Abstract
Post-translational modifications on various receptor proteins have significant effects on receptor activation. For the Transient Receptor Potential family V type 1 (TRPV1) receptor, phosphorylation of certain serine/threonine amino acid residues sensitizes the receptor to activation by capsaicin and heat. Although Protein Kinase C (PKC) phosphorylates TRPV1 on certain serine/threonine residues, it is not completely understood how PKC functionally associates with TRPV1. Recent studies have reported that the A-kinase Anchoring Protein 150 (AKAP150) mediates PKA phosphorylation of TRPV1 in several nociceptive models. Here, we demonstrate that AKAP150 also mediates PKC-directed phosphorylation and sensitization of TRPV1. In cultured rat trigeminal ganglia, immunocytochemical analyses demonstrate co-localization of AKAP150 and PKC isoforms α, δ, ε, and γ in TRPV1-positive neurons. Additional biochemical evidence supports immunocytochemical results, indicating that AKAP150 preferentially associates with certain PKC isoforms in rat trigeminal ganglia neurons. Employing siRNA-mediated knock-down of AKAP150 expression, we demonstrate that PKC-mediated phosphorylation of TRPV1 and sensitization to a capsaicin response is dependent upon functional expression of the AKAP150 scaffolding protein. Furthermore, PKC-induced sensitization to a thermal stimulus is abrogated in AKAP150 knock-out animals relative to wild-type. Collectively, results from these studies indicate that the AKAP150 scaffolding protein functionally modulates PKC-mediated phosphorylation and sensitization of the TRPV1 receptor in rat sensory neurons, suggesting the scaffolding protein to be an integral regulator of peripheral inflammatory hyperalgesia.
Keywords: TRPV1, AKAP, Trigeminal, Pain, PKC, Hyperalgesia
Introduction
The TRPV1 receptor is a member of a larger TRP family of ligand-gated ion channels that activate in response to multiple environmental stimuli. TRPV1 is primarily expressed on c- and Aδ-type nociceptive fibers and is activated upon exposure to capsaicin, heat (> 43°C) [8], protons (pH < 5.9) [32], and certain cannabinoids [27,29] to stimulate neuronal activation and nociception [7]. Recent characterizations have also identified TRPV1 as a voltage-gated ion channel [20,23,34]. In response to injury, TRPV1 undergoes biochemical modification(s) that reduces the normal threshold for activation, known as sensitization. Specifically, the inflammatory mediators prostaglandin E2, (PGE2, [4,22]) and bradykinin (BK, [3,10,26]) stimulate kinase signaling cascades that phosphorylate and sensitize TRPV1. Various serine/threonine amino acid residues of TRPV1 serve as phosphorylation targets for kinases that are activated following exposure to inflammatory mediators, including Ser 116 [4], Thr 144, Thr 370, and Ser 502 [28], and sites Ser 502 and Ser 800 [3]. Recently, Zhang and McNaughton have suggested that scaffolding proteins bring PKA and PKC to the plasma membrane to act on certain TRP receptors, including TRPV1 [37].
Scaffolding proteins function as regulators of signal transduction by bringing effectors and substrates into close spatial proximity. AKAP79/150 (AKAP79:human, AKAP150:rodent), first identified as a scaffolding protein for the RII subunit of PKA in post-synaptic densities [6], has since been found to scaffold various signaling molecules, including other kinases and phosphatases (for review, see [35]. Recent studies have demonstrated AKAP79/150 to be an important mediator of PKA phosphorylation of TRPV1 [15,28,30]. In light of these reports, it is hypothesized that AKAP150 also targets other signaling kinases to TRPV1, including PKC [17]. Amino acid analysis of the TRPV1 primary sequence suggests that an associative protein is required to target PKC to the receptor. Furthermore, several studies have demonstrated the role of AKAPs in modulating the PKC-dependent activation status of several receptor channels, including KCNQ 2 potassium channels [14] and epithelial Na+ channels [2]. Certainly, AKAP-mediated targeting of PKC to TRPV1 would increase efficiency of the signaling pathway, as it does for PKA.
In this current analysis, multidisciplinary approaches were employed to determine whether AKAP150 mediates PKC-dependent phosphorylation and sensitization of TRPV1. Indeed, others have recently demonstrated in vitro evidence suggesting that AKAP79 mediates the effects of PKC on human TRPV1 [37]. Current work by our research group utilizes primary trigeminal cultures and genetically modified animals to demonstrate that AKAP150 is required to support PKC-mediated sensitization of TRPV1.
Materials & Methods
Tissue Culture
All procedures utilizing animals were approved by the Institutional Animal Care and Use Committee at the University of Texas Health Science Center at San Antonio and were conducted in accordance with policies for the ethical treatment of animals established by the National Institutes of Health. Trigeminal ganglia (TG) were cultured as previously described [16] from Sprague-Dawley rats 200–250g in weight (Jackson Laboratories, Ban Harbor, ME), or from C57/Bl6 WT or AKAP150 knock-out mice 4–8 weeks of age (generated as previously described [12]). Cultures were maintained at 37°C, 5% CO2, and grown in 10 cm poly-D-lysine coated plates for 5 – 7 days for phosphorylation experiments, 48 hr for electrophysiology experiments. Chinese hamster ovary (CHO) cells were utilized for heterologous expression of cDNA constructs. They were maintained at 37°C, 5% CO2 and transfected using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) following manufacturer’s instructions.
Immunohistochemistry
Cultured rat TG cells were grown on poly-D lysine coated coverslips for 5–7 days in normal media. Coverslips were rinsed with phosphate buffered saline (PBS), and fixed with 4% paraformaldehyde in 0.1M PBS for 10 min at 25°C. Following fixation, coverslips were rinsed twice with PBS, and incubated with 5% normal goat serum, 0.5% Triton X-100 in PBS for 30 min at 25°C. Coverslips were then incubated with antisera directed specifically towards AKAP150 (1:250, Millipore, Billerica, MA), TRPV1 (1:10,000, Neuromics, Edina, MN, catalog# GP14100), and either PKC α, δ, ε, or γ isoforms (1:250, BD Transduction, San Diego, CA) overnight at 4°C. Coverslips were then rinsed three times and incubated with species-appropriate Alexa-Fluor secondary antibodies (Molecular Probes, Eugene, OR) for one hour at 25°C. Following rinsing three times with PBS, coverslips were mounted to microscope slides with Vectashield (Vector Labs, Burlingame, CA) and stored at 4°C until viewed with a Nikon C1si laser scanning confocal microscope equipped with three lasers. Three different coverslips were triple-stained with antibodies against AKAP, TRPV1 and each of the four different PKC isoforms evaluated. Representative images were obtained with a 40X oil-immersion objective lens of 10–15 neurons within the coverslips in each group to evaluate staining relationships.
Western Blot and 32P Autoradiography
The lysis of TG cells, quantification of protein concentration, immunoprecipitation, and Western blotting were conducted as previously described [16]. Antibodies used for Western blotting were AKAP150 (1:1000, Upstate, Lake Placid, NY), PKC α, δ, ε and γ isoforms (1:500, BD Biosciences), and TRPV1 (1:1000, Calbiochem, La Jolla, CA, clone Ab-1, catalog# PC420). Densitometry data analyzed by one-way ANOVA, *p<0.05, **p<0.01, NS=not significant, results are representative of 3–5 independent trials.
siRNA Design and Transfection
Specific siRNA duplexes designed to knock down AKAP150 were utilized as before [15].
PKC Activity Assay
PKC activity from TG neurons transfected with AKAP150-specific siRNA, scrambled control siRNA (Scr siRNA, Ambion, Austin, TX) or mock-transfected were collected in ice-cold PBS, washed twice with PBS, and suspended in 1X sample preparation buffer (50- mM Tris-HCl, 10 mM benzamidine, 5 mM EDTA, 10 mM EGTA, pH 7.5). Samples were sonicated five times for 10 sec each, and centrifuged at 100,000 × g for 60 min at 4°C. The resulting supernatant was collected and quantified by Bradford analysis [5]. Aliquots (50 μg) of sample supernatants were assayed for PKC activity following manufacturer’s instructions (Calbiochem). Results are representative of 4 independent trials conducted in triplicate. Data analyzed by one-way ANOVA, *p<0.05.
Electrophysiology
All recordings were made in a whole-cell perforated patch clamp configuration at 22– 24°C from small-to-medium sized (20–35 pF) cultured TG neurons (24–48 h post-transfection). A holding potential (Vh) is −60 mV. Recordings and following analysis were carried out using an Axopatch 200B amplifier and pCLAMP9.0 software (Axon Instruments, Union City, CA). Data were filtered at 0.5 kHz and samples at 2 kHz. Borosilicate pipettes (Sutter, Novato, CA) were polished to resistances of 8–10 MW in perforated patch pipette solution. If necessary, access resistance (Rs) was compensated by 40–80% to 15–20 MWCell diameters were calculated using d=√[100*Cm/π], where d (mm) is cell diameter and Cm (pF) is membrane capacitance.
All recordings are made in the presence of 2 mM Ca+2 in external solution. Standard external solution (SES) contained (in mM): 140 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 D-glucose and 10 HEPES, pH 7.4. The pipette solution consisted of (in mM): 110 K- methanesulfonate, 30 KCl, 1 MgCl2, 10 HEPES pH 7.3 and 250 mg/ml amphotericin B (Sigma, St. Louis, MO). Drugs were applied using a computer controlled pressure-driven 8-channel system (ValveLink8; AutoMate Scientific, San Francisco, CA). FITC-labeled AKAP150-1 siRNA (Qiagen) used to positively identify tranfected neurons. Data were analyzed by one-way ANOVA, ***p<0.001, NS=not significant.
Calcium Imaging
CHO cells were transfected as outlined above (Tissue Culture) with pEGFP-N1 (Clontech, Mountain View, CA, to identify transfected cells) and cDNA vectors containing inserts corresponding to rat TRPV1 (generously provided by David Julius, UCSF, San Francisco, CA), rat AKAP150 wt and AKAP150DPKC (Δ amino acids 31–52 [17], generously provided by John D. Scott, Vollum Institute, OHSU, Portland OR). To measure intracellular [Ca+2] levels, the dye Fura-2 AM (2 μM; Molecular Probes, Carlsbad, CA) was loaded for 30 min at 37°C into cells in the presence of 0.05% Pluronic (Calbiochem). Fluorescence was detected with a Nikon Eclipse TE 2000-U microscope fitted with a 20x/0.8 NA Fluor objective. Fluorescence images from 340 nm and 380 nm excitation wavelengths were collected and analyzed with the MetaFluor Software (MetaMorph, Universal Imaging Corporation (a subsidiary of Molecular Devices), Downingtown, PA). The net change in Ca+2 (ΔF340/380) was calculated by subtracting the basal F340/380 Ca+2 level (mean value collected for 60 s prior to agonist addition) from the peak F340/380 Ca+2 level achieved after exposure to the agonists. For each transfection/treatment group, 27–43 cells were imaged. Data were analyzed by one-way ANOVA analysis, *p<0.05, ***p<0.001.
Behavioral Test for Inflammatory Hyperalgesia
Bradykinin-evoked thermal paw withdrawal latencies (PWL) were measured as described [13,31]. Male mice 4–8 weeks of age were acclimated on the Hargreaves’ apparatus for 1 hr, and then, baseline readings were collected. After collection of basal PWL, wild-type (WT, AKAP150+/+) and AKAP150 knock-out (AKAP150 KO, AKAP150−/−) mice were intraplantarly injected (20 μl) with saline vehicle or bradykinin (10 nM) were administered into the right rear hindpaw. PWL was measured 20 min later by blinded observers. AKAP150 KO mice were developed in the laboratory of G. Stanley McKnight (University of Washington, Seattle, Washington, USA) [12]. Data were analyzed with n=6–8 animals/group, employing 2-way ANOVA with Bonferroni correction, *p<0.05.
Results
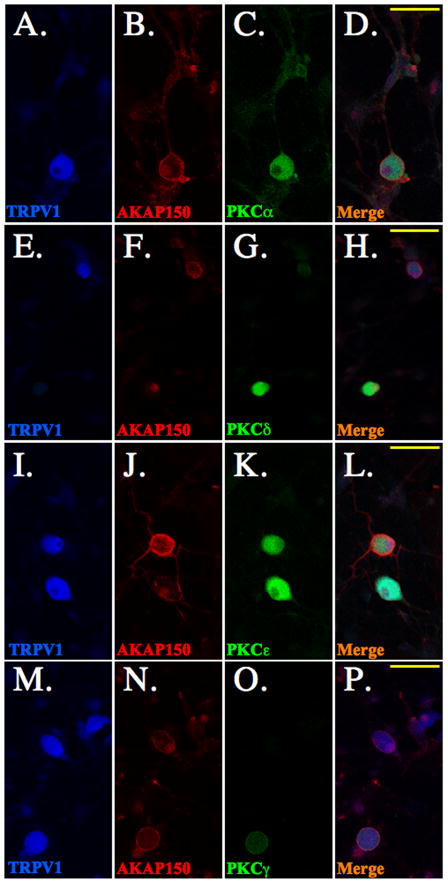
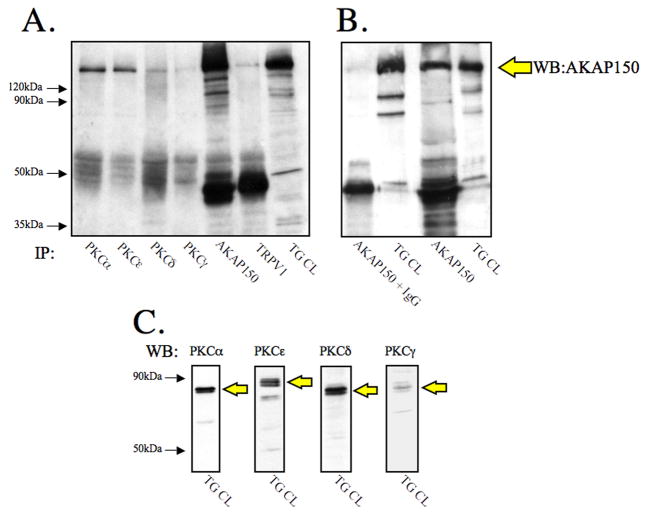
Various kinases, including protein kinas A (PKA), protein kinase C (PKC), calcium/calmodulin-dependent kinase II (CaMKII), src, and cyclin dependent kinase 5 (cdk5), have demonstrated the ability to phosphorylate TRPV1. The role of PKC in the phosphorylation and sensitization of TRPV1 has led to the identification of multiple PKC isoforms that may be involved, including ε and α [3,11,24,26,33]. In order to determine whether AKAP150 modulates PKC-dependent phosphorylation and sensitization of TRPV1, we first conducted immunocytochemical analyses to discern specific PKC isoform co-expression with AKAP150 in TRPV1-expressing cultured trigeminal ganglia (TG) neurons. In Figure 1, TG neurons were probed with antisera specific for rat TRPV1 (Fig. 1A, E, I, M), rat AKAP150 (Fig. 1B, F, J, N), and either rat PKC αFig. 1C), δ Fig 1G, εFig. 1K, or γ (Fig. 1O) isoforms. Confocal microscopy results from multiple images indicate higher levels of co-expression of PKA α and ε with AKAP150 in TRPV1-positive TG neurons, in contrast to lower-levels of PKC δ and γco-expression with AKAP150. Biochemical analyses employing co-immunoprecipitation verify immunocytochemical results, as shown in Figure 2. Both PKC isoforms α and e preferentially co-immunoprecipitated with AKAP150 from TG cultures more so than PKC isoforms δ and γ (Fig. 2A). Western blot analysis of TG cell lysates demonstrated that PKC γ isoform expression is low relative to PKC α, ε, or δ isoforms (Fig. 2B), similar to studies performed in hippocampal neurons [9]. These results suggest that PKC isoforms α and ε preferentially associate with AKAP150 in TRPV1-positive TG neurons.
Figure 1. PKC isoform co-expression with AKAP150 in TRPV1 (+) trigeminal.
neurons. Cultured TG neurons were analyzed for PKC isoform, AKAP150, and TRPV1 expression relationships using immunofluorescence confocal microscopy. TG neurons were probed for TRPV1 (blue, A, E, I, M), AKAP150 (red, B, F, J, N) and PKCα (green, C), PKCδ (green, G), PKCε (green, K) or PKCγ (green, O), with merged images (yellow, D, H, L, P) indicating co-expression (yellow bar = 50 μm). Results are representative of staining relationships observed in 10–15 neurons from 3 coverslips that were stained with each antibody combination.
Figure 2. AKAP150 Co-immunoprecipitates with specific PKC isoforms.
A. Cultured TG neurons were harvested, and 350 mg aliquots were immunoprecipitated with antibodies directed against PKCα, PKCε, PKCδ, PKCγ, AKAP150, and TRPV1. Immunoprecipitates and TG cell lysate (TG CL) were resolved by SDS-PAGE, transferred to PVDF, and probed for AKAP150 expression.
B. IgG immunoprecipitation control demonstrates specificity of AKAP150 antibody.
C. TG CL samples were also probed for PKC isoforms α, ε, δ, and γ to observe relative expression levels in TG cultures.
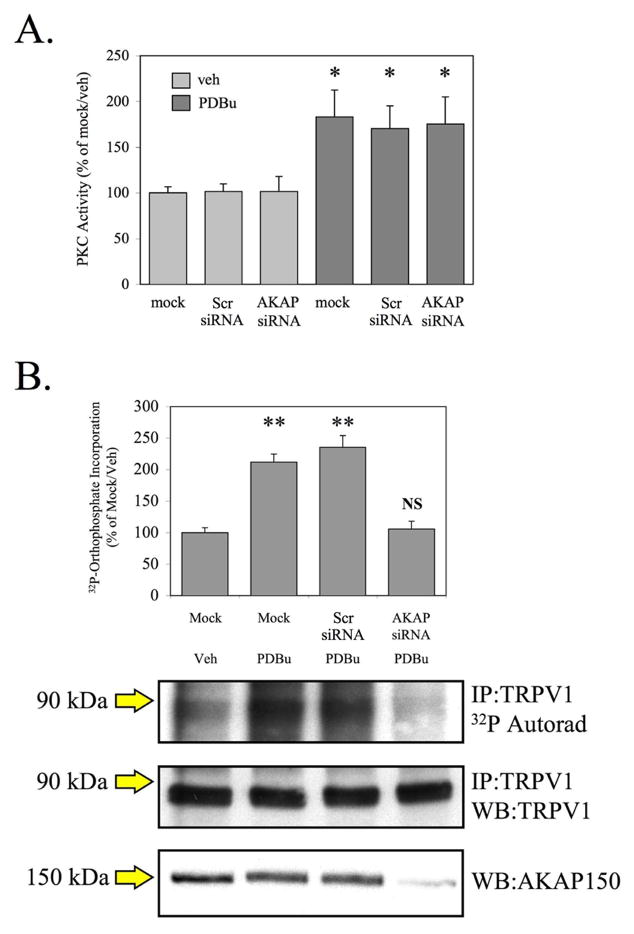
To determine whether PKC phosphorylation of TRPV1 is dependent upon AKAP150 expression, we utilized an approach using siRNA specifically targeted to AKAP150 [15]. Preliminary studies of cultured TG neurons that were mock-transfected, or transfected with scrambled control siRNA (Scr siRNA) or AKAP150-specific siRNA (AKAP siRNA), revealed that AKAP150 siRNA transfection had no significant effect on basal or phorbol 12, 13-dibutyrate (PDBu, 1 μM)-stimulated PKC activity (Fig. 3A). Previously published work demonstrates the specificity of the AKAP150 siRNA [15]. We utilized the siRNA approach to determine whether PKC could phosphorylate TRPV1 in cultured TG neurons following siRNA-mediated knock-down of AKAP150 protein expression. As shown in Figure 3B, PDBu treatment (1 μM, 5 min) significantly increased the ratio of phosphorylated TRPV1:total TRPV1 in mock-transfected and scrambled siRNA- transfected neurons (p<0.01, one-way ANOVA). However, in TG neurons transfected with AKAP150 siRNA, PDBu did not stimulate an increase in TRPV1 phosphorylation compared to mock-transfected, vehicle-treated neurons. These data indicate that AKAP150 expression is required for PKC-mediated phosphorylation of TRPV1 in cultured TG neurons.
Figure 3. PKC-mediated phosphorylation of TRPV1 is dependent upon AKAP150 expression.
A. Cultured TG neurons were transfected in a mock setting, with scrambled control siRNA, or with AKAP150-specific siRNA. In vitro PKC kinase activity was assayed from cell lysates of cultured TG neurons treated with vehicle (0.1% ethanol) or PDBu (1 mM, 5 min).
B. Following siRNA transfection, TG neurons were loaded with 32P orthophosphate and treated with either veh or PDBu (1 μM, 5 min), and immunoprecipitated TRPV1 was analyzed for 32P incorporation. Representative autoradiographic and Western blot results are shown. Results are representative of 4 individual trials. * p<0.05, ** p<0.01, NS: no significance, as determined by one-way ANOVA.
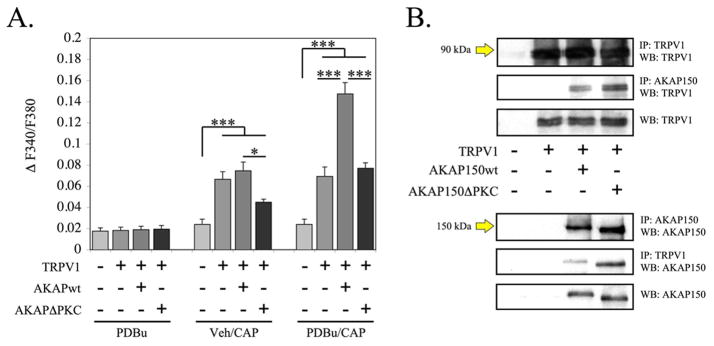
To determine whether PKC association with AKAP150 is necessary for PDBu to stimulate TRPV1 sensitization, we utilized an AKAP150 deletion mutant with the PKC-binding sequence deleted, termed AKAP150DPKC [14]. As depicted in Figure 4A, PDBu (1 μM, 5 min) alone failed to elicit a significant accumulation of calcium in TRPV1-expressing Chinese Hamster Ovary (CHO) cells, in agreement with previous findings [3]. However, capsaicin (CAP, 50 nM)-stimulated calcium accumulation was observed for all TRPV1-expressing CHO cells. Furthermore, PDBu-pretreatment stimulated a significant increase in CAP-stimulated calcium accumulation in CHO cells expressing TRPV1 and full length AKAP150 over cells solely expressing TRPV1. Of importance, CHO cells expressing TRPV1 and the mutant AKAP150DPKC were not sensitized by PDBU pre-treatment. Interestingly, CAP-stimulated calcium accumulation following PDBu pre-treatment in CHO cells expressing TRPV1 and AKAP150ΔPKC was not significantly different from those cells expressing TRPV1 alone. Co-immunoprecipitates of transfected CHO cell lysates revealed equal association between TRPV1 and full length AKAP150 or AKAP150ΔPKC (Figure 4B). These data demonstrate that PKC is unable to sensitize TRPV1 activity if co-expressed AKAP150 does not contain the PKC binding site.
Figure 4. PKC-mediated sensitization of capsaicin (CAP)-induced calcium influx is dependent upon the PKC-binding domain of AKAP150.
A. Cummulative measurements of calcium accumulation in CHO cells transiently transfected with either AKAP150wt or AKAP150ΔPKC with rTRPV1. Cells were pre-treated with vehicle or PDBu (1 μM, 5 min) and stimulated with CAP (50 nm) to measure calcium accumulation. Results are representative of 45–60 cells per transfection/treatment.
B. Co-immunoprecipitation and Western blot analysis of transfected CHO cells (as shown). Results are representative of 3 individual trials. * p<0.05, *** p<0.005, as determined by one-way ANOVA.
We next evaluated the ability of PDBu to sensitize CAP-current in cultured TG neurons either transfected with AKAP150-specific siRNA, or from AKAP150 WT and KO mice (Figure 5). In mock-transfected TG neurons and those transfected with scrambled control siRNA, PDBu pre-treatment (1 μM, 5 min) significantly increased CAP (50 nM)-induced current, indicating sensitization of the endogenously expressed TRPV1 channel (p<0.005, Student’s t-test). However, in TG neurons transfected with siRNA specific for rat AKAP150, PDBu pre-treatment was unable to sensitize CAP-current (Figure 5A–B). Using C57/Bl6 mice lacking AKAP150 expression (AKAP150 KO, [12]), we sought to determine the functional validity of AKAP150-specific siRNA. PDBu-sensitization of CAP-current was measured in TG neurons cultured from AKAP150 KO and WT littermates, and revealed similar results to experiments utilizing AKAP150-specific siRNA (Figure 5C–D). Importantly, the general PKC inhibitor, GF109203x (GFX), was unable to significantly inhibit non-significant effects of PDBu treatment on CAP-current in TG neurons isolated from AKAP150 KO animals. These results indicate that either knockdown or knockout of AKAP150 expression prevents PDBu-stimulated, PKC-mediated sensitization of TRPV1 activity.
Figure 5. PKC-mediated sensitization of capsaicin(CAP)-induced inward current is dependent upon AKAP150 expression.
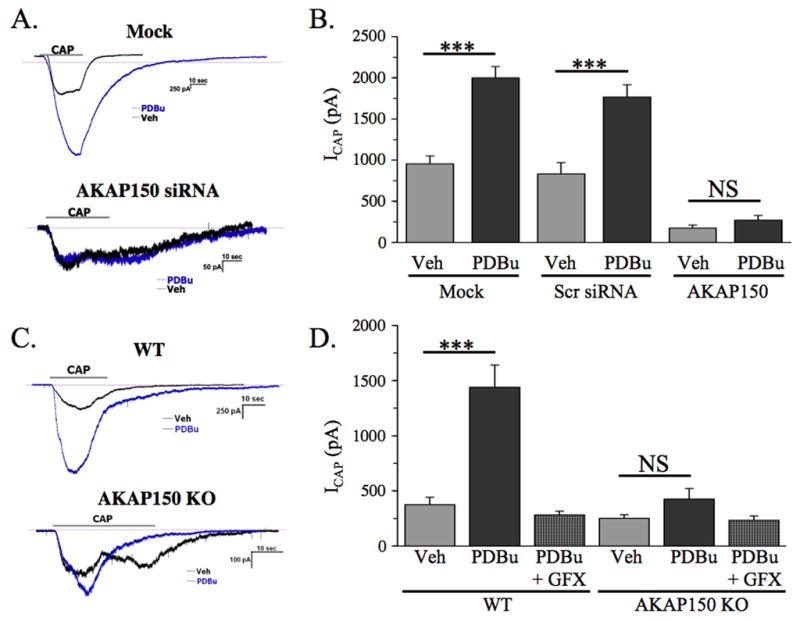
A. Sample CAP-current traces from mock transfected and FITC AKAP150 siRNA-transfected rat TG neurons pre-treated with vehicle (black) or PDBu (blue, 1 μM, 5 min) prior to CAP treatment.
B. Cummulative measurements of CAP current from rat TG neurons transfected in a mock setting, with scrambled control siRNA (Scr siRNA), or with FITC-labeled AKAP150-specific siRNA. Results are representative of 6–8 neurons per transfection/treatment.
C. Sample CAP-current traces from TG neurons isolated from wild-type (WT) or AKAP150 knock-out (AKAP150 KO) mice, pre-treated with vehicle (black) or PDBu (blue, 1 μM, 5 min) prior to CAP treatment.
D. Cummulative measurements of CAP current from TG neurons isolated from wild-type (WT) or AKAP150 knock-out (AKAP150 KO) mice. GF109203x (GFX, 3 μM), a general PKC inhibitor, was co-treated with PDBu, as indicated. Results are representative of 6– 10 neurons per treatment.
*** p<0.005, NS: no significance, as determined by student’s t-test.
To determine whether the genetic ablation of AKAP150 has a negative impact on TRPV1 in vivo, we measured thermal paw withdrawal latencies in animals following intraplantar administration of PDBu, or the inflammatory mediator bradykinin, which is known to indirectly stimulate PKC activity in nociceptive neurons through bradykinin type 2 receptor-driven phospholipase Cβ activation [10,18]. For these studies, we employed C57/Bl6 mice lacking AKAP150 expression (AKAP150 KO) that have undergone previous genotypic characterization [12]. Wild-type littermates of similar age were used for control purposes. In wild-type mice, PDBu (1nmol/20μl) of bradykinin injection (10 nM/20 μl) into the hindpaw resulted in a significant reduction in paw withdrawal latency upon exposure to heat (p<0.05, two-way ANOVA). In contrast, AKAP150 KO mice experienced no significant decrease in paw withdrawal latency in response to a heat following PDBu or bradykinin injection (Figure 6). These data indicate that the development of heat hyperalgesia following exposure to PDBu or bradykinin requires AKAP150 expression.
Figure 6. Genetic ablation of functional AKAP150 protein reduces Protein Kinase C-induced sensitization to thermal hyperalgesia.
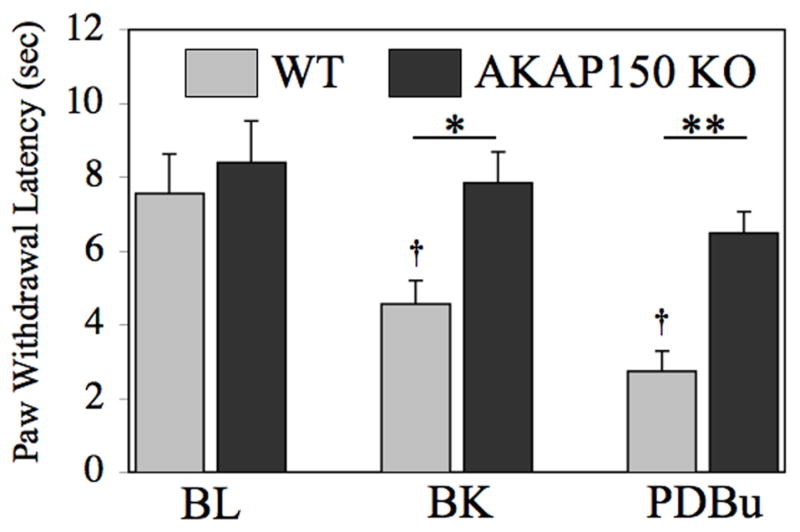
WT and AKAP150KO mice were injected with 20 μl of 10nM bradykinin (BK) or 1 nmol PDBu in the right rear hindpaw, upon which thermal paw withdrawal latencies were measured by blinded observation using a Hargreaves’ apparatus. Data are depicted as paw withdrawal latency (sec). All plotted data expressed as mean ± SEM, n= 6–8 male animals per group. * p<0.05, ** p<0.01, † p<0.05, as determined by two-way ANOVA. Asterisks denote significance within treatment groups, while crosses denote significance between treatments.
Discussion
The release of inflammatory mediators following tissue injury sensitizes primary afferent nociceptors, which can develop into inflammatory hyperalgesia. A complete understanding of the signaling pathways involved with inflammatory sensitization of terminal afferent endings has yet to be developed, although new molecules and their signaling roles are continually identified. In this sense, AKAP150 scaffolding with TRPV1 comprises an important new model for understanding inflammatory sensitization. In this collection of studies, we have demonstrated the co-expression of certain PKC isoforms and AKAP150 in TRPV1-positive TG neurons, in conjunction with co-immunoprecipitation studies that demonstrate PKC α and ε isoform association with AKAP150. Importantly, the use of AKAP150-specific siRNA and mutant AKAP150DPKC cDNA indicate that TRPV1 cannot be phosphorylated or sensitized by PKC without functional expression of full-length AKAP150. Furthermore, thermal hyperalgesia that develops following PDBu or bradykinin exposure in wild type mice was not observed in AKAP150 KO mice. Collectively, these data suggest that AKAP150 modulates PKC phosphorylation and sensitization of TRPV1.
The family of PKC kinases is sub-divided into three distinct groups: conventional isoforms that require both Ca+2 and diacylglycerol (DAG) for activation (α, β1, β2, and γ isoforms), novel isoforms that only require DAG for activation (δ, ε, η, and θ), and atypical isoforms that do not require Ca+2 or DAG for activation (ζ, μ, and ι) (for review, see [21]. Faux et al. reported previously that AKAP150 co-purifies with PKC isoforms α, β1, β2, δ, ε, ζ, and ι from rat brain extracts [9]. Results presented in these studies indicate similar results, demonstrating PKC α and ε co-expression with AKAP150 in TRPV1-positive TG neurons and co-immunoprecipitation with AKAP150, although the tissue source was significantly different. Importantly, others have shown that PKC isoforms α and ε contribute to TRPV1 sensitization in several models [1,11,24,25,36]. In contrast, we observed little to no PKC δ or γ co-expression or association with AKAP150 in our studies, which supports others who demonstrate no effects of the PKC isoforms δ or γ on TRPV1 activity [1,25]. Collectively, past and present results indicate that AKAP150 mediates sensitization of TRPV1 by targeting certain PKC isoforms to the vicinity of the receptor channel.
Behavioral measures of heat hyperalgesia following exposure to inflammatory mediators provide an important in vivo correlate to in vitro studies. We report that the genetic ablation of AKAP150 prevents PDBu or bradykinin from sensitizing mice to heat stimulation. In vehicle treated animals, there was no significant difference in paw withdrawal latency in response to thermal stimulation, indicating that basal activation of heat-responsive receptor-channels does not require functional AKAP150 expression. However, in current and previous electrophysiology studies utilizing AKAP150-specific siRNA, the knock-down in scaffolding protein expression had a significant effect on CAP current [15]. Interestingly, CAP-current density in TRPV1-expressing HEK-293 cells transfected with AKAP150-specific siRNA is significantly reduced relative to mock-transfected cells [37]. Also, certain cultured DRG neurons from transgenic mice that express AKAP150 with the PKA binding site deleted (AKAP150 D36) failed to display calcium influx in response to 100 nM CAP [30]. Although compensatory effects could account for differences in control measurements between siRNA-mediated knock-down models and genetic knock-out models, both lines of evidence reported here indicate that AKAP150 expression is required for PKC sensitization of TRPV1 activity.
An interaction site between the human species of AKAP150 (AKAP79) and TRPV1 was proposed to exist within the intracellular C-terminus of the receptor-channel [37]. It is also noted that the N-terminus of TRPV1 also co-precipitated AKAP in this study, although to a lesser extent than the C-terminus of TRPV1. This may be of importance, especially in light of recent observations by Lishko et al., who document ATP association within the ankyrin repeat domain of TRPV1 [19]. Similar to results presented above, Zhang et al. observed a biochemical association between AKAP79 and hTRPV1 in transfected HEK293 cells that modulated PKC-sensitization of TRPV1 activity [37]. However, studies presented here comprise the first report on the physiological importance of AKAP150 in PKC-mediated thermal hyperalgesia using AKAP150−/− mice. Taken together, results presented here identify the AKAP150 scaffolding protein as an important mediator of PKC-mediated inflammatory hyperalgesia, in so much as TRPV1 sensitization is involved. Recent studies identifying the TRPA1 receptor-channel complex as another associative partner for AKAP150 [37] highlights future avenues of research into the importance of scaffolding proteins and peripheral nociception.
Acknowledgments
We thank Abirami Ramalingam, Ruben Gomez, Griffin Perry, and Gabriella Helesic for their expert technical assistance. Research funded by NIH grant NS061884 (NAJ), and NIH grant NS043394 and AHA grant 0755071Y (MSS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Amadesi S, Cottrell GS, Divino L, Chapman K, Grady EF, Bautista F, Karanjia R, Barajas-Lopez C, Vanner S, Vergnolle N, Bunnett NW. Protease-activated receptor 2 sensitizes TRPV1 by protein kinase Cepsilon- and A-dependent mechanisms in rats and mice. J Physiol. 2006;575:555–571. doi: 10.1113/jphysiol.2006.111534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bengrine A, Li J, Awayda MS. The A-kinase anchoring protein 15 regulates feedback inhibition of the epithelial Na+ channel. Faseb J. 2007;21:1189–1201. doi: 10.1096/fj.06-6046com. [DOI] [PubMed] [Google Scholar]
- 3.Bhave G, Hu HJ, Glauner KS, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RWT. Protein kinase C phosphorylation sensitizes but does not activate the capsaicin receptor transient receptor potential vanilloid 1 (TRPV1) Proc Natl Acad Sci U S A. 2003;100:12480–12485. doi: 10.1073/pnas.2032100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RWT. cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron. 2002;35:721–731. doi: 10.1016/s0896-6273(02)00802-4. [DOI] [PubMed] [Google Scholar]
- 5.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 6.Carr DW, Stofko-Hahn RE, Fraser ID, Cone RD, Scott JD. Localization of the cAMP-dependent protein kinase to the postsynaptic densities by A-kinase anchoring proteins. Characterization of AKAP 79. J Biol Chem. 1992;267:16816–16823. [PubMed] [Google Scholar]
- 7.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 8.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 9.Faux MC, Rollins EN, Edwards AS, Langeberg LK, Newton AC, Scott JD. Mechanism of A-kinase-anchoring protein 79 (AKAP79) and protein kinase C interaction. Biochem J. 1999;343(Pt 2):443–452. [PMC free article] [PubMed] [Google Scholar]
- 10.Ferreira J, Da Silva GL, Calixto JB. Contribution of vanilloid receptors to the overt nociception induced by B2 kinin receptor activation in mice. Br J Pharmacol. 2004;141:787–794. doi: 10.1038/sj.bjp.0705546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferreira J, Triches KM, Medeiros R, Calixto JB. Mechanisms involved in the nociception produced by peripheral protein kinase c activation in mice. Pain. 2005;117:171–181. doi: 10.1016/j.pain.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Hall DD, Davare MA, Shi M, Allen ML, Weisenhaus M, Mcknight GS, Hell JW. Critical role of cAMP-dependent protein kinase anchoring to the L-type calcium channel Cav1.2 via A-kinase anchor protein 150 in neurons. Biochemistry. 2007;46:1635–1646. doi: 10.1021/bi062217x. [DOI] [PubMed] [Google Scholar]
- 13.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 14.Hoshi N, Zhang JS, Omaki M, Takeuchi T, Yokoyama S, Wanaverbecq N, Langeberg LK, Yoneda Y, Scott JD, Brown DA, Higashida H. AKAP150 signaling complex promotes suppression of the M-current by muscarinic agonists. Nat Neurosci. 2003;6:564–571. doi: 10.1038/nn1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeske NA, Diogenes A, Ruparel NB, Fehrenbacher JC, Henry M, Akopian AN, Hargreaves KM. A-kinase anchoring protein mediates TRPV1 thermal hyperalgesia through PKA phosphorylation of TRPV1. Pain. 2008;138:604–616. doi: 10.1016/j.pain.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeske NA, Patwardhan AM, Gamper N, Price TJ, Akopian AN, Hargreaves KM. Cannabinoid WIN 55,212-2 Regulates TRPV1 Phosphorylation in Sensory Neurons. J Biol Chem. 2006;281:32879–32890. doi: 10.1074/jbc.M603220200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klauck TM, Faux MC, Labudda K, Langeberg LK, Jaken S, Scott JD. Coordination of three signaling enzymes by AKAP79, a mammalian scaffold protein. Science. 1996;271:1589–1592. doi: 10.1126/science.271.5255.1589. [DOI] [PubMed] [Google Scholar]
- 18.Lee SY, Lee JH, Kang KK, Hwang SY, Choi KD, Oh U. Sensitization of vanilloid receptor involves an increase in the phosphorylated form of the channel. Arch Pharm Res. 2005;28:405–412. doi: 10.1007/BF02977669. [DOI] [PubMed] [Google Scholar]
- 19.Lishko Pv, Procko E, Jin X, Phelps Cb, Gaudet R. The ankyrin repeats of TRPV1 bind multiple ligands and modulate channel sensitivity. Neuron. 2007;54:905–918. doi: 10.1016/j.neuron.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 20.Matta JA, Ahern GP. Voltage is a partial activator of rat thermosensitive TRP channels. J Physiol. 2007;585:469–482. doi: 10.1113/jphysiol.2007.144287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mellor H, Parker PJ. The extended protein kinase C superfamily. Biochem J. 1998;332 ( Pt 2):281–292. doi: 10.1042/bj3320281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohapatra DP, Nau C. Desensitization of capsaicin-activated currents in the vanilloid receptor TRPV1 is decreased by the cyclic AMP-dependent protein kinase pathway. J Biol Chem. 2003;278:50080–50090. doi: 10.1074/jbc.M306619200. [DOI] [PubMed] [Google Scholar]
- 23.Nilius B, Talavera K, Owsianik G, Prenen J, Droogmans G, Voets T. Gating of TRP channels: a voltage connection? J Physiol. 2005;567:35–44. doi: 10.1113/jphysiol.2005.088377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Numazaki M, Tominaga T, Toyooka H, Tominaga M. Direct phosphorylation of capsaicin receptor VR1 by protein kinase Cepsilon and identification of two target serine residues. J Biol Chem. 2002;277:13375–13378. doi: 10.1074/jbc.C200104200. [DOI] [PubMed] [Google Scholar]
- 25.Olah Z, Karai L, Iadarola MJ. Protein kinase C(alpha) is required for vanilloid receptor 1 activation. Evidence for multiple signaling pathways. J Biol Chem. 2002;277:35752–35759. doi: 10.1074/jbc.M201551200. [DOI] [PubMed] [Google Scholar]
- 26.Premkumar LS, Ahern GP. Induction of vanilloid receptor channel activity by protein kinase C. Nature. 2000;408:985–990. doi: 10.1038/35050121. [DOI] [PubMed] [Google Scholar]
- 27.Price TJ, Patwardhan A, Akopian AN, Hargreaves KM, Flores CM. Modulation of trigeminal sensory neuron activity by the dual cannabinoid-vanilloid agonists anandamide, N-arachidonoyl-dopamine and arachidonyl-2-chloroethylamide. Br J Pharmacol. 2004;141:1118–1130. doi: 10.1038/sj.bjp.0705711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rathee PK, Distler C, Obreja O, Neuhuber W, Wang GK, Wang SY, Nau C, Kress M. PKA/AKAP/VR-1 module: A common link of Gs-mediated signaling to thermal hyperalgesia. J Neurosci. 2002;22:4740–4745. doi: 10.1523/JNEUROSCI.22-11-04740.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ross RA, Gibson TM, Brockie HC, Leslie M, Pashmi G, Craib SJ, Di Marzo V, Pertwee RG. Structure-activity relationship for the endogenous cannabinoid, anandamide, and certain of its analogues at vanilloid receptors in transfected cells and vas deferens. Br J Pharmacol. 2001;132:631–640. doi: 10.1038/sj.bjp.0703850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schnizler K, Shutov LP, Van Kanegan MJ, Merrill MA, Nichols B, Mcknight GS, Strack S, Hell JW, Usachev YM. Protein kinase A anchoring via AKAP150 is essential for TRPV1 modulation by forskolin and prostaglandin E2 in mouse sensory neurons. J Neurosci. 2008;28:4904–4917. doi: 10.1523/JNEUROSCI.0233-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schuligoi R, Donnerer J, Amann R. Bradykinin-induced sensitization of afferent neurons in the rat paw. Neuroscience. 1994;59:211–215. doi: 10.1016/0306-4522(94)90111-2. [DOI] [PubMed] [Google Scholar]
- 32.Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 33.Vellani V, Mapplebeck S, Moriondo A, Davis JB, Mcaughton PA. Protein kinase C activation potentiates gating of the vanilloid receptor VR1 by capsaicin, protons, heat and anandamide. J Physiol. 2001;534:813–825. doi: 10.1111/j.1469-7793.2001.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Voets T, Droogmans G, Wissenbach U, Janssens A, Flockerzi V, Nilius B. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature. 2004;430:748–754. doi: 10.1038/nature02732. [DOI] [PubMed] [Google Scholar]
- 35.Wong W, Scott JD. AKAP signalling complexes: focal points in space and time. Nat Rev Mol Cell Biol. 2004;5:959–970. doi: 10.1038/nrm1527. [DOI] [PubMed] [Google Scholar]
- 36.Zhang H, Cang CL, Kawasaki Y, Liang LL, Zhang YQ, Ji RR, Zhao ZQ. Neurokinin-1 receptor enhances TRPV1 activity in primary sensory neurons via PKCepsilon: a novel pathway for heat hyperalgesia. J Neurosci. 2007;27:12067–12077. doi: 10.1523/JNEUROSCI.0496-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang X, Li L, Mcnaughton PA. Proinflammatory mediators modulate the heat-activated ion channel TRPV1 via the scaffolding protein AKAP79/150. Neuron. 2008;59:450–461. doi: 10.1016/j.neuron.2008.05.015. [DOI] [PubMed] [Google Scholar]