Abstract
To delineate the inflammatory potential of the 3 pathogenic species of Borrelia burgdorferi sensu lato, we stimulated monocyte-derived macrophages from healthy human donors with 10 isolates each of B. burgdorferi, B. afzelii, or B. garinii recovered from erythema migrans (EM) skin lesions of Lyme borreliosis patients from the United States or Slovenia. U.S. B. burgdorferi isolates induced macrophages to secrete significantly higher levels of IL-8, CCL3, CCL4, IL-6, IL-10 and TNF than B. garinii or B. afzelii isolates. Consistent with this response in cultured macrophages, the cytokine levels in sera of patients from whom the isolates were obtained were significantly greater in B. burgdorferi-infected patients than in B. afzelii- or B. garinii-infected patients. These results demonstrate in vitro and in vivo that B. burgdorferi has greater inflammatory potential than B. afzelii and B. garinii, which may account in part for variations in the clinical manifestations of Lyme borreliosis.
Keywords: Lyme disease, Borrelia, inflammation, macrophages, cytokines, chemokines
Lyme borreliosis, the most common vector-borne disease in North America, Europe and Asia [1], is caused primarily by 3 pathogenic species of Borrelia burgdorferi sensu lato [2]. In the United States (U.S.), B. burgdorferi is the sole agent of the disease, whereas Borrelia afzelii and Borrelia garinii are more prevalent in Europe. Variations in the clinical manifestations of the disease have been noted with each species [1]. For example, in the U.S., untreated B. burgdorferi infection often leads to arthritis, whereas in Europe, B. afzelii infection usually remains localized to the skin and B. garinii is associated with a range of neurologic complications.
With all 3 species, the first sign of infection is often an expanding skin lesion, called erythema migrans (EM), sometimes accompanied by non-specific symptoms, such as headache, fatigue, malaise, arthralgias, or myalgias [1, 3-6]. However, U.S. B. burgdorferi infection is associated with faster lesion expansion, more disease-associated symptoms, and more frequent hematogenous dissemination compared with European B. afzelii infection [3, 7, 8]. In Europe, B. garinii causes faster expansion and larger size of EM lesions than B. afzelii [9, 10], but both species are thought to expand more slowly than B. burgdorferi in the U.S. [7, 10]. Both microbial and host factors may play a role in these differences in disease expression.
In an effort to understand host responses to Borrelia species early in the infection, several previous studies examined cytokine or chemokine mRNA profiles in the EM skin lesions of U.S. and Austrian patients with Lyme borreliosis [3, 11-13]. Predominant cytokines in EM lesions were the pro-inflammatory cytokine IFN-γ and the anti-inflammatory cytokine IL-10 [11, 13]. However, in many cases, a number of additional pro-inflammatory cytokines were present, including IL-6, TNF, and IL-1β, which are associated with the activation of macrophages and other innate immune cells [11, 13]. Similarly, inflammatory chemokines were significantly greater in lesional skin than in normal skin, including the neutrophil chemoattractant CXCL1, the macrophage chemoattractants, CCL2, CCL3, and CCL4, and the T-cell chemoattractants, CXCL9 and CXCL10 [12]. In a recent study, the EM skin lesions of B. burgdorferi-infected U.S. patients had higher mRNA levels of cytokines and chemokines associated with macrophage activation than the lesions of B. afzelii-infected Austrian patients [3]. However, multiple resident and immune cells are involved in an effective immune response to Borrelia infection and the inflammatory potential of the 3 pathogenic Borrelia species has not been studied directly in macrophages.
In this study, we stimulated macrophages from healthy human donors with isolates of B. burgdorferi, B. afzelii or B. garinii, recovered from EM skin lesions of patients with Lyme borreliosis from the U.S. or Slovenia. B. burgdorferi induced normal macrophages to secrete higher levels of chemokines and cytokines than B. afzelii or B. garinii. This shows clearly that B. burgdorferi induces a greater inflammatory response in macrophages than B. afzelii or B. garinii.
MATERIALS AND METHODS
Patients
Between 1998 and 2001, 93 isolates of B. burgdorferi were recovered from biopsy samples of EM skin lesions in a study of U.S. patients with Lyme borreliosis from Rhode Island or Connecticut [14]. The Human Investigation Committee at Tufts Medical Center (1998-2001) and Massachusetts General Hospital (MGH, 2002-2008) approved the study. After obtaining informed consent, the study physicians (Dr. Nitin Damle, RI and Dr. Vijay Sikand, CT) performed a clinical assessment of signs and symptoms. In addition to skin biopsy samples for culturing, blood samples were obtained for PCR and serologic testing, and serum samples were frozen for subsequent determinations.
In Slovenia, EM skin biopsies from Lyme borreliosis patients were obtained from 1999 through 2006. Borrelia species were identified as previously described [7]. Nearly 80% of these isolates were B. afzelii and approximately 15% were B. garinii [15]. The study physician made a clinical assessment of signs and symptoms. In addition to skin biopsy samples, serum samples were obtained for serologic testing and subsequent determinations. The Medical Ethics Committee of Slovenia approved the study.
For this study, in which normal macrophages were stimulated with patients’ isolates, 10 isolates of B. burgdorferi from EM skin lesions of U.S. patients and 10 isolates each of B. afzelii or B. garinii from EM lesions of Slovenian patients were selected for testing. In an effort to assess isolates with possible differences in virulence, B. burgdorferi isolates from the U.S. were chosen randomly from 5 patients who had hematogenous dissemination, based on a positive PCR test for RecA gene segment in blood, and from 5 patients who lacked evidence of disseminated disease [14]. In Slovenia, where B. afzelii and B garinii less often cause hematogenous dissemination [16, 17], 5 isolates each of B. afzelii or B. garinii were selected randomly from patients who had ≥2 symptoms, and 5 each from patients who lacked associated symptoms. The clinical findings regarding EM expansion and the number of associated symptoms in patients from whom the isolates were obtained were consistent with those in larger patient series [3-7].
Preparation of spirochetal isolates
Because it is difficult to count motile spirochetes reliably and because all 30 cultures needed to be ready at the same time, the numbers of organisms in each culture were determined by optical density (OD) and the concentration of organisms was adjusted based on a carefully constructed standard curve. To construct the curve, the numbers of organisms in a control culture (N40) were first counted 4 times and averaged using a Petroff-Hauser chamber with darkfield microscopy. The number of oganisms was validated by QPCR targeting the RecA gene, as previously described [18]. Serial dilutions of the control culture were then used to generate a standard curve of OD values, as determined by spectrophotometer (Beckman DU 520 spectrophotometer, Beckman Coulter) at 600nm.
In preparation for experiments with macrophages, each of the 30 low passage (≤5) isolates were grown to late-log phase in complete BSK medium (Sigma Aldrich) containing 6% rabbit serum [19]. After washing twice, the OD value of each isolate was determined, and the standard curve was used to adjust the concentration of each isolate to 1.25 × 108 spirochetes/ml. As a final confirmation, the total protein concentration (DC Protein Assay, BioRad) of each isolate was shown to be similar.
Macrophage cell culture
Human macrophages were differentiated from peripheral blood mononuclear cells (PBMC) obtained from 9 healthy donors by the MGH Blood-Component laboratory. To assure that the donor was healthy, he or she was required to answer a questionnaire, provide a vaccination report, and undergo a physical examination. In addition, the blood samples were tested for markers of infectious diseases, including syphilis, HBV, HCV, HIV 1 / 2, HTLV I/II and West Nile Virus. If any of the tests were positive, the blood sample was not used.
Macrophages were derived as previously described [20-22]. Briefly, PBMC were re-suspended in RPMI-1640 (Invitrogen) containing 10% human serum (Mediatech) and seeded in flasks for 1 h to allow attachment of monocytes. Detached cells were removed by washing, and adherent cells were allowed to differentiate into macrophages by 6-day culture in RPMI-1640 supplemented with 25% human serum, 2mM L-glutamine, 100μg/ml streptomycin and 100μ/ml of penicillin at 37°C and 5% CO2. Before stimulation with borrelial isolates, adherent macrophages were washed 3 times, transferred to 96-well culture plates at 1×105 cells/well and deprived of serum and antibiotics for 12 h to remove growth factors. Because the inflammatory response in macrophages from frozen PBMC stocks was lower than in freshly derived macrophages, only fresh macrophages were used in subsequent experiments.
Macrophage markers
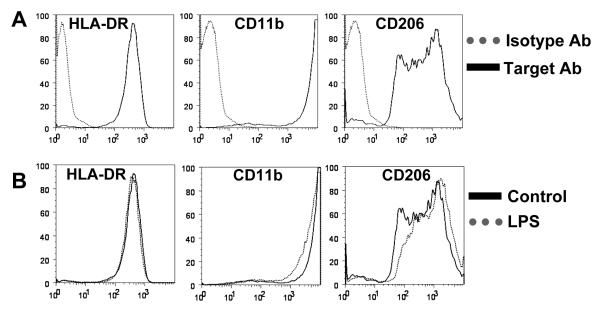
Pilot experiments using B. burgdorferi strain N40, demonstrated that maximum expression of the macrophage-derived cytokines and chemokines occurred within 48 h at a multiplicity of infection (MOI) of 25 organisms per cell (data not shown). These conditions were used in all subsequent experiments. Monocyte-derived macrophages were >95% pure as evidenced by their expression of cell-surface markers HLA-DR (BioLegend), CD11b and CD206 (BD Biosciences) following incubation in medium alone or LPS (50 ng/ml) for 36 h (FACSCalibur, BD Biosciences).
Biologic activity of macrophages
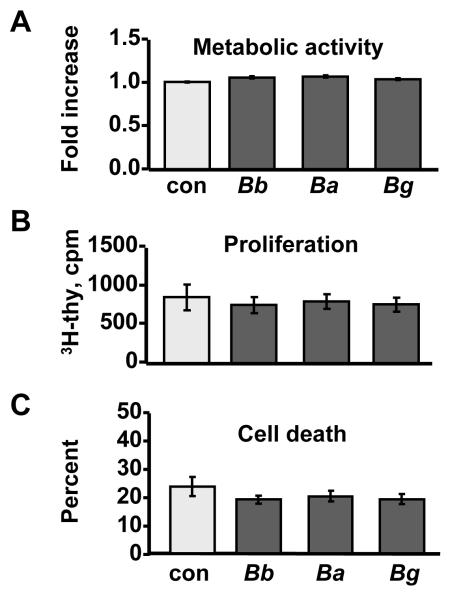
To assess whether live Borrelia were cytotoxic to macrophages in culture, metabolic activity, proliferation and cell death were measured after 48 h culture with each of the 30 borrelial isolates. The global metabolic activity of macrophages was assessed using the CellTiter Aqueous Cell Proliferation Assay (Promega), according to manufacturer’s protocol. Proliferation was determined by culturing macrophages with each of the borrelial isolates for 30 h, prior to addition of 0.5μCi of 3H-thymidine (Perkin Elmer) for 18 h and measurement of 3H-thymidine incorporation. Macrophage cell death was assessed via colorimetric CytoTox-96 Cytotoxicity Assay (Promega) following manufacturer’s Total-Cell-Number protocol. The percent cell death was a ratio of the absorbance values of LDH released into culture medium, a sign of cell death, divided by the absorbance value of total LDH released by homogenizing the remaining cells in the well.
Detection of chemokines and cytokines from normal macrophages stimulated with patients’ Borrelia isolates
Macrophages were cultured with each of the 10 isolates of B. burgdorferi, B. afzelii or B. garinii (MOI of 25) for 48 h in medium devoid of serum and antibiotics. All 30 isolates were tested with macrophages from each of the 9 healthy donors in 9 independent experiments. The expression of macrophage-derived chemokines, IL-8, CCL2, CCL3 and CCL4, and cytokines, TNF, IL-1β, IL-6, and IL-10, was assessed in culture supernatants (1:25) using 3 separate bead-based Multiplex assays from Millipore coupled with the Luminex-200 System (Luminex), following manufacturer’s overnight protocol. Mean fluorescence intensity was converted to pg/ml by the Upstate Beadview software (Millipore). The results for each chemokine and cytokine from all 9 experiments were averaged for analysis.
Detection of chemokines and cytokines in patient and control serum samples
Serum samples, obtained at the time of EM skin biopsy, were available from 22 of the 30 patients from whom Borrelia isolates were recovered; 10 were from B. burgdorferi—infected U.S. patients, 8 from B. afzelii-infected and 4 from B. garinii-infected Slovenian patients. In these samples, and in those from 9 healthy controls, the levels of chemokines (IL-8, CCL2, CCL3, CCL4 and CXCL-10) and cytokines (IL-6, IL-10, TNF, IL-1β and IFNγ) were assessed at the same time using the bead-based Multiplex assays (Millipore). Due to limited amounts of serum from patients, cytokine and chemokine levels in the samples were only determined once.
Statistical Analysis
Differences between groups of borrelial isolates were assessed using the Mann-Whitney rank-sum test. Statistical analyses were conducted using the Sigma Stat version 3.0.1 software from SPSS.
RESULTS
Characteristics and biologic activity of macrophages from healthy human donors
Following differentiation, virtually all (>95%) monocyte-derived cells expressed high levels of HLA-DR, CD11b and CD206 cell-surface markers, confirming their macrophage lineage (Fig. 1A). Stimulation with LPS did not significantly alter the expression of these surface markers (Fig. 1B). Thus, culturing of blood-derived monocytes in serum-enriched medium was sufficient to generate macrophages in absence of other stimuli [20-22].
Figure 1. Characterization of macrophage cell surface markers.
A) Monocyte-derived macrophages from normal human donors were cultured in medium alone for 12 h and then incubated with antibodies specific for cell surface markers HLA-DR, CD11b and CD206 (solid line), or with isotype control antibodies (dotted line). B) Macrophages from the same human donors were stimulated for 36 h with LPS (50ng/ml; dotted line), or left untreated (solid line) before incubation with antibodies specific for HLA-DR, CD11b and CD206. Approximately 95% of monocyte-derived macrophages from normal human donors expressed high levels of HLA-DR, CD11b and CD206, which is indicative of their macrophage lineage. The data shown are representative of 2 independent experiments.
As expected for mature, differentiated cells, no significant differences were observed in the total metabolic activity (Fig. 2A), proliferation (Fig. 2B) or viability (Fig. 2C) of macrophages during the 48-hour culture period with isolates from different species. Since Borrelia did not adversely affect the proliferation and viability of macrophages, we next assessed chemokine and cytokine secretion from these cells after stimulation with each of the 3 Borrelia species.
Figure 2. Biological activity of macrophages.
Macrophages from normal human donors were stimulated for 48 h with 10 B. burgdorferi isolates from EM lesions of U.S. patients, or 10 isolates each of B. afzelii or B.garinii from EM lesions of Slovenian patients. Unstimulated cells served as a control (con). A) Metabolic activity was expressed as fold increase above unstimulated control cells. B) Proliferation was assessed by the amount (cpm) of 3H-thymidine incorporated into DNA. C) Percent cell death was determined as a ratio of LDH released into culture medium divided by the total LDH released after homogenizing all cells. The data shown are representative of 2 independent experiments.
Chemokine and cytokine secretion by normal human macrophages stimulated with Borrelia isolates
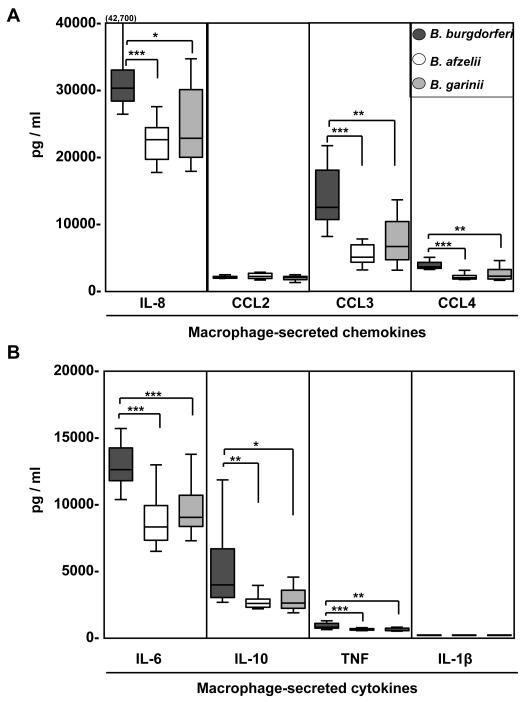
All 30 borrelial isolates induced the secretion of all chemokines and cytokines tested, but U.S. B. burgdorferi isolates stimulated macrophages to secrete significantly higher levels of IL-8, CCL3, CCL4, IL-6, TNF, and IL-10 than European B. afzelii or B. garinii isolates (Fig. 3). Among these inflammatory mediators, B. burgdorferi induced the highest secretion of IL-8 (median value, 30,088 pg/ml; range, 26,248-42,700), CCL3 (12,270 pg/ml; 7923-21441) and IL-6 (12,540 pg/ml; 10,273-15,620). In contrast, the 3 Borrelia species did not differ in induction of CCL2 or IL-1β (Fig. 3). Furthermore, no statistically significant differences in cytokine and chemokine secretion were observed between macrophages stimulated with B. afzelii or B. garinii. Among the U.S. B. burgdorferi isolates, the levels of chemokines and cytokines did not correlate with dissemination, and among the B. afzelii or B. garinii isolates the levels of these inflammatory mediators did not correlate with whether or not the patient had associated symptoms (data not shown). In comparison with Borrelia-activated macrophages, unstimulated cells secreted only low levels of IL-8 (median value, 173 pg/ml), CCL2 (405 pg/ml) and CCL3 (11 pg/ml), and undetectable levels of the other inflammatory mediators measured here (data not shown). We concluded that U.S. B. burgdorferi isolates induced macrophages to secrete higher levels of inflammatory chemokines and cytokines than Slovenian B. afzelii or B. garinii isolates.
Figure 3. Comparison of U.S. B. burgdorferi isolates and Slovenian B. afzelii and B. garinii isolates.
Normal human macrophages were stimulated for 48 h with 10 B. burgdorferi isolates from EM lesions of U.S. patients, or 10 B. afzelii isolates or 10 B. garinii isolates from EM lesions of Slovenian patients. A) Chemokine and B) cytokine levels were determined in culture supernatants using bead-based Multiplex assays. All 30 isolates were tested with macrophages from each of the 9 healthy donors. The values from the 9 experiments were then averaged. The box in the graph represent 25th and 75th percentiles, the line in the box is the median value and the lines outside the box represent 5th and 95th percentiles. Except for CCL2 and IL-1β, B. burgdorferi isolates induced significantly higher levels of chemokines and cytokines than B. afzelii and B. garinii isolates. *P ≤ 0.05, **P ≤ 0.01 ***P ≤ 0.001.
Chemokine and cytokine levels in serum samples from patients from whom the Borrelia isolates were obtained
Frozen serum samples, but not EM skin biopsy samples, were available from 22 of the 30 patients, including all 10 patients with B. burgdorferi infection, 8 with B. afzelii infection, and 4 with B. garinii infection. Because the results were similar in Slovenian patients infected with B. afzelii or B. garinii and because of small numbers, particularly in the B. garinii group, the results in these patients were combined for presentation (Fig. 4).
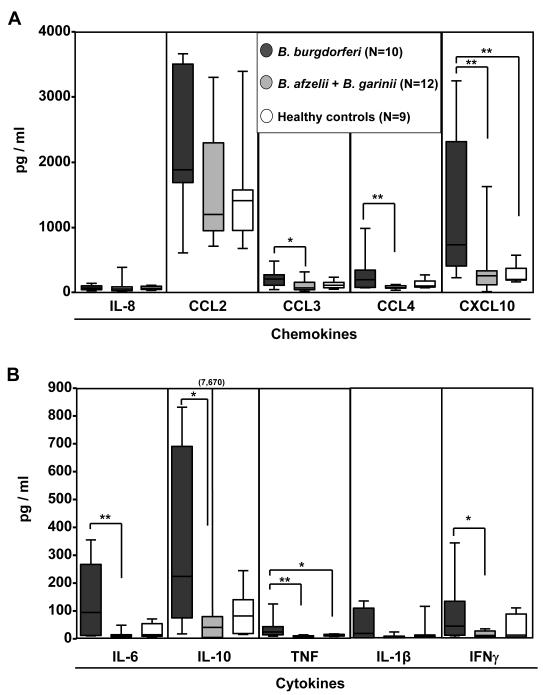
Figure 4. Serum levels of chemokines and cytokines.
Chemokine and cytokine levels were assessed in serum samples from 22 of the 30 patients from whom the Borrelia isolates were obtained and from 9 healthy donors with no history of Lyme borreliosis. Of the 22 patient sera, 10 were from U.S. patients with B. burgdorferi infection, and 12 were from Slovenian patients, 8 with B. afzelii infection and 4 with B. garinii infection. A) Chemokine and B) cytokine expression was determined using bead-based multiplex assays. The box in the graph represents 25th and 75th percentiles, the horizontal line in the box is the median and the lines outside the box are the 5th and 95th percentiles. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
Consistent with the response of macrophages to Borrelia stimulation in culture, the levels of CCL3, CCL4, IL-6, IL-10, and TNF were significantly higher in serum of B. burgdorferi-infected U.S. patients than in serum of B. afzelii- or B. garinii-infected Slovenian patients or normal control subjects. A similar trend was observed for CCL2 and IL-1β (Fig. 4). In addition, serum levels of the T-cell-derived cytokine, IFN-γ and the IFN-γ-inducible chemokines, CCL2 and CXCL10 were high, particularly in B. burgdorferi-infected patients. Among the American patients, there was not a significant correlation between the presence of B. burgdorferi DNA in blood and the levels of chemokines and cytokines (data not shown). Chemokine and cytokine levels in the sera of Slovenian patients were similar to those in normal control subjects. Thus, the presence of chemokines and cytokines in serum is a phenomenon associated with B. burgdorferi infection in the US.
DISCUSSION
Previous studies have suggested that B. burgdorferi may be more virulent than B. afzelii or B. garinii [3, 7]. Compared with the 2 European Borrelia species, B. burgdorferi in the U.S. causes faster expansion of EM skin lesions, is associated with more symptoms, and more often disseminates hematogenously [7, 8, 16, 17]. In addition, in our previous study, the EM lesions of B. burgdorferi-infected U.S. patients had higher mRNA expression of inflammatory chemokines and cytokines, including those associated with macrophage activation, than the lesions of B. afzelii-infected patients [3]. In both the human infection and in murine models, macrophages are critical in the early innate immune response [23-27]. However, the possibility that the 3 pathogenic Borrelia species differ in their ability to activate macrophages had not been assessed. We tested this hypothesis by stimulating macrophages from normal human donors with isolates of the 3 Borrelia species, and measured chemokine and cytokine responses in culture supernatants.
A number of steps were taken to standardize and validate the study methods. Since individual isolates of each Borrelia species may vary in virulence, we tested 10 isolates each of the 3 pathogenic Borrelia species obtained from patients’ EM skin lesions. In preliminary experiments, we confirmed that monocytes differentiated into macrophages, and showed that the global metabolic activity, proliferation and viability of these cells were not adversely affected by culture with Borrelia species. In an effort to hold host factors constant, we tested all 30 isolates with fresh macrophages from each of the 9 healthy donors. We timed the experiments so that isolates reached late-log phase at the same time that fresh macrophages differentiated to maturity. The numbers of organisms in each culture were determined by optical density (OD) and the concentration of organisms was adjusted based on a carefully constructed standard curve so that macrophages were stimulated with similar numbers of spirochetes. Because spirochetes begin to die within hours when removed from serum-enriched BSK medium [28], we do not think that the numbers of spirochetes increased in the macrophage cell cultures. Finally, we utilized bead-based multiplex assays to simultaneously measure the protein expression of multiple chemokines and cytokines from 1 complete experiment. This optimized culture system clearly showed that U.S. B. burgdorferi isolates stimulate macrophages to secrete higher levels of IL-8, CCL3, CCL4, IL-6, TNF, and IL-10 than European B. afzelii or B. garinii isolates.
Consistent with the response of macrophages to stimulation with Borrelia species, the levels of these chemokines and cytokines in the serum samples of patients from whom the isolates were obtained were greater in B. burgdorferi-infected patients than in B. afzelii- or B. garinii-infected patients. This shows that the higher inflammatory potential of U.S. B. burgdorferi isolates is not just an in vitro phenomenon observed with macrophages; it also occurs during the course of the natural infection. However, in contrast with cell culture, serum reflects global innate and adaptive immune responses of multiple cell types. In addition to the macrophage-derived chemokines and cytokines, sera from patients with B. burgdorferi infection contained significantly higher levels of the T-cell-derived cytokine, IFN-γ and the IFN-γ-inducible chemokines CCL2 and CXCL10, than sera from B. afzelii- or B. garinii-infected patients. We do not yet know whether B. burgdorferi activates other immune cells directly and to a greater degree than B. garinii or B. afzelii, or whether macrophages mediate the greater global immune responses associated with B. burgdorferi infection. Regardless, the analysis of sera, which is consistent with the results from EM skin lesions [3, 12], shows that U.S. B. burgdorferi isolates activate directly or indirectly both innate and adaptive immune responses to a greater degree than European B. afzelii or B. garinii isolates.
Why do U.S. B. burgdorferi isolates have greater inflammatory potential than European B. garinii or B. afzelii isolates? Since B. burgdorferi induces greater secretion of the same cytokines and chemokines as B. afzelii or B. garinii, it may simply express higher levels of one or more of the same lipoproteins. Alternately, B. burgdorferi may express one or more different lipoproteins, which could trigger additional intracellular signaling cascades, leading to higher cytokine and chemokine production. Several studies demonstrate the heterogeneity of lipoprotein expression among and within Borrelia species [29, 30]. We believe that our macrophage cell-culture system provides an important model to characterize the effects of these differences on immune cells.
The greater inflammatory potential of U.S. B. burgdorferi isolates probably accounts for certain differences in the clinical manifestations of Lyme borreliosis in the United States and Europe. For example, the greater inflammatory responses to B. burgdorferi in American patients may well explain the larger number of symptoms associated with EM skin lesions [3, 7] and the greater swelling of joints [1] compared with B. garinii or B. afzelii infection in Europe. However, it is not yet clear how these differences in host immune responses influence hematogenous dissemination, tropism to particular tissues, or persistence of the infection. B. burgdorferi in the U.S. is more commonly associated with hematogenous dissemination than B. afzelii or B. garinii in Europe [3, 4, 7, 16, 17]. Surprisingly, in the 10 American patients in our study, the presence of B. burgdorferi DNA in blood did not correlate with the levels of chemokines and cytokines. However, not all patients with hematogenous dissemination have a positive PCR result in blood [8, 14], and the possible presence of patients with dissemination in the PCR-negative group may blur distinctions between groups. What is clear is that patients infected with U.S. B. burgdorferi isolates, whether they had PCR evidence of dissemination or not, had significantly higher levels of chemokines and cytokines in blood than those infected with European Borrelia isolates.
In summary, our results show that B. burgdorferi directly stimulates macrophages to secrete higher levels of chemokines and cytokines than B. garinii and B. afzelii. In addition, the analysis of sera shows that B. burgdorferi activates both innate and adaptive immune responses to a greater degree than the 2 European Borrelia species. These results show unequivocally that B. burgdorferi has greater inflammatory potential than B. afzelii and B. garinii, which is likely to account in part for variations in the clinical manifestations of Lyme borreliosis.
Acknowledgments
We thank Dr. Nitin Damle and Dr. Vijay Sikand for obtaining the skin biopsy and serum samples from patients with EM, Dr. Xin Li for help with culture of borrelial isolates, Dr. Lisa Glickstein for review of the manuscript, and Colleen Squires for help with preparation of the manuscript.
Financial support: The National Institutes of Health (AR-20358), the Centers for Disease Control and Prevention (CCU110291), the English, Bonter, Mitchell Foundation, the Eshe Fund, and the Lyme/Arthritis Research Fund at Massachusetts General Hospital. K.S. received support from a scholarship from the Walter J. and Lille A. Berbecker Foundation for the study of Lyme disease. F.S. received funding from the Ministry of Science and Technology of the Republic of Slovenia (P3-0296).
Footnotes
1Conflict of interest: The authors do not have associations that may cause a conflict of interest.
Meeting information: A part of the information in this manuscript was presented at the 11th International Conference on Lyme Borreliosis and Other Tick-borne Diseases, Oct 2008, Irvine, CA
References
- 1.Steere AC. Lyme disease. N Engl J Med. 2001;345:115–25. doi: 10.1056/NEJM200107123450207. [DOI] [PubMed] [Google Scholar]
- 2.Baranton G, Postic D, Saint-Girons I, et al. Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov., and group VS461 associated with Lyme borreliosis. Int J Syst Bacteriol. 1992;42:378–83. doi: 10.1099/00207713-42-3-378. [DOI] [PubMed] [Google Scholar]
- 3.Jones KL, Muellegger RR, Means TK, et al. Higher mRNA levels of chemokines and cytokines associated with macrophage activation in erythema migrans skin lesions in patients from the United States than in patients from Austria with Lyme borreliosis. Clin Infect Dis. 2008;46:85–92. doi: 10.1086/524022. [DOI] [PubMed] [Google Scholar]
- 4.Nadelman RB, Nowakowski J, Forseter G, et al. The clinical spectrum of early Lyme borreliosis in patients with culture-confirmed erythema migrans. Am J Med. 1996;100:502–8. doi: 10.1016/s0002-9343(95)99915-9. [DOI] [PubMed] [Google Scholar]
- 5.Smith RP, Schoen RT, Rahn DW, et al. Clinical characteristics and treatment outcome of early Lyme disease in patients with microbiologically confirmed erythema migrans. Ann Intern Med. 2002;136:421–8. doi: 10.7326/0003-4819-136-6-200203190-00005. [DOI] [PubMed] [Google Scholar]
- 6.Strle F, Nelson JA, Ruzic-Sabljic E, et al. European Lyme borreliosis: 231 culture-confirmed cases involving patients with erythema migrans. Clin Infect Dis. 1996;23:61–5. doi: 10.1093/clinids/23.1.61. [DOI] [PubMed] [Google Scholar]
- 7.Strle F, Nadelman RB, Cimperman J, et al. Comparison of culture-confirmed erythema migrans caused by Borrelia burgdorferi sensu stricto in New York state and by Borrelia afzelii in Slovenia. Ann of Intern Med. 1999;130:32–6. doi: 10.7326/0003-4819-130-1-199901050-00006. [DOI] [PubMed] [Google Scholar]
- 8.Wormser GP, McKenna D, Carlin J, et al. Brief communication: hematogenous dissemination in early Lyme disease. Ann Intern Med. 2005;142:751–5. doi: 10.7326/0003-4819-142-9-200505030-00011. [DOI] [PubMed] [Google Scholar]
- 9.Carlsson SA, Granlund H, Jansson C, Nyman D, Wahlberg P. Characteristics of erythema migrans in Borrelia afzelii and Borrelia garinii infections. Scand J Infect Dis. 2003;35:31–3. doi: 10.1080/0036554021000026978. [DOI] [PubMed] [Google Scholar]
- 10.Logar M, Ruzic-Sabljic E, Maraspin V, et al. Comparison of erythema migrans caused by Borrelia afzelii and Borrelia garinii. Infection. 2004;32:15–9. doi: 10.1007/s15010-004-3042-z. [DOI] [PubMed] [Google Scholar]
- 11.Mullegger RR, McHugh G, Ruthazer R, Binder B, Kerl H, Steere AC. Differential expression of cytokine mRNA in skin specimens from patients with erythema migrans or acrodermatitis chronica atrophicans. J Investig Dermatol. 2000;115:1115–23. doi: 10.1046/j.1523-1747.2000.00198.x. [DOI] [PubMed] [Google Scholar]
- 12.Mullegger RR, Means TK, Shin JJ, et al. Chemokine signatures in the skin disorders of Lyme borreliosis in Europe: predominance of CXCL9 and CXCL10 in erythema migrans and acrodermatitis and CXCL13 in lymphocytoma. Infect Immun. 2007;75:4621–8. doi: 10.1128/IAI.00263-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salazar JC, Pope CD, Sellati TJ, et al. Coevolution of markers of innate and adaptive immunity in skin and peripheral blood of patients with erythema migrans. J Immunol. 2003;171:2660–70. doi: 10.4049/jimmunol.171.5.2660. [DOI] [PubMed] [Google Scholar]
- 14.Jones KL, Glickstein LJ, Damle N, Sikand VK, McHugh G, Steere AC. Borrelia burgdorferi genetic markers and disseminated disease in patients with early Lyme disease. J Clin Microbiol. 2006;44:4407–13. doi: 10.1128/JCM.01077-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruzic-Sabljic E, Maraspin V, Lotric-Furlan S, et al. Characterization of Borrelia burgdorferi sensu lato strains isolated from human material in Slovenia. Wien Klin Wochenschr. 2002;114:544–50. [PubMed] [Google Scholar]
- 16.Arnez M, Ruzic-Sabljic E, Ahcan J, Radsel-Medvescek A, Pleterski-Rigler D, Strle F. Isolation of Borrelia burgdorferi sensu lato from blood of children with solitary erythema migrans. Pediatr Infect Dis J. 2001;20:251–5. doi: 10.1097/00006454-200103000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Maraspin V, Ruzic-Sabljic E, Cimperman J, et al. Isolation of Borrelia burgdorferi sensu lato from blood of patients with erythema migrans. Infection. 2001;29:65–70. doi: 10.1007/s15010-001-0154-6. [DOI] [PubMed] [Google Scholar]
- 18.Morrison TB, Ma Y, Weis JH, Weis JJ. Rapid and sensitive quantification of Borrelia burgdorferi-infected mouse tissues by continuous fluorescent monitoring of PCR. J Clin Microbiol. 1999;37:987–92. doi: 10.1128/jcm.37.4.987-992.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barbour AG. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984;57:521–5. [PMC free article] [PubMed] [Google Scholar]
- 20.el Khoury J, Thomas CA, Loike JD, Hickman SE, Cao L, Silverstein SC. Macrophages adhere to glucose-modified basement membrane collagen IV via their scavenger receptors. J Biol Chem. 1994;269:10197–200. [PubMed] [Google Scholar]
- 21.Hickman SE, el Khoury J, Greenberg S, Schieren I, Silverstein SC. P2Z adenosine triphosphate receptor activity in cultured human monocyte-derived macrophages. Blood. 1994;84:2452–6. [PubMed] [Google Scholar]
- 22.Loike JD, Somes M, Silverstein SC. Creatine uptake, metabolism, and efflux in human monocytes and macrophages. Am J Physiol. 1986;251:C128–35. doi: 10.1152/ajpcell.1986.251.1.C128. [DOI] [PubMed] [Google Scholar]
- 23.Brown CR, Blaho VA, Loiacono CM. Susceptibility to experimental Lyme arthritis correlates with KC and monocyte chemoattractant protein-1 production in joints and requires neutrophil recruitment via CXCR2. J Immunol. 2003;171:893–901. doi: 10.4049/jimmunol.171.2.893. [DOI] [PubMed] [Google Scholar]
- 24.Montgomery RR, Malawista SE. Entry of Borrelia burgdorferi into macrophages is endon and leads to degradation in lysosomes. Infect Immun. 1996;64:2867–72. doi: 10.1128/iai.64.7.2867-2872.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Talkington J, Nickell SP. Role of Fc gamma receptors in triggering host cell activation and cytokine release by Borrelia burgdorferi. Infect and Immun. 2001;69:413–9. doi: 10.1128/IAI.69.1.413-419.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weis JJ, McCracken BA, Ma Y, et al. Identification of quantitative trait loci governing arthritis severity and humoral responses in the murine model of Lyme disease. J Immunol. 1999;162:948–56. [PubMed] [Google Scholar]
- 27.Wooten RM, Ma Y, Yoder RA, et al. Toll-like receptor 2 is required for innate, but not acquired, host defense to Borrelia burgdorferi. J Immunol. 2002;168:348–55. doi: 10.4049/jimmunol.168.1.348. [DOI] [PubMed] [Google Scholar]
- 28.Alban PS, Johnson PW, Nelson DR. Serum-starvation-induced changes in protein synthesis and morphology of Borrelia burgdorferi. Microbiology. 2000;146(Pt 1):119–27. doi: 10.1099/00221287-146-1-119. [DOI] [PubMed] [Google Scholar]
- 29.Alitalo A, Meri T, Comstedt P, et al. Expression of complement factor H binding immunoevasion proteins in Borrelia garinii isolated from patients with neuroborreliosis. Eur J Immunol. 2005;35:3043–53. doi: 10.1002/eji.200526354. [DOI] [PubMed] [Google Scholar]
- 30.Liang FT, Nelson FK, Fikrig E. DNA microarray assessment of putative Borrelia burgdorferi lipoprotein genes. Infect Immun. 2002;70:3300–3. doi: 10.1128/IAI.70.6.3300-3303.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]