Abstract
While studies have found influenza vaccination to be cost-effective in older adults (65 years or older), they have not looked at how the vaccine's economic value may vary with the timing of vaccine administration. We developed a set of computer simulation models to evaluate the economic impact of vaccinating older adults at different months. Our models delineated the costs and utility losses in delaying vaccination past October and suggest that policy makers and payors may consider structuring incentives (≤ $2.50 per patient) to vaccinate in October. Our results also suggest that vaccination is still cost-effective through the end of February.
Keywords: Influenza Vaccine, Vaccine Administration, Computer Simulation, Economics, Older Adults
INTRODUCTION
While studies have found influenza vaccination to be a cost-effective means of preventing seasonal influenza in older adults (65 years or older), they have not looked at how the vaccine's cost-effectiveness may vary with the timing of vaccine administration.[1-5] Presumably, vaccinating a patient earlier rather than later provides more value by protecting the patient for a greater proportion of the influenza season, assuming that vaccine conferred-immunity does not wane over a short period of time (<6 months) and the seasonal flu does not strike later than usual. However, quantifying the value of vaccinating patients at different times in the year can help health care workers and policy makers answer some important questions. For example, how aggressively should earlier vaccination be pushed, what is the impact of delays in vaccine availability, and how late during the influenza season should vaccination still be offered? Knowing the answers to these questions can help schedule vaccine ordering, delivery, and administration as well as determine whether incentives should be offered to get patients vaccinated earlier.
We developed two sets of computer simulation models to predict the potential economic impact of vaccinating older adults at different months of the year. The aim of the first set was to quantify the incremental economic value of vaccinating an older adult earlier in the influenza season and the incremental cost of delaying vaccination. The goal of the second set was to determine how late in the influenza season is vaccination still cost effective.
METHODS
Model Structures
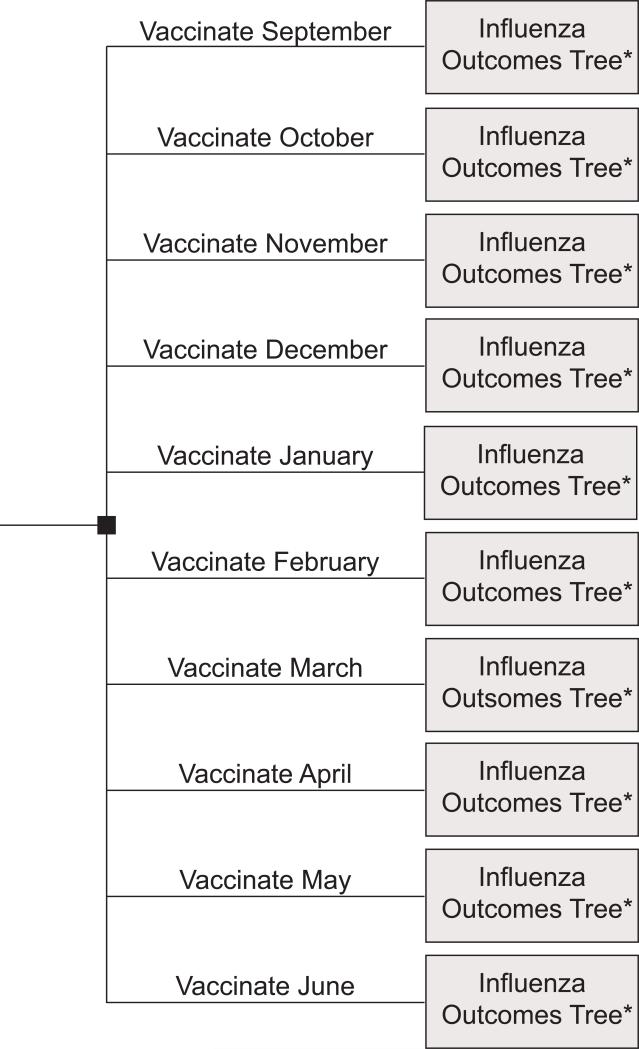
Using TreeAge Pro 2009 (TreeAge Software, Williamstown, Massachusetts), we constructed a set of decision analytic computer simulation models, with probabilistic sensitivity analyses. The first type of model, the Vaccination Timing Model (depicted in Figures 1 and 2) compared the administration of influenza vaccine to an older adult at different months of the year and the resulting incremental morbidity, mortality, and cost-effectiveness. The second type of model, the Monthly Vaccination versus No Vaccination Decision Model represented the decision of whether to vaccinate a patient in a given month (e.g., if you see an unvaccinated patient in March, should you vaccinate the patient) and the resulting incremental morbidity, mortality, and cost-effectiveness of each choice. We created a model for each month of the year from September to June.
FIGURE 1.
Influenza Vaccination Timing Model Base Structure
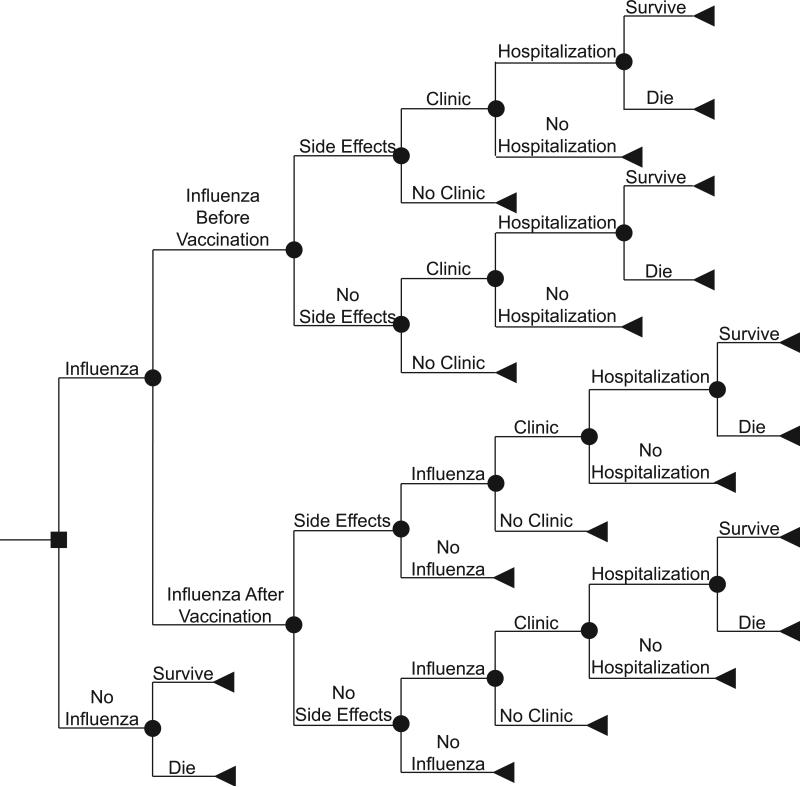
FIGURE 2.
Influenza Outcomes Tree Structure
The time frame for each of the models was one year, i.e., a single influenza season. For each model, the base case scenario assumed the societal perspective and accounted for direct and indirect costs of illness, and an additional scenario took the third-party payer perspective, considering only the direct costs of illness.
Figure 2 shows the different possible outcomes that each patient traveling through the model may have. After vaccination, a patient may develop local side effects, which would require one day of ibuprofen treatment or systemic side effects, which would require 3 days of ibuprofen treatment. We assumed that influenza vaccine would take at least 2 weeks to provide clinical protection. The patient's risk of subsequently contracting influenza is a function of how much time remains in the influenza season. Of the patients who develop influenza, the probability of requiring hospitalization depends on whether they were vaccinated and the effectiveness of the vaccine. Those who do not require hospitalization either just treat themselves with over-the-counter medications or visit an outpatient medical clinic, where 50% received prescriptions for anti-viral medications. Hospitalized patients have a probability of not surviving, dependent on whether they were vaccinated and the effectiveness of the vaccine. The model assumed that all older adult patients would first be hospitalized before they die from influenza. While in real life, some may pass away without being hospitalized, the majority would likely seek medical care; in fact, a percentage of older adults may undergo lengthy hospitalizations with multiple complications before succumbing (which would accrue additional costs not considered in our model). Excluding these costs may compensate for attributing hospitalizations to influenza victims who are never hospitalized.
To account for uncertainty and stochasticity, we used distributions for most of our data inputs and performed a probabilistic (Monte Carlo) sensitivity analysis, in which we simultaneously varied all parameters.
Data Inputs
Table 1 lists the various data inputs for our model (dividing them into probabilities, costs, and utilities) and the corresponding distributions and data sources used. We used beta distributions for all of our utility variables and normal distributions for all other variables. Where possible, data inputs came from published meta-analyses.
TABLE 1.
Data Inputs for Model Variables
| 95% Range | ||||
|---|---|---|---|---|
| Description (units) | Mean | Source | ||
| Lower Limit | Upper Limit | |||
| COSTS | ||||
| Influenza Vaccine ($US) | 15.00 | - | - | [23] |
| Vaccine Administration ($US) | 5.00 | - | - | Estimate |
| Anti-Viral medication: Oseltamivir ($US) | 99.32 | 77.32 | 121.32 | [23] |
| Influenza Treatment: | ||||
| Over the Counter Medications ($US) | 15.61 | 10.30 | 20.91 | [23] |
| Outpatient Visit ($US) | 151.38 | 114.94 | 187.81 | [24] |
| Productivity Loss for Outpatient Visit ($US) | 64.08 | 54.00 | 74.16 | [25] |
| Hospitalization ($US), 65-84 years old | 4723 | 4382 | 5064 | [26] |
| Hospitalization ($US), >85 years old | 5146 | 5406 | 5886 | [26] |
| Death in Hospital ($US) | 5,000 | - | - | [27] |
| Treatment of Vaccine Side Effects ($US) | 0.76 | 0.68 | 3.82 | [28] |
| DURATIONS | ||||
| Influenza (days) | 7 | - | - | [27, 29, 30] |
| Clinic Visit (hours) | 4 | - | - | [27, 29, 30] |
| UTILITIES | ||||
| One Year of Life for Older Adult (QALY) | 0.84 | - | - | [10] |
| Utility/Day | ||||
| Influenza no Hospitalization (QALY) | 0.65 | 0.49 | 0.81 | [11, 31] |
| Influenza with Hospitalization (QALY) | 0.50 | 0.38 | 0.63 | [11, 31] |
| Vaccine Side Effects (QALY) | 0.95 | 0.71 | 1.00 | [11] |
| PROBABILITIES | ||||
| Clinical Outcomes without Vaccination | ||||
| Influenza throughout the Year | 0.125 | 0.05 | 0.20 | [14] |
| Clinic Visit Given Influenza | 0.40 | - | - | [14] |
| Hospitalization Given Influenza | 0.04 | 0.01 | 0.07 | [14] |
| Clinical Outcomes with Vaccination | ||||
| Reduction in Hospitalization | 0.565 | 0.45 | 0.68 | [14] |
| Reduction in Mortality | 0.21 | 0 | 0.42 | [14] |
| Side Effects | 0.05 | - | - | [14] |
| Decrease in Attack Rate from Earlier Vaccination | 0.00 | 0.00 | 0.25 | [13, 14] |
Using the Centers for Disease Control and Prevention (CDC) monthly influenza surveillance data from 2000 to 2008 (Table 2), we created a risk distribution of influenza cases occurring each month.[6] This distribution was stochastic to mimic variability from influenza season to season. So for each patient entering the model, the per month risk of developing influenza may be drawn from any year between 2000 and 2008. Determining the relationship between vaccine coverage and influenza activity among the overall adult population is challenging. Many other factors (e.g., vaccine coverage of children and strain matching) may play a role. Therefore, in addition to focusing on the individual rather than the overall population, we designed our simulation experiments so that they would randomly draw from one of the 2000-2007 influenza seasons. Such a time window served as a sample of years that would have enough variation in important factors such as vaccine availability, strain matching and coverage. Running millions of realizations helped minimize the effects of a single outlier influenza season.
TABLE 2.
Monthly Distribution of Influenza Cases, 2000-2008
| Influenza Season | October | November | December | January | February | March | April | May |
|---|---|---|---|---|---|---|---|---|
| 2007-2008 | 0.44% | 1.01% | 3.34% | 13.21% | 45.49% | 32.54% | 3.97% | 0.00% |
| 2006-2007 | 1.23% | 2.53% | 10.35% | 13.08% | 38.14% | 27.07% | 6.29% | 1.30% |
| 2005-2006 | 0.34% | 0.82% | 11.72% | 16.27% | 25.15% | 35.76% | 8.32% | 1.62% |
| 2004-2005 | 0.41% | 1.12% | 8.97% | 23.98% | 40.67% | 21.81% | 2.68% | 0.36% |
| 2003-2004 | 2.87% | 18.99% | 69.44% | 7.22% | 0.99% | 0.29% | 0.18% | 0.03% |
| 2002-2003 | 0.07% | 0.40% | 4.87% | 14.81% | 44.46% | 30.82% | 3.91% | 0.65% |
| 2001-2002 | 0.23% | 0.66% | 4.18% | 19.40% | 42.26% | 27.07% | 5.14% | 1.06% |
| 200-2001 | 23.23% | 22.87% | 27.80% | 17.78% | 7.11% | 1.16% | 0.05% | 0.00% |
All costs were in 2009 U.S. dollars. In the base case scenario, a 3% discount rate, the standard rate for time preference discounting, converted all costs from other years into 2009 dollars.[7-9]
Our model measured effectiveness in quality adjusted life-years (QALY). Patients who did not develop vaccine side effects or influenza throughout our model time frame accrued 0.84 QALYs, based on the quality of life utility obtained by Gold et al for persons 65 years or older with no health conditions.[10] Vaccine side effects, influenza, and hospitalization each caused different decrements in QALY.[11] The expected loss of QALYs from death came from the life expectancy in QALYs of the patient at that age. Life expectancy estimates came from the Human Mortality Database.[12]
Sensitivity Analyses
Sensitivity analyses determined the effects of varying different parameter values individually throughout the ranges listed in Table 1. Multi-dimensional sensitivity analyses were performed on selected parameters. In particular, we examined the effects of varying patient ages and levels of financial incentive ($1, $2.50, $5, $7.50, and $10 per patient) to get patients vaccinated earlier. Starting from a $0 incentive per patient, we systematically increased this incentive by $0.50 at a time until vaccination in October was no longer cost-effective. In other words, the goal was to find how high a per patient incentive could go for vaccination in October (versus later months) to remain cost-effective. In addition, we conducted probabilistic (Monte Carlo) sensitivity analyses.
Older Adult Population Model
To be conservative about the benefits of earlier vaccination, our base-case scenario model focused on the individual patient and did not consider the potential added benefits of getting a greater percentage of the overall older adult population vaccinated earlier. Earlier vaccine coverage of the population may provide herd immunity benefits, thereby decreasing the influenza attack rate. An additional scenario attempted to capture these effects. A search of the literature (including a 2006, Cochrane systematic review) found no clear correlation between influenza vaccination rates and influenza attack rates among the elderly.[13, 14] Therefore, without clear guidance on how to adjust the attack rate with earlier vaccination, we ran sensitivity analyses varying the effect that earlier vaccination would have on the influenza attack rate. In other words, this set of sensitivity analyses examined scenarios in which earlier vaccination would decrease the influenza attack rate by 1%, 5%, 10%, and 25%.
RESULTS
Influenza Vaccination Timing Model
Each simulation run sent 1,000 simulated older adults (age 65 and above) 5,000 times (i.e., 5,000,000 trials) through the Optimal Vaccination Timing Model. We calculated the incremental cost-effectiveness ratio (ICER) of vaccinating in September versus all other months (October, November, etc.), October versus all other months, November versus all other months, etc. The following equation calculated the ICER:
From both the societal and the third party payor perspectives, October was the optimal month to administer the vaccine, dominating (i.e., resulting in less cost and greater utility) other months. September yielded identical costs and effectiveness and therefore offered no advantage over October vaccination.
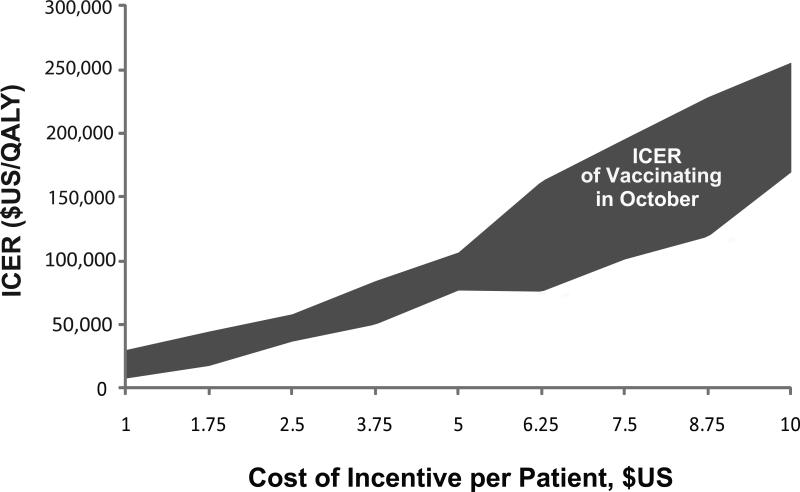
We also explored the effects of offering different levels of per patient financial incentives to get him or her vaccinated earlier. Using the $50,000 per QALY threshold for acceptable cost-effectiveness, vaccinating in October with a $2.50 per patient incentive remained cost-effective from the societal perspective for patients ages 65 to 85 (the ICER ranged from $37,609 to $45,194 per QALY in multiple runs). In other words, it was still cost-effective from the societal perspective to invest up to $2.50 per patient in getting older adults vaccinated in October. In Figure 3, the gray bands represents how the ICER varies by per patient financial incentive from the societal perspective. Simulating patients of ages from 65 to 85 years old generated the band. From the third payor perspective, it was still cost-effective to invest up to $2.50 per patient in getting older adults vaccinated in October (the ICER ranged from $33,161 to $62,515 per QALY).
FIGURE 3.
Incremental Cost-Effectiveness Ratio (ICER) of Vaccinating in October (versus November) from the Societal Perspective
Table 3 displays the potential effects per patient of delaying vaccination from the societal and third party payor perspectives. As shown in the table, delaying vaccination until November will cost society an additional $0.40 to $0.49 per patient (third party payors $0.33 to $0.50) and result in a loss of 0.00004 to 0.00009 QALYs (0.000032 to 0.00011 from the third party perspective. So, delaying vaccination for 1,000 patients will cost society $400 to $490 and 0.04 to 0.09 QALYs. It will cost third party payors $330 to $500 and 0.032 to 0.11 QALYs. Based on U.S. census bureau statistics, in July 2003, 35.9 million people were aged 65 and older in the United States (with 33.2 million were between age 65 and 85 years of age).[15] Therefore, delaying vaccination from October to November for 10% of the older adult population would cost society $1.33 to $1.63 million and third party payors $1.10 to $1.66 million. It also would cost society 120 to 299 QALYs. Increasing the delay to 25% of the older adult population would cost society $3.32 to $4.07 million and third party payors $2.74 to $4.15 million. It also would cost society 299 to 747 QALYs. An October to November delay for the entire U.S. older adult population would cost society $13.28 to $16.27 million (and 1195 to 2988 QALYs) and third party payors $10.96 to $16.50 million.
TABLE 3.
Incremental Cost and Incremental Effectiveness of Delaying Influenza Vaccination (versus Vaccinating in October)
| Societal Perspective | Third Party Payor Perspective | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vaccinate in*... | Incremental Cost ($US) |
Incremental Effectiveness (QALYs) |
Incremental Cost ($US) |
Incremental Effectiveness (QALYs) |
||||||||
| October Vaccination Does not Decrease Influenza Attack Rate | ||||||||||||
| November | $ 0.40 | to | $ 0.49 | −0.000036 | to | −0.000090 | $ 0.33 | to | $ 0.50 | −0.000032 | to | −0.000110 |
| December | $ 1.07 | to | $ 1.26 | −0.000105 | to | −0.000192 | $ 1.00 | to | $ 1.24 | −0.000075 | to | −0.000173 |
| January | $ 2.87 | to | $ 3.74 | −0.000248 | to | −0.000413 | $ 2.79 | to | $ 3.23 | −0.000254 | to | −0.000439 |
| February | $ 4.62 | to | $ 5.72 | −0.000402 | to | −0.000673 | $ 4.59 | to | $ 5.13 | −0.000384 | to | −0.000750 |
| March | $ 7.81 | to | $ 9.37 | −0.000692 | to | −0.001160 | $ 7.91 | to | $ 8.83 | −0.000732 | to | −0.001215 |
| October Vaccination Decreases Influenza Attack Rate by 1% | ||||||||||||
| November | $ 0.61 | to | $ 0.66 | −0.00005 | to | −0.000063 | $ 0.58 | to | $ 0.63 | −0.000047 | to | −0.000051 |
| December | $ 1.26 | to | $ 1.33 | −0.00011 | to | −0.00014 | $ 1.20 | to | $ 1.30 | −0.00011 | to | −0.00017 |
| January | $ 3.10 | to | $ 3.45 | −0.00030 | to | −0.00044 | $ 3.07 | to | $ 3.40 | −0.00031 | to | −0.00050 |
| February | $ 4.91 | to | $ 5.41 | −0.00049 | to | −0.00071 | $ 4.96 | to | $ 5.25 | −0.00051 | to | −0.00080 |
| March | $ 8.15 | to | $ 9.20 | −0.00079 | to | −0.0013 | $ 8.47 | to | $ 8.84 | −0.00078 | to | −0.0014 |
| October Vaccination Decreases Influenza Attack Rate by 5% | ||||||||||||
| November | $ 1.53 | to | $ 1.69 | −0.000075 | to | −0.000080 | $ 1.09 | to | $ 1.56 | −0.000028 | to | −0.00010 |
| December | $ 2.17 | to | $ 2.40 | −0.00015 | to | −0.00021 | $ 1.52 | to | $ 2.22 | −0.000072 | to | −0.00024 |
| January | $ 4.04 | to | $ 4.68 | −0.00038 | to | −0.00047 | $ 2.29 | to | $ 4.18 | −0.00013 | to | −0.00058 |
| February | $ 5.62 | to | $ 6.54 | −0.00054 | to | −0.00067 | $ 4.64 | to | $ 5.80 | −0.00039 | to | −0.00086 |
| March | $ 9.13 | to | $ 10.33 | −0.00090 | to | −0.0013 | $ 6.40 | to | $ 9.05 | −0.00057 | to | −0.0014 |
| October Vaccination Decreases Influenza Attack Rate by 10% | ||||||||||||
| November | $ 2.95 | to | $ 3.10 | −0.00011 | to | −0.00012 | $ 2.46 | to | $ 2.78 | −0.000091 | to | −0.00011 |
| December | $ 3.62 | to | $ 3.89 | −0.00018 | to | −0.00022 | $ 3.06 | to | $ 3.55 | −0.00016 | to | −0.00018 |
| January | $ 5.48 | to | $ 5.99 | −0.00043 | to | −0.00056 | $ 4.80 | to | $ 5.84 | −0.00032 | to | −0.00050 |
| February | $ 7.25 | to | $ 7.97 | −0.00063 | to | −0.00081 | $ 6.51 | to | $ 7.73 | −0.00048 | to | −0.00080 |
| March | $ 10.65 | to | $ 11.70 | −0.00090 | to | −0.0013 | $ 9.77 | to | $ 11.57 | −0.00082 | to | −0.0013 |
| October Vaccination Decreases Influenza Attack Rate by 25% | ||||||||||||
| November | $ 6.63 | to | $ 7.10 | −0.00017 | to | −0.00026 | $ 5.91 | to | $ 6.36 | −0.00018 | to | −0.00025 |
| December | $ 7.33 | to | $ 7.87 | −0.00023 | to | −0.00036 | $ 6.60 | to | $ 7.12 | −0.00022 | to | −0.00039 |
| January | $ 9.28 | to | $ 10.19 | −0.00044 | to | −0.00064 | $ 8.43 | to | $ 9.34 | −0.00039 | to | −0.00063 |
| February | $ 10.90 | to | $ 12.13 | −0.00061 | to | −0.00085 | $ 10.05 | to | $ 11.15 | −0.00056 | to | −0.00093 |
| March | $ 14.17 | to | $ 15.81 | −0.00091 | to | −0.0013 | $ 13.48 | to | $ 14.93 | −0.00088 | to | −0.0015 |
Compared to vaccinating in October.
Older Adult Population Model
When we assumed that earlier vaccination would decrease the influenza attack rate, the value of October vaccination increased. We re-explored the effects of offering different levels of per patient financial incentives when varying the influenza attack rates. Having October vaccination result in a 5% decrease in influenza attack rate meant that it was still cost-effective from the societal perspective to invest up to $5.00 per patient to get older adults vaccinated in October (the ICER ranged from $35,217 to $45,257 per QALY in multiple runs). This also held for the third payor perspective (the ICER ranged from $29,243 to $48,211 per QALY).
Table 3 shows how the change in attack rate affects the incremental cost and incremental effectiveness of later (versus October) vaccination. Even if October vaccination were to decrease the influenza attack rate by only 5%, delaying vaccination until November will cost society $1.53 to $1.69 per patient (third party payors $1.09 to $1.56). Therefore, delaying vaccination from October to November for 10% of the older adult population would cost society $5.08 to $5.61 million and third party payors $3.62 to $5.18 million. An October to November delay for the entire U.S. older adult population would cost society $50.80 to $56.11 million and third party payors $36.19 to $51.80 million.
Monthly Vaccination versus No Vaccination Decision Model
Each monthly simulation run involved sending 1,000 simulated older adults 5,000 times (i.e., 5,000,000 trials) through each monthly model. Vaccinating the patient remained a cost-effective option (ICER<$50,000/QALY) for ages 65, 75, and 85 years old) until the end of February (Table 4). Table 4 lists the ICERs of vaccinating compared with not vaccinating for each month. For example, vaccinating in January has an ICER of $15,400 to $21,096 compared to not vaccinating. This suggests that should a patient enter a clinic in January, it is still cost effective to vaccinate the patient. This choice holds in February too. From March on, it is no longer to vaccinate an unvaccinated patient. (After February, vaccination has an ICER greater than $50,000 per QALY, represented by the shaded area.) This relatively large jump in ICERs occurs because the chance of having influenza from March onwards is fairly low.
TABLE 4.
Incremental Cost Effectiveness Ratio (ICER) of Vaccinating (versus Not Vaccinating) an Unvaccinated Patient Each Month
| Unvaccinated Patient Enters Clinic in Month... | Societal Perspective ($US/QALY) | Third Party Payor Perspective ($US/QALY) | ||
|---|---|---|---|---|
| Low | High | Low | High | |
| October | 5,468 | 8,333 | 5,263 | 7,566 |
| November | 7,488 | 10,549 | 6,358 | 10,186 |
| December | 8,111 | 13,041 | 9,291 | 12,944 |
| January | 15,400 | 21,096 | 16,623 | 19,513 |
| February | 34,413 | 50,297 | 31,776 | 53,124 |
| March | 185,110 | 306,298 | 168,581 | 215,208 |
| April | 375,715 | 420,860 | 353,037 | 666,058 |
DISCUSSION
Our results indicate that the timing of annual influenza vaccination does make a difference, i.e., vaccinating later in the influenza season is not equivalent to vaccinating earlier in the influenza season. While this finding is not surprising, quantifying the economic value of earlier vaccination may help with vaccination logistics planning. For example, investing up to $5.00 per patient to get patients vaccinated in October rather November or later may be worthwhile. Such an investment could come in many forms: ranging from paying each patient to get vaccinated earlier to health care worker incentive to funding programs that will enable earlier vaccination.[16, 17] For instance, it may be justifiable to invest up to $5,000 in a program that gets an additional 1,000 older adults vaccinated in October. Our study may provide benchmarks to policy makers and administrators planning such programs.
These findings could have implications for vaccine production and supply logistics. Vaccine supply chain disruptions (e.g., production or shipment problems) that delay vaccine arrivals until November or later may be substantially detrimental to the older adult population.[18, 19] Leaving the elderly population unprotected for even just a month can have serious consequences. It may be worthwhile to invest millions of dollars each year to ensure that the vaccine supply chain effectively gets vaccine to older adults on time.[20-22]
Each year physicians and other health care workers encounter unvaccinated patients late in the influenza season and must decide whether to still offer the influenza vaccine. Our monthly models suggest that vaccination is still worthwhile through the end of February. So while earlier vaccination is still preferable, up to the end of February, vaccination is still more cost-effective than no vaccination.
Limitations
Every computer model is a simplification of real life. No model can fully represent every single event and outcome that may ensue in the year after an influenza vaccination. For example, our model did not fully represent the older population's heterogeneity and accompanying co-morbidities. Co-morbidities (e.g., severe chronic obstructive pulmonary disease) such as may increase a person's risk of influenza and influenza-related complications and his or her corresponding resource-use (e.g., intubation and mechanical ventilation). Resource use and probabilities of hospitalization and death may vary among those in the community versus those in nursing homes and by race, ethnicity, and socioeconomic status. Moreover, the model did not include the potential effects of vaccination on transmission, i.e., earlier vaccination may build local herd immunity sooner and confer indirect protection to those other than the patient. All of these simplifications could in fact underestimate the cost-effectiveness gains of earlier vaccination in the Timing Model and later vaccination in the Monthly Vaccination versus No Vaccination Model.
Conclusions and Future Directions
There is value in getting patients vaccinated by the end of October versus later in the influenza season. Policy makers and payors may consider structuring incentives to get patients vaccinated earlier. Understanding the economic impact of delaying vaccination may be useful for influenza vaccine production and supply chain decisions. Our results also suggest that vaccination is still of substantial use through the end of February. Therefore, health care workers and clinics may want to plan on offering vaccine late into the influenza season. Future studies may look at specific incentives and programs to motivate earlier vaccination and strategies to mitigate production and supply chain delays.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Allsup S, Haycox A, Regan M, Gosney M. Is influenza vaccination cost effective for healthy people between ages 65 and 74 years? A randomised controlled trial. Vaccine. 2004 Dec 16;23(5):639–45. doi: 10.1016/j.vaccine.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Hoshi SL, Kondo M, Honda Y, Okubo I. Cost-effectiveness analysis of influenza vaccination for people aged 65 and over in Japan. Vaccine. 2007 Aug 29;25(35):6511–21. doi: 10.1016/j.vaccine.2007.05.067. [DOI] [PubMed] [Google Scholar]
- 3.Nichol KL, Goodman M. Cost effectiveness of influenza vaccination for healthy persons between ages 65 and 74 years. Vaccine. 2002 May 15;20(Suppl 2):S21–4. doi: 10.1016/s0264-410x(02)00124-x. [DOI] [PubMed] [Google Scholar]
- 4.Nichol KL, Margolis KL, Wuorenma J, Von Sternberg T. The efficacy and cost effectiveness of vaccination against influenza among elderly persons living in the community. N Engl J Med. 1994 Sep 22;331(12):778–84. doi: 10.1056/NEJM199409223311206. [DOI] [PubMed] [Google Scholar]
- 5.Schoenbaum SC. Economic impact of influenza. The individual's perspective. Am J Med. 1987 Jun 19;82(6A):26–30. doi: 10.1016/0002-9343(87)90557-2. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention (CDC) [2009 April 15];Flu activity and surveillance. 2009 Available from: http://www.cdc.gov/flu/weekly/fluactivity.htm.
- 7.Discounting and the Treatment of Uncertainty in Natural Resource Damage Assessment: Technical Paper 99-1. NOAA (National Oceanic and Atmospheric Administration); Silver Spring, MD.: 1999. [Google Scholar]
- 8.Sloan FA. Valuing health care: costs, benefits, and effectiveness of pharmaceuticals and medical technologies. Cambridge University Press; New York: 1995. [Google Scholar]
- 9.Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-Effectiveness in Health and Medicine. Oxford University Press; New York: 1996. [Google Scholar]
- 10.Gold MR, Franks P, McCoy KI, Fryback DG. Toward consistency in cost-utility analyses: using national measures to create condition-specific values. Med Care. 1998 Jun;36(6):778–92. doi: 10.1097/00005650-199806000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Tengs TO, Wallace A. One thousand health-related quality-of-life estimates. Med Care. 2000 Jun;38(6):583–637. doi: 10.1097/00005650-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Wilmoth JR, Shkolnikov V. [2008 January 21, 2008];Human Mortality Database. 2008 doi: 10.1093/ije/dyv105. Available from: www.mortality.org or www.humanmortality.de. [DOI] [PMC free article] [PubMed]
- 13.Nunes B, Falcao I, Machado A, Rodrigues E, Falcao JM. Influenza vaccine coverage and the attack rate of influenza-like illness among the elderly in Portugal: is there a correlation? Euro Surveill. 2007 May;12(5):E070517 2. doi: 10.2807/esw.12.20.03195-en. [DOI] [PubMed] [Google Scholar]
- 14.Rivetti D, Jefferson T, Thomas R, Rudin M, Rivetti A, Di Pietrantonj C, et al. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev. 2006;3:CD004876. doi: 10.1002/14651858.CD004876.pub2. [DOI] [PubMed] [Google Scholar]
- 15.Wan He, Manisha Sengupta, Victoria A. Velkoff, and, DeBarros KA. 65+ in the United States: 2005. U.S. Department of Health and Human Services and U.S. Department of Commerce; 2005. [Google Scholar]
- 16.Kouides RW, Bennett NM, Lewis B, Cappuccio JD, Barker WH, LaForce FM. Performance-based physician reimbursement and influenza immunization rates in the elderly. The Primary-Care Physicians of Monroe County. Am J Prev Med. 1998 Feb;14(2):89–95. doi: 10.1016/s0749-3797(97)00028-7. [DOI] [PubMed] [Google Scholar]
- 17.Moran WP, Nelson K, Wofford JL, Velez R, Case LD. Increasing influenza immunization among high-risk patients: education or financial incentive? Am J Med. 1996 Dec;101(6):612–20. doi: 10.1016/S0002-9343(96)00327-0. [DOI] [PubMed] [Google Scholar]
- 18.Lu P, Bridges CB, Euler GL, Singleton JA. Influenza vaccination of recommended adult populations, U.S., 1989-2005. Vaccine. 2008 Mar 25;26(14):1786–93. doi: 10.1016/j.vaccine.2008.01.040. [DOI] [PubMed] [Google Scholar]
- 19.Chi RC, Neuzil KM, Lipsky BA, Reiber GE. Where high-risk adults receive influenza vaccine during a shortage. Arch Intern Med. 2007 Nov 26;167(21):2366–8. doi: 10.1001/archinte.167.21.2366. [DOI] [PubMed] [Google Scholar]
- 20.Uscher-Pines L, Barnett DJ, Sapsin JW, Bishai DM, Balicer RD. A systematic analysis of influenza vaccine shortage policies. Public Health. 2008 Feb;122(2):183–91. doi: 10.1016/j.puhe.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Ransom J, Bashir Z, Phillips C. Local health department responses during the 2004-2005 influenza vaccine shortage. J Community Health. 2007 Aug;32(4):283–97. doi: 10.1007/s10900-007-9049-5. [DOI] [PubMed] [Google Scholar]
- 22.Mody L, Langa KM, Malani PN. Impact of the 2004-2005 influenza vaccine shortage on immunization practices in long-term care facilities. Infect Control Hosp Epidemiol. 2006 Apr;27(4):383–7. doi: 10.1086/503179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.PDR . Red Book 2009. Thompson Healthcare, Inc.; Montvale, NJ: 2009. [Google Scholar]
- 24.Centers for Medicare & Medicaid Services [2009 January 10];2009 Available from: http://www.cms.hhs.gov/ [PubMed]
- 25.National Compensation Survey . occupational wages in the United States, June 2006. U.S. Department of Labor; Washington, DC: 2007. [Google Scholar]
- 26.Levit K, Ryan K, Elixhauser A, Stranges E, Kassed C, Coffey R. [2009 February 1];HCUP facts and figures: Statistics on hospital-based care in the United States in 2007. 2009 Available from: www.hcup-us.ahrq.gov/reports.jsp.
- 27.Smith KJ, Roberts MS. Cost-effectiveness of newer treatment strategies for influenza. Am J Med. 2002 Sep;113(4):300–7. doi: 10.1016/s0002-9343(02)01222-6. [DOI] [PubMed] [Google Scholar]
- 28.PDR . Red Book 2008. Thompson Healthcare, Inc.; Montvale, NJ: 2008. [Google Scholar]
- 29.Rothberg MB, Bellantonio S, Rose DN. Management of influenza in adults older than 65 years of age: cost-effectiveness of rapid testing and antiviral therapy. Ann Intern Med. 2003 Sep 2;139(5 Pt 1):321–9. doi: 10.7326/0003-4819-139-5_part_1-200309020-00007. [DOI] [PubMed] [Google Scholar]
- 30.Rothberg MB, Rose DN. Vaccination versus treatment of influenza in working adults: a cost-effectiveness analysis. Am J Med. 2005 Jan;118(1):68–77. doi: 10.1016/j.amjmed.2004.03.044. [DOI] [PubMed] [Google Scholar]
- 31.Sackett DL, Torrance GW. The utility of different health states as perceived by the general public. J Chronic Dis. 1978;31(11):697–704. doi: 10.1016/0021-9681(78)90072-3. [DOI] [PubMed] [Google Scholar]