Summary
Carbon nanotubes have fibre-like shape1 and stimulate inflammation at the surface of the peritoneum when injected into the abdominal cavity of mice2, raising concerns that inhaled nanotubes3 may cause pleural fibrosis and/or mesothelioma4. Here we show that multi-walled carbon nanotubes reach the sub-pleura in mice after a single inhalation exposure of 30 mg/m3 for 6 hours. Nanotubes were embedded in the sub-pleural wall and within sub-pleural macrophages. Mononuclear cell aggregates on the pleural surface increased in number and size after 1 day and nanotube-containing macrophages were observed within these foci. Sub-pleural fibrosis increased after 2 and 6 weeks following inhalation. None of these effects were seen in mice that inhaled carbon black nanoparticles or a lower dose of nanotubes (1 mg/m3). This work advances a growing literature on pulmonary toxicology of nanotubes5 and suggests that minimizing inhalation of nanotubes during handling is prudent until further long term assessments are conducted.
Letter
The lungs are the most likely route of exposure to carbon nanotubes (CNTs)5. CNTs have a high aspect (length to width) ratio, a property shared with asbestos fibers, which has led to concern that inhalation of CNTs may cause similar lung pathology1. These include pulmonary fibrosis (scarring of the lung tissue), and mesothelioma, a cancer of the pleura and peritoneum4. The administration of multi-walled CNTs (MWCNT)6 and single-walled CNTs (SWCNT)7,8 by intratracheal instillation or aspiration9,10 in rodents causes lung injury. However, it is not known whether CNTs delivered to the lung reach the pleura, the outer mesothelial lining of the lung, to cause injury and disease.
Inhalation is the most relevant method for determining toxicity of CNTs for several reasons. First, airborne particles are subject to the physics of impaction, sedimentation, and diffusion, whereas instilled particles in aqueous suspension are not11. Second, inhaled nano-sized particles reach the distal regions of the lung5. Third, inhaled CNTs are more dispersed and less agglomerated as compared to an instilled bolus dose in aqueous liquid3,12,13. In addition, inhalation3,12 is much more relevant than injection of materials into the body cavity2, which bypass the lung and its defense and clearance mechanisms entirely. Therefore, inhalation studies more accurately model deposition and pathologic responses to ‘real world’ exposure scenarios.
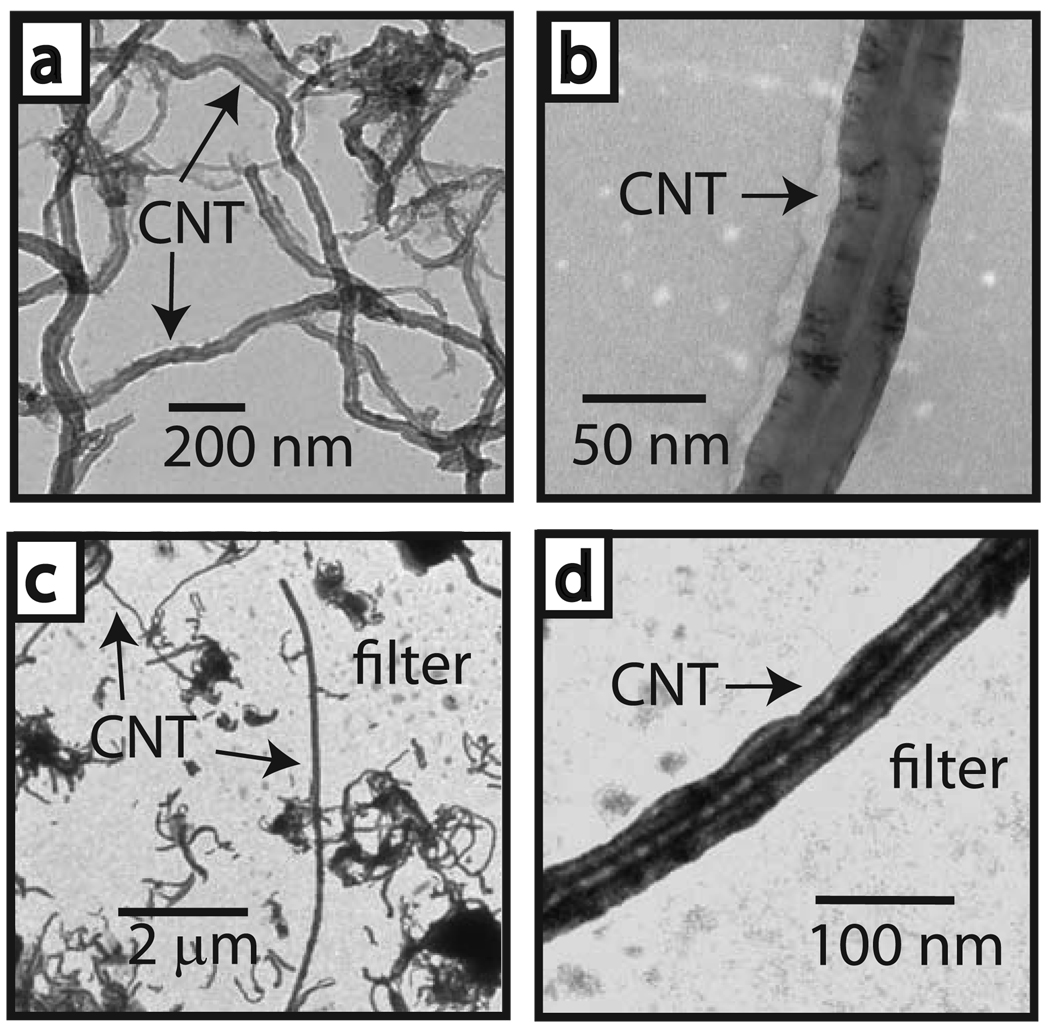
We exposed male C57BL6 mice to an aerosol of MWCNT (1 mg/m3 or 30 mg/m3) or carbon black nanoparticles (CB, 30 mg/m3) for 6 h and then collected lung tissues at 1 dy, 2 wk, 6 wk, and 14 wk post-inhalation exposure. The physicochemical parameters of these MWCNT have been reported by our laboratory3 and are summarized in Table S1). A schematic illustration and photograph of our inhalation setup is provided (Fig. S1 and S2) and has been described3. Transmission electron microscopy (TEM) of the bulk MWCNT showed a width range of 10–50 nm (Fig. 1a,b) confirmed by independent analysis (Table S1). Aerosolized MWCNT were a mixture of agglomerated and individual nanotubes with lengths of <100 nm to >10 µm (Fig. 1c,d). High dose MWCNT (30 mg/m3), low dose MWCNT (1 mg/m3), and CB (30 mg/m3) had mass median aerodynamic diameters of 183 nm, 164 nm, and 209 nm, respectively, with a geometric standard deviation<2 (Fig. S3). The calculated high and low MWCNT doses were 4 mg/kg and 0.2 mg/kg, respectively. Dose was based on a 10% estimated deposition fraction11.
Figure 1. Aerosolization of carbon nanotubes.
a, Transmission electron micrograph (TEM) of bulk MWCNT prior to aerosolization. b, Higher magnification of an individual CNT in the bulk sample. c. Aerosolized CNT captured by electrostatic precipitation on a filter located within the inhalation tower port (see supplementary information). d. Higher magnification of an aerosolized precipitated CNT on filter.
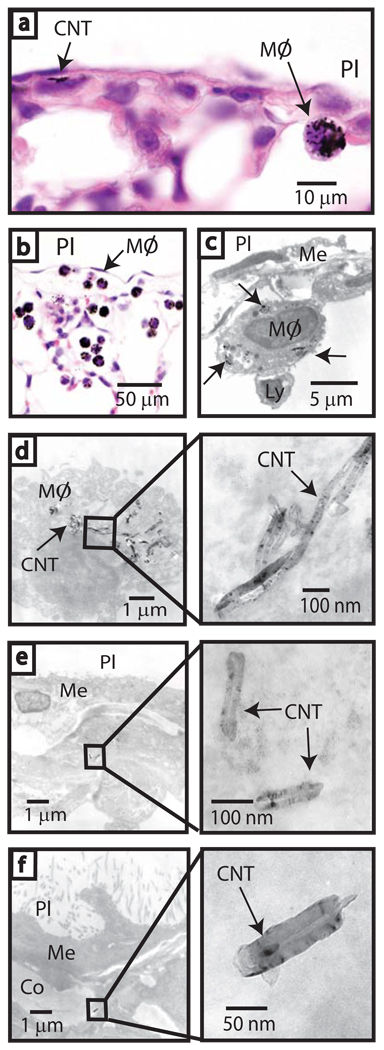
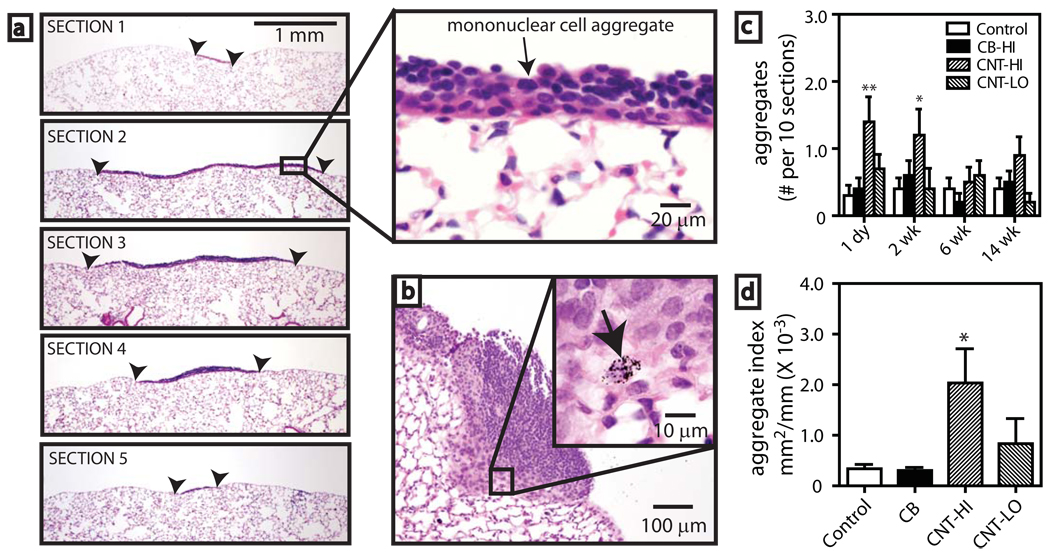
Inhaled MWCNT were engulfed by macrophages which migrated to the subpleural region (Fig. 2a–d). MWCNT were observed in macrophages (Fig. 2c,d) and within subpleural mesenchymal cells and the collagen matrix of the subpleura 1 day after inhalation (Fig. 2e,f). Serial sectioning of lung tissue revealed mononuclear cell aggregates, comprised mainly of lymphocytes with some monocytes/macrophages, on the pleural surface of mice exposed to MWCNT (Fig. 3a). Macrophages containing MWCNT were present in these foci (Fig. 3b). Occasional minor mononuclear cell aggregates were present in all exposure groups (data not shown). High dose MWCNT significantly increased the incidence (Table S2) and number (Fig. 3c) of aggregates (1 to 2 per 10 sections) at 1 day and 2 wks post-exposure. The size of mononuclear cell aggregates was significantly increased by high dose MWCNT (Fig. 3d) as determined by image analysis2 and a point counting method14 (see a comparison of methods in Supplementary Information and Figs. S4, S5).
Figure 2. Inhaled carbon nanotubes reach the sub-pleura in mice.
a, Light microscopy of CNTs in sub-pleura and macrophage (MØ) indicated by arrows (hematoxylin and eosin, 1000X). b, Sub-pleural macrophages (MØ) with CNT 1 day after high dose inhalation (hematoxylin and eosin, 200X). c, TEM showing CNTs within a macrophage (arrows) beneath the pleura (PL). Relative location of the mesothelium (Me) and a lymphocyte (Ly) are indicated. d, TEM of CNT within a sub-pleural macrophage (inset shows detail). e, TEM of CNTs in a sub-pleural cell (inset shows detail). f, CNT in collagen (Co) beneath the mesothelium (Me) (see inset for detail).
Figure 3. Pleural immune response after carbon nanotube inhalation in mice.
a, Sequential sections 100 µm apart through a pleural mononuclear cell aggregate (bordered by arrowheads) 1 day post-CNT inhalation (30 mg/m3) (hematoxylin and eosin, 40X). Inset shows detail (400X). b, Mononuclear aggregate 2 wk after CNT exposure (100X). Inset shows a macrophage with CNT indicated by arrow (1000X). c, Numbers of pleural aggregates after exposure to saline aerosol (control), 30 mg/m3 carbon black nanoparticles (CB), 30 mg/m3 CNT-(HI), or 1 mg/m3 CNT-(LO). Data are aggregates per 10 sections from each lung (N=10 animals). *P<0.05 or **P<0.001 compared to control. d, Aggregate size index measured by image analysis (see supplementary information). *P<0.05 compared to control determined by one-way ANOVA with post-hoc Tukey test.
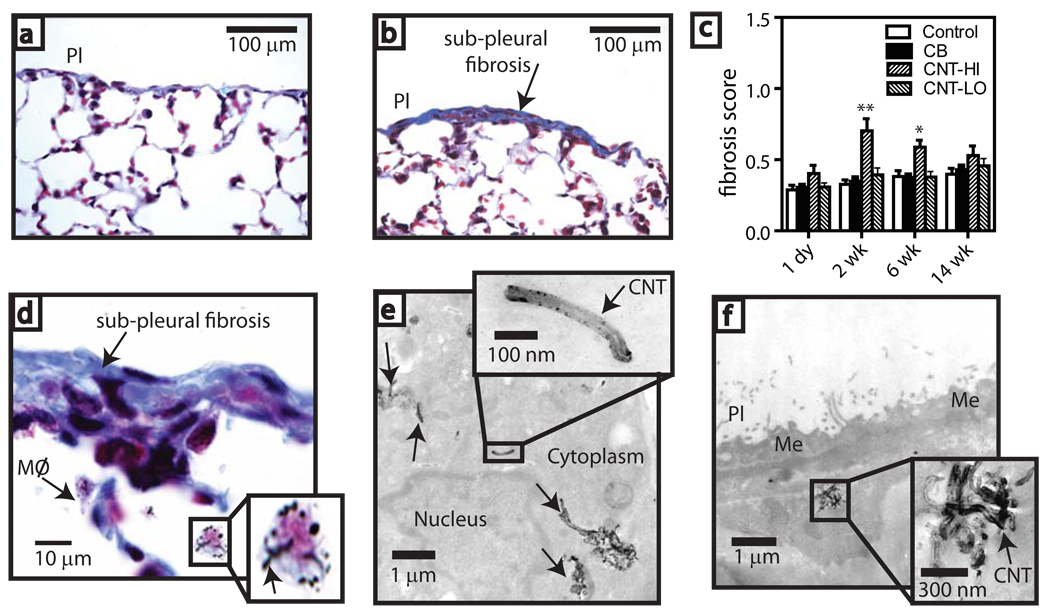
Sub-pleural fibrosis was also observed in mice that inhaled MWCNT (Fig. 4a–d). There was a high incidence (9 out of 10 mice) of fibrotic lesions in this group (Table S3). A point counting method14 was modified to derive a fibrosis score (Fig. S6). High dose MWCNT exposure caused a significant increase in fibrosis at 2 wk and 6 wk post-inhalation (Fig. 4c). MWCNT-laden macrophages were present beneath these sub-pleural lesions (Fig. 4d–f). A caveat is that the fibrosis score relied on trichrome staining which, although commonly used, could stain other cell matrix components and contribute to the observed pleural wall thickness. It is also important to note that most of the sub-pleura in mice exposed to MWCNT was normal as fibrotic lesions were focal and regional. Nevertheless, sub-pleural fibrotic lesions were only observed in the high dose MWCNT group and maximal thickness of these lesions ranged from 20 to 70 µm compared to normal sub-pleural thickness which ranged from 3 to 15 µm.
Figure 4. Sub-pleural fibrosis in mice after carbon nanotube inhalation.
Microscopy of trichrome-stained lung from a, saline aerosol-exposed (control) and b, CNT-exposed (30 mg/m3) mice 2 wk after inhalation (200X). c, Fibrosis score derived from a point-counting method after exposure to saline aerosol (control), 30 mg/m3 carbon black nanoparticles (CB), 30 mg/m3 CNT-(HI), or 1 mg/m3 CNT-(LO). *P<0.05 or **P<0.001 compared to control, CB, or CNT-LO determined by ANOVA with post-hoc Tukey test. d, Sub-pleural fibrotic lesion showing macrophages with CNT (inset shows detail). e, TEM of CNT within sub-pleural macrophage (arrows) at 2 wk. (Inset shows detail). f, TEM of CNT agglomerate in a sub-pleural cell at 6 wk (inset shows detail).
Our study was motivated by reports of peritoneal inflammation in the abdominal cavities of mice injected with MWCNT that led investigators to propose “asbestos-like pathogenicity”2,15. These investigators reported granulomas on the surface of the diaphragm, while we observed mononuclear cell aggregates at the pleura. Since minor aggregates were occasionally observed in control animals, these likely represent lymphoid tissue that is increased in number and size by MWCNT. We hypothesize that activated macrophages containing MWCNT traffic through the pleural lymphatic drainage and stimulate recruitment of mononuclear cells to enlarge these focal aggregates. Indeed, we observed macrophages containing MWCNT within mononuclear cell aggregates (Fig. 3b). Increased migration of mononuclear cells to the pleura is consistent with our previous finding that the monocyte chemokine CCL2 (MCP-1) is increased in the lungs of mice after MWCNT inhalation3. Bromodeoxyuridine labeling was not observed in these foci (data not shown), indicating that the mononuclear cells were not proliferating.
The development of sub-pleural fibrosis in mice suggest MWCNT could behave like asbestos in that they (1) are deposited at the alveolar level17 (Fig. 2), (2) reach the subpleura after inhalation18 (Fig. 2), and (3) remain in the sub-pleura intact for weeks to stimulate fibrosis19 (Fig. 4). However, the pathology we observed with MWCNT appears different from pleural pathology induced by asbestos fibers as MWCNT caused focal sub-pleural fibrosis and mononuclear cell aggregates, whereas asbestos causes pleural inflammation (granulomas) and diffuse pleural fibrosis18,20. It is therefore important that further work compare CNTs and asbestos to carefully address these important pathologic issues and elucidate whether there are similar mechanisms of action for asbestos and CNTs. For example, both asbestos and MWCNT increase platelet-derived growth factor (PDGF) in the lungs of mice3,16 and PDGF is an important mediator of fibrosis21 and mesothelioma22. Investigation of other fibrotic mediators should provide insight into the molecular mechanisms underlying the pathologic responses to CNTs.
The issue of whether MWCNT cause mesothelioma cannot be answered from the results of this study. We showed no indication of neoplasia nor did we use asbestos as a positive control. However, mesothelioma does not readily develop in mice after asbestos inhalation, except in mice that are deficient in the p53 tumor suppressor gene20. Asbestos and MWCNT both induce p5323,24. Therefore, further inhalation studies should be performed with p53-deficient mice using relevant exposure concentrations of MWCNT.
It is unknown whether other types of MWCNT would cause the same response that we report here. Metal catalysts used in the manufacturing process of CNTs could be important determinants of pleural toxicity. The MWCNT used in our study contained nickel which is known to elicit immune responses and pleural fibrosis14. In addition, agglomeration could affect lung deposition and toxicity of inhaled MWCNT. We used pluronic detergent to disperse the MWCNT, but it appears that the MWCNT were widely deposited in all aspects of the lung as would be expected when humans inhale such nanoparticles.
In our study some of the inhaled nanotubes remained in the sub-pleural wall for at least 14 wks, although most appeared to be cleared by this time and this could explain why fibrosis and mononuclear cell aggregates diminished. However, we did not carry out quantitative measures of clearance dynamics. Elgrabli et al recently showed that the majority of MWCNT (~65%) instilled into the lungs of rats were eliminated after 3 months, and ~15% of instilled MWCNT remained in the lungs after 6 months25. High aspect (length to width) ratio can influence biopersistence19. Poland et al2 showed that fiber length was important for inducing granulomas on the peritoneal surface of the diaphragm after intraperitoneal injection of MWCNT. Our study used MWCNT that possessed a length range from 0.5 to 50 µm (Table S1). Therefore, further inhalation studies should compare purified long and short MWCNT.
Whether CNTs cause pulmonary disease in humans is not known. There is currently no epidemiologic data to indicate that CNTs cause pulmonary fibrosis, mesothelioma, or other adverse effects. The individuals at most risk for inhalation exposure are those working with nanomaterials in occupational settings26. We previously found that inhaled MWCNT caused airway fibrosis in mice with pre-existing allergic inflammation3, suggesting that individuals with asthma are at greater risk.
In summary, inhaled MWCNT increased pleural mononuclear cell accumulation and sub-pleural fibrosis in mice. To our knowledge, this is the first report demonstrating that inhaled carbon nanotubes migrate to the sub-pleura to cause pathologic effects. These pathological changes could be unique to this type of MWCNT and may or may not persist under conditions of repeated, low-dose occupational exposures. Given this uncertainty, precautions minimizing inhalation during handling and use of MWCNT would be prudent until further studies can assess longer term responses of the lung and pleura after repeated exposures to MWCNT.
Materials and Methods
MWCNT characterization
Multi-walled carbon nanotubes (MWCNT) of “standard” length (0.5–40 µm) synthesized by carbon vapor deposition (CVD) with nickel and lanthanum catalysts were purchased from Helix Material Solutions, Inc., Richardson, TX. Characterization of the size, purity, and elemental composition of the MWCNT was provided by Helix, Inc. We verified these parameters by independent analysis (Millennium Research Laboratories Inc., Woburn, MA). Size was characterized by TEM and SEM. Purity was determined by thermogravimetric analysis (TGA). Elemental composition was performed by both ICP-AES and energy dispersive x-ray analysis (EDX). EDX data were expressed as the average and SEM of three different spots for each element. Physico-chemical characteristics of MWCNT are provided in Table S1 and have been published previously3.
Assay for LPS Contamination
Endotoxin (LPS) was measured by a Limulus amebocyte lysate (LAL) assay kit according to manufacturer's specifications (Associates of Cape Cod, East Falmouth, MA). MWCNT were sonicated in vehicle (0.1% Pluronic surfactant in PBS) for 60 min at RT prior to performing LAL assay. The maximum sensitivity of this assay is 0.005 EU/ml. MWCNT were negative for endotoxin within this detection limit.
Animals
Pathogen-free adult male C57BL6 mice at 6–8 weeks of age (Charles River Laboratories, Raleigh, NC) were randomized by weight and divided into treatment groups. Animals were individually housed in polycarbonate cages with Alphi-dri cellulose bedding (Shepherd Specialty Papers, Kalamazoo, MI) in a temperature and humidity controlled environment with a 12 h light/dark cycle at an International Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC)-accredited facility. Mice were given food (NIH-07 ground meal, Zeiger Brothers, Gardners, PA) and water ad libitum when not in inhalation chambers. All mice were acclimated to inhalation chambers one week prior to aerosol exposure by adaptation to chambers from 1 h to 6 h over a five day period. All procedures requiring animal use were approved by the Institutional Animal Care and Use Committee (IACUC) at The Hamner Institutes for Health Sciences.
Experimental design
Mice (10/group) were exposed to aerosolized MWCNT (1 mg/m3 or 30 mg/m3) or CB nanoparticles (30 mg/m3) or saline vehicle for 6 h in nose-only inhalation chambers and returned to the vivarium until necropsy. Animals were anesthetized with 90 mg/kg pentobarbital (i.p.) and euthanized by exsanguination at 1 dy, 2 wks, 6 wks, or 14 wks post-inhalation exposure for collection of lung tissue.
MWCNT aerosol generation and characterization
Autoclaved MWCNT were dry-milled in a Retsch Mixer Mill (Retsch Inc., Newtown, PA) for 5 minutes at 30 cycles per second. The milled MWCNT were suspended in a sterile, biocompatible nonionic surfactant, 1% pluronic F-68 (Sigma Aldrich, St. Louis, MO) in sterile Dulbecco’s phosphate buffered saline (DPBS). The MWCNT suspensions were further diluted with DPBS to achieve the desired final concentration of approximately 5 mg/ml MWCNT in 0.1% pluronic F-68. The inhalation setup used in this study was the same as we reported in a previous study3. Detailed methods, illustration and photograph of the inhalation setup are provided in supplementary information Fig. S1, S2).
Dose Calculation
Dose was calculated by the following equation:
where RMV is the respiratory minute volume equal to 0.0273 L/min for a mouse, DF is deposition fraction estimated at 10% at the alveolar region11 for particles with an MMAD of ~100 nm, T is time (6 h), C is concentration (30 mg/m3 or 1 mg/m3), and M is the mass of the animal (~24 gm for a mouse).
Lung histopathology
The left lung was insufflated with 10% neutral buffered formalin, fixed for 72 h, transferred to 70% ethanol, and embedded in parafin. Five µm sections were processed for histopathology with Masson’s trichrome stain or hematoxylin and eosin stain.
Morphometry
A semi-quantitative index for pleural mononuclear cell aggregates was evaluated using two methods; image analysis as described by Poland et al2 and a modified point-counting method described by Ogami et al14. The details of these methods and representative data are shown in supplementary information methods and Fig. S6. In addition, the number of mononuclear cell aggregates per 10 serial sections (100 µm apart) of each lung was also evaluated at each time point and for each exposure group (N=10 animals per group).
Electron microscopy of lung tissue
Lung tissues were post-fixed in 1% osmium tetroxide in 0.1 M sodium phosphate buffer, pH 7.2, dehydrated through graded ethanol solutions, cleared in acetone, and then infiltrated and embedded in Spurr’s resin. Unstained thin sections were mounted on copper grids and then examined on a Philips EM208S transmission electron microscope.
Data and statistical analysis
All graphs were constructed and statistical analysis performed using GraphPad Prism® software v. 5.00 (GraphPad Software, Inc., San Diego, CA). A one-way ANOVA with a post-hoc Tukey test was used to identify significant differences among treatment groups. Significance was set at p<0.05 unless otherwise stated.
Supplementary Material
Acknowledgements
This study was funded by the American Chemistry Council’s Long Range Research Initiative provided to The Hamner Institutes for Health Sciences, The National Institutes of Environmental Health Sciences grant R21-ES015801-01, North Carolina State University College of Agricultural and Life Sciences, and the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences. ACS/LRRI provided funds only and played no role in study design, data gathering and interpretation, authoring of the manuscript, or decision to publish. The authors declare no conflict of interest. Special thanks to Betsy Gross-Bermudez and the pathology staff at The Hamner Institutes for excellent technical assistance. Special thanks to Alexia Taylor at NCSU for critical reading of the manuscript.
Footnotes
Author Contributions
J.P.R. and J.C.B. initiated, directed and performed all experiments and took responsibility for planning and writing the manuscript. J.P.R., M.E.A., B.A.W., O.M., and J.C.B designed the inhalation exposure experiment. B.W. and E.W.T. designed the inhalation exposure apparatus and performed testing for dosimetery. M.F.C., A.R.B., J.E., and J.C.B. evaluated pathology for identification and verification of pleural lesion identity. J.K.S. performed electron microscopy for identification of nanotubes in lung tissue. A.R.B. and M.E.A. provided intellectual support on mesothelioma and risk assessement.
Additional Information
Supplementary information accompanies this paper at www.nature.com/naturenanotechnology. Reprints and permission information is available online at http://npg.nature.com/reprintsandpermssion/.
References
- 1.Donaldson K, et al. Carbon nanotubes: a review of their properties in relation to pulmonary toxicology and workplace safety. Toxicol. Sci. 2006;92:5–22. doi: 10.1093/toxsci/kfj130. [DOI] [PubMed] [Google Scholar]
- 2.Poland CA, et al. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat. Nanotech. 2008;3:423–428. doi: 10.1038/nnano.2008.111. [DOI] [PubMed] [Google Scholar]
- 3.Ryman-Rasmussen JP, et al. Inhaled multiwalled carbon nanotubes potentiate airway fibrosis in a murine model of allergic asthma. Am. J. Resp. Cell. Mol. Biol. 2009;40:349–358. doi: 10.1165/rcmb.2008-0276OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mossman BT, Churg A. Mechanisms in the pathogenesis of asbestosis and silicosis. Am. J. Resp. Crit. Care. Med. 1998;157:1666–1680. doi: 10.1164/ajrccm.157.5.9707141. [DOI] [PubMed] [Google Scholar]
- 5.Card JW, Zeldin DC, Bonner JC, Nestman ER. Pulmonary applications and toxicity of engineered nanoparticles. Am. J. Physiol. 2008;295:L400–L411. doi: 10.1152/ajplung.00041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muller J, et al. Respiratory toxicity of multi-wall carbon nanotubes. Toxicol. Appl. Pharmacol. 2005;207:221–231. doi: 10.1016/j.taap.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Lam CW, James JT, McCluskey R, Hunter RL. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Toxicol. Sci. 2004;77:126–134. doi: 10.1093/toxsci/kfg243. [DOI] [PubMed] [Google Scholar]
- 8.Warheit DB, et al. Comparative pulmonary toxicity assessment of single-wall carbon nanotubes in rats. Toxicol. Sci. 2004;77:117–125. doi: 10.1093/toxsci/kfg228. [DOI] [PubMed] [Google Scholar]
- 9.Mercer RR, et al. Alteration of deposition pattern and pulmonary response as a result of improved dispersion of aspirated single-walled carbon nanotubes in a mouse model. Am. J. Physiol. 2008;294:L87–L97. doi: 10.1152/ajplung.00186.2007. [DOI] [PubMed] [Google Scholar]
- 10.Shvedova AA, et al. Unusual inflammatory and fibrogenic pulmonary responses to single-walled carbon nanotubes in mice. Am. J. Physiol. 2005;289:L698–L708. doi: 10.1152/ajplung.00084.2005. [DOI] [PubMed] [Google Scholar]
- 11.Miller FJ. Dosimetry of particles in laboratory animals and humans in relationship to issues surrounding lung overload and human health risk assessment: a critical review. Inhal. Toxicol. 2000;12:19–57. doi: 10.1080/089583700196536. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell LA, et al. Pulmonary and systemic immune response to inhaled multiwalled carbon nanotubes. Toxicol. Sci. 2007;100:203–214. doi: 10.1093/toxsci/kfm196. [DOI] [PubMed] [Google Scholar]
- 13.Li JG, et al. Comparative study of pathological lesions induced by multiwalled carbon nanotubes in lungs of mice by intratracheal instillation and inhalation. Environ. Toxicol. 2007;22:415–421. doi: 10.1002/tox.20270. [DOI] [PubMed] [Google Scholar]
- 14.Ogami A, et al. Pathological features of different sizes of nickel oxide following intratracheal instillation in rats. Inhal. Toxicol. 2009;19:1–7. doi: 10.1080/08958370802499022. [DOI] [PubMed] [Google Scholar]
- 15.Takagi A, et al. Induction of mesothelioma in p53+/− mouse by intraperitoneal application of multi-wall carbon nanotube. J. Toxicol. Sci. 2008;33:105–116. doi: 10.2131/jts.33.105. [DOI] [PubMed] [Google Scholar]
- 16.Mangum JB, et al. Single-walled carbon nanotube (SWCNT)-induced interstitial fibrosis in the lungs of rats is associated with increased levels of PDGF mRNA and the formation of unique intercellular carbon structures that bridge alveolar macrophages in situ. Part. Fibre. Toxicol. 2006;3:15. doi: 10.1186/1743-8977-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brody AR, Hill LH, Adkins B, Jr, O'Connor RW. Chrysotile asbestos inhalation in rats: deposition pattern and reaction of alveolar epithelium and pulmonary macrophages. Am. Rev. Respir. Dis. 1981;123:670–679. doi: 10.1164/arrd.1981.123.6.670. [DOI] [PubMed] [Google Scholar]
- 18.Choe N, et al. Pleural macrophage recruitment and activation in asbestos-induced pleural injury. Environ. Health. Perspect. 1997;105:1257–1260. doi: 10.1289/ehp.97105s51257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coin PG, Roggli VL, Brody AR. Persistence of long, thin chrysotile asbestos fibers in the lungs of rats. Environ. Health Perspect. 1994;5:197–199. doi: 10.1289/ehp.94102s5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kane A. Animal models of malignant mesothelioma. Inhal. Toxicol. 2006;18:1001–1004. doi: 10.1080/08958370600835393. [DOI] [PubMed] [Google Scholar]
- 21.Bonner JC. Regulation of PDGF and its receptors in fibrotic diseases. Cytokine Growth Factor Rev. 2004;15:255–273. doi: 10.1016/j.cytogfr.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 22.Walker C, et al. Characterization of platelet-derived growth factor and platelet-derived growth factor receptor expression in asbestos-induced rat mesothelioma. Cancer Res. 1992;52:301–306. [PubMed] [Google Scholar]
- 23.Mishra A, Liu JY, Brody AR, Morris GF. Inhaled asbestos fibers induce p53 expression in the rat lung. Am. J. Respir. Cell. Mol. Biol. 1997;16:479–485. doi: 10.1165/ajrcmb.16.4.9115760. [DOI] [PubMed] [Google Scholar]
- 24.Zhu L, Chang DW, Dai L, Hong Y. DNA damage induced by multiwalled carbon nanotubes in mouse embryonic stem cells. Nano. Lett. 2007;7:3592–3597. doi: 10.1021/nl071303v. [DOI] [PubMed] [Google Scholar]
- 25.Elgrabli D, et al. Biodistribution and clearance of instilled carbon nanotubes in rat lung. Part. Fibre. Toxicol. 2008;5:20. doi: 10.1186/1743-8977-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maynard AD, et al. Exposure to carbon nanotube material: aerosol release during the handling of unrefined single-walled carbon nanotube material. J. Toxicol. Environ. Health A. 2004;67:87–107. doi: 10.1080/15287390490253688. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.