Abstract
Rationale
Apoptosis signal-regulating kinase 1 (ASK1) is a central upstream kinase in the greater mitogen-activated protein kinase (MAPK) cascade that mediates growth and death decisions in cardiac myocytes in response to diverse pathologic stimuli.
Objective
However, the role that ASK1 plays in regulating the cardiac hypertrophic response in vivo remains controversial.
Methods and Results
Here we generated mice with cardiac-specific and inducible overexpression of ASK1 in the heart to assess its gain-of-function effect. ASK1 transgenic mice exhibited no induction of cardiac hypertrophy or pathology at 3 and 12 months of age, and these mice showed an identical hypertrophic response to controls following 2 weeks of pressure overload stimulation or isoproterenol infusion. While ASK1 overexpression did not alter the cardiac hypertrophic response, it promoted cardiomyopathy and greater TUNEL following pressure overload stimulation and myocardial infarction. Indeed, ASK1 transgenic mice showed a greater than 2-fold increase in ischemia reperfusion-induced injury to the heart compared with controls. Examination of downstream signaling showed a prominent activation of MKK4/6 and JNK1/2 (but not p38 or extracellular signal-regulated kinases (ERKs)), inhibition of calcineurin-nuclear factor of activated T-cells (NFAT), and induction of Bax in the hearts of ASK1 transgenic mice following 1 and 8 weeks of pressure overload stimulation. Mechanistically, cardiomyopathy associated with ASK1 overexpression after 8 weeks of pressure overload was significantly reduced in the calcineurin Aβ−/− background.
Conclusions
These results indicate that ASK1 does not directly regulate the cardiac hypertrophic response in vivo, but it does alter cell death and propensity to cardiomyopathy, in part, through a calcineurin-dependent mechanism.
Keywords: myocardial infarction, apoptosis, hypertrophy, MAPK, signaling
Introduction
Cardiac hypertrophy is typically characterized by enlargement of the heart and an increase in myocyte cell volume that occurs in response to diverse pathophysiologic stimuli such as hypertension, ischemic heart disease, valvular insufficiency, infectious agents, exercise, or mutations in sarcomeric genes.1 Pathologic hypertrophy of the myocardium temporarily preserves pump function, although prolongation of this state is a leading predictor for the development of arrhythmias and sudden death, as well as dilated cardiomyopathy and heart failure.2,3 The hypertrophic growth of the myocardium is typically initiated by neuro-endocrine factors and membrane-bound receptors that integrate their responses through nodal intracellular signal transduction pathways such as the mitogen-activated protein kinase (MAPK) cascade, calcineurin-nuclear factor of activated T cells (NFAT), insulin-like growth factor-I (IGF-I)-phosphatidylinositol 3-kinase (PI3K)-Akt/protein kinase B (PKB), and many others.4
MAPK signaling is comprised of a series of successively acting kinases that function as central regulators of cell growth, differentiation, transformation, and death.5,6 MAPK signaling pathways culminate in the activation of extracellular signal-regulated protein kinases 1/2 (ERK1/2), the c-Jun NH2-terminal kinases (JNK), p38, and ERK5.5,6 ERK, p38 and JNK are each directly activated by a family of dual-specificity kinases referred to as MAPK kinases, which themselves are activated by specific upstream kinases referred to as MAPK kinase kinases. Apoptosis signal-regulating kinase 1 (ASK1) is a MAPK kinase kinase that is activated by oxidative stress resulting in MKK3/6 and MKK4/7 activation, which in turn mediates p38 and JNK activation, respectively.7
ASK1 is unique among the MAPK kinase kinases in that it appears to directly regulate cell death through p38, JNK, and other effectors.7 For example, expression of a constitutively active mutant of ASK1 in a wide variety of celltypes induced apoptosis, while expression of dominant negative ASK1 blocked tumor necrosis factor-α- (TNF-α)-, oxidative stress-, anti-cancer agent-, and growth factor withdrawal-induced cell death.8–13 Mouse embryonic fibroblasts generated from Ask1−/ − mice were also resistant to TNF-α- and H2O2-induced cell death.14 In the heart, ASK1 has been implicated as a mediator of cellular remodeling and apoptotic and necrotic cell death. Indeed, Ask1−/− mice exhibited reduced ventricular remodeling in response to angiotensin II infusion, myocardial infarction (MI), and pressure overload stimulation.15,16 Cardiomyocytes generated from Ask1−/− mice were also resistant to H2O2-induced apoptosis.16 In vivo, Ask1−/− mice showed less apoptotic and necrotic cell death following ischemia-reperfusion (I/R) injury to the heart.17
While ASK1 is prominently activated by hypertrophic stimuli in vitro and in vivo, such as pressure overload, I/R, and agonist treatments,16–18 its mechanistic involvement in directing the cardiac growth program has become an area of controversy in the literature. For example, Ask1−/− mice showed reduced hypertrophy following angiotensin II infusion, suggesting that ASK1 positively regulates cardiac growth,15 while studies in cultured neonatal myocytes with activated and dominant negative ASK1 suggested a growth inhibitory effect.19 c-Raf-1 null mice showed abundant ASK1 activity in the heart without corresponding hypertrophy,20 and pressure overload induced by transverse aortic constriction (TAC) did not result in less cellular hypertrophy in Ask−/− mice compared with wildtype (WT) mice.16 More recently, Ask1−/− mice were reported to develop more cardiac hypertrophy following 4 weeks of swimming exercise, suggesting that it is normally anti-hypertrophic to this form of physiologic stimulation.21 Thus, the role of ASK1 as a hypertrophic regulator is far from settled, despite its prominent effect in controlling p38 and JNK signaling in the heart.
Materials and Methods
Animals
A tetracycline-responsive binary α-myosin heavy chain (α-MHC) transgene system permitted temporally regulated expression of ASK1 in myocytes of the heart.22 TAC was performed as previously described.23 Transthoracic echocardiography to measure cardiac dimensions and pressure gradients across the aortic constriction was performed as described previously.24 Azlet 1002 osmotic minipumps (Cupertino, CA) either filled with isoproterenol(60 mg/kg/day in phosphate-buffered saline) or phosphate-buffered saline were implanted under the skin for 2 weeks with a routine surgical procedure. The surgical procedure for I/R or MI injury in the mouse and analysis of injury area were described previously.25 Luciferase assays from NFAT-luciferase reporter mice were performed as described previously.23 Calcineurin Aβ−/− mice were described previously.26
Histological Analysis, TUNEL, Western Blotting, and Kinase Assays
Assessment of TUNEL from paraffin sections was performed with TMR Red In Situ Death Detection Kit (Roche Diagnostics)according to the manufacturer’s instructions (Roche Diagnostics).24 Protein extraction from mouse heart and subsequent Western blotting followed by enhanced chemiluminescence detection was performed as previously described.23,25 ASK1 activity assays with recombinant MKK6 was described previously.19
An expanded Materials and Methods section can be found in the online data supplement at http://circres.ahajournals.org
Results
Generation of ASK1 inducible transgenic mice
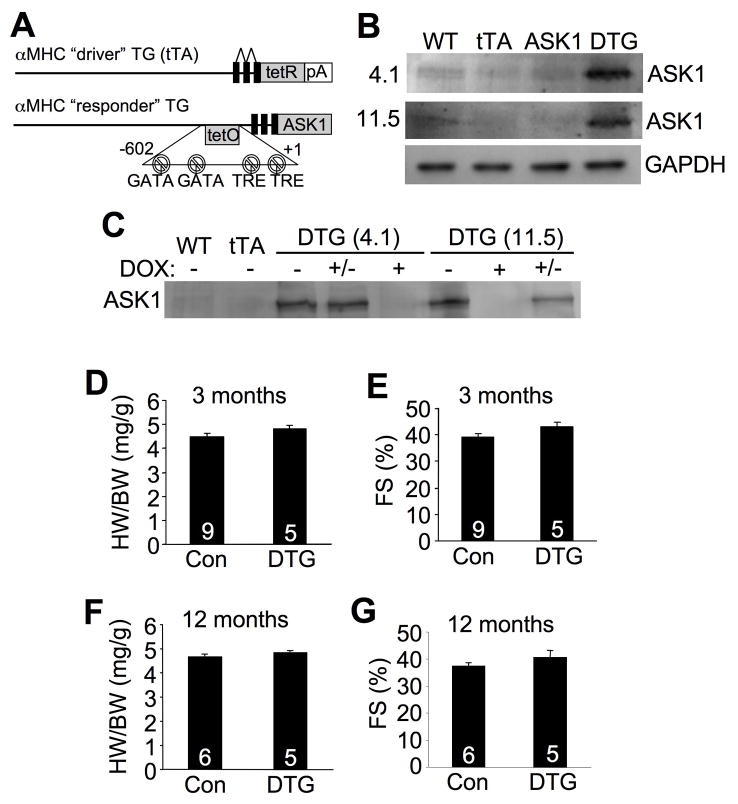
To further examine the function of ASK1 in vivo we generated inducible, cardiac-specific transgenic mice expressing mouse ASK1. Heart-specific and inducible expression was achieved with a binary αMHC promoter-based transgene strategy (Figure 1A).22 Two independent responder transgenic lines were generated (4.1 and 11.5) that each permitted expression of ASK1 in the heart only in the presence of the driver transgene encoding the tetracycline transactivator (tTA) protein (double transgenic, DTG) when Dox is absent (Figure 1B). Administration of Dox completely eliminated expression of ASK1 protein in DTG mice in both lines (Figure 1C). Most subsequent experiments were performed with Dox administration for the first 3 weeks of life, which blocks developmental expression, followed by removal of Dox thereafter to selectively permit transgene expression beginning only in young adulthood and thereafter (Figure 1C). Despite relatively high levels of ASK1 protein overexpression in the adult heart, no hypertrophy or reductions in cardiac ventricular performance were noted at 3 and 12 months of age (Figure 1D,E,F,G), nor were signs of histopathology observed (data not shown). These results indicate that ASK1 overexpression in the heart is without appreciable effect.
Figure 1.
Characterization of cardiac-specific ASK1-inducible transgenic mice. A, Schematic of the bitransgenic inducible expression system used to regulate ASK1 expression in the mouse heart. B, Western blot for ASK1 expression at 3 months of age in DTG mice (off Dox) from 2 different lines (4.1 and 11.5). C, Western blot for ASK1 protein in the hearts of the indicated mice on Dox (+), off Dox (−), or breeding on Dox until weaning, then off (+/−). D and E, Heart weight/body weight ratio (HW/BW) and echocardiographic analysis of ventricular fractional shortening (FS) in ASK1 transgenic line 4.1 at 3 months of age. F and G, HW/BW and FS in ASK1 transgenic line 4.1 at 12 months of age. The number of mice analyzed is shown in the bars of each panel.
ASK1 overexpression does not enhance stimuli-induced hypertrophy
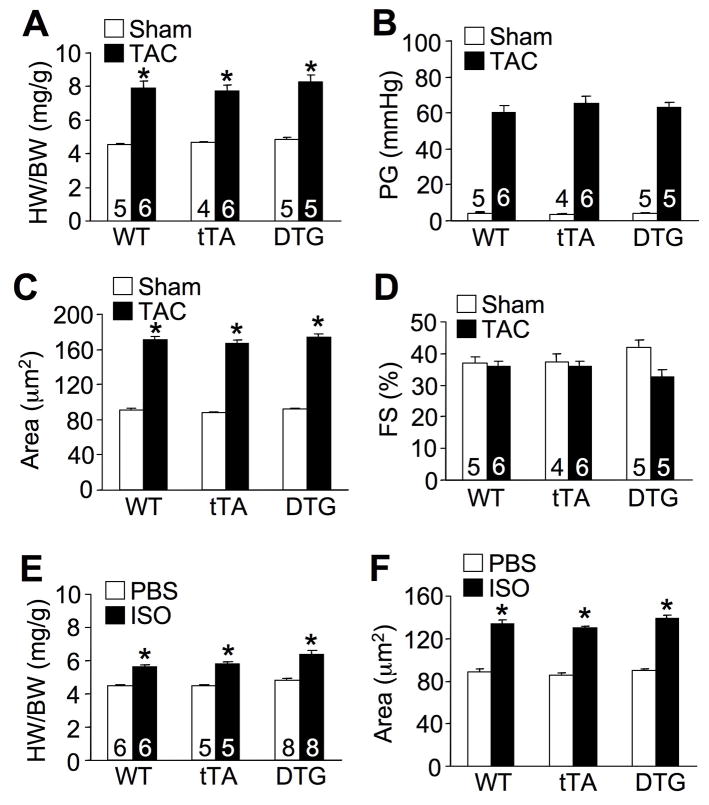
ASK1 is not normally activated in unstimulated hearts, hence overexpression of this protein might require co-stimulation to achieve activity given exquisite silencing by co-regulatory proteins in vivo. Since ASK1 is strongly induced by pressure overload,16 we predicted that TAC stimulation would unmask silencing of ASK1, permitting alteration of the hypertrophic response due to its overexpression. However, 2 weeks of TAC stimulation in ASK1 DTG mice at 12 weeks of age showed an identical hypertrophic response to WT or tTA control mice, with equivalent pressure gradients across the aortic constriction (Figure 2A,B). Measurement of cell surface area also showed no difference in hypertrophy between the 3 groups of mice, nor was ventricular performance significantly altered after 2 weeks of TAC stimulation (Figure 2C,D). Isoproterenol was used as another hypertrophic stimuli to extend and confirm the pressure overload results. Once again, ASK1 DTG mice showed no difference in cardiac hypertrophic growth at the whole organ or cellular level compared with WT or tTA control mice (Figure 2E,F). Thus, greater ASK1 expression did not alter the baseline growth of the heart or significantly alter stimulus-induced hypertrophy.
Figure 2.
Examination of the cardiac hypertrophic response in ASK1 TG mice following stimulation. A, Quantitation of HW/BW in 3 month-old Wt, tTA, or DTG mice subjected to TAC or sham procedure for 2 weeks. Number of mice analyzed is shown in the bars. *P< 0.05 versus respective sham group. B, Systolic pressure gradient (PG) across the aortic constriction. C, Myocyte surface area from heart histological sections of the mice shown in A. Surface areas of 500 cells per mouse were measured in random fields in 3 mice per group. *P< 0.05 versus sham. D, Fractional shortening (FS) of the mice shown in A. E, Quantitation of HW/BW in 3 month-old WT, tTA, or DTG mice implanted with PBS or isoproterenol (ISO) containing Alzet mini-pumps (60 mg/kg/day) for 2 weeks. *P< 0.05 versus PBS. F, Myocyte surface area from histological sections of the mice shown in E. Surface areas of 500 cells per mouse were measured in random fields in 3 mice per group. *P < 0.05 versus PBS.
ASK1 DTG mice are more susceptible to ischemic injury
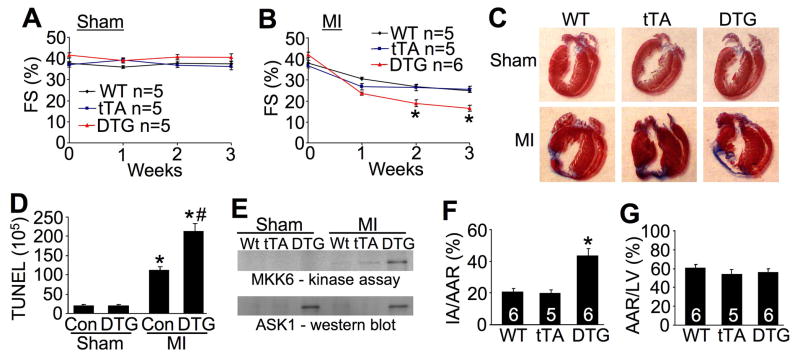
While ASK1 overexpression had no effect on the hypertrophic response, it did alter myocyte death following ischemic injury, indicating that the transgene is functional and that ASK1 can have an important biologic effect in the heart. Specifically, ASK1 DTG mice showed worse ventricular performance 2 and 3 weeks after MI compared with WT and tTA control groups, although sham mice were unaffected (Figure 3A,B). This greater reduction in ventricular performance in ASK1 DTG mice was associated with more expansive and thinner scars in the left ventricle as assessed from histological sections (Figure 3C). More importantly, careful quantitation of TUNEL in the peri-infarct region from histological sections 21 days after MI showed nearly a 2-fold increase in DTG mice versus control mice (mix of WT and tTA) (Figure 3D). At this same 21-day time point DTG mice showed a much greater induction of ASK1 activity as measured with a direct kinase assay in vitro (Figure 3E). The MI model was performed by permanent ligation of the left coronary artery to analyze secondary remodeling due to enhanced ASK1 activity over extended periods of time. We also performed an acute cell death model involving I/R injury by coronary artery occlusion for 60 minutes followed by 24 hrs of reperfusion. ASK1 DTG mice showed a greater than 2-fold increase in total area of infarction in this acute assay, while the area at risk was not different from WT or tTA controls (Figure 3F,G). Taken together, these results indicate that ASK1 can directly enhance myocyte death following MI or I/R injury.
Figure 3.
ASK1 overexpression alters apoptosis and cardiac compensation following MI. A and B, Echocardiographic analysis of FS in WT, tTA, or DTG mice subjected to sham or MI surgical procedure for 3 weeks. *P< 0.01 versus WT or tTA MI. C, Masson’s trichrome-stained histological sections of hearts from WT, tTA, or DTG mice subjected to MI or a sham procedure for 3 weeks. The scar area is in blue. D, Immunohistochemical assessment of TUNEL-positive nuclei in the hearts of control (WT or tTA) or DTG mice as shown in C. *P< 0.05 versus respective sham (at least 100,000 nuclei were counted). #P< 0.05 versus Con MI. E, Assessment of ASK1 kinase activity in vitro with recombinant MKK6 protein with immunoprecipitation of ASK1 from heart extracts of the indicated groups. The ASK1 DTG mice after MI show increase activity in the heart. F, Assessment of infarct area normalized to area at risk (IA/AAR) in the hearts of WT, tTA, or DTG mice after 60 min of ischemia followed by 24 h of reperfusion. *P< 0.05 versus WT or tTA. G, Assessment of area at risk (AAR) normalized to the perfused area of the left ventricle (LV) in WT, tTA, or DTG mice. Number of mice analyzed is shown in the bars of panels E and F.
ASK1 DTG mice exhibit cardiomyopathy with long-term pressure overload
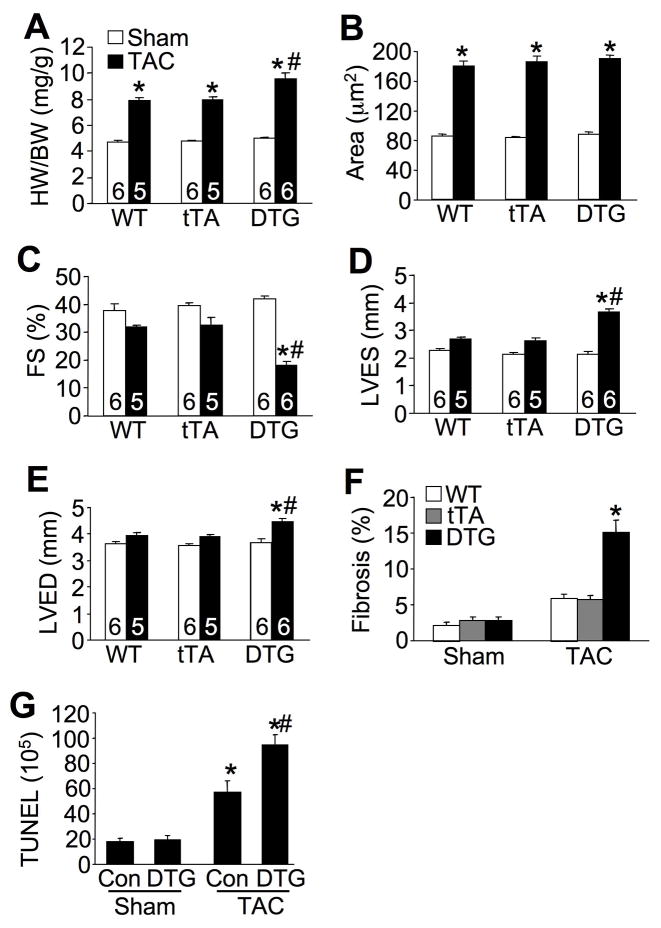
While ventricular performance in ASK1 DTG mice was unaffected after 2 weeks of TAC stimulation, 8 weeks of TAC uncovered a greater propensity towards heart failure and cell death. ASK1 DTG mice showed significantly greater increases in heart weight normalized to body weight compared with WT and tTA mice after 8 weeks of TAC (Figure 4A). However, careful assessment of cardiomyocyte cross-sectional surface areas showed no greater cellular hypertrophy compared with controls, suggesting that the greater increase in heart weight was due to dilation and failure (Figure 4B). Consistent with this interpretation, ASK1 DTG mice exhibited a significant reduction in ventricular fractional shortening and chamber dilation in systole and diastole (Figure 4C,D,E). Hearts from ASK1 DTG mice also developed significant fibrosis assessed by Masson’s trichrome staining of histological sections, which was not significantly increased in hearts from WT and tTA mice (Figure 4F). This increase in fibrosis was also associated with significantly greater levels of TUNEL after TAC stimulation in hearts of DTG mice (Figure 4G). Thus, increased ASK1 expression predisposed the heart to cardiomyopathy associated with greater levels of TUNEL and fibrosis after 8 weeks of pressure overload stimulation, but not more cellular hypertrophy.
Figure 4.
Enhanced cardiac decompensation in ASK1 TG mice after long-term TAC. A, Quantitation of HW/BW in 3 month-old WT, tTA, or DTG mice subjected to TAC or a sham procedure for 8 weeks. *P< 0.05 versus respective sham group. #P< 0.05 versus WT or tTA TAC. B, Myocyte cross-sectional areas of the mice as shown in A. Surface areas of 500 cells per mouse were measured in random fields in 3 mice per group. *P< 0.05 versus respective sham. C, D, and E, Echocardiographic analysis of fractional shortening (FS), left ventricular diastolic dimension (LVED), and left ventricular systolic dimension (LVES) in the indicated groups of mice subjected to TAC or a sham procedure for 8 weeks. *P< 0.05 versus sham; #P< 0.05 versus WT or tTA TAC. F, Quantitation of cardiac fibrotic area after 8 weeks of TAC or a sham procedure in WT, tTA, or DTG mice. *P< 0.05 versus WT or tTA TAC. G, Measurement of TUNEL positive nuclei in cardiac histological sections from the indicated mice subjected to TAC or a sham surgical procedure for 8 weeks. *P< 0.05 versus respective sham; #P< 0.05 versus Con TAC. Number of mice analyzed is shown in the bars of each panel.
ASK1 regulates JNK and calcineurin signaling in the heart
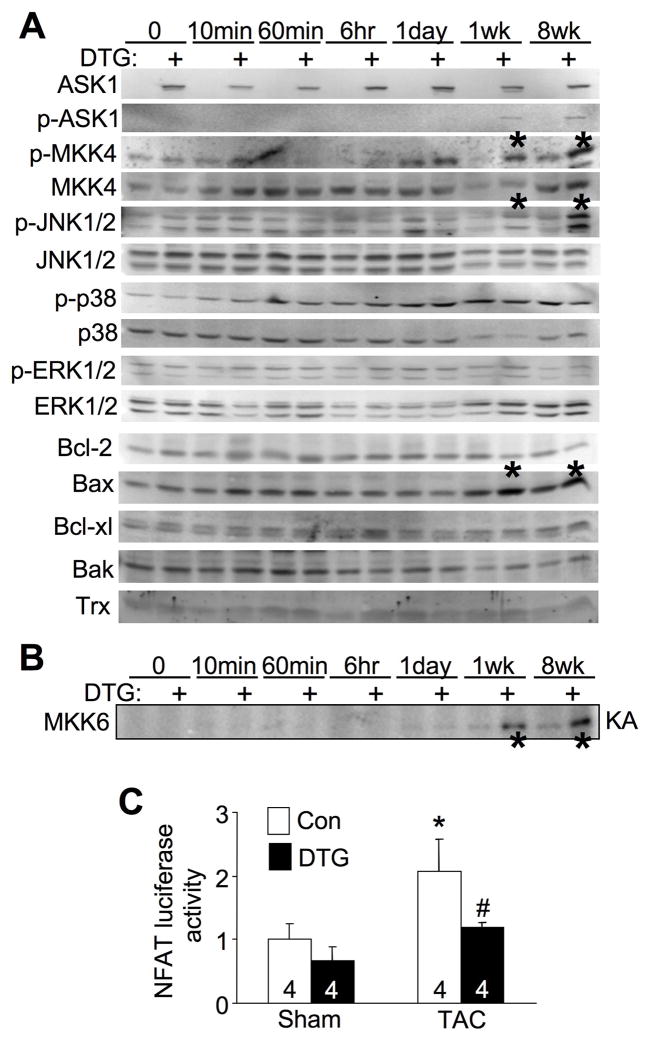
In addition to regulating JNK and p38, we previously demonstrated that ASK1 could alter calcineurin-NFAT activation in cardiomyocytes.16,19,27 Thus, it was critical to investigate what signaling effectors might be altered by ASK1 overexpression in the heart to gain insight into its mechanism of action. Consistent with the lack of a baseline phenotype, ASK1 overexpression in unstimulated hearts had no effect on activation of any MAPKK or MAPK factor at baseline, nor was calcineurin activity altered in NFAT-luciferase transgenic mice crossed to contain the ASK1 inducible transgenes (Figure 5A,C). We also performed direct ASK1 kinase assays (KA) with MKK6 protein as an in vitro substrate, and once again ASK1 overexpression did not induce activity at baseline (Figure 5B). However, TAC stimulation showed substantial induction of ASK1 kinase activity at 1 and 8 weeks, and much greater phosphorylation of MKK4 and JNK1/2 in the hearts of ASK1 DTG mice compared with controls (Figure 5A,B). No activation was observed at 10 minutes, 60 minutes, 6 hrs, or 1 day of TAC, nor was p38 or ERK1/2 appreciably activated by ASK1 overexpression at any time point investigated (Figure 5A). Proteins that alter the cellular apoptotic response were also investigated, revealing a significant increase in Bax protein levels in ASK1 DTG mice at 1 and 8 weeks of TAC, but no alteration in Bak, Bcl-2 or Bcl-xl (Figure 5A). Thioredoxin protein levels were not changed by ASK1 overexpression at baseline or after TAC (Figure 5A). However, ASK1 overexpression did significantly reduced the increase in NFAT-luciferase activity following TAC stimulation (Figure 5C), consistent with our previous observations that ASK1 can antagonize calcineurin-NFAT signaling in cardiomyocytes.19 Thus, ASK1 overexpression selectively increases MKK4/6 and JNK1/2 activity in the heart after at least 1 week of TAC stimulation, as well as antagonizes calcineurin-NFAT signaling and leads to increased Bax protein levels.
Figure 5.
Enhanced JNK signaling and reduced NFAT transcriptional activity in ASK1 transgenic mice following pressure overload. A, Western blot assessment of MAPK phosphorylation and apoptotic regulatory proteins from hearts at baseline or after TAC for the indicated times in control and DTG mice. The entire time course was repeated in separate mice with similar results. The asterisks show increased MKK4 and JNK1/2 phosphorylation. B, An autoradiogram of an ASK1 kinase assay (KA) for MKK6 phosphorylation in vitro from heart protein extracts from the indicated mice. C, Measurement of NFAT luciferase activity from control and DTG mice containing the NFAT luciferase reporter transgene after 2 weeks of TAC or a sham operation. *P< 0.05 versus sham; #P< 0.05 versus Con TAC.
ASK1 functions, in part, through calcineurin in the heart
Calcineurin is a Ca2+-activated protein phosphatase that directly dephosphorylates members of the NFAT transcription factor family in the cytoplasm, promoting their translocation into the nucleus where they participate in the transcriptional induction of various genes with specific inducible functions.28 The calcineurin-NFAT signaling circuit has been shown to play a central role in regulating the hypertrophic growth response and survival versus apoptotic decisions of cardiomyocytes.29 We previously demonstrated that calcineurin is required to activate ASK1 in cultured cardiomyocytes by dephosphorylation of serine 967, leading to disassociation of 14-3-3 proteins.19
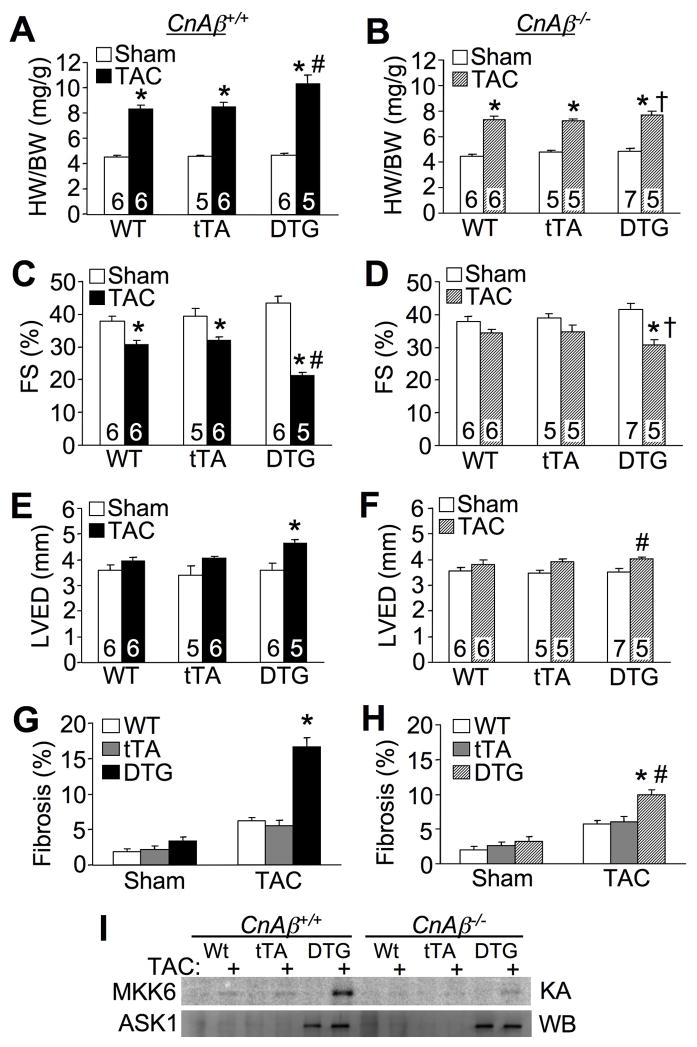
Here we crossed the ASK1 inducible transgenes into the CnAβ−/− background to examine the hypothesis that reductions in calcineurin activity would render ASK1 less functional in overexpressing DTG mice. Indeed, when crossed into the CnAβ−/− background, ASK1 overexpression no longer promoted greater increases in heart weights normalized to body weight after 8 weeks of TAC stimulation, while DTG control mice from the same cross that had 2 WT CnAβ alleles showed proportionately greater increases in heart-weight normalized to body-weight (Figure 6A,B). Deletion of CnAβ also partially protected ASK1 DTG mice from loss of ventricular performance and chamber dilation compared with DTG mice in the CnAβ+/+ background (Figure 6C,D,E,F). The increase in myocardial fibrosis following 8 weeks of TAC in ASK1 DTG mice was also significantly less in the CnAβ−/− background (Figure 6G,H). Finally, we also measured ASK1 kinase activity in hearts, which again showed increases only in ASK1 DTG mice after TAC stimulation in the CnAβ+/+ background, and a substantial reduction in the CnAβ−/− background (Figure 6I). The results suggest that calcineurin participates in controlling the effectiveness of ASK1 signaling in vivo, consistent with our previous in vitro results.19
Figure 6.
Loss of calcineurin Aβ reduced heart failure propensity in ASK1 DTG mice after long-term TAC. A and B, Assessment of HW/BW from the indicated mice in the CnAβ+/+ or CnAβ−/− background subjected to TAC or sham procedure for 8 weeks. *P< 0.05 versus respective sham group. #P< 0.05 versus WT or tTA TAC. †P< 0.05 versus CnAβ+/+ DTG TAC. C and D, Echocardiographic analysis of FS from the indicated mice in the CnAβ+/+ (C) or CnAβ−/− (D) background subjected to TAC or sham procedure for 8 weeks. *P< 0.05 versus respective sham group. #P< 0.05 versus WT or tTA TAC. †P< 0.05 versus CnAβ+/+ DTG TAC. E and F, Echocardiographic analysis of left ventricular diastolic dimension (LVED) from the indicated mice in the CnAβ+/+ (E) or CnAβ−/− (F) background subjected to TAC or a sham procedure for 8 weeks. *P< 0.05 versus sham. #P< 0.05 versus CnAβ+/+ DTG TAC. G and H, Histological assessment of fibrosis from indicated mice in the CnAβ+/+ (G) or CnAβ−/− (H) background subjected to TAC or a sham procedure for 8 weeks. Multiple sections from at least 3 mice were analyzed per group. *P< 0.05 versus WT or tTA TAC. #P< 0.05 versus CnAβ+/+ DTG TAC. I, Autoradiogram of an ASK1 kinase assay (KA) from hearts of the indicated mice, and a western blot (WB) for total ASK1 protein. Number of mice analyzed is shown in the bars of each panel.
Discussion
ASK1 has emerged as a kinase of central importance in cardiac myocytes given its dominant role in regulating MAPK signaling and subsequent control of cell death. To gain additional insight into the full range of ASK1 function in the heart we generated inducible transgenic mice to overexpress this protein in the adult heart. While overexpression approaches in the mouse heart can produce non-physiologic effects, numerous kinases and phosphatases have been overexpressed as a means of gaining functional insight. For example, overexpression of activated calcineurin or activated MEK1 in the mouse heart provided important mechanistic data regarding the function of these signaling factors in regulating the cardiac hypertrophic response.29,30 Also to be considered, not all overexpressed signaling proteins in the heart produce a phenotype, as ERK2 overexpression with the αMHC promoter was without effect, nor did it enhance pressure overload hypertrophy.30 In a similar manner, ASK1 transgenic mice had no discernable phenotype up to 12 months of age, despite very high levels of overexpression.
ASK1 overexpression in the heart was likely without baseline effect given the many levels of regulation imposed on this kinase. ASK1 is uniquely activated by phosphorylation of threonine 845, while dephosphorylation of serine 83, serine 967, and serine 1034 results in activation.27 We previously determined that calcineurin enhances ASK1 activation through direct dephosphorylation of serine 967 and an indirect increase in threonine 845 phosphorylation.19 ASK1 is also bound to and inhibited by thioredoxin and glutaredoxin, which upon oxidative stress are released allowing ASK1 activation.31,32 Even though we failed to identify increases in thioredoxin protein in the hearts of ASK1 DTG mice at baseline or after TAC, endogenous levels of thioredoxin may be in excess and fully capable of silencing overexpressed ASK1.
The most prominent biologic effect observed in ASK1 overexpressing mice was a sensitization to cell death following stimulation. ASK1 transgenic mice exhibited greater cardiac TUNEL after MI and following 8 weeks of pressure overload stimulation. ASK1 DTG mice also exhibited a more than 2-fold increase in I-R injury compared with control mice. The I-R model is particularly relevant given ROS generation during reperfusion, which should liberate ASK1 from thioredoxin and glutaredoxin. Previous work showed that Ask1−/− mice had reduced cardiomyocyte apoptosis and TUNEL in response to angiotensin II infusion,15 or in response to 4 weeks of pressure overload stimulation or 4 weeks after MI.16 Cardiomyocytes generated from Ask1−/− mice were also resistant to H2O2- and calcium overload-induced apoptosis.16,17 Finally, deletion of Ask1 in the mouse rescued cardiomyopathy and the increase in cardiac apoptosis associated with a cardiac-specific deletion of the c-Raf-1 gene.20 Thus, our results in ASK1 overexpressing TG mice are consistent with data obtained in Ask1−/− mice, together indicting that ASK1 plays a critical role in regulating cardiomyocyte death, possibly due to increased JNK1/2 activity and upregulated Bax.
In contrast to the cell death observations, ASK1 transgenic mice showed no increase in myocyte or whole organ hypertrophy following TAC or isoproterenol stimulation for 2 weeks compared with controls. Even after 8 weeks of TAC, ASK1 transgenic mice showed no increase in myocyte cross-sectional areas compared with controls. Importantly, pressure overload stimulation is known to potently activate ASK1 in the adult mouse heart,16 so if ASK1 truly functioned as a hypertrophic regulator, the overexpressing mice should have shown enhancement in this process. Ironically, we previously observed increased cardiomyocyte hypertrophy by adenoviral infection with a dominant negative ASK1 mutant, while overexpression of WT ASK1 suppressed hypertrophy due to calcineurin, phenylephrine and FBS stimulation.19 Not surprisingly, results in cultured neonatal myocytes are sometimes at odds with results obtained in genetically modified mouse models, in part because of the variability in the culture model itself. Indeed, other studies in cultured myocytes suggest that overexpression of activated ASK1 actually induced cardiomyocyte hypertrophy in culture, while overexpression of dominant negative ASK1 attenuated hypertrophy.18
Previous results in genetically modified mouse models are similarly unclear in defining the role of ASK1 in regulating cardiac hypertrophy. For example, Ask1−/− mice showed reduced hypertrophy following angiotensin II infusion, suggesting that ASK1 could positively regulate cardiac growth in vivo.15 However, TAC stimulation did not result in less cellular hypertrophy in Ask−/− mice compared with WT mice, suggesting that ASK1 is not required in vivo for successful pressure overload hypertrophy.16 More recently, Ask1−/− mice were actually shown to have enhanced physiologic hypertrophy following swimming exercise, suggesting that ASK1 antagonizes the adaptive growth response.21 Our results in ASK1 overexpressing TG mice suggest that ASK1 is not a central regulator of the pathologic hypertrophy response. The discordance in results from the various studies in Ask1−/− mice and in cultured myocytes may reflect secondary effects associated with increased cell death or greater propensity towards cardiomyopathy. Indeed, 8 weeks of TAC stimulation produced increased heart-weights normalized to body-weight in ASK1 transgenic mice, although more careful inspection of these mice revealed greater ventricular dilation as the causative factor in affecting total heart weights. It is even more complicated when one attempts to invoke an underlying mechanism for an effect on hypertrophy, as ASK1 transgenic mice showed inhibition NFAT activity following TAC stimulation, but hypertrophy was not inhibited. To explain this effect, it is likely that ASK1 regulates other growth effecting pathways that might counteract this anti-hypertrophic effect, such as alterations in MAPK signaling. Second, ASK1 overexpression induces greater cell death with some degree of cardiomyopathy that likely secondarily enhances the cardiac hypertrophic response through greater neuroendocrine dysfunction. Thus, ASK1 is likely a disease modifying kinase that can secondarily impacts cardiac hypertrophy and heart failure through a primary mechanism involving cell death, ventricular remodeling, and other uncharacterized effects.
We previously observed that ASK1 overexpression in neonatal cardiomyocytes induced activation of p38 and JNK, as well as inhibition of calcineurin-NFAT signaling.19 Otsu and colleagues similarly observed that TAC stimulation in Ask1−/− mice resulted in defective cardiac JNK activition.16 We failed to observe an increase in p38 activation in the heart with ASK1 overexpression after TAC stimulation, although we did observe enhanced activation of MKK4/6 and JNK1/2. It is possible that p38 was not induced in the hearts of ASK1 DTG mice because stimuli other than TAC are needed to induce coupling between ASK1 and the p38 branch of the MAPK cascade when ASK1 is in abundance. Indeed, ASK1 might serve a scaffold function, such that its overexpression affects p38 signaling in a different manner from that observed in Ask1−/− mice. However, ASK1 overexpression did show coupling to the JNK1/2 signaling branch in the heart after TAC stimulation, an effect known to alter the cell death response.33
The interconnectivity between calcineurin-NFAT and ASK1 signaling circuits is even more intricate, as calcineurin appears to be required for ASK1 activation through dephosphorylation of serine 967 in cultured cardiomyocytes.19 Here we extended this later observation in our transgenic mice. Specifically, we crossed the ASK1 transgene into the CnAβ−/− background as a way of reducing total calcineurin activity in the heart, as we have previously characterized.26 Consistent with our in vitro loss-of-function experiments, the CnAβ−/− background attenuated ASK1’s ability to promote cardiomyopathy upon pressure overload stimulation. Calcineurin is known to play a critical role in altering the decision of death versus survival of cardiomocytes in response to stress stimulation.23 Thus, ASK1 is a highly interconnected signaling effector that holds potential therapeutic relevance, such that inhibitors against this kinase might be cardioprotective in response to diverse disease stimuli that result in cardiomyopathy.
Supplementary Material
Acknowledgments
Sources of funding
This work was supported by grants from the National Institutes of Health (J.D.M), the Fondation Leducq (Heart failure network grant to J.D.M), and the Howard Hughes Medical Institute (J.D.M.). Q.L was supported by a postdoctoral grant from the Ohio Valley Affiliate, American Heart Association.
Non-standard abbreviations
- ASK1
Apoptosis signal-regulating kinase 1
- α-MHC
α-myosin heavy chain
- ERK
extracellular signal-regulated kinases
- I/R
ischemia-reperfusion
- JNK
c-Jun NH2-terminal kinase
- MAPK
mitogen-activated protein kinase
- MI
myocardial infarction
- MKK
MAPK kinase
- NFAT
nuclear factor of activated T-cellsl
- TAC
transverse aortic constriction
- WT
wildtype
Footnotes
Disclosures: None
Codes [15] [138] [145] [147]
References
- 1.Lorell BH, Carabello BA. Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation. 2000;102:470–479. doi: 10.1161/01.cir.102.4.470. [DOI] [PubMed] [Google Scholar]
- 2.Ho KK, Levy D, Kannel WB, Pinsky JL. The epidemiology of heart failure: The Framingham study. J Am Coll Cardiol. 1993;22:6–13. doi: 10.1016/0735-1097(93)90455-a. [DOI] [PubMed] [Google Scholar]
- 3.Lloyd-Jones DM, Larson MG, Leip EP, Beiser A, D’Agostino RB, Kannel WB, Murabito JM, Vasan RS, Benjamin EJ, Levy D. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106:3068–3072. doi: 10.1161/01.cir.0000039105.49749.6f. [DOI] [PubMed] [Google Scholar]
- 4.Molkentin JD, Dorn GW., 2nd Cytoplasmic signaling pathways that regulate cardiac hypertrophy. Annu Rev Physiol. 2001;63:391–426. doi: 10.1146/annurev.physiol.63.1.391. [DOI] [PubMed] [Google Scholar]
- 5.Garrington TP, Johnson GL. Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr Opin Cell Biol. 1999;11:211–218. doi: 10.1016/s0955-0674(99)80028-3. [DOI] [PubMed] [Google Scholar]
- 6.Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79:143–180. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- 7.Takeda K, Matsuzawa A, Nishitoh H, Ichijo H. Roles of MAPKKK ASK1 in stress-induced cell death. Cell Struct Funct. 2003;28:23–29. doi: 10.1247/csf.28.23. [DOI] [PubMed] [Google Scholar]
- 8.Chang HY, Nishitoh H, Yang X, Ichijo H, Baltimore D. Activation of apoptosis signal-regulating kinase 1 (ASK1) by the adapter protein Daxx. Science. 1998;281:1860–1863. doi: 10.1126/science.281.5384.1860. [DOI] [PubMed] [Google Scholar]
- 9.Hatai T, Matsuzawa A, Inoshita S, Mochida Y, Kuroda T, Sakamaki K, Kuida K, Yonehara S, Ichijo H, Takeda K. Execution of apoptosis signal-regulating kinase 1 (ASK1)-induced apoptosis by the mitochondria-dependent caspase activation. J Biol Chem. 2000;275:26576–26581. doi: 10.1074/jbc.M003412200. [DOI] [PubMed] [Google Scholar]
- 10.Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K, Gotoh Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- 11.Kanamoto T, Mota M, Takeda K, Rubin LL, Miyazono K, Ichijo H, Bazenet CE. Role of apoptosis signal-regulating kinase in regulation of the c-Jun N-terminal kinase pathway and apoptosis in sympathetic neurons. Mol Cell Biol. 2000;20:196–204. doi: 10.1128/mcb.20.1.196-204.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang TH, Popp DM, Wang HS, Saitoh M, Mural JG, Henley DC, Ichijo H, Wimalasena J. Microtubule dysfunction induced by paclitaxel initiates apoptosis through both c-Jun N-terminal kinase (JNK)-dependent and -independent pathways in ovarian cancer cells. J Biol Chem. 1999;274:8208–8216. doi: 10.1074/jbc.274.12.8208. [DOI] [PubMed] [Google Scholar]
- 14.Tobiume K, Matsuzawa A, Takahashi T, Nishitoh H, Morita K, Takeda K, Minowa O, Miyazono K, Noda T, Ichijo H. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2001;2:222–228. doi: 10.1093/embo-reports/kve046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Izumiya Y, Kim S, Izumi Y, Yoshida K, Yoshiyama M, Matsuzawa A, Ichijo H, Iwao H. Apoptosis signal-regulating kinase 1 plays a pivotal role in angiotensin II-induced cardiac hypertrophy and remodeling. Circ Res. 2003;93:874–883. doi: 10.1161/01.RES.0000100665.67510.F5. [DOI] [PubMed] [Google Scholar]
- 16.Yamaguchi O, Higuchi Y, Hirotani S, Kashiwase K, Nakayama H, Hikoso S, Takeda T, Watanabe T, Asahi M, Taniike M, Matsumura Y, Tsujimoto I, Hongo K, Kusakari Y, Kurihara S, Nishida K, Ichijo H, Hori M, Otsu K. Targeted deletion of apoptosis signal-regulating kinase 1 attenuates left ventricular remodeling. Proc Natl Acad Sci U S A. 2003;100:15883–15888. doi: 10.1073/pnas.2136717100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watanabe T, Otsu K, Takeda T, Yamaguchi O, Hikoso S, Kashiwase K, Higuchi Y, Taniike M, Nakai A, Matsumura Y, Nishida K, Ichijo H, Hori M. Apoptosis signal-regulating kinase 1 is involved not only in apoptosis but also in non-apoptotic cardiomyocyte death. Biochem Biophys Res Commun. 2005;333:562–567. doi: 10.1016/j.bbrc.2005.05.151. [DOI] [PubMed] [Google Scholar]
- 18.Hirotani S, Otsu K, Nishida K, Higuchi Y, Morita T, Nakayama H, Yamaguchi O, Mano T, Matsumura Y, Ueno H, Tada M, Hori M. Involvement of nuclear factor-kappaB and apoptosis signal-regulating kinase 1 in G-protein-coupled receptor agonist-induced cardiomyocyte hypertrophy. Circulation. 2002;105:509–515. doi: 10.1161/hc0402.102863. [DOI] [PubMed] [Google Scholar]
- 19.Liu Q, Wilkins BJ, Lee YJ, Ichijo H, Molkentin JD. Direct interaction and reciprocal regulation between ASK1 and calcineurin-NFAT control cardiomyocyte death and growth. Mol Cell Biol. 2006;26:3785–3797. doi: 10.1128/MCB.26.10.3785-3797.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamaguchi O, Watanabe T, Nishida K, Kashiwase K, Higuchi Y, Takeda T, Hikoso S, Hirotani S, Asahi M, Taniike M, Nakai A, Tsujimoto I, Matsumura Y, Miyazaki J, Chien KR, Matsuzawa A, Sadamitsu C, Ichijo H, Baccarini M, Hori M, Otsu K. Cardiac-specific disruption of the c-raf-1 gene induces cardiac dysfunction and apoptosis. J Clin Invest. 2004;114:937–943. doi: 10.1172/JCI20317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taniike M, Yamaguchi O, Tsujimoto I, Hikoso S, Takeda T, Nakai A, Omiya S, Mizote I, Nakano Y, Higuchi Y, Matsumura Y, Nishida K, Ichijo H, Hori M, Otsu K. Apoptosis signal-regulating kinase 1/p38 signaling pathway negatively regulates physiological hypertrophy. Circulation. 2008;117:545–552. doi: 10.1161/CIRCULATIONAHA.107.710434. [DOI] [PubMed] [Google Scholar]
- 22.Sanbe A, Gulick J, Hanks MC, Liang Q, Osinska H, Robbins J. Reengineering inducible cardiac-specific transgenesis with an attenuated myosin heavy chain promoter. Circ Res. 2003;92:609–616. doi: 10.1161/01.RES.0000065442.64694.9F. [DOI] [PubMed] [Google Scholar]
- 23.Wilkins BJ, Dai YS, Bueno OF, Parsons SA, Xu J, Plank DM, Jones F, Kimball TR, Molkentin JD. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ Res. 2004;94:110–118. doi: 10.1161/01.RES.0000109415.17511.18. [DOI] [PubMed] [Google Scholar]
- 24.Oka T, Maillet M, Watt AJ, Schwartz RJ, Aronow BJ, Duncan SA, Molkentin JD. Cardiac-specific deletion of Gata4 reveals its requirement for hypertrophy, compensation, and myocyte viability. Circ Res. 2006;98:837–845. doi: 10.1161/01.RES.0000215985.18538.c4. [DOI] [PubMed] [Google Scholar]
- 25.Kaiser RA, Liang Q, Bueno O, Huang Y, Lackey T, Klevitsky R, Hewett TE, Molkentin JD. Genetic inhibition or activation of JNK1/2 protects the myocardium from ischemia-reperfusion-induced cell death in vivo. J Biol Chem. 2005;280:32602–32608. doi: 10.1074/jbc.M500684200. [DOI] [PubMed] [Google Scholar]
- 26.Bueno OF, Wilkins BJ, Tymitz KM, Glascock BJ, Kimball TF, Lorenz JN, Molkentin JD. Impaired cardiac hypertrophic response in Calcineurin Abeta -deficient mice. Proc Natl Acad Sci U S A. 2002;99:4586–4591. doi: 10.1073/pnas.072647999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishida K, Otsu K. The role of apoptosis signal-regulating kinase 1 in cardiomyocyte apoptosis. Antioxid Redox Signal. 2006;8:1729–1736. doi: 10.1089/ars.2006.8.1729. [DOI] [PubMed] [Google Scholar]
- 28.Hogan PG, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003;17:2205–2232. doi: 10.1101/gad.1102703. [DOI] [PubMed] [Google Scholar]
- 29.Wilkins BJ, Molkentin JD. Calcineurin and cardiac hypertrophy: where have we been? Where are we going? J Physiol. 2002;541:1–8. doi: 10.1113/jphysiol.2002.017129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Molkentin JD, Robbins J. With great power comes great responsibility: using mouse genetics to study cardiac hypertrophy and failure. J Mol Cell Cardiol. 2009;46:130–136. doi: 10.1016/j.yjmcc.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song JJ, Lee YJ. Differential role of glutaredoxin and thioredoxin in metabolic oxidative stress-induced activation of apoptosis signal-regulating kinase 1. Biochem J. 2003;373:845–853. doi: 10.1042/BJ20030275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dhanasekaran DN, Reddy EP. JNK signaling in apoptosis. Oncogene. 2008;27:6245–6251. doi: 10.1038/onc.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.