- 67.Sharma RA, et al. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin. Cancer Res. 2004;10:6847–6854. doi: 10.1158/1078-0432.CCR-04-0744. [DOI] [PubMed] [Google Scholar]
- 68.Sharma RA, et al. Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin. Cancer Res. 2001;7:1894–1900. [PubMed] [Google Scholar]
- 69.Vareed SK, et al. Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol. Biomarkers Prev. 2008;17:1411–1417. doi: 10.1158/1055-9965.EPI-07-2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sotanaphun U, et al. Application of Scion image software to the simultaneous determination of curcuminoids in turmeric (Curcuma longa) Phytochem. Anal. 2009;20:19–23. doi: 10.1002/pca.1086. [DOI] [PubMed] [Google Scholar]
- 71.Yodkeeree S, et al. Curcumin, demethoxycurcumin and bisdemethoxycurcumin differentially inhibit cancer cell invasion through the down-regulation of MMPs and uPA. J. Nutr. Biochem. 2009;20:87–95. doi: 10.1016/j.jnutbio.2007.12.003. [DOI] [PubMed] [Google Scholar]