Abstract
Ethnopharmacological relevance
Nutmeg, the seeds of Myritica fragrans (family Myristicaceae), is a well known kitchen spice with a long-standing reputation as a psychoactive herb. Nutmeg at high doses is considered a cheap substitute to several drugs of abuse. Earlier reports have attributed amphetamine-like activities to nutmeg.
Aim of the study
To characterize the neuropharmacological effects of different nutmeg extracts, administered orally and intraperitoneally, in comparison to Δ9-terahydrocannabinol, amphetamine, and morphine.
Materials and methods
Methanolic (ME), dichloromethane (DE), and hexane (HE) extracts were obtained from a chromatographically fingerprinted batch of nutmeg. Biological evaluation was conducted in sets of 6–8 mice in the tetrad assay at doses ranging from 100–500 and 500–1000 mg/kg for i.p. and oral administration, respectively.
Results
While oral administration of all the nutmeg extracts at 500 mg/kg caused a significant increase in locomotor activity, the i.p. administration of DE showed significant reduction in rectal temperature along with a significant increase in tail flick latency at 300 mg/kg. A significant decrease in core body temperature was observed with HE at 100 mg/kg, while higher doses caused significant increases in hot plate latency.
Conclusion
Different behavioral effects were observed that varied by the type of extract as well as by the route of administration.
Keywords: Nutmeg, Myristica fragrans, Mouse tetrad assay, Amphetamine, Δ9-Tetrahydrocannabinol, Morphine, HPLC
1. Introduction
Nutmeg is the dried seed kernel of Myristica fragrans Houtt (Family Myristicaceae). Nutmeg contains volatile oils, fats, starch, and mucilage. The fixed oil contains trimyristin and myristic acid, while the volatile oil is comprised of a mixture of terpenes and alkenylbenzene derivatives. Myristicin, safrole, and elimicin constitute about 80% of the alkenylbenzene derivatives (Janssen and Lackman, 1990; Evans, 1996). In addition to its common use as a kitchen spice, in alternative medicine, nutmeg has been used as a stimulant, antidiarrheal, carminative, stomachic, tonic, and as aphrodisiac (Evans, 1996; Nadkarni, 1998). Both in vitro and in vivo studies have resulted in a wide array of pharmacological actions attributed to nutmeg including analgesic (Sonavane et al., 2001), antifungal (Nadkarni, 1998), antimicrobial (Takikawa et al., 2002), anti-inflammatory (Olajide et al., 1999), as well as hepatoprotective (Morita et al., 2003) activities.
Nutmeg in sufficient dosage is reported to be psychoactive. The psychoactivity has been described since the Middle Ages, with hallucinations, feelings of euphoria, unreality, and delusions documented (Beck and Marty, 2001). Because of its euphoric and hallucinogenic effects, nutmeg has been widely abused as a cheap substitute for narcotic drugs since the 12th century (Shafran, 1976; Quin et al., 1998; Sangalli and Chiang, 2000). Recently, several cases of nutmeg ingestion have been reported, particularly in adolescents, all of which were in attempt to achieve an euphoric state at low cost (Scholefield, 1995; Kelly et al., 2003; Demetriades et al., 2005). In all cases, symptoms presented predominantly involved the central nervous system. Despite its widespread use and reported psychoactive properties in humans, the neuro-pharmacological actions of nutmeg have not been evaluated in depth. In 1966, Shulgin proposed that the psychoactivity of nutmeg might be due to the metabolic conversion of myristicin to amphetamine-like compounds (Shulgin, 1966). However, this theory has been recently questioned due to inconsistent animal findings and lack of detection of the proposed amphetamine-like metabolites in biological fluids of nutmeg abuse cases or experimentally administered nutmeg (Braun, 1973; Solheim and Scheline, 1973; Beyer et al., 2006).
Numerous CNS activities have been reported for nutmeg. Olajide et al. (Olajide et al., 1999) reported that oral administration of the chloroform extract of nutmeg (50–200 mg/kg) possessed a potent analgesic effect against acetic acid-induced writhings in mice. On the other hand, Sonavane et al. reported that trimyristin and the acetone insoluble fraction of the hexane extract of the seed demonstrated anxiogenic activity in mice (10–100 mg/kg and 30–300 mg/kg, i.p., respectively) when tested in the elevated plus maze and the hole-board paradigms (Sonavane et al., 2002). An antidepressant activity of the seed hexane extract (10 mg/kg, 3 d, p.o.) was also observed in mice using the forced swim and tail suspension tests. Interaction of the extract with adrenergic, dopaminergic, and serotonergic receptors has been proposed to mediate the antidepressant (Dhingra and Sharma, 2006) and anticonvulsant actions observed in rodents. Oral administration of the hexane seed extract at 5 mg/kg dose for 3 successive days improved learning and memory in both young and aged mice as well as reversed the diazepam and scopolamine-induced learning and memory impairment (Parle et al., 2004). Inhibition of acetylcholinesterase activity has been suggested as potential mechanism for memory enhancement based on both in vitro and in vivo studies (Dhingra et al., 2006; Mukherjee et al., 2007). Recently, Abdul Wahab et al. reported that i.p. administration of nutmeg oil resulted in anticonvulsant activity against pentylene tetrazole (PTZ)-induced seizure and hind limb tonic extension phase in the maximal electric shock (MES) seizure test (Abdul Wahab et al., 2008).
As evident from the literature, various neurological activities have been reported for nutmeg extracts. Moreover, studies conducted so far report a diversity of actions with different extracts and different routes of administration, mainly oral (p.o.) and intraperitoneal (i.p.). With the ease of obtaining nutmeg and the legality of its use, it constitutes a popular low cost substitute for drugs of abuse. Recently, psychotropic and rare fatal effects have been reported in cases of high nutmeg intake (Servan et al., 1998; Sangalli and Chiang, 2000; Stein et al., 2001; McKenna et al., 2004; Forrester, 2005; Demetriades et al., 2005). Thus the need for detailed pharmacological evaluation of the neurological effects of nutmeg and proper understanding of the mechanism of action of its constituents is ever increasing.
In an attempt to understand the reputed psychopharmacology of nutmeg, the primary objective of this study was to evaluate the neuro-pharmacological activity of different nutmeg extracts in mice using two routes of administration (p.o. and i.p.). Earlier reports list nutmeg as a recreational drug commonly used by teenagers as well as in prison settings primarily as a cheap marijuana substitute. In fact anecdotal reports compare the feeling experienced by nutmeg intake to a “medium marijuana buzz”. Additionally, the pain relieving capacity of nutmeg has been historically utilized to ward off pain, and as such has been commonly used to substitute morphine narcotic drugs (Rudgley, 1998). As mentioned earlier, the reputed psychoactivity of nutmeg has always been associated with the hypothesis of potential metabolic activation of nutmeg constituents to amphetamine-like compounds. Hence, this study compared the neuro-pharmacological actions of different nutmeg extracts to the actions of these commonly abused drugs, Δ9-tetrahydrocannabinol, morphine, and amphetamine. The tetrad assay was utilized as a platform to achieve this objective.
2. Materials and methods
2.1. Preparation of nutmeg extracts
The different extracts were prepared according to the following general procedure. In each of three 125 mL conical flasks, 10 g of freshly ground whole nutmeg kernels (Sharp Labs, Port Orange, FL) were soaked overnight in 75 mL of the specified organic solvent. The extract of each solvent was filtered and the residue was subjected to two additional extraction cycles. The three extracts were combined and concentrated at 45 °C under vacuum in a rotary evaporator. Extract yields were 2.1, 1.4 and 1.4 g for methanol (ME), dichloromethane (DE) and n-hexane (HE), respectively. The waxy component (myristic acid and its triglyceryl ester, trimyristin) was separately isolated by storing 100 mL of the total methanolic extract of nutmeg (10 g powder nutmeg) in a freezer and filtering the off-white residue (WR) that separated overnight (1.5 g). The remaining methanolic solution was concentrated under vacuum to yield a dark brown oily residue (DME, 0.5 g). Each extract was fingerprinted by HPLC as described below.
2.2. HPLC analysis
A Shimadzu Prominence® system composed of a quaternary pump, autoinjector and PDA detector was used for fingerprinting the tested extracts. The system is controlled by LCSolution ver. 1.22 running under MS Windows XP. A Luna C18 column (150 × 4.6 mm, 5u) with a SecurityGuard® cartridge (Phenomenex, Torrance, CA) was used for the analysis. A gradient elution was run at 1 mL/min with 40% acetonitrile in 0.1% aqueous formic acid to 100% acetonitrile over 40 minutes followed by a 5 min washing/re-equilibriation period. Samples (20 mg of each extract) were dissolved in methanol (1.00 mL), passed through 45u membrane filters and 10 uL injected on the column. Chromatograms were recorded at 280 nm. Initial HPLC profiling of different nutmeg samples revealed no major variations in the fingerprint analysis (data not shown). Thus the study focused on preparing extracts from the sample comprised of whole (unpowdered) nutmeg kernels, which as such was less subject to uncontrolled manipulation by the supplier. Additionally, such sample reflects the more common form of nutmeg consumed by human population.
2.3. Pharmacological experiments
2.3.1. Animals
Experiments were performed using eight week old mice. Male Swiss Webster mice (Harlan, IN, USA) weighing 24–30 g at the time of testing were used. The mice were housed in groups of five with a 12 h light/12 h dark cycle. Food and water were provided ad libitum. All mice were randomly selected for each treatment group. Procedures involving animals were performed according to the guidelines approved by the Institutional Animal Care and Use Committee (IACUC).
2.3.2. Mouse tetrad assay
The mouse tetrad assay is a four point behavioral assay that characterizes the effect on locomotor activity, catalepsy, body temperature, and nociception. It is well established that the psychoactive properties exerted by Δ9-THC and other cannabinoids manifest in the mouse tetrad assay as classical cannabimimetic activity of decreased locomotor activity, catalepsy, hypothermia, and antinociceptive effects (Martin et al., 1994; Varvel et al., 2005; Pertwee et al., 2007). Twenty four hours prior to testing, animals were acclimated to experimental settings (ambient temperature 22–24 °C) and rectal probe insertion. At test day, pre-injection control values for rectal temperature, catalepsy, tail flick, and hot plate latencies were determined. Animals were then injected orally (p.o.) or intraperitoneally (i.p.) with either the vehicle control, Δ9-THC (10–40 mg/kg, i.p.), amphetamine (5–20 mg/kg, i.p.), morphine (5–20 mg/kg, i.p.) or nutmeg extract (100–1000 mg/kg, p.o. or i.p.). Thirty minutes following i.p. injection or sixty minutes following oral dosing, animals were individually placed on a ring immobility apparatus and the latency to drop was recorded in sec with a maximum of 180 sec latency. Rectal temperature was then recorded using a digital rectal probe and was expressed as the difference between basal and post injection temperatures. Tail flick and hot plate latencies were measured with a maximum tail flick latency of 15 s and hot plate latency of 45 s to avoid tissue damage. Animals were then individually placed in activity chambers (San Diego Instruments, San Diego, CA) where the locomotor activity was automatically monitored for thirty minutes. Total activity was expressed as the total number of interruptions of 16 cell photobeam per chamber.
2.3.3. Data analysis
All values were presented as mean ± S.E.M. with n = 6–8 animals/group. Antinociception was expressed as the percent maximal effect (% MPE=[(Post drug latency − basal latency)/(cutoff latency−basal)] × 100). All data were analyzed using One Way ANOVA followed by Dunnett’s post hoc test to determine significant difference from vehicle control at p<0.05.
3. Results
3.1. HPLC analysis of nutmeg extracts
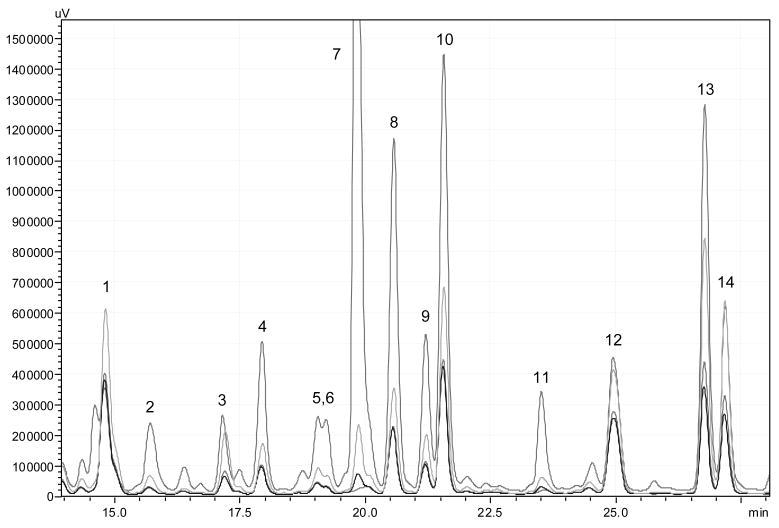
All extracts showed the same HPLC profile at 280 nm (Fig. 1) with at least 11 characteristic components present (yet to be identified). Based on sample weight, however, each of DE and HE had ca. 50% total concentration of the main UV-active components while DME had ca. 200% as ME (calculated by comparing the total peak areas of each extract). In contrast to ME, a brownish waxy residue, both DE and HE appeared as yellowish white semi-solids while DME was a brown oil.
Figure 1.
HPLC fingerprints of the tested extracts showing similar qualitative profiles (peaks 1–14) and different levels of UV-active components.
3.2. Behavioral effects of Δ9-THC, amphetamine, and morphine in mouse tetrad assay
As shown in table 1, Δ9-THC exerted the typical cannabimimetic activity whereby it caused significant reduction in locomotor activity (F[3,39] = 17.41; p<0.0001), catalepsy (F[3,45] = 5.06; p=0.004), significant hypothermic effect (F[3,36] = 21.94; p<0.0001), as well as antinociceptive action in both the hot plate and tail flick assays (F[3,50] = 14.74; p<0.0001 and F[3,45]=9.97; p<0.001, respectively). Dunnett’s post hoc comparison revealed that the decrease in locomotor activity caused by 10 (q=5.53, p<0.001), 20 (q=4.61, p<0.001), and 40 (q=5.71, p<0.001) mg/kg doses of Δ9-THC was statistically significant compared to the vehicle control. Similarly, the increase in catalepsy latency induced by the 20 (q=3.38, p<0.01) and 40 (q=2.83, p<0.05) mg/kg doses of Δ9-THC was statistically significant. Both the 20 (q=2.44, p<0.001) and 40 (q=7.02, p<0.001) mg/kg doses of Δ9-THC significantly reduced the animals’ core body temperature. The classical cannabinoid Δ9-THC caused a dose dependent increase in hot plate and tail flick withdrawal latencies confirming its antinociceptive effect. The increase in hot plate latency was statistically different from the vehicle control at the 10 (q=3.90, p<0.001), 20 (q=5.67, p<0.001), and 40 (q=4.81, p<0.001) mg/kg doses. A dose dependant increase was observed in the tail flick latency, with the changes induced by the 20 (q=3.44, p<0.01) and 40 (q=4.58, p<0.001) mg/kg doses being significantly different from the vehicle control.
Table 1.
Behavioral effects of the intraperitoneal administration of Δ9-tetrahydrocannabinol (Δ9-THC), amphetamine, and morphine in the mouse tetrad assay
| Treatment | Locomotor | Catalepsy | Rectal Temperature Change (°C) | Tail-Flick Latency (%MPE) | Hotplate Latency (%MPE) |
|---|---|---|---|---|---|
| Δ9-THC (mg/kg) | |||||
| 0 | 1329 ± 175.9 | 1.44 ± 0.28 | − 0.73 ± 0.12 | 1.50 ± 1.85 | 6.37 ± 2.51 |
| 10 | 190.9 ± 33.8*** | 14.40 ± 4.16 | − 2.05 ± 0.25 | 6.52 ± 2.00 | 29.04 ± 5.37* |
| 20 | 260.4 ± 41.4*** | 47.88 ± 20.04*** | −3.87 ± 0.45*** | 28.24 ± 11.12* | 36.33 ± 6.72*** |
| 40 | 194.0 ± 49.2*** | 37.70 ± 14.64*** | −3.48 ± 0.28*** | 51.96 ± 13.22*** | 38.18 ± 4.05*** |
| Amphetamine (mg/kg) | |||||
| 0 | 1676 ± 238.6 | 1.88 ±0.58 | −0.39 ±0.12 | 5.92 ± 2.45 | 11.12 ± 1.17 |
| 5 | 5337 ± 713.7 *** | 1.63 ± 0.50 | 1.11 ± 0.15*** | 65.63 ±12.60** | 19.29 ± 2.70* |
| 20 | 1682 ± 423.3 | 2.63 ±0.65 | 1.61 ± 0.25*** | 78.63 ± 15.65*** | 27.84 ±2.66*** |
| Morphine (mg/kg) | |||||
| 0 | 1676 ± 238.6 | 1.88 ± 0.58 | −0.39 ± 0.12 | 5.92 ± 2.45 | 11.12 ± 1.17 |
| 5 | 688.7 ± 136.9*** | 2.25 ±0.86 | −0.71 ±0.18 | 60.24 ± 19.00** | 16.01 ± 2.97 |
| 10 | 274.3 ± 44.68*** | 2.17 ± 0.98 | −0.73 ± 0.13 | 89.54 ±10.46*** | 30.86 ± 2.92* |
| 20 | 271.4 ±39.71*** | 1.63 ±0.42 | −2.83 ±0.36*** | 100.00 ± 0.00*** | 69.66 ± 8.06*** |
p<0.05,
p<0.01,
p<0.001 versus the vehicle, Dunnett’s post hoc test
Administration of Amphetamine to mice caused a significant increase in locomotor activity (F[2,19] = 17.21; p<0.0001), significant increase in core body temperature (F[2,21] = 80.61; p<0.0001), as well antinociceptive action in both the hot plate and tail flick assays (F[2,20] = 13.38; p=0.0002 and F[2,14]=15.11; p =0.003, respectively). No catalepsy was noted in any of the animals (F[2,21] = 1.14; p =0.04) (Table 1). Post hoc analysis revealed that the enhanced locomotor activity was significant only at the 5 mg/kg dose (q=5.03, p<0.001), while the hyperthermic effect was significant at both the 5 (q=7.36, p<0.001) and 20 (q=12.64, p<0.001) mg/kg doses. A dose dependant antinociceptive action was observed in both the hot plate (q=2.62, p<0.05; q=5.17, p<0.001 for the 5 and 20 mg/kg doses, respectively) and tail flick assays (q=4.43, p<0.01; q=4.78, p<0.001 for the 5 and 20 mg/kg doses, respectively).
Morphine administration resulted in significant decrease in locomotor activity (F[3,21] = 19.89; p<0.0001) at the 5 (q=4.64, p<0.001), 10 (q=6.59, p<0.001), and 20 (q=6.59, p<0.001) mg/kg doses. No cataleptic effect was observed at any of the used doses (F[3,26] = 0.16; p=0.92). A significant hypothermic effect (F[3,27] = 25.37; p<0.0001) was evident only at the 20 mg/kg dose (q=7.79, p<0.001). As expected, morphine demonstrated a strong antinocipetive action in both the tail flick (F[3,22] = 25.87; p<0.0001) and hot plate tests (F[3,28] = 33.60; p<0.0001). Post hoc analysis showed that the prolongation of tail flick latency was significant at the 5 (q=4.21, p<0.01), 10 (q=6.80, p<0.001), and 20 (q=8.22, p<0.001) mg/kg doses, while hot plate latency prolongation was significant only at the 10 (q=3.05, p<0.05) and 20 (q=9.05, p<0.001) mg/kg doses. Collected data are summarized in table 1.
3.2. Behavioral effects of i.p. administration of different nutmeg extracts in the mouse tetrad assay
As shown in table 2, DE did not have a significant effect on the locomotor activity of the animals (F[3,25]=1.08, p=0.38), catalepsy (F[3,25]=0.58, p=0.63), or tail flick latency (F[3,25]=0.63, p=0.60). On the other hand, a significant hypothermic effect (F[3,30]=29.43, p<0.0001) and delay in hot plate latency(F[3,28]=5.83, p=0.0032) were observed. Dunnett’s post hoc analysis showed that the 100, 300, and 500 mg/kg doses of DE all possess significant hypothermic effect (q=2.50, p<0.05; q=5.18, p<0.01; q=5.94, p<0.01, respectively), while only the 300 mg/kg dose showed significant antinociceptive effect in the hot plate assay.
Table 2.
Behavioral effects of the intraperitoneal administration of nutmeg extracts in the mouse tetrad assay
| Treatment | Locomotor | Catalepsy | Rectal Temperature | Tail-Flick | Hotplate |
|---|---|---|---|---|---|
| Vehicle | 829.4±232.9 | 1.29±0.18 | −0.65±0.10 | 5.29±7.86 | 2.16±2.99 |
| Dichloromethane Extract (DE) | |||||
| 100mg/kg | 909.5±265 | 1.13±0.13 | −1.54±0.40* | 2.89±3.744 | 2.45±3.57 |
| 300mg/kg | 1304±200.7 | 1.86±0.86 | −1.18±0.23*** | 8.45±5.95 | 17.84±2.65** |
| 500mg/kg | 1210±88.18 | 1.86±0.55 | −1.53±0.31*** | −2.76±3.97 | 9.35±2.96 |
| Methanolic Extract (ME) | |||||
| 100mg/kg | 1343±123.1 | 1.38±0.38 | −1.54±0.40 | −1.19±3.44 | −0.99±1.62 |
| 300mg/kg | 671.4±147.7 | 3.00±1.02 | −1.36±0.39 | −4.84±3.50 | 8.65±2.36 |
| 500mg/kg | 1025±328.2 | 1.60±0.50 | −2.22±0.59* | 4.92±1.70 | 19.22±6.54* |
| n-Hexane Extract (HE) | |||||
| 100mg/kg | 630.6±199.8 | 1.25±0.16 | −2.21±0.37*** | 0.45±3.59 | 6.551±3.592 |
| 300mg/kg | 1046±121.5 | 3.25±1.05 | −1.68±0.38* | 9.57±11.49 | 20.47±3.134*** |
| 500mg/kg | 1054±114.2 | 1.00±0.00 | −0.51±0.10 | 3.82±2.81 | 14.34±2.531* |
| Defatted Methanolic Extract (DME) | |||||
| 100mg/kg | 1646±145.10 ** | 1.25±0.16 | −0.23±0.06 | 10.59±1.854 | 25.16±2.86*** |
| 300mg/kg | 285±137.70** | 1.375±0.38 | −1.31±0.37 | 6.37±2.45 | 18.41±2.99* |
| 500mg/kg | 196.6±77.26** | 1.67±0.49 | −3.38±0.53*** | 53.91±19.17*** | 30.37±4.88*** |
p<0.05,
p<0.01,
p<0.001 versus the vehicle, Dunnett’s post hoc test
Similarly, HE had no significant effect on locomotor activity F[3,26]=1.25, p=0.31), catalepsy F[3,29]=2.57, p=0.073), or tail flick latency F[3,28]=0.27, p=0.85), but it caused significant overall decrease in core body temperature F[3,31]=10.28, p<0.0001) and prolongation of hot plate latency (F[3,28]=6.97, p=0.0012). Interestingly, regarding the hypothermic effect, only the 100 (q=4.51, p<0.001) and 300 mg/kg (q=2.95, p<0.05) doses were statistically different from the vehicle control. The antinociceptive action observed in the hot plate assay was significant at the 300 (q=4.20, p<0.001) and 500 (q=2.79, p<0.05) mg/kg doses only.
While the i.p. administration of ME had no significant effect on locomotor activity (F[3,28]=1.11, p=0.36), catalepsy (F[3,38]=1.87, p=0.15), or tail flick assays (F[3,30]=1.20, p=0.33), a mildly significant hypothermic (F[3,33]=18.75, p<0.0001) and enhanced hot plate latency (F[3,29]=4.46, p=0.01) were observed at the 500 mg/kg dose (q=5.32, p<0.01; q=2.82, p<0.05, respectively). On the other hand, DME caused significant reduction in locomotor activity (F[3,23]=68.58, p<0.0001), significant decrease in body temperature (F[3,28]=18.05, p<0.0001), as well as antinociceptive action in both the tail flick (F[3,20]=8.41, p=0.0008) and hot plate (F[3,27]=12.19, p<0.0001) tests. The decrease in locomotor activity was significant at the 100 (q=4.26, p<0.01), 300 (q=11.34, p<0.01), and 500 (q=12.32, p<0.01) mg/kg doses. The hypothermic effect was only significant at the 500 mg/kg dose (q=5.86, p<0.01). The increase in tail flick latency was significant only at the 500 mg/kg dose (q=4.76, p<0.01), while all three doses caused significant increase in hot plate latency with a 30 % increase induced by the 500 mg/kg dose (q=5.68, p<0.01).
3.2. Behavioral effects of p.o. administration of different nutmeg extracts in the mouse tetrad assay
Table 3 summarizes the effects exerted by the oral administration of the different nutmeg extracts in the mouse tetrad assay. The only effect exerted by DE in the assay was a significant increase in locomotor activity (F[2,20]=13.52, p=0.0002), no effect was observed on catalepsy (F[2,20]=1.50, p=0.25), body temperature (F[2,21]=0.45, p=0.65), or latency in hot plate (F[2,21]=1.91 p=0.17), or tail flick (F[2,20]=1.95, p=0.17) assays. The locomotor stimulant action was only significant at the 500 mg/kg dose (q=4.71, p<0.001) and not the 1000 mg/kg oral dose of DE.
Table 3.
Behavioral effects of oral administration of nutmeg extracts in mouse tetrad assay
| Treatment | Locomotor | Catalepsy | Rectal Temperature | Tail-Flick | Hotplate |
|---|---|---|---|---|---|
| Vehicle | 1139±309.0 | 1±0 | −0.50±0.24 | −4.51±5.17 | 12.91±1.90 |
| Dichloromethane Extract (DE) | |||||
| 500mg/kg | 4320±739.5*** | 1.25±0.25 | −0.73±0.10 | 5.62±2.29 | 20.43±1.47 |
| 1000mg/kg | 1379±111.4 | 1.71±0.47 | −0.48±0.20 | 1.42±2.82 | 16.25±4.06 |
| Methanolic Extract (ME) | |||||
| 500mg/kg | 5516±875.7*** | 1.38±0.38 | −0.38±0.08 | 3.18±4.19 | 5.36±1.24 |
| 1000mg/kg | 2339±515.4 | 1.25±0.25 | −0.45±0.13 | 5.08±3.50 | 9.61±2.78 |
| n-Hexane Extract (HE) | |||||
| 500mg/kg | 3123±674.8** | 1.38±0.26 | −0.73±0.12 | 3.62±3.02 | 19.27±1.40* |
| 1000mg/kg | 2323±206.4 | 1.38±0.38 | −0.48±0.11 | 9.02±4.02 | 9.32±1.54 |
| Defatted Methanolic Extract (DME) | |||||
| 500mg/kg | 852.7±205.6 | 1.13±0.13 | −0.23±0.09 | 1.54±2.46 | 11.08±2.04 |
| 1000mg/kg | 908.2±207.6 | 2.43±1.13 | −0.23±0.06 | 7.41±6.46 | 10.28±0.96 |
p<0.05,
p<0.01,
p<0.001 versus the vehicle, Dunnett’s post hoc test
Similar to the DE effect, oral administration of HE resulted in a locomotor stimulant effect (F[2,19]=6.24, p=0.0082) that was significant only at the 500 mg/kg dose (q=3.46, p<0.01). The extract did not cause any catalepsy (F[2,21]=0.67, p=0.52), change in body temperature (F[2,20]=0.72, p=0.50), or prolongation of tail flick latency (F[2,21]=2.68, p=0.09). A slight increase in hot plate latency (F[2,21]=9.60, p=0.0011), suggestive of a mild analgesic effect, was observed with the 500 mg/kg dose (q=2.77, p<0.05).
As evident in table 3, oral administration of ME caused significant hyperlocomotion (F[2,21]=13.60, p=0.0002) at the 500 mg/kg dose (q=5.05, p<0.001). The same dose caused a mild but significant decrease in hot plate latency (F[2,21]=3.34, p=0.055; q=2.58, p<0.05). The extract did not have any effect on catalepsy (F[2,21]=0.54, p=0.59), rectal temperature (F[2,21]=0.15, p=0.86), or tail flick latency (F[2,20]=1.40, p=0.27). On the other hand, DME had no effect on locomotor activity (F[2,17]=1.28, p=0.30), catalepsy (F[2,18]=1.43, p=0.27), rectal temperature (F[2,17]=0.61, p=0.50), tail flick (F[2,19]=0.47, p=0.63), or hot plate (F[2,17]=3.01, p=0.08) latencies.
4. Discussion
The current study was aimed at evaluating the neuropharmacological activity of nutmeg extracts in the mouse tetrad assay. The assay is a panel of four tests that determine the effect on locomotor activity, core body temperature, induction of catalepsy, and antinociceptive action using the hot plate and tail flick tests. Historically, the tetrad assay has been used to characterize cannabinoid-induced activity, particularly of Δ9-THC. Typically, Δ9-THC administration in mice results in reduced locomotor activity, hypothermia, catalepsy, and antinociceptive action evidenced by a prolonged latency in both the hot plate and tail flick tests (Wiley and Martin, 2003). Since nutmeg is commonly used as a substitute for several drugs of abuse, the effects of nutmeg extract in mouse tetrad assay was compared to the effects exerted by Δ9-THC, amphetamine, and morphine, using both i.p. and p.o. administration routes. Nutmeg powder was extracted by solvents of varying polarities in an attempt to control the polar/non-polar constituent ratio in each extract. To ensure conformity of extracts, an HPLC method was developed to fingerprint each extract based on its UV-active constituents (aromatic phenylpropanoids, such as myristicin, safrole, eugenol and others) (Bruneton, 1999). The variation in the specific total concentration of the UV-active constituents between extracts was also used as a guide to the specific total concentration of non-UV-active constituents, mainly terpenes, myristic acid and trimyristin. As expected, although the HPLC fingerprint of all extracts was qualitatively similar the consistency of each extract was different depending on the type of solvent and/or extraction procedure. Hence, extracts with the highest lipid content (HE, DE and WR) were waxy and light in color as opposed to more polar extracts (ME and DME) which were semisolid or oily and brownish in color. Although the lipid and terpene constituents were invisible in the HPLC fingerprints, the gradual decrease in the total UV-active constituents towards more lipophilic extracts together with the increased waxy consistency reflected the higher lipophilicity of such extracts.
In accordance with previous reports, Δ9-THC administration resulted in typical cannabimimetic activity: reduction in locomotor activity, catalepsy, hypothermia, and antinociception (Martin et al., 1995). Such effects have been primarily attributed to agonist action on the cannabinoid CB1 receptor (Chaperon and Thiobot, 1999). The psychostimulant amphetamine, on the other hand, caused a biphasic response in locomotor activity, with significant increase observed at low dose (5 mg/kg) that disappeared at the high (20 mg/kg) dose. Yates et al reported a similar biphasic effect of amphetamine-induced locomotor behavior in C57Bl/6 mice at doses similar to those used in the current study. Amphetamine caused a significant enhancement of locomotor activity at doses between 2–6 mg/kg followed by locomotor suppression at doses between 12–20 mg/kg. At such high doses, the enhanced locomotor activity was replaced by enhanced stereotypy (Yates et al., 2007). The observed hyperthermic effect of amphetamine is also consistent with previously published reports (Salminen Jr. et al., 1997; Krasnova et al., 2001) and the classical symptoms of psychostimulant toxicity (Callaway and Clark, 1994). Similar to previous findings, amphetamine, up to 20 mg/kg dose did not induce catalepsy (Geffen et al., 2009). A significant antinociceptive effect exerted by amphetamine was recorded at both the 5 and 20 mg/kg doses. However, such effect was more pronounced in the tail flick (78.6 % MPE) than the hot plate (27.8 % MPE). Such data suggest the involvement of a supraspinal rather than a spinal mechanism of antinociception (Chapman et al., 1985). Similar to our findings, Connor et al reported that a low dose of amphetamine (1.8 mg/kg) had significant analgesic effect in the tail flick but not the hot plate assay (Connor et al., 2000). Analogous to amphetamine, morphine did not exhibit a typical cannabinoid activity in all the tests. However, it did cause significant reduction in locomotor activity, significant hypothermia, and analgesic action in both the hot plate and tail flick tests. An earlier study evaluated the effect of morphine in tetrad assay and reported significant hypothermia and analgesic effect. However, the reduction in locomotor activity was not observed in that study (Wiley and Martin, 2003).
The present study demonstrated that administration of nutmeg extracts did not produce a typical cannabinoid activity in all four tests. The effects observed varied by route of administration as well as type of extract. Oral administration of all extracts, except DME, resulted in significant stimulant effect on locomotor activity at the 500 mg/kg dose, while the higher 1000 mg/kg dose did not exert such stimulant action. The observed U-shaped effect on locomotor activity is similar to the behavioral profile observed for the psychostimulant drug amphetamine. In addition to the observed locomotor stimulant action, the HE produced a mild but significant antinociceptive effect in the hot plate assay indicating action on a supraspinal analgesic pathway.
On the other hand, the i.p. administration of the nutmeg extracts lacked any effect on locomotor activity, except DME, which had a significant depressant action. Previously reported human and animal data with pure nutmeg compounds suggest CNS depressant activity as well (Truitt et al., 1961; Engelbrecht et al., 1972; de Mello et al., 1973; Sangalli and Chiang, 2000). Similar to our findings, Sonavane et al (Sonavane et al., 2002) reported that the i.p. administration of the acetone insoluble fraction of the n-hexane extract of nutmeg in mice caused significant reduction in locomotor activity and rearing in an open field test coupled to an anxiogenic effect. The observed hypothermic effect exerted by the extracts also confirms a CNS depressant action. A mild but significant antinociceptive action was also observed for the extracts given i.p. Interestingly, the i.p. administration of DE, ME, as well as HE caused significant antinociception in the hot plate but not the tail flick assay. The hot plate test involves two types of responses: paw licking and jumping. Both responses integrate at supraspinal structures with the C and Aδ type I and II sensitive fibers participating in this model (Lopes et al., 2009). Accordingly, the data suggest that the observed antinociceptive action of nutmeg extracts might involve an analgesic mechanism at the supraspinal level. On the other hand, the 500 mg/kg dose of DME showed significant analgesic effect in both the hot plate and tail flick tests suggesting enrichment in components (primarily non-lipid and/or aromatic compounds) that might activate a spinally-mediated analgesic pathway. Further mechanistic studies are needed to better understand the analgesic action of nutmeg.
An interesting finding of this study is the qualitative difference demonstrated between the oral and i.p. routes of administration. Such differences might be attributed to lower bioavailability and/or greater metabolism for the oral versus i.p. routes. The data suggest that the compounds responsible for CNS depressant action are ineffective orally, possibly due to metabolic inactivation. On the other hand, the observed stimulant effect of oral versus i.p. routes indicates possible metabolism of certain nutmeg constituents to stimulant compounds. Although the early hypothesis of metabolic amination/transmination of nutmeg phenylpropanoids to amphetamine-like compounds has not been advocated by human metabolic studies (Shulgin, 1966; Braun, 1973; Solheim and Scheline, 1973), the possibility that nutmeg compounds and their metabolites might be responsible for such psychoactivity still exists. Similar to classic hallucinogens, both myristicin and elemicin have been reported to elicit psychoactivity as well as binding to 5-HT receptors (Glennon, 1990; Sangalli and Chiang, 2000). Moreover, structural similarity of compounds isolated from the terpenic fraction of nutmeg (e.g. camphene, borneol) to known CNS stimulants has been noted (Glennon, 1990). However, mechanistic studies are still needed to determine the effect of these compounds on central neurotransmitter release and activity. Moreover, the obvious CNS depressant, hypothermic, and analgesic actions exerted when the extracts were administered i.p. suggest that the extracts are enriched with CNS depressant compounds. However, the actions of these compounds become masked following biotransformation orally to CNS stimulant ones. A comprehensive pharmacokinetic study coupled to characterization of the CNS actions of nutmeg extracts as well as purified components would certainly address these issues and provide insight about nutmeg and its controversial status as a drug of abuse.
In summary, nutmeg extracts displayed different activities in the mouse tetrad assay. The observed activities were not typically cannabinoid-like in nature and they varied with the type of extract and the route of administration. HPLC fingerprinting showed that the tested extracts had different hydrophilic/lipophilic constituent ratios, which may be a factor in the varied pharmacological effect. Further mechanistic studies are warranted for the extracts and the pure constituents. The development of a validated quantitative analytical method is also needed for monitoring extract quality and to ensure reproducibility of data. Finally, the essential oil of nutmeg, which was not included in this study, should be the scope of similar future investigation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdul Wahab, Ul Haq R, Ahmed A, Khan R, Raza M. Anticonvulsant activities of nutmeg oil of Myristica fragrans. Phytotherapy Research. 2009;23(2):153–158. doi: 10.1002/ptr.2548. [DOI] [PubMed] [Google Scholar]
- Beck T, Marty H. Die Nervenkekse der Hildgard von Bingenkeine harmlose Nascherei. Schweiz Med Forum. 2001;51/52:1287–1288. [Google Scholar]
- Beyer J, Ehlers D, Maurer HH. Abuse of nutmeg (Myristica fragrans Houtt.): studies on the metabolism and the toxicologic detection of its ingredients Elemicin, Myristicin, and Safrole in rat and human urine using gas chromatography/mass spectrometry. Therapeutic Drug Monitoring. 2006;28(4):568–575. doi: 10.1097/00007691-200608000-00013. [DOI] [PubMed] [Google Scholar]
- Braun UKD. Evidence for the biogenic formation of amphetamine derivatives from components of nutmeg. Pharmacology. 1973;9:312–316. doi: 10.1159/000136402. [DOI] [PubMed] [Google Scholar]
- Bruneton J. Pharmacognosy, Phytochemistry, Medicinal Plants. 2. Lavoisier Publishing Inc; Secaucus, NJ: 1999. [Google Scholar]
- Callaway C, Clark R. Hyperthermia in psychostimulant overdose. Annals of Emergency Medicine. 1994;24(1):68–76. doi: 10.1016/s0196-0644(94)70165-2. [DOI] [PubMed] [Google Scholar]
- Chaperon F, Thiebot MH. Behavioral effects of cannabinoid agents in animals. Critical Reviews in Neurobiology. 1999;13(3):243–281. doi: 10.1615/critrevneurobiol.v13.i3.20. [DOI] [PubMed] [Google Scholar]
- Chapman C, Casey K, Dubner R, Foley K, Gracely R, Reading A. Pain measurement: an overview. Pain. 1985;22:1–31. doi: 10.1016/0304-3959(85)90145-9. [DOI] [PubMed] [Google Scholar]
- Connor J, Makonnen E, Rostom A. Comparison of analgesic effects of khat (Catha edulis Forsk) extract, d-amphtemine, and ibuprofen in mice. Journal of Pharmacy and Pharmacology. 2000;52:107–110. doi: 10.1211/0022357001773580. [DOI] [PubMed] [Google Scholar]
- de Mello A, Carlini E, Dressler K, Green J, Kang S, Margolis S. Behavioral observations on compounds found in nutmeg. Psychopharmacologia. 1973;31:349–363. doi: 10.1007/BF00421279. [DOI] [PubMed] [Google Scholar]
- Demetriades AK, Wallman PD, McGuiness A, Gavalas MC. Low cost, high risk: accidental nutmeg intoxication. Emergency Medicinal Journal. 2005;22:223–225. doi: 10.1136/emj.2002.004168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra D, Parle M, kulkarni S. Comparative brain cholinesterase-inhibiting activty of Glycyrrhiza glabra, Myritica fragrans, acorbic acid, and metrofinate in mice. Journal of Medicinal Food. 2006;9(2):281–283. doi: 10.1089/jmf.2006.9.281. [DOI] [PubMed] [Google Scholar]
- Dhingra D, Sharma A. Antidepressant-like activity of n-hexane extarct of nutmeg (Myristica fragrans) seeds in mice. Journal of Medicinal Food. 2006;9(1):84–89. doi: 10.1089/jmf.2006.9.84. [DOI] [PubMed] [Google Scholar]
- Engelbrecht J, Long J, Nichols D, Barfknecht C. Pharmacologic evaluation of 3,4-dimethoxyphenylpropenes and 3,4-dimethoxyphenylpropanediols. Archives Internationales de Pharmacodynamie et de Therapie. 1972;2:226–244. [PubMed] [Google Scholar]
- Evans WC. Treese and Evans Pharmacognosy. 14. Harcourt Brace & Co; Asia, Singapore: 1996. p. 273. [Google Scholar]
- Forrester MB. Nutmeg intoxication in Texas, 1998–2004. Human and Experimental Toxicology. 2005;24:563–566. doi: 10.1191/0960327105ht567oa. [DOI] [PubMed] [Google Scholar]
- Geffen YNA, Gil-Ad I, Rephaeli A, Huang M, Savitsky K, Klapper L, Winkler I, Meltzer H, Weizman A. BL-1020: A novel antipsychotic drug with GABAergic activity and low catalepsy, is efficacious in a rat model of schizophrenia. European Neuropsychopharmacol. 2009;19:1–13. doi: 10.1016/j.euroneuro.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Glennon R. Do classical hallucinogens act as 5-HT2 agonists or antagonists? Neuropsychopharmacology. 1990;3:509–517. [PubMed] [Google Scholar]
- Janssen J, Lackman G. Nutmeg oil: identification and quantification of its most active constituents as inhibitors of platelet aggregation. Journal of Ethnopharmacology. 1990;29:179–188. doi: 10.1016/0378-8741(90)90054-w. [DOI] [PubMed] [Google Scholar]
- Kelly BD, Clarke MM, Gavin BE, Lane AA, Larkin CC. Nutmeg and psychosis. Schizophrenia Research. 2003;60:95–96. doi: 10.1016/s0920-9964(02)00204-9. [DOI] [PubMed] [Google Scholar]
- Krasnova I, Ladenheim B, Jayanthi S, Oyler J, Moran T, Huestis M, Cadet J. Amphetamine-induced toxicity in dopamine terminals in CD-1 and C57BL/6J mice: complex role for oxygen-based species and temperature regulation. Neuroscience. 2001;107(2):265–274. doi: 10.1016/s0306-4522(01)00351-7. [DOI] [PubMed] [Google Scholar]
- Lopes L, Pereira S, Silva L, Figueiredo K, Moura B, Almeida F, Sousa F. Antinociceptive effect of topiramate in models of acute pain and diabetic neuropathy in rodents. Life Sciences. 2009;84:105–110. doi: 10.1016/j.lfs.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Martin BR, Compton DR, Prescott WR, Barret RL, Razdan RK. Pharmacological evaluation of dimethylheptyl analogs of delta9-THC: reassessment of the putative three-point cannabinoid-receptor interaction. Drug and Alcohol Dependence. 1994;37:231–240. doi: 10.1016/0376-8716(94)01081-u. [DOI] [PubMed] [Google Scholar]
- McKenna A, Nordt S, Ryan J. Acute nutmeg poisoning. European Journal of Emergency Medicine. 2004;11(240):241. doi: 10.1097/01.mej.0000127649.69328.a5. [DOI] [PubMed] [Google Scholar]
- Morita T, Jinno K, Kawagishi H, Arimoto Y, Suganuma H, Inakuma T, Sugiyama K. Hepatoprotective effect of myristicin from nutmeg (myristica fragrans) on lipopolysaccharide/D-galactosamine-induced liver injury. Journal of Agricultural and Food Chemistry. 2003;51:1560–1565. doi: 10.1021/jf020946n. [DOI] [PubMed] [Google Scholar]
- Mukherjee P, Kumar V, Houghton P. Screening of Indian medicianl plants for acetylcholinesterase inhibitory activity. 2007:1142–1145. doi: 10.1002/ptr.2224. [DOI] [PubMed] [Google Scholar]
- Nadkarni K. Indian materia medica. 3. Bombay Popular Prakashan; Mumbai: 1998. p. 830. [Google Scholar]
- Olajide OA, Ajayi FF, Ekhelar AI, Awe SO, Makinde JM, Alada ARA. Biological effects of myristica fragrans (nutmeg) extract. Phytotherapy Research. 1999;13:344–345. doi: 10.1002/(SICI)1099-1573(199906)13:4<344::AID-PTR436>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Parle M, Dhingra D, kulkarni S. improvement of mouse memory by Myristica fragrans seeds. J Med Food. 2004;7(2):157–161. doi: 10.1089/1096620041224193. [DOI] [PubMed] [Google Scholar]
- Pertwee RG, Thomas A, Stevenson LA, Ross RA, Varvel SA, Lichtman AH, Martin BR, Razdan RK. The psychoactive plant cannabinoid, delta9-tetrahydrocannabinol, is antagonized by delta8-and delta9-tetrahydrocannabivarin in mice in vivo. British Journal of Pharmacology. 2007;150:586–594. doi: 10.1038/sj.bjp.0707124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quin G, Fanning N, Plunkett P. Nutmeg intoxication. Letters, Notice, Book reviews. 1998:287–288. doi: 10.1136/emj.15.4.287-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudgley R. Thomas Dunne Books. St. Martin’s Griffin; 1998. The Encyclopedia of Psychoactive Substances. [Google Scholar]
- Salminen W, Jr, Voellmy R, Roberts S. Protection against hepatotoxicity by a single dose of amphetamine: the potential role of heat shock protein adduction. Toxicology and Applied Pharmacology. 1997;147:247–258. doi: 10.1006/taap.1997.8290. [DOI] [PubMed] [Google Scholar]
- Sangalli BC, Chiang W. Toxicology of nutmeg abuse. Clinical Toxicology. 2000;38(6):671–678. doi: 10.1081/clt-100102020. [DOI] [PubMed] [Google Scholar]
- Scholefield JH. Nutmeg-an unusual overdose. Letters to the editor. 1995:154–155. doi: 10.1136/emj.3.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servan J, Cochon F, Dulocos H. Hallucinations after voluntary ingestion of nutmeg: an unrecognized drug abuse. Reviews in Neurological Diseases (Paris) 1998;154:708. [PubMed] [Google Scholar]
- Shafran I. Letter: nutmeg toxicity. The New England Journal of Medicine. 1976;294:849. [PubMed] [Google Scholar]
- Shulgin AT. Possible implication of myristicin asa psychotropic substance. Nature. 1966;210:380–384. doi: 10.1038/210380a0. [DOI] [PubMed] [Google Scholar]
- Solheim E, Scheline R. Metabolism of alkylbenzene derivatives in the rat. I. p-Methoxyallylbenzene (Estragole) and p-methoxypropenylbenzene (Anethole) Xenobiotica. 1973;3:493–510. doi: 10.3109/00498257309151538. [DOI] [PubMed] [Google Scholar]
- Sonavane GS, Sarveiya VP, Kasture VS, Kasture SB. Behavioral actions of Myristica fragrans seeds. Indian Journal of Pharmacology. 2001;33:417–424. [Google Scholar]
- Sonavane GS, Sarveiya VP, Kasture VS, Kasture SB. Anxiogenic activity of myristica fragrans seeds. Pharmacology, Biochemistry, and Behavior. 2002;71:247–252. doi: 10.1016/s0091-3057(01)00660-8. [DOI] [PubMed] [Google Scholar]
- Stein U, Greyer H, Hentschel H. Nutmeg (myristicin) poisoning-report on a fatal case and a series of cases recorded by a poison information centre. Forensic Science International. 2001;118:87–90. doi: 10.1016/s0379-0738(00)00369-8. [DOI] [PubMed] [Google Scholar]
- Takikawa A, Abe K, Yamamoto M, Ishimaru S, Yasui M, Okubo Y, Yokoigawa K. Anti microbial Activity of Nutmeg against Escherichia coli O157. Journal of Bioscience and Bioenginering. 2002;94(4):315–320. doi: 10.1263/jbb.94.315. [DOI] [PubMed] [Google Scholar]
- Truitt E, Callaway EBM, Krantz J. The pharmacology of myristicin: a contribution to the psychopharmacology of nutmeg. Journal of Neuropsychiatry. 1961;2:205–210. [PubMed] [Google Scholar]
- Varvel SA, Bridgen DT, Tao Q, Thomas BF, Martin BR, Lichtman AH. Delta9-tetrahydrocannbinol accounts for the antinociceptive, hypothermic, and cataleptic effects of marijuana in mice. The Journal of Pharmacology and Experimental Therapeutics. 2005;314(1):329–337. doi: 10.1124/jpet.104.080739. [DOI] [PubMed] [Google Scholar]
- Wiley J, Martin B. Cannabinoid pharmacological properties common to other centrally acting drugs. European Journal of Pharmacology. 2003;471:185–193. doi: 10.1016/s0014-2999(03)01856-9. [DOI] [PubMed] [Google Scholar]
- Yates J, Meij J, Sullivan J, Richtand N, Yu L. Bimodal effect of amphetamine on motor behaviors in C57BL/6 mice. Neuroscience Letters. 2007;427:66–70. doi: 10.1016/j.neulet.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]