Abstract
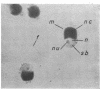
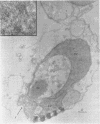
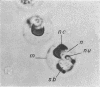
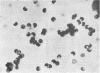
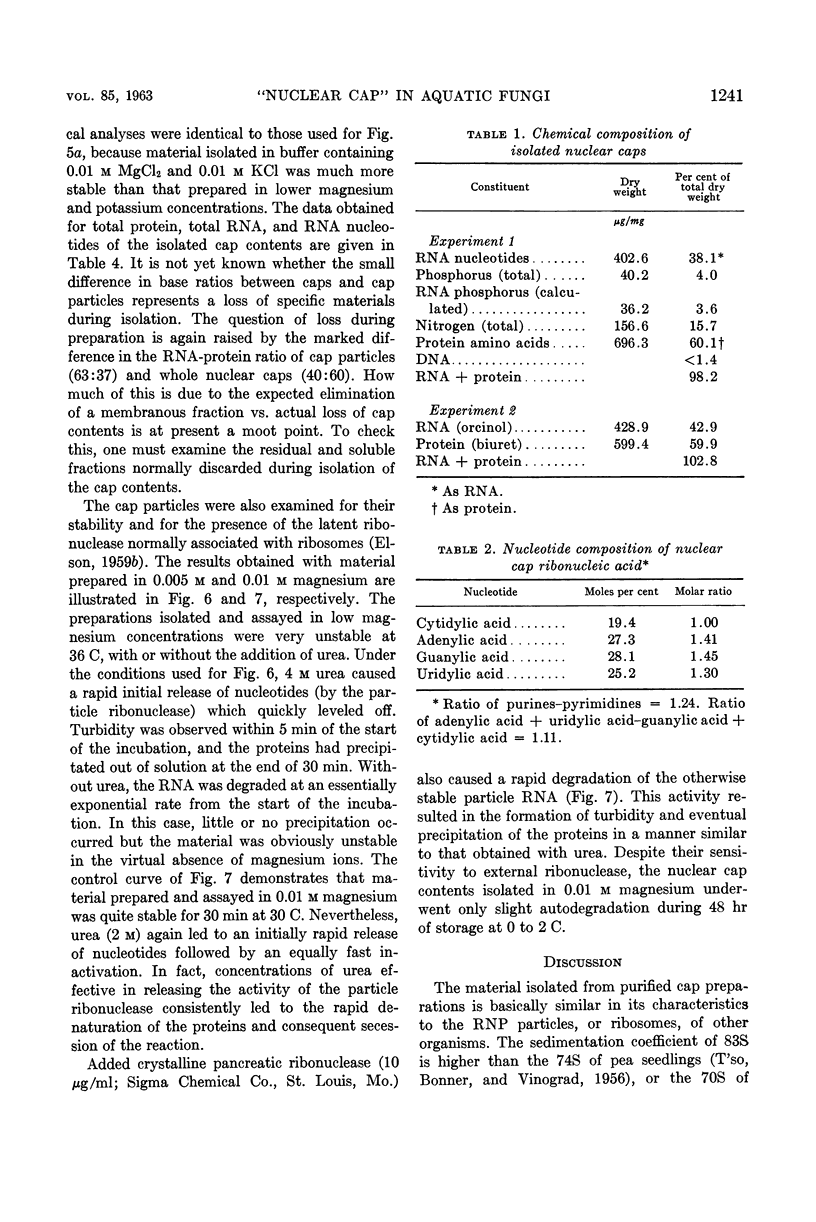
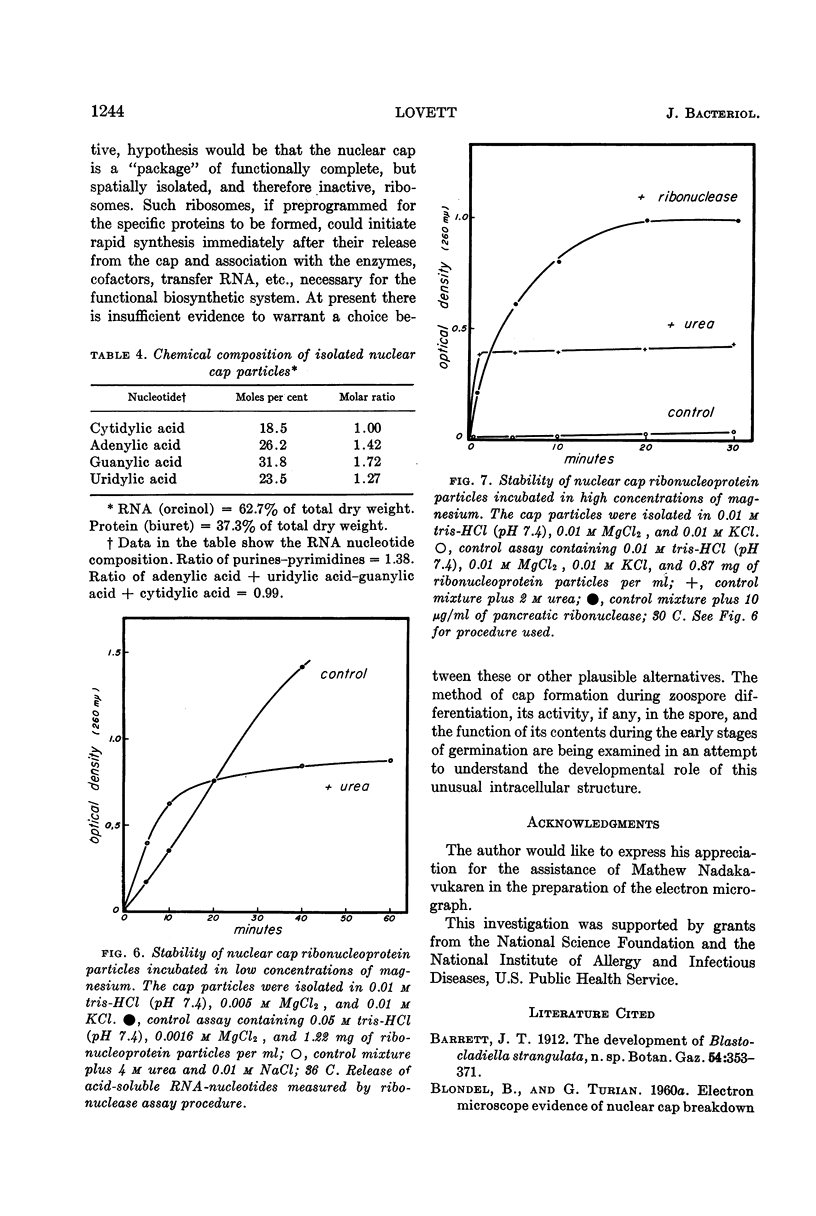
Lovett, James S. (Purdue University, West Lafayette, Ind.). Chemical and physical characterization of “nuclear caps” isolated from Blastocladiella zoospores. J. Bacteriol. 85:1235–1246. 1963.—Electron micrographs of Blastocladiella zoospores have shown the nuclear cap to contain essentially all of the small (250 to 300 A) electron-dense particles of the cell. Preparations of clean, whole nuclear caps were isolated to study the composition of the intact organelles and their particulate contents. The cap is strongly basophilic, and is composed of 60% protein and 40% ribonucleic acid (RNA). It represents 18% of the dry weight, and contains 69% of the total RNA, of the spore. The amino acid composition of cap proteins is similar to the ribosomal protein of other organisms. The nuclear cap contents have been extracted and isolated by high-speed centrifugation. More than 95% of the material has a sedimentation coefficient of 83S in 0.005 m Mg. The 83S particles form aggregates at higher Mg concentrations and dissociate to yield 63S and 41S peaks at low Mg concentrations. Purified cap particles contain 37% protein and 63% RNA. The RNA has a nucleotide composition (in moles per cent) of 18.5% cytidylic, 26.2% adenylic, 31.8% guanylic, and 23.5% uridylic acid. The particles contain a latent ribonuclease, which can be activated by urea, and are susceptible to degradation by added pancreatic ribonuclease. The available evidence supports a concept of the zoospore nuclear cap as an unusual intracellular “packet” of ribosomes.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLONDEL B., TURIAN G. Relation between basophilia and fine structure of cytoplasm in the fungus Allomyces macrogynus Em. J Biophys Biochem Cytol. 1960 Feb;7:127–134. doi: 10.1083/jcb.7.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAO F. C. Dissociation of macromolecular ribonucleoprotein of yeast. Arch Biochem Biophys. 1957 Aug;70(2):426–431. doi: 10.1016/0003-9861(57)90130-3. [DOI] [PubMed] [Google Scholar]
- CHAO F. C., SCHACHMAN H. K. The isolation and characterization of a macro-molecular ribonucleoprotein from yeast. Arch Biochem Biophys. 1956 Mar;61(1):220–230. doi: 10.1016/0003-9861(56)90334-4. [DOI] [PubMed] [Google Scholar]
- DAVIDSON J. N., SMELLIE R. M. S. Phosphorus compounds in the cell. II. The separation by ionophoresis on paper of the constituent nucleotides of ribonucleic acid. Biochem J. 1952 Dec;52(4):594–599. doi: 10.1042/bj0520594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELSON D. Latent enzymic activity of a ribonucleoprotein isolated from Escherichia coli. Biochim Biophys Acta. 1959 Dec;36:372–386. doi: 10.1016/0006-3002(59)90179-9. [DOI] [PubMed] [Google Scholar]
- ELSON D. Preparation and properties of a ribonucleoprotein isolated from Escherichia coli. Biochim Biophys Acta. 1959 Dec;36:362–371. doi: 10.1016/0006-3002(59)90178-7. [DOI] [PubMed] [Google Scholar]
- GLAUERT A. M., GLAUERT R. H. Araldite as an embedding medium for electron microscopy. J Biophys Biochem Cytol. 1958 Mar 25;4(2):191–194. doi: 10.1083/jcb.4.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMILTON M. G., PETERMANN M. L. Ultracentrifugal studies on ribonucleoprotein from rat liver microsomes. J Biol Chem. 1959 Jun;234(6):1441–1446. [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER E., TURIAN G. Ultrastructure du corps paranucléaire, des mitochondries et de la membrane nucléaire des gamètes d'Allomyces macrogynus. Exp Cell Res. 1956 Aug;11(2):417–422. doi: 10.1016/0014-4827(56)90117-3. [DOI] [PubMed] [Google Scholar]
- KIHARA H. K., HU A. S., HALVORSON H. O. The identification of a ribosomal-bound beta-glucosidase. Proc Natl Acad Sci U S A. 1961 Apr 15;47:489–497. doi: 10.1073/pnas.47.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIURA K. I. The nucleotide composition of ribonucleic acids of soluble and particle fractions in several species of bacteria. Biochim Biophys Acta. 1962 Jan 22;55:62–70. doi: 10.1016/0006-3002(62)90931-9. [DOI] [PubMed] [Google Scholar]
- MORGAN R. S. A new form of ribosome from yeast. J Mol Biol. 1962 Feb;4:115–117. doi: 10.1016/s0022-2836(62)80043-6. [DOI] [PubMed] [Google Scholar]
- McCurdy H. D., Cantino E. C. Isocitritase, Glycine-Alanine Transaminase, and Development in Blastocladiella Emersonii. Plant Physiol. 1960 Jul;35(4):463–476. doi: 10.1104/pp.35.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NELSON C. A., HUMMEL J. P. Reversible denaturation of pancreatic ribonuclease by urea. J Biol Chem. 1962 May;237:1567–1574. [PubMed] [Google Scholar]
- PIEZ K. A., MORRIS L. A modified procedure for the automatic analysis of amino acids. Anal Biochem. 1960 Nov;1:187–201. doi: 10.1016/0003-2697(60)90045-2. [DOI] [PubMed] [Google Scholar]
- PLESNER P. Changes in ribosome structure and function during synchronized cell division. Cold Spring Harb Symp Quant Biol. 1961;26:159–162. doi: 10.1101/sqb.1961.026.01.020. [DOI] [PubMed] [Google Scholar]
- ROREM E. S., MACHLIS L. The ribonucleoprotein nature of large particles in the meiosporangia of Allomyces. J Biophys Biochem Cytol. 1957 Nov 25;3(6):879–888. doi: 10.1083/jcb.3.6.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TISSIERES A., WATSON J. D. Ribonucleoprotein particles from Escherichia coli. Nature. 1958 Sep 20;182(4638):778–780. doi: 10.1038/182778b0. [DOI] [PubMed] [Google Scholar]
- TS'O P. O., BONNER J., DINTZIS H. On the similarity of amino acid composition of microsomal nucleoprotein particles. Arch Biochem Biophys. 1958 Jul;76(1):225–227. doi: 10.1016/0003-9861(58)90137-1. [DOI] [PubMed] [Google Scholar]
- TURIAN G., CANTINO E. C. The stimulatory effect of light on nucleic acid synthesis in the mould Blastocladiella emersonii. J Gen Microbiol. 1959 Dec;21:721–735. doi: 10.1099/00221287-21-3-721. [DOI] [PubMed] [Google Scholar]
- TURIAN G. Sur la nature ribonucléique du corps paranucléaire et ses relations avec la différenciation du sexe chez Allomyces javanicus. C R Hebd Seances Acad Sci. 1955 Jun 13;240(24):2344–2346. [PubMed] [Google Scholar]
- WANG T. Y. The chemical composition of nuclear ribosomes of calf thymus. Arch Biochem Biophys. 1962 May;97:387–392. doi: 10.1016/0003-9861(62)90094-2. [DOI] [PubMed] [Google Scholar]