Summary
Epithelial branching morphogenesis is critical to formation of various organs such as the vasculature, mammary glands, lungs and kidneys in vertebrate embryos. One fascinating aspect of branching morphogenesis is to understand how a simple epithelial tube grows by reiterative branching to form a complex epithelial tree structure. Recent studies combining mouse genetics and chimeric analysis with live imaging have uncovered the molecular networks and interactions that govern kidney branching morphogenesis. This review focuses on ureteric bud formation and epithelial branching during kidney development. The invasion of the metanephric mesenchyme by the ureteric bud is a fundamental step towards establishing the cyto-architecture of the kidney and determining the number of nephrons, which form the filtration units of the adult kidney.
Introduction
Embryonic development of the vertebrate urogenital system requires progressive formation of three types of kidneys by the intermediate mesoderm: the pronephros, the mesonephros and the metanephros. In amniotes like mammals, the pronephros is a vestigial structure rapidly replaced by the mesonephros, which functions during embryonic development. The mesonephros will degenerate or differentiate into part of the reproductive system in the male [1]. In mouse, the Wolffian Duct (WD) grows rostro-caudally along the embryonic axis. As it elongates caudally it first induces the pronephros, then the mesonephros and finally the metanephros at the level of the midhind limb [1]. Metanephric kidney development is initiated around midgestation (E10.5) when the caudal part of the Wolffian duct (WD) starts to swell and an epithelial thickening called the ureteric bud (UB) forms and elongates to invade the adjacent metanephric mesenchyme (mm). Interactions between the metanephric mesenchyme and the tip of the UB lead to the establishment of complex epithelial-mesenchymal (e-m) signaling interactions [1–4]. These interactions are critical to initiation of outgrowth and repetitive branching of the UB that will generate the definitive renal collecting system and induce formation of renal vesicles. The renal vesicles form by a mesenchyme to epithelium conversion and are the precursors of the nephrons [1,3,4]. Nephrons are the basic functional units of the kidney and are essential for regulating the water content and removing soluble waste substances by filtering the blood [4].
Understanding the molecular mechanisms underlying UB formation and branching morphogenesis is very important with respect to gaining insight into the etiology of human congenital malformations and diseases. Indeed, urinary tract malformations are among the most common birth defects in humans and represent almost 1% of all birth defects [5–7]. Outgrowth and correct positioning of the UB is critical as anomalies in this process account for the majority of all developmental defects affecting kidneys [7]. Moreover, the number of nephrons is primarily determined by the amount of branches generated during metanephric kidney morphogenesis. Therefore, defects in metanephric kidney branching morphogenesis may predispose people to future, chronic renal disease [4]. This review will focus on recent studies exploring the role of Ret/GDNF signaling during UB induction and the control of branching morphogenesis.
The molecular networks controlling ureteric bud formation
Kidney development is controlled by e-m signaling interactions that are mediated in large parts by the Ret/GDNF signaling pathway [2,8]. Indeed, genetic inactivation of Gdnf or its receptor complex results in complete renal agenesis or severe reduction in kidney size in mouse embryos [9–14]. Gdnf is expressed by the metanephric mesenchyme and signals through a receptor complex consisting of the receptor tyrosine kinase (RTK) Ret and the co-receptor GRFα, both expressed initially by the WD epithelium and later by the tips of the UB [13,15]. Upon stimulation by GDNF, Ret activates several downstream effectors pathways including the ERK MAPK, PI3Kinase/Akt and PLC pathways [2].
Recently, several groups have shown that β-catenin, a key component of canonical WNT signal transduction [16], is involved in the regulation of Ret expression. Using a specific Cre recombinase transgenic mouse strain, it was shown that targeted inactivation of β-catenin specifically in the epithelial cells forming the WD and UB results in either aberrant UB budding along the entire WD [17] or rapid cessation of branching morphogenesis, which causes renal agenesis [18]. These different effects after inactivating β-catenin in the epithelium are a likely consequence of incomplete mosaic inactivation, which results in an epithelium consisting of both wild type and mutant cells. Interestingly, it was shown that Ret expression is lost from β-catenin deficient WD cells, which also resulted in premature activation of genes normally expressed by differentiated epithelial cells such as the water channel proteins or the tight junction protein ZO1 [17]. On the contrary, cells that continue to express β-catenin also retain Ret expression, which reveals the cell autonomous effects of β-catenin. In summary, WNT/β-catenin appears important regulators of Ret expression in WD/UB cells and seems to maintain the epithelium in an undifferentiated stage that allows progression of morphogenesis. However, the nature of the involved Wnt ligand remains unknown as none of the Wnt loss-of-function mutations reproduces the kidney phenotypes of β-catenin deficient mouse embryos [19–22].
Another important transcriptional regulator involved in the control of Ret expression is Gata3, a Zn-finger transcription factor. Genetic inactivation of Gata3 results in formation of ectopic buds, renal agenesis and/or severe hypoplasia with associated loss of Ret expression [23•]. The observed phenotypes are strikingly similar to the ones observed upon conditional deletion of β-catenin from the WD epithelium (see above) and [17]. Indeed, Gata3 expression is down-regulated in β-catenin mutant WD epithelial cells, while β-catenin expression is retained in the WD epithelium of Gata3 deficient mouse embryos. In conclusion, these data suggest that Gata3 acts downstream of β-catenin in activation of Ret expression and prevents precocious differentiation of the WD epithelium, thereby allowing continued morphogenesis [17,18,23•].
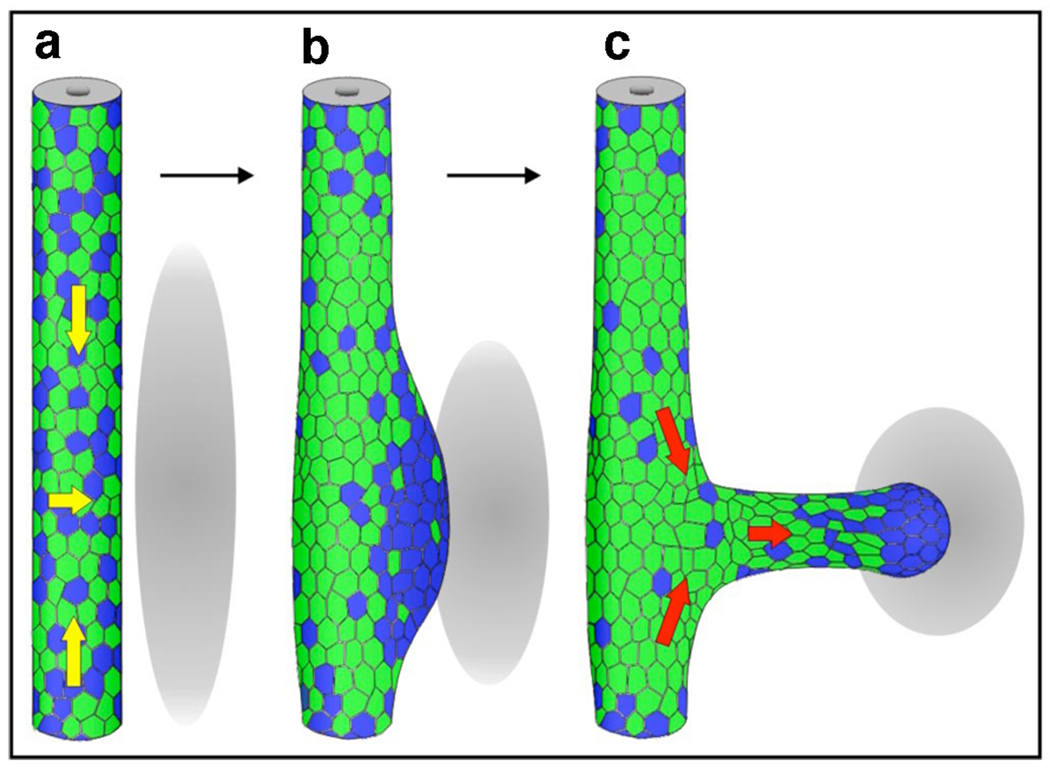
The first step in UB formation is the swelling of the caudal part of the WD at the prospective budding site. It has been shown that caudal WD cells have a greater proliferative index than WD cells located more rostrally [24]. From mainly this observation, it was hypothesized that the swelling of the WD was due to locally increased cell proliferation. However a recent study established that UB formation is mainly initiated as a consequence of directed cell movements within the WD epithelium rather than altered proliferation rates [25••]. Although Ret is expressed all along the entire WD epithelium prior to localized induction of the swelling, Ret activity is clearly heterogeneous as judged by using the pERK antigen as readout of Ret-mediated signal transduction. In response to such localized stimulation by GDNF induced Ret signal transduction, WD cells compete for inclusion in the forming primary ureteric bud based on their Ret activity levels. WD cells with high Ret signal transduction will out-compete cells with lower activity and move towards the region that will swell to form the ureteric bud. At the end of this process, the caudal WD epithelium is composed of two compartments: the swelling region that is composed of cells with high Ret activity and the adjacent region, which is mostly composed of cells with low or no Ret activity. The Ret positive cells included in the swelling then go on to form the UB that elongates toward the source of GDNF [25••] (Figure 1). This study begins to reveal the molecular and cellular mechanisms that underlie formation of the UB and shows that an essential step in this process involves cell competition based on Ret activity levels.
Figure 1. Cell movements and heterogeneous Ret signaling during ureteric bud formation.
Diagram illustrating rearrangement of Ret “positive” (blue) and Ret “negative” cells (green). Gray ovals represent the metanephric mesenchyme. Initially Ret “positive” cells are dispersed along the WD (A) and start to move (yellow arrows) to form the primary ureteric bud (UB) tip domain (B). As the UB grows out, these cells “lead” to form the distal tip, while Ret “negative” cells follow (C, red arrows). Reproduced with permission from [25]
However, it is important to note that during this process the entire WD expresses Ret [15] and several studies using mouse embryos lacking particular genes [26–28••] in combination with in vitro kidney culture experiments [29,30•] have established that the caudal WD has the capability to form UB along its entire length. Therefore, additional levels of regulation must restrict formation of the ureteric bud to one single site.
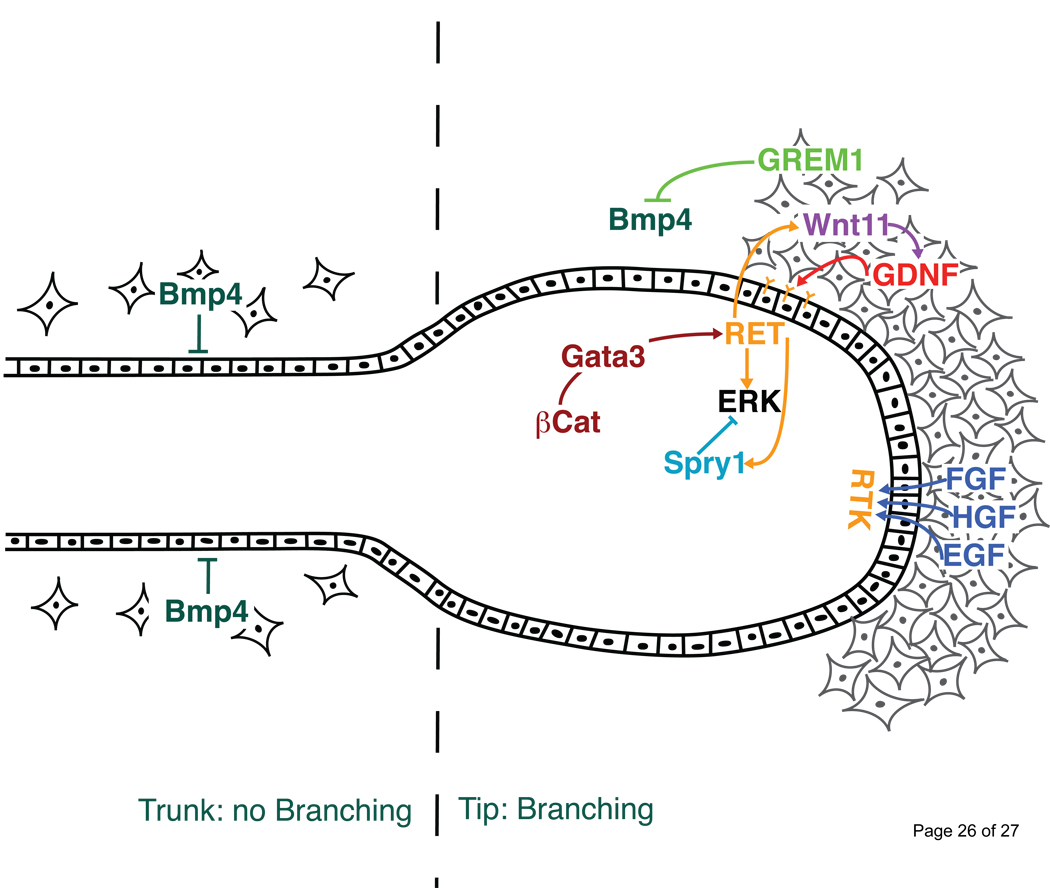
There are different ways to inhibit UB formation by the WD such as inhibiting Ret activity by intra-cellular inhibitors directly in the epithelium or production of mesenchymal-derived signaling inhibitors that exert their effects on the epithelium. With respect to the latter, BMP4, a member of the TGFβ–super family, is expressed by the mesenchyme surrounding the WD and was shown to inhibit UB outgrowth in culture. Addition of recombinant BMP4 to kidney explants in culture inhibits UB outgrowth [31] and mouse embryo heterozygous for a loss-of-function Bmp4 mutation display ectopic buds at low frequency [32]. Interestingly, Gremlin1, a secreted BMP antagonist, is also dynamically expressed in a restricted mesenchymal region around the future budding site. Moreover, mouse embryos lacking Gremlin1 display complete renal agenesis due to a failure of the UB to invade the metanephric mesenchyme [30•,33]. Initially, all genes required for initiation of UB outgrowth are normally expressed and a UB forms in Gremlin1 deficient mouse embryos albeit its shape being rather abnormal. However, initiation of its outgrowth is blocked resulting in down-regulation of Gdnf expression in the adjacent mesenchyme. This block results in massive mesenchymal cell death due to the failure to induce the e-m signaling interaction between the UB and the metanephric mesenchyme [33]. Interestingly, reduction of the Bmp4 gene dosage completely restores UB outgrowth and epithelial branching in Gremlin1 deficient mouse embryos in agreement with the proposal that high BMP activity represses UB outgrowth and branching morphogenesis [30•] (Figure 2). These results place Gremlin1 in a critical position during induction of kidney branching morphogenesis as its activity in the mesenchyme enables the Ret positive epithelial tip, i.e. the UB, to invade the metanephric mesenchyme and initiate branching morphogenesis.
Figure 2. Molecular interactions of the signals and antagonists participating in kidney epithelial branching morphogenesis.
The Ret/GDNF pathway is restricted to the tip of the developing UB epithelium to insure progression of branching morphogenesis. Correct activity of Ret is assured by positive (Wnt11) and negative (Sprouty1) feedback signaling. Other RTK signal pathways such as FGF, EGF and HGF also positively contribute to this feedback loop. Along the ureteric stalk, BMP4 signaling exerts an inhibitory effect on branching morphogenesis. Inhibition of BMP4 by Gremlin 1 in the mesenchyme surrounding the UB tip region could enables branching morphogenesis.
Furthermore, Sprouty1 plays an important function in inhibition of Ret-mediated signal transduction directly in the WD epithelium. Sprouty1 is an intra-cellular inhibitor of RTK signaling. Although, the exact mechanism by which Sprouty1 acts is not clear, it was shown that Sprouty1 inhibits ERK signal transduction [34]. Inactivation of Sprouty1 results in aberrant formation of extra buds along the caudal WD in mouse embryos [28••]. Interestingly, reduction of the Gdnf gene dosage in Sprouty1 deficient mouse embryos inhibits the formation of additional buds [28••]. Taken together, these results indicate that Sprouty1 inhibits Ret activity probably via inhibition of ERK phosphorylation, which contributes to restricting UB formation to one single site. In summary, UB formation requires cell movements within the WD epithelium that are mediated by high Ret activity. Therefore, tight regulation of Ret-mediated signal transduction is critical to normal metanephric kidney development.
Branching morphogenesis and formation of the UB tree
During invasion of the metanephric mesenchyme, the UB generates “tip-trunk” identities and tip cells that function as a signaling center under control of Ret activity [29]. In fact, most proliferation during epithelial branching occurs in the tip region, which expresses genes regulating branching morphogenesis [35]. At this stage, tip cells engage in rapid expansion, which initiates outgrowth of the UB and is followed by bifurcation of its tip [36] (Figure 3).
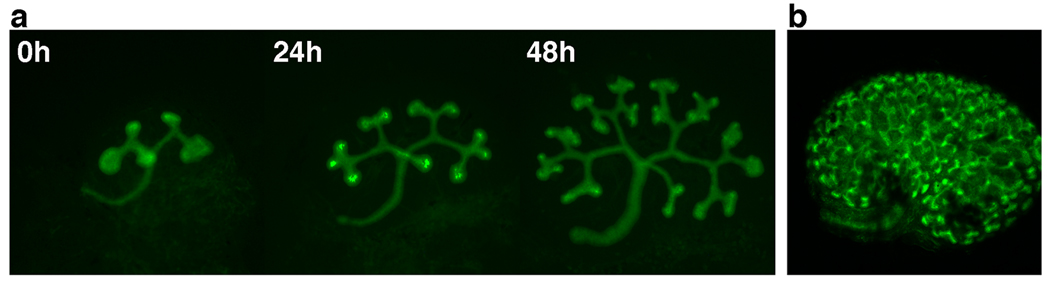
Figure 3. In vivo visualization of UB branching morphogenesis.
(A) A metanephric kidney rudiment (E12.5) cultured on a Transwell filter at the air/surface interface at 37°C for 48 hours. The epithelium is marked by GFP expression using the HoxB7-mVenus transgene [52]. (B) The epithelial branching tree visualized in an E15.0 kidney by confocal microscopy to reveal the complex 3D architecture of the UB tree.
During branching, GDNF continues to play a pivotal role and its importance has been analyzed in detail. For example, only 50% of all kidneys develop normally in Gdnf heterozygote mice, the others display branching defects ranging from reduced kidney size to complete renal aplasia [2]. In contrast, UB tips are aberrantly enlarged and abnormal branching patterns in association with increased Gdnf expression are detected in developing kidneys of Sprouty1 deficient mouse embryos [37]. Interestingly, genetic reduction of Gdnf rescues most of the branching defects observed in Sprouty1 deficient kidneys. Moreover, the hypomorphic phenotype (smaller kidneys) observed in Gdnf heterozygous mice is also rescued by reducing the Sprouty1 gene dosage [37]. These experiments point to an important aspect of signaling during kidney branching morphogenesis, namely that is not a particular signal that is decisive, but the balance between activating (GDNF) and inhibitory (Sprouty1) signals received by cells.
During branching morphogenesis, other RTK signaling pathways also participate in regulating branching of the UB. FGF signaling via FGFR2 plays a significant role as epithelial branching is reduced in mouse embryos lacking Fgfr2 in the WD/UB epithelium [38]. Similarly, branching morphogenesis is also reduced in Fgf7 and Fgf10 deficient mouse embryos [39,40]. Moreover, HGF/Met and EGF/EGFR were recently shown to participate in branching morphogenesis [41•]. Although, inhibition of these growth factors in kidney rudiment cultures inhibits UB branching [42–44], their genetic inactivation does not impair branching morphogenesis significantly [45–47]. However, Met deficient mouse embryos are characterized by increased Egfr expression during kidney development [41•]. Interestingly, the generation of the Met-EGFR double mutant causes more severe branching defects than the one observed in single mutant embryos. These results indicate that Met and EGFR interact synergistically during kidney epithelial branching morphogenesis. In conclusion, this study points to compensatory effects among different growth factors during kidney morphogenesis. Such compensatory mechanisms could explain why the addition of recombinant factors to kidney rudiments in culture causes more significant alterations than expected from the rather subtle phenotypes following their genetic inactivation in mouse embryos (see above) [41•]. Last but not least, these studies reveal that other RTK signaling pathways are required for normal kidney epithelial branching morphogenesis albeit Ret/GDNF signaling being the key regulatory pathway. These pathways may act in parallel or sequentially to Ret/GDNF signaling but additional analysis is required to identify the relevant interactions and underlying networks that orchestrate branching morphogenesis.
For example, the functions of Wnt signaling during initiation (see above) and progression of branching morphogenesis remain largely elusive. To date only Wnt11 is known to be directly required for normal branching morphogenesis. Mice deficient for Wnt11 display reduced kidney sizes, which correlates with reduced Ret/GDNF signaling [22]. In Wnt11 deficient mouse embryos, Gdnf expression is reduced in the mesenchyme and conversely, Wnt11 expression is down-regulated in Ret deficient kidney rudiments [22]. It was concluded that GDNF activates Wnt11 expression in the Ret expressing UB tip cells and that Wnt11 in turn maintains and reinforces Gdnf expression in the adjacent metanephric mesenchyme [22]. Therefore, Wnt11 is part of a positive feedback loop that potentiates GDNF signaling to enable progression of epithelial branching morphogenesis (Figure 2).
An important aspect of UB branching morphogenesis is specification of the branching pattern over space and time. GDNF has long been thought to be the factor that determines where the next branches will be generated [2,48]. However, several attempts to verify this hypothesis have failed to provide conclusive insights and it remains unclear if and how GDNF would exerts its function as a chemo-attractant that would direct UB tip outgrowth toward the GDNF source [49]. In this respect, the specific restriction of the expression of e.g. Ret and Wnt11 to the tip of the UB might have significant implications in terms of regulating the epithelial branching pattern. Another way to control the branching pattern could be imposed by external forces independent of Ret/GDNF signaling. Interestingly, Bmp4 and Gremlin1 remain expressed in a complementary pattern during progression of branching. Bmp4 is expressed along the ureter and UB stalks and Gremlin1 in the metanephric mesenchyme surrounding the UB tips ([30•] and unpublished). At this stage, BMP4 is required for differentiation of the ureter formed by the proximal part of the UB stalk that does not invade the metanephric mesenchyme [50•]. Moreover, addition of BMP4 to kidney rudiments in culture inhibits branching morphogenesis [31]. Conversely, supplementing kidney rudiments in culture with Gremlin1 causes aberrant extra budding from the WD and distal UB stalk and perturbs the regular epithelial branching pattern [30]. In agreement, mouse embryos lacking the BMP4 receptor Alk3 display an initial increase in branching, which is followed by a marked reduction resulting again in abnormal branching [51]. Therefore, the Gremlin1/BMP pathway maybe part of the genetic network that regulates the branching pattern in time and space.
Conclusions
Although much is still to be learnt with respect to the molecular and cellular mechanisms that underlie establishment of the UB epithelial branching network, the development of genetic tools such as mouse mutants that allow generation and analysis of kidney development in chimeric embryos has begun to facilitate these studies. Moreover, the results obtained from such clonal analysis in combination with 3D imaging of branching morphogenesis make the developing kidney an attractive model system to identify and study the basic developmental mechanisms and networks that govern epithelial branching morphogenesis in vertebrate embryos. The analysis of different epithelial branching modes is not only important to uncover the extent of evolutionary conservation of the underlying mechanisms, but is necessary to understand how possibly rather similar principles are used to generate organ specific branching patterns.
Acknowledgements
The author thanks Frank Costantini for helpful discussions while writing the manuscript. Many thanks to Antonella Galli for input and help in designing the figures and Rolf Zeller for editing the manuscript. I was supported by a fellowship from the National Kidney Foundation. I apologize to all colleagues, whose research could not be discussed or cited due to space limitations
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The author has no conflict of interests.
References and recommended reading
- 1.Saxén L. In: Organogenesis of the Kidney. Barlow PW, Green PB, Wylie CC, editors. Cambridge: Cambridge University Press; 1987. [Google Scholar]
- 2.Costantini F. Renal branching morphogenesis: concepts, questions, and recent advances. Differentiation. 2006;74:402–421. doi: 10.1111/j.1432-0436.2006.00106.x. [DOI] [PubMed] [Google Scholar]
- 3.Dressler GR. The cellular basis of kidney development. Annu Rev Cell Dev Biol. 2006;22:509–529. doi: 10.1146/annurev.cellbio.22.010305.104340. [DOI] [PubMed] [Google Scholar]
- 4.Schedl A. Renal abnormalities and their developmental origin. Nat Rev Genet. 2007;8:791–802. doi: 10.1038/nrg2205. [DOI] [PubMed] [Google Scholar]
- 5.Scott JE. Fetal ureteric reflux. Br J Urol. 1987;59:291–296. doi: 10.1111/j.1464-410x.1987.tb04634.x. [DOI] [PubMed] [Google Scholar]
- 6.Scott JE, Renwick M. Antenatal diagnosis of congenital abnormalities in the urinary tract. Results from the Northern Region Fetal Abnormality Survey. Br J Urol. 1988;62:295–300. doi: 10.1111/j.1464-410x.1988.tb04351.x. [DOI] [PubMed] [Google Scholar]
- 7.Mendelsohn C. Using mouse models to understand normal and abnormal urogenital tract development. Organogenesis. 2009;5:306–314. doi: 10.4161/org.8173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costantini F, Shakya R. GDNF/Ret signaling and the development of the kidney. Bioessays. 2006;28:117–127. doi: 10.1002/bies.20357. [DOI] [PubMed] [Google Scholar]
- 9.Moore MW, Klein RD, Farinas I, Sauer H, Armanini M, Phillips H, Reichardt LF, Ryan AM, Carver-Moore K, Rosenthal A. Renal and neuronal abnormalities in mice lacking GDNF. Nature. 1996;382:76–79. doi: 10.1038/382076a0. [DOI] [PubMed] [Google Scholar]
- 10.Pichel JG, Shen L, Sheng HZ, Granholm AC, Drago J, Grinberg A, Lee EJ, Huang SP, Saarma M, Hoffer BJ, et al. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature. 1996;382:73–76. doi: 10.1038/382073a0. [DOI] [PubMed] [Google Scholar]
- 11.Sanchez MP, Silos-Santiago I, Frisen J, He B, Lira SA, Barbacid M. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature. 1996;382:70–73. doi: 10.1038/382070a0. [DOI] [PubMed] [Google Scholar]
- 12.Schuchardt A, D'Agati V, Larsson-Blomberg L, Costantini F, Pachnis V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature. 1994;367:380–383. doi: 10.1038/367380a0. [DOI] [PubMed] [Google Scholar]
- 13.Enomoto H, Araki T, Jackman A, Heuckeroth RO, Snider WD, Johnson EM, Jr, Milbrandt J. GFR alpha1-deficient mice have deficits in the enteric nervous system and kidneys. Neuron. 1998;21:317–324. doi: 10.1016/s0896-6273(00)80541-3. [DOI] [PubMed] [Google Scholar]
- 14.Sainio K, Suvanto P, Davies J, Wartiovaara J, Wartiovaara K, Saarma M, Arumae U, Meng X, Lindahl M, Pachnis V, et al. Glial-cell-line-derived neurotrophic factor is required for bud initiation from ureteric epithelium. Development. 1997;124:4077–4087. doi: 10.1242/dev.124.20.4077. [DOI] [PubMed] [Google Scholar]
- 15.Pachnis V, Mankoo B, Costantini F. Expression of the c-ret proto-oncogene during mouse embryogenesis. Development. 1993;119:1005–1017. doi: 10.1242/dev.119.4.1005. [DOI] [PubMed] [Google Scholar]
- 16.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 17.Marose TD, Merkel CE, McMahon AP, Carroll TJ. Beta-catenin is necessary to keep cells of ureteric bud/Wolffian duct epithelium in a precursor state. Dev Biol. 2008;314:112–126. doi: 10.1016/j.ydbio.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bridgewater D, Cox B, Cain J, Lau A, Athaide V, Gill PS, Kuure S, Sainio K, Rosenblum ND. Canonical WNT/beta-catenin signaling is required for ureteric branching. Dev Biol. 2008;317:83–94. doi: 10.1016/j.ydbio.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 19.Yu J, Carroll TJ, Rajagopal J, Kobayashi A, Ren Q, McMahon AP. A Wnt7b-dependent pathway regulates the orientation of epithelial cell division and establishes the cortico-medullary axis of the mammalian kidney. Development. 2009;136:161–171. doi: 10.1242/dev.022087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell. 2005;9:283–292. doi: 10.1016/j.devcel.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 21.Karner CM, Chirumamilla R, Aoki S, Igarashi P, Wallingford JB, Carroll TJ. Wnt9b signaling regulates planar cell polarity and kidney tubule morphogenesis. Nat Genet. 2009;41:793–799. doi: 10.1038/ng.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Majumdar A, Vainio S, Kispert A, McMahon J, McMahon AP. Wnt11 and Ret/Gdnf pathways cooperate in regulating ureteric branching during metanephric kidney development. Development. 2003;130:3175–3185. doi: 10.1242/dev.00520. [DOI] [PubMed] [Google Scholar]
- •23.Grote D, Boualia SK, Souabni A, Merkel C, Chi X, Costantini F, Carroll T, Bouchard M. Gata3 acts downstream of beta-catenin signaling to prevent ectopic metanephric kidney induction. PLoS Genet. 2008;4:e1000316. doi: 10.1371/journal.pgen.1000316. Inactivation of Gata3 specifically in the Wolffian duct epithelium results in loss of Ret expression. Moreover, this study demonstrate that Gata3 acts downstream of β-catenin to activate/maintain Ret expression during kidney development.
- 24.Michael L, Davies JA. Pattern and regulation of cell proliferation during murine ureteric bud development. J Anat. 2004;204:241–255. doi: 10.1111/j.0021-8782.2004.00285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••25. Chi X, Michos O, Shakya R, Riccio P, Enomoto H, Licht JD, Asai N, Takahashi M, Ohgami N, Kato M, et al. Ret-dependent cell rearrangements in the Wolffian duct epithelium initiate ureteric bud morphogenesis. Dev Cell. 2009;17:199–209. doi: 10.1016/j.devcel.2009.07.013. Analysis of the formation of the primary ureteric bud in mouse kidney using live imaging and chimeric analysis. The authors demonstrate that the formation of the primary UB is controlled by competive cell movements based on Ret activity.
- 26.Grieshammer U, Le M, Plump AS, Wang F, Tessier-Lavigne M, Martin GR. SLIT2-mediated ROBO2 signaling restricts kidney induction to a single site. Dev Cell. 2004;6:709–717. doi: 10.1016/s1534-5807(04)00108-x. [DOI] [PubMed] [Google Scholar]
- 27.Kume T, Deng K, Hogan BL. Murine forkhead/winged helix genes Foxc1 (Mf1) and Foxc2 (Mfh1) are required for the early organogenesis of the kidney and urinary tract. Development. 2000;127:1387–1395. doi: 10.1242/dev.127.7.1387. [DOI] [PubMed] [Google Scholar]
- ••28.Basson MA, Akbulut S, Watson-Johnson J, Simon R, Carroll TJ, Shakya R, Gross I, Martin GR, Lufkin T, McMahon AP, et al. Sprouty1 is a critical regulator of GDNF/RET-mediated kidney induction. Dev Cell. 2005;8:229–239. doi: 10.1016/j.devcel.2004.12.004. The Sprouty proteins are important intracellular inhibitor of Receptor Tyrosine Kinase signaling. This study shows that inactivation of Sprouty1 during kidney development results in formation of extrabud, which results in abnormal kidney formation. This includes hydroureters, hydronephrosis and multiple kidneys. Moreover, the authors demontrate that in the context of kidney development, Sprouty1 inhibits mainly the Ret/GDNF pathway.
- 29.Shakya R, Watanabe T, Costantini F. The role of GDNF/Ret signaling in ureteric bud cell fate and branching morphogenesis. Dev Cell. 2005;8:65–74. doi: 10.1016/j.devcel.2004.11.008. [DOI] [PubMed] [Google Scholar]
- •30.Michos O, Goncalves A, Lopez-Rios J, Tiecke E, Naillat F, Beier K, Galli A, Vainio S, Zeller R. Reduction of BMP4 activity by gremlin 1 enables ureteric bud outgrowth and GDNF/WNT11 feedback signalling during kidney branching morphogenesis. Development. 2007;134:2397–2405. doi: 10.1242/dev.02861. Mice deficient for Gremlin1 display complete renal agenesis due to failure of UB outgrowth. Interestingly, reduction of the Bmp4 gene dosage rescues kidney development in Gremlin1 mutant demonstrating the inhibitory activity of BMP on UB outgrowth.
- 31.Bush KT, Sakurai H, Steer DL, Leonard MO, Sampogna RV, Meyer TN, Schwesinger C, Qiao J, Nigam SK. TGF-beta superfamily members modulate growth, branching, shaping, and patterning of the ureteric bud. Dev Biol. 2004;266:285–298. doi: 10.1016/j.ydbio.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 32.Miyazaki Y, Oshima K, Fogo A, Hogan BL, Ichikawa I. Bone morphogenetic protein 4 regulates the budding site and elongation of the mouse ureter. J Clin Invest. 2000;105:863–873. doi: 10.1172/JCI8256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Michos O, Panman L, Vintersten K, Beier K, Zeller R, Zuniga A. Gremlin-mediated BMP antagonism induces the epithelial-mesenchymal feedback signaling controlling metanephric kidney and limb organogenesis. Development. 2004;131:3401–3410. doi: 10.1242/dev.01251. [DOI] [PubMed] [Google Scholar]
- 34.Mason JM, Morrison DJ, Basson MA, Licht JD. Sprouty proteins: multifaceted negative-feedback regulators of receptor tyrosine kinase signaling. Trends Cell Biol. 2006;16:45–54. doi: 10.1016/j.tcb.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt-Ott KM, Yang J, Chen X, Wang H, Paragas N, Mori K, Li JY, Lu B, Costantini F, Schiffer M, et al. Novel regulators of kidney development from the tips of the ureteric bud. J Am Soc Nephrol. 2005;16:1993–2002. doi: 10.1681/ASN.2004121127. [DOI] [PubMed] [Google Scholar]
- 36.Watanabe T, Costantini F. Real-time analysis of ureteric bud branching morphogenesis in vitro. Dev Biol. 2004;271:98–108. doi: 10.1016/j.ydbio.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 37.Basson MA, Watson-Johnson J, Shakya R, Akbulut S, Hyink D, Costantini FD, Wilson PD, Mason IJ, Licht JD. Branching morphogenesis of the ureteric epithelium during kidney development is coordinated by the opposing functions of GDNF and Sprouty1. Dev Biol. 2006;299:466–477. doi: 10.1016/j.ydbio.2006.08.051. [DOI] [PubMed] [Google Scholar]
- 38.Zhao H, Kegg H, Grady S, Truong HT, Robinson ML, Baum M, Bates CM. Role of fibroblast growth factor receptors 1 and 2 in the ureteric bud. Dev Biol. 2004;276:403–415. doi: 10.1016/j.ydbio.2004.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qiao J, Uzzo R, Obara-Ishihara T, Degenstein L, Fuchs E, Herzlinger D. FGF-7 modulates ureteric bud growth and nephron number in the developing kidney. Development. 1999;126:547–554. doi: 10.1242/dev.126.3.547. [DOI] [PubMed] [Google Scholar]
- 40.Ohuchi H, Hori Y, Yamasaki M, Harada H, Sekine K, Kato S, Itoh N. FGF10 acts as a major ligand for FGF receptor 2 IIIb in mouse multi-organ development. Biochem Biophys Res Commun. 2000;277:643–649. doi: 10.1006/bbrc.2000.3721. [DOI] [PubMed] [Google Scholar]
- •41.Ishibe S, Karihaloo A, Ma H, Zhang J, Marlier A, Mitobe M, Togawa A, Schmitt R, Czyczk J, Kashgarian M, et al. Met and the epidermal growth factor receptor act cooperatively to regulate final nephron number and maintain collecting duct morphology. Development. 2009;136:337–345. doi: 10.1242/dev.024463. Met mutant embryos are characterized by a mild kidney phenotype associated with an upregulation of the EGF receptor. Moreover, the Met/EGFR double mutant embryos display a severe reduction in branching morphogenesis. This study identifies the synergestic requirement of Met/EGFR signaling during kidney development.
- 42.Santos OF, Barros EJ, Yang XM, Matsumoto K, Nakamura T, Park M, Nigam SK. Involvement of hepatocyte growth factor in kidney development. Dev Biol. 1994;163:525–529. doi: 10.1006/dbio.1994.1169. [DOI] [PubMed] [Google Scholar]
- 43.Woolf AS, Kolatsi-Joannou M, Hardman P, Andermarcher E, Moorby C, Fine LG, Jat PS, Noble MD, Gherardi E. Roles of hepatocyte growth factor/scatter factor and the met receptor in the early development of the metanephros. J Cell Biol. 1995;128:171–184. doi: 10.1083/jcb.128.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sakurai H, Tsukamoto T, Kjelsberg CA, Cantley LG, SK N. EGF receptor ligands are a large fraction of in vitro branching morphogens secreted by embryonic kidney. Am J Physiol. 1997;273(3 Pt 2):F463–F472. doi: 10.1152/ajprenal.1997.273.3.F463. [DOI] [PubMed] [Google Scholar]
- 45.Bladt F, Riethmacher D, Isenmann S, Aguzzi A, Birchmeier C. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature. 1995;376:768–771. doi: 10.1038/376768a0. [DOI] [PubMed] [Google Scholar]
- 46.Schmidt C, Bladt F, Goedecke S, Brinkmann V, Zschiesche W, Sharpe M, Gherardi E, Birchmeier C. Scatter factor/hepatocyte growth factor is essential for liver development. Nature. 1995;373:699–702. doi: 10.1038/373699a0. [DOI] [PubMed] [Google Scholar]
- 47.Uehara Y, Minowa O, Mori C, Shiota K, Kuno J, Noda T, Kitamura N. Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature. 1995;373:702–705. doi: 10.1038/373702a0. [DOI] [PubMed] [Google Scholar]
- 48.Sariola H, Sainio K. The tip-top branching ureter. Curr Opin Cell Biol. 1997;9:877–884. doi: 10.1016/s0955-0674(97)80091-9. [DOI] [PubMed] [Google Scholar]
- 49.Shakya R, Jho EH, Kotka P, Wu Z, Kholodilov N, Burke R, D'Agati V, Costantini F. The role of GDNF in patterning the excretory system. Dev Biol. 2005;283:70–84. doi: 10.1016/j.ydbio.2005.04.008. [DOI] [PubMed] [Google Scholar]
- •50.Brenner-Anantharam A, Cebrian C, Guillaume R, Hurtado R, Sun TT, Herzlinger D. Tailbud-derived mesenchyme promotes urinary tract segmentation via BMP4 signaling. Development. 2007;134:1967–1975. doi: 10.1242/dev.004234. Bmp4 produced by the tailbud-derived mesenchyme is required for differentiation the definitive ureter. Ectopic Bmp4 induces ureter formation from regions of the UB that normaly do not differentiate into ureter.
- 51.Hartwig S, Bridgewater D, Di Giovanni V, Cain J, Mishina Y, Rosenblum ND. BMP receptor ALK3 controls collecting system development. J Am Soc Nephrol. 2008;19:117–124. doi: 10.1681/ASN.2007010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chi X, Hadjantonakis AK, Wu Z, Hyink D, Costantini F. A transgenic mouse that reveals cell shape and arrangement during ureteric bud branching. Genesis. 2009;47:61–66. doi: 10.1002/dvg.20452. [DOI] [PMC free article] [PubMed] [Google Scholar]