Abstract
To more fully characterize the sickness response in young (3 mo) and older (24 mo) rats, we measured core body temperature (CBT), activity level, and body weight changes for seven days following a peripheral immune challenge with E. coli. CBT increases were delayed and blunted during the 12 h following infection in older rats. Indeed, in aging subjects the initial response was hypothermia, but this was followed by a significant and prolonged elevation in CBT lasting 3 days. Young rats, in contrast, generated a rapid and robust CBT elevation lasting just over a day. Activity level was significantly reduced only on the day of E. coli administration in both young and older rats. Body weight loss was equivalent in both age groups one day after E. coli administration, although there was a trend for older rats to continue losing more weight across the next six days than in young rats. This is the first study to examine CBTs in young and older rats for a protracted amount of time, thereby revealing that aging rats do have an exaggerated, albeit delayed, fever which is in keeping with other exaggerated sickness behavioral responses observed in aging rodents.
Introduction
An immune challenge with either LPS or E. coli produces a potentiated and prolonged pro-inflammatory response in the brains of aged rodents (Barrientos et al., 2009; Foster et al., 1992; Godbout et al., 2005; Huang et al., 2008). Pro-inflammatory cytokines (e.g., TNFa, IL-6, and IL-1) within the brain stimulate all aspects of the constellation of behaviors referred to as the sickness response (Dantzer et al., 1998). These behaviors include fever, decreased food/water intake, decreased motor activity and social interaction, and deficits in cognition (Dantzer et al., 1998; Hart, 1988). This pattern is thought to be adaptive. Fever is often viewed as the critical change as it serves as a primary host defense response against the replication of bacterial and viral pathogens (Hart, 1988; Kluger, 1991). At least some of the behavioral alterations function to facilitate the efficiency of fever, conserving vital energy resources for use in elevating core body temperature (CBT) (Hart, 1988). Decreased locomotor activity, decreased social exploration, anorexia (Godbout et al., 2005; Huang et al., 2008), and long-term memory impairments (Barrientos et al., 2009; Barrientos et al., 2006) have been shown to be exaggerated and/or prolonged in aging rodents following an immune challenge, compared to younger cohorts. These exaggerated sickness behaviors are concordant with the exaggerated proinflammatory response found in the brain of aging animals. In contrast, increases in CBT in response to a peripheral immune challenge, have been repeatedly shown to be blunted or absent in aging rodents (Buchanan et al., 2008; Foster et al., 1992; Miller et al., 1991; Scarpace et al., 1992). These findings are obviously inconsistent with the exaggerated proinflammatory profile and sickness behaviors of aging rodents. However, a common element in all of the reported studies was that CBT was measured for no longer than 12 hr following the challenge. It seemed possible that even though CBT changes in aging animals might be blunted initially, that nevertheless fever might still be more prolonged. Because fever has been assigned such a critical role in the sickness pattern this issue is worthy of resolution. To more fully characterize the sickness response in young and aging rats, we measured CBT, activity level, and body weight changes for seven days following an E. coli infection.
2.1. Subjects
Subjects were male F344xBN F1 rats obtained from the National Institute on Aging (Bethesda, MD). Older rats (n=5) were 24 mo old and weighed 609 g (±5.47 SEM). Young rats (n=7) were 3 mo old and weighed 342 g (±7.78 SEM). All rats were housed individually in cages (52L × 20W × 21H, cm). The animal colony was a dedicated room that was only used for one fever study at a time. The experimenter was the only person to enter the room and did so only once a day, at the same time every day, to minimize stress-induced fevers. The colony was maintained at 22±1°C on a 12-h light/dark cycle (lights on at 07:00 h). All rats were allowed free access to food and water and were given at least 1 week to acclimate to colony conditions before experimentation began. All experiments were conducted in accordance with protocols approved by the University of Colorado Animal Care and Use Committee. All efforts were made to minimize the number of animals used and their suffering.
2.2. Immune challenge and experimental procedure
All rats received an intraperitoneal (i.p.) injection of sterile phosphate buffered saline (PBS) (250 μL) between 8–9 am, on the first day. Temperatures that were recorded on this day served as the baseline values. The next day (day 0), all animals received an i.p. injection of Escherichia coli (E. coli), also between 8–9 am. Temperature recordings began just before the baseline day and continued for 7 days.
One day prior to injection with E. coli, stock E. coli cultures (ATCC 15746; American Type Culture Collection, Manassas, VA) were thawed and cultured overnight (15–20 h) in 40 mL of brain-heart infusion (BHI; DIFCO Laboratories, Detroit, MI) in an incubator (37°C, 95% air + 5% CO2). The number of bacteria in culture was quantified by extrapolating from previously determined growth curves. Cultures were then centrifuged for 15 min at 4°C, 3000 RPM, supernatants discarded, and bacteria were resuspended in PBS to achieve a dose of 1.0 × 1010 CFU. A volume of 250 μL was injected.
2.4. Sickness Behavior (Fever, Activity, Food/Water Intake)
Core body temperature (CBT) and activity were measured remotely at minute intervals continuously from the day before bacterial challenge (baseline) to 7 days after challenge with the use of standard biotelemetry procedures. Precalibrated radio thermistors (Series 4000, Minimitter, Sun River, OR) were aseptically implanted in the peritoneal cavity of each rat while under isoflurane anesthesia. Rats were allowed ~3 weeks to recover before the start of the experiment. Animals remained in their cages, while the frequencies emitted by the thermistors were monitored. These frequencies were then converted to temperature (°C) and activity (number of times rat crossed grid in cage) values based on each thermistor’s calibration data. Hourly averages were then calculated from the raw temperatures and activity counts to aid in the analysis of the data. Every morning at 9:00 am, the experimenter entered the room to check body weights. The whole procedure took about 12–15 minutes.
Statistical Analysis
Because of the marked circadian rhythm in CBT, data for light and dark phases were analyzed separately. Repeated measures ANOVAs were employed to determine baseline differences between young and old rats, for each circadian phase. Repeated measures ANOVAs were also employed with time (at each circadian phase) as a within subjects variable and group (pre-E. coli CBT vs. post-E. coli CBT) as the between subjects variable. Where appropriate, Scheffe’s post hoc analyses were conducted. Alpha was set at 0.05.
3. Results
Baseline CBTs (one day before E. coli administration) were not very different between young and aging rats across a 24 h period (Fig. 1A). A repeated measures ANOVA on the light phase data showed no main effect of age (P > 0.05), but an age × time interaction (F(11, 110) = 3.59, P = 0.0002). Post hoc tests revealed that older rats had a higher CBT at 1pm and 2pm, and a lower CBT at 6pm. Dark phase data showed no main effect of age (P > 0.05), and no age × time interaction (P > 0.05).
Figure 1.

Baseline core body temperature (CBT) in young and aging rats (A). Following E. coli administration young rats showed alterations in CBT from baseline on day 0 (B) and day 1 (C), and aging rats showed alterations from baseline on day 0 (D), day 1 (E), day 2 (F), and day 3 (G). Error bars represent ±SEM. Dark bark represents the dark phase of day. *=P < 0.05.
On the day of the E. coli injection (day 0, light phase), young rats exhibited a fever starting within an hour after administration and lasting 6 hr (Fig. 1B). Repeated measures ANOVA indicated a main effect of group (pre-E. coli CBT vs. post E. coli CBT) (F(1,12) = 7.36, P = 0.02); and a significant group × time interaction (F(11, 132) = 17.04, P < 0.0001). Post hoc tests revealed that E. coli treatment resulted in a significant drop in CBT at 9am, followed immediately by a significant rise in CBT lasting 6 hr. During the dark phase, there were no differences in CBT on this day compared to baseline CBTs. That is, there was no main effect of group (P > 0.05), and no group × time interaction (P > 0.05). The next day (day 1, light phase), young rats had a significantly higher CBT compared to baseline CBTs (Fig. 1C). There was a main effect of group (F(1,12) = 8.53, P = 0.01), and a group × time interaction (F(11,132) = 2.91, P = 0.002). Post hoc tests revealed that E. coli treatment significantly elevated CBT starting around 10am and remained elevated for 7hr. CBTs in young rats returned to baseline levels during the dark phase of day 1, and remained there through the end of the experiment, on day 6 (figures not shown). There were no main effects of group (P> 0.05), or group × time interactions (P> 0.05).
Older rats had a very different response to the E. coli treatment. On the day of the E. coli injection (day 0, light phase), older rats exhibited unstable thermoregulation, with a short hypothermic response ~30 min after E. coli administration, followed by a short fever (Fig. 1D). There was no main effect of group (P > 0.05), but a significant group × time interaction (F(11, 88) = 18.87, P < 0.0001). Post hoc tests revealed that E. coli treatment significantly reduced CBT (starting at 8am), for 3hr. CBT then significantly increased (starting at 1pm), for 3hr. During the dark phase of the same day, old rats experienced a marked hypothermic response lasting 12 h (Fig. 1D). There was a main effect of group (F(1, 8) = 57.77, P < 0.0001) and a group × time interaction (F(11,88) = 2.04, P = 0.03). Interestingly, the following day (day 1, light phase), the older rats exhibited a fever lasting ~9 h (Fig. 1E). There was a significant main effect of group (F(1, 8) = 42.46, P = 0.0002) and a group × time interaction (F(11, 88) = 8.43, P < 0.0001). During the dark phase of day 1 (Fig. 1E), and the light phase of day 2 (Fig. 1F) and day 3 (Fig. 1G), E. coli treatment resulted in overall higher CBTs than baseline CBTs. There were main effects of group [(F(1, 8) = 9.16, P = 0.02); (F(1, 8) = 15.11, P = 0.005); (F(1, 8) = 8.78, P = 0.02), respectively], but no group × time interactions (P > 0.05). CBTs in old rats returned to baseline levels during the dark phase of day 4, and remained equivalent to baseline CBTs through the end of the experiment, on day 6 (figures not shown). There were no main effects of group (P> 0.05), or group × time interactions (P> 0.05).
Baseline activity counts (one day before E. coli administration) were not different between young and old rats during the light phase, but young rats had significantly higher activity counts than did old rats during the dark phase (Fig. 2A). A repeated measures ANOVA showed no main effect of age (P > 0.05), and no age × time interaction (P > 0.05) during the light phase. During the dark phase there was a main effect of age (F(1,10) = 18.52, P = 0.002), but no age × time interaction (P > 0.05).
Figure 2.

Baseline activity counts in young and aging rats (A). Following E. coli administration, young (B) and aging (C) rats showed decreases in activity only on day 0. Error bars represent ±SEM. Dark bark represents the dark phase of day. *=P < 0.05.
On Day 0, E. coli induced a drop in activity ~2 h before the onset of the dark phase in young rats, and this reduction continued through the end of the dark phase on day 0 (Fig. 2B). A repeated measures ANOVA showed no main effect of group (P > 0.05); but a group × time interaction (F(11, 132) = 2.57, P = 0.006) for the light phase of day 0. There was a main effect of group (F(1, 12) = 14.38, P = 0.003); but no group × time interaction (P > 0.05) for the dark phase of day 0. By day 1, young rats had returned to their baseline activity and there were no main effects of group and no group × time interactions (P > 0.05) through the end of the experiment, on day 6 (Figures not shown).
Older rats did not exhibit a significant difference in activity in response to E. Coli compared to their baseline activity counts during the light phase of day 0, but exhibited a significant reduction in activity during the dark phase of the same day (Fig. 2C). There was no main effect of group (P > 0.05), and no group × time interaction (P > 0.05), during the light phase. During the dark phase, there was a main effect of group (F(1, 8) = 14.24, P = 0.005), but no group × time interaction (P > 0.05). By day 1 older rats had returned to their baseline activity, and there were no main effects of group and no group × time interactions (P > 0.05) through the end of the experiment, on day 6 (Figures not shown).
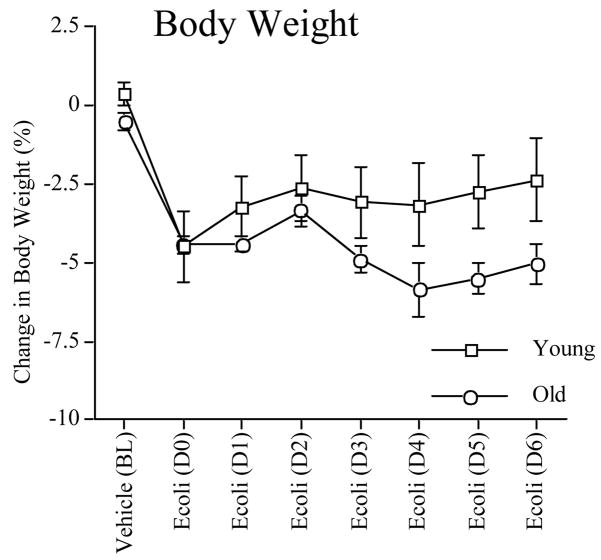
Body weight changes following the vehicle or E. coli injection were not different between the two age groups (P > 0.05; Fig. 3). Both groups lost ~4% of their body weight 24 h after E. coli administration. The six days following infection however, showed a trend that aging rats continued to lose more body weight than did young rats, although this did not reach statistical significance on any day. A repeated measures analysis showed no main effect of age (P > 0.05), a main effect of day (F(1, 6) = 5.74, P < 0.0001), and a significant age × day interaction (F(6, 60) = 6.73, P < 0.0001).
Figure 3.
Body weight changes in young and aging rats following a vehicle injection, an E. coli injection, and the subsequent six days. Error bars represent ±SEM.
Discussion
The CBT data are consistent with what others have found (Buchanan et al., 2008; Foster et al., 1992; Miller et al., 1991; Scarpace et al., 1992). That is, aging rats exhibited a blunted and delayed rise in CBT within the first twelve hours following infection with E. coli. Interestingly, the time period beyond 12 hr revealed a very different pattern. For the next 12 h aging rats showed a marked and long-lasting hypothermic response. And, for the three days thereafter, they had significantly elevated CBTs during the light phase of the day. These finding offer a quite different view of aging rats’ CBT responses to an immune challenge than would be derived from prior work. It is clearly a delayed response, yet it is exaggerated in keeping with the other exaggerated and/or prolonged sickness responses observed in aging animals (Barrientos et al., 2009; Godbout et al., 2005; Huang et al., 2008). This pattern of results was very different to the CBT response in young rats. Although baseline activity level was lower in aging rats than in young rats during the dark phase, the pattern of activity changes between the age groups was not different after E. coli treatment. That is, both groups exhibited a significant reduction in activity from their own baseline levels during the dark phase on day 0, but returned to baseline levels the next day. While some studies have found activity levels to be significantly reduced for longer periods in aging animals than in young animals (Godbout et al., 2005; Huang et al., 2008), others have not (Kinoshita et al., 2008).
Although body weight loss was equivalent in young and older rats one day after E. coli infection, older rats showed a trend of losing more weight over time than did young rats. These data are consistent with prior studies (Godbout et al., 2005).
The aging-induced prolongation of CBT changes following E. coli could be mediated by aging-induced increases in the duration of either peripheral or central signals, such as cytokines. However, the source is not likely to be peripheral because spleen and serum IL-1 responses to E. coli do not differ between young and old F344/BN rats (Barrientos et al., 2009). Although it should be noted that neither other cytokines or proinflammatory proteins in the liver, an immune organ shown to be instrumental in relaying a proinflammtory signal to the brain (Campbell et al., 2005), were examined. It has also been shown that bacterial clearance is not impaired in aging rats (Barrientos et al., 2009), so it is less likely that the exaggerated levels of brain cytokines and resulting behaviors are due to an exaggerated peripheral cytokine response.
With regard to central cytokine signals, the aging process has been suggested to prime microglia, resulting in increases in antigenic and morphological markers of activation (Frank et al., 2006; Godbout et al., 2005). Upon stimulation, with an immune challenge for example, these primed microglia release exaggerated levels of proinflammatory cytokines within the brain (Barrientos et al., 2009; Barrientos et al., 2006; Dilger and Johnson, 2008; Godbout et al., 2005; Perry et al., 2003). In aging animals similar to those used in the present study this exaggerated response lasts at least eight days (Barrientos et al., 2009). Thus, glial priming and the consequent exaggerated neuroinflammatory response may be at the core of these exaggerated and prolonged sickness behavioral responses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barrientos RM, Frank MG, Hein AM, Higgins EA, Watkins LR, Rudy JW, Maier SF. Time course of hippocampal IL-1 beta and memory consolidation impairments in aging rats following peripheral infection. Brain Behav Immun. 2009;23:46–54. doi: 10.1016/j.bbi.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Higgins EA, Biedenkapp JC, Sprunger DB, Wright-Hardesty KJ, Watkins LR, Rudy JW, Maier SF. Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiol Aging. 2006;27:723–732. doi: 10.1016/j.neurobiolaging.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Buchanan JB, Peloso E, Satinoff E. A warmer ambient temperature increases the passage of interleukin-1beta into the brains of old rats. Am J Physiol Regul Integr Comp Physiol. 2008;295:R361–368. doi: 10.1152/ajpregu.00104.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell SJ, Perry VH, Pitossi FJ, Butchart AG, Chertoff M, Waters S, Dempster R, Anthony DC. Central nervous system injury triggers hepatic CC and CXC chemokine expression that is associated with leukocyte mobilization and recruitment to both the central nervous system and the liver. Am J Pathol. 2005;166:1487–1497. doi: 10.1016/S0002-9440(10)62365-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Bluthe RM, Gheusi G, Cremona S, Laye S, Parnet P, Kelley KW. Molecular basis of sickness behavior. Ann N Y Acad Sci. 1998;856:132–138. doi: 10.1111/j.1749-6632.1998.tb08321.x. [DOI] [PubMed] [Google Scholar]
- Dilger RN, Johnson RW. Aging, microglial cell priming, and the discordant central inflammatory response to signals from the peripheral immune system. J Leukoc Biol. 2008;84:932–939. doi: 10.1189/jlb.0208108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster KD, Conn CA, Kluger MJ. Fever, tumor necrosis factor, and interleukin-6 in young, mature, and aged Fischer 344 rats. Am J Physiol. 1992;262:R211–215. doi: 10.1152/ajpregu.1992.262.2.R211. [DOI] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Biedenkapp JC, Rudy JW, Watkins LR, Maier SF. mRNA up-regulation of MHC II and pivotal pro-inflammatory genes in normal brain aging. Neurobiol Aging. 2006;27:717–722. doi: 10.1016/j.neurobiolaging.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. Faseb J. 2005;19:1329–1331. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- Hart BL. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- Huang Y, Henry CJ, Dantzer R, Johnson RW, Godbout JP. Exaggerated sickness behavior and brain proinflammatory cytokine expression in aged mice in response to intracerebroventricular lipopolysaccharide. Neurobiol Aging. 2008;29:1744–1753. doi: 10.1016/j.neurobiolaging.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita D, Cohn DW, Costa-Pinto FA, de Sa-Rocha LC. Behavioral effects of LPS in adult, middle-aged and aged mice. Physiol Behav. 2008 doi: 10.1016/j.physbeh.2008.10.018. [DOI] [PubMed] [Google Scholar]
- Kluger MJ. Fever: role of pyrogens and cryogens. Physiol Rev. 1991;71:93–127. doi: 10.1152/physrev.1991.71.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D, Yoshikawa T, Castle SC, Norman D. Effect of age on fever response to recombinant tumor necrosis factor alpha in a murine model. J Gerontol. 1991;46:M176–179. doi: 10.1093/geronj/46.5.m176. [DOI] [PubMed] [Google Scholar]
- Perry VH, Newman TA, Cunningham C. The impact of systemic infection on the progression of neurodegenerative disease. Nat Rev Neurosci. 2003;4:103–112. doi: 10.1038/nrn1032. [DOI] [PubMed] [Google Scholar]
- Scarpace PJ, Borst SE, Bender BS. The association of E. coli peritonitis with an impaired and delayed fever response in senescent rats. J Gerontol. 1992;47:B142–145. doi: 10.1093/geronj/47.4.b142. [DOI] [PubMed] [Google Scholar]