Abstract
Nesfatin-1 is a newly discovered peptide that was reported to reduce food intake when injected centrally. We recently described its wide distribution in rat brain autonomic nuclei which implies potential recruitment of nesfatin-1 by stress. We investigated whether restraint, a mixed psychological and physical stressor activates nesfatin-1-immunoreactive (ir) neurons in the rat brain. Male Sprague-Dawley rats were either subjected to 30 min restraint or left undisturbed and 90 min later brains were processed for double immunohistochemical labeling of Fos and nesfatin-1. Restraint induced significant Fos expression in neurons of the supraoptic nucleus (SON), paraventricular nucleus (PVN), locus coeruleus (LC), rostral raphe pallidus (rRPa), nucleus of the solitary tract (NTS) and ventrolateral medulla (VLM). Double Fos/nesfatin-1 labeling revealed that Fos-ir neurons comprised 95% of nesfatin-1-ir cells in the SON, 90% in the VLM, 80% in the LC, 48% in the caudal NTS, 57% in the rRPa, 48% in the anterior parvicellular PVN, 27% in the medial magnocellular PVN, 18% in the lateral magnocellular PVN and 10% in the medial parvicellular PVN. These data demonstrate that nesfatin-1 neurons are part of the hypothalamic and hindbrain neuronal cell groups activated by restraint suggesting a possible role of nesfatin-1 in the response to stress.
Keywords: Fos, nesfatin-1, NUCB2, restraint, hypothalamus, medulla
1. Introduction
The recently discovered 82 amino acid peptide nesfatin-1 and its precursor nucleobindin2 (NUCB2) were first described to induce dose-dependent anorexigenic effects in rats and mice upon 3rd ventricular or intraperitoneal injection during the dark phase (Oh-I et al., 2006; Shimizu et al., 2009). In addition, we showed that nesfatin-1 acts centrally to inhibit food intake through the activation of brain corticotropin-releasing factor (CRF)2-receptor (Stengel et al., 2009c). There is also a report that central injection of nesfatin-1 induces anxiety- and fear-related behaviors in the rat (Merali et al., 2008). Recent neuroanatomical studies depicted the occurrence of nesfatin-1 immunoreactivity in cell bodies of autonomic regulatory nuclei in the forebrain, hindbrain and spinal cord along with other forebrain nuclei involved in stress response and cognitive function (Foo et al., 2008; Goebel et al., 2009). Collectively, these data suggest a potential role of the peptide in stress-recruited circuitries which so far has not been explored. Emotional or psychological stressors (processive) are integrated by the brain and activate cortical limbic and brainstem structures that then impact on the hypothalamus (Dayas et al., 2001b; Senba and Ueyama, 1997). Restraint is considered primarily an emotional/processive stress paradigm that does not induce pain or direct physical insult (Dayas et al., 1999; Herman and Cullinan, 1997). The combination of a given stressor and successive Fos immunohistochemistry in the brain is a widespread approach to visualize neuronal activation and to characterize brain areas involved in the processing of stressors (Dayas et al., 2001a; Dragunow and Faull, 1989; Sagar et al., 1988).
In the present study we investigated whether acute exposure to restraint, known to alter gut function through central modulation of autonomic outflow (Taché and Bonaz, 2007), activates nesfatin-1 immunoreactive (ir) neurons with an emphasis on hypothalamic and hindbrain nuclei involved in autonomic regulation of visceral function (Saper, 2002; Taché et al., 1995). This was achieved using double immunohistochemical detection of the immediate early gene Fos and nesfatin-1 immunoreactivity with an antiserum raised against the nesfatin-1 fragment corresponding to rat NUCB2 amino acid residues 1-82.
2. Results
2.1 Restraint stress activates nesfatin-1-immunoreactive neurons in hypothalamic nuclei in conscious ad libitum fed rats
As expected, Fos immunostaining was low in the rat forebrain of undisturbed freely fed control rats (Figs. 1-3). Restraint for 30 min significantly increased the number of Fos-ir neurons/section compared to non-stressed controls in the medial parvicellular paraventricular nucleus of the hypothalamus (mpPVN, 91.7 ± 18.8 vs. 1.1 ± 0.6; p < 0.001; Figs. 1C, 2E-F) and lateral magnocellular PVN (lmPVN, 45.2 ± 16.0 vs. 0.7 ± 0.3; p < 0.05; Figs. 1D, 2E-F) and to a smaller extent in the medial magnocellular PVN (mmPVN, 28.9 ± 7.7 vs. 0.3 ± 0.2; p < 0.01; Figs. 1B, 2C-D), anterior parvicellular PVN (apPVN, 13.9 ± 1.4 vs. 1.5 ± 0.7; p < 0.001; Figs. 1A, 2A-B) and supraoptic nucleus (SON, 21.0 ± 6.7 vs. 2.4 ± 1.4; p < 0.05; Fig. 3) as monitored 90 min after the end of the stress. Restraint did not significantly increase Fos immunoreactivity in neurons of the ventral bed nucleus of the stria terminalis (BNST) and of subcortical and cortical limbic structures such as the septum and cingulate, piriform and entorhinal cortex and in the amygdaloid nuclei (data not shown because no double labeling was observed in these areas).
Fig. 1.
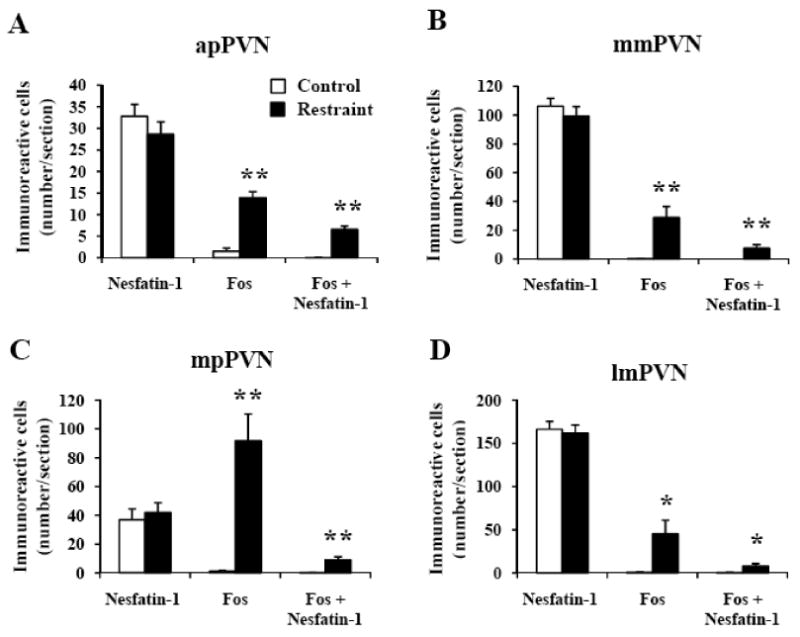
Restraint stress induced Fos expression in a small population of nesfatin-1-positive neurons in the paraventricular nucleus of the hypothalamus in conscious rats. Unilateral cell count/section in (A) the anterior parvicellular part of the paraventricular nucleus (apPVN), (B) medial magnocellular part of the PVN (mmPVN), (C) medial parvicellular part of the PVN (mpPVN) and (D) lateral magnocellular part of the PVN (lmPVN) 90 min after a 30 min restraint exposure. Data are mean ± SEM of 6 rats/group. * p < 0.05; ** p < 0.01 compared with the respective control.
Fig. 3.
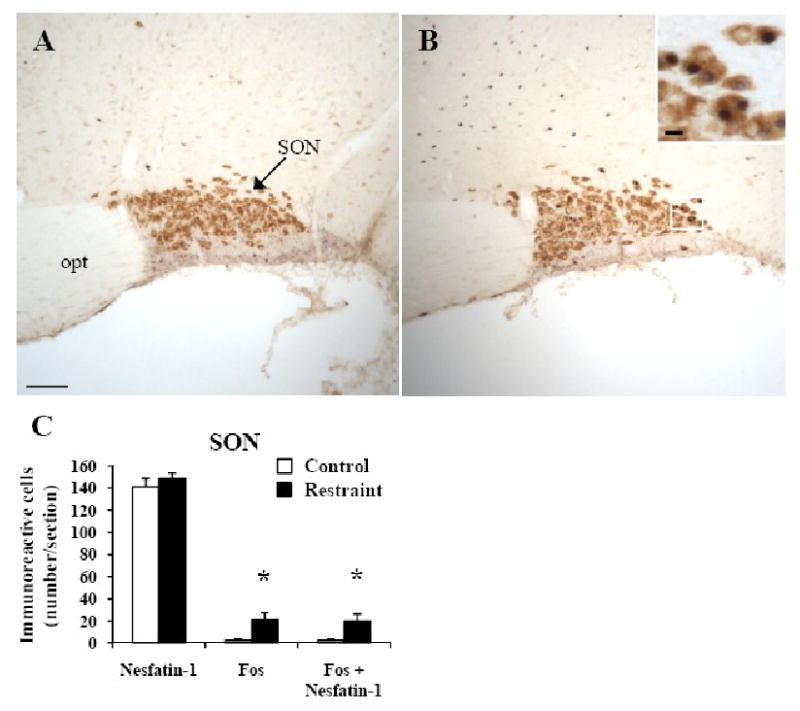
Restraint induced Fos expression in nesfatin-1-positive neurons in the rat supraoptic nucleus (SON). Non-fasted conscious rats were left undisturbed (A) or exposed for 30 min to restraint stress (B) and 90 min later, animals were euthanized. Brain was processed for immunohistochemical detection of Fos (dark blue, B) and nesfatin-1 (brown, A, B) in the SON. The insert in B shows a higher magnification of neurons with Fos immunoreactivity co-localizing with nesfatin-1 immunoreactivity. The scale bar represents 100 μm in A and 10 μm in the insert. Unilateral cell count/section in the SON (C) as mean ± SEM of 6 rats/group. ** p < 0.05 compared with respective control. Other abbreviations: opt: optic tract.
Fig. 2.
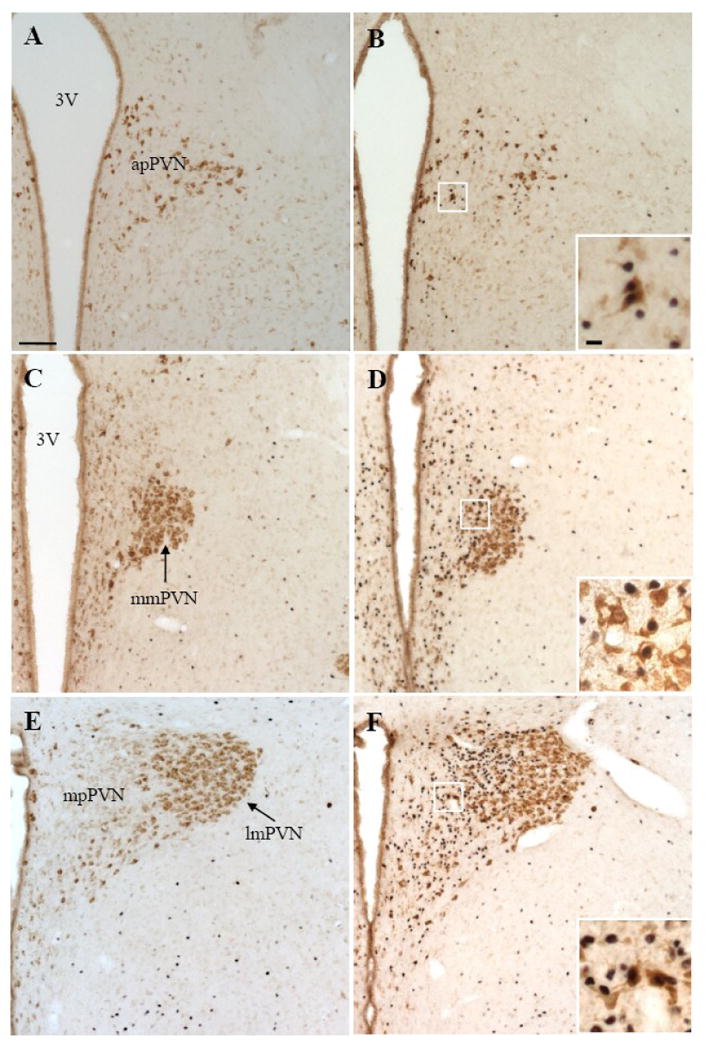
Representative double immunohistochemical staining for Fos (dark blue) and nesfatin-1 (brown) in the anterior parvicellular part of the PVN (apPVN, A and B), medial magnocellular part of the PVN (mmPVN, C and D), medial parvicellular part of the PVN (mpPVN, E and F) and lateral magnocellular part of the PVN (lmPVN, E and F) in control rats (A, C, E) and 90 min after a 30 min restraint exposure (B, D, F). The inserts in B, D and F show higher magnification of neurons with Fos immunoreactivity co-localizing with nesfatin-1 immunoreactivity in the apPVN (insert B), mmPVN (insert D) and mpPVN (insert F). The scale bar in A is 100 μm representing the scale for all the panels and 10 μm in the insert in B, and all the inserts have the same magnification. Other abbreviations: 3V: third ventricle.
In control rats, nesfatin-1-ir neurons were prominently localized (number/section) in the lmPVN (166.3 ± 9.1, Figs. 1D, 2E), SON (141.0 ± 7.8, Fig. 3), mmPVN (106.3 ± 5.5, Figs. 1B, 2C), and to a lesser extent in the mpPVN (36.9 ± 7.6, Figs. 1C, 2E). Rats exposed for 30 min to restraint had similar numbers of nesfatin-1-positive neurons as the control group monitored 90 min after the end of stress (Figs. 1-3). Restraint significantly increased the number of double labeled cells in the SON (19.8 ± 6.6 vs. 2.2 ± 1.3, p < 0.05; Fig. 3C), apPVN (6.6 ± 0.8 vs. 0.0 ± 0.0, p < 0.001; Fig. 1A), mmPVN (7.7 ± 2.4 vs. 0.0 ± 0.0; p < 0.01; Fig. 1B), lmPVN (8.0 ± 2.8 vs. 0.5 ± 0.3, p < 0.05; Fig. 1D) and in the mpPVN (9.0 ± 2.2 vs. 0.1 ± 0.1, p < 0.01; Fig. 1C) compared to controls. Double Fos/nesfatin-1 labeling showed that of the Fos-positive cells, 95% were nesfatin-1-ir in the SON (Fig. 3), 48% in the apPVN (Figs. 1A, 2B), 27% in the mmPVN (Figs. 1B, 2D), 18% in the lmPVN (Figs. 1D, 2F) and 10% in the mpPVN (Figs. 1C, 2F).
2.2 Restraint stress activates nesfatin-1-immunoreactive neurons in pontine and medullary nuclei in conscious ad libitum fed rats
Fos immunostaining was low (≤5) in all pontine and medullary nuclei in undisturbed freely fed control rats (Figs 4-6). Restraint for 30 min significantly increased the number of Fos-ir neurons/section compared to non-stressed controls in the locus coeruleus (LC, 11.0 ± 1.7 vs. 0.0 ± 0.0; p < 0.001; Fig. 4), rostral raphe pallidus (rRPa, 17.6 ± 2.3 vs. 1.2 ± 0.4; p < 0.001; Figs. 5A-C), ventrolateral medulla (VLM, 10.3 ± 0.9 vs. 0.3 ± 0.0; p < 0.001; Figs. 5D-F) and caudal part of the NTS (cNTS, 19.7 ± 2.3 vs. 3.7 ± 1.3; p < 0.001; Fig. 6) while neurons of the dorsal motor nucleus of the vagus nerve (DMN) were not activated (Fig. 6B).
Fig. 4.
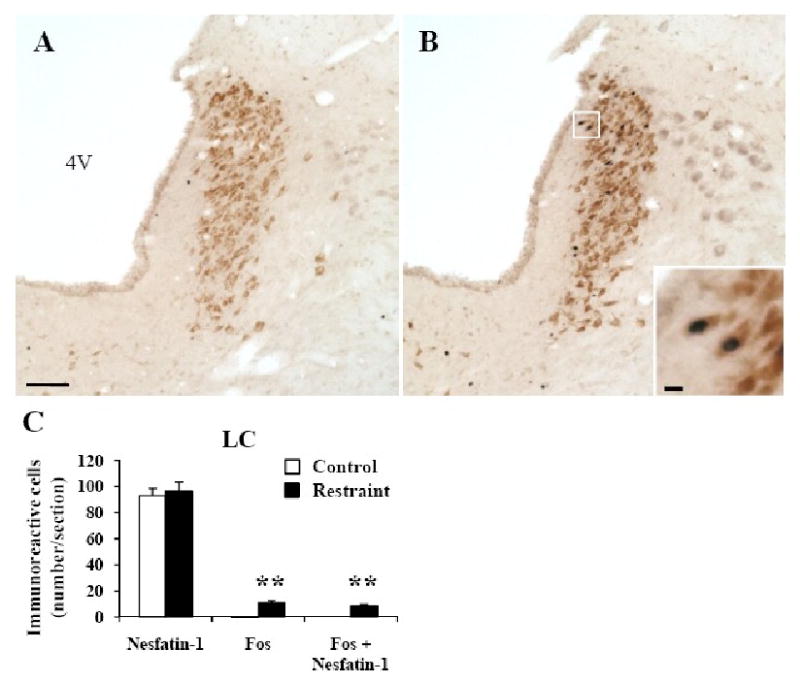
Double immunohistochemical staining for Fos (dark blue) and nesfatin-1 (brown) in the locus coeruleus (LC, A-C) of control rats (A, C) and 90 min after a 30 min restraint stress (B, C). The insert in B shows a higher magnification of neurons with Fos immunoreactivity co-localizing with nesfatin-1 immunoreactivity. The scale bar represents 100 μm in A and 10 μm in the insert. Unilateral cell count/section in the LC (C) expressed as mean ± SEM of 6 rats/group. ** p < 0.001. Other abbreviations: 4V: fourth ventricle.
Fig. 6.
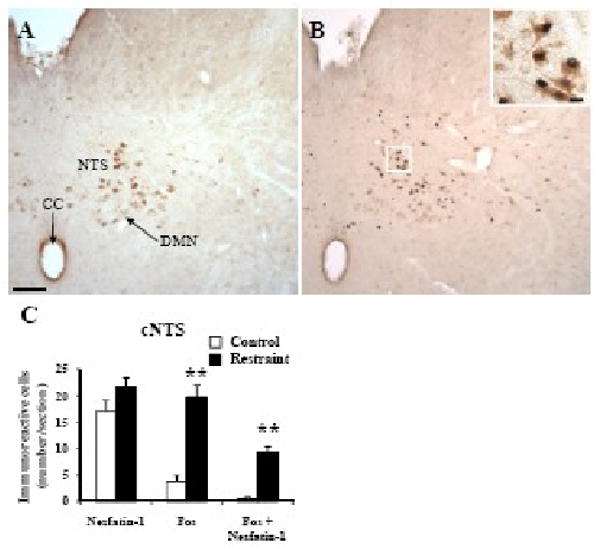
Double immunohistochemical staining for Fos (dark blue) and nesfatin-1 (brown) in the caudal part of the nucleus of the solitary tract (NTS) in control rats (A) and 90 min after a 30 min immobilization restraint (B). The insert in B shows higher magnification of activated neurons co-localizing with nesfatin-1 in the cNTS. The scale bar represents 100 μm in A and 10 μm in the insert. Cell count per section (unilateral) in the caudal NTS (C) expressed as mean ± SEM of 6 rats/group. ** p < 0.001. Other abbreviations: AP: area postrema; CC: central canal; DMN: dorsal motor nucleus of vagus.
Fig. 5.
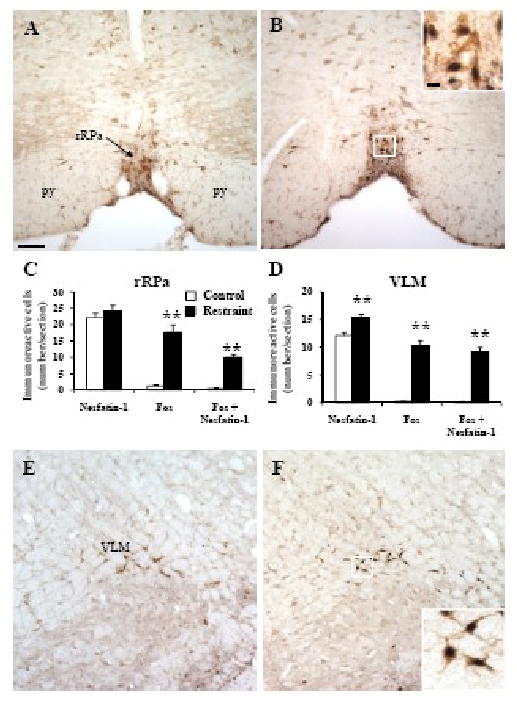
Double immunohistochemical staining for Fos (dark blue) and nesfatin-1 (brown) in the rostral raphe pallidus (rRPa, A-C) and ventrolateral medulla (VLM, D-F) in control rats (A, E) and 90 min after a 30 min restraint (B, F). The inserts in B and F show higher magnification of neurons with Fos immunoreactivity co-localizing with nesfatin-1 immunoreactivity in the rRPa (insert B) and VLM (insert F). The scale bar in A is 100 μm representing the scale for all the panels and 10 μm in the insert in B, and all the inserts have the same magnification. Unilateral cell count/section in the rRPa (C) and VLM (D). Data are mean ± SEM of 6 rats/group. ** p < 0.001. Other abbreviations: py: pyramidal tract.
In control rats, nesfatin-1 immunoreactive neurons were prominently localized (number/section) in the LC (93.1 ± 5.6, Figs. 4A and C) and to a lesser extent in the rRPa (22.1 ±1.4, Figs. 5A and C) and cNTS (17.0 ± 2.1, Figs. 6A and C). These numbers were not significantly different in rats exposed for 30 min to restraint monitored 90 min after the end of stress (Figs. 4-6) except in the VLM where the number of nesfatin-1-ir neurons was higher compared to control rats (15.3 ± 0.7 vs. 12.1 ± 0.5; p < 0.01, Fig. 5D-E). Nesfatin-1 immunoreactivity in the midbrain and medulla was also found in several other nuclei namely the Edinger-Westphal nucleus (EW), dorsal raphe nuclei or DMN as previously described (Foo et al., 2008; Goebel et al., 2009). Restraint significantly increased the number of double labeled cells in the VLM (9.2 ± 0.7 vs. 0.2 ± 0.1, p < 0.001; Fig. 5D), LC (8.8 ± 1.1 vs. 0.0 ± 0.0, p < 0.001; Fig. 4C), rRPa (10.0 ± 0.9 vs. 0.4 ± 0.2, p < 0.001; Fig. 5C) and cNTS (9.4 ± 1.0 vs. 0.5 ± 0.3, p < 0.001; Fig. 6C) compared to controls. Double Fos/nesfatin-1 labeling revealed that of the Fos-positive cells, 90% in the VLM (Figs. 5D and F), 80% in the LC (Figs. 4B-C), 57% in the rRPa (Figs. 5B-C) and 48% in the cNTS at the level below obex (Fig. 6) were nesfatin-1-ir.
Although restraint increased the number of Fos-positive neurons in the medial NTS (mNTS) at the level of the area postrema and the rostral part of the NTS (rNTS), this did not reach statistical significance. However, 41% of Fos-positive neurons in the mNTS and 16% in the rNTS were also nesfatin-1-ir which was significantly increased compared to controls (data not shown).
Following pre-absorption of the anti-nesfatin-1 antibody by rat nesfatin-1 peptide as described before (Stengel et al., 2009b), no immunostaining could be detected in any brain nucleus (data not shown).
3. Discussion
The present study indicates that a large percentage of activated neurons, in response to 30 min of restraint, encompasses nesfatin-1-ir cells located in the SON (95%), VLM (90%), LC (80%), cNTS (48%) and rRPa (57%) with a smaller percentage found in the PVN (10-20%) except for the apPVN (48%) as shown by double Fos/nesfatin-1 labeling 90 min after the end of restraint in conscious freely fed rats. These data identify a novel phenotype of neurons activated by acute restraint stress and also establish nesfatin-1-containing neurons in specific hindbrain and hypothalamic nuclei as part of the neuroanatomical circuitry activated by a processive stressor. While the specificity of the antibody was shown by the lack of nesfatin-1 immunolabeling in these brain nuclei when the antibody was pre-absorbed with synthetic nesfatin-1 peptide, the antibody also recognizes the full length NUCB2, but does not cross-react with other neuropeptides including nesfatin-2 and nesfatin-3 (Brailoiu et al., 2007; Foo et al., 2008). Therefore, the activated neurons are likely to reflect nesfatin-1/NUCB2 immunoreactivity consistent with the expression of the gene encoding NUCB2 that mirrors the distribution of cell bodies exhibiting nesfatin-1 immunolabeling in various brain nuclei (Foo et al., 2008; Goebel et al., 2009; Oh-I et al., 2006).
A small population of neurons in the hypothalamic magnocellular neurosecretory system responded to a restraint that encompasses a prominent pool of nesfatin-1-ir neurons in the SON nucleus and to a lesser extent in the magnocellular (m) PVN. The SON displays intense nesfatin-1 labeling consistent with other studies (Brailoiu et al., 2007; Foo et al., 2008; Goebel et al., 2009; Kohno et al., 2008; Oh-I et al., 2006), which was previously identified to overlap with 35% of oxytocin- and 28% of arginine-vasopressin (AVP)-positive neurons (Kohno et al., 2008). Utilization of restraint stress for 30 min did not alter the number of nesfatin-1-ir neurons and led to Fos immunoreactivity in SON neurons localized more prominently in the dorsal part containing the majority of oxytocin neurons (Engelmann et al., 2004; Hoffman et al., 1993; Hou-Yu et al., 1986; Rhodes et al., 1981). Of these Fos-positive neurons, 90% also labeled for nesfatin-1/NUCB2 indicating that the majority of restraint-activated neurons in the SON expressed nesfat-in-1 immunoreactivity. Previous reports showed that acute restraint in rats induces Fos labeling in the SON with the intensity of labeling related to the duration of restraint reaching a peak at 120 min (Miyata et al., 1995; Pacak and Palkovits, 2001). Other studies also established that Fos protein induced by 1- or 3-h restraint occurred in nearly 50% of oxytocin-synthesizing cells in the SON (Miyata et al., 1995) and is accompanied by increased oxytocin secretion into the systemic blood circulation without changes in AVP gene expression in the SON or circulating levels of AVP in restrained rats (Hashimoto et al., 1989; Herman, 1995; Laguna-Abreu et al., 2005). Taken together, these data suggest that at least a subpopulation of nesfatin-1 neurons in the SON responding to restraint also co-expresses oxytocin. As both nesfatin-1 and oxytocin suppress food intake when injected centrally (Douglas et al., 2007; Oh-I et al., 2006; Stengel et al., 2009c), activation of a population of these neurons may have relevance in the hypophagic response to restraint (Kinzig et al., 2008; Miragaya and Harris, 2008). The other neurosecretory system localized in the mPVN (Swanson and Kuypers, 1980) also contains nesfatin-1-ir cells in the medial and lateral subdivisions (Brailoiu et al., 2007; Foo et al., 2008; Goebel et al., 2009; Oh-I et al., 2006) and their numbers were not influenced by restraint (present study). This population of nes-fatin-1-ir neurons is extensively colocalized with oxytocin, AVP and cocaine- and amphetamine related transcript (CART) (Brailoiu et al., 2007; Foo et al., 2008; Kohno et al., 2008). Restraint stress induced Fos in a small proportion of mPVN neurons in agreement with previous reports (Ceccatelli et al., 1989; Dayas et al., 2001b; Laguna-Abreu et al., 2007; Miyata et al., 1995). Of the Fos-positive neurons in the mPVN, 18% in the lmPVN and 27% in the mmPVN also co-labeled for NUCB2/nesfatin-1. The lower percentage of activated nesfatin-1 neurons in the lmPVN and mmPVN compared to SON is unlikely to be related to differences in the number of Fos-positive neurons induced by restraint or the extent of nesfatin-1-containing cells between the SON and mPVN which are within the same range. This may reflect a dissociation in the population of nesfatin-1 neurons activated by restraint in the SON and mPVN as observed in neuroendo-crine secretory AVP and oxytocin SON/mPVN neurons in response to various stressors including restraint (Engelmann et al., 2004; Laguna-Abreu et al., 2007).
Restraint stress induced robust neuronal activation in the parvicellular part of the PVN in line with previous studies (Laguna-Abreu et al., 2007; Rotllant et al., 2007). Restraint-activated neurons in the mpPVN have been identified to overlap with CRF-containing neurons (Ceccatelli et al., 1989; Pacak and Palkovits, 2001; Rotllant et al., 2007) and many neurons in the apPVN contain CRF (Sawchenko et al., 1993). Restraint also activates a population of nesfatin-1-ir neurons in the mpPVN as shown by 10% of these cells which co-labeled for Fos and nesfatin-1 and 48% of the Fos-ir neurons in the apPVN were also nesfatin-1-ir. The small percentage of Fos/nes-fatin-1 double-labeled neurons may be related to the more sparse distribution of nesfatin-1 immunoreactivity (Brailoiu et al., 2007); present study) along with more prominent induction of Fos in the mpPVN compared to the magnocellular PVN. Of all nesfatin-1-ir neurons in the mpPVN, 64% have been reported to contain also CART and 24% CRF immunoreactivity (Foo et al., 2008). Whether the nesfatin-1-containing cells activated by restraint in the mpPVN include these populations or different cell groups needs to be further investigated. However, the involvement of the mpPVN to mediate several aspects of the stress response (Pacak and Palkovits, 2001) combined with recent evidence that nesfatin-1 influences the excitability of a large population of neurons in the mpPVN (Price et al., 2008) suggests a possible modulatory role of nesfatin-1/NUCB2 in the endocrine stress response. Other studies have shown that re-feeding after a 48-h fast results in a 2.7-fold increase in the total amount of nesfatin-1 immunoreactivity and expression of NUCB2 mRNA in the SON compared to fasted rats (Kohno et al., 2008) suggestive that nesfatin-1 expression can be modulated by environmental conditions.
Restraint in rats following our protocol did not result in a significant induction of Fos in neurons of the BNST and central amygdala consistent with previous reports showing no Fos im-munoreactivity in response to 15 to 60 min of immobilization in a plastic restraint device (Arnold et al., 1992; Chowdhury et al., 2000). More severe conditions of restraint by taping rat limbs to a board result however in Fos induction in the central amygdala, after 1 hour while 30 min did not induce any changes (Arnold et al., 1992; Chowdhury et al., 2000; Honkaniemi et al., 1992; Senba and Ueyama, 1997). Previous studies have established that the central amygdala is preferentially activated by physical stressors (immune challenge or hemorrhage) (Dayas et al., 1999; Dayas et al., 2001a).
The LC noradrenergic system is activated by a number of stressors including restraint (Valentino and Van Bockstaele, 2008). Likewise in our study, restraint for 30-min induces Fos expression in a prominent number of LC neurons. We also found a widespread distribution of nesfatin-1 immunoreactivity in LC neurons of which 80% were activated by restraint. Expression of nesfatin-1 in the LC has been less investigated than in the hypothalamus, however consistent with our immunohistochemistry data, one previous report revealed a moderate to high expression of NUCB2 mRNA as well as nesfatin-1-like immunoreactivity in the LC (Foo et al., 2008). The LC norepinephrine system is well established to promote aspects of cognitive processes of the stress response (Valentino and Van Bockstaele, 2008). In particular, recent evidence also supports the involvement of LC noradrenergic neurons in anxiety (Itoi, 2008). The activation of 80% of LC nesfatin-1-ir neurons by restraint and the reported central action of nesfatin-1 to increase anxiety in rats (Merali et al., 2008), may suggest the participation of nesfatin-1 along with the LC noradrenergic system in the stress-related behavioral response.
In line with our recent report (Goebel et al., 2009) and others (Brailoiu et al., 2007; Oh-I et al., 2006), we found nesfatin-1 immunoreactivity in medullary catecholaminergic cell groups including the ventrolateral medulla (A1/C1) and caudal NTS (A2/C2). A large population of these nesfatin-1-positive cells was activated by restraint reaching 90% in the VLM and 48% in the caudal NTS. Induction of Fos expression in response to restraint has been observed to occur mainly in VLM A1 and caudal NTS A2 noradrenergic cells while there is a minor recruitment of adrenergic cells in the VLM and none in the NTS (Dayas et al., 2001b; Dayas et al., 2004). Other studies showed that the majority of nesfatin-1 immunoreactivity in the NTS is localized in cat-echolaminergic neurons shown by tyrosine hydroxylase- (TH) immunoreactivity (Brailoiu et al., 2007). Collectively these data are indicative that restraint activates a subpopulation of nesfatin-1/noradrenergic neurons in the caudal NTS and VLM. They also raise the possibility of their participation in the neuroendocrine response to restraint (Dayas et al., 2001b) and/or to be part of brainstem multifunctional reflex centers of the autonomic nervous system and short term satiety (Blessing, 1997). We found a slight but significant increase in the number of nesfatin-1-ir neurons in the VLM. The functional relevance of this observation remains to be further investigated.
The raphe pallidus nucleus is part of the serotonergic system which also co-expresses nes-fatin-1 (Brailoiu et al., 2007). We observed that 57% of restraint-activated neurons of the raphe pallidus were immunolabeled with nesfatin-1. Such activation may have implications in the autonomic visceral response to stress. The raphe pallidus sends direct projections to the dorsal vagal complex and intermediolateral cell column of the spinal cord (Taché et al., 1995). In particular, these neurons mediate prominent tachycardia in response to emotional or psychological stress, possibly via direct disinhibitory projections to the spinal cardiac sympathetic preganglionic neurons (Cao and Morrison, 2003). They also influence the activity of DMN neurons regulating gastric function (Taché et al., 1995) and may be involved in restraint-related vagus mediated suppression of gastric emptying (Taché and Bonaz, 2007).
In summary, restraint for 30 min activates a large proportion of nesfatin-1 neurons in pontine (LC)/medullary nuclei most likely catecholaminergic neurons (caudal NTS and VML), along with raphe pallidus neurons, SON neurons and to a smaller extent, neurons in the magno- and parvicellular subdivisions of the PVN. Such a pattern underlies a possible extended role of nesfatin-1 in stress-related behavioral, endocrine and autonomic responses that may improve the understanding of the physiological stress response.
4. Experimental procedures
4.1 Animals
Adult male Sprague-Dawley rats (Harlan, San Diego, CA, body weight: 280–350 g) were group housed (four animals/cage) under conditions of controlled illumination (12:12 h light/dark cycle, lights on/off: 06.00 h/18.00 h), humidity, and temperature (22 ± 2 °C). Animals were fed with a standard rodent diet (Prolab RMH 2500; LabDiet, PMI Nutrition, Brentwood, MO) and tap water ad libitum. Animal care and experimental procedures followed institutional ethic guidelines and conformed to the requirements of the federal authority for animal research conduct. All procedures were approved by the Animal Research Committees at Veterans Affairs Greater Los Angeles Healthcare System (animal protocol number 99127-07).
4.2 Restraint Stress
Ad libitum fed rats (n=6) were restrained individually in immobilization bags with ventilation holes (DecapiCones, Braintree Scientific, Inc. Braintree, MA) for 30 min between 9.00 and 10.00 am as described before (Radley et al., 2008) and then returned to their group home cage with free access to food and water until euthanasia 90 min later. The control group (n=6) consisted of rats left undisturbed in their housing cages for 120 min before sacrifice.
4.3 Tissue processing and Fos and nesfatin-1 double immunohistochemistry
Ninety min after the end of the 30-min restraint, stressed and non-stressed control rats were deeply anesthetized with sodium pentobarbital (70 mg/kg, intraperitoneally, Nembutal, Abbott Lab.) and transcardially perfused as described before (Wang et al., 2000). Briefly, the thoracic cavity was opened and a cannula inserted into the ascending aorta via the left heart ventricle. Perfusion consisted of a quick flush with sodium chloride (0.9% NaCl) and 500 ml of 4% paraformaldehyde, 14% saturated picric acid in 0.1 M phosphate buffer (pH 7.2). Brains were removed and post-fixed overnight in the same fixative, rinsed and cryoprotected in 10% sucrose for 24 h and snap-frozen in dry ice-cooled 2-methylbutane. Rat brains from the two treatment groups were processed together to ensure consistency. Coronal sections (25 μm) of the whole brain were cut using a cryostat (Microm International GmbH, Walldorf, Germany). Free-floating brain sections were incubated in rabbit anti-c-Fos serum (1:10,000, Catalog No. PC#38, Oncogene, Cambridge, MA) as primary antibody followed by biotinylated goat anti-rabbit Fab fragment (1:1000, Catalog No. 111-067-003, Jackson ImmunoResearch Laboratories Inc., West Grove, PA) and avidin-biotin-peroxidase complex (ABC, Vector, Vermont, CA) method as previously described (Wang et al., 2000). Staining was visualized with 3,3′-diaminobenzidine tetrachloride (DAB) and nickel ammonium sulfate. For double-labeling, sections were thereafter incubated in rabbit anti-rat nesfatin-1 antibody (1:10,000, Catalog No. H-003-22, Phoenix Pharmaceuticals, Burlingame, CA) followed by biotinylated goat anti-rabbit antibody (1:1000, Catalog No. 111-065-144, Jackson ImmunoResearch Laboratories Inc.) and ABC method and developed with DAB. Cells with dark blue nuclear staining were Fos-ir and cells with strong brown cytoplasmic staining were nesfatin-1-ir.
The anti-nesfatin-1 antibody was raised in rabbit against rat nesfatin-1 corresponding to rat NUCB2 amino acid residues 1-82 (Phoenix Pharmaceuticals). This antibody has been shown to stain a Western Blot band corresponding to NUCB2, the precursor of nesfatin-1 which also contains the epitope (manufacturer's technical information). We recently showed by Western blot that the antibody also stains rat nesfatin-1 (10kDa band) (Stengel et al., 2009b). Moreover, we recently demonstrated that nesfatin-1 immunoreactivity corresponded to NUCB2 mRNA expression as shown e.g. in the nucleus accumbens, cerebellar cortex, and paraventricular nucleus of the hypothalamus (Goebel et al., 2009). According to the editorial of Saper (Saper, 2005) in the absence of a nesfatin-1/NUCB2 knockout model, pre-absorption of the antibody (1ml, 1:10,000) with synthetic rat nesfatin-1 (10 μg, Phoenix Pharmaceuticals) was performed to further assess specificity. After 24-h incubation the solution was centrifuged and the supernatant used for immunostaining. No immunosignals could be detected after pre-absorption.
4.4 Cell counting
Fos and nesfatin-1-ir cells were observed by light microscopy (Axioscop II, Carl Zeiss, Germany) and counted unilaterally (right side). Unilateral cell count was chosen based on the observation that no hemispheric differences were observed within animals and groups as assessed in the right and left NTS. For quantitative assessment, the number of immunoreactive cells was counted unilaterally in the right brain hemisphere in several sections of selected brain nuclei as in our previous studies (Stengel et al., 2009a; Wang et al., 1998; Wang et al., 2002; Wang et al., 2009). Nucleus coordinates were identified with the Paxinos and Watson's atlas (Paxinos and Watson, 2007) and are given in mm from bregma: SON, 12 sections: -0.6 to -1.56; apPVN, 4 sections: -1.08 to -1.32; mmPVN, 3 sections: -1.44 to -1.72; mpPVN, 3 sections: -1.72 to -1.92; lmPVN, 3 sections: -1.72 to -1.92; LC, 7 sections: -9.48 to -10.08; rRPa, 8 sections: -13.8 to -13.2; VLM (also named A1/C1), 12 sections: -13.38 to -14.28; rostral NTS, 5 sections: -13.56 to -13.2; NTS at the level of the area postrema, 8 sections: -13.68 to -14.28 and caudal NTS, 5 sections: -14.4 to -14.76. Images were acquired by a digital camera (Hamamatsu, Bridgewater, NJ) using the image acquisition system SimplePCI (Hamamatsu Corporation, Sewickley, PA).
The average number of single or double labeled Fos-ir and nesfatin-1-ir cells/section for each animal was calculated. Since no consecutive sections were used for the detection of the same neuronal marker, no corrections for double counting were applied. No differences were observed between different batches of rats. The investigator was blinded to the treatment.
4.5 Statistical analysis
Data were analyzed by analysis of variance (ANOVA) followed by all pair wise multiple comparison procedures (Tukey post hoc test). Differences were considered significant when p < 0.05. Data are expressed as mean ± SEM.
Acknowledgments
This work was supported by German Research Foundation fellowship grants GO 1718/1-1 (M.G.) and STE 1765/1-1 (A.S.), the VA Research Career Scientist Award, Department of Veterans Affairs, NIHDK 33061 (Y.T.) and Center grant DK-41301 (Animal Core, Y.T.). We are grateful to Mrs. Honghui Liang for her excellent technical support and Ms. Eugenia Hu for reviewing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnold FJ, De Lucas Bueno M, Shiers H, Hancock DC, Evan GI, Herbert J. Expression of c-fos in regions of the basal limbic forebrain following intracerebroventricular corticotropin-releasing factor in unstressed or stressed male rats. Neuroscience. 1992;51:377–90. doi: 10.1016/0306-4522(92)90322-s. [DOI] [PubMed] [Google Scholar]
- Blessing WW. The lower brainstem and bodily homeostasis. Oxford University Press; 1997. [Google Scholar]
- Brailoiu GC, Dun SL, Brailoiu E, Inan S, Yang J, Chang JK, Dun NJ. Nesfatin-1: distribution and interaction with a G protein-coupled receptor in the rat brain. Endocrinology. 2007;148:5088–94. doi: 10.1210/en.2007-0701. [DOI] [PubMed] [Google Scholar]
- Cao WH, Morrison SF. Disinhibition of rostral raphe pallidus neurons increases cardiac sympathetic nerve activity and heart rate. Brain Res. 2003;980:1–10. doi: 10.1016/s0006-8993(03)02981-0. [DOI] [PubMed] [Google Scholar]
- Ceccatelli S, Villar MJ, Goldstein M, Hokfelt T. Expression of c-Fos immunoreactivity in transmitter-characterized neurons after stress. Proc Natl Acad Sci U S A. 1989;86:9569–73. doi: 10.1073/pnas.86.23.9569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury GM, Fujioka T, Nakamura S. Induction and adaptation of Fos expression in the rat brain by two types of acute restraint stress. Brain Res Bull. 2000;52:171–82. doi: 10.1016/s0361-9230(00)00231-8. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Day TA. Neuroendocrine responses to an emotional stressor: evidence for involvement of the medial but not the central amygdala. Eur J Neurosci. 1999;11:2312–22. doi: 10.1046/j.1460-9568.1999.00645.x. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Crane JW, Xu Y, Day TA. Stressor categorization: acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. Eur J Neurosci. 2001a;14:1143–52. doi: 10.1046/j.0953-816x.2001.01733.x. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Day TA. Medullary neurones regulate hypothalamic corticotropin-releasing factor cell responses to an emotional stressor. Neuroscience. 2001b;105:707–19. doi: 10.1016/s0306-4522(01)00213-5. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Day TA. Hypothalamic paraventricular nucleus neurons regulate medullary catecholamine cell responses to restraint stress. J Comp Neurol. 2004;478:22–34. doi: 10.1002/cne.20259. [DOI] [PubMed] [Google Scholar]
- Douglas AJ, Johnstone LE, Leng G. Neuroendocrine mechanisms of change in food intake during pregnancy: a potential role for brain oxytocin. Physiol Behav. 2007;91:352–65. doi: 10.1016/j.physbeh.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Dragunow M, Faull R. The use of c-fos as a metabolic marker in neuronal pathway tracing. J Neurosci Methods. 1989;29:261–5. doi: 10.1016/0165-0270(89)90150-7. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Landgraf R, Wotjak CT. The hypothalamic-neurohypophysial system regulates the hypothalamic-pituitary-adrenal axis under stress: an old concept revisited. Front Neuroendocrinol. 2004;25:132–49. doi: 10.1016/j.yfrne.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Foo K, Brismar H, Broberger C. Distribution and neuropeptide coexistence of nucleobindin-2 mRNA/nesfatin-like immunoreactivity in the rat CNS. Neuroscience. 2008;156:563–79. doi: 10.1016/j.neuroscience.2008.07.054. [DOI] [PubMed] [Google Scholar]
- Goebel M, Stengel A, Wang L, Lambrecht NWG, Taché Y. Nesfatin-1 immunoreactivity in rat brain and spinal cord autonomic nuclei. Neurosci Lett. 2009;452:241–246. doi: 10.1016/j.neulet.2009.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Murakami K, Takao T, Makino S, Sugawara M, Ota Z. Effect of acute ether or restraint stress on plasma corticotropin-releasing hormone, vasopressin and oxytocin levels in the rat. Acta Med Okayama. 1989;43:161–7. doi: 10.18926/AMO/30888. [DOI] [PubMed] [Google Scholar]
- Herman JP. In situ hybridization analysis of vasopressin gene transcription in the paraventricular and supraoptic nuclei of the rat: regulation by stress and glucocorticoids. J Comp Neurol. 1995;363:15–27. doi: 10.1002/cne.903630103. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Hoffman GE, Smith MS, Verbalis JG. c-Fos and related immediate early gene products as markers of activity in neuroendocrine systems. Front Neuroendocrinol. 1993;14:173–213. doi: 10.1006/frne.1993.1006. [DOI] [PubMed] [Google Scholar]
- Honkaniemi J, Kainu T, Ceccatelli S, Rechardt L, Hokfelt T, Pelto-Huikko M. Fos and jun in rat central amygdaloid nucleus and paraventricular nucleus after stress. Neuroreport. 1992;3:849–52. doi: 10.1097/00001756-199210000-00007. [DOI] [PubMed] [Google Scholar]
- Hou-Yu A, Lamme AT, Zimmerman EA, Silverman AJ. Comparative distribution of vasopressin and oxytocin neurons in the rat brain using a double-label procedure. Neuroendocrinology. 1986;44:235–46. doi: 10.1159/000124651. [DOI] [PubMed] [Google Scholar]
- Itoi K. Ablation of the central noradrenergic neurons for unraveling their roles in stress and anxiety. Ann N Y Acad Sci. 2008;1129:47–54. doi: 10.1196/annals.1417.012. [DOI] [PubMed] [Google Scholar]
- Kinzig KP, Hargrave SL, Honors MA. Binge-type eating attenuates corticosterone and hypophagic responses to restraint stress. Physiol Behav. 2008;95:108–13. doi: 10.1016/j.physbeh.2008.04.026. [DOI] [PubMed] [Google Scholar]
- Kohno D, Nakata M, Maejima Y, Shimizu H, Sedbazar U, Yoshida N, Dezaki K, Onaka T, Mori M, Yada T. Nesfatin-1 neurons in paraventricular and supraoptic nuclei of the rat hypothalamus coexpress oxytocin and vasopressin and are activated by refeeding. Endocrinology. 2008;149:1295–301. doi: 10.1210/en.2007-1276. [DOI] [PubMed] [Google Scholar]
- Laguna-Abreu MT, Koenigkam-Santos M, Colleta AM, Elias PC, Moreira AC, Antunes-Rodrigues J, Elias LL, Castro M. Time course of vasopressin and oxytocin secretion after stress in adrenalectomized rats. Horm Metab Res. 2005;37:84–8. doi: 10.1055/s-2005-861159. [DOI] [PubMed] [Google Scholar]
- Laguna-Abreu MT, Margatho L, Germano CM, Antunes-Rodrigues J, Elias LL, de Castro M. The effect of adrenalectomy on Fos expression in vasopressinergic and oxytocinergic neurons in response to stress in the rat. Stress. 2007;10:332–41. doi: 10.1080/10253890701287614. [DOI] [PubMed] [Google Scholar]
- Merali Z, Cayer C, Kent P, Anisman H. Nesfatin-1 increases anxiety- and fear-related behaviors in the rat. Psychopharmacology (Berl) 2008;201:115–23. doi: 10.1007/s00213-008-1252-2. [DOI] [PubMed] [Google Scholar]
- Miragaya JR, Harris RB. Antagonism of Corticotrophin-Releasing Factor Receptors in the Fourth Ventricle Modifies Responses to Mild but not Restraint Stress. Am J Physiol Regul Integr Comp Physiol. 2008;295:R404–16. doi: 10.1152/ajpregu.00565.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata S, Itoh T, Lin SH, Ishiyama M, Nakashima T, Kiyohara T. Temporal changes of c-fos expression in oxytocinergic magnocellular neuroendocrine cells of the rat hypothalamus with restraint stress. Brain Res Bull. 1995;37:391–5. doi: 10.1016/0361-9230(95)00017-8. [DOI] [PubMed] [Google Scholar]
- Oh-I S, Shimizu H, Satoh T, Okada S, Adachi S, Inoue K, Eguchi H, Yamamoto M, Imaki T, Hashimoto K, Tsuchiya T, Monden T, Horiguchi K, Yamada M, Mori M. Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature. 2006;443:709–12. doi: 10.1038/nature05162. [DOI] [PubMed] [Google Scholar]
- Pacak K, Palkovits M. Stressor specificity of central neuroendocrine responses: implications for stress-related disorders. Endocr Rev. 2001;22:502–48. doi: 10.1210/edrv.22.4.0436. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Vol. San Diego: Academic Press; 2007. [Google Scholar]
- Price CJ, Hoyda TD, Samson WK, Ferguson AV. Nesfatin-1 influences the excitability of paraventricular nucleus neurones. J Neuroendocrinol. 2008;20:245–50. doi: 10.1111/j.1365-2826.2007.01641.x. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Williams B, Sawchenko PE. Noradrenergic innervation of the dorsal medial prefrontal cortex modulates hypothalamo-pituitary-adrenal responses to acute emotional stress. J Neurosci. 2008;28:5806–16. doi: 10.1523/JNEUROSCI.0552-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes CH, Morrell JI, Pfaff DW. Immunohistochemical analysis of magnocellular elements in rat hypothalamus: distribution and numbers of cells containing neurophysin, oxytocin, and vasopressin. J Comp Neurol. 1981;198:45–64. doi: 10.1002/cne.901980106. [DOI] [PubMed] [Google Scholar]
- Rotllant D, Nadal R, Armario A. Differential effects of stress and amphetamine administration on Fos-like protein expression in corticotropin releasing factor-neurons of the rat brain. Dev Neurobiol. 2007;67:702–14. doi: 10.1002/dneu.20345. [DOI] [PubMed] [Google Scholar]
- Sagar SM, Sharp FR, Curran T. Expression of c-fos protein in brain: metabolic mapping at the cellular level. Science. 1988;240:1328–31. doi: 10.1126/science.3131879. [DOI] [PubMed] [Google Scholar]
- Saper CB. The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annu Rev Neurosci. 2002;25:433–69. doi: 10.1146/annurev.neuro.25.032502.111311. [DOI] [PubMed] [Google Scholar]
- Saper CB. An open letter to our readers on the use of antibodies. J Comp Neurol. 2005;493:477–8. doi: 10.1002/cne.20839. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Imaki T, Potter E, Kovacs K, Imaki J, Vale W. The functional neuroanatomy of corticotropin-releasing factor. Ciba Found Symp. 1993;172:5–21. doi: 10.1002/9780470514368.ch2. [DOI] [PubMed] [Google Scholar]
- Senba E, Ueyama T. Stress-induced expression of immediate early genes in the brain and peripheral organs of the rat. Neurosci Res. 1997;29:183–207. doi: 10.1016/s0168-0102(97)00095-3. [DOI] [PubMed] [Google Scholar]
- Shimizu H, Oh-I S, Hashimoto K, Nakata M, Yamamoto S, Yoshida N, Eguchi H, Kato I, Inoue K, Satoh T, Okada S, Yamada M, Yada T, Mori M. Peripheral Administration of Nesfatin-1 Reduces Food Intake in Mice: The leptin-independent mechanism. Endocrinology. 2009;150:662–71. doi: 10.1210/en.2008-0598. [DOI] [PubMed] [Google Scholar]
- Stengel A, Goebel M, Million M, Stenzel-Poore MP, Kobelt P, Mönnikes H, Taché Y, Wang L. CRF over-expressing mice exhibit reduced neuronal activation in the arcuate nucleus and food intake in response to fasting. Endocrinology. 2009a;150:153–60. doi: 10.1210/en.2008-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stengel A, Goebel M, Yakubov I, Wang L, Witcher D, Coskun T, Taché Y, Sachs G, Lambrecht NW. Identification and characterization of nesfatin-1 immunoreactivity in endocrine cell types of the rat gastric oxyntic mucosa. Endocrinology. 2009b;150:232–8. doi: 10.1210/en.2008-0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stengel A, Goebel M, Wang L, Rivier J, Kobelt P, Mönnikes H, Lambrecht NWG, Taché Y. Central Nesfatin-1 Reduces Dark Phase Food Intake and Gastric Emptying in Rats: Differential Role of Corticotropin-Releasing Factor2 Receptor. Endocrinology. 2009c doi: 10.1210/en.2009-0578. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Kuypers HG. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol. 1980;194:555–70. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- Taché Y, Yang H, Kaneko H. Caudal raphe-dorsal vagal complex peptidergic projections: role in gastric vagal control. Peptides. 1995;16:431–5. doi: 10.1016/0196-9781(94)00212-o. [DOI] [PubMed] [Google Scholar]
- Taché Y, Bonaz B. Corticotropin-releasing factor receptors and stress-related alterations of gut motor function. J Clin Invest. 2007;117:33–40. doi: 10.1172/JCI30085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Van Bockstaele E. Convergent regulation of locus coeruleus activity as an adaptive response to stress. Eur J Pharmacol. 2008;583:194–203. doi: 10.1016/j.ejphar.2007.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Martinez V, Barrachina MD, Taché Y. Fos expression in the brain induced by peripheral injection of CCK or leptin plus CCK in fasted lean mice. Brain Res. 1998;791:157–66. doi: 10.1016/s0006-8993(98)00091-2. [DOI] [PubMed] [Google Scholar]
- Wang L, Martinez V, Vale W, Taché Y. Fos induction in selective hypothalamic neuroendocrine and medullary nuclei by intravenous injection of urocortin and corticotropin-releasing factor in rats. Brain Res. 2000;855:47–57. doi: 10.1016/s0006-8993(99)02200-3. [DOI] [PubMed] [Google Scholar]
- Wang L, Saint-Pierre DH, Taché Y. Peripheral ghrelin selectively increases Fos expression in neuropeptide Y - synthesizing neurons in mouse hypothalamic arcuate nucleus. Neurosci Lett. 2002;325:47–51. doi: 10.1016/s0304-3940(02)00241-0. [DOI] [PubMed] [Google Scholar]
- Wang L, Martinez V, Larauche M, Taché Y. Proximal colon distension induces Fos expression in oxytocin-, vasopressin-, CRF- and catecholamines-containing neurons in rat brain. Brain Res. 2009;1247:79–91. doi: 10.1016/j.brainres.2008.09.094. [DOI] [PMC free article] [PubMed] [Google Scholar]


