Abstract
Nonarteritic anterior ischemic optic neuropathy (NAION) results from isolated anterior optic nerve (ON)-axonal ischemia near the retina–optic nerve junction. We utilized a rodent model of NAION (rAION) to study the in vivo inflammatory response after pure axonal ischemic infarct. ON ischemia was generated using laser-coupled rose Bengal dye photoactivation, and the infarct localized using tetrazolium red and histology. ON inflammation was evaluated following infarct using extrinsic macrophage (ED1) and microglial (isolated Iba1) cell markers. In naive ONs, some ED1(+)/Iba1(+) cells, representing extrinsic macrophages, were present in intraretinal ON region, but not in the retroscleral (isolated ON) region. Numerous ED1(−)/Iba1(+) cells, likely representing intrinsic microglia, were present throughout the entire ON. One day post-stroke, slight increases in both ED1(+) and Iba1(+) cells were apparent in the eye region immediately surrounding the anterior ON. Three days post-stroke, there was marked infiltration and aggregates of ED1(+)/Iba1(+) cells, with axon structural disruption in the region of the ischemic infarct. ED1(+) and Iba1(+) cells were present in the portion of the ON surrounding the infarct, possibly representing a penumbral region similar to that seen in ischemic brain infarcts. Although ED1(+) cells decreased by 7–14 days post-stroke, large numbers of Iba1(+) cells persisted in the anterior ON. Similar to other CNS ischemic strokes, pure axonal ischemia results in the early recruitment of extrinsic macrophages to the ischemic region. Manipulation of the inflammatory response may be an important variable that could potentially improve visual outcome.
Keywords: Optic nerve, AION, Inflammation, Stroke, Ischemic infarction, White matter
1. Introduction
Nonarteritic anterior ischemic optic neuropathy (NAION) is a pure white-matter, central nervous system (CNS) stroke and is the leading cause of sudden optic nerve (ON)-related vision loss in the elderly worldwide (Hattenhauer et al., 1997). NAION is believed to result from thrombotic or hypoperfusion-associated ischemia of the anterior portion of the ON (Arnold, 1995). Immediately following ischemia, the ON head swells, presumably caused by axon transport blockade (Miller, 1982; Tesser et al., 2003). ON ischemia causes permanent vision loss due to primary damage to retinal ganglion cell (RGC) axons and subsequent RGC death from apoptosis (Levin and Louhab, 1996). Because the ON is a CNS-fiber tract, NAION is actually an isolated CNS axonal infarct. NAION is pathologically similar to axonal strokes that occur in other CNS regions. Thus, treatments that are effective for NAION may also be valuable in treating other types of CNS white-matter ischemia.
A major problem associated with the study of NAION and other forms of CNS white-matter ischemia are a lack of early clinical material. This lack hampers our understanding of the pathophysiology of these conditions. Few NAION histological studies are available (Lieberman et al., 1978; Knox et al., 2000), and only one early clinical histopathological study is available, from an ON of a patient developing NAION 20 days prior to death (Tesser et al., 2003). This study digitally reconstructed the region of the ischemic lesion (Tesser et al., 2003). The NAION lesion was shown to be an infarct confined to the region of the lamina: an intrascleral junctional structure separating the intraretinal (prelaminar) portion of the ON and the more posterior (post-laminar) ON.
In order to better study events occurring during NAION, we recently developed a rodent animal model of NAION (rAION). In this model, the initial damage is restricted to the ON axons, with RGC loss first detectable on the seventh day and increasing significantly over the subsequent 14 days (Bernstein et al., 2003; Slater et al., 2008). Rodent AION does not appear to damage to the outer retina (Bernstein et al., 2003). The rAION model is the first in vivo model of isolated white-matter stroke.
In addition to the ischemic event itself, post-ischemic inflammation appears to play an important role in brain ischemic injury (Castellanos et al., 2002). Thus, prevention of further injury as well as treatment of the injury may require modulation of several aspects of post-ischemic inflammation. For example, recruitment of extrinsic macrophages and inflammation can enhance spinal cord regeneration following trauma and protection of RGC death in an animal model of glaucoma (David and Ousman, 2002; Schori et al., 2001), possibly by oncomodulin secretion (Yin et al., 2006). Indeed, oncomodulin can stimulate ON regeneration and RGC survival in an in vitro retinal culture system as well as in an in vivo ON crush model. Degenerating CNS axons release solubilized myelin fragments such as Nogo, that can inhibit axon regeneration (He et al., 2003; Filbin, 2003). Extrinsic (hematogenous) macrophage recruitment can clear soluble Nogo and thus also may be important in nerve regeneration (Fry et al., 2007). Finally, resident CNS macrophages (microglia) are known to enhance remyelination by removal of degenerated myelin (Selvaraju et al., 2004).
Spontaneous clinical recovery of ON function following NAION has been documented in as many as 40% of patients (IONDT study group, 2000). It was previously assumed that the initial visual loss in patients with NAION, and subsequent progression or improvement resulted from non-inflammatory mechanisms. While the previous histological study of an early case of NAION did not identify pathologic evidence of an inflammatory response (Tesser et al., 2003), the investigators did not utilize any specialized stains to identify inflammatory cell involvement. The purpose of this study was to determine if there are previously unrecognized inflammatory responses following acute ON ischemia that may contribute to ON damage, or conversely, improve the potential for post-ischemic remyelination and axon regeneration.
2. Results
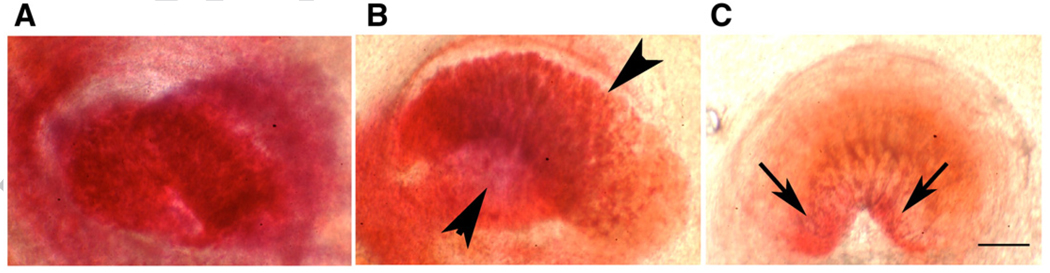
The intrascleral portion of the ON perforates the posterior portion of the eye, and emerges as the intra-orbital ON. This ON region is variable in appearance, ranging from ovoid (Figs. 1A and B) to crescentic (Fig. 1C). Triphenyl-tetrazolium chloride (TTC) vital staining of control intrascleral ON generates a uniform red color (Fig. 1A), revealing that there is no ON ischemia in uninduced eyes. In contrast, TTC-stained ON from eyes induced for 8 s (Fig. 1B) reveal ischemic regions characterized by a lack of color (arrowheads). The remainder of the ON is deep red, representing axons with normal metabolism. TTC stained ON from eyes induced for 12 s are pale (Fig. 1C), with only two small remaining regions of relatively normal TTC staining (arrows, Fig. 1C). The degree of rAION-induced ON ischemia is therefore related to the induction time.
Fig. 1.
Tetrazolium red (TTC) analysis of the degree of focal ischemia in the intrascleral portion of the ON one day following rAION. TTC staining was performed on optic nerves one day post rAION induction as described in Experimental procedures. The intrascleral portion of the rat ON has a variable shape, ranging from ovoid to crescentic. (A) Naive (uninduced) ON. There is intense red staining of the entire ON portion. (B) Mild induction (8 s). Red staining is less intense, and there is a relatively pale region, indicated by arrowheads, adjoining a more energetically active area, shown as dark red. (C) Severe induction (12 s). The entire ON portion is relatively pale, with red staining reduced to two small areas (arrows). Scale bar: 50 µm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
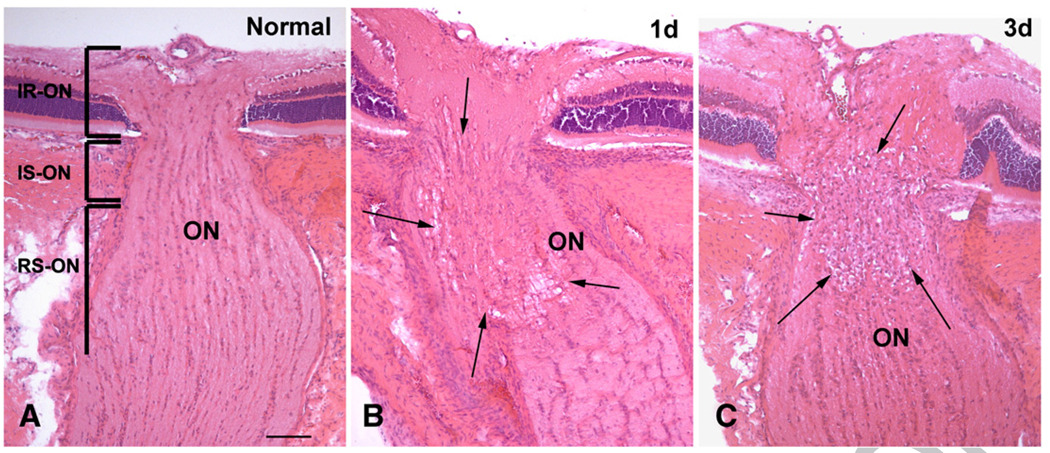
The normal rat ON has a highly structured appearance with normal longitudinally arranged columns of nerve fibers and associated astrocytes and oligodendrocytes (Fig. 2A). One day after induction of ON ischemia, there is a focal area of tissue edema in the intrascleral and adjacent retroscleral portions of the nerve (Fig. 2B). Three days after induction of ON ischemia, the anterior ON showed significant edema both grossly and histopathologically (Fig. 2C). During this latter time period, cell aggregates are present, infiltrating the intrascleral as well as the pre- (intraretinal) and post-scleral ON regions (Fig. 2C).
Fig. 2.
Histopathological changes in normal, one and three days post-stroke rat ON. H&E staining, frozen sections. (A) In the normal ON, the nerve bundles, astrocytes, and oligodendrocytes are arranged in highly structured, longitudinal bundles separated by fibrovascular pial septae. The optic nerve is divided into three regions: the intraretinal portion (IR-ON), intrascleral portion (IS-ON) and retroscleral portion (RS-ON). The diameter of the ON becomes substantially increased in the RS-ON due to myelination of the nerve fibers. (B) One day post-ON infarct, an area of tissue edema is present in the IS-ON and RS-ON areas (between arrows). (C) Three days after the ON infarct, cell aggregates are present in the ON, predominantly in the IS-ON but also in the IR-ON and RS-ON areas (between arrows). Note that significant disc edema is also present at three days post-ischemia. Bar=50 µm.
Iba1 immunolabeling identifies both extrinsic macrophages and resident microglia (Ito et al., 2001; Ahmed et al., 2007), while ED1 immunopositivity is exclusive to recently generated blood borne extrinsic macrophages (Priller et al., 2001).
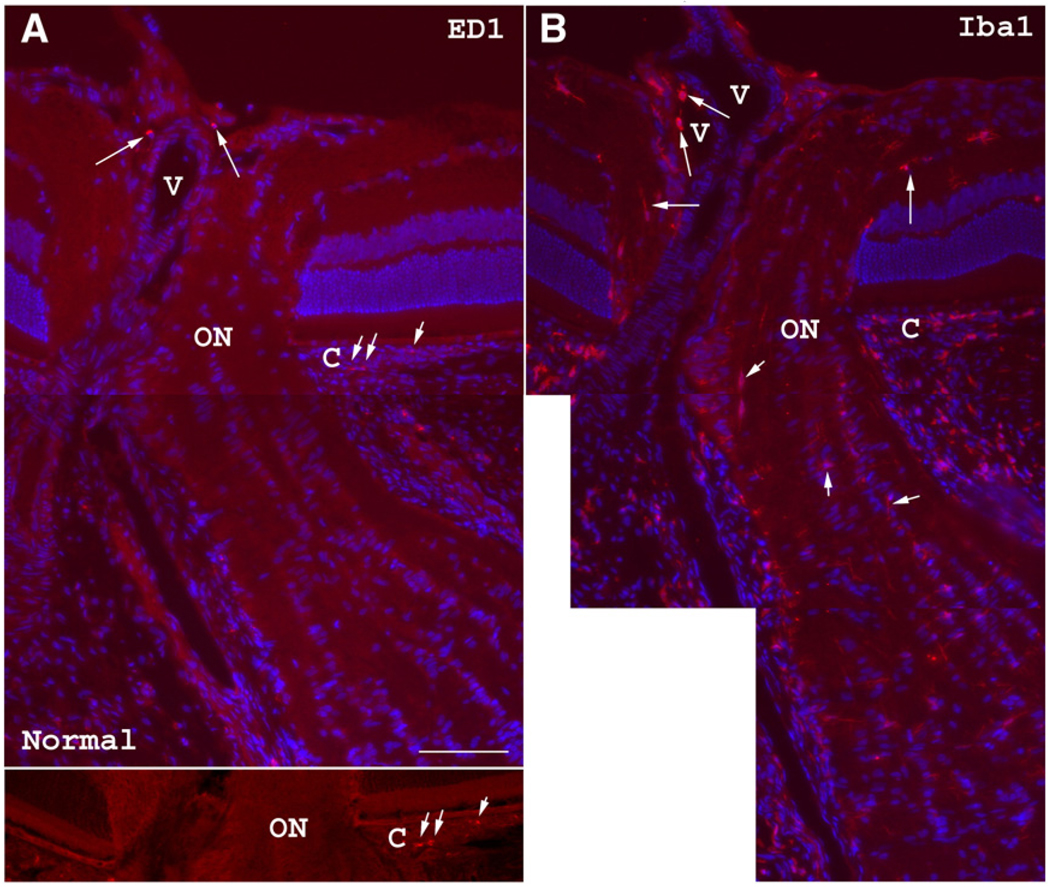
In naive ONs, rare ED1(+) cells are present only in the most anterior (intraretinal) portion of the nerve and appear as oval or round cells that typically surround blood vessels (Fig. 3A). No ED1(+) cells are present in the intrascleral segment of the ON (the part of the nerve in rodents corresponding to the laminar region of the nerve in humans) or any ED1(+) cells in the immediate retroscleral ON. However, many Iba1(+) cells, which can represent both extrinsic macrophages as well as microglia (Ito et al., 2001), are present in the anterior, intrascleral, and immediate retroscleral portions of the ON (Fig. 3B). These cells are either ovoid in appearance or possess more ramified morphology with slender cellular processes (Fig. 3B, arrows). The peripapillary choroid and retina of normal rat eyes typically contain a few ED1(+) cells (not shown); however, Iba1(+) cells are fairly abundant in the naive peripapillary choroid.
Fig. 3.
ED1 (extrinsic macrophage) and Iba1 (macrophage/microglia) immunohistochemistry of a normal control rat optic nerve. (A) Occasional ED1(+) cells (arrows) are seen in the anterior ON surrounding a central blood vessel (V) as well as in the peripapillary choroid (C; short arrows). The lower panel shows a better view of the scattered peripapillary choroidal (C) ED1(+) cells without Hoechst counterstaining. (B) Iba1 immunostaining shows many positive cells in the ON head (arrows). Some Iba1(+) cells surround blood vessels (V). The peripapillary choroid (C) contains many more Iba1(+) cells than ED1(+) cells. Although Iba1(+) cells are also present in the intrascleral and posterior ON segments (short arrows), no ED1(+) cells are seen in these areas. Bar=50 µm.
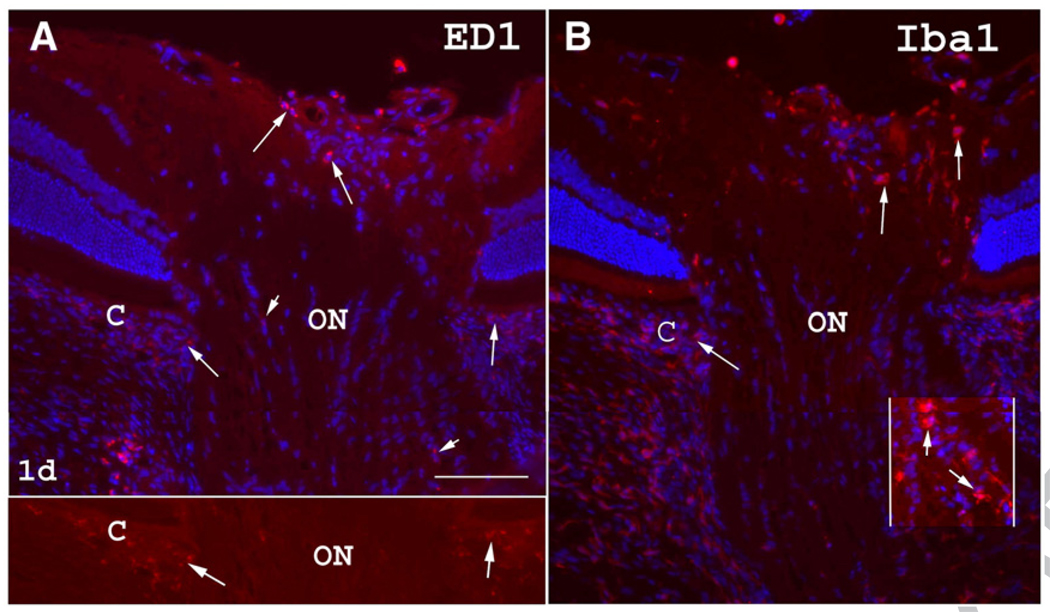
One day post-induction of ON ischemia, the region of the choroid (the vascular tissue bed directly under the retina), which lies adjacent to the ON (the peripapillary choroid) showed increased numbers of ED1(+) cells (C in Fig. 4A; arrows) compared with normal controls (compare Fig. 3A with Fig. 4A). ED1(+) cells are also present in the anterior, intrascleral, and adjacent retroscleral portions of the ON (Fig. 4A). Iba1(+) cells are also increased in the anterior ON (Fig. 4B) and in the peripapillary choroid, and appear more ameboid and activated in the posterior ON.
Fig. 4.
Inflammatory cells in the rat ON one day post-ON ischemia. (A) ED1 immunoreactivity. Scattered ED1(+) cells are detectable in the anterior ON as well as in the peripapillary choroid (C; long arrows). A few ED1(+) cells are also seen scattered throughout the intrascleral and adjacent posterior portions of the ON (short arrows). The lower panel shows a better view of the increased choroidal (C) ED1(+) cells without Hoechst counterstaining, compared with the ED1 staining in normal peripapillary choroid in Fig. 3A. (B) Iba1 immunoreactivity. There is a moderate increase in Iba1(+) cells in the anterior ON (arrows) and in the peripapillary choroid (C). In the posterior ON, Iba1(+) cells appeared more amoeboid and less ramified (enhanced contrast inset: short arrows). Bar=50 µm.
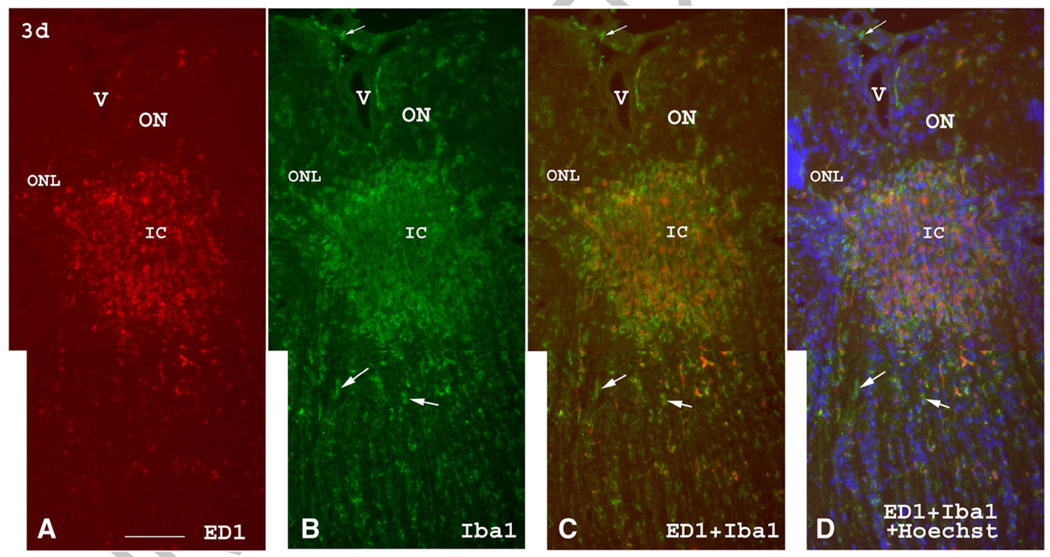
Three days post-induction of rAION, ED1 and Iba1 immunostaining of the anterior ON reveals a significant ED1(+) and Iba1(+) cellular accumulation in the intrascleral portion of the ON (Fig. 5). This region corresponds to the region of ischemia following rAION induction, both by India ink vascular imaging (Bernstein et al., 2003), as well as by tetrazolium red vital staining (also see Figs. 1B and C).
Fig. 5.
ED1 (red) and Iba1 (green) immunolabeling of the anterior, intrascleral, and postscleral portions of the ON three days post-induction of rAION. (A) There is an intense accumulation of ED1(+) cells in the intrascleral and immediate posterior portions of the ON (corresponding to the ischemic core, IC). Posterior to the highly infiltrated ED1(+) area, a few scattered ED1(+) cells are present. (B) Iba1 immunostaining shows a similar accumulation of Iba1(+) round or ameboid cells in the intrascleral portion of the ON and in the ischemic core, as well as some Iba1(+) cells posterior to the core lesion. Most Iba1(+) cells are ED1(−) (arrows) behind the ischemic core. (C) Merged view. The accumulated ED1(+)/Iba1(+) cells are present in the ischemic core (IC). (D) Merged view with nuclear (Hoechst) counterstain. ED1(+)/Iba1(+) cells are present in the anterior ON and are interspersed among the oligodendrocyte nuclei (blue) posterior to the ischemic core, with more Iba1(+) cells than ED1(+) cells being present. The Iba1(+)/ED1(−) cells (arrows) posterior to the ischemic core are probably resident microglia. V: blood vessel; ONL: outer nuclear layer; IC: ischemic core. Bar=50 µm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
A few scattered ED1(+) cells are also detectable immediately posterior to this core region (Fig. 5A). Few ED1(+) cells are present in the far posterior ON. Iba1 immunostaining showed a slightly different picture. Iba1(+) cells (Fig. 5B) are also present in the intrascleral ON, in the ischemic core, and immediately posterior to the ischemic core but, in addition, are also present in the far posterior ON without significant increase in number (Fig. 5B). Three days post-induction, double immunolabeling with both ED1 and Iba1 antibodies (Figs. 5C and D) reveals that most Iba1(+) cells in the ischemic core are also ED1(+); however, Iba1(+)/ED1(−) cells are also present in adjacent anterior and posterior nerve portions, which may represent the penumbra region seen in brain
To determine whether the degree of axonal ischemia correlates with the intensity of inflammation, we quantified ED1(+) cell numbers in rAION-induced ONs, from eight second (mild induction; n=3), and twelve second (severe induction; n=3) eyes, three days post-induction. ON sections from mildly induced (8 s) eyes revealed an average of 43.8±21.7 ED1(+) cells/section, compared with 86.3±39.9 cells/section in the severe induction group. In contrast, few or no ED1(+) cells were found in ON sections from control (uninduced) eyes. This suggests that the level of ON-extrinsic inflammation varies with the degree of ON ischemia.
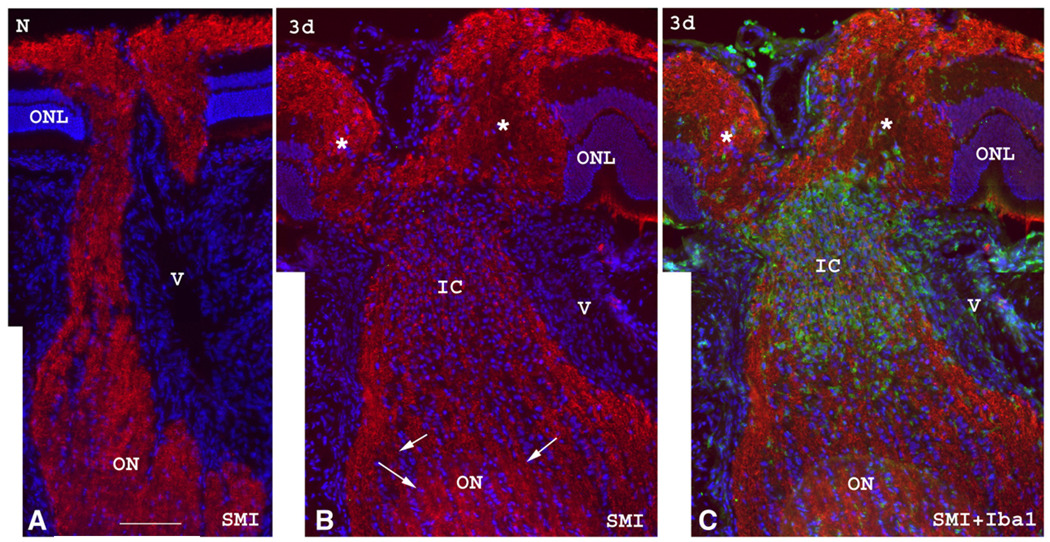
SMI312 immunostaining, which identifies intact neurofilaments (Fig. 6), reveals a significant axonal structural disruption in the intrascleral and immediate retroscleral portions of the nerve with heavy infiltration of Iba1(+) cells at three days post-ischemia. Far distal to the primary lesion (Fig. 6B), the axonal structures appear intact compared with the normal control ON (Fig. 6A). The focal accumulation of macrophages/microglia and acute axon damage likely represent the primary ischemic core region affected by rAION and is located in the intrascleral region and adjacent retroscleral portion of ON ischemic stroke. Similar findings are demonstrable in specimens with mildly induced (8 s) partial ON infarcts (data not shown), where there is partial maintenance of normal axon integrity, a pattern similar to that seen in human clinical specimens (Tesser et al., 2003; Lieberman et al., 1978; Knox et al., 2000). In these cases, longitudinal sections of the affected ON show a partial infarct that does not involve the entire nerve area, with both ED1(+) and Iba1(+) cells present in the ischemic core and the anterior ON. Again, more Iba1(+) than ED1(+) cells are seen in all of these areas.
Fig. 6.
SMI312 (red) and Iba1 (green) immunolabeling of the ON in a control ON (A) and an rAION-induced ON three days after induction (B and C). Sections are counterstained with Hoechst for the cell nuclei. (A) The normal ON shows intact neurofilaments characterized by intense SMI312 immunostaining in the anterior, intrascleral and retroscleral portions of the ON. (B and C). In a rAION-induced ON, SMI312 staining is intense in the anterior and retroscleral ON (arrows in panel B), but there is significant disruption of labeled neurofilaments in the axons in the intrascleral and immediate retroscleral portion of the ON (IC, ischemic core in panel B), with heavy infiltration of Iba1(+) macrophage/microglia (green in panel C). This likely represents the ischemic infarct region. Significant disc edema (*) is also noted in the anterior portion of the ON at 3d post ischemia. The juxtapapillary ONL layer is displaced laterally from the ON edema caused by the infarct in the intrascleral and immediate retroscleral region. (ONL: outer nuclear layer; V: blood vessel.) Bar=50 µm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
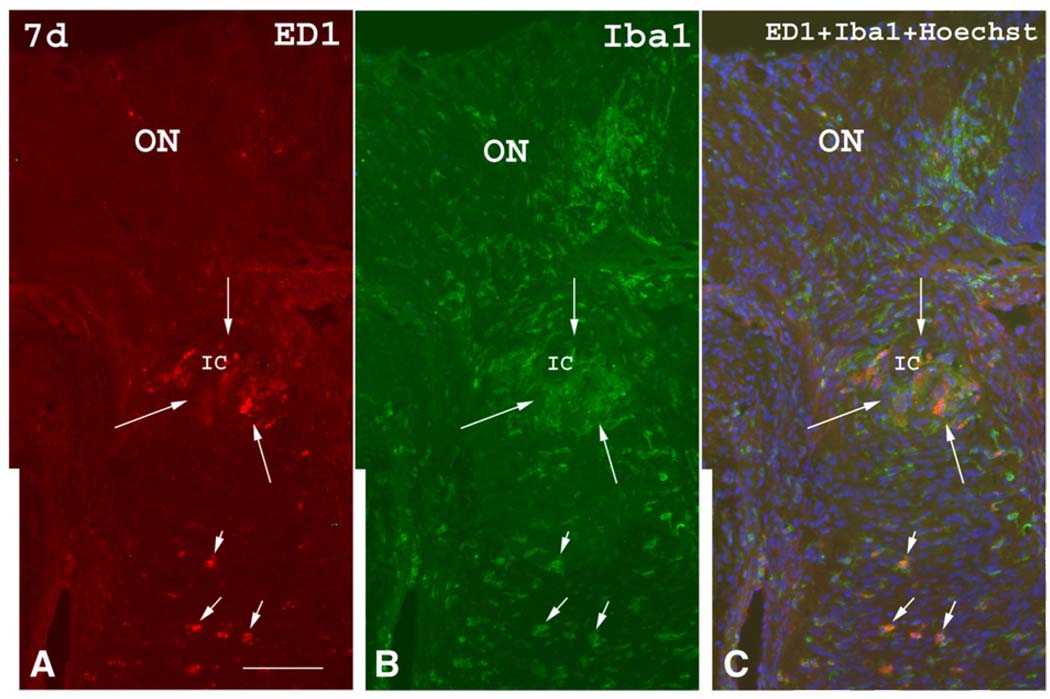
By seven days post-induction of rAION (Fig. 7), optic disc edema has resolved, and ED1(+) and Iba1(+) cell aggregates that represent the ischemic infarct in the intrascleral and immediate retroscleral portions of the ON, have become much smaller in size. While ED1(+) cells decrease in number by seven days, Iba1(+) cell numbers qualitatively increase in a more diffuse pattern. By fourteen days (not shown) post induction, aggregates of ED1(+) and Iba1(+) cells in the intrascleral and immediate retroscleral portions of the ON have largely disappeared, although some inflammatory cells remain scattered throughout these areas.
Fig. 7.
ED1 (red) and Iba1 (green) immunostaining seven days post-rAION induction. (A) ED1 immunostaining. The aggregates of ED1(+) cells previously present within the ischemic core (IC, between long arrows) have decreased in size although scattered ED1(+) cells are still present in the anterior and posterior ON (short arrows). (B) Iba1 immunostaining shows a similar pattern as ED1 immunostaining, but many more Iba1(+) cells are seen in the anterior and posterior ON. (C) Double labeling with ED1 and Iba1 antibodies shows that the ED1(+) cells co-localize with Iba1(+) cells (short arrows) in the posterior ON (orange color), whereas ED1(−)/Iba1(+) cells show the green color only. Bar=50 µm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3. Discussion
Our results show that soon after ON ischemia, there is a rapid and early cellular immune response that includes both an extrinsic (hematogenous) macrophage component and a microglial (intrinsic macrophage) component. These components are sequentially activated. The level of ON ischemic severity is directly related to the level of induction (compare Figs. 1B and C with the TTC staining of naive ON seen in Fig. 1A). We have previously determined that, using this model, the degree of RGC loss and axonal dropout is directly related to the degree of ON stroke induction (data not shown).
Optic disc swelling, indicative of axonal-ON edema, is an important component of human NAION (Knox et al., 2000). In this study, we also observed early tissue edema in the intrascleral portion of the ON beginning one day after the ischemic insult. In our rodent model, ON edema is maximal by the third day after induction of ischemia and resolves by the fifth day (Bernstein et al., 2003). This process is approximately seven times as rapid as that seen in humans, which usually requires about 4–6 weeks. Previous investigators have postulated that during human NAION, optic disc edema may result in compression and additional ON axonal compromise within the laminar portion of the ON. This compression potentially causes additional RGC loss and further loss of vision (Tesser et al., 2003). Our findings indicate that inflammatory processes are not only concurrent with early edema but persist after resolution of discernable edema.
The current study using our rAION model reveals that there is extrinsic inflammation by one day following ON stroke. This is demonstrable by a mild accumulation of ED1(+) extrinsic macrophages within the anterior portion of the ON and peripapillary choroid. These cells are recently generated blood-borne monocytes or macrophages of the same type that have also been shown to migrate into the CNS in the setting of both brain and retinal ischemia (Priller, 2003; Zhang et al., 2005). Macrophages are increased first in the peripapillary choroid and then intensely accumulate within the intrascleral and adjacent retroscleral portions of the ON by three days post-rAION induction.
While edema is a feature of blood–brain barrier (BBB) breakdown, other mechanisms also occur, including macrophage migration into the affected area. By three days post-ON infarct, BBB breakdown is associated with heavy infiltration of extrinsic (ED1[+]) macrophages within the intrascleral ON region and in the core of the ischemic lesion. Significant macrophage accumulation does not occur earlier than two days post-stroke (data not shown). There are fewer ED1(+) macrophages in ONs with a mild (8 s ) ischemic lesion, than that seen with a more severe (12 s) ischemic lesion. Therefore, the degree of extrinsic macrophage infiltration appears to be directly related to the degree of ON ischemia generated.
There are also fewer detectable extrinsic macrophages within the ischemic core region at times after seven days, suggesting that extrinsic macrophage migration represents a response to localized BBB breakdown in the region of the infarct. This finding is similar to that seen in other CNS ischemic models. For example, following middle cerebral artery (MCA) occlusion, ED1(+)/IBA1(+) cells appear in the ischemic core by one day post-insult and reach a peak by 4–7 days (Ito et al., 2001). These cells likely represent extrinsic recruited monocytes, since ED1(−)/Iba1(+)microglia are present in high numbers in the peri-ischemic (penumbral) area. Similarly, in a rat model of retinal ischemia induced by increased intraocular pressure, massive ED1(+) cellular infiltration occurs one day post-ischemia (Zhang et al., 2005) due to the breakdown of the blood–retinal barrier.
Human NAION most probably results from flow impairment to the anterior optic nerve (Arnold, 2003), which generates ON ischemia. A number of mechanisms have been proposed to cause this, including vascular auto-dysregulation (Arnold, 2003), compartmentation syndrome (Tesser et al., 2003), and embolism (Lieberman et al., 1978; Knox et al., 2000). In our rAION model, Swartz et al. (unpublished data) used intra-cardiac perfusion of fluorescent markers to show a massive loss of vascularity in the ON one day after stroke, with the area of ischemia extending into the intrascleral and adjacent retroscleral regions 500 µm distally from the primary site of damage in the anterior ON. Thus, the model associated, laser-induced injury likely occurs in the small vessels in the intrascleral and immediate retroscleral portions of the ON. During the early inflammatory response in the ON following ischemia, blood-borne ED1(+) macrophages may reach the anterior ON via the injured small branches, as well as the adjacent vascular network generated by the posterior ciliary artery (PCA) and central retinal artery (CRA) vessels. This network occurs at the level of the intrascleral region as well as the peripapillary (area surrounding the optic nerve) choroid. The damaged region in these clinical specimens is therefore similar in location to the infarct seen in our rodent model.
There is some evidence that cellular inflammation may also be a significant factor in clinical NAION (Knox et al., 2000). Although previous clinical specimens did not show the type of localized inflammatory response in the infarct region seen in our model, these specimens were obtained at later times following stroke occurrence. Thus, any localized response may be present earlier but subsequently masked by a more diffuse response. Indeed, in rAION the intense accumulation of macrophages within and adjacent to the area of the ischemic infarct occurring by three days post-induction largely resolves by 7–14 days. Thus, the macrophage accumulation appears to represent a delayed but still early and transient response to the ischemia.
Some Iba1(+) cells are present in control ONs, but these cells are more prominent in ONs one day post-induction, suggesting early microglial activation following ON ischemia. Unlike the rapid decrease in ED1(+) cells seven days post-rAION, there are qualitatively more ED1(−)/Iba1(+) cells in ONs from rAION eyes at 7–14 days compared with control ONs. These findings suggest continued long-term cellular immune activity following rAION induction. While a relatively rapid disappearance of ED1(+)cells can be interpreted to suggest that extrinsic macrophages may only briefly target infarcted ON tissue, extrinsic macrophages can also differentiate into long-term resident [ED1(−)] microglia having typical CNS morphology (Priller et al., 2001). Long-term residence within the CNS may down-regulate ED1 immunoactivity over time. In a previous study utilizing the mouse MCA model, animals receiving isogenic bone marrow transplantation from GFP-transgenic animals showed a significant increase in ED1(+) cells in the stroke region 1 day after ischemic insult (Priller et al., 2001; Furuya et al., 2003). On the fourteenth day after ischemic insult, these cells had differentiated into cells with a ramified morphology, consistent with microglia. These results suggest that by one day post-ischemia, blood-borne monocytes can infiltrate affected brain parenchymal tissue, and then can further differentiate into ramified microglial cells. In rAION, similar to many other CNS and ocular models of ischemia (Ito et al., 2001; Priller, 2003), there is therefore both early extrinsic macrophage recruitment and intrinsic macrophage/microglial activation following ON ischemia.
Our results demonstrate that, similar to other CNS ischemic infarcts, ON ischemia results in early recruitment of extrinsically recruited macrophages in the ischemic infarct core. Although the initiating insult is different in rAION than in human NAION, the gross appearance is identical to that seen in human NAION and the infarct location is histologically similar to the few reported cases of human NAION. These results suggest that rAION is an appropriate model for human NAION. Our finding of an inflammatory response within the ON shortly after production of ON ischemia is also likely to be applicable to the human condition. Post-ischemic inflammation with macrophage infiltration into the ON may have two opposing roles: Inflammation may cause increased tissue destruction, edema, and compression of adjacent vessels and axons, further compromising visual sensory function and reducing likelihood of significant visual recovery. Alternatively, extrinsic macrophages can enhance neuronal survival, play a major role in phagocytosis and remove myelin debris after ischemic axonal degeneration. This latter role can thus potentially enhance potential remyelination and subsequent axon regeneration. Therefore after ON ischemia, a number of types of inflammatory processes occur that may work at cross purposes. By selectively manipulating this inflammatory response, it may be possible to develop new treatment modalities for NAION and other ischemic optic nerve diseases.
4. Experimental procedures
4.1. Animal model
All animal experiments were conducted in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) revised 1996. Sprague–Dawley outbred rats (weight 120–150 g; Harlan Corp; Indianapolis, IN) were kept under a twelve hr light–dark cycle and allowed free access to food and water. Optic nerve ischemia was generated essentially as described previously, in deeply anesthetized animals (ketamine/xylazine, 80 mg/5 mg/kg) (Bernstein et al., 2003). Briefly, 0.15 ml intravenous Rose Bengal (RB) (2.5 mM) was injected via tail vein and RB was photoactivated in the vessels of the optic nerve using a 535 nm wavelength laser light with a 500 µm spot size for 12 s. This induction typically results in a loss of >60% of RGCs by 30 days post-induction (Bernstein et al., 2007). Three additional animals were induced for a shorter interval (8 s), to determine whether reduced severity of optic nerve stroke results in reduced ON ischemia and inflammatory cell infiltration, At 1 and 3 days post-induction and prior to euthanasia, rat retinae were evaluated clinically through pupils dilated with 1% tropicamide.
For in vivo ON ischemia analysis, animals were terminally anesthetized 1 day post-induction with 100 mg/kg Pentobarbital (Nembutal), and perfused with 4% 2,3,5-Triphenyl-2H-tetrazolium chloride (TTC; Sigma-Aldrich) in D-PBS (pH 7.4). Eyes were isolated, and ONs further incubated in 2% TTC in D-PBS at 37 °C and fixed in 4% PF-PBS. The intra-scleral portion of the optic nerve was isolated and photographed. Animals used for histology were euthanized at 1, 2, 3, 5, 7 and 14 days post-induction using CO2 inhalation. Four rats were used in each time point. The right eye of each animal received laser treatment; the left eye was used for a normal control.
4.2. Specimen preparation
Eyes used for histology were removed from freshly euthanized animals and fixed in freshly-prepared 4% paraformaldehyde in phosphate-buffered saline (PF-PBS) for 1 h. The anterior segments were then removed, with remaining posterior segments post-fixed in the same fixative for 12 additional hours, transferred to 20% sucrose for cryo-protection, and then embedded in Tissue-Tek OCT. (Sakura, Tokyo) within chilled methyl-butane in dry ice. The blocks were kept in −80 °C until sectioning. The posterior segments, including about 3 mm of the ON stump were serially sectioned at 10 µm thickness.
ED1(+) inflammatory cell counts were performed on 3–10 µm thick sections from groups of two animals induced at either 8 or 12 s. The center of each optic nerve sectioned longitudinally from retina to optic nerve, to include both the intra-retinal and 1 mm of intra-orbital optic nerve.
4.3. Immunohistochemistry
Sections were air-dried at room temperature for 10 min and successively incubated with 5% blocking serum in PBS for 30 min, followed by a primary antibody overnight at 4 °C, and, then a secondary fluorescent antibody conjugated with either Cy-2 or Cy-3 (Jackson ImmunoRes, West Grove, PA; 1:100) for 1 h at room temperature. The primary antibodies used were mouse monoclonal ED1 (Serotec, Raleigh, NC; 1:400), rabbit polyclonal Iba1 (Wako Chemicals USA, Inc., Richmond, VA; 1:800), and SMI312 mouse monoclonal antibody (Sternberg Monoclonals, Baltimore, MD; 1:500). ED1 antibody reacts against recently blood-borne (hematogenous) or extrinsic macrophages. Iba1 antibody recognizes the ionized calcium-binding receptor membrane protein specifically expressed in inflammatory cells, including macrophages/microglia (Ito et al., 2001). SMI312 antibody labels neurofilaments of the ON axons. For double-labeling, the sections were incubated with the two primary antibodies overnight and then two secondary fluorescent antibodies conjugated with either Cy-2 (green, donkey anti-rabbit; 1:300) or Cy-3 (red, donkey anti-mouse; 1:100) respectively. Slides were finally incubated with Hoechst nuclear stain (Molecular Probe, Eugene, OR; 1:2000) for 30 s and mounted with Gel Mount Aqueous Mounting Medium (Sigma, St. Louis). Sections were visualized with a fluorescent digital microscope (Zeiss Axioscop).
Acknowledgments
The authors wish to thank Mr. Bernard Slater, UMAB Department of Ophthalmology, for providing the stereological quantification of inflammatory cell numbers. Grant Support: The Donegan Fund for Ischemic Optic Neuropathy Research (NRM); NIH grant EY-015304 (SLB), and an unrestricted grant by Research to Prevent Blindness (RPB) to the Department of Ophthalmology and Visual Sciences of the University of Maryland at Baltimore.
Abbreviations
- NAION
nonarteritic anterior ischemic optic neuropathy
- rAION
rodent Anterior ischemic optic neuropathy
- ON
optic nerve
- RGC
retinal ganglion cell
- TTC
Triphenyl-tetrazolium chloride
REFERENCES
- Ahmed ZZ, Shaw G, Sharma VP, Yang C, McGowan E, Dickson DW. Actin-binding proteins coronin-1a and IBA-1 are effective microglial markers for immunohistochemistry. J. Histochem. Cytochem. 2007;55:687–700. doi: 10.1369/jhc.6A7156.2007. [DOI] [PubMed] [Google Scholar]
- Arnold AC. Anterior ischemic optic neuropathy. Semin. Ophthalmol. 1995;10:221–233. doi: 10.3109/08820539509060976. [DOI] [PubMed] [Google Scholar]
- Arnold AC. Pathogenesis of nonarteritic anterior ischemic optic neuropathy. J. Neuro-Ophthalmol. 2003;23:157–163. doi: 10.1097/00041327-200306000-00012. [DOI] [PubMed] [Google Scholar]
- Bernstein SL, Guo Y, Kelman SE, Flower RW, Johnson MA. Functional and cellular responses in a novel rodent model of anterior ischemic optic neuropathy. Investig. Ophthalmol. Vis. Sci. 2003;44:4153–4162. doi: 10.1167/iovs.03-0274. [DOI] [PubMed] [Google Scholar]
- Bernstein SL, Mehrabian Z, Guo Y, Moianie N. Estrogen is not neuroprotective in a rodent model of optic nerve stroke. Mol. Vis. 2007;13:1920–1925. [PMC free article] [PubMed] [Google Scholar]
- Castellanos M, Castillo J, Garcia MM, Leira R, Serena J, Chamorro A, Davalos A. Inflammation-mediated damage in progressing lacunar infarctions: a potential therapeutic target. Stroke. 2002 Apr.33:982–987. doi: 10.1161/hs0402.105339. 2002. [DOI] [PubMed] [Google Scholar]
- David S, Ousman SS. Recruiting the immune response to promote axon regeneration in the injured spinal cord. Neuroscientist. 2002;8:33–41. doi: 10.1177/107385840200800108. [DOI] [PubMed] [Google Scholar]
- Filbin MT. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat. Rev. Neurosci. 2003;4:703–713. doi: 10.1038/nrn1195. [DOI] [PubMed] [Google Scholar]
- Fry EJ, Ho C, David S. A role for Nogo receptor in macrophage clearance from injured peripheral nerve. Neuron. 2007;53:649–662. doi: 10.1016/j.neuron.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Furuya T, Tanaka R, Urabe T, Hayakawa J, Migita M, Shimada T, Mizuno Y, Mochizuki H. Establishment of modified chimeric mice using GFP bone marrow as a model for neurological disorders. NeuroReport. 2003;14:629–631. doi: 10.1097/00001756-200303240-00021. [DOI] [PubMed] [Google Scholar]
- Hattenhauer MG, Leavitt JA, Hodge DO, Grill R, Gray DT. Incidence of nonarteritic anterior ischemic optic neuropathy. Am. J. Ophthalmol. 1997;123:103–107. doi: 10.1016/s0002-9394(14)70999-7. [DOI] [PubMed] [Google Scholar]
- He XL, Bazan JF, McDermott G, Park JB, Wang K, Tessier-Lavigne M, He Z, Garcia KC. Structure of the Nogo receptor ectodomain: a recognition module implicated in myelin inhibition. Neuron. 2003;38:177–185. doi: 10.1016/s0896-6273(03)00232-0. [DOI] [PubMed] [Google Scholar]
- IONDT study group. Ischemic optic neuropathy decompression trial: twenty-four-month update. Arch. Ophthalmol. 2000;118:793–798. [PubMed] [Google Scholar]
- Ito D, Tanaka K, Suzuki S, Dembo T, Fukuuchi Y. Enhanced expression of Iba1, ionized calcium-binding adapter molecule 1, after transient focal cerebral ischemia in rat brain. Stroke. 2001;32:1208–1215. doi: 10.1161/01.str.32.5.1208. [DOI] [PubMed] [Google Scholar]
- Knox DL, Kerrison JB, Green WR. Histopathologic studies of ischemic optic neuropathy. Trans. Am. Ophthalmol. Soc. 2000;98:202–203. [PMC free article] [PubMed] [Google Scholar]
- Levin LA, Louhab A. Apoptosis of retinal ganglion cells in anterior ischemic optic neuropathy. Arch. Ophthalmol. 1996:488–491. doi: 10.1001/archopht.1996.01100130484027. [DOI] [PubMed] [Google Scholar]
- Lieberman MF, Shahi A, Green WR. Embolic ischemic optic neuropathy. Am. J. Ophthalmol. 1978;86:206–210. doi: 10.1016/s0002-9394(14)76813-8. [DOI] [PubMed] [Google Scholar]
- Miller N. Anterior ischemic optic neuropathy. In: Miller NR, editor. Walsh and Hoyts Neuro-ophthalmology, 1. Baltimore: Williams and Wilkins; 1982. pp. 212–226. [Google Scholar]
- Priller J. Robert feulgen prize lecture. Grenzganger: adult bone marrow cells populate the brain. Histochem. Cell Biol. 2003;120:85–91. doi: 10.1007/s00418-003-0559-7. [DOI] [PubMed] [Google Scholar]
- Priller J, Flugel A, Wehner T, Boentert M, Haas CA, Prinz M, Fernandez-Klett F, Prass K, Bechmann I, de Boer BA, Frotscher M, Kreutzberg GW, Persons DA, Dirnagl U. Targeting gene-modified hematopoietic cells to the central nervous system: use of green fluorescent protein uncovers microglial engraftment. Nat. Med. 2001;7:1356–1361. doi: 10.1038/nm1201-1356. [DOI] [PubMed] [Google Scholar]
- Schori H, Kipnis J, Yoles E, Wolde Mussie E, Ruiz G, Wheeler LA, Schwartz M. Vaccination for protection of retinal ganglion cells against death from glutamate cytotoxicity and ocular hypertension: implications for glaucoma. Proc. Natl. Acad. Sci. U. S. A. 2001;98:3398–3403. doi: 10.1073/pnas.041609498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraju R, Bernasconi L, Losberger C, Graber P, Kadi L, Avellana-Adalid V, Picard-Riera N, Van Evercooren AB, Cirillo R, Kosco-Vilbois M, Feger G, Papoian R, Boschert U. Osteopontin is upregulated during in vivo demyelination and remyelination and enhances myelin formation in vitro. Mol. Cell Neurosci. 2004;25:707–721. doi: 10.1016/j.mcn.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Slater BJ, Mehrabian Z, Guo Y, Hunter A, Bernstein SL. Rodent anterior ischemic optic neuropathy (rAION) induces regional retinal ganglion cell apoptosis with a unique temporal pattern. Investig. Ophthalmol. Vis. Sci. 2008;49:3671–3676. doi: 10.1167/iovs.07-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesser RA, Niendorf ER, Levin LA. Themorphology of an infarct in nonarteritic anterior ischemic optic neuropathy. Ophthalmology. 2003;110:2031–2035. doi: 10.1016/S0161-6420(03)00804-2. [DOI] [PubMed] [Google Scholar]
- Yin Y, Henzl MT, Lorber B, Nakazawa T, Thomas TT, Jiang F, Langer R, Benowitz LI. Oncomodulin is a macrophage-derived signal for axon regeneration in retinal ganglion cells. Nat. Neurosci. 2006;9:843–852. doi: 10.1038/nn1701. [DOI] [PubMed] [Google Scholar]
- Zhang C, Lam TT, Tso MO. Heterogeneous populations of microglia/macrophages in the retina and their activation after retinal ischemia and reperfusion injury. Exp. Eye Res. 2005;81:700–709. doi: 10.1016/j.exer.2005.04.008. [DOI] [PubMed] [Google Scholar]