Abstract
Spinal proinflammatory cytokines are powerful pain-enhancing signals that contribute to pain following peripheral nerve injury (neuropathic pain). Recently, one proinflammatory cytokine, interleukin-1, was also implicated in the loss of analgesia upon repeated morphine exposure (tolerance). In contrast to prior literature, we demonstrate that the action of several spinal proinflammatory cytokines oppose systemic and intrathecal opioid analgesia, causing reduced pain suppression. In vitro morphine exposure of lumbar dorsal spinal cord caused significant increases in proinflammatory cytokine and chemokine release. Opposition of analgesia by proinflammatory cytokines is rapid, occurring ≤5 minutes after intrathecal (perispinal) opioid administration. We document that opposition of analgesia by proinflammatory cytokines cannot be accounted for by an alteration in spinal morphine concentrations. The acute anti-analgesic effects of proinflammatory cytokines occur in a p38 mitogen-activated protein kinase and nitric oxide dependent fashion. Chronic intrathecal morphine or methadone significantly increased spinal glial activation (toll-like receptor 4 mRNA and protein) and the expression of multiple chemokines and cytokines, combined with development of analgesic tolerance and pain enhancement (hyperalgesia, allodynia). Statistical analysis demonstrated that a cluster of cytokines and chemokines was linked with pain-related behavioral changes. Moreover, blockade of spinal proinflammatory cytokines during a stringent morphine regimen previously associated with altered neuronal function also attenuated enhanced pain, supportive that proinflammatory cytokines are importantly involved in tolerance induced by such regimens. These data implicate multiple opioid-induced spinal proinflammatory cytokines in opposing both acute and chronic opioid analgesia, and provide a novel mechanism for the opposition of acute opioid analgesia.
Keywords: proinflammatory cytokine, chemokine, morphine, tolerance, glia, microglia, p38 MAP kinase, nitric oxide, toll like receptor-4
Introduction
Evidence has recently accrued that repeated morphine administration induces the release of the proinflammatory cytokine, interleukin-1β (IL-1β), which opposes morphine analgesia (Johnston et al., 2004; Shavit et al., 2005). This is generally believed to be due to a progressive activation of spinal cord glia in response to repeated morphine (Song and Zhao, 2001; Watkins et al., 2005). Whether the release of IL-1 or other such proinflammatory mediators within spinal cord also contributes to the opposition of acute morphine analgesia is currently unknown. Likewise, the mechanism(s) by which acute opioids may induce proinflammatory mediators is unknown. While nitric oxide (NO) induced p38 mitogen-activated protein kinase (MAPK) activation in microglia has been implicated in decreased analgesia following repeated morphine (Cui et al., 2006; Liu et al., 2006), no parallel information exists as to the acute effects of morphine.
The present series of experiments explores several questions regarding the spinal proinflammatory effects of morphine, the prototypical opioid. Firstly, is a proinflammatory response restricted to morphine, a 4,5-epoxymorphinan, versus may it be a more generalized response to opioids from other opioid structural classes? Secondly, how rapidly does opioid-induced proinflammatory cytokine responses occur and to what extent does this oppose opioid analgesia? Thirdly, what proinflammatory mediators are responsible for opposing acute and chronic opioid analgesia and do they oppose analgesia by altering morphine pharmacokinetics? Finally, are these proinflammatory mediators correlationally or causally linked with reductions in opioid analgesia? The present work seeks to address these questions firstly using a behavioral approach which tests the effects of antagonists of the proinflammatory mediators hypothesized to be involved in opposing opioid analgesia, and analyses of mRNA and protein changes in response to acute and repeated opioids. In addition, the development of analgesic tolerance (loss of pain suppression following repeated opioid exposure), opioid-induced hyperalgesia (enhanced responsivity to radiant heat stimuli as a consequence of repeated opioid exposure) and opioid-induced allodynia (enhanced responsivity to touch/pressure stimuli as a consequence of repeated opioid exposure) is examined with respect to which proinflammatory products are mediating these chronic opioid-induced changes. Lastly, the pharmacological characteristics of the response are examined to clarify whether proinflammatory cytokines alter the analgesic efficacy of morphine by altering its pharmacokinetics.
Materials and Methods
Subjects
Pathogen-free adult male Sprague–Dawley rats (300–375 g; Harlan Labs, Madison, WI) were used in all experiments. Rats were housed in temperature (23±3 °C) and light (12 h:12 hr light:dark cycle; lights on at 0700) controlled rooms with standard rodent chow and water available ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Colorado at Boulder. Each study involves n = 6 per group.
Drugs
Morphine sulfate was kindly gifted by Mallinckrodt, Inc. (St. Louis, MO, USA). Methadone base (only the [−]-isomer) was obtained from the National Institute on Drug Abuse (Research Triangle Park, NC, USA). Endotoxin-free solutions of recombinant met-human interleukin-1 (IL-1) receptor antagonist, its vehicle and lyophilized tumor necrosis factor-α (TNF-α) soluble receptor (endotoxin-free polyethylene glycol recombinant-met-human soluble TNF-α receptor type 1) were kindly supplied by Amgen (Thousand Oaks, CA, USA). Neutralizing rabbit antibodies against the rat fractalkine receptor (α-CX3CR1) were purchased from Torrey Pines Biolabs (Houston, TX, USA). L-NAME, Fc-IL-10, minocycline, SB203508, MK-801 and placebo rabbit IgG antibodies (normal IgG) were purchased from Sigma (St. Louis, MO, USA). Ac.YVAD-cmk (YVAD) was purchased from Calbiochem (San Diego CA, USA). Endotoxin-free sheep anti-rat IL-6 neutralizing antibody was kindly provided by the European Cytokine Network. Where applicable, drugs were prepared and are reported as free base concentrations.
Catheter implantation
The method of constructing and implanting the indwelling intrathecal catheters in rats was based on that described previously (Milligan et al., 1999). Briefly, intrathecal catheters were implanted under anesthesia (isoflurane; Phoenix Pharmaceuticals, St. Joseph, MO, USA) by threading sterile polyethylene-10 tubing (PE-10 Intramedic Tubing; Becton Dickinson Primary Care Diagnostics, Sparks, MD, USA) guided by an 18-gauge needle between the L5 and L6 vertebrae. The catheter was threaded rostrally such that the proximal catheter tip lay over the lumbosacral enlargement. The needle was removed and the catheter was sutured to the superficial musculature of the lower back and the exterior end led subcutaneously to exit through a small incision at the nape of the neck. For acute intrathecal drug experiments (Experiment 1, Experiment 2 and Experiment 3), the catheters were preloaded with drugs at the distal end in a total volume of no greater than 25 µl. The catheters were 90 cm in length, allowing remote drug delivery without touching or otherwise disturbing the rats during the testing. Acute and chronic intrathecal drug experiments began 2 hr and 7 days after surgery, respectively. The surgery for chronic indwelling intrathecal catheters was exactly the same as for the acute intrathecal catheter, except that after the catheter was led subcutaneously to the nape of the neck the catheter was heat sealed with approximately 3 cm of the catheter protruding to allow easy access for subsequent dosing. These catheters were not preloaded with drug and were instead filled with injection saline.
For the subcutaneous catheter, a 90 cm length of PE-10 tubing was sutured to the superficial musculature of the lower back at the same time as the intrathecal catheter was implanted in these animals. The exterior end of the subcutaneous catheter paralleled the intrathecal catheter out of the same incision in the nape of the neck, allowing for remote subcutaneous administration without disturbance of the animals.
Behavioral measures
Hargreaves tests for analgesia and hyperalgesia
Rats received at least three 60 min habituations to the test environment prior to behavioral testing. Latencies for behavioral response to radiant heat stimuli applied to the plantar surface of each hind-paw and tail were assessed using a modified Hargreaves test (Hargreaves et al., 1988). All testing was conducted blind with respect to group assignment. Pilot studies determined that intrathecal catheter surgery did not affect baseline responses after 2 hr or 7 days recovery from surgery, compared to latencies recorded prior to surgery. Briefly, baseline withdrawal values were calculated from an average of 2 consecutive withdrawal latencies of the tail and the left and the right hind-paws, measured at 15 min intervals. The intensity of the radiant heat stimuli were adjusted across tests so to obtain either short or long baseline latencies. This allowed quantification of analgesia (lengthening of the latency, relative to baseline, in response to pain suppressive procedures) and hyperalgesia (shortening of the latency, relative to baseline, in response to pain enhancing procedures), respectively. Latencies for the short baseline latency Hargreaves stimuli at baseline ranged from 2 to 3 s, and a cut-off time of 10 s was imposed to avoid tissue damage. Latencies for the long baseline latency Hargreaves stimuli at baseline ranged from 8 to 10 s, and a cut-off time of 20 s was imposed to avoid tissue damage. While it is possible that these two stimuli may activate different sensory afferents (Yeomans and Proudfit, 1996), the need for two different stimuli is due to the type and direction of the anticipated behavioral response, and are standardly used for this purpose in pain research. The order of paw and tail testing varied randomly. Nociceptive assessments for acute administration experiments were then made at 0 (immediately following remote drug delivery), 5 min, 15 min and every 10 min thereafter until completion of the experiment only using the short baseline latency Hargreaves stimuli. For the chronic drug delivery experiments short and long baseline latency Hargreaves stimuli were employed on alternating time points, and in these experiments the rats were tested pre dose and over a 2 hr post dose timecourse on days 1, 4 and 7 of dosing. For twice daily injections the nociceptive response to the morning dose was assessed.
Von Frey test for mechanical allodynia
Rats received at least three 60 min habituations to the test environment prior to behavioral testing. Response thresholds to calibrated light pressure stimuli applied to the plantar surface of the paws was measured using the von Frey test (Chaplan et al., 1994). The test was performed using 0.406–15.136 g calibrated Semmes-Weinstein monofilaments (von Frey hairs; Stoelting, Wood Dale, IL, USA) as described in detail previously (Milligan et al., 2000). Briefly, rats were first assessed for baseline response thresholds (average of three consecutive withdrawal assessments) from each paw at 15 min intervals, and the average response threshold from both feet was calculated. All testing was conducted blind with respect to group assignment. The behavioral responses were used to calculate the absolute threshold, by fitting a Gaussian integral psychometric function using a maximum-likelihood fitting method (Harvey, 1986; Treutwein and Strasburger, 1999), as described in detail previously (Milligan et al., 2000). Allodynia was assessed pre and post intrathecal drug delivery on days 1 and 4 and pre dose on day 7 of chronic dose regimens. In experiment 4 and Experiment 5 where von Frey testing was employed, the same animals that were tested on von Frey also received Hargreaves testing prior to and following the Hargreaves timecourse.
Drug administration
For acute intrathecal indwelling catheters employed in Experiment 1 (Figure 1), morphine, methadone, or vehicle (saline) was dosed at 15 µg in 1 µl with a 10 µl flush of saline. This initial dose of opioid was followed by IL-1ra 100 µg or vehicle in 1 µl (Figures 1A,B,C) with a 10 µl flush of saline. Subcutaneous morphine (4 mg/kg in a dose volume of 1 ml/kg in saline) was followed by intrathecal administration of IL-1ra (100 µg in 1 µl with a 10 µl flush saline) in Figure 1D. Intrathecal dosing in Experiment 2 consisted of intrathecal co-administration of morphine and intrathecal IL-1ra or vehicle (0.1 to 15 µg morphine in 1 µl and IL-1ra 100 µg in 1 µl with 10 µl flush) (Figure 2A,B,C). For the quantification of spinal morphine levels, morphine was co-administered with IL-1ra or vehicle (15 µg morphine in 1 µl plus 100 µg IL-1ra in 1 µl with a 10 µl flush saline). Figures 3A and B of Experiment 3 involved intrathecal injections of morphine (15 µg in 1 µl with a 10 µl flush saline) following by intrathecal TNF-α soluble receptor (300 µg in 5 µl as previously published by Milligan et al. (2001) with a 10 µl flush, Figure 3A) or intrathecal IL-6 neutralizing antibody (0.325 µg; in 5 µl as previously published by Milligan et al. (2005; 2003) with a 10 µl flush saline). For Figures 3C,D,E,F, morphine (15 µg in 1 µl with a 10 µl flush saline) was co-administrated intrathecally with minocycline (100 µg in 3 µl as previously published by Ledeboer et al. (2005) at time of catheter implant and 33.3 µg in 1 µl with a 10 µl flush saline with morphine), fractalkine receptor antibody (α-CX3CR1 10 µg in 1 µl as previously published by Johnston et al. (2004) at time of catheter implant and then again co-administered with morphine in 1 µl with a 10 µl flush saline), L-NAME (5 µg in 1 µl as previously published by Holguin et al. (2004) in 1 µl with a 10 µl flush) and SB203508 (10 µg in 1 µl as previously published by Wu et al. (2006) with morphine in 1 µl with a 10 µl flush). In Experiment 4, animals were administered once daily 15 µg morphine, 15 µg methadone or saline (in 1 µl with a 25 µl flush) intrathecally for 7 days via an indwelling catheter. For Experiment 5, animals received 20 µg morphine (in 1 µl with a 25 µl flush) twice daily via an indwelling intrathecal catheter, co-administered with IL-1ra (100 µg in 1 µl) plus Fc-IL-10 (250 ng in 5 µl), IL-1ra (100 µg in 1 µl) plus YVAD (500 ng in 5 µl), morphine co-administered with vehicles, or saline co-administered with vehicles.
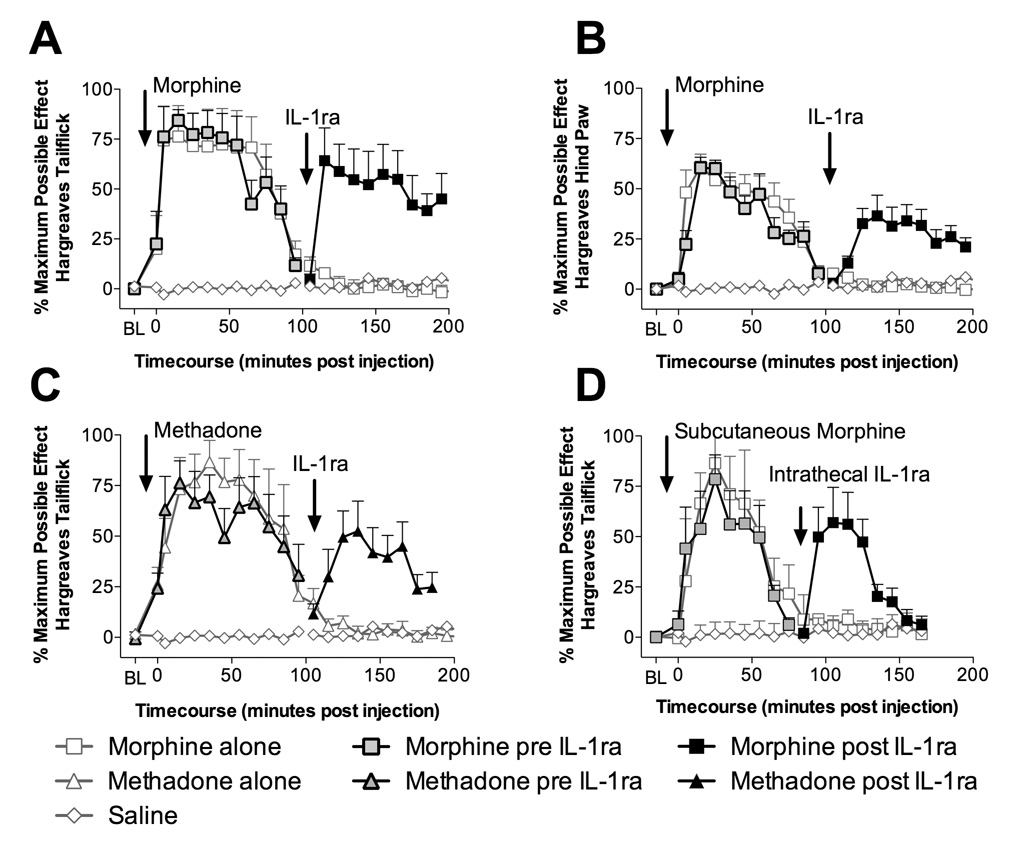
Figure 1. Intrathecal interleukin-1 receptor antagonist "unmasks" both intrathecal and systemic opioid analgesia.
A: Intrathecal injection of 15 µg of morphine (in 1 µl with a 10 µl flush; black square with gray center) produces significant increases in tailflick latencies (5 min to 85 min compared to vehicle treated animals or saline plus IL-1ra treated animals [gray diamond with white center]; P < 0.05) which dissipated by ~100 min, following which intrathecal IL-1ra (100 µg in 1 µl with a 10 µl flush; black square) unmasked continuing analgesia (115 min to 175 min compared to morphine followed by vehicle treated animals [gray square with white center] and vehicle followed by IL-1ra [gray diamond with white center]; P < 0.05). B: Intrathecal injection of 15 µg of morphine (in 1 µl with a 10 µl flush; black square with gray center) produces significant increases in hind paw withdraw latencies (15 min to 85 min compared to vehicle treated animals or saline plus IL-1ra treated animals [gray diamond with white center]; P < 0.05) which dissipated by ~100 min, following which intrathecal IL-1ra (100 µg in 1 µl with a 10 µl flush; black square) unmasked continuing analgesia (125 min to 165 min compared to morphine followed by vehicle treated animals [gray square with white center] and vehicle followed by IL-1ra [gray diamond with white center]; P < 0.05). C: Intrathecal injection of 15 µg of methadone (in 1 µl with a 10 µl flush; black triangle with gray center) produces significant analgesia (5 min to 85 min compared to vehicle treated animals or saline plus IL-1ra treated animals [gray diamond with white center]; P < 0.01) which dissipates by ~100 min, following which intrathecal IL-1ra (100 µg in 1 µl with a 10 µl flush; black triangle) unmasked continuing analgesia (125 min to 165 min; P < 0.05 compared to methadone followed by vehicle treated animals [gray triangle with white center] and vehicle followed by IL-1ra [gray diamond with white center]) in a similar fashion to the IL-1ra unmasking of morphine analgesia. D: Subcutaneous morphine (4 mg/kg in a dose volume of 1 ml/kg; black square with gray center) produced significant analgesia (5 min to 55 min compared to vehicle treated animals or saline plus IL-1ra treated animals [gray diamond with white center]; P < 0.05) dissipating by ~75 min. Intrathecal administration of IL-1ra (100 µg in 1 µl with a 10 µl flush; black square) unmasked significant continuing analgesia (95 min to 125 min compared to morphine followed by vehicle treated animals [gray square with white center] and vehicle followed by IL-1ra [gray diamond with white center]; P < 0.01) that lasted for a further 50 min.
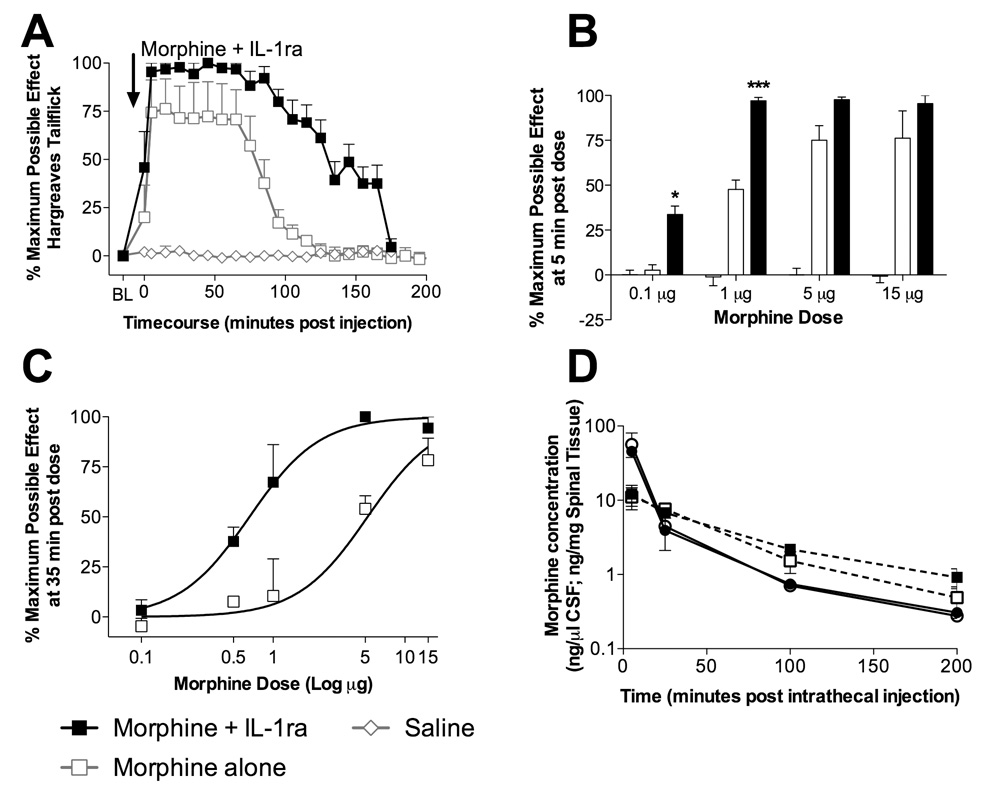
Figure 2. Intrathecal interleukin-1 receptor antagonist potentiates morphine analgesia, independent of a change in pharmacokinetics.
A: Intrathecal co-administration of morphine and intrathecal IL-1ra (15 µg morphine in 1 µl and IL-1ra 100 µg in 1 µl with 10 µl flush; black square) produced significant analgesia lasting for 175 min (0 min to 165 min compared to vehicle plus IL-1ra treated animals [gray diamond with white center]; P < 0.001) which was significantly greater than morphine alone (85 to 145 min; gray square with white center; P < 0.05). B: Responses to the Hargreaves test were assessed 5 min after intrathecal administration of morphine alone (0.1 µg, 0.5 µg, 1 µg, 5 µg and 15 µg in 1 µl with a 10 µl flush; open bars), morphine co-administered with IL-1ra (0.1 µg, 0.5 µg, 1 µg, 5 µg and 15 µg morphine in 1 µl plus 100 µg IL-1ra in 1 µl followed by a 10 µl flush; filled bars) and vehicle treated animals (gray bars). Co-administration of IL-1ra with morphine significantly potentiated 0.1 µg and 1 µg morphine analgesia 5 min following drug administration, demonstrating the speed of onset of IL-1 opposition of morphine analgesia. C: Responses to the Hargreaves test were assessed 35 min after intrathecal administration of morphine alone (0.1 µg, 0.5 µg, 1 µg, 5 µg and 15 µg in 1 µl with a 10 µl flush; black square with white center) and morphine co-administered with IL-1ra (0.1 µg, 0.5 µg, 1 µg, 5 µg and 15 µg morphine in 1 µl plus 100 µg IL-1ra in 1 µl followed by a 10 µl flush; black square). Morphine alone produced an dose response curve with an EC50 of 4.9 µM, while morphine co-administered with IL-1ra caused a leftward shift in the dose response function with an EC50 of 0.66 µM. D: CSF concentrations of morphine (ng morphine per µl CSF) over time following an intrathecal injection of morphine alone (15 µg in 1 µl with a 10 µl flush; black circle with white center) and morphine co-administered with IL-1ra (15 µg morphine in 1 µl plus 100 µg IL-1ra in 1 µl with a 10 µl flush; black circle) were quantified. Dorsal spinal cord concentrations of morphine over time following an intrathecal injection of morphine alone (15 µg in 1 µl with a 10 µl flush; black square with white center) and morphine co-administered with IL-1ra (15 µg morphine in 1 µl plus 100 µg IL-1ra in 1 µl with a 10 µl flush; black square) were also quantified. No significant differences in morphine CSF or dorsal spinal cord tissue concentration were observed at any time point (P > 0.05).
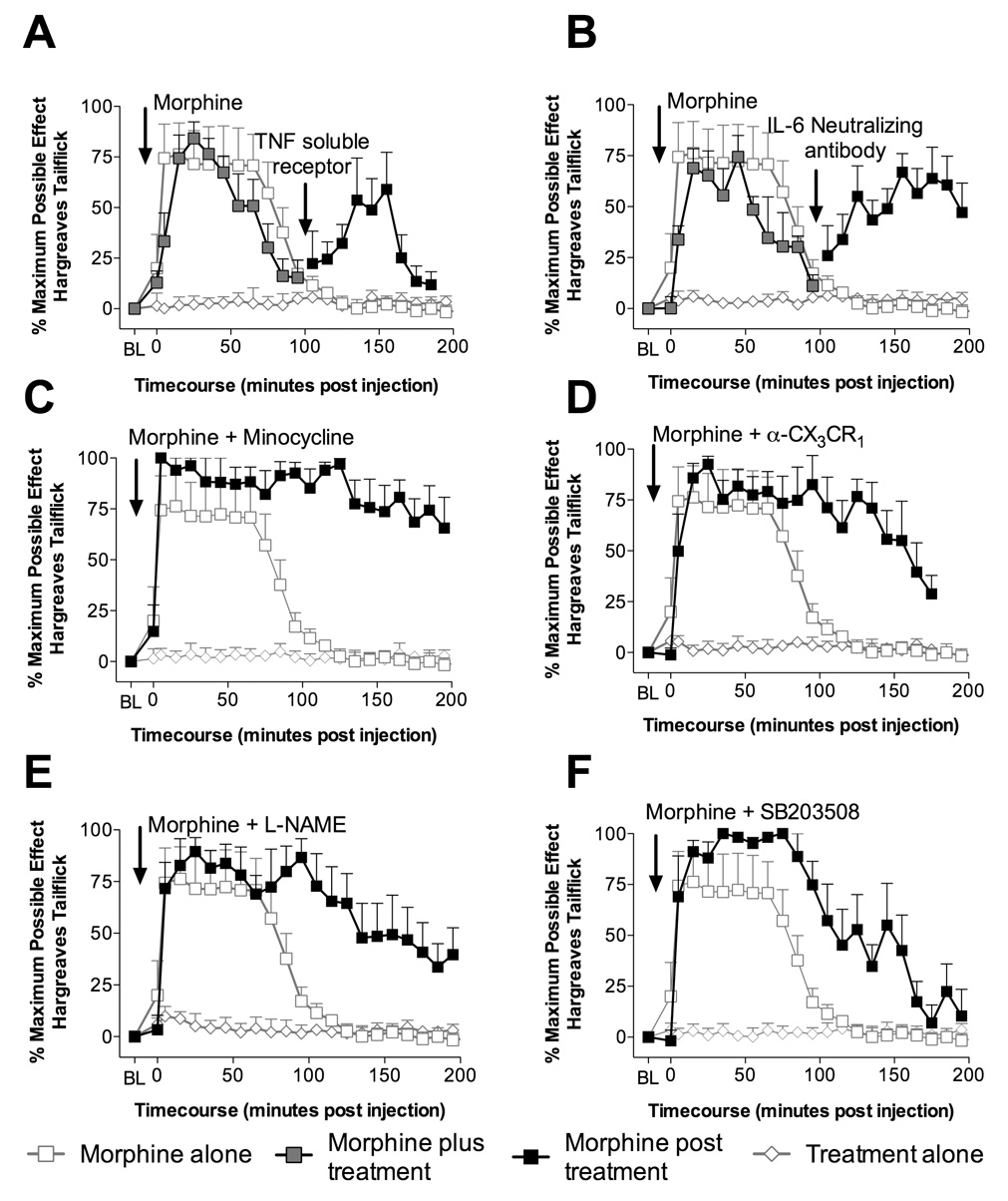
Figure 3. Characterization of potential mediators opposing acute morphine analgesia.
A: Intrathecal injection of morphine (15 µg in 1 µl with a 10 µl flush; black square with gray center) produces analgesia that dissipated by ~100 min, following which intrathecal TNF-α soluble receptor (300 µg in 5 µl as previously published by Milligan et al. (2001) with a 10 µl flush; black square) unmasked significant continuing analgesia (135 min and 155 min compared to morphine control gray square with white center; P < 0.05), whilst IgG control or TNF-α soluble receptor alone had no effect (controls average, gray diamond with white center). B: Intrathecal injection of morphine (15 µg in 1 µl with a 10 µl flush; black square with gray center) produces analgesia dissipating by ~100 min, following which intrathecal IL-6 neutralizing antibody (0.325 µg; in 5 µl as previously published by Milligan et al. (2005; 2003) with a 10 µl flush; black square) unmasked significant continuing analgesia (125 min to 185 min compared to morphine control gray square with white center; P < 0.05), whilst IgG or IL-6 neutralizing antibody alone had no effect (controls average, gray diamond with white center). C: Intrathecal co-administration of morphine (15 µg in 1 µl with a 10 µl flush; black square) and minocycline (100 µg in 3 µl as previously published by Ledeboer et al. (2005) at time of catheter implant and 33.3 µg in 1 µl with 15 µg morphine in 1 µl with a 10 µl flush) produced significantly potentiated analgesia compared to morphine alone (gray square with white center; 85 min to 245 min; P < 0.05), whilst minocycline alone had no effect (gray diamond with white center). D: Intrathecal co-administration of morphine (15 µg in 1 µl with a 10 µl flush; black square) and fractalkine receptor antibody (α-CX3CR1 10 µg in 1 µl as previously published by Johnston et al. (2004) at time of catheter implant and then again co-administered with 15 µg morphine in 1 µl with a 10 µl flush) produced significantly potentiated analgesia compared to morphine alone (gray square with white center; 95 min to 155 min; P < 0.05), whilst fractalkine receptor antibody alone had no effect (gray diamond with white center). E: Intrathecal co-administration of morphine (15 µg in 1 µl with a 10 µl flush; black square) and L-NAME (5 µg in 1 µl as previously published by Holguin et al. (2004) with 15 µg morphine in 1 µl with a 10 µl flush) produced significantly potentiated analgesia compared to morphine alone (gray square with white center; 95 min to 155 min; P < 0.05), whilst L-NAME administration alone had no significant effect (gray diamond with white center). F: Intrathecal co-administration of morphine (15 µg in 1 µl with a 10 µl flush; black square) and SB203508 (10 µg in 1 µl as previously published by Wu et al. (2006) with 15 µg morphine in 1 µl with a 10 µl flush) produced significantly potentiated analgesia compared to morphine alone (gray square with white center; 85 min to 155 min; P < 0.05), whilst SB203508 alone had no effect (gray diamond with white center).
Spinal cord tissue and cerebrospinal fluid (CSF) collection
Under pentobarbital anesthesia (50 mg/kg), CSF was collected by threading sterile PE-10 tubing guided by an 18-gauge needle between the L5 and L6 vertebrae on the side contralateral to the chronically implanted catheter. The catheter used to collect CSF was inserted rostrally such that the catheter tip lay over the lumbosacral enlargement. Approximately 20–30 µl of CSF was withdrawn and immediately flash-frozen in liquid nitrogen. Rats were then transcardially perfused with chilled saline. The lumbosacral enlargement of the spinal cord was exposed by laminectomy, and after verifying intrathecal catheter placement by visual inspection, the lumbosacral spinal cord was then dissected free and placed on an ice-chilled glass plate. The dorsal aspects of these tissues were dissected, divided into left and right halves, and separately flash-frozen in liquid nitrogen. Left and right halves of the dorsal spinal cord were randomly assigned to mRNA or protein quantification. The entire procedure required a maximum of 15–20 min per animal. Samples were stored at −80°C until the time of assay.
In vitro lumbar dorsal spinal cord preparation
Whether morphine induces the release cytokines and/or chemokines was tested using an in vitro assay in a fashion similar to that previously described (Johnston et al., 2004). This in vitro assay was chosen as it allows release of proteins into the supernatant to be sensitively assessed, as proteins accumulate in the closed system rather than diffusing away as occurs with in vivo sampling of cerebrospinal fluid. Assay of spinal cord tissue is inappropriate for addressing this issue as chemokine/cytokine levels in tissue cannot distinguish between intracellular content and released protein. Thus, the lumbar spinal cord was rapidly exposed from unanaesthetized decapitated rats using aseptic technique, and then the cord was placed in a 70% ethanol rinse. The dorsal spinal cord was isolated and rinsed with sterile HBSS, and a 1.75 cm section of the lumbar enlargement was isolated. Tissue was placed in 25 µl of incubation medium (DMEM, supplemented with 2 mM L-glutamine, 100 U penicillin, 100 µg streptomycin and 10 mM HEPES buffer (Sigma)), inside a sterile modified 500 µl Eppendorf centrifuge tube. The tube was modified by removal of the snap cap and creation of a 2 mm wide slit down one side to within 7 mm of the base. Once the tissue was inserted into the tube, dorsal side up, media and drug were added such that the total volume was 200 µl. Tissues were incubated with 10 µM and 100 µM morphine equivolume vehicle and incubated for 3 hr at 37°C, 5% CO2-95% air. After incubation, supernatants were collected and assayed for cytokines and chemokines using a custom rat multiplex described below (IL-1β, IL-6, IL-10, Fractalkine, GRO/KC, MIP-1α, MCP-1, RANTES and TNF-α). One half of the tissue was also collected for TLR4 protein analysis by western blot (described below).
Cytokine, chemokine and TLR4 receptor quantification
Procedures for tissue processing for protein quantification were similar to those described in detail previously (Hansen et al., 2000a; Hansen et al., 2000b; Johnston et al., 2004). Multiplex protein quantification (Thermo Scientific SearchLight Multiplex Sample Testing Service, Woburn, MA, USA) was utilized to quantify 16 rat analytes from single samples. Specifically the cytokines: tumor necrosis factor-α (TNF-α; limit of quantification [LOQ] 0.39 pg/ml), granulocyte macrophage colony stimulating factor (GMCSF; LOQ 0.39 pg/ml), interferon -γ (IFN-γ; LOQ 0.78 pg/ml), interleukin-10 (IL-10; LOQ 0.1 pg/ml), interleukin-1α (IL-1α; LOQ 0.2 pg/ml), interleukin-1β (IL-1β; LOQ 0.78 pg/ml), interleukin-2 (IL-2; LOQ 0.78 pg/ml), interleukin-4 (IL-4; LOQ 0.78 pg/ml) and interleukin-6 (IL-6; LOQ 1.56 pg/ml); and chemokines: GRO/KC (CXCL1; LOQ 0.1 pg/ml), monocyte chemotatic protein-1 (MCP-1, CCL2; LOQ 0.1 pg/ml), macrophage inflammatory protein-1α (MIP-1α; LOQ 0.05 pg/ml), macrophage inflammatory protein-2 (MIP-2; LOQ 0.05 pg/ml), macrophage inflammatory protein-3α (MIP-3α; LOQ 0.39 pg/ml), Regulated upon Activation Normal T-cell Expressed and Secreted (RANTES; LOQ 0.1 pg/ml); were quantified from one half of the dorsal spinal cord at the level of the lumbar enlargement and CSF collected over this region. Dorsal spinal cord samples from the remaining hemisphere were prepared for real-time reverse transcription-PCR cytokine mRNA quantification in the same fashion as previously reported. Both protein and mRNA were analyzed blind with respect to group assignment. The following targets were investigated and expressed relative to the levels of the housekeeping gene GAPDH (M17701; forward: GTTTGTGATGGGTGTGAACC; reverse: TCTTCTGAGTGGCAGTGATG); IL-1β (M98820; forward: GAAGTCAAGACCAAAGTGG; reverse: TGAAGTCAACTATGTCCCG), TNF-α (D00475; forward: CTTCAAGGGACAAGGCTG; reverse: GAGGCTGACTTTCTCCTG), IL-6 (NM_012589; forward: ACTTCACAGAGGATACCAC; reverse: GCATCATCGCTGTTCATAC), IL-1 converting enzyme (D85899; forward: TGGTCTTGTGACTTGGAGG; reverse: CCTTTCAGTGGTTGGCATC) and Toll-like receptor 4 (NM_019178; forward: CAGAGGAAGAACAAGAAGC; reverse: CCAGATGAACTGTAGCATTC). Primers were purchased from Proligo (Boulder, CO, USA). mRNA data were expressed as relative to the house keeping gene (GAPDH) using the ΔΔCT method. TLR4 protein was examined by Western blot. Spinal cord tissue from the in vitro lumber dorsal cord experiment and supernatants from the 16 multiplex were assayed for total protein content via BCA assay (Pierce, Rockford, IL). Each sample (60 µg) and positive controls (lysates from HEK-TLR4 cells, Invivogen, San Diego, CA, USA) were subjected to SDS-PAGE and Western blot analysis. Briefly, samples were electrophoresed through 6% polyacrylamide gels. Proteins were transferred onto a nitrocellulose 0.45-µm membrane (BioRad, Hercules, CA, USA) and blocked on a rotating table for 1hr at room temperature (RT) in Tris-buffered saline-TWEEN-20 (TBS-T), 5% milk (24 mM Tris-HCl, 137 mM NaCl, 5% non-fat dry milk, pH 7.4). After blocking, the membranes were incubated overnight at 4°C with affinity purified goat anti-rat TLR4 (1:400 dilution) antibody (Santa Cruz Antibodies, Santa Cruz, CA) in TBS plus 0.1% TWEEN-20 (TBS-T) with 5% milk. The membranes were washed 3 times (20 min per wash) in TBS-T and subsequently incubated in a peroxidase-conjugated affinity purified donkey anti-goat IgG (H+L) (1:10,000) (Jackson ImmunoResearch, West Grove, PA, USA) in TBS-T, 5% milk for 1 hr at RT. The membranes were washed as before and developed for 1 min with ECL/ECLplus kit (GE Healthcare, Waukesha, WI, USA). Membranes were exposed Kodak X-OMAT film (Kodak, Rochester, NY, USA) and developed on a Kodak X-OMAT 1000-A processor. Band density was analyzed using ImageJ (v1.39i). Data were expressed relative to the integrated density of the TLR4 positive control run on each gel.
Spinal morphine quantification following intrathecal administration
CSF and lumbosacral dorsal spinal cord tissue morphine concentrations were quantified by a modification of a high-performance liquid chromatographic electrochemical detection method previously described (Doverty et al., 2001; van Crugten et al., 1997). The system consisted of an ESA 5600A Coularray detector with an ESA 5014B analytical cell and an ESA 5020 guard cell. The column was an ESA MD-150 (C-18, 3 µm, 150 × 3.2 mm), and the mobile phase was ESA buffer MD-TM (Chelmsford, MA, USA). The analytical cell potentials were kept at −100 mV and +250 mV and the guard cell at +300 mV. Lumbosacral dorsal spinal cord tissue was weighed and then sonicated in 1 ml of de-ionized water, while CSF was diluted in water for a total volume of 1 ml. Samples were then alkalinized with 500 µl of sodium bicarbonate buffer (500 mM; pH 9.6) and extracted with chloroform (6 ml) for 120 s on vortex followed by centrifugation (1700 × g; 10 min). The upper aqueous layer was aspirated to waste followed by a further addition of the sodium bicarbonate buffer. Samples were then vortexed (10 s) and centrifuged (1700 × g; 10 min). After aspirating the aqueous layer to waste, morphine was back extracted from 5 ml of chloroform into 300 µl NaH2PO4 (50 mM; pH 2) by vortexing for 120 s. After centrifugation, an aliquot (100 µl) of the aqueous phase was injected onto the system. Calibration standards ranged from 0.25 ng/ml to 400 ng/ml, and samples above this were diluted with water. High (300 ng/ml) and low (1 ng/ml) quality control samples were assayed with each assay, and were within 10% of the nominal concentrations. The limit of quantification was 0.25 ng/ml with inter assay variability of the bottom standard 8.3%. The slope and intercept of the 1/Y weighted line of best fit varied 6.2% and 7.4%, respectively. There were no interfering peaks in the chromatography.
Statistics
The analgesic responses were calculated, as is standard in the pain field (Carmody, 1995; Grumbach et al., 1977), as the % of maximal possible effect (%MPE) using the following equation (Carmody, 1995). For short baseline latency Hargreaves stimuli a cut-off of 10 s was used while for long baseline latency Hargreaves stimuli a 20 s cut-off was employed. Baseline latencies for the Hargreaves tests ranged from 2–3 s for the short baseline latency stimuli and 8–10 seconds for the long baseline latency stimuli. The morphine dose-response function was fitted using a Hill equation where the minimum value was constrained to zero and the maximum was constrained to 100. Statistical significance was assessed using a two-way repeated ANOVA with Bonferroni post hoc test when comparing the analgesic responses of intrathecal treatments vs their vehicle controls.
Statistical mediation analysis of nociceptive behaviors (change from baseline in tolerance, hyperalgesia and allodynia) by select dorsal spinal cord and CSF cytokines and chemokines was conducted. In order to select candidate cytokines and chemokines from both dorsal spinal cord and CSF, hierarchical cluster analysis was used to identify molecular endpoints that clustered with behaviors. The data was first standardized to remove the inherent variability between different cytokine and chemokine levels and the behavioral response values, thereby removing numerical magnitude bias from correlations. Hierarchical clustering was then used to analyze the absolute correlations between the cytokine and chemokine levels from dorsal spinal cord and CSF and nociceptive behaviors using the R Project package pvclust and 1000 bootstraps (Suzuki and Shimodaira, 2006). Analyses of this nature with similar datasets generated from DNA arrays has been performed extensively and is a well accepted method for identifying targets and clusters of targets of interest (Suzuki and Shimodaira, 2006). The cytokines and chemokines that clustered with behaviors in morphine and methadone treatment groups were selected by inspection and the standardized values for only these chosen protein values was then summed. Sobel simple mediation analysis with 10000 bootstraps was then conducted on the summed cluster values and behavioral results using the SPSS Sobel syntax script as previously described (Preacher and Hayes, 2004). Multivariate analysis was conducted using treatment as a fixed factor controlling for the summed cytokine cluster variable.
Analyses and calculations were conducted with Excel 2003 SP2 (Microsoft, Redmond, CA, USA), Prism 4.03 (GraphPad, San Diego, CA, USA), R Project (R Development Core Team, 2006) and SPSS 14 for Windows (SPSS Inc., Chicago, IL, USA). Data are presented as mean ± SEM. Significance was set at P < 0.05.
Results
Experiment 1: Intrathecal interleukin-1 receptor antagonist (IL-1ra) “unmasks” opioid analgesia
In all experiments, both hindpaw withdrawal and tailflick data were collected and were consistent in the results found. For simplicity, tailflick data are primarily shown (e.g. Figure 1A). Examples of hindpaw data are presented in Figure 1B for morphine, and statistical analyses incorporated into the text for each study. Intrathecal morphine (15 µg) analgesia dissipated by ~100-min (Figure 1A). At this time, intrathecal IL-1ra (100 µg), which blocks the actions of IL-1, unveiled further analgesia, which lasted for at least an additional 100 min (Figure 1A), while vehicle or saline had no effect. Intrathecal IL-1ra or vehicle (data not shown) following intrathecal saline at time 0, produced no significant change in withdrawal latencies. Together, these data imply that the effect of acute intrathecal morphine was opposed by the action of spinal IL-1, thereby masking it and prematurely returning withdrawal latencies to baseline. The phenomenon of spinal IL-1 modulation of opioid analgesia is not one restricted to morphine, since intrathecal IL-1ra following the completion of intrathecal methadone (15 µg) analgesia also unmasked further tailflick (Figure 1C) and hind paw (P < 0.05) analgesia.
Whether spinal IL-1 opposes systemic analgesia is unknown. Given the complexities of brain-to-spinal cord circuitries activated in response to systemic morphine, it cannot simply be assumed a priori that results obtained from intrathecal morphine would apply to systemic morphine. Therefore, we wished to determine whether the analgesia from an acute systemic morphine administration is also opposed by spinal IL-1 and hence whether intrathecal IL-1ra unmasks analgesia once systemic morphine analgesia has apparently dissipated. To do this, subcutaneous morphine (4 mg/kg) induced analgesia that dissipated by ~75 min (Figure 1D), at which time intrathecal IL-1ra resulted in a rebound in tailflick and hind paw analgesia that lasted a further 45 min. Intrathecal vehicle administered after subcutaneous morphine analgesia dissipated produced no change in nociceptive latencies; neither did IL-1ra after subcutaneous saline. These data suggest that acute systemic morphine is also opposed by spinal IL-1. Whether these effects occur because morphine increases spinal IL-1 release or is the result of basal IL-1 action cannot be resolved by these data.
Therefore, lumbar dorsal spinal cord sections were incubated for 3 hr in vitro with morphine and the release of proinflammatory cytokines and chemokines was quantified. Changes in toll like receptor 4 (TLR4) expression were also quantified in sections of the lumbar dorsal spinal cord tissue as a index of glial activation as glia but not neurons express TLR4 (Tanga et al., 2005). Morphine increased the release of IL-1β, IL-6, Fractalkine, GRO/KC, MIP-1α, MCP-1 and TNF-α (for each, P < 0.05), without modifying IL-10 or RANTES (Table 1). TLR4 protein expression was significantly elevated by the 3 hr in vitro morphine exposure (Media control: 0.54±0.06 vs. 100 µM morphine 0.88±0.1; P < 0.05).
Table 1.
Acute in vitro morphine-induced increases in proinflammatory cytokine and chemokine release.
| Control | Morphine 10 µM | Morphine 100 µM | ||||
|---|---|---|---|---|---|---|
| (pg/ml) | (pg/ml) | (pg/ml) | ||||
| Mean | SEM | Mean | SEM | Mean | SEM | |
| IL-1β | 11.3 | 1.1 | 15.4 | 2.0 | 19.0* | 2.5 |
| IL-6 | 163.1 | 19.4 | 345.9 | 60.1 | 381.3* | 55.7 |
| IL-10 | 0.9 | 0.3 | 1.7 | 0.3 | 1.9 | 0.5 |
| Fractalkine | 44.6 | 1.0 | 74.0* | 7.0 | 73.6* | 7.5 |
| GRO/KC | 317.2 | 93.5 | 1232.5 | 520.9 | 2477.2* | 500.4 |
| MIP-1α | 4.5 | 0.4 | 18.3* | 3.3 | 23.8* | 3.6 |
| MCP-1 | 258.8 | 18.3 | 400.3 | 43.6 | 441.4* | 57.3 |
| RANTES | 7.7 | 1.0 | 7.5 | 0.6 | 11.3 | 1.2 |
| TNF-α | 8.1 | 1.3 | 16.6 | 2.8 | 26.3* | 2.0 |
Proinflammatory cytokine and chemokine release from lumbar dorsal spinal cord sections incubated for 180 min in vitro with morphine (10 µM and 100 µM) compared to media alone
P < 0.05.
Experiment 2: Intrathecal IL-1ra rapidly potentiates analgesia without affecting intrathecal morphine pharmacokinetics
From Experiment 1, it is clear that the opposition of analgesia by IL-1 developed at some point during the first 90 min following morphine administration. How rapidly IL-1-induced masking occurs is unknown, but could be revealed by co-administering IL-1ra with an opioid agonist. The rapidity of IL-1 masking is important if it is the case that morphine leads to the release of IL-1 (discussed below), as a substantial delay in masking could suggest a downstream effect dependent on IL-1 transcription and/or translation, contrary to the rapid liberation of pre-existing pro-IL-1 stores envisioned here as negative feedback circuitry. An intrathecal morphine tailflick analgesia dose-response function was established with co-administered IL-1ra versus vehicle, allowing us to quantify the enhancement of morphine analgesia by minimizing IL-1 actions.
As an example of the potentiation of morphine analgesia by co-administered intrathecal IL-1ra, Figure 2A illustrates that intrathecal morphine (15 µg) + IL-1ra produced significant analgesia lasting for ~175 min. In contrast, saline + IL-1ra produced no change in nociceptive latencies (Figure 2A). Comparing analgesia achieved by morphine (15 µg) + IL-1ra versus morphine (15 µg) alone (Figure 2A) revealed significant potentiation of morphine analgesia by morphine (15 µg) + IL-1ra from 85 to 145 min. Analysis of the area under the curve of these data confirmed that intrathecal co-administration of IL-1ra potentiated and prolonged morphine analgesia (area under the analgesia curve of intrathecal morphine then intrathecal saline 6,792 ± 1199 versus morphine plus IL-1ra 13,281 ± 720; P = 0.0009). Once again this phenomenon is not restricted to morphine as methadone + IL-1ra also resulted in a significant increase in analgesia ([−]-methadone alone: 5991 ± 2571; [−]-methadone + IL-1ra: 8699 ± 1388; P < 0.05).
As a ceiling effect prevented a clear analysis of the speed of IL-1 effects following the 15 µg morphine dose above, lower doses of morphine were examined. The speed of onset of IL-1 action was assessed by examining the withdrawal latencies 5 min after intrathecal administration of a range of morphine doses given alone or in combination with IL-1ra (Figure 2B). Morphine analgesia at 0.1 µg and 1 µg was significantly potentiated by IL-1ra co-administration even at this early (5 min) timepoint (P < 0.05). The action of IL-1ra at higher morphine doses could not be observed due to the ceiling effect of the Hargreaves test cut-off. These analyses implicate a rapid onset of IL-1 action following opioid administration and hence the rapid onset of the opposing process.
To pharmacologically characterize whether the potentiation of analgesia by IL-1ra would result in a leftward shift in the morphine dose-response function, an intrathecal morphine dose-response function was generated in the presence versus absence of IL-1ra for responses at the peak of the morphine effect (35 min following administration; Figure 2C). IL-1ra caused a leftward shift in the morphine dose-response function with a shift in the ED50 from 4.9 µg for morphine alone to 0.66 µg when morphine was co-administered with IL-1ra. There was no significant difference in the Hill slope of the equation between the 2 sets of data (F-test P = 0.69) so the same Hill slope value of 1.353 was used to fit the 2 data sets (morphine alone R2 = 0.79 and morphine co-administered with IL-1ra R2 = 0.86). When the Hill slope was kept constant, an F-test revealed that the ED50 values between the two conditions are significantly different (P < 0.0001). Therefore, co-administration of IL-1ra with morphine produced a nearly 8-fold (4.9 µg divided by 0.66 µg) increase in analgesic efficacy.
One potential explanation for potentiation of morphine analgesia by IL-1ra would be a simple modification in the pharmacokinetic clearance or distribution of morphine, rather than a pharmacodynamic change in efficacy. However, analysis of cerebrospinal fluid and dorsal spinal cord tissue (Figure 2D) revealed no change in morphine concentrations with the co-administration of IL-1ra. Thus altered morphine clearance from the intrathecal compartment or spinal tissue cannot account for the potentiation of morphine analgesia observed as a consequence of IL-1ra co-administration, meaning that IL-1ra likely produced a pharmacodynamic enhancement by antagonizing the actions of IL-1 that oppose morphine analgesia.
Experiment 3: Several opioid-induced proinflammatory mechanisms oppose opioid analgesia
To date, only IL-1 has been implicated in modulating the effects of opioids (Johnston et al., 2004; Shavit et al., 2005). However, IL-1 is but one member of a family of proinflammatory cytokines. Johnston et al. (2004) demonstrated spinal increases in IL-1, tumor necrosis factor-α (TNF-α) and IL-6 following chronic intrathecal morphine, but provided no data to implicate a functional role of any of these cytokines in response to acute morphine, as ceiling effects were produced by the dose of morphine used. Shavit et al. (2005) potentiated acute systemic morphine analgesia by systemic IL-1ra in mice; whether IL-1ra exerted its effects at spinal sites of action in that study is unknown and unlikely given that IL-1ra is a large protein with limited, at best, access to CNS after systemic administration. Thus, there are no prior acute opioid exposure data demonstrating the influence of proinflammatory cytokines on morphine analgesia, as presented here.
Here soluble TNF-α receptor (300 µg; Figure 3A) and IL-6 neutralizing antibody (0.325 µg; Figure 3B) were administered intrathecally either alone or after morphine analgesia had returned to baseline. In all cases when the antagonist was administered alone, no changes in nociceptive latencies were observed. Neutralizing the action of TNF-α after the dissipation of morphine analgesia resulted in unmasking of significant tailflick analgesia from 135 min to 155 min (Figure 3A). Neutralizing IL-6 also unmasked significant analgesia from 125 min to 185 min (Figure 3B). Unmasking of significant hind paw analgesia by neutralizing IL-6 and TNF-α was also observed (P < 0.05). These data implicate IL-6 and TNF-α, in addition to IL-1, in opposing opioid analgesia.
An initial assessment of a potential cellular source of the products that result in the opposition of acute opioid analgesia was undertaken. The potential of morphine-induced microglial involvement was directly assessed by co-administration of morphine plus the putative microglial inhibitor minocycline (100 µg prior and then 33.3 µg with morphine). This significantly potentiated tailflick (Figure 3C) and hind paw analgesia compared to morphine alone. These data are suggestive that the opposition of acute morphine analgesia may be dependent, at least in part, on microglia. To further test this microglial hypothesis, we explored whether fractalkine signaling may be involved in the opposition of acute morphine analgesia. Under normal conditions, fractalkine (CX3CL1) expression in spinal cord appears to be limited to neurons, whereas its receptor (CX3CR1) appears limited to microglia (Verge et al., 2004). This neuron-to-microglia signal induces, at minimum, IL-1 release from spinal cord (Johnston et al., 2004). While chronic intrathecal morphine-induced release of fractalkine contributes to morphine tolerance, allodynia and hyperalgesia, whether fractalkine contributes to the opposition of acute morphine analgesia is unknown (Johnston et al., 2004). CX3CR1 neutralizing antibody (10 µsg) prior to intrathecal morphine significantly potentiated tailflick (Figure 3D) and hind paw analgesia (P < 0.05). This result suggests that acute opposition of morphine analgesia is fractalkine dependent agreeing with the microglial dependent nature of the opposition of acute morphine analgesia. The sum of the minocycline and fractalkine data suggests that microglia may be key cellular mediators of the opposition of morphine analgesia.
Beyond exploring proinflammatory cytokines and their potential cellular sources, we also wished to assess the potential second messenger cascades involved in the opioid-induced proinflammatory cytokine signaling. The first candidate to be explored was nitric oxide (NO), as it is a key mediator of proinflammatory cytokine production (Holguin et al., 2004). Intrathecal co-administration of the NO synthase inhibitor L-NAME (5 µg) with morphine significantly potentiated tailflick (Figure 3E) and hind paw analgesia compared to morphine alone. L-NAME is not a selective inhibitor of a particular cell type NO synthase and would inhibit both glial and neuronal NO sources.
The second candidate explored was p38 mitogen-activated protein kinase (MAPK), based in part on the work of Wu and colleagues. Wu et al. have demonstrated that an initial ultra low dose of morphine induces glial activation, via a p38 MAPK dependent mechanism (Wu et al., 2006), and that this activation of p38 opposes subsequent morphine analgesia (Wu et al., 2005). Therefore, the p38 MAPK inhibitor SB203508 (10 µg) was co-administered intrathecally with morphine to ascertain if similar mechanisms were involved in this model. Given that p38 MAPK activation is well documented to lead to proinflammatory cytokine production and release (Svensson et al., 2003; Wu et al., 2006), this would also predict a likely involvement in opposing morphine analgesia based on the results of Experiment 1. Indeed, inhibition of p38 MAPK activation potentiated tailflick (Figure 3F) and hind paw analgesia (P < 0.05) compared to morphine alone. As with L-NAME, SB203508 is a non-selective p38 MAPK inhibitor so has potential to be acting at neuronal as well as glial p38 MAPK.
Experiment 4: Opioid-induced glial activation and production of proinflammatory mediators contributes to opioid-induced tolerance, hyperalgesia and allodynia
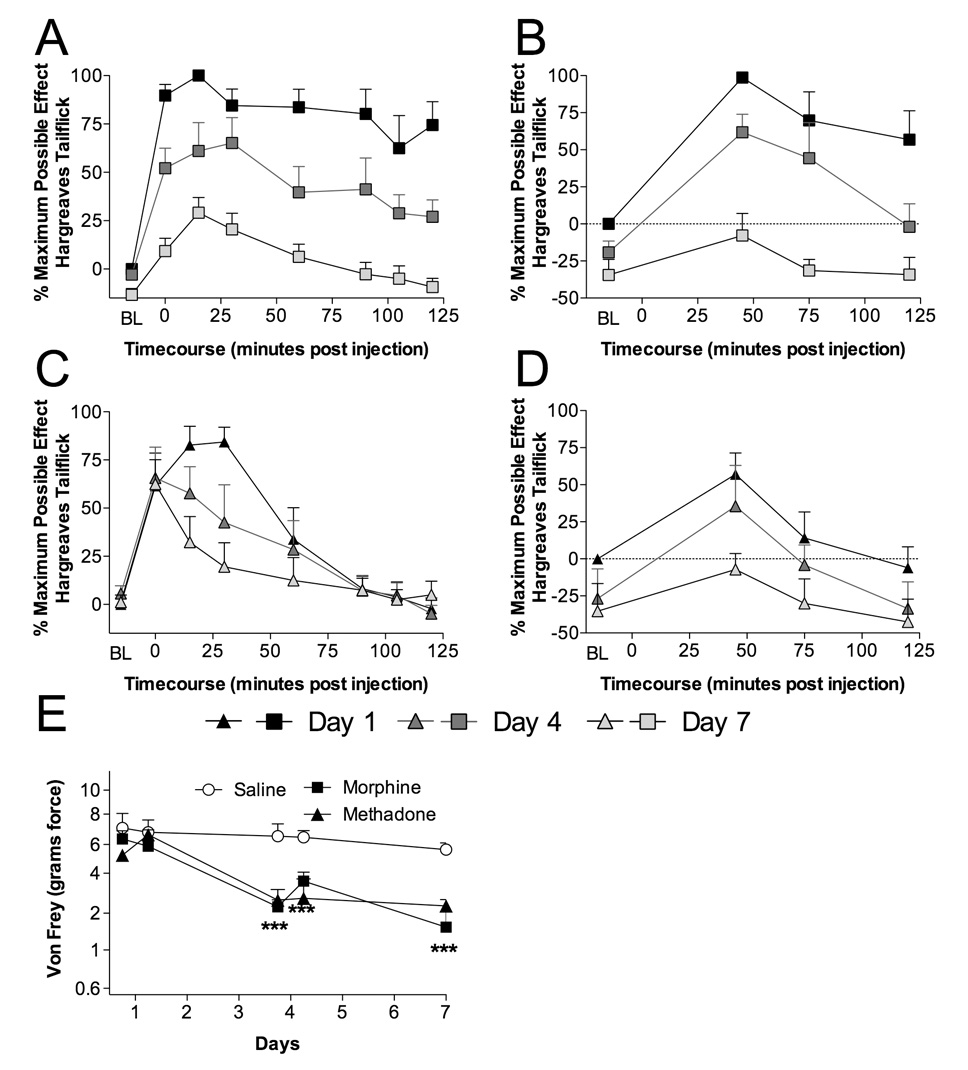
Repeated opioid administration results in the development of analgesic tolerance. Beyond the loss of analgesic efficacy, chronic opioids can increase nociception, producing heightened responses to painful stimuli (hyperalgesia) (Doverty et al., 2001; Johnston et al., 2004) and elicit painful responses from stimuli that were previously not perceived as painful (allodynia) (Johnston et al., 2004). The results of Experiment 1, Experiment 2 and Experiment 3 suggest that proinflammatory cytokines may be involved in the development of these unwanted and detrimental exaggerated pain states. To investigate these issues, animals were implanted with chronic intrathecal catheters and received once daily intrathecal morphine, methadone or saline administration, with behavioral testing to verify the development of analgesic tolerance (short baseline latency tailflick Hargreaves stimuli; Figure 4A,C), hyperalgesia (long baseline latency tailflick Hargreaves stimuli; Figure 4B,D) and allodynia (von Frey test; Figure 4E). CSF (protein) and dorsal spinal cord tissue (protein and mRNA) cytokine levels were quantified from these animals following nociceptive testing on day 7. In a significant departure from previous studies (Johnston et al., 2004), a far broader survey of proinflammatory cytokine and chemokine proteins was measured by utilizing multiplex analysis. In addition, TLR4 protein was quantified from the supernatants of the dorsal spinal cord sonicates following multiplex protein quantification. Significant elevations in dorsal spinal cord IL-1 and IL-6 mRNA were observed in both morphine and methadone treated groups compared to saline vehicle, while TNF-α and IL-1 converting enzyme were unaffected by either treatment (Table 2). Toll-like receptor (TLR) 4 mRNA levels were increased by both opioid treatments demonstrating a significant elevation of glial activation following intrathecal opioid administration, as glia but not neurons express TLR4 (Tanga et al., 2005). Analysis of CSF protein demonstrated that morphine and methadone treatment caused significant elevations of chemokines (fractalkine, GRO/KC, MIP-1α) as well as cytokines (IL-1β, IL-6, TNF-α, and IL-10) (Table 3A). Corresponding increases were observed in dorsal spinal cord IL-1β, IL-1α, IL-6, and TNF-α levels (Table 3B) and relative TLR4 protein levels (saline: 0.85± 0.03; morphine: 1.1±0.04; (−)-methadone: 1.0±0.03). The opioid treatment did not significantly alter dorsal spinal cord GM-CSF, GRO/KC, IFN-γ, IL-10, IL-2, IL-4, MCP-1, MIP-1α, MIP-2, MIP-3a or RANTES levels, while for CSF the levels of GM-CSF, IFN-γ, IL-1α, IL-2, IL-4, MCP-1, MIP-2, MIP-3a and RANTES remained constant (Table 3A,B).
Figure 4. Chronic intrathecal morphine and methadone administration produce significant tolerance, hyperalgesia, allodynia, and alterations in mRNA and/or protein levels of cytokines, chemokines and a glial activation marker.
Animals were administered once daily 15 µg morphine (A,B), 15 µg methadone (C,D) or saline (in 1 µl with a 25 µl flush) intrathecally for 7 days via an indwelling catheter. On days 1 (black square and triangle), 4 (black square and triangle with dark gray center) and 7 (black square and triangle with light gray center) animals were tested for short baseline latency Hargreaves stimuli (A,C) to assess analgesia and long baseline latency Hargreaves stimuli (B,D) to assess hyperalgesia over a 2 hr timecourse following drug administration. Significant reductions in analgesia were observed across the multiple days dosing, indicating the development of tolerance (A,C) and hyperalgesia (B,D). Mechanical allodynia (E) was assessed using von Frey filaments 60 min prior and 2 hr after administration on day 1 and day 4 and only 60 min prior to drug administration on day 7 for morphine (black square), methadone (black triangle) or saline (black circle with white center). *** P < 0.001
Table 2.
Chronic morphine and methadone increase proinflammatory mRNA expression in lumbar dorsal spinal cord.
| Saline | Morphine | Methadone | ||||
|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | Mean | SEM | |
| IL-1β | 2.1 | 0.4 | 8.3* | 2.7 | 10.4* | 3.0 |
| IL-6 | 3.2 | 0.6 | 10.3* | 3.6 | 15.5* | 4.0 |
| TNF-a | 3.0 | 1.0 | 5.9 | 2.4 | 7.9 | 2.4 |
| ICE | 2.4 | 0.4 | 4.4 | 1.8 | 3.4 | 1.6 |
| TLR4 | 3.7 | 0.5 | 11.4* | 2.1 | 12.2* | 3.4 |
Relative mRNA expression in lumbar dorsal spinal cord following 7 days intrathecal morphine, methadone or saline administration in vivo
P < 0.05.
Table 3.
| Table 3A: Chronic morphine and methadone increase CSF proinflammatory cytokine and chemokine levels. | ||||||
|---|---|---|---|---|---|---|
| CSF | Saline (pg/µl) |
Methadone (pg/µl) |
Morphine (pg/µl) |
|||
| Mean | SEM | Mean | SEM | Mean | SEM | |
| Fractalkine | 59.8 | 3.7 | 167.9* | 23.6 | 154.3* | 2.6 |
| GMCSF | 122.1 | 30.9 | 192.3 | 25.3 | 187.9 | 5.6 |
| GRO/KC | 428.1 | 96.0 | 3420.5* | 1229.6 | 1953.4* | 248.0 |
| IFN-γ | 207.6 | 73.1 | 401.6 | 45.5 | 360.9 | 14.2 |
| IL-10 | 7.6 | 1.3 | 43.1* | 9.4 | 28.5* | 0.4 |
| IL-1α | 40.8 | 7.2 | 116.4* | 37.8 | 117.4* | 47.6 |
| IL-1β | 115.7 | 18.0 | 378.4 | 56.2 | 473.9 | 106.5 |
| IL-2 | 206.3 | 75.5 | 369.5 | 34.9 | 359.9 | 15.5 |
| IL-4 | 13.2 | 4.8 | 20.5 | 3.7 | 24.1 | 1.0 |
| IL-6 | 348.1 | 72.5 | 3560.5* | 865.0 | 1315.5* | 53.8 |
| MCP-1 | 796.5 | 112.3 | 1211.2 | 159.7 | 1124.6 | 118.6 |
| MCP-1α | 10.5 | 2.0 | 52.0* | 14.4 | 32.9* | 3.8 |
| MIP-2 | 137.7 | 54.8 | 623.7 | 417.7 | 297.4 | 82.4 |
| MIP-3α | 95.4 | 26.4 | 478.4 | 202.0 | 124.0 | 11.9 |
| RANTES | 153.8 | 29.6 | 246.6 | 63.5 | 147.7 | 18.6 |
| TNF-α | 279.7 | 121.1 | 799.3* | 109.5 | 747.7* | 33.6 |
| Table 3B: Chronic morphine and methadone increase lumbar dorsal spinal cord proinflammatory cytokine and chemokine levels. | ||||||
|---|---|---|---|---|---|---|
| Lumbar | Saline | Methadone | Morphine | |||
| DSC | (pg/mg) | (pg/mg) | (pg/mg) | |||
| Mean | SEM | Mean | SEM | Mean | SEM | |
| Fractalkine | 123.2 | 6.4 | 157.7 | 27.3 | 105.1 | 14.7 |
| GMCSF | 62.8 | 8.6 | 106.1 | 22.9 | 67.2 | 6.1 |
| GRO/KC | 33.8 | 9.5 | 130.6 | 59.4 | 74.3 | 17.1 |
| IFN-γ | 27.1 | 6.7 | 52.9 | 13.1 | 39.5 | 6.4 |
| IL-10 | 3.6 | 0.7 | 7.0 | 2.0 | 4.2 | 0.6 |
| IL-1α | 10.0 | 1.9 | 47.0* | 13.9 | 26.4* | 1.1 |
| IL-1β | 28.9 | 2.1 | 94.5* | 26.0 | 72.9* | 3.2 |
| IL-2 | 42.7 | 5.5 | 86.5 | 14.1 | 66.1 | 9.4 |
| IL-4 | 12.7 | 2.1 | 12.0 | 1.2 | 16.8 | 3.3 |
| IL-6 | 287.9 | 29.7 | 865.8* | 266.2 | 568.5* | 59.3 |
| MCP-1 | 218.2 | 43.9 | 574.3 | 206.5 | 232.3 | 23.9 |
| MIP-1α | 12.2 | 4.4 | 43.6 | 20.4 | 10.4 | 2.1 |
| MIP-2 | 11.6 | 2.8 | 55.0 | 38.4 | 10.3 | 1.5 |
| MIP-3α | 43.8 | 2.3 | 108.5 | 48.7 | 46.0 | 5.2 |
| RANTES | 10.5 | 1.3 | 22.5 | 5.9 | 14.7 | 1.3 |
| TNF-α | 74.0 | 6.9 | 217.8* | 37.5 | 126.6* | 18.5 |
CSF cytokine and chemokine protein levels following 7 days intrathecal morphine, methadone or saline administration
P < 0.05.
Lumbar dorsal spinal cord protein levels following 7 days intrathecal morphine, methadone or saline administration
P < 0.05.
Given the results from Experiment 3, which demonstrated that multiple cytokines and fractalkine work in parallel to produce a behavioral response, an analysis that allowed examination of the sum of these factors was required. Furthermore, a method for selecting which molecular endpoints to include was also needed since a priori hypotheses would be biased by past publications. Therefore, a cluster analysis, using bootstrapping to increase the power of the data set owing to its degrees of freedom limitations, of all behavioral (delta baseline to day 7), protein and mRNA data was performed to ascertain which molecular endpoints co-varied with each behavior. This method of analysis identified seven molecular endpoints from the CSF and nine from dorsal spinal cord tissue, which clustered with nociceptive behaviors consistently from both morphine and methadone treated animals. Specifically, in CSF fractalkine, GRO/KC, IL-1β, IL-6, MIP-1α, MIP-3α and MIP-2 were identified. In dorsal spinal cord tissue IL-1β (protein and mRNA), IL-6 (protein and mRNA), MCP-1, MIP-1α, TNF-α mRNA and TLR4 (protein and mRNA) clustered significantly with the nociceptive behaviors. The sum of the standardized CSF and dorsal spinal cord chemokine, cytokine and glial activation values identified significant treatment effects of morphine and methadone compared to saline treated animals (P < 0.05). A multivariate analysis with treatment as a fixed factor revealed that when no co-variate was specified there was a significant morphine and methadone treatment effect on each of the nociceptive behaviors when compared to saline control (P < 0.05), as already reported. However, when the sum of clusters value was specified as a co-variate the treatment effect was no longer significant (P > 0.5). Importantly, however, the clusters effect as a covariate was significant (P < 0.05). These data suggest that these cytokines, chemokines and glial activation may be involved as at least partial mediators of the change in the development of tolerance, allodynia and hyperalgesia following chronic intrathecal methadone and morphine treatment.
Experiment 5: Blockade of proinflammatory cytokine action and production potentiates intrathecal high dose morphine analgesia and reduces the development of tolerance, hyperalgesia and allodynia
The data presented thus far implicate proinflammatory cytokines in the enhanced nociception (both thermal hyperalgesia and tactile allodynia) associated with acute and chronic opioid exposure. An opportunity now exists to experimentally potentiate opioid analgesia and reduce the severity of these opioid-induced nociceptive side effects by targeting proinflammatory cytokines and their production. In a departure from similar experiments conducted previously using quite mild morphine dosing regimens (Johnston et al., 2004; Raghavendra et al., 2004), we chose a more rigorous morphine dosing regimen that has previously been shown to induce neuronal changes associated with opioid tolerance and, as such, is a regimen much more typically used in studies of morphine tolerance (Shui et al., 2007). Therefore, if an anti-inflammatory treatment were to reduce opioid tolerance, hyperalgesia or allodynia under these more challenging conditions it would suggest that proinflammatory mediators are not simply involved in response to mild opioid regimens. Rather, proinflammatory products may potentially contribute to the previously reported neuronal changes and/or mediate different portions of the response. Specifically, animals received 20 µg morphine twice daily via an indwelling intrathecal catheter, co-administered with IL-1ra plus Fc-IL-10 (a stabilized human IL-10 which has an extended half-life; IL-10 is a powerful anti-inflammatory cytokine), or IL-1 receptor antagonist plus YVAD (a caspase-1 inhibitor, the enzyme that cleaves IL-1 into its mature form; YVAD will reduce the levels of mature active IL-1). The aim of Fc-IL-10 and YVAD treatments were to reduce the production of proinflammatory cytokines by producing an anti-inflammatory environment (Fc-IL-10) and by blocking the production of mature IL-1 (YVAD). IL-1ra was included with these anti-inflammatory mediators, to produce an immediate blockade of IL-1 actions.
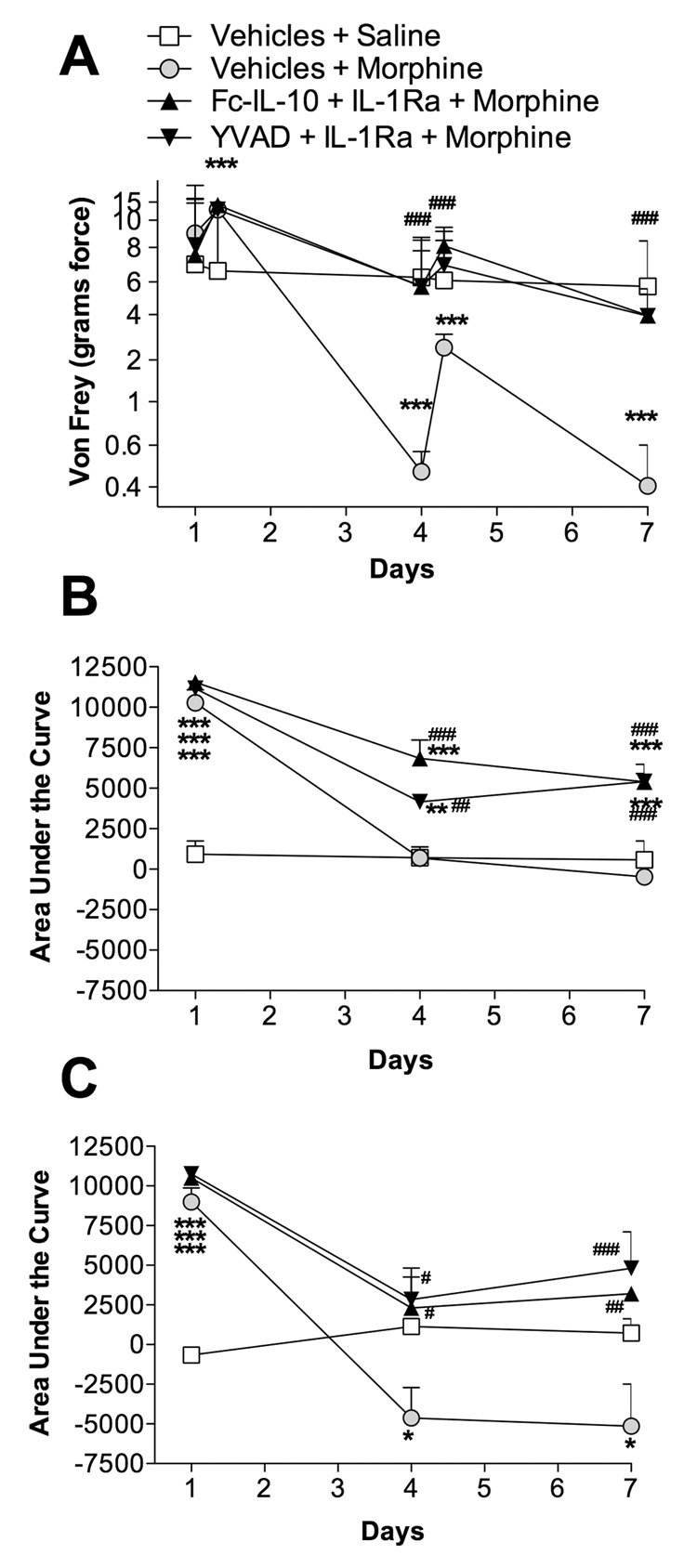
Morphine plus vehicles produced significant tailflick and hind paw analgesia following the first intrathecal morphine dose which was detected by all three nociceptive measures: von Frey test (Figure 5A), short baseline latency tailflick Hargreaves stimuli area under the curve (Figure 5B) and long baseline latency tailflick Hargreaves stimuli area under the curve (Figure 5C). However, after six doses of morphine, significant allodynia developed. This is apparent in Figure 5A on the pre dose von Frey assessment (day 4). On day 4 and day 7, tolerance had developed to such an extent that no significant tailflick (Figure 5B) or hind paw analgesia was observed and this was accompanied by the development of significant hyperalgesia (Figure 5C). In contrast, co-administration of either anti-inflammatory treatment resulted in the complete blockade of allodynia (Figure 5A), attenuation of the development of tolerance (Figure 5B) and reduction in the development of hyperalgesia (Figure 5C). These data again implicate opioid-induced proinflammatory cytokines in the development of tolerance using a rigorous morphine dosing regimen previously associated with neuronal adaptation.
Figure 5. Chronic intrathecal co-administration of anti-inflammatory treatments blunts the development of morphine tolerance, hyperalgesia and allodynia.
Animals received 20 µg morphine (in 1 µl with a 25 µl flush) twice daily via an indwelling intrathecal catheter, co-administered with IL-1ra (100 µg in 1 µl) plus Fc-IL-10 (250 ng in 5 µl; black triangle, point up), IL-1ra (100 µg in 1 µl) plus YVAD (500 ng in 5 µl; black triangle, point down), morphine co-administered with vehicles (black circle with gray center), or saline co-administered with vehicles (black square with white center). Fc-IL-10 is a stabilized human IL-10 which has an extended half-life. YVAD is a caspase-1 inhibitor, the enzyme that cleaves IL-1 into its mature form. YVAD will reduce the levels of mature active IL-1. The aim of Fc-IL-10 and YVAD treatments were to reduce the production of proinflammatory cytokines by producing an anti-inflammatory environment (Fc-IL-10) and by blocking the production of mature IL-1 (YVAD). IL-1ra was included to provide an immediate block of IL-1 actions. Animals were tested on days 1, 4 and 7 prior to, during and after the morning drug administration. The development of allodynia (von Frey; A), tolerance (short baseline latency Hargreaves stimuli; B) and hyperalgesia (long baseline latency Hargreaves stimuli; C) were measured. A two-way ANOVA with Bonferroni post hoc test was conducted to assess statistical significance between saline co-administered with vehicles versus the animals receiving morphine treatments (# P < 0.05, ##, P < 0.01 and ### P < 0.001), as well as the difference between morphine plus vehicles versus other treatments (* P < 0.05, ** P < 0.01 and *** P < 0.001).
Discussion
These studies demonstrate the novel findings that, ≤5 minutes after intrathecal opioids, endogenous IL-1 reduces morphine analgesia. Intrathecal morphine analgesia was reduced 8-fold by spinal IL-1. IL-1 is not the only spinal proinflammatory cytokine involved, as endogenous TNF-α and IL-6 exert similar effects. Spinal IL-1 also opposes systemic opioid analgesia. This effect is not limited to morphine, but rather occurs with the structurally dissimilar opioid, methadone, as well. Importantly, reduction of opioid analgesia by endogenous IL-1 cannot be accounted for by alterations in morphine’s pharmacokinetics. This implies that IL-1 is altering the actions of morphine rather than its clearance or distribution. Furthermore, the analgesia unmasked is opioid in nature as we (Hutchinson Unpub. Obs.) and others (Shavit et al., 2005) observed naloxone sensitivity of such rebound analgesia. Based on studies of a putative microglial inhibitor (minocycline) and of an inhibitor of fractalkine receptors (CX3CR1 neutralizing antibody) predominantly expressed by microglia in the spinal cord, the data suggest that the opposition of acute opioid analgesia is likely dependent on spinal microglia. Additional inhibitor studies revealed dependence on p38 MAPK activation and NO. Several CSF and dorsal spinal cord proinflammatory mediators were generated in response to acute and chronic opioid administration. Mediation statistical analysis demonstrated that the sum of these proinflammatory changes was related to the changes in nociception following chronic opioid administration. Analyses of this nature, combined with bootstrapping, with similar datasets generated from DNA arrays has been performed extensively and is a well accepted method for identifying targets and clusters of targets of interest (Suzuki and Shimodaira, 2006). We substantiated these findings by demonstrating that anti-inflammatory treatments enhanced morphine analgesia and attenuated the development of hyperalgesia and allodynia under a rigorous morphine administration regimen previously associated with neuronal adaptation. These data demonstrate that opioid-induced proinflammatory mediators contribute significantly to the opposition of morphine analgesia even upon the first exposure to opioids. As proinflammatory mediators often synergize and interact, it is not surprising that multiple proinflammatory mediators have been identified in the present study as contributing to the effects under study.
Previous studies have focused on the elevation of glial activation markers and proinflammatory mediators following chronic morphine (Cui et al., 2006; Johnston et al., 2004; Liu et al., 2006; Raghavendra et al., 2002; Raghavendra et al., 2004; Song and Zhao, 2001). Here we demonstrate that acute morphine and methadone administration causes a proinflammatory cytokine-mediated opposition of acute intrathecal and/or systemic opioid analgesia. Moreover, this may prove to be a general opioid phenomenon with regard to opioids, given it occurred in response to both the 4,5-epoxymorphinan opiate morphine and the fully synthetic 3,3-diphenylpropylamine opioid methadone.
Given that IL-1ra potentiates morphine analgesia in less than 5 minutes, this suggests an action of pre-existing stores of cytokines, rather than de novo synthesis, at early timepoints. In vitro studies revealed that morphine reliably elevated proinflammatory cytokines and chemokines in the supernatants relative to vehicle controls. In addition, TLR4 protein was elevated demonstrating glial activation resulting from in vitro morphine as TLR4 is expressed predominantly by microglia, and some astrocytes, but not neurons (Tanga et al., 2005). As in previous reports (Johnston et al., 2004; Shavit et al., 2005), basal proinflammatory cytokine release did not control basal nociception as TNF-α, IL-1 and IL-6 inhibitor/antagonists were without effect in the absence of opioids. Furthermore, these data suggest that proinflammatory cytokines alone may not be responsible for the reduction in opioid efficacy, but are required for it. These data extend the only other publication investigating acute proinflammatory opposition of morphine analgesia by Shavit and colleagues. They demonstrated that systemic IL-1ra potentiated acute systemic morphine analgesia (Shavit et al., 2005). Moreover, they demonstrated, at a single timepoint, that systemic administration of IL-1ra unveiled continuing analgesia following the dissipation of systemic morphine analgesia. Importantly, the data presented here demonstrate a spinal site of action of proinflammatory cytokine opposition of morphine analgesia, irrespective of morphine delivery route. Whether supraspinal sites may additionally contribute to such effects is at present unknown.
These data are also informative regarding mechanisms by which opioid-induced inflammatory changes result in the opposition of opioid analgesia. We established that the major proinflammatory cytokines (IL-1, IL-6 and TNF-α) oppose acute opioid analgesia. Their effects may interact since suppressing the action of any single cytokine unmasked continuing analgesia. Cytokine interactions have been described peripherally and in CNS (Milligan et al., 2003; Sweitzer et al., 2001; Szelenyi, 2001). The present study is the first suggestion that this may occur in spinal cord following opioid administration.
The spinal cellular source of the proinflammatory signal was also of interest. Studies of normal spinal cord document the basal expression of proinflammatory cytokines in glia (Sweitzer et al., 1999) and to some extent in neurons (Fu et al., 2006) suggesting that under acute conditions both glial and neuronal stores may be available to cause the opposition of opioid analgesia. The potentiation of acute morphine analgesia by minocycline suggests that microglia may prove to be a significant source of mediators that opposes opioid analgesia. Moreover, enhancement of morphine analgesia by fractalkine blockade again suggests microglia since, in spinal cord, fractalkine receptors are predominantly, if not exclusively, expressed by microglia (Verge et al., 2004). Fractalkine is an important neuron-to-microglia signal in spinal cord (Milligan et al., 2005; Verge et al., 2004) that contributes to chronic morphine-induced tolerance, allodynia and hyperalgesia (Johnston et al., 2004). Importantly, the present data are the first evidence of acute opposition of opioid analgesia by fractalkine.
As both the NO synthase inhibitor L-NAME and the p38 MAPK inhibitor SB203508 potentiated morphine analgesia, this suggests that both NO and p38 MAPK activation are required for opposing acute morphine analgesia. Activation of microglial p38 MAPK is integral to the development of morphine tolerance (Cui et al., 2006), an effect dependent on NO (2006). The opposition of opioid analgesia by p38 MAPK is intriguing as p38 MAPK activation is pivotal to the release of several proinflammatory cytokines (Svensson et al., 2003) and has been implicated in glial mediated morphine anti-analgesia (Wu et al., 2005). The fact that fractalkine induces microglial activation and consequent activation of p38 MAPK and release of IL-1, IL-6 and NO (Clark et al., 2007; Johnston et al., 2004; Milligan et al., 2005; Zhuang et al., 2006) is strikingly parallel to the results found in the present study in response to opioids.
The reduction in analgesic efficacy that occurs upon repeated opioid exposure has previously been hypothesized to occur solely as a result of neuronal adaptations. Despite being hypothesized nearly 20 years ago (Ronnback and Hansson, 1988), it has only been within the past 6 years that data have accrued, supportive of a role of glia as well (Cui et al., 2006; Johnston et al., 2004; Liu et al., 2006; Raghavendra et al., 2002; Raghavendra et al., 2004; Shavit et al., 2005; Song and Zhao, 2001; Watkins et al., 2005; Watkins et al., 2006). Here we both statistically and mechanistically demonstrate that glial activation, proinflammatory cytokines and chemokines including fractalkine contribute to the development of opioid tolerance, opioid-induced hyperalgesia and allodynia. Furthermore, since both the 4,5-epoxymorphinan opiate morphine and the fully synthetic 3,3-diphenylpropylamine opioid methadone cause glial activation (as reflected by upregulation of glially-selective TLR4 protein and mRNA), it appears that opioids may generally cause glial activation, rather than only morphine. In support of this, ongoing studies document that acute proinflammatory cytokine release occurs in response to other opioids of diverse structural classes as well (Hutchinson et al., Unpub. Obs.). Together, these data demonstrate that opioid-induced proinflammatory mediators significantly contribute to the decline in opioid analgesic efficacy. Furthermore, we establish a potential role for additional chemokines that have not previously been implicated in the opioid-glial literature. Specifically, we have identified from both in vitro and in vivo opioid exposure significantly increase GRO/KC, MIP-1α, MIP-3α and MIP-2 and MCP-1, in addition to confirming roles of fractalkine, IL-1β and IL-6.
Interestingly, the opioid-induced elevations of TLR4 protein and mRNA, while indicating general glial activation resulting from chronic opioid administration, also provide a parallel to recent developments in the neuropathic pain literature. Tanga et al. (2005) demonstrated that TLR4 mRNA is upregulated in spinal cord in response to peripheral nerve injury and that TLR4 activation is pivotal for the expression and development of neuropathic pain. We (Hutchinson et al., in press) have recently extended this work by demonstrating the importance of spinal TLR4 in maintaining neuropathic pain. Indeed, we now have evidence that TLR4 is a final common pathway used by cells such as microglia to become activated not only by endogenous danger signals released under conditions of neuropathic pain, but by opioids such as morphine as well (Hutchinson et al., 2007).
While cytokines are released in response to pathological conditions, there is growing recognition of cytokine actions in normal physiology, such as regulating sleep (Kapsimalis et al., 2005) and learning and memory (Maier and Watkins, 2003). Their production and release by glia in the central nervous system is increased physiologically in response to peripheral immune activation, forming the central nervous system component of immune-to-brain communication that drives sickness responses such as fever, increased sleep, changes in endocrine function and increased pain (Watkins and Maier, 2000). Therefore, their release is not triggered just in response to pathology. Elevations in cytokine action in response to opioid administration may be part of a physiological negative feedback circuit, meant to maintain nociceptive homeostasis. This has precedence in the analgesia literature, where a number of anti-opioids, such as FMRF-amide and cholecystokinin, have been documented to be released in response to the presence of opioids and act to suppress opioid analgesia and contribute to morphine tolerance (King et al., 2005).
In conclusion, these data demonstrate that opioid actions are opposed by proinflammatory mediators following both acute and chronic administration. This suggests the potential therapeutic utility of targeting opioid-induced proinflammatory mediators to attenuate the development of opioid tolerance, opioid-induced allodynia and hyperalgesia.
Acknowledgements
This work was supported by an International Association for the Study of Pain International Collaborative grant, American Australian Association Merck Company Foundation Fellowship, National Health and Medical Research Council CJ Martin Fellowship (ID 465423) and NIH Grants DA023132, DA015642, DA017670 and DE017782. Thanks to the Debra Berkelhammer, Dr Sondra Bland, Dr Jose Amat, Dr Erin Milligan, Chris Altman, Todd Carlson, Anita Brzeski, Sonica Patel, Kaly Warner, Nicole Crysdale, Lindsey Chao, Mitesh Shridhar, Amanda Ellis and Kimberley Brown for their invaluable technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Carmody J. Avoiding fallacies in nociceptive measurements. Pain. 1995;63:136. doi: 10.1016/0304-3959(95)90018-7. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Clark AK, Yip PK, Grist J, Gentry C, Staniland AA, Marchand F, Dehvari M, Wotherspoon G, Winter J, Ullah J, Bevan S, Malcangio M. Inhibition of spinal microglial cathepsin S for the reversal of neuropathic pain. Proc Natl Acad Sci U S A. 2007;104:10655–10660. doi: 10.1073/pnas.0610811104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Chen Y, Zhi JL, Guo RX, Feng JQ, Chen PX. Activation of p38 mitogen-activated protein kinase in spinal microglia mediates morphine antinociceptive tolerance. Brain Res. 2006;1069:235–243. doi: 10.1016/j.brainres.2005.11.066. [DOI] [PubMed] [Google Scholar]
- Doverty M, Somogyi AA, White JM, Bochner F, Beare CH, Menelaou A, Ling W. Methadone maintenance patients are cross-tolerant to the antinociceptive effects of morphine. Pain. 2001;93:155–163. doi: 10.1016/S0304-3959(01)00306-2. [DOI] [PubMed] [Google Scholar]
- Fu D, Guo Q, Ai Y, Cai H, Yan J, Dai R. Glial Activation and Segmental Upregulation of Interleukin-1b (IL-1b) in the Rat Spinal Cord after Surgical Incision. Neurochem Res. 2006;31:333–340. doi: 10.1007/s11064-005-9032-4. [DOI] [PubMed] [Google Scholar]
- Grumbach K, Baringer JR, Klassen T. Herpes-simplex antigen in rabbit cerebrospinal fluid. Lancet. 1977;1:149. doi: 10.1016/s0140-6736(77)91749-4. [DOI] [PubMed] [Google Scholar]
- Hansen MK, Nguyen KT, Fleshner M, Goehler LE, Gaykema RP, Maier SF, Watkins LR. Effects of vagotomy on serum endotoxin, cytokines, and corticosterone after intraperitoneal lipopolysaccharide. Am J Physiol Regul Integr Comp Physiol. 2000a;278:R331–R336. doi: 10.1152/ajpregu.2000.278.2.R331. [DOI] [PubMed] [Google Scholar]
- Hansen MK, Nguyen KT, Goehler LE, Gaykema RP, Fleshner M, Maier SF, Watkins LR. Effects of vagotomy on lipopolysaccharide-induced brain interleukin-1beta protein in rats. Auton Neurosci. 2000b;85:119–126. doi: 10.1016/s1566-0702(00)00230-7. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Harvey LO. Efficient estimation of sensory thresholds. Behav Res Methods Instr Comp. 1986;18:623–632. [Google Scholar]
- Holguin A, O'Connor KA, Biedenkapp J, Campisi J, Wieseler-Frank J, Milligan ED, Hansen MK, Spataro L, Maksimova E, Bravmann C, Martin D, Fleshner M, Maier SF, Watkins LR. HIV-1 gp120 stimulates proinflammatory cytokine-mediated pain facilitation via activation of nitric oxide synthase-I (nNOS) Pain. 2004;110:517–530. doi: 10.1016/j.pain.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Hutchinson MR, Bland ST, Johnson KW, Rice KC, Maier SF, Watkins LR. Opioid-induced glial activation: mechanisms of activation and implications for opioid analgesia, dependence, and reward. TheScientificWorldJournal. 2007;7:98–111. doi: 10.1100/tsw.2007.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Zhang Y, Brown KD, Coats BD, Shridhar M, Sholar PW, Patel SJ, Crysdale NY, Harrison JA, Maier SF, Rice KC, Watkins LR. Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: Involvement of toll-like receptor 4 (TLR4) Eur J Neurosci. doi: 10.1111/j.1460-9568.2008.06321.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston IN, Milligan ED, Wieseler-Frank J, Frank MG, Zapata V, Campisi J, Langer S, Martin D, Green P, Fleshner M, Leinwand L, Maier SF, Watkins LR. A role for proinflammatory cytokines and fractalkine in analgesia, tolerance, and subsequent pain facilitation induced by chronic intrathecal morphine. J Neurosci. 2004;24:7353–7365. doi: 10.1523/JNEUROSCI.1850-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapsimalis F, Richardson G, Opp MR, Kryger M. Cytokines and normal sleep. Curr Opin Pulm Med. 2005;11:481–484. doi: 10.1097/01.mcp.0000183062.98665.6b. [DOI] [PubMed] [Google Scholar]
- King T, Ossipov MH, Vanderah TW, Porreca F, Lai J. Is paradoxical pain induced by sustained opioid exposure an underlying mechanism of opioid antinociceptive tolerance? Neurosignals. 2005;14:194–205. doi: 10.1159/000087658. [DOI] [PubMed] [Google Scholar]
- Ledeboer A, Sloane EM, Milligan ED, Frank MG, Mahony JH, Maier SF, Watkins LR. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain. 2005;115:71–83. doi: 10.1016/j.pain.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Liu W, Wang CH, Cui Y, Mo LQ, Zhi JL, Sun SN, Wang YL, Yu HM, Zhao CM, Feng JQ, Chen PX. Inhibition of neuronal nitric oxide synthase antagonizes morphine antinociceptive tolerance by decreasing activation of p38 MAPK in the spinal microglia. Neurosci Lett. 2006;410(0):174–177. doi: 10.1016/j.neulet.2006.08.091. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Immune-to-central nervous system communication and its role in modulating pain and cognition: Implications for cancer and cancer treatment. Brain Behav Immun. 2003;17 Suppl 1:S125–S131. doi: 10.1016/s0889-1591(02)00079-x. [DOI] [PubMed] [Google Scholar]
- Milligan E, Zapata V, Schoeniger D, Chacur M, Green P, Poole S, Martin D, Maier SF, Watkins LR. An initial investigation of spinal mechanisms underlying pain enhancement induced by fractalkine, a neuronally released chemokine. Eur J Neurosci. 2005;22:2775–2782. doi: 10.1111/j.1460-9568.2005.04470.x. [DOI] [PubMed] [Google Scholar]
- Milligan ED, Hinde JL, Mehmert KK, Maier SF, Watkins LR. A method for increasing the viability of the external portion of lumbar catheters placed in the spinal subarachnoid space of rats. J Neurosci Methods. 1999;90:81–86. doi: 10.1016/s0165-0270(99)00075-8. [DOI] [PubMed] [Google Scholar]
- Milligan ED, Mehmert KK, Hinde JL, Harvey LO, Martin D, Tracey KJ, Maier SF, Watkins LR. Thermal hyperalgesia and mechanical allodynia produced by intrathecal administration of the human immunodeficiency virus-1 (HIV-1) envelope glycoprotein, gp120. Brain Res. 2000;861:105–116. doi: 10.1016/s0006-8993(00)02050-3. [DOI] [PubMed] [Google Scholar]
- Milligan ED, O'Connor KA, Nguyen KT, Armstrong CB, Twining C, Gaykema RP, Holguin A, Martin D, Maier SF, Watkins LR. Intrathecal HIV-1 envelope glycoprotein gp120 induces enhanced pain states mediated by spinal cord proinflammatory cytokines. J Neurosci. 2001;21:2808–2819. doi: 10.1523/JNEUROSCI.21-08-02808.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan ED, Twining C, Chacur M, Biedenkapp J, O'Connor K, Poole S, Tracey K, Martin D, Maier SF, Watkins LR. Spinal glia and proinflammatory cytokines mediate mirror-image neuropathic pain in rats. J Neurosci. 2003;23:1026–1040. doi: 10.1523/JNEUROSCI.23-03-01026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. 2004;36:717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Austria: Vienna; 2006. [Google Scholar]
- Raghavendra V, Rutkowski MD, DeLeo JA. The role of spinal neuroimmune activation in morphine tolerance/hyperalgesia in neuropathic and sham-operated rats. J Neurosci. 2002;22:9980–9989. doi: 10.1523/JNEUROSCI.22-22-09980.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendra V, Tanga FY, DeLeo JA. Attenuation of morphine tolerance, withdrawal-induced hyperalgesia, and associated spinal inflammatory immune responses by propentofylline in rats. Neuropsychopharmacology. 2004;29:327–334. doi: 10.1038/sj.npp.1300315. [DOI] [PubMed] [Google Scholar]
- Ronnback L, Hansson E. Are astroglial cells involved in morphine tolerance? Neurochem Res. 1988;13:87–103. doi: 10.1007/BF00973320. [DOI] [PubMed] [Google Scholar]
- Shavit Y, Wolf G, Goshen I, Livshits D, Yirmiya R. Interleukin-1 antagonizes morphine analgesia and underlies morphine tolerance. Pain. 2005;115:50–59. doi: 10.1016/j.pain.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Shui HA, Ho ST, Wang JJ, Wu CC, Lin CH, Tao YX, Liaw WJ. Proteomic analysis of spinal protein expression in rats exposed to repeated intrathecal morphine injection. Proteomics. 2007;7:796–803. doi: 10.1002/pmic.200600699. [DOI] [PubMed] [Google Scholar]
- Song P, Zhao ZQ. The involvement of glial cells in the development of morphine tolerance. Neurosci Res. 2001;39:281–286. doi: 10.1016/s0168-0102(00)00226-1. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Shimodaira H. Pvclust: an R package for assessing the uncertainty in hierarchical clustering. Bioinformatics. 2006;22:1540–1542. doi: 10.1093/bioinformatics/btl117. [DOI] [PubMed] [Google Scholar]
- Svensson CI, Marsala M, Westerlund A, Calcutt NA, Campana WM, Freshwater JD, Catalano R, Feng Y, Protter AA, Scott B, Yaksh TL. Activation of p38 mitogen-activated protein kinase in spinal microglia is a critical link in inflammation-induced spinal pain processing. J Neurochem. 2003;86:1534–1544. doi: 10.1046/j.1471-4159.2003.01969.x. [DOI] [PubMed] [Google Scholar]
- Sweitzer S, Martin D, DeLeo JA. Intrathecal interleukin-1 receptor antagonist in combination with soluble tumor necrosis factor receptor exhibits an anti-allodynic action in a rat model of neuropathic pain. Neuroscience. 2001;103:529–539. doi: 10.1016/s0306-4522(00)00574-1. [DOI] [PubMed] [Google Scholar]
- Sweitzer SM, Colburn RW, Rutkowski M, DeLeo JA. Acute peripheral inflammation induces moderate glial activation and spinal IL-1beta expression that correlates with pain behavior in the rat. Brain Res. 1999;829:209–221. doi: 10.1016/s0006-8993(99)01326-8. [DOI] [PubMed] [Google Scholar]
- Szelenyi J. Cytokines and the central nervous system. Brain Res Bull. 2001;54:329–338. doi: 10.1016/s0361-9230(01)00428-2. [DOI] [PubMed] [Google Scholar]
- Tanga FY, Nutile-McMenemy N, DeLeo JA. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc Natl Acad Sci U S A. 2005;102:5856–5861. doi: 10.1073/pnas.0501634102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treutwein B, Strasburger H. Fitting the psychometric function. Percept Psychophys. 1999;61:87–106. doi: 10.3758/bf03211951. [DOI] [PubMed] [Google Scholar]
- van Crugten JT, Somogyi AA, Nation RL, Reynolds G. Concentration-effect relationships of morphine and morphine-6 beta-glucuronide in the rat. Clin Exp Pharmacol Physiol. 1997;24:359–364. doi: 10.1111/j.1440-1681.1997.tb01202.x. [DOI] [PubMed] [Google Scholar]
- Verge GM, Milligan ED, Maier SF, Watkins LR, Naeve GS, Foster AC. Fractalkine (CX3CL1) and fractalkine receptor (CX3CR1) distribution in spinal cord and dorsal root ganglia under basal and neuropathic pain conditions. Eur J Neurosci. 2004;20:1150–1160. doi: 10.1111/j.1460-9568.2004.03593.x. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Johnston IN, Maier SF. Glia: novel counter-regulators of opioid analgesia. Trends Neurosci. 2005;28:661–669. doi: 10.1016/j.tins.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Ledeboer A, Wieseler-Frank J, Milligan ED, Maier SF. Glia as the "bad guys": Implications for improving clinical pain control and the clinical utility of opioids. Brain Behav Immun. 2006;21:131–146. doi: 10.1016/j.bbi.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Maier SF. The pain of being sick: implications of immune-to-brain communication for understanding pain. Annu Rev Psychol. 2000;51:29–57. doi: 10.1146/annurev.psych.51.1.29. [DOI] [PubMed] [Google Scholar]
- Wu HE, Sun HS, Cheng CW, Tseng LF. p38 Mitogen-activated protein kinase inhibitor SB203580 reverses the antianalgesia induced by dextro-morphine or morphine in the mouse spinal cord. Eur J Pharmacol. 2006;550:91–94. doi: 10.1016/j.ejphar.2006.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HE, Thompson J, Sun HS, Terashvili M, Tseng LF. Antianalgesia: stereoselective action of dextro-morphine over levo-morphine on glia in the mouse spinal cord. J Pharmacol Exp Ther. 2005;314:1101–1108. doi: 10.1124/jpet.105.087130. [DOI] [PubMed] [Google Scholar]
- Yeomans DC, Proudfit HK. Nociceptive responses to high and low rates of noxious cutaneous heating are mediated by different nociceptors in the rat: electrophysiological evidence. Pain. 1996;68:141–150. doi: 10.1016/S0304-3959(96)03177-6. [DOI] [PubMed] [Google Scholar]
- Zhuang ZY, Kawasaki Y, Tan PH, Wen YR, Huang J, Ji RR. Role of the CX3CR1/p38 MAPK pathway in spinal microglia for the development of neuropathic pain following nerve injury-induced cleavage of fractalkine. Brain Behav Immun. 2006 doi: 10.1016/j.bbi.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]