Abstract
Autoimmune retinopathies (AR) are uncommon retinal degenerations with vision loss associated with unique clinical symptoms and findings and with serum anti-retinal autoantibodies. The experimental and clinical studies corroborate that autoantibodies in high titers can penetrate into the retina affecting function of the target antigens, which leads to retinal dysfunction and degeneration. Anti-recoverin and anti-enolase α-enolase autoantibodies were more frequently recognized in AR but autoantibodies with other specificities have also been documented, indicating immunological heterogeneity. Our goal was to examine the associations of anti-retinal autoantibodies with retinopathy in order to identify molecular biomarkers for better diagnosis and prognosis of retinopathies. In these studies we examined 39 patients (10 with cancers) of average age of ∼57 years old with sudden onset of unexplained progressive vision loss and the presence of circulating serum autoantibodies against 40-kDa retinal protein. The patients presented the retinal phenotype characterized by defects in visual fields and reduced scotopic ERG responses. Anti-40-kDa autoantibodies had specificity to the amino terminus of transducin-α. None of the normal subjects' sera had anti-40-kDa autoantibodies. In conclusion, clinical phenotype of patients with anti-transducin-α autoantibodies differed from other phenotypes of AR. These patients, often women at a ratio ∼2:1, had defects in rod (scotopic) photoreceptor function and typically did not have cancers, whereas anti-recoverin phenotype is associated with cancer and severe loss of rod and cones function, and anti-enolase retinopathy typically presents with cone dysfunction and is equal in cancer and non-cancer patients. Our studies suggest that anti-transducin autoantibodies can serve as molecular biomarkers for retinal phenotypes and could be used for progression of retinal dysfunction and degeneration.
Keywords: biomarker, retinopathy, autoantibody, autoimmunity, transducin
Introduction
The study of autoimmune retinopathies (AR), including cancer-associated retinopathy (CAR) and melanoma-associated retinopathy (MAR), is a relatively new field of retinal degeneration research and our understanding of the pathogenicity of autoimmune retinopathy is not fully elucidated. The disorder for these patients is characterized by cone or rod dysfunction associated with photosensitivity, progressive loss of vision and color perception, central or ring scotomas, night blindness, and attenuation of photopic and scotopic responses in the ERG (Jacobson, 1996). These disorders are mediated by autoimmune mechanisms, are associated with serum anti-retinal autoantibodies (AAbs), and may be associated with several different systemic neoplasms, including lung, breast, prostate, and colon cancer (Adamus, 2009). AR resembles CAR but patients with AR do not present with cancer at the initial ophthalmological examination or the cancer may be in such an early stage that is difficult to find by the conventional methods (Adamus et al., 2004). However, tumors may be detected in subsequent years and the retinopathy may precede recognition of cancer by months to years (up to 5 years, in our experience) (Adamus, 2009). Although the role of autoimmunity in retinal degeneration has not been fully explained, experimental and clinical studies corroborate that AAbs in high titers can penetrate into the retina, affecting function of the target antigens and, in turn, leading to retinal dysfunction and degeneration through activation of cell apoptosis after prolonged exposure of the retina to autoantibodies (Adamus, 2003; Adamus, 2006; Adamus and Karren, 2009; Adamus et al., 1997; Maeda et al., 2001; Ohguro and Nakazawa, 2002). Anti-recoverin (23-kDa) and anti-enolase (46-kDa) AAbs were frequently associated with CAR, but AAbs with other retinal specificities were also documented (Adamus, 2006; Adamus et al., 2004; Khan et al., 2006). Moreover, CAR, MAR, and AR are heterogeneous, may produce a number of ocular symptoms, and may be associated with different anti-retinal AAbs (Weleber et al., 2005). These autoantibodies could persist over long periods of time in the circulation, and may be associated with a stable or progressive visual course (Adamus et al., 1998; Adamus et al., 2004; Kobayashi et al., 2007; Mantel et al., 2008; Oohira, 2007).
In recent years, it has become clear that different phenotypes of AR may be related to the heterogeneity in antigenic recognition and the targeting of different cells in the retina, which may also explain the variation and complexity of clinical symptoms in patients with AR. A unique correlation between the presence of some specific antibodies, such as recoverin or enolase, is associated with a distinctive ocular presentation (Keltner and Thirkill, 1999; Ohguro et al., 2004; Weleber et al., 2005). Anti-recoverin (23-kDa) AAbs have been historically associated with CAR and very aggressive, severe retinopathy (Adamus et al., 1993; Polans et al., 1991; Thirkill et al., 1992). Anti-α-enolase (46-kDa) AAbs can be found equally in patients with or without cancer (Adamus et al., 1996). This observation implies that retinal autoantigens could be used as biomarkers for different subtypes of AR to help with better diagnosis and prognosis of visual loss. Thus, in our laboratory we seek to correlate the presence of specific retinal autoantigens with clinical symptoms, findings on examination, and ERG results to determine not only their role in retinal degeneration but also their usefulness as molecular biomarkers in pathology. In this paper, we describe a group of 39 patients with autoantibodies against a unique retinal antigen of the molecular weight of 40-kDa that we identified as transducin-α. We report that the presence of these specific AAbs against transducin is associated with a distinctive retinal dysfunction.
Methods and Materials
Patient Selection
Patients were ascertained through the Ocular Immunology Laboratory Oregon Health & Science University (OHSU). The studies were approved by the OHSU Institutional Review Board, and Informed Consent for research was obtained from subjects. We identified 39 retinopathy patients with autoantibodies against a 40-kDa retinal antigen, who presented with acquired retinal dysfunction, no active inflammation, abnormal ERG. Ten patients had diagnosed cancer at the time of antibody testing. An average age at diagnosis was 56±6 ranging from 27 to 87 years old. We also obtained 18 sera of normal consented age-matched subjects of an average age 54.5.
Antibody Evaluation - Western blotting
Human retinal proteins were extracted from a donor retina with 2% octyl glucoside in phosphate buffer, pH 7.2 and 10 μg protein was used for electrophoresis using Bio-Rad Criterion gels followed by transfer to PVDF membrane (Adamus et al., 2004). As positive controls, we used a reference human serum containing anti-enolase autoantibodies at 1:100, anti-enolase MAb at 1:2000, and anti-recoverin antiserum at 1:50000 dilutions. A negative control contained secondary antibodies only. The reactivity with a suspected known protein was confirmed by an additional Western blotting using an appropriated purified protein. For the identification of 40-kDa proteins we used pre-purified transducin α and β (a gift from Dr. Rehwa Lee) and rabbit antiserum to transducin α synthetic peptide 17-30 (Ab3504; Abcam) and anti-transducin β636 (from Dr. Rehwa Lee).
Antibody evaluation – Immunohistochemistry (IHC)
Fresh human retina was obtained from a donor and fixed in 4% paraformaldehyde and 30% sucrose and was frozen in OCT as previously described (Polans et al., 1995). Twelve microns cryosections were prepared for incubation with human serum (Adamus et al., 2004). As positive control we used a reference human serum containing anti-recoverin autoantibodies diluted 1:50. A negative control contained secondary antibodies only.
Titration of specific autoantibodies by ELISA
Microtiter plates were coated with 0.1 μg of the human rod transducin-α peptide in 0.1 M Tris-HCl buffer, pH 9.0 overnight at room temperature. The peptide of amino acid residues 17-30 KLKEDAEKDARTVK was synthesized by Celtek BioSciences (Nashville, TN). After coating, the plates were washed and then blocked with 1% bovine serum albumin and 1% normal goat serum in PBS for 1 hr. Then, after washing, the wells were incubated with serial dilutions of human sera for 1 hr followed by the goat anti-human IgG conjugated to HRP (Invitrogen). The color reaction was developed with the HRP substrate: ABTS in 0.1M citrate-phosphate buffer, pH 4.5 and 0.3% H2O2 for 30 min, and the absorbance was immediately measure at 0D415 using an ELISA Reader.
Results
Clinical Characterization
We identified 39 patients who showed presence of serum anti-40-kDa AAbs associated with a unique clinical presentation. The average age of these patients was 56±6 years old and they were represented by a majority of women at a ratio close to 2:1 (25 women to 14 males). Those patients had the following clinical symptoms and findings: sudden, progressive bilateral visual loss that was painless except for 1 patient (Case #9), photopsias, blurred vision, central scotomas, progressive worsening of visual fields, and abnormalities on ERG testing. The analysis of their ocular symptoms and findings (Table 1) shows that vision loss was sudden and progressive and was bilateral within weeks or months in all patients. Some patients reported blurred or dimmed vision; others reported flashing lights before the eyes or persistent images, night blindness, photopsias and photophobia. Overall, acuities ranged between 20/20 and 20/40 in the majority, although 4 patients had significantly reduced acuity to from 20/200 to count fingers. As an example, Fig. 1 illustrates the fundus examination and visual fields of 2 typical patients in this group: Patient #5: 73-year old female: 20/40 OU; Patient #14: 82-year old female: 20/40 OD and 20/25 OS. The visual field defects were reported for 23 patients and showed central scotoma, generalized or peripheral constriction, general depression, arcurate and altitudinal defects with patchy severe loss of retinal sensitivity (Fig. 1). Optic disc and retina were normal in the majority of patients. Specifically, pigmentary changes, e.g., bone spicules were mostly absent. Eight patients presented with loss of color vision.
Table 1.
Symptoms and findings of patients in the study
| Case | Sex | Age | Eye Affected |
Primary Complains | Color Vision | Best Recorded VA |
Visual Fields | ffERG | mfERG |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 63 | OU | Visual problems with light flashes | concentric attenuation | scotopic: lower b-amplitudes, a-wave normal; photopic: slightly lower b-amplitudes with longer implicit times at lower intensities; 30Hz response amplitude reduced | |||
| 2 | F | 70 | OU | Progressive loss of vision OU | 20/60 OU, | ring scotoma OS, unable to perform OD | extinguished ERG OU | ||
| 3 | F | 45 | OU | Decrease vision with small difficulties with dark adaptation | 20/40 OD, 20/40 OS | defect in central visual fields OU, small difficulties with dark adaptation | |||
| 4 | F | 48 | OU | Decreased vision OU | extinguished b-wave | ||||
| 5 | F | 73 | OU | Unexplained vision loss OU | Decreased | 20/400 OD, 20/30 OS | regions of central and peripheral loss of sensitivity, central scotomas OU on kinetic visual field | Mildly subnormal response of dark-adapted cone and 30 Hz flicker, moderately subnormal scotopic bright flash cone responses, cone implicit times prolonged | OD-diffuse loss of amplitude density throughout with mildly prolonged implicit times; OS-similar loss of amplitude with more patchy, mostly peripheral time delays |
| 6 | F | 89 | OU | Dramatic decrease in vision OS; blurry vision | Normal | 20/50 OD, 20/40 OS | constriction of visual fields OU | mild scotopic retinal dysfunction | |
| 7 | F | 50 | OU | Progressive loss of vision, CARlike symptoms | abnormal | ||||
| 8 | F | 71 | OU | Bilateral unexplained vision loss and vitreous debris OD; mild vitritis, VEP non-recordable; | 20/50; always poor vision OS | mild generalized constriction OU with some crossing of the isopters OD | scotopic ERG 30% reduced including the bright white flash, photopic normal | ||
| 9 | F | 33 | OU | Extreme photophobia, 3 months later symptoms worsen, persistent visual images, eye pain OU | Normal | 20/15 OU | Normal | normal | abnormal |
| 10 | F | 27 | OU | Loss of vision, night blindness with photopsias OU | Global peripheral retinal dysfunction | ||||
| 11 | F | 64 | OU | Progressive of color vision loss; blind spot OS than OD | Progressive loss | Far OD 20/25, OS 20/50 near 20/200 OU | severe bicentral scotoma | responses in central are diminished OU | |
| 12 | F | 59 | OU | Progressive loss of vision | Color blind | ||||
| 13 | F | 52 | OU | Visual loss; low tension glaucoma; dry eyes | 11/12 OD, 10/12 OS | 20/25+ OU | fields full to finger counting OU; nasal loss progressive to one central spot | ||
| 14 | F | 83 | OU | Sudden onset of photopsias, photophobia, decrease in distance vision | Normal | 20/25 OD, 20/40 OS | mild to moderate constriction for all isopters tested, patchy static fields, severe loss of retinal sensitivity throughout central 30 degrees | mild to moderate subnormal responses of rods, dark adapted cones, scotopic bright flash responses, and 30 Hz flicker responses w/prolonged rod and cone implicit times, mildly subnormal photopic cone response OD and low normal OS | |
| 15 | F | 68 | OU | loss of vision, diffuse RPE loss | 50% reduced diffusely | ||||
| 16 | F | 71 | OU | 1-2 years of unexplained photopsias | Normal | abnormal cone and rod | |||
| 17 | F | 52 | OU | Unexplained visual acuity loss OU | rod responses decreased | ||||
| 18 | F | 59 | OU | Progressive visual field loss and photopsias | Normal | 20/20 and J1+ OU | confrontational fields were intact OD but were impaired temporally OS to finger counting | abnormal | |
| 19 | F | 40 | OU | Progressive visual symptoms, photophobia, flashing lights | defects OD – inferioronasal defect | reduced scotopic and photopic a decrease in the 30Hz flicker implicit time | reduced scotopic and photopic | ||
| 20 | F | 37 | OD→OS | Unilateral pigmentary retinopathy OD; OS with sparkling photopsias | non-recordable OD | ||||
| 21 | F | 43 | OS | Unexplained vision loss OS, floaters, distortion and areas of missing, scrambled vision, decreased night vision and photophobia | Normal | 20/20 OD, 20/40 OS | Normal | mild to moderate scotopic dysfunction: borderline to mildly subnormal responses of rods, dark-adapted cones, scotopic 30 Hz flicker, moderately subnormal scotopic bright flash, normal implicit times, light-adapted cone responses normal | normal |
| 22 | F | 48 | OU | Acquired night blindness and peripheral visual loss | |||||
| 23 | F | 42 | OU | Rapid visual loss over 18 months | reduction in VF | ||||
| 24 | F | 73 | OD | Decreased vision | 20/60 OD, CF 2′ OS | reduced OU | |||
| 25 | F | 48 | OS>OD | Severe visual loss OS>OD, photophobia for about 6 months | |||||
| 26 | M | 69 | OU | Unexplained loss of vision | 20/25; 20/25 | abnormal scotopic responses OU | |||
| 27 | M | 54 | OD→OS | Visual sensations OD and OS, poor dark vision, nyctalopia | 20/100 and 20/30 OS | Defects | b-wave reduction in amplitude under scotopic condition to a bright intensity (MAR-like response) | ||
| 28 | M | 75 | OU | Bilateral several visual loss of sudden onset; normal VER with low amplitudes OU | CF OU | central scotomas; severe four quadrate loss OD | subnormal photopic OU -normal in amplitude but increased in implicit time | ||
| 29 | M | 80 | OU | Loss of vision and visual hallucinations | 20/25 OD, 20/25 OS | superior and inferior arcuate scotoma OD | |||
| 30 | M | 67 | OU | Photosensitivity | 10/14 OD, 11/14 OS | Normal | abnormal scotopic responses | ||
| 31 | M | 54 | OD→OS | Rapid progressive loss of vision OU, spontaneous photopsia OD | 1/20 OD, 6/10 OS | photopic response reduced | almost completely flat OU | ||
| 32 | M | 55 | OS→OD | Graduated decrease in vision in OS then OD | Poor | 20/200 OD, 5/200 OS | central scotomas | photopic response reduced | |
| 33 | M | 47 | OD | Visual field defects | central defect | OD normal, OS reduced | |||
| 34 | M | 42 | OU | Light sensitivity, and poor night vision | 20/20 OU | paracentral scotoma incorporating the blind spot, no far peripheral loss. | OD - 25% below normal minimum, cone 60% below, and moderate delay, weak OPs | weak across entire posterior pole, and striking delays | |
| 35 | M | 49 | OD | Visual problems | 20/30 OD 20/50 OS | slightly decreased scotopic amplitude on ERG | |||
| 36 | M | 43 | OD>OS | Poor night vision, reduced peripheral vision and glare | OD 20/70, OS 20/30 (Lasik OS) | central scotoma OD, OS better | unrecordable OD, small residual ERG OS | ||
| 37 | M | 46 | OS>OD | Progressive loss of vision, blurry vision more OS than OD, hypersensitivity to light, better dark vision | Severe deficit OU | OD 20/100, OS 20/40 | indicate retinal damage | ||
| 38 | M | 59 | OD>OS | Loss of vision | No color OD, half a control OS | CF OD, 20/300 OS | worsening in OD, slightly worse OS | ||
| 39 | M | 59 | OD→OS | Sudden onset of cloudiness in front of OD and 4 month later OS | 20/125 OD, 20/30 OS | central scotoma OD and an early central scotoma OS | symmetrically depressed values OU | ||
OD – right eye, OS – left eye, OU – both eyes, CF – count fingers, ERG – electroretinogram, ffERG – full field ERG, mf ERG - multifocal ERG, VEP – visual evoked response
Figure 1.
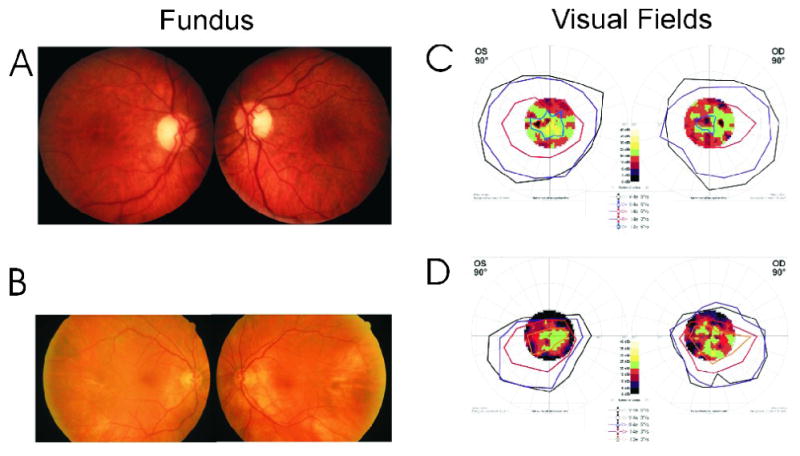
Fundus photographs and visual field tests of 2 female patients with anti-transducin autoantibodies–Patient #5, age 73-years (A and C) and Patient #14, age 82-years (B and D): A: Mild temporal pallor of the disc. B: Normal fundus appearance. Bottom: Octopus 101 (Interzeag, Berne-Koniz, Switzerland) kinetic perimetry with overlying static fields showing mild (C) and moderate (D) constriction for all isopters tested with patchy, more severe loss of central retinal sensitivity on static testing.
The patients with anti-40-kDa antibodies showed retinal functional changes as determined by full field ERG (ffERG) and showed a reduction in ERG a-wave and b-wave amplitudes, suggesting defects in rod photoreceptor function and retinal degeneration. Fig. 2 shows the mildly to moderately subnormal responses of rods, dark adapted cones, scotopic bright flash responses, and 30 Hz flicker responses with prolonged rod and cone implicit times for 2 patients (Patients # 5 and 14, Table 1). The multifocal ERG (mfERG) for patient #5 showed loss of amplitudes with patchy, peripheral and central prolongations of implicit times. In general, the abnormal scotopic responses were reduced by 30% to unrecordable (18 patients). In summary, abnormalities included reduced scotopic responses and abnormal photopic cone- and 30-Hz flicker responses, with prolonged rod and cone implicit times or extinguished ERG indicating global retinal damage.
Figure 2.
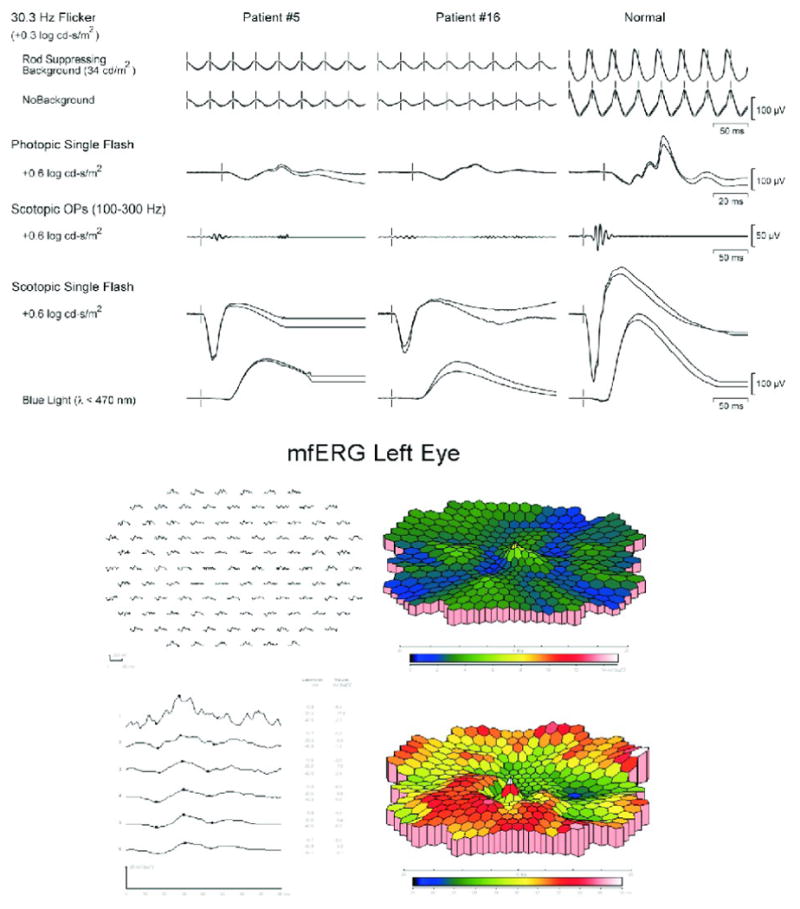
Representative Electroretinograms: A: Full field ERG for the 2 patients in Figure 1 showing mildly to moderately subnormal responses of rods, dark-adapted cones, and scotopic bright flash responses. Rod and cone implicit times were mildly prolonged. B: Multifocal ERG for the right eye of patient #5 showing loss of amplitude with patchy prolonged implicit times, both peripherally and centrally.
The clinical history revealed that 10 (25 %) patients (Table 2) had been previously diagnosed with cancer, including skin melanomas (5 patients) and carcinomas of lung (2 patients), breast (1 patient) uterine (1 patient), and prostate (1 patient) - all with the diagnosis of cancer months to up seven years before visual presentation (Table 2). One patient (Patient #14) developed colon cancer more that a year after initial visual presentation and presence of autoantibodies. History of systemic illnesses was unrevealing of any trend. Seven patients (6 women and 1 man) had history of systemic autoimmune diseases, including lupus, multiple sclerosis, rheumatoid arthritis, polymyalgia rheumatic, rheumatic fever, and vitiligo. Five of 14 men (36%) and one woman (1/27, <0.1%) had smoked for more than 25 year. Five patients had history of migraine headaches.
Table 2.
The relationship between the development of visual symptoms, the presence of anti-transducin-α autoantibodies and diagnosis of cancer in our cohort of patients
| Case # | Sex/Age | Time of Antibody Testing | Onset of Visual Symptoms | Onset of Cancer | Time of Cancer Diagnosis to Retinopathy Onset | Cancer Type |
|---|---|---|---|---|---|---|
| 1 | F/63 | 5/2006 | 2006 | 1999 | 7 years | breast metastatic small cell carcinoma of |
| 2 | F/70 | 3/2007 | 1/2007 | 2007 | 1 month | the lung |
| 3 | F/45 | 5/2004 | 2003 | 2002 | 1 year | skin |
| 4 | F/48 | 3/2007 | 2007 | NA | melanoma metastatic | |
| 5 | F/73 | 3/2007 | 2005 | 2005 | months | skin |
| 6 | F/89 | 4/2006 | 2006 | 2005 | 1 year | uterine |
| 14 | F/86 | 3/2006 | 2005 | 2006 | - 1 year | colon |
| 26 | M/69 | 10/2000 | 2000 | NA | colon, skin | |
| 27 | M/54 | 6/2000 | 1/2000 | 1994 | 6 years | skin non-small carcinoma of |
| 28 | M/75 | 9/2006 | 2006 | 2002 | 4 years | the lung |
| 29 | M/80 | 2/2007 | 2007 | 2004 | 3 years | prostate |
NA – not available; Case numbers as in Table 1
Antibody Analysis
The presence of serum autoantibodies was determined by Western blotting and IHC. We identified 39 sera that strongly reacted with a protein band of human 40-kDa protein out of over 1000 proteins present on the blot containing human retinal protein extracts. Figure 3 shows representative results from Western blotting and IHC. The reactive sera with anti-40-kDa AAbs were incubated a candidate protein of the same molecular weight - purified retinal transducin-α. All 39 sera reacted with transducin-α on the blot confirming their specificity (Fig. 3). None of the 19 control sera from normal subjects showed antibody binding to 40-kDa protein on the blot containing retinal proteins although 4 subjects had antibodies against other retinal proteins (not shown). Twenty four sera showed a single reactivity with transducin-α and 15 sera reacted with one of the additional protein bands such as α-enolase (46-kDa), carbonic anhydrase II (30-kDa), transducin-β (35-kDa), or other unidentified proteins (44-kDa and 33-kDa). The IHC results showed that autoantibodies from patients labeled mostly outer and inner segments of photoreceptors and the cytoplasm of ganglion cells in a fashion similar to antibodies generated against the amino-terminal transducin-α peptide containing the amino acid sequence 17-30 specific for rod photoreceptor transducin, which we used as control in our experiments (Fig. 3C). Thus, next we determined whether patients' sera reacted with this immunogenic transducin peptide using ELISA. Fig. 4A shows that all patients sera recognized the peptide and that anti-transducin autoantibodies persist in significantly higher levels than the normal controls (p=0.001). As additional controls we included 2 groups of patients with visual problems – patients with intraocular inflammation (uveitis) and patients with age related macula degeneration. As is seen in Fig. 4A, sera of those patients did not have a significant level of anti-peptide AAbs. To further determine the specificity of anti-transducin antibodies the inhibition ELISA was performed for 5 patients sera that were available in quantities needed for the assay. Figure 4B shows that the peptide 17-30 inhibited antibody binding to the peptide on the plate in a dose depended manner, confirming the specificity of autoantibody binding to the amino terminus of rod transducin-α.
Figure 3.
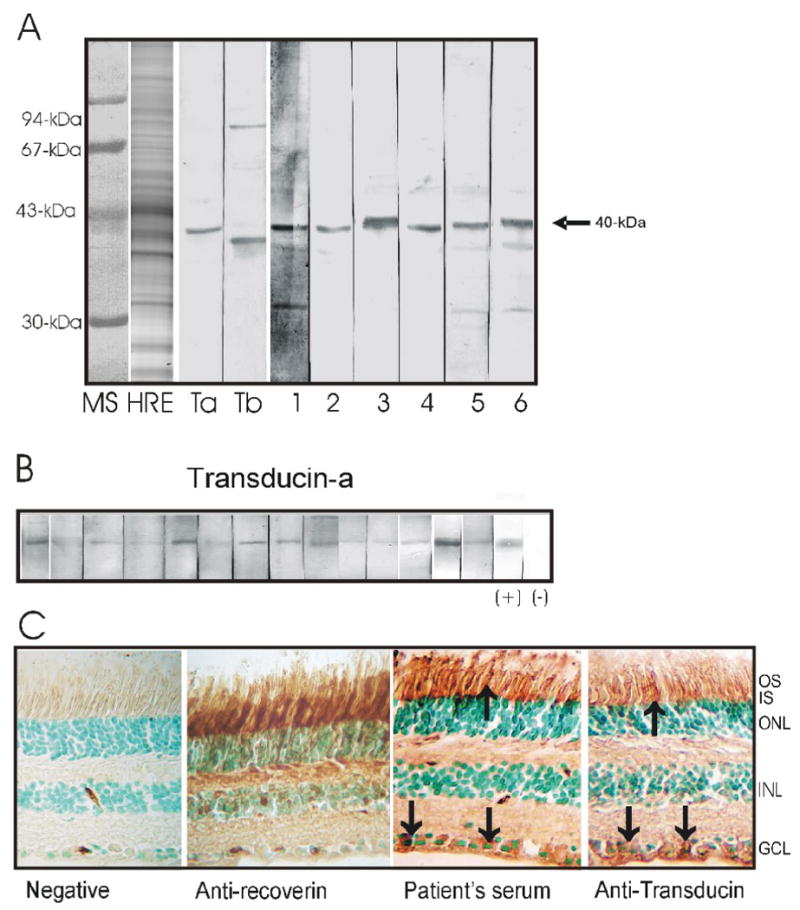
Immunostaining of human retinal proteins by Western blotting and immunocytochemistry: A: The sera from 6 representative patients (on the right) reacted with a 40-kDa retinal protein on the blot. MS – protein molecular standards, HRE- human retinal proteins stained with Coomassie brilliant blue; Controls for anti-transducin antibodies are shown on the left –Ta - anti-transducin-α and Tb - anti-transducin-β antibodies. B: Immunostaining of transducin-α by sera from selected patients. C: Representative staining of human retina with patient's anti-transducin-α serum, note labeling of the outer (OS) and inner segments (IS) of photoreceptors in a similar pattern as specific anti-transducin antiserum. The cytoplasm of ganglion cells was also labeled (arrows). Anti-recoverin antibodies were used as a positive control. ONL –outer nuclear layer, INL –inner nuclear layer, GCL – ganglion cell layer.
Figure 4.

A. Levels of anti-transducin-α peptide autoantibodies in AR patients (n=39) and control subjects without visual problems (n=18), patients with uveitis (n=12) and age related macular degeneration (ARMD, n=10) measured by ELISA were significantly different among the groups (Man Whitney test, p<0.001) with the control group having the smallest median deviation (A). One ELISA unit is defined as A405 = 0.1. B: Inhibition of binding to transducin-α peptide by anti-transducin-α antibodies from 5 representative patients, as measured by the inhibition ELISA test. Numbers on the left site of the graph indicate the patient's number as in Table 1. For both tests 0.1 μg of peptide, containing amino acid rod transducin-α sequence 17-30, was coated on the ELISA plate.
Discussion
Autoimmune retinopathies, including CAR and MAR, are immunologically heterogeneous. An important conclusion from our studies is that the targeting of multiple autoantigens in the retina by autoantibodies may lead to different ocular manifestations in AR. Photosensitivity, ring scotoma, attenuated retinal arteriole, decreased ERG response, in addition to the presence of serum autoantibody to retinal antigens are considered to be crucial for diagnosis of CAR (Heckenlively and Ferreyra, 2008; Jacobson et al., 1990; Mantel et al., 2008). The current studies revealed a group of patients with anti-transducin-α autoantibodies who have bilateral defects in rod (scotopic) photoreceptor function and typically do not have cancers. In contrast, anti-recoverin autoantibodies are associated with cancer and show severe almost equal ERG abnormalities of the rod and cone system, indicating severe widespread dysfunction (Ohguro et al., 2004; Weleber et al., 2005). Anti-enolase retinopathy typically presents with cone dysfunction and is equal in cancer and non-cancer patients (Weleber et al., 2005). Therefore, the diversity in serum autoantibodies implies that ocular presentation reflects targeted retina-specific autoantigens. Thus, we propose that some retinal autoantigens can be used as molecular biomarkers to distinguish different subtypes of AR and progression of the retinal dysfunction and degeneration. Table 3 summarizes clinical phenotype for anti-transducin retinopathy in four major criteria: visual manifestations, clinical findings, ERGs, and presence of cancer and compare it to the previously published anti-recoverin and anti-enolase phenotypes (Ohguro et al., 2004; Weleber et al., 2005).
Table 3.
Characteristics of Anti-transducin Retinal Phenotype in Comparison to Anti-Recoverin and Anti-Enolase Phenotypes
| Features | Anti-recoverin | Anti-enolase | Anti-transducin-α |
|---|---|---|---|
| Average Age * | 69 | 64 | 57 |
| Onset | Acute, sudden | Subacute, chronic | Sudden progressive |
| Ocular symmetry | Usually symmetric | Often symmetric | Symmetric |
| Presentation | Severe central and peripheral vision loss | Mostly central vision loss | Mild, patchy to global acuity and field loss |
| Course | Rapid rod and cone loss | Mild to severe progression | Mild progression |
| Full Field ERG | Severe equal rod and cone loss | Normal to severe cone loss | Reduced rod more than cone dysfunction |
| Multifocal ERG | Severely abnormal | Mildly to severely abnormal | Decreased amplitudes, Delayed timing |
| Association with cancer | Almost 100% | About 40% | About 25 % |
| Relationship of cancer diagnosis of retinopathy onset | Retinopathy precedes diagnosis of cancer by months | Retinopathy develops after diagnosis of cancer by months to years | Retinopathy develops after diagnosis of cancer by months to years** |
Average age of patients tested in our laboratory
In one patient, the presence of autoantibodies preceded diagnose with cancer
To understand how autoantibodies can affect vision, one has to appreciate the function of the target autoantigen (Adamus, 2003). Mammalian rods and cones are known to contain different transducins (Ma et al., 2001). Transducin-α is a member of guanine nucleotide–binding (GTP) proteins, also known as G-proteins or transducins, that mediate signal transduction triggered by light, hormones, neurotransmitters, and sensory stimuli and are found in all eukaryotes (Navon and Fung, 1988). Transducins are composed of three protein subunits – α, β, and γ. The transducin α-subunit of retinal rod cells (Gt1α) and cone cells (Gt2α), which were present on our blots show strong conservation of the amino acid sequences in each group of the Gα subfamily with greater than 98% homologies in the overall structure (Itoh et al., 1988). The autoantibodies found in our group of patients were specific against the amino-terminus of rod transducin-α. Although we have not determined all reactive transducin epitopes it is clear that the sequence 17-30 belongs to one of the major epitopes for rod transducin-α recognized by patients in this cohort of patients. The sequences of the amino terminal region of Gα corresponding to amino acid 1-33, including our immunoreactive peptide 17-30, has considerable differences among the G proteins (Meurs et al., 1987). The BLAST analysis of the peptide 17-30 showed specificity to rod transducin-α. Hence, it is unlikely that autoantibodies against 40-kDa were originally generated to the Gα of a different tissue than the retina, and now these autoantibodies cross-react with ocular transducin-α. Moreover, phototransduction proteins, including transducins can be aberrantly expressed in cancer cells, including melanoma or lung, similarly to the expression of recoverin in tumors (Bazhin et al., 2004; Bazhin et al., 2007; Polans et al., 1991; Tan et al., 2004). When released and taken up by dendritic cells, these antigens could induce anti-transducin autoimmunization in humans that in turn, ultimately lead to development of retinal degeneration. Furthermore, our examination of MAR patients tested for anti-retinal antibodies showed 5 out 13 seropositive patients had anti-transducin-α antibodies (Adamus, unpublished data). Earlier published studies demonstrated transducin-β as a novel autoantigen in a MAR patient (Potter et al., 2002), an antigen that is also aberrantly expressed in melanocytes (Bazhin et al., 2007).
Transducin-α has not been reported as a target autoantigen associated with autoimmune visual loss. Although two published reports showed anti-40-kDa autoantibodies, those antigens seem to be different from the retinal antigens in the current studies. Peek et al reported on anti-40-kDa autoantibodies in 2 patients with cone and rod dystrophy with colon adenocarcinoma and prostate carcinoma surgically removed preceding the ocular symptoms (Peek et al., 2002). In contrast to our soluble 40-kDa antigen, their 40-kDa antigen was insoluble and localized in the membranes of photoreceptor outer segments. In a different study, anti-40-kDa antibodies were found in a patient with cone dystrophy and laryngeal carcinoma, which was also surgically removed 18 months prior to presenting with photophobia and decreased vision in both eyes (Parc et al., 2006). This patient had bilateral central scotomas as demonstrated by Goldmann perimetry but peripheral vision was normal. The cone dysfunction was confirmed by mfERG. Because the reactive autoantigen was a photoreceptors soluble protein, it resembles transducin-α in our studies. We hypothesize that, in the cases, where a tumor was removed prior to the occurrence of visual problems and the presence of autoantibodies, transducin might have been released from cancer cells during surgery, thus, allowing exposure to the immune system and generation of an anti-tumor response in the pre-retinopathy stage.
In photoreceptor cells, transducin-α is activated by the exchange of GDP for GTP, accompanied by dissociation of the GTP-bound α-subunit from the βγ-transducin complex, and the resulting phototransduction cascade produces a hyperpolarization of the photoreceptor (Ridge and Palczewski, 2007). Inactivation of transducin-α by autoantibodies might reduce signaling in photoreceptors and lead to changes in intracellular calcium and apoptosis. Recent findings from animal studies on anti-protein G autoantibodies from sera of diabetic BB/W rats with neuropathy support this hypothesis (Ristic et al., 1998). The exposure of neurons from the nondiabetic rat to autoimmune serum from the diabetic rat caused an impaired regulation of the inhibitory G protein-calcium channel complex in those neurons. Such impaired regulation resulted in enhanced calcium influx, suggesting that these autoantibodies influenced G-protein function.
To date, transducin subunits α (Gsα and Gtα) have been associated with ocular diseases (Lania et al., 2006). Loss-of-function mutations of the Gtα gene (GNAT1) cause autosomal-dominant stationary night blindness called Nougaret syndrome (Dryja et al., 1996). These patients are unable to see in the dark and have no a-wave in the ERG, consistent with a defect in rod-photoreceptor transducin. A subset of genetic mutations in photoreceptor-specific genes results in abnormally prolonged activation of transducin-mediated photosignaling in rod cells. In human disease and animal models, these mutations cause visual dysfunctions ranging from a mild stationary night blindness to severe, early-onset retinal degeneration (Lem and Fain, 2004).
In summary, testing for autoantibodies provides information important for the molecular diagnosis of autoimmune retinopathies because specific anti-retinal autoantibodies can be associated with unique clinical presentations, symptoms, phenotypic findings, and ERG patterns. Anti-transducin phenotype differs from anti-recoverin and anti-enolase phenotypes and characterizes by bilateral visual loss of sudden onset, defects in visual fields, and reduced ERG scotopic responses and typically do not have cancers (Table 3). Anti-recoverin phenotype is associated with cancer and almost equal severe loss of rod and cones system, indicating widespread dysfunction. Anti-enolase retinopathy typically presents with cone dysfunction and is equal in cancer and non-cancer patients. Although five out 11 patients with cancer had been diagnosed with skin melanoma, anti-transducin antibodies may not be a good predictor of association with other forms of neoplasm. Our study suggests that anti-transducin autoantibodies can serve as molecular biomarkers for retinal phenotypes and may be used for progression of retinal dysfunction and degeneration.
Acknowledgments
Rule of the Funding Source: The studies were supported in part by the NIH grant EY13053 (GA), and the Foundation Fighting Blindness (RGW) and Unrestricted Departmental Grant from the Research to Prevent Blindness.
Footnotes
Disclosure Statements
Grazyna Adamus – no conflict of interest
Lori Brown – no conflict of interest
Richard G Weleber – no conflict of interest
The role of authors: Grazyna Adamus – study design, data analysis and interpretation, manuscript preparation and submission for publication; Lori Brown – data collection and analysis, manuscript preparation; Richard G Weleber - data analysis and interpretation, manuscript preparation
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Grazyna Adamus, Casey Eye Institute, Department of Ophthalmology, School of Medicine Mail code L467, Oregon Health & Science University, 3181 SW Sam Jackson Park Rd, Portland, OR 97239, USA.
Lori Brown, Casey Eye Institute, Department of Ophthalmology, School of Medicine Mail code L467, Oregon Health & Science University, 3181 SW Sam Jackson Park Rd, Portland, OR 97239, USA, Mosgrove@ohsu.edu.
Richard G. Weleber, Casey Eye Institute, Department of Ophthalmology, School of Medicine, Mail code CEI, Oregon Health & Science University, 3181 SW Sam Jackson Park Rd, Portland, OR 97239, USA, weleberr@ohsu.edu
References
- Adamus G. Autoantibody-induced apoptosis as a possible mechanism of autoimmune retinopathy. Autoimmunity Rev. 2003;2:63–69. doi: 10.1016/s1568-9972(02)00127-1. [DOI] [PubMed] [Google Scholar]
- Adamus G. The role of recoverin in autoimmunity. In: Philippov PP, Koch KW, editors. Neuronal Calcium Sensor Protein. Nova Science Publisher, Inc.; New York: 2006. pp. 181–200. [Google Scholar]
- Adamus G. Autoantibody Targets and their Cancer Relationship in the Pathogenicity of Paraneoplastic Retinopathy. Autoimmun Rev. 2009;8(5):410–414. doi: 10.1016/j.autrev.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamus G, Amundson D, MacKay C, Gouras P. Long-term persistence of anti-recoverin autoantibodies in endometrial cancer-associated retinopathy. Arch Ophthalmol. 1998;116:251–253. [PubMed] [Google Scholar]
- Adamus G, Aptsiauri N, Guy J, Heckenlively J, Flannery J, Hargrave PA. The occurrence of serum autoantibodies against enolase in cancer-associated retinopathy. Cli Immunol Immunopath. 1996;78:120–129. doi: 10.1006/clin.1996.0021. [DOI] [PubMed] [Google Scholar]
- Adamus G, Guy J, Schmied JL, Arendt A, Hargrave PA. Role of anti-recoverin autoantibodies in cancer-associated retinopathy. Invest Ophthalmol Vis Sci. 1993;34:2626–2633. [PubMed] [Google Scholar]
- Adamus G, Karren L. Autoimmunity against carbonic anhydrase II affects retinal cell functions in autoimmune retinopathy. J Autoimmun. 2009;32(2):133–139. doi: 10.1016/j.jaut.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamus G, Machnicki M, Seigel GM. Apoptotic retinal cell death induced by autoantibodies of cancer associated retinopathy. Inves Ophthal Vis Sci. 1997;38:283–291. [PubMed] [Google Scholar]
- Adamus G, Ren G, Weleber RG. Autoantibodies against retinal proteins in paraneoplastic and autoimmune retinopathy. BMC Ophthalmol. 2004;4(1):5. doi: 10.1186/1471-2415-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazhin AV, Savchenko MS, Shifrina ON, Demoura SA, Chikina SY, Jaques G, Kogan EA, Chuchalin AG, Philippov PP. Recoverin as a paraneoplastic antigen in lung cancer: the occurrence of anti-recoverin autoantibodies in sera and recoverin in tumors. Lung Cancer. 2004;44(2):193–198. doi: 10.1016/j.lungcan.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Bazhin AV, Schadendorf D, Willner N, Smet CD, Heinzelmann A, Tikhomirova NK, Umansky V, Philippov PP, Eichmüller SB. Photoreceptor proteins as cancer-retina antigens. Int J Cancer. 2007;120(6):1268–1276. doi: 10.1002/ijc.22458. [DOI] [PubMed] [Google Scholar]
- Dryja TP, Hahn LB, Reboul T, Arnaud B. Missense mutation in the gene encoding the [alpha] subunit of rod transducin in the Nougaret form of congenital stationary night blindness. Nat Genet. 1996;13(3):358–360. doi: 10.1038/ng0796-358. [DOI] [PubMed] [Google Scholar]
- Heckenlively J, Ferreyra H. Autoimmune retinopathy: A review and summary. Sem Immunopathol. 2008;30(2):127–134. doi: 10.1007/s00281-008-0114-7. [DOI] [PubMed] [Google Scholar]
- Itoh H, Toyama R, Kozasa T, Tsukamoto T, Matsuoka M, Kaziro Y. Presence of three distinct molecular species of Gi protein alpha subunit. Structure of rat cDNAs and human genomic DNAs. J Biol Chem. 1988;263(14):6656–6664. [PubMed] [Google Scholar]
- Jacobson DM. Paraneoplastic disorders of neuro-ophthalmologic interest. Curr Opin Ophthalmol. 1996;7:30–38. doi: 10.1097/00055735-199612000-00005. [DOI] [PubMed] [Google Scholar]
- Jacobson DM, Thirkill CE, Tipping SJ. A clinical triad to dignose paraneoplastic retinopathy. Ann Neurol. 1990;28:162–167. doi: 10.1002/ana.410280208. [DOI] [PubMed] [Google Scholar]
- Keltner JL, Thirkill CE. The 22-kDa antigen in optic nerve and retinal diseases. J Neuroophthalmol. 1999;19:71–83. [PubMed] [Google Scholar]
- Khan N, Huang JJ, Foster CS. Cancer associated retinopathy (CAR): An autoimmune-mediated paraneoplastic syndrome. Semin Ophthalmol. 2006;21(3):135–141. doi: 10.1080/08820530500350662. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Ikezoe T, Uemura Y, Ueno H, Taguchi H. Long-term survival of a patient with small cell lung cancer associated with cancer-associated retinopathy. Lung Cancer. 2007;57(3):399–403. doi: 10.1016/j.lungcan.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Lania AG, Mantovani G, Spada A. Mechanisms of Disease: mutations of G proteins and G-protein-coupled receptors in endocrine diseases. Nature Clinical Practice Endocrinol Metabol. 2006;2(12):681–693. doi: 10.1038/ncpendmet0324. [DOI] [PubMed] [Google Scholar]
- Lem J, Fain GL. Constitutive opsin signaling: night blindness or retinal degeneration? Trends Mol Med. 2004;10(4):150–157. doi: 10.1016/j.molmed.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Ma Jx, Znoiko S, Othersen KL, Ryan JC, Das J, Isayama T, Kono M, Oprian DD, Corson DW, Cornwall MC, Cameron DA, Harosi FI, Makino CL, Crouch RK. A Visual Pigment Expressed in Both Rod and Cone Photoreceptors. Neuron. 2001;32(3):451–461. doi: 10.1016/s0896-6273(01)00482-2. [DOI] [PubMed] [Google Scholar]
- Maeda T, Maeda A, Maruyama I, Ogawa KI, Kuroki Y, Sahara H, Sato N, Ohguro H. Mechanisms of photoreceptor cell death in cancer-associated retinopathy. Invest Ophthalmol Vis Sci. 2001;42(3):705–712. [PubMed] [Google Scholar]
- Mantel I, Ramchand KV, Holder GE, Ohbayashi M, Morohoshi K, Patel N, Toda M, Fitzke FW, Bird AC, Ono SJ. Macular and retinal dysfunction of unknown origin in adults with normal fundi: Evidence for an autoimmune pathophysiology. Exp Mol Pathol. 2008;84(2):90–101. doi: 10.1016/j.yexmp.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Meurs KPV, Angus CW, Lavu S, Kung HF, Czarnecki SK, Moss J, Vaughan M. Deduced Amino Acid Sequence of Bovine Retinal G{oalpha}: Similarities to Other Guanine Nucleotide-Binding Proteins. Proc Natl Acad Sci USA. 1987;84(10):3107–3111. doi: 10.1073/pnas.84.10.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navon S, Fung B. Characterization of transducin from bovine retinal rod outer segments. Use of monoclonal antibodies to probe the structure and function of the subunit. J Biol Chem. 1988;263(1):489–496. [PubMed] [Google Scholar]
- Ohguro H, Nakazawa M. Pathological roles of recoverin in cancer-associated retinopathy. Adv Exp Med Biol. 2002;514:109–124. doi: 10.1007/978-1-4615-0121-3_7. [DOI] [PubMed] [Google Scholar]
- Ohguro H, Yokoi Y, Ohguro I, Mamiya K, Ishikawa F, Yamazaki H, Metoki T, Takano Y, Ito T, Nakazawa M. Clinical and immunologic aspects of cancer-associated retinopathy. Am J Ophthalmol. 2004;137(6):1117–1119. doi: 10.1016/j.ajo.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Oohira A. Fifteen-year follow-up of patient with cancer-associated retinopathy. Jpn J Ophthalmol. 2007;51(1):74–75. doi: 10.1007/s10384-006-0396-z. [DOI] [PubMed] [Google Scholar]
- Parc CE, Azan E, Bonnel S, Sahel JA, Kaplan J, Thirkill CE. Cone dysfunction as a paraneoplastic syndrome associated with retinal antigens approximating 40 kiloDalton. Ophthalmic Genet. 2006;27(2):57–61. doi: 10.1080/13816810600678097. [DOI] [PubMed] [Google Scholar]
- Peek R, Dijkstra BG, Meek B, Kuijpers RW. Autoantibodies to photoreceptor membrane proteins and outer plexiform layer in patients with cancer-associated retinopathy. Clin Exp Immunol. 2002;128(3):498–503. doi: 10.1046/j.1365-2249.2002.01834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polans A, Witkowska D, Haley T, Amundson D, Baizer L, Adamus G. Recoverin, a photoreceptor-specific calcium-binding protein, is expressed by the tumor of a patient with cancer-associated retinopathy. Proc Natl Acad Sci USA. 1995;92:9176–9180. doi: 10.1073/pnas.92.20.9176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polans AS, Buczylko J, Crabb J, Palczewski K. A photoreceptor calcium binding protein is recognized by autoantibodies obtained from patients with cancer-associated retinopathy. J Cell Biol. 1991;112(5):981–989. doi: 10.1083/jcb.112.5.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter MJ, Adamus G, Szabo SM, Lee R, Mohaseb K, Behn D. Autoantibodies to transducin in a patient with melanoma-associated retinopathy. Am J Ophthalmol. 2002;134(1):128–130. doi: 10.1016/s0002-9394(02)01431-9. [DOI] [PubMed] [Google Scholar]
- Ridge KD, Palczewski K. Visual Rhodopsin Sees the Light: Structure and Mechanism of G Protein Signaling. J Biol Chem. 2007;282(13):9297–9301. doi: 10.1074/jbc.R600032200. [DOI] [PubMed] [Google Scholar]
- Ristic H, Srinivasan S, Hall KE, Sima AAF, Wiley JW. Serum From Diabetic BB/W Rats Enhances Calcium Currents in Primary Sensory Neurons. J Neurophysiol. 1998;80(3):1236–1244. doi: 10.1152/jn.1998.80.3.1236. [DOI] [PubMed] [Google Scholar]
- Tan E, Ding XQ, Saadi A, Agarwal N, Naash MI, Al-Ubaidi MR. Expression of cone-photoreceptor-specific antigens in a cell line derived from retinal tumors in transgenic mice. Invest Ophthalmol Vis Sci. 2004;45(3):764–768. doi: 10.1167/iovs.03-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirkill CE, Tait RC, Tyler NK, Roth AM, Keltner JL. The cancer-associated retinopathy antigen is a recoverin-like protein. Invest Ophthalmol Vis Sci. 1992;33:2768–2772. [PubMed] [Google Scholar]
- Weleber RG, Watzke RC, Shults WT, Trzupek KM, Heckenlively JR, Egan RA, Adamus G. Clinical and electrophysiologic characterization of paraneoplastic and autoimmune retinopathies associated with antienolase antibodies. Am J Ophthalmol. 2005;139(5):780–794. doi: 10.1016/j.ajo.2004.12.104. [DOI] [PubMed] [Google Scholar]


