Abstract
In daily activities, humans must attend and respond to a range of important items and inhibit and not respond to unimportant distractions. Our current understanding of these processes is largely based on perceptually simple stimuli. This study investigates the interaction of conceptual-semantic categorization and inhibitory processing using Event Related Potentials (ERPs). Participants completed three Go-NoGo tasks that increased systematically in the degree of conceptual-semantic information necessary to respond correctly (from single items to categories of objects and animals). Findings indicate that the N2 response reflects inhibitory processing but does not change significantly with task difficulty. The P3 NoGo amplitude, on the other hand, is attenuated by task difficulty. Further, the latency of the peak of the P3 NoGo response elicited by the most difficult task is significantly later than are the peaks detected during performance of the other two tasks. Thus, the level of complexity of conceptual-semantic representations influences inhibitory processing in a systematic way. This inhibition paradigm may be a key for investigating inhibitory dysfunction in patient populations.
Keywords: Event Related Potentials, inhibition, N2-P3, Go-NoGo, object-animal categorization, visual categorization, conceptual difficulty
Everyday functioning requires the ability to successfully inhibit irrelevant stimuli, thoughts, and behaviors (Logan & Cowan, 1984; Posner & DiGirolamo, 1998). To date, research on response inhibition has started to localize some of the basic neural processes associated with this behavior (Folstein & van Petten, 2008 or Mostofsky & Simmonds, 2008 for review). However, despite the real world implications of successful and unsuccessful inhibition, as reported in Attention Deficit Hyperactivity Disorder (Barkley, 1997; Luu & Tucker, 2002) and healthy aging (Hasher & Quig, 1997), little work has focused on how response inhibition changes as tasks become conceptually more abstract: for example, knowing to stop the car for red lights, small children, or a stray dog, but not for a few leaves blowing across the street. Amongst the most commonly documented manifestations of inhibitory processing are the Event Related Potentials (ERPs) associated with the Go-NoGo task, in which participants press a button for one type of stimuli and withhold a button response for a second type of stimuli. This task provides a reliable index of inhibitory processes (Perner, Lang & Kloo, 2002; Simpson & Riggs, 2006; Weintraub, 2000) and elicits predictable changes in the N2 and P3 ERP components. Further, the Go-NoGo task has been used to effectively measure abstract and rapid object categorization (Kincses, Chadaide, Varga, Antal, & Paulus, 2006; Siakaluk, Buchanan, & Westbury, 2003; VanRullen & Thorpe, 2001). The goal of this study is to investigate how inhibitory processing changes as the cognitive demands necessary to respond become systematically less perceptual and more conceptual-semantic in nature.
To date, ERP research has identified two components that relate to inhibitory processing, the N2 and P3 components. Both of these components display larger amplitudes when inhibiting a motor response compared to what is elicited during execution of the response. The N2 is found over fronto-central areas, peaking around 250 ms after stimulus presentation. The P3 is a fronto-central component peaking around 300 ms after stimulus presentation. The relationship between the N2, the P3, and inhibitory processing is debated (Bruin, Wijers, & van Staveren, 2001; Lavric, Pizzagalli, & Forstmeier, 2004; Smith, Johnstone, & Barry, 2006, 2007, 2008). Some argue that the inhibitory processes are manifested in the N2 (Ciesielski, Harris, & Cofer, 2004; Jodo & Kayama, 1992; Kopp, Mattler, Goertz, & Rist, 1996; van Veen & Carter, 2002), while others argue that the P3 is a more likely candidate as a measure of inhibition (Bruin, Wijers, & van Staveren, 2001; Smith, Johnstone, & Barry, 2006, 2007, 2008). There is, however, a general consensus that both are markers of inhibition to some degree (Smith, Johnstone & Barry, 2007). The present study does not aim to differentiate these components, but to uncover whether and how each of them is influenced by conceptual-semantic processing.
The literature addressing the influence of stimulus type and stimulus presentation on the inhibitory N2 and P3 components has been varied in regard to methodologies and outcomes. Increasing task difficulty by methodological manipulations such as increasing the speed with which participants had to respond (Jodo & Kayama, 1992) resulted in an increase in the N2 NoGo amplitude but no change in the P3. Similarly, Nieuwenhuis, Yeung, and Cohen (2004) found that as the perceptual overlap between the Go and NoGo stimuli increased (for example, discriminating T from F) the amplitude of the N2 NoGo response also increased. When the task difficulty has been more semantic as opposed to perceptual, i.e. stopping on the word “go” and going on the word “stop”, a decrease in the N2 and P3 amplitudes has been reported (Schapkin, Falkenstein, Marks, & Griefahn, 2006). Thus, the limited research to date indicates that while increases in perceptual difficulty may result in increases in the N2 responses, conceptual and semantic increases in task demand appear to result in a decrease in the amplitudes of the N2 and P3 responses.
The goal of this study is to delineate how inhibitory processes are influenced by conceptual-semantic processing in a systematic way. To investigate this question we used object categorization, a core cognitive ability that calls on both perceptual and conceptual-semantic knowledge (French, 1995; French, Mareschal, Mermillod, & Quinn, 2004; Goldstone, & Barsalou, 1998; Murphy & Kaplan, 2000; Schyns, Goldstone & Thibaut, 1998). Categories at the basic level, such as dogs, horses, and birds can be formed and differentiated from one another based on perceptual features (French et al., 2004). In fact, it has been found that pre-lingual infants as young as 3–4 months of age can group dogs as distinct from cats based on quite complex commonalities and differences in their component visual features (French et al., 2004). However, often times we need categories that extend beyond perceptual similarities. One example would be the category ‘animals’, which includes disparate perceptual entities such as a snake and an elephant. In forming these equally important categories humans must rely more heavily on abstract conceptual and semantic processes.
Using ERP responses to the Go-NoGo paradigm for abstract categories of objects and animals, Thorpe and Fize (1996), reported differences between Go (objects) and NoGo (animals) trials by about 150 ms over frontal areas, even using animals in complex scenes compared to a wide range of non-animal target items as the stimuli. The authors concluded that this was a marker of the human capacity to categorize perceptually dissimilar items in to conceptually meaningful categories at an “ultra-rapid” pace. Given the use of a Go-NoGo paradigm, it is likely that the differences reported in this study were also related to the N2 inhibitory process, meaning that the two processes of inhibition and categorization operate contemporaneously, and are mediated in the same or closely approximated brain regions. However, the study did not employ multiple levels of categorization from perceptually-based to abstract. As a result, the nature of this interaction remains unclear.
In this study, we address the issue of how inhibitory processes and conceptual-semantic complexity interact by using three different inhibition Go-NoGo tasks which each require different levels of semantic abstraction to make a correct response. Each includes Go items presented 80% of the time and NoGo items presented 20% of the time. The “Single” task includes one image of a car (Go) and one image of a dog (NoGo). Because the identical images are repeated, the perceptual properties of the items stay identical, limiting the need to categorize across distinct images to respond correctly. The “Multiple” task contains multiple pictures of cars (Go) and multiple pictures of dogs (NoGo) that vary in orientation and subordinate type of item (e.g., SUVs, trucks, convertibles, beagles, great danes, golden retrievers). Thus, correctly responding requires some item-level identification across category exemplars that can be accomplished by focusing on common perceptual features of the items (legs, eyes, wheels, windshields) and then grouping them based on their semantic item level representation (dog or car). It is important to note that responses to this task can also be made on the supra-ordinate level of object or animal, but only one basic item from each supra-ordinate (i.e., only dogs, as opposed to dogs in addition to other animals) category was used. The “Semantic” task is the least perceptual, most conceptual-semantic task. It includes a wide range of perceptually dissimilar non-animals (Go), from the categories of clothing, tools, furniture, and vehicles and a wide range of animals (NoGo), including a spider, a worm, a lobster, and a dog. Although there are some perceptual features that can be used to identify the animals as distinct from all other items, such as eyes and legs, these were kept to a minimum, biasing toward a focus on the semantic categorization of items. In addition, we have included a more standard Go-NoGo design (“Standard”), using arrows (Go) and octagons (NoGo) which should elicit pre-learned inhibitory responses as a check that the expected inhibitory responses are being elicited.
This design allows us to address our primary question: do inhibitory processes as measured by the N2 and P3 components vary based on the amount of perceptual and conceptual-semantic information necessary to correctly respond? We predict that each of these tasks, regardless of difficulty, will require inhibitory processing that will result in larger amplitudes for the NoGo items compared to Gos in the N2 and P3. By comparing across tasks we can investigate the interaction between inhibitory processes and conceptual-semantic information. We predict that if the differences between Single, Multiple, and Semantic are the result of a difficulty in distinguishing the Go items from NoGo items on a perceptual level, the N2 NoGo amplitudes will decrease systematically with difficulty. Specifically, Semantic would be significantly larger than Multiple which would be significantly larger than Single. In this case, there would be no corresponding changes in the P3. However, we predict that these differences will not be perceptual, but conceptual in nature, which would influence the P3 amplitude, particularly a decrease with semantic-conceptual difficulty as well as a decrease in the N2 amplitude.
Methods
Subjects
Thirty-five subjects were recruited from the University of Texas at Dallas community via word of mouth and web-based advertising. All subjects were between the ages of 18 and 31. The subjects were all college students with at least 12 years of education. Subjects were screened, per exclusion criteria, to be free from history of traumatic brain injury and other significant neurological issues (CVA, seizure disorders, history of high fevers, tumors, or learning disabilities). Exclusion criteria also included left-handedness, use of alcohol or other controlled substances within twenty-four hours of EEG administration, and medications other than over-the-counter analgesics and birth-control pills.
Subjects were not included in the final analyzed data set if one or more tasks included major movement artifact, failure to comprehend the instructions, errors of omission or commission above 20%, or excess noise (N = 3). Thus, data from 32 subjects across all four tasks were fully analyzed. The pool of included subjects was 14 males and 18 females. Informed written consent was collected from each subject according to the rules of the Institutional Review Board of The University of Texas at Dallas. This study was conducted according to the Good Clinical Practice Guidelines, the Declaration of Helsinki, and the U.S. Code of Federal Regulations.
Stimuli
Four different tasks were developed, each following the basic Go-NoGo paradigm. In each task, there were 160 (80%) ‘Go’ stimuli for which the subject was to press a button and 40 (20%) ‘NoGo’ stimuli for which the subject was instructed to withhold a response. In each of the four tasks stimuli were presented for 300 milliseconds followed by a fixation point (+) for 1700 milliseconds. All of the stimuli were black line drawings fitted to a white 600 × 600 pixel square.
As can be seen in Figure 1, in the first task (Standard) the Go stimulus was an arrow and the NoGo stimulus was an octagon. It was predicted that these images would include semantic information about the required response, with an arrow to press a button and an octagon (like a stop sign) to not press the button. Thus, the items were predicted to be cognitively rudimentary compared to the stimuli used in the subsequent tasks. In the second task (Single), the Go stimulus was a line drawing of a car and the NoGo stimulus was a line drawing of a dog. Because the exact same images were repeated multiple times, no categorization of perceptually variable items was necessary for making a response. As such, this is the most perceptually based condition. In the third task (Multiple), the Go stimuli included 40 cars and the NoGo stimuli included 10 dogs, which were each repeated 4 times each. To increase the amount of perceptual similarity, all of the images of cars and dogs were drawn as side views. The images of cars included at least 2 wheels, front seat and backseat windows and side-view mirrors. The images of dogs included at least 2 legs, an eye, ears, a nose and a tail. Unlike the Single condition the size and orientation of the items differed across stimuli, but each exemplar of the dog and car categories includes these perceptual cues. Each subject saw each of the cars and the dogs presented in a random order.
Figure 1.
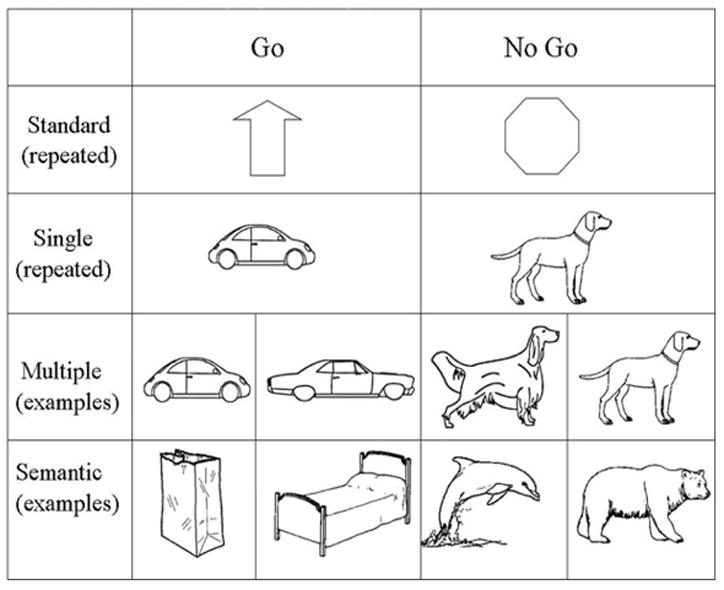
Sample of stimuli used across all four inhibition tasks. Go items were shown 160 times, or 80%, while NoGo were shown 40 or 20%. For the Standard and Single tasks these specific items were repeated 160 and 40 times. For the Multiple and Semantic conditions there are example items taken from the larger set.
In the final task, (Semantic), the Go stimuli consisted of 160 objects (40 food items, 40 cars, 20 clothing items, 20 kitchen items, 20 body parts, and 20 tools) and the NoGo stimuli consisted of 40 animals of varying visual typicality. These ranged from animals seen frequently in daily life, such as dogs and cats, to those seen less frequently such as lobsters and elephants. To decrease the reliance on perceptual cues when performing this task, features often seen in animals were decreased in presentation frequency in the animal condition and increased in the object condition. For example, some of the animals (worm, butterfly, fish) had no legs, while some of the objects (chair, table, a pair of pants) did have legs. Similarly multiple animals without discernable eyes were included, such as the worm, the fly and the lobster. By limiting the perceptual cues that are available in the Multiple and Single conditions participants were required to rely more on semantic-conceptual information to make category responses.
The images were from the standardized picture sets Snodgrass and Vanderwart (1980) and the Boston Naming Test (Kaplan, Goodglass & Weintraub, 1983) or were created in-house. All attempts were made to keep the images in a similar style and the line thickness of all pictures was adapted to range between 5 pixels and 14 pixels to maintain similarity in visual complexity.
Six randomizations were created to eliminate order effect within each task and the order in which the subjects performed the tasks was counterbalanced to mitigate order effects. The instructions were identical across tasks with only the important object names changed, i.e., “You are going to see pictures of cars (objects) and dogs (animals). You are to press the button for all cars (objects) but do not press the button for anything else”. Instructions were given verbally and then displayed on the computer screen prior to each task.
Data Acquisition and Preprocessing
Continuous EEG was recorded from 64 silver/silver-chloride electrodes mounted within an elastic cap (Neuroscan Quickcap) which are placed according to the Interanatioal 10–20 electrode placement standard (Compumedics, Inc.). The data was collected using a Neuroscan SynAmps2 amplifier and Scan 4.3.2 software sampled at 1 kHz with impedances typically below 10 kΩ. Blinks and eye movement were monitored via two electrodes, one mounted above the left eyebrow and one mounted below the left eye. The data were processed to remove ocular and muscle artifacts in the following way. First, poorly functioning electrodes were identified visually and removed. Second, eye blink artifacts were removed by a spatial filtering algorithm in the Neuroscan Edit software using the option to preserve the background EEG. Third, time segments containing significant muscle artifacts or eye movements were rejected. The continuous EEG data were band-pass filtered from 0.15 Hz to 30 Hz for analysis. The EEG data were segmented offline into 2 second epochs spanning 100 ms before to 1500 ms after the presentation of the visual stimuli.
Reference Correction
The data were recorded with the ground at AFz and the reference electrode located near the vertex, resulting in small amplitudes over the top of the head. In order to eliminate this effect, the data were re-referenced to the average potential over the entire head, which approximates the voltages relative to infinity (Nunez, 1981). In order to minimize a small bias in the electrode-based average reference (Junghöfer et al., 1999), a spline-based estimate of the average scalp potential (Ferree, 2006) was computed using spherical splines (Perrin et al., 1989). Placing the electrode cap on a realistic phantom head, the electrode coordinates were digitized (Polhemus, Inc.), and these coordinates were used to fit the splines for each subject. In subjects with a small number of bad electrodes, the splines were used to interpolate those electrodes, to yield a total of 62 data channels in every subject.
ERP Calculation
For each trial and electrode, the mean amplitude of the prestimulus interval (−100 ms − 0 ms) was subtracted from each time point and those data were averaged across trials to create the ERP. Only those items to which correct responses were given were included in the analysis. The waveforms for a midline frontal electrode (Fz) can be seen in Figures 2 and 3.
Figure 2.
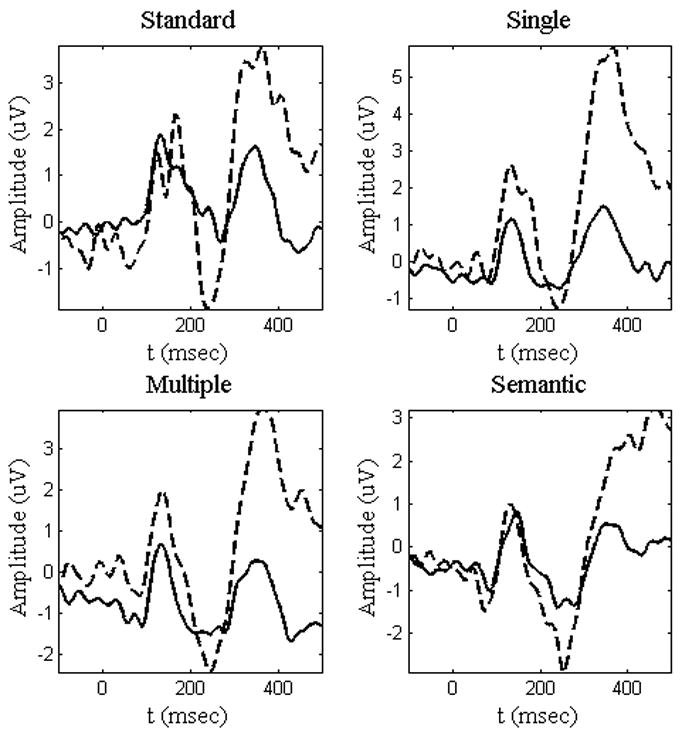
N2 and P3 Go and NoGo responses at Fz for Go (solid) and NoGo (dashed) conditions across all tasks. The traditional N2 and P3 components, which display a larger amplitude for NoGo than Go responses, can be seen for each condition.
Figure 3.
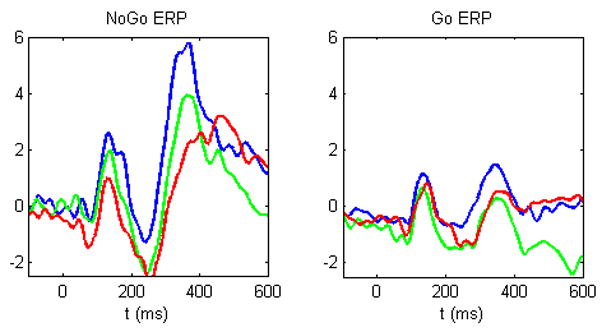
Grand average at Fz across the three inhibition tasks: Single (blue), Multiple (green), and Semantic (red). As can be seen in this depiction there is a decrease in the amplitude of the P3 with task difficulty. A similar trend in the N2.
Peak Analysis
Figure 4 depicts the grand mean average across the scalp for Multiple, which is typical of all of the studies. Of note is that the N2 and P3 effects are distinctly visible. The N2 is focused over frontal midline and bilateral sites. The P3, on the other hand, is maximal over a wide fronto-central area.
Figure 4.
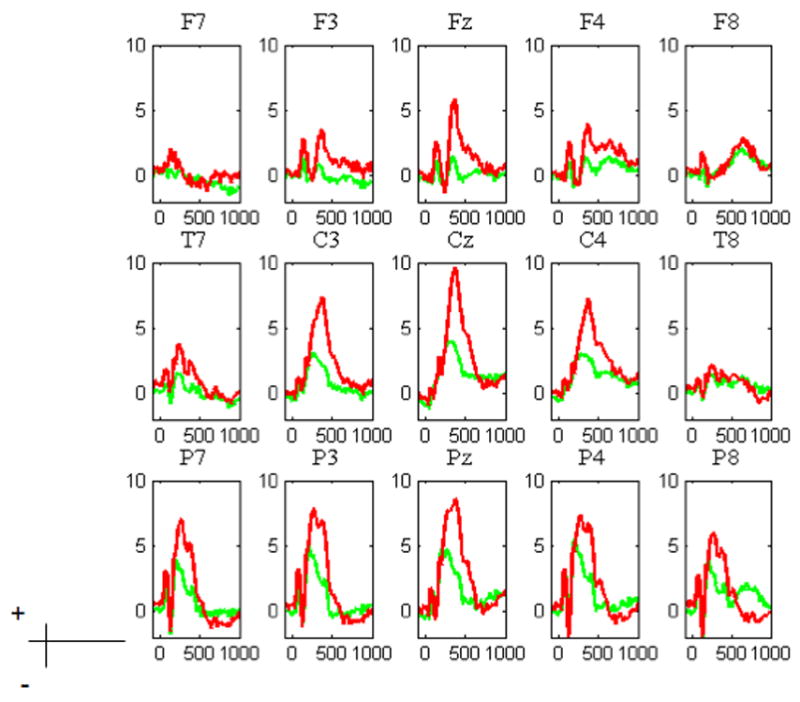
Grand average of selected electrodes of interest for the Multiple Task: Go (green) NoGo (red). As can be seen in the figure, the inhibitory Go-NoGo effect was largest over the frontal areas.
The ERP topographies for N2 and P3 responses separately were averaged across sets of electrodes displaying maximal responses. Based on previous literature and visual inspection of individual and group data across the four tasks, we defined two time areas of interest. For the N2 component we calculated the peak amplitudes over frontal areas by identifying the peak amplitude between 150–300 msec for midline frontal electrodes (FPz, FP1, FP2, AF3, AF4, Fz, F1, F2) for each condition for each participant then averaging across participants. For the P3 effect, the mean peak voltage at central sites (FCz, FC1, FC2, Cz, C1, C2, CPz, CP1, CP2) was calculated between 300–600 msec.
Results
Behavioral Error Rates: Errors of Omission
An error of omission is a failure to respond to a Go item. To determine this tendency across tasks, a 4 (task: Standard, Single, Multiple, Semantic) X 2 (condition: Go, NoGo) repeated-measures ANOVA on the percentage of correct Go responses was calculated. A lower percentage represents more errors of omission. This analysis revealed a significant task difference, F(3, 93) = 3.88, p = .012. Further there was a significant linear trend, F(1, 30) = 6.82, p = .014 in which percent correct decreased with task difficulty, as is depicted to Table 1. Following this trend, directed pair-wise comparisons were performed between each task and the next most difficult (Standard to Single, Single to Multiple, Multiple to Semantic). On a pair-wise comparison level, none of the comparisons were statistically significant after Bonferroni corrections were applied.
Table 1.
Means and Standard Deviations of Behavioral Results Across Tasks
| Experiment | Go Correct | NoGo Correct | Reaction Times ms |
|---|---|---|---|
| Standard | 97.5% (4.3) | 82.7% (13.4) | 307.7 (9.9) |
| Single | 93.9% (17.6) | 82.7% (18.9) | 293.7 (5.9) |
| Multiple | 93.1% (17.9) | 83.3% (18.6) | 307.7 (5.8) |
| Semantic | 90.8% (18.2) | 82.5% (18.5) | 361.1 (10.7) |
Error Rates: Errors of Commission
An error of commission is responding with a button press to a NoGo item. In this case a higher percentage indicates fewer errors. To calculate this tendency across tasks a 4 (task: Standard, Single, Multiple, Semantic) X 2 (condition: Go, NoGo) repeated-measures ANOVA on the percent of correct NoGos revealed no significant differences across tasks. These means are also listed in Table 1.
Reaction Times
The reaction times for the Semantic task were longer than the others by approximately 50 ms. To investigate the significance of processing differences between the tasks we performed a one-way ANOVA of the reaction times for Go items. The ANOVA revealed a main effect of task, F(3, 93) = 11.32, p < .0001. Subsequent paired samples t-tests with Bonferroni corrections revealed significant differences between Semantic and each of the other tasks, Standard, t(31) = 3.61, p = .001, Single, t(31) = 4.74, p < .001 and Multiple, t(31) = 3.68, p < .001. Other comparisons did not reach significance.
Event Related Potentials
A comparison of Go and NoGo waveforms for each task can be seen in Figure 2, with a comparison across tasks for Go and NoGo responses in Figure 3. In creating the ERPs only correct responses were included and participants had to have at least 20 artifact-free and correct-response epochs per condition. Across tasks there were no significant differences in the number of trials included based on a 2 × 3 repeated-measures ANOVA, p > 0.50. The mean number of epochs included in the participants’ average ERP per condition is as follows, Goes: Single 120.65, Multiple 130.09, Semantic 122.21, NoGoes: Single 28.94, Multiple 30.09, Semantic 30.28. Thus, although there is large variability in the error rates of participants, this did not differentially influence the number of items included in the analysis of each study.
N2
The first question was whether the Go-NoGo N2 inhibitory effects elicited by each of these tasks were similar across tasks. The Standard task was assumed to be a baseline against which to compare responses recorded during performance of the three tasks of interest. To determine this for each task the N2 Go and NoGo amplitudes were compared at mid-frontal sites between 150 and 300ms. As can be seen in Table 2 all of the tasks of interest elicited significantly higher NoGo N2 effects compared to Go effects, in a pattern similar to the Standard task. The only exception is the Semantic task, in which the effect is significant at p = 0.01 without corrections for multiple comparisons, but is not quite significant when these corrections are taken into account.
Table 2.
N2 Amplitude Findings across Experiments
| Experiment | Go Amp | No Go Amp | t-stat | p-value |
|---|---|---|---|---|
| Standard | −3.13 | −5.40 | 3.68 | < 0.0001* |
| Single | −3.34 | −4.62 | 3.00 | 0.0022* |
| Multiple | −3.57 | −4.79 | 2.77 | 0.0005* |
| Semantic | −3.13 | −4.18 | 2.45 | 0.01 |
p < alpha (0.006) with corrections for multiple comparisons
The next question was how the N2 components were influenced by semantic level of representation. Because the Standard task was a control that is conceptually dissimilar to the three semantic inhibition tasks, in that abstract symbols with preconceived meanings were used instead of objects and animals, it was removed from these comparative analyses. A 3 (task: Single, Multiple, Semantic) × 2 (condition: Go, NoGo) ANOVA performed on the midline frontal electrodes for the average peak amplitude for the time points between 150–300ms revealed a significant interaction, F(2, 62) = 17.78, p = 0.04. There was also a main effect of task, F(2, 62) = 17.08, p = 0.008, and the main effect of condition nearly reached significance, F(2, 62) = 3.77, p = 0.06. To assess the nature of this interaction two ANOVAs were performed comparing across tasks within each condition, Go and NoGo, but neither ANOVA revealed statistically significant interactions.
P3
As with the N2 effects, we first tested the hypothesis that the expected Go-NoGo inhibition effect was elicited by each of our tasks. To do so, t-tests were performed on the mean peak amplitudes of the Go conditions compared to the NoGo condition for each condition between 300 ms and 600 ms over fronto-central sites. As can be seen in Table 3, all of the NoGos elicited significantly larger P3 amplitudes than the Gos, all p’s < 0.0001.
Table 3.
P3 Amplitude Findings across Experiments
| Experiment | Go Amp | No Go Amp | t-stat | p-value |
|---|---|---|---|---|
| Standard | 2.93 | 6.04 | 7.21 | < 0.0001* |
| Single | 3.04 | 7.35 | 5.60 | < 0.0001* |
| Multiple | 1.89 | 5.75 | 5.71 | < 0.0001* |
| Semantic | 2.26 | 5.04 | 5.07 | < 0.0001* |
p < alpha (0.006) with corrections for multiple comparisons
Once the expected inhibition effect was verified in comparison to the Standard task, the Standard task was removed from further analyses. To determine how task influenced inhibitory processing as measured by the P3 Go-NoGo a 3 (task: Single, Multiple, Semantic) × 2 (condition: Go, NoGo) ANOVA was performed. The ANOVA revealed an interaction, F(2, 62) = 21.30, p < 0.0001, as well as main effects of task and condition, all p’s < 0.002. To follow up on the interaction two ANOVAs were performed comparing across tasks within each condition. The one way ANOVA on Go amplitudes revealed no significant differences between tasks. However there were significant differences in the NoGo condition, F(2, 62) = 5.77, p = 0.005. As can be seen in Table 4, subsequent t-tests revealed significant differences between Single and Multiple, and Single and Semantic, but not between Multiple and Semantic.
Table 4.
NoGo P3 Amplitude Differences between Tasks
| Comparison | df | t-value | p-value |
|---|---|---|---|
| Single – Multiple | 31 | 2.40 | 0.001* |
| Single – Semantic | 31 | 3.29 | 0.001* |
| Multiple – Semantic | 31 | 0.99 | 0.16 |
p < alpha (0.012) with corrections for multiple comparisons.
Because the P3 Go effect is often reported to be maximal at central compared to fronto-central areas and this was confirmed based on visual inspection of our grand averages across the tasks, we also performed this analysis on the 300–600 ms epoch over centro-parietal electrodes. To determine how task influenced inhibitory processing as measured by the P3 Go-NoGo, a 3 (task: Single, Multiple, Semantic) × 2 (condition: Go, NoGo) ANOVA was performed at midline centro-parietal sites from 300–600ms after stimulus presentation, which revealed no significant interaction.
Visual inspection of the data also seemed to show that there were large latency differences between tasks for the NoGo conditions (See Figure 3). As a result, we also performed a one way between task ANOVA for the NoGo peak latencies between 300–600 ms over the fronto-central regions, which revealed significant task differences, F(2, 31) = 11.51, p = 0.0005. As can be seen in Table 5, subsequent t-tests revealed that there were significant differences between Single and Semantic, and Multiple and Semantic, but not Single and Multiple. The latency for the Semantic NoGo P3 (M = 449.42 ms, SD = 68.07) was significantly longer than for Single (M = 404.30 ms, SD = 54.00) or Multiple (M = 403.33 ms, SD = 48.91).
Table 5.
NoGo P3 Latency Differences between Tasks
| Comparison | df | t-value | p-value (one-tailed) |
|---|---|---|---|
| Single – Multiple | 31 | 0.08 | 0.94 |
| Single – Semantic | 31 | 2.99 | 0.002* |
| Multiple – Semantic | 31 | 3.94 | 0.0002* |
p < alpha (0.012) with corrections for multiple comparisons.
Discussion
The focus of this study was to investigate how increased levels of conceptual-semantic processing influence inhibitory processing. We investigated how behavioral measures and known neural markers of inhibitory processing (the N2 and the P3 ERPs) differed across three Go-NoGo tasks that varied systematically based on conceptual-semantic processing necessary to make the inhibitory response. Overall, we found that all three paradigms elicited inhibitory responses similar to our control task of arrows and octagons, but did so in ways that increase our understanding of how inhibitory processes and conceptual-semantic processing interact. Specifically, both the N2 and P3 decreased with task difficulty, though only the P3 does so significantly. This trend suggests the influence of an increase in semantic difficulty as opposed to perceptual difficulty (Schapkin et al., 2003). Had perceptual differences been driving our effects we would have expected an increase in N2 amplitude and no change in P3, as Nieuwenhuis et al (2004) reported after manipulating stimulus discrimination in a Go-NoGo task.
The known inhibition-related phenomena associated with the N2, i.e., larger amplitude for NoGo compared to Go responses, occurred in all of our semantic inhibition tasks. This is essential to addressing our question of how inhibition and categorization interacted. If there were no N2 NoGo differences, then we would have to conclude that task difficulty slowed the process to such a degree that stopping a response no longer required the invocation of active inhibitory processes. Thus, all of our tasks elicited an inhibitory N2 effect regardless of difficulty, which supports previous literature.
Although there was a trend towards a decrease in the N2 amplitude with task difficulty, post hoc analyses did not reveal any statistically significant differences. In general, however, the trend was as expected.
The amplitude of the P3 to the NoGo stimuli decreased systematically, from Single to Multiple to Semantic, as the conceptual-semantic information needed to make a response became more complex. There are several findings of interest here. First, it was the NoGos and not the Gos that were statistically different across tasks, indicating that inhibitory processes more than attentional ones are influenced. The second point of interest is that the NoGo amplitude decreases, as opposed to increasing, with difficulty. This pattern was also reported by Schapkin et al. (2006). Their task included pressing the button for the word “go” and withholding for “stop” compared to the more difficult, Stroop-like condition in which “go” and “stop” were reversed. The task difference under investigation in the current study is also a conceptual-semantic one, more so than changes in speed (Jodo & Kayama, 1992) or perceptual similarity between stimuli (Nieuwenhuis et al., 2004). Thus, the fact that the P3 decreases in amplitude, as opposed to becoming larger with task difficulty, is consistent with the idea that the increased difficulty was conceptual as opposed to perceptual.
There are other possible explanations for this effect. The first is that decreased P3 across tasks is caused by different levels of expectation or response preparation across the tasks. Response preparation can result in the changes in the P3 amplitude independently (Smith, Johnstone, & Barry, 2007; Stadler, Klimesch, Pouthas, & Ragot, 2006) and because the P3 may overlap with the CNV or Contingent Negative Variation (Kok, 1988; Simson, Vaughan & Ritter, 1977). Either cause would result in the same outcome. The higher the predictability of the coming response, the higher the P3 amplitude will be (Bruin et al., 2001; Ruchkin et al., 1975; Stadler et al., 2006). Although the probability of target to non-target items is identical in all three tasks, these effects may be perceptually driven such that as the visual predictability increases, so does the amplitude of the P3. In this case, the Single task has only two stimuli repeated multiple times resulting in a high level of expectancy about any given trial. The Semantic task, on the other hand, has 200 different images, making the predictability of a given trial very low. The Multiple task falls between these two because each stimuli is repeated 4 times and the items within each category necessarily share important perceptual features. Thus, the decrease in P3 amplitude across these three tasks could be driven by decreased amounts of perceptual similarity within Go and NoGo conditions.
To some degree this argument is similar to our own. We agree that the decrease in P3 amplitude is driven by a decrease in the amount of perceptual information available to form a response. However we argue that this decrease in perceptual cues results in an increase in semantic-conceptual processing which in turn elicits smaller P3 amplitudes. The current data cannot distinguish between increased semantic-conceptual processing and response preparation. The only evidence that we have of an influence beyond stimulus predictability is that in the current study the N2 also displays a slight decrease in amplitude with task difficulty. Response preparation often only influences the amplitude of the P3 with no corresponding N2 difference (Bruin et al., 2001; Smith, Johnstone, & Barry, 2007). Future studies may be able to tease these factors apart by varying item predictability with cues before the stimulus onset, or varying the inter-stimulus interval.
The second alternative explanation for the decrease in P3 amplitude across tasks is that the increase in reaction time, reaction time variability, or peak latency increased variability in the individual P3 peak amplitudes, resulting in a smearing effect and thus a smaller peak amplitude. However, there are reasons to discount this possibility. For one, the Single and Multiple tasks did not differ significantly in reaction times, but the corresponding P3 amplitudes do differ. Secondly, studies focusing on the factors influencing the P3 effect have shown that, based on single trial latency adjustment procedures, reaction time and corresponding P3 latency differences are less influential in determining the P3 amplitude than is task difficulty (see Kok, 2001 for review). As a result, it seems that the systematic decrease in P3 NoGo amplitude from Single to Multiple to Semantic is the effect of increased conceptual-semantic complexity across the tasks.
The relative decreases in the N2 and P3 are especially interesting given the potential effects of repetition. The Single task repeats the identical stimuli 160 and 40 times respectively. In the Multiple task the stimuli are each repeated 4 times and the Semantic task has no repetitions. If repetition alone were driving the N2 and P3 effects we would actually see the opposite trend, in which with repetition the N2 and P3 amplitudes decrease (Courchesne et al., 1978; Polich, 1989; Ravden & Polich, 1998).
The other interesting finding with regard to the P3 was the difference in NoGo peak latency. The latencies were almost identical for Single and Multiple tasks, with a significant increase in the Semantic task. Interestingly, this NoGo latency difference was mirrored in the reaction times for Go responses, in which the Semantic task was significantly longer than the two others. Other studies have reported a difference in P3 latency with stimulus complexity (McCarthy & Donchin, 1981).
Overall, the findings indicate that conceptual-semantic processing does influence inhibitory responses in quite specific and predicable ways. In particular, it appears that inhibition tasks engaging categorization at a superordinate level influence the P3 effect differently than item/object level inhibitory tasks. We remain undecided in the ongoing N2 and P3 inhibitory debate. The fact that N2 is not significantly affected but that P3 is influenced means that by about 300 ms, higher level semantic processing is influencing the inhibitory responses on a neural level. These findings are also of importance because there is a growing body of research focused on applying inhibitory tasks to clinical populations in order to discern neural markers of inhibitory dysfunction. Thus, it is vital that the interactions between conceptual-semantic processing and inhibition be taken into consideration, or systematically tested, when designing studies and interpreting inhibitory processes across populations. Uncovering the range of influence of inhibitory processing deficits in disorders such as Attention Deficit Hyeractivity Disorder, dyslexia, autism, and degenerative diseases will likely necessitate tasks that require higher level cognitive processing to make inhibitory responses. This paper presents a broader range of inhibitory tasks than have typically been used in studies of populations with proposed inhibitory deficits. In addition, using tasks that are more complex than a simple perceptual decision has the potential to address the real world implications of inhibitory deficits evident in disorders such as ADHD.
Acknowledgments
The authors would like to thank Joshua White, Sandra Chapman, and Jacque Gamino for their comments and help. The funding for this project was provided by a grant from the Sparrow Foundation for the CAARTE program and NIH (NINDS) K02NS044850 and R01NS047781
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Bruin KJ, Wijers AA, van Staveren ASJ. Response priming in a go/nogo task: do we have to explain the go/nogo N2 in terms of response activation instead of inhibition? Clinical Neurophysiology. 2001;112(9):1660–1671. doi: 10.1016/s1388-2457(01)00601-0. [DOI] [PubMed] [Google Scholar]
- Ciesielski KT, Harris RJ, Cofer LF. Posterior brain ERP patterns related to the go/no-go task in children. Psychophysiology. 2004;41:882–892. doi: 10.1111/j.1469-8986.2004.00250.x. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Courchesne RY, Hillyard SA. The effects of stimulus deviation on P3 waves to easily recognized stimuli. Neuropsychologia. 1978;16:189–199. doi: 10.1016/0028-3932(78)90106-9. [DOI] [PubMed] [Google Scholar]
- Ferree TC. Spherical splines and average referencing in scalp EEG. Brain Topography. 2006;19(12):43–52. doi: 10.1007/s10548-006-0011-0. [DOI] [PubMed] [Google Scholar]
- French R. The subtlety of sameness: A theory and computer model of analogy-making. Cambridge, MA: MIT Press; 1995. [Google Scholar]
- French R, Mareschal D, Mermillod M, Quinn PC. The role of bottom-up processing in perceptual categorizaton by 3- to 4-month-old infants: Simulations and Data. Journal of Experimental Psychology: General. 2004;133(3):382–397. doi: 10.1037/0096-3445.133.3.382. [DOI] [PubMed] [Google Scholar]
- Folstein JR, van Petten C. Influence of cognitive control and mismatch on the N2 component of the ERP: A review. Psychophysiology. 2008;45:152–170. doi: 10.1111/j.1469-8986.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstone RL, Barsalou LW. Reuniting perception and conception. Cognition. 1998;65:231–262. doi: 10.1016/s0010-0277(97)00047-4. [DOI] [PubMed] [Google Scholar]
- Hasher L, Quig MB. Inhibitory control over no-longer-relevant information: adult age differences. Memory and Cognition. 1997;25:286–295. doi: 10.3758/bf03211284. [DOI] [PubMed] [Google Scholar]
- Jodo E, Kayama Y. Relation of negative ERP component to response inhibition in a Go/No-go task. Electroencephalography and Clinical Neurophysiology. 1992;82:477–482. doi: 10.1016/0013-4694(92)90054-l. [DOI] [PubMed] [Google Scholar]
- Junghöfer M, Elbert T, Tucker DM, Braun C. The polar average reference effect a bias in estimating the head surface integral in EEG recording. Clinical Neurophysiology. 1999;110(6):1149–1155. doi: 10.1016/s1388-2457(99)00044-9. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. 2. Lea & Febiger; Philadelphia: 1983. [Google Scholar]
- Kincses TZ, Chadaide Z, Varga ET, Antal A, Paulus W. Task-related temporal and topographical changes of cortical activity during ultra-rapid visual categorization. Brain Research. 2006;1112:191–200. doi: 10.1016/j.brainres.2006.07.044. [DOI] [PubMed] [Google Scholar]
- Kok A. Overlap between P300 and Movement-related potentials: A response to Verleger. Biological Psychology. 1988;27:51–58. doi: 10.1016/0301-0511(88)90005-1. [DOI] [PubMed] [Google Scholar]
- Kok A. On the utility of P3 amplitude as a measure of processing capacity. Psychophysiology. 2001;38:557–577. doi: 10.1017/s0048577201990559. [DOI] [PubMed] [Google Scholar]
- Kopp B, Mattler U, Goertz R, Rist F. N2, P3, and the lateralized readiness potential in a no-go task involving selective response priming. Electroencephalography and Clinical Neurophysiology. 1996;99:19–27. doi: 10.1016/0921-884x(96)95617-9. [DOI] [PubMed] [Google Scholar]
- Lavric A, Pizzagalli DA, Forstmeier S. When go and nogo are equally frequent: ERP components and cortical tomography. European Journal of Neuroscience. 2004;20(9):2483–2488. doi: 10.1111/j.1460-9568.2004.03683.x. [DOI] [PubMed] [Google Scholar]
- Logan GD, Cowan WB. On the ability to inhibit thought and action: A theory of an act of control. Psychological Review. 1984;91:295–327. doi: 10.1037/a0035230. [DOI] [PubMed] [Google Scholar]
- Luu P, Tucker DM. Regulating action: alternating activation of midline frontal and motor cortical networks. Clinical Neurophysiology. 2001;112:1295–1306. doi: 10.1016/s1388-2457(01)00559-4. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Donchin E. A metric for thought: a comparison of P300 latency and reaction time. Science. 1981;211:77–80. doi: 10.1126/science.7444452. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Simmonds DJ. Response inhibition and response selection: Two sides of the same coin. Journal of Cognitive Neuroscience. 2008;20:751–761. doi: 10.1162/jocn.2008.20500. [DOI] [PubMed] [Google Scholar]
- Murphy GL, Kaplan AS. Feature distribution and background knowledge in category learning. Quarterly Journal of Experimental Psychology: Human Experimental Psychology. 2000;53A:962–982. doi: 10.1080/713755932. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Yeung N, Cohen JD. Stimulus modality, perceptual overlap and the go/no-go N2. Psychophysiology. 2004;41:157–160. doi: 10.1046/j.1469-8986.2003.00128.x. [DOI] [PubMed] [Google Scholar]
- Nunez PL. A study of origins of the time dependencies of scalp EEG: ii-experimental support of theory. IEEE Transactions on Bio-Medical Engineering. 1981;28:281–288. doi: 10.1109/TBME.1981.324701. [DOI] [PubMed] [Google Scholar]
- Perner J, Lang B, Kloo D. Theory of mind and self-control: More than a common problem of inhibition. Child Development. 2002;73:752–767. doi: 10.1111/1467-8624.00436. [DOI] [PubMed] [Google Scholar]
- Perrin F, Pernier J, Betrand O, Echallier JF. Spherical splines for scalp potential and current density mapping. Electroencephalography and Clinical Neurophysiology. 1989;72:184–187. doi: 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
- Posner MI, DiGirolamo GJ. Executive attention: Conflict, target detection and cognitive control. In: Parasuraman R, editor. The Attentive Brain. Cambridge, MA: MIT Press; 1998. pp. 401–424. [Google Scholar]
- Polich J. Habituation of P300 from auditory stimuli. Psychobiology. 1989;17:19–28. [Google Scholar]
- Ravden D, Polich J. Habituation of P300 from visual stimuli. International Journal of Psychophysiology. 1998;30:359–365. doi: 10.1016/s0167-8760(98)00039-7. [DOI] [PubMed] [Google Scholar]
- Ruchkin DS, Sutton S, Tueting P. Emitted and evoked P300 potentials and variation in stimulus probability. Psychophysiology. 1975;12:592–595. doi: 10.1111/j.1469-8986.1975.tb00052.x. [DOI] [PubMed] [Google Scholar]
- Schapkin SA, Falkenstein M, Marks A, Griefahn B. Executive brain functions after exposure to nocturnal traffic effects of task difficulty and sleep quality. European Journal of Applied Physiology. 2006;96:693–702. doi: 10.1007/s00421-005-0049-9. [DOI] [PubMed] [Google Scholar]
- Schyns PG, Goldstone RL, Thibaut JP. The development of features in object concepts. Behavioral and Brain Sciences. 1998;21:1–54. doi: 10.1017/s0140525x98000107. [DOI] [PubMed] [Google Scholar]
- Siakaluk PD, Buchanan L, Westbury C. The effect of semantic distance in yes/no and go/no-go semantic categorization tasks. Memory and Cognition. 2003;31:100–113. doi: 10.3758/bf03196086. [DOI] [PubMed] [Google Scholar]
- Simpson A, Riggs KJ. Conditions under which children experience inhibitory difficulty with a “button-press” go/no-go task. Journal of Experimental Child Psychology. 2006;94:18–26. doi: 10.1016/j.jecp.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Simson R, Vaughan HG, Jr, Ritter W. The scalp topography of potentials in auditory and visual Go-NoGo tasks. Electroencephalography and Clinical Neurophysiology. 1977;43:864–875. doi: 10.1016/0013-4694(77)90009-8. [DOI] [PubMed] [Google Scholar]
- Smith JL, Johnstone SJ, Barry RJ. Effects of pre-stimulus processing on subsequent events in a warned Go/NoGo paradigm: Response preparation, execution and inhibition. International Journal of Psychophysiology. 2006;61(2):121–133. doi: 10.1016/j.ijpsycho.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Smith JL, Johnstone SJ, Barry RJ. Response priming in the Go/NoGo task: The N2 reflects neither inhibition nor conflict. Clinical Neurophysiology. 2007;118(2):343–355. doi: 10.1016/j.clinph.2006.09.027. [DOI] [PubMed] [Google Scholar]
- Smith JL, Johnstone SJ, Barry RJ. Movement-related potentials in the Go/NoGo task: The P3 reflects both cognitive and motor inhibition. Clinical Neurophysiology. 2008;119:704–714. doi: 10.1016/j.clinph.2007.11.042. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: Norms for name agreement, image agreement, familiarity, and visual complexity. Journal of Experimental Psychology: Human Learning and Memory. 1980;6:1461–1492. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- Stadler W, Klimesch W, Pouthas V, Ragot R. Differential effects of the stimulus sequence of CNV and P300. Brain Research. 2006;1123:157–167. doi: 10.1016/j.brainres.2006.09.040. [DOI] [PubMed] [Google Scholar]
- Thorpe SJ, Fize D. Speed of processing in the human visual system. Nature. 1996;381:520–522. doi: 10.1038/381520a0. [DOI] [PubMed] [Google Scholar]
- VanRullen R, Thorpe SJ. Is it a bird? Is it a plane? Ultra-rapid visual categorisation of natural and artifactual objects. Perception. 2001;30:655–668. doi: 10.1068/p3029. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter SC. The timing of action-monitoring processes in the anterior cingulated cortex. Journal of Cognitive Neuroscience. 2002;14:593–602. doi: 10.1162/08989290260045837. [DOI] [PubMed] [Google Scholar]
- Weintraub S. Neuropsychological assessment of mental state. In: Mesulam MM, editor. Principles of Behavioral and Cognitive Neurology. Oxford University Press; New York: 2000. pp. 121–173. [Google Scholar]


