Abstract
Purpose
Despite the strong association between malignant disease and thromboembolic disorders, the molecular and cellular basis of this relationship remains uncertain. We evaluated the hypothesis that tumor-derived tissue factor-bearing microparticles in plasma contribute to cancer-associated thrombosis.
Experimental Design
We developed impedance-based flow cytometry to detect, quantitate and size microparticles in platelet-poor plasma. We evaluated the number of tissue factor-bearing microparticles in a cohort of cancer patients of different histologies (N=96) and performed a case control study of 30 cancer patients diagnosed with an acute venous thromboembolic event (VTE) compared to 60 cancer patients of similar age, stage, sex, diagnosis without known VTE as well as 22 patients with an idiopathic VTE.
Results
Tissue factor-bearing microparticles were detected in patients with advanced malignancy including two thirds of patients with pancreatic carcinoma. Elevated levels of tissue factor-bearing microparticles were associated VTE in cancer patients (adjusted OR 3.72, 95% CI 1.18-11.76, P=0.01). In cancer patients without VTE, a retrospective analysis revealed a one-year cumulative incidence of VTE of 34.8% in patients with tissue factor-bearing microparticles versus 0% those without detectable tissue factor-bearing microparticles (Gray test p-value=0.002). The median number of tissue factor-bearing microparticles in the cancer VTE cohort (7.1 × 104 microparticles/μl) was significantly greater than both the idiopathic VTE and cancer-no VTE groups (P=0.002 and P=0.03, respectively). Pancreatectomy in three patients eliminated or nearly eliminated these microparticles which co-expressed the epithelial tumor antigen, MUC-1.
Conclusion
We conclude that tumor-derived tissue factor-bearing microparticles are associated with VTE in cancer patients and may be central to the pathogenesis of cancer-associated thrombosis.
Introduction
Thromboembolic disease is a recognized complication of cancer (1-8). Patients with cancer experience venous thromboembolic events (VTE) at a rate 4 to 7 times higher than that of the general population (9). Although all tumor types are associated with thrombosis, glioblastoma and carcinomas of the ovary and pancreas are consistently associated with the highest incidence of VTE (10, 11). Despite the strong association between malignant disease and thromboembolic disorders, the molecular and cellular basis of this relationship remains uncertain. Hypotheses regarding the mechanism have included the activation of blood coagulation by: tissue factor in tumors (12), a factor X-activating cysteine protease (13), mucinous glycoproteins (8), and MET oncogene activation (14). Laboratory markers of coagulation activation are often elevated in patients with cancer (15-21) but are of little clinical utility in assessing individual risk for thrombosis.
Tissue factor, a receptor protein constitutively expressed on the plasma membrane of most non-vascular cells and inducibly expressed on monocytes and endothelial cells, initiates blood coagulation in vivo (22). In addition to its expression in the vessel wall, tissue factor is associated with cell-derived vesicles in blood that bind to the developing thrombus and play a role in fibrin propagation (23, 24). These vesicles, known as microparticles based on their submicron diameter, express protein surface components from the parent cell from which they are derived. Monocyte-derived microparticles that express tissue factor accumulate in the developing thrombus through the interaction of P-selectin and PSGL-1, thus delivering tissue factor to the site of vascular injury and facilitating fibrin propagation (24). Microparticles derived from blood and vascular cells circulate in the blood of normal subjects, and these populations are altered qualitatively and quantitatively in specific disease states (25-28). Tissue factor-bearing microparticles have been observed in the plasma of cancer patients, and a possible role for these microparticles in cancer-associated thrombosis has been proposed (29, 30). To evaluate whether tissue factor-bearing microparticles are a risk factor in and a possible cause of cancer-associated thrombosis, we quantified tissue factor-bearing microparticles in cancer patients using impedance-based flow cytometry and analyzed their association with acute venous thromboembolic disease. We show that impedance-based flow cytometry allows identification and quantitation of tissue factor-bearing microparticles, that most of these tissue factor-bearing microparticles are tumor-derived, and that the presence of detectable tissue factor-bearing-microparticles is associated with an increased risk of thromboembolic disease accompanying malignant disease.
Methods
Materials
Humanized monoclonal antibody, cH36, against human tissue factor was the generous gift from Dr. Hing Wong (Altor Biosciences, Miramar FL). Antibodies against MUC1 were generously provided by Dr. Donald Kufe (Dana Farber Cancer Institute, Boston MA); these antibodies were labeled with Alexa 488 using standard methods. ASPC-1, a human pancreatic carcinoma cell line, was obtained from the ATCC (Rockville MD). Goat anti-human Ig coupled to Alexa 488 was obtained from Invitrogen (Carlsbad CA), anti-CD41a-FITC antibody and PE-conjugated mouse anti-human monoclonal TF antibody from BD Biosciences (San Diego CA) and all other immunochemical reagents from Sigma-Aldrich (St. Louis MO). Fluorescently labeled polystyrene microspheres were purchased from Bangs Laboratories (Fishers IN). These microspheres are homogeneous with regard to size by 10%.
Generation of tissue factor-bearing microparticles via cell culture
ASPC-1 cells were incubated at 37° in RPMI media for 72 hours. The cells were removed from conditioned media by centrifugation twice at 2100 xg. Aliquots (10 μl) of supernatant were labeled with (0.25μg) humanized anti-tissue factor monoclonal antibody cH36 and labeled with a goat anti-human IgG-Alexa 488 antibody.
Flow cytometry analyses of platelets and platelet microparticles
Platelet-rich plasma was centrifuged for 2 min at 2100 xg. A 40 μl aliquot of plasma, containing both platelets and platelet microparticles, was incubated with 0.125 μg of anti-CD41a-FITC antibody for 30 minutes. The plasma samples were diluted 1:50 in NPE Iso-Diluent with 0.78 μm polystyrene microspheres at a final concentration 6000/μl). Samples were analyzed on a Beckman Coulter Quanta flow cytometer equipped with a 40 μm flow cell, a Becton Dickinson LSRII flow cytometer and a Becton Dickinson FACSCalibur flow cytometer.
Impedance-based flow cytometry for the detection of microparticles
A Quanta (NPE Systems, Pembroke Pines FL) or Beckman Coulter SC Quanta flow cytometer (Beckman Coulter, Miami FL) were used to measure particle size by impedance. Both flow cytometry instruments required post-market installation of smaller flow cells (25 μm or 40 μm, respectively) in order to analyze particles between 0.3-1.0 μm in diameter, A 0.5 μg aliquot of humanized monoclonal antibody cH36 against human tissue factor or a purified human IgG antibody control was added to platelet-poor plasma, and the sample incubated for 30 min at room temperature. Subsequently, a secondary goat anti-human IgG coupled to Alexa 488 was added and the sample incubated for 30 min at room temperature. An aliquot of the antibody-treated platelet-poor plasma (10μl) was diluted 1:100 into filtered NPE Iso-Diluent (Beckman-Coulter, Miami FL) and assayed in triplicate in the Quanta flow cytometer. Microparticle concentration, particle size and particle fluorescence were recorded. Fluorescent 0.78 μm microspheres were used for instrument calibration. The cell-free supernatant of ASPC-1 pancreatic cells containing tissue factor-bearing microparticles served as positive controls. The lower limit of detection of tissue factor-bearing microparticles at these dilutions was estimated to be 1 × 104 microparticles per μl of plasma. Dual label experiments were performed using 5 μl of platelet poor plasma and 0.05 μg PE-conjugated mouse anti-human monoclonal TF antibody (BD Biosciences, San Diego, CA) and 0.5 μg mouse-anti human MUC-1 monoclonal antibody labeled with Alexa 488 (Invitrogen). Isotype matched mouse IgG controls were similarly labeled.
Survey of patients with advanced malignancy for plasma tissue factor-bearing microparticles
The plasma of cancer-free subjects and subjects with surgically unresectable or metastatic cancer were assayed for tissue factor-bearing microparticles. Malignancy diagnosis required histological documentation as well as radiographic evidence of disease. Cancer diagnoses included pancreatic carcinoma, non-small cell lung cancer, ovarian carcinoma, colorectal cancer and breast cancer. Cancer-free controls were cancer-free by history, without a history of VTE, and could not be taking warfarin for any indication.
Clinical trial to determine whether tissue factor-bearing microparticles in cancer patients are more likely associated with those with thrombosis than those free of thrombosis
Patients with an active malignancy who presented with symptomatic proximal deep venous thrombosis or pulmonary embolism were enrolled. A qualifying VTE required radiographic evidence of proximal deep vein thrombosis by compression ultrasound and/or a pulmonary embolism by high probability ventilation/perfusion lung scan or CT angiogram of the pulmonary arteries. Patients who experienced a thrombotic event within 30 days of a surgical procedure or following trauma were excluded.
The control population was a cohort of cancer patients without evidence of venous thromboembolic disease in the previous 5 years, matched for cancer diagnosis, cancer stage at time of blood draw (equal stage or higher), age (± 10 years), and sex with each cancer patient with acute VTE. Two control patients were enrolled for each cancer patient with acute venous thromboembolic disease. This provided a control group with a similar distribution of diagnoses, age, and sex. Radiotherapy or chemotherapy administered within 30 days prior to enrollment was documented. For patients in the cancer-no VTE group, a retrospective analysis was performed to evaluate the incidence of VTE; all radiographic reports for the cancer-no VTE group were analyzed by a reviewer blinded to microparticle status. Only documented evidence of a new proximal extremity deep vein thrombosis or pulmonary embolism was included in the analysis. A third comparator group included patients who presented with an acute idiopathic symptomatic VTE. An idiopathic event was defined as not occurring within 30 days of surgery or trauma and no history of malignancy within the last 5 years. Patients with hereditary thrombophilias were not specifically excluded.
All venipunctures were performed within 72 hours of initial diagnosis of acute VTE. Blood anticoagulated in citrate buffer was centrifuged at 2100 × g for 20 min twice to obtain platelet-poor plasma and stored at -80°C. All study protocols were approved by the Human Investigation Review Board at Beth Israel Deaconess Medical Center and the University of Southern California prior to the enrollment of study subjects. All patients signed informed consent prior to laboratory testing and personal health information was protected in accordance with HIPAA guidelines.
Statistical Analysis
Based on our preliminary data that tissue factor-bearing microparticles were measurable in patients with prothrombotic malignancies (31), the study was powered to detect a difference of 40 percentage points between the percentage of individuals in the cancer-VTE group versus the cancer-no VTE group who had measurable tissue factor-bearing microparticles. We anticipated enrollment of a minimum of 21 patients with cancer-acute VTE with a 2:1 matched enrollment of no VTE-cancer controls (N=42) in order to achieve a statistical power of 80% with a two-sided alpha level of 0.05%. Matching was implemented to assure similar age, sex, and cancer diagnosis distribution in the VTE and no VTE groups; there was no plan to perform a matched analysis. We also anticipated comparing the cancer-VTE group to an idiopathic VTE group, with planned enrollment of 21 patients with acute idiopathic VTE. In order to account for this second comparison involving the cancer-VTE group, significance by Fisher exact testing was defined a priori as P≤ 0.025.
The determination of significance between groups based on the presence or absence of detectable tissue factor-bearing microparticles was performed using Fisher exact analysis. The odds ratio with 95% confidence intervals for thrombotic risk attributable to tissue factor-bearing microparticles was calculated by logistic regression; stepwise logistic regression was used to identify factors which significantly contributed to thromboembolic risk in addition to tissue factor-bearing microparticles. Absolute differences in tissue factor-bearing microparticles were analyzed by the Wilcoxon rank-sum test as the median values for the cancer-no VTE control and idiopathic VTE groups fell within the undetectable range. The incidence of VTE among cancer patients without VTE at the time of blood sampling was estimated by the method of cumulative incidence, identifying death without VTE as a competing risk; only patients alive without VTE were censored. Differences in the time to VTE using the cumulative incidence method were assessed by the Gray test.
Results
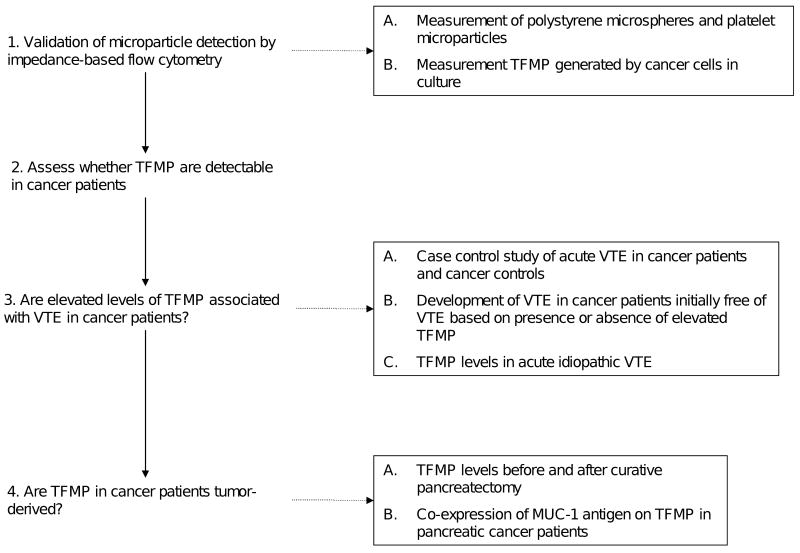
In order to investigate whether tissue factor bearing microparticles are associated with VTE in cancer patients, we first evaluated impedance-based flow cytometry as an accurate method for microparticle detection and subsequently performed a series of clinical investigations to establish a link between elevated tissue factor bearing microparticles and cancer-associated thrombosis as outlined in Figure 1 (supplemental data).
Figure 1.
Flow diagram of studies performed to validate microparticle detection by impedance based flow cytometry and investigate the association between tumor-derived tissue factor bearing microparticles and VTE in cancer patients. (TFMP – tissue factor bearing microparticles)
We previously demonstrated in a mouse model of thrombosis that tissue factor-bearing microparticles accumulate in the developing thrombus in an interaction that is mediated by platelet P-selectin and microparticle PSGL-1 (24). However, our efforts to detect, quantitate and size these tissue factor-bearing microparticles by light scattering using flow cytometry were limited by conventional methods. When particles are of the same order of magnitude as the incident wavelength, the angular distribution of forward light scatter is not dependent on particle size (32, 33). Moreover, the sizing of particles by light scatter is influenced by the refractive index, internal structure, and presence of absorptive material on the particle surface. The absolute sizing of cells or particles by flow cytometry is typically performed by referencing the light scatter characteristics of a uniform population of sized beads, ignoring the fact that the refractive index of polystyrene microspheres is much greater than cellular membranes (33). These issues have been recognized by others but, for lack of better alternatives, light scattering-based flow cytometry has remained a standard method for microparticle detection and measurement.
Impedance-based flow cytometry: application to fluorescent beads of defined size
For these reasons, we have explored a novel method for identifying, sizing and quantitating microparticles utilizing impedance-based flow cytometry. This system, based on the Coulter principle, determines the electronic volume of a particle based on the fact that the electronic volume is proportional to the change in the impedance associated with the displacement of electrolyte in a flow cell by the particle of interest. The system is calibrated using fluorescent polystyrene microspheres of uniform size. In the example shown, polystyrene microspheres of 520 nm in diameter were monitored by impedance-based flow cytometry. A dominant population with a diameter of 520 nm is observed in the histogram (Figure 2A) as are smaller populations of microsphere aggregates. A plot of fluorescence against particle size illustrates the size distribution of fluorescently-labeled microspheres (Figure 2B). Using polystyrene microspheres of known concentration, analyses of solutions containing these microspheres determined concentration within 5% of the theoretical concentration value. The mean coefficient of variation for microspheres with a diameter of 0.78 μm included an electronic volume and fluorescence of 25.2% and 9.2%, respectively (N=15). Populations of polystyrene microspheres of two sizes can be resolved from each other based on their diameter, and independently quantitated (Figure 2C).
Figure 2.
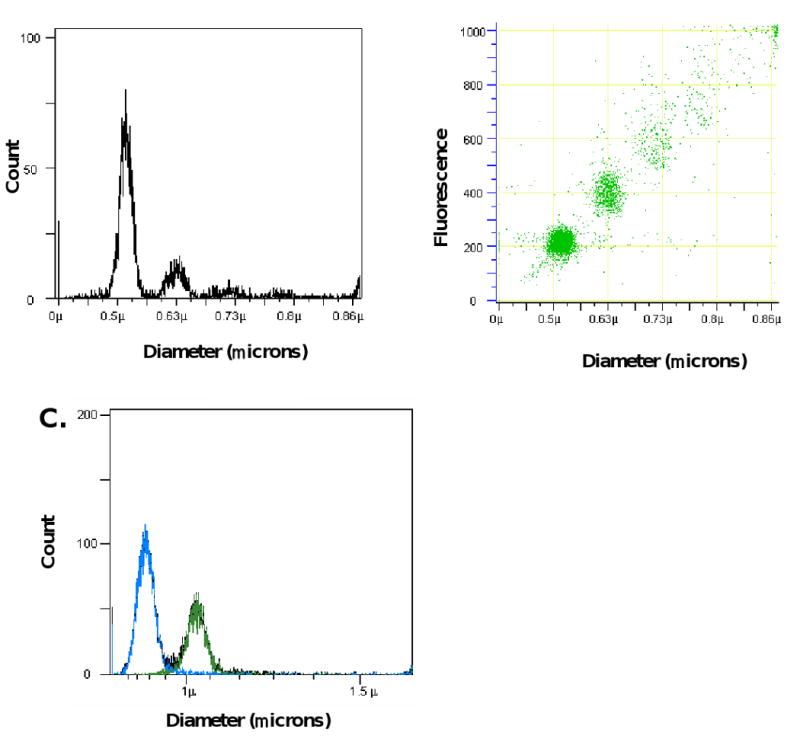
Analysis of fluorescent microspheres using impedance-based flow cytometry. A. Histogram of 0.52 μm polystyrene fluorescent microspheres showing particle distribution as a function of particle size. B. Dot plot of particle diameter versus relative fluorescence for 0.52 μm microspheres. Some aggregates were observed. C. Histograms generated by measurement of electronic volumes of a mixed population of microspheres with diameters of 0.78 μm (blue) and 1.01 μm (green) microspheres showing full resolution of the two populations. Fluorescence was monitored at 488 nm.
To compare microsphere and microparticle sizing, we measured platelet microparticles, calibrated microspheres, and platelets in human plasma both by impedance- and light scatter-based flow cytometry. When characterized by forward light scatter, there is poor size discrimination between platelets and platelet microparticles (Figure 3A-B). Furthermore, the platelet population and the platelet microparticle population overlap with the 0.78 μm microspheres. Using impedance-based flow cytometry, the dot plot of the diameter versus fluorescence demonstrated a platelet microparticle population measuring less than the 0.78 μm microspheres that is completely resolved from the population of platelets (Figure 3C).
Figure 3.
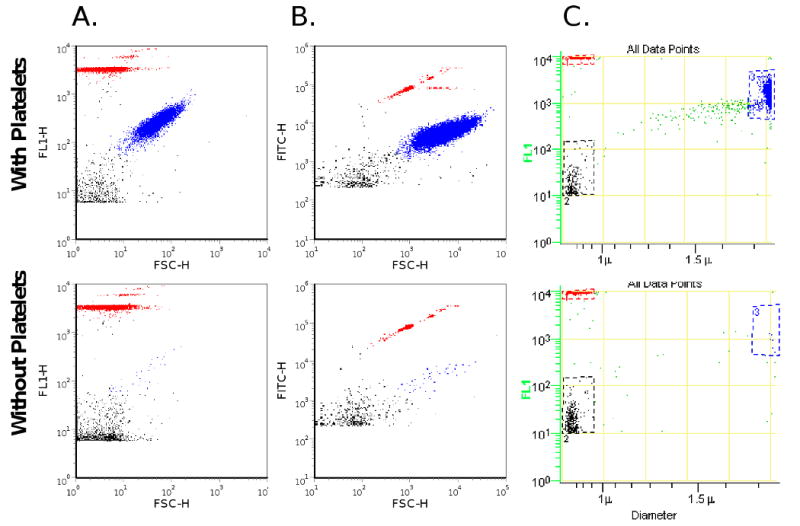
Particle size comparisons. Platelet microparticles, platelets and 0.78 μm fluorescent microspheres were analyzed using impedance-based and light scatter-based flow cytometry (top panel). Platelets were removed by centrifugation; platelet microparticles and 0.78 μm microspheres alone were detected (bottom panel). A. Light scatter flow cytometry with Becton Dickinson FACSCalibur. Platelets (blue) and platelet microparticles (black) were partially resolved and both overlap with 0.78 μm microspheres (red). The platelet microparticles appear within the tail of the platelet population (x-axis forward scatter, y-axis fluorescence). B. Light scatter flow cytometry with the Becton Dickinson LSRII. Platelets (blue) and platelet microparticles (black) were partially resolved, and both overlap with the population of 0.78 μm microspheres (red) (x-axis forward scatter, y-axis fluorescence). C. Impedance-based flow cytometry. Platelets (blue) and platelet microparticles (black) were resolved (x-axis, diameter; y-axis, fluorescence), and can be compared to the 0.78 μm microspheres (red). Platelets and platelet microparticles were labeled with CD41a-FITC antibody. Platelets (blue), platelet microparticles (blue), and platelet microparticles (black). Histograms depict events accumulated over 30 seconds.
Impedance-based flow cytometry: application for detection of tissue factor-bearing microparticles
Dvorak has previously shown that procoagulant microvesicles are released from malignant cells in culture (34). Based on this observation, we examined conditioned media from ASPC-1 pancreatic cells in culture for the presence of tissue factor-bearing microparticles. Using a monoclonal antibody specific for human tissue factor and a FITC-labeled secondary antibody directed against the anti-tissue factor antibody, tissue factor-bearing microparticles were detected, sized and quantitated by impedance-based flow cytometry (Figures 4A). Tissue factor-bearing microparticles derived from the ASPC-1 pancreatic cells varied in size from about 800 nm to 300 nm. As a control, purified human IgG was used in place of the anti-tissue factor antibody (Figure 4B).
Figure 4.
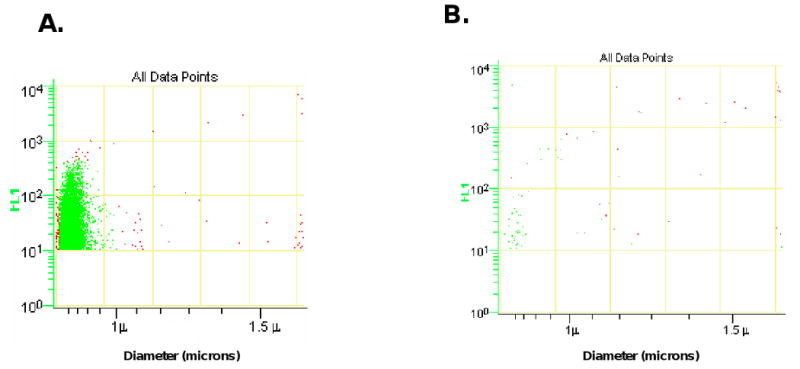
Tissue factor-bearing microparticles were measured in the conditioned media from the ASPC-1 pancreatic cancer cell line. The x-axis presents particle diameter and the y-axis presents fluorescence using a humanized anti-TF monoclonal antibody. The scatter plots for anti-tissue factor-Alexa 488 (A) and IgG-Alexa 488 control (B) are shown.
Tissue factor-bearing microparticles in cancer patients
To determine whether tissue factor-bearing microparticles could be detected and quantitated in platelet-poor plasma from cancer patients, blood samples were obtained from patients with advanced disease for an initial survey. Four histograms from the analysis of samples from patients with metastatic breast carcinoma, non-small cell lung carcinoma and pancreatic carcinoma are shown. Tissue factor-bearing microparticles were detected in these four samples, and the microparticle size varied from 0.30 μm to 0.75 μm, with a median value of about 0.40-0.45 μm (Figure 5A).
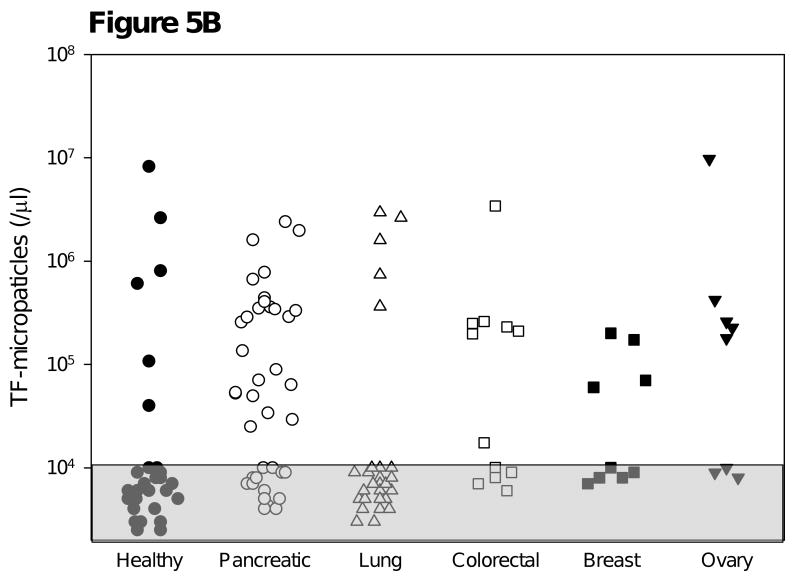
Figure 5.
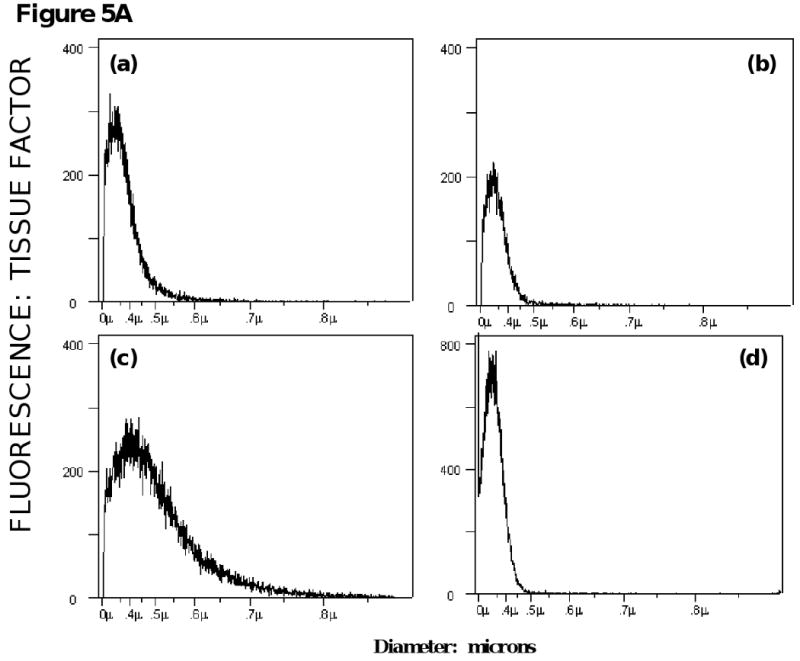
Survey of tissue factor-bearing microparticles in cancer patients. A. Histograms of tissue factor-bearing microparticle detected in four cancer patients. Tissue factor microparticle count versus particle diameter; the X axis is linear for particle volume but is presented in particle diameter in micrometers (0 to 1.0 μm). The patients had advanced disease and samples were drawn prior to a cycle of chemotherapy. The cancer diagnoses were (a) breast (b) pancreatic (c) pancreatic and (d) non-small cell lung cancer. Microparticle size varied from 0.3 μm (the lower limits of detection) to 500-800 μm. B. Tissue factor-bearing microparticle concentrations in advanced malignant disease. The percentage of patients with elevated levels of tissue factor bearing microparticles was significantly greater for pancreatic carcinoma (25 of 39) and colorectal carcinoma (7 of 12) compared with cancer-free subjects (6 of 31, P<.001 and P=0.03 respectively). Significant differences were not observed between cancer-free subjects and non-small cell lung carcinoma (5 of 28), breast carcinoma (4 of 9) and ovarian cancer (5 of 8). Tissue factor-bearing microparticle concentrations less than 1×104/μl were considered undetectable (gray). The P values were calculated using Fishers exact test and compared cancer-free controls with each cancer category (pancreatic, non-small cell lung cancer, colorectal, breast, and ovary).
Blood samples were randomly collected from a variety of cancer patients as well as a cohort of cancer-free controls to provide preliminary information concerning the prevalence of tissue factor-bearing microparticles in these disorders. These data were subsequently used to adequately power case-control study. All cancer patients had metastatic or locally advanced disease whereas the control population was cancer-free by history. Tissue factor-bearing microparticles were detected in the platelet-poor plasma of subjects in all categories (Figure 5B). Tissue factor-bearing microparticles were detected in 68% of patients with pancreatic cancer, seven of whom had previously incurred a venous thromboembolic event. About half of the patients with metastatic ovarian carcinoma, breast carcinoma and colorectal carcinoma had detectable tissue factor-bearing microparticles. The concentration of tissue factor-bearing microparticles varied from 20,000 to 10 million per μl, with the lower limits of detection at 10,000 microparticles/μl. Tissue factor-bearing microparticles were detectable in significantly fewer cancer-free controls and patients with non-small cell lung carcinoma compared with patients with advanced pancreatic cancer (P<.001) or colorectal cancer (P=0.03). These data demonstrate that tissue factor-bearing microparticles can be measured in a significant percentage of cancer populations using impedance-based flow cytometry.
Tissue factor-bearing microparticles in cancer patients with and without venous thromboembolic disease
Given that tissue factor-bearing microparticles can be detected in a high percentage of patients with malignant diseases using impedance-based flow cytometry and that these microparticles are derived from the underlying tumor, we explored the statistical association between tissue factor-bearing microparticles and thromboembolic disease in patients with cancer. A case control study was performed to establish whether there is a relationship between the presence of tissue factor-bearing microparticles and cancer-associated thrombosis.
Tissue factor-bearing microparticles were measured in 30 cancer patients with acute VTE and 60 cancer patients without VTE selected to reflect a similar distribution of age, stage, sex, and diagnosis (Table 1). The median age in this cohort was 60. In the cancer-VTE group, one-half of qualifying events were pulmonary emboli and five patients presented with both a proximal DVT and pulmonary embolus at time of enrollment. Nine patients were enrolled with an isolated deep vein thrombosis; three were of the lower extremity and one patient was simultaneously diagnosed with upper and lower extremity DVT. A single patient was enrolled with a large inferior vena cava thrombus. The cancer stage of the cancer-VTE group were as follows: two myeloma patients were stage I by International Staging System, one case of chronic lymphocytic leukemia (Rai stage II), three patients with non-Hodgkins lymphoma were stage III and one with one stage II, and the patient with ovarian cancer had active recurrent disease. The patient with glioblastoma had active disease and all of the other solid tumor malignancies were metastatic except one patient with stage I breast cancer. A greater percentage of controls were actively receiving either chemotherapy or radiation therapy. With regard to anti-angiogenesis agents associated with thrombosis, one individual in the acute VTE group was receiving lenalidomide while two control patients were taking thalidomide. Bevacizumab was administered to one individual in the acute VTE group and four in the control group. The detection of tissue factor-bearing microparticles was not associated with gender, active therapy, smoking, or diabetes by Fisher's exact test (p>0.05), or with age, complete blood count parameters (white cell count, hematocrit, platelet count) by Wilcoxon rank sum analysis (P>.05).
Table 1.
Characteristics of cancer patients with acute VTE compared with matched cancer controls.
| Cancer VTE (N=30) |
Cancer Controls (N=60) |
P* | |
|---|---|---|---|
| Median Age (years) | 59.5 | 59 | 0.93 |
| Female Sex (%) | 60 | 58 | 0.94 |
| Cancer Diagnosis: | |||
| Breast | 4 | 8 | |
| Non-Hodgkin's Lymphoma | 4 | 8 | |
| Ovarian | 4 | 8 | |
| Renal Cell | 4 | 8 | |
| Non-Small Cell Lung Cancer | 2 | 4 | |
| Myeloma | 2 | 4 | |
| Pancreatic | 2 | 4 | |
| Colon | 1 | 2 | |
| Rectal | 1 | 2 | |
| Thyroid | 1 | 2 | |
| Glioblastoma | 1 | 2 | |
| Hepatocellular | 1 | 2 | |
| Gastric | 1 | 2 | |
| Chronic Lymphocytic Leukemia | 1 | 2 | |
| Uterine | 1 | 2 | |
| White blood cell count (× 109/L) | 6.4 | 6.1 | 0.90 |
| Hemoglobin (g/dL) | 10.8 | 12.8 | <.01 |
| Platelet Count (× 109/L) | 220 | 295 | 0.01 |
| Active cancer therapy | 16 (53%) | 47 (78%) | 0.02 |
| Diabetes | 6 (20%) | 9 (15%) | 0.56 |
| Current Smoker | 2 (6.7%) | 6 (10%) | 0.71 |
Age and complete blood counts were compared by Wilcoxon rank sum analysis
In this case-control study, tissue factor-bearing microparticles were identified in 60% (18 of 30) of patients with cancer-associated VTE compared with 27% (16 of 60) of patients with cancer without VTE (P=0.01). This corresponded to greater than a 4-fold increase risk of thrombosis associated with detectable tissue factor-bearing microparticles (OR 4.13, 95% CI 1.63-10.43). Multivariable models suggest that in addition to the presence of tissue factor-bearing microparticles, lower platelet count, active therapy for underlying disease, and lower hemoglobin were also significantly associated with VTE. The adjusted OR for the effect of detectable tissue factor-bearing microparticles on occurrence of VTE in this model is 3.72 (95% CI 1.18 -11.76).
Microparticles predict the development of thrombosis in cancer patients initially free of venous thromboembolic disease
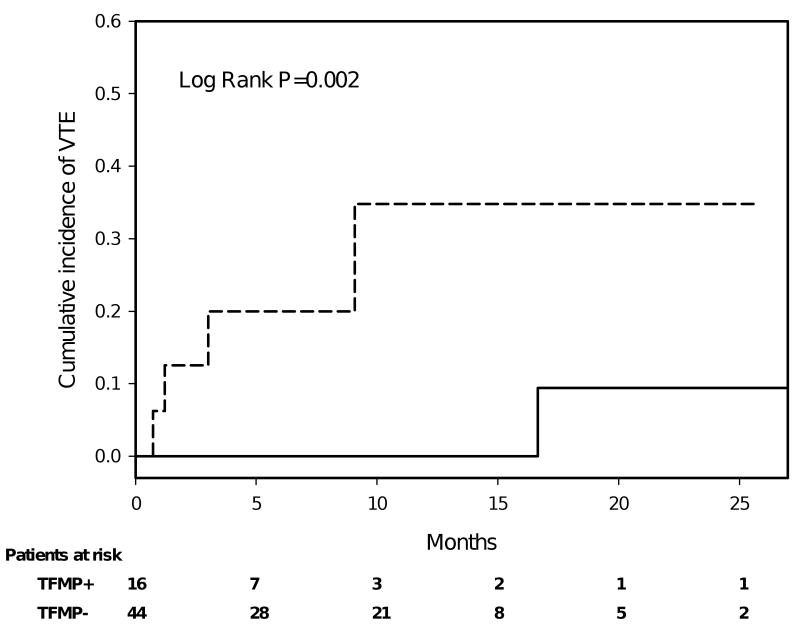
To further assess the relationship between tissue factor-bearing microparticles and thrombosis, we performed a retrospective analysis of deep venous thrombosis or pulmonary emboli diagnosed in the cancer-no VTE group in the 2 years following enrollment. Only documented evidence of a new proximal extremity deep vein thrombosis or pulmonary embolism was included in the analysis. Sixteen of the 60 patients in this group had measurable tissue factor-bearing microparticles, 4 of whom subsequently developed radiographic evidence of VTE within 12 months of enrollment (Figure 6). One thrombotic event was recorded among the 44 patients without detectable tissue factor-bearing microparticles, but after 15 months following enrollment into the study. The tissue factor-bearing microparticle-positive group and the tissue factor-bearing microparticle-negative group did not differ significantly for age, sex, active cancer treatment, smoking status, diabetes, or the presence of metastatic disease at time of enrollment. Identifying death without VTE as a competing risk, the one-year estimate of the rate of VTE in cancer patients with detectable tissue factor-bearing microparticles was 34.8%; among the same group without detectable tissue factor-bearing microparticles, the 1-year rate was 0% (Gray test p-value=0.002). The presence of tissue factor-bearing microparticles in cancer patients initially thrombosis-free predicted a 7-fold increased risk of thrombosis over cancer patients who were negative for tissue factor-bearing microparticles (OR 7.00, 95% CI 0.85-82.74, P=0.02). Elevated levels of tissue factor bearing microparticles were associated with a sensitivity of 0.80 and specificity of 0.78 for the development of VTE with a positive predictive value of 0.25 and negative predictive value of 0.97 for a population with disease site distribution that matches that of our study.
Figure 6.
Cumulative incidence of VTE for cancer patients initially without of VTE according to the presence of tissue factor-bearing microparticles. Tissue factor-bearing microparticle-positive (dashed line, N=16) and tissue factor-bearing microparticle-negative (solid line, N=44) cancer patients were assessed for radiographic evidence of thromboembolic disease. In the year following enrollment, thromboembolic disease only developed in a subset of patients who had detectable tissue factor-bearing microparticles. Median follow up was 8.9 months (range 1.4 to 27.9 months), and 75% of the patients were followed for 5 months or more. There were 11 deaths on record, at a median of 4 months after study entry (range 4 days to 23 months). Dashed line represents cancer patients with high levels of tissue factor bearing microparticles (TFMP+), and solid line represents those with undetectable levels (TFMP-).
Tissue factor-bearing microparticles in idiopathic VTE
A total of 22 patients with idiopathic venous thromboembolic disease without cancer were compared with the 30 cancer patients complicated by acute venous thromboembolic disease to determine whether the presence of tissue factor-bearing microparticles was a feature of venous thromboembolic disease. The median age in the idiopathic VTE group was 55.5 years, which was younger than 59.5 years in the cancer VTE group (P=0.01), and 54.5% subjects were female (12 of 22, P=0.47). Nine patients were diagnosed with a pulmonary embolism; this number did not differ significantly from that of the cancer VTE group (P=0.71). Tissue factor-bearing microparticles were detectable in 23% patients with acute idiopathic VTE (5 of 22), significantly fewer than the 60% (18 of 30) in the cancer patients with acute thromboembolic disease (P=0.01). The median number of tissue factor-bearing microparticles in the cancer VTE cohort (7.1 × 104 microparticles/μl) was significantly greater than both the idiopathic VTE and cancer-no VTE groups whose levels fell within the undetectable range (P=0.002 and P=0.03, respectively). These results confirm that the high prevalence of tissue factor-bearing microparticles in patients who present with an acute VTE is associated with cancer patients and not VTE alone.
Decrease in tissue factor-bearing microparticles following cancer resection
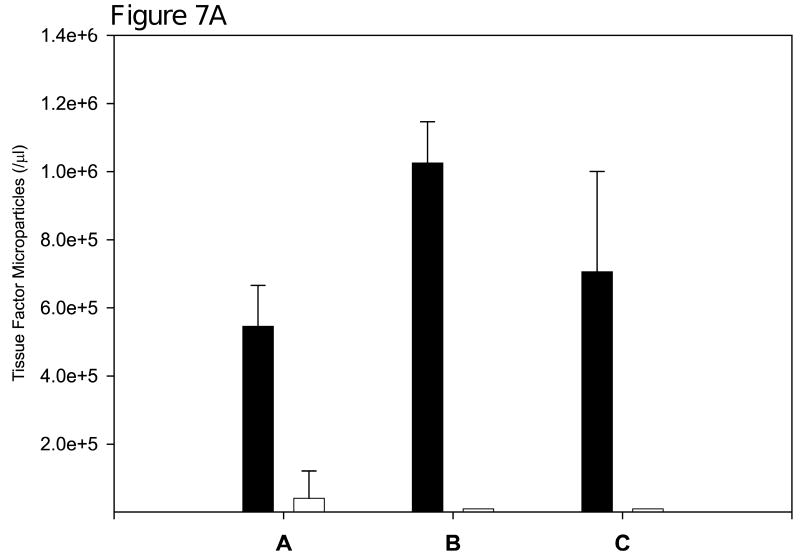
To examine whether the measured tissue factor-bearing microparticles are derived from the underlying malignancy, we measured tissue factor-bearing microparticle concentrations before and after definitive surgery in three patients with pancreatic carcinoma. In one patient, the preoperative concentration of tissue factor-bearing microparticles was 5.40 × 105/μl. Forty-four days following distal pancreatectomy, there was over a 10-fold reduction of these microparticles to 3.92 × 104/μl (Figure 7A). In a second patient, the preoperative concentration of tissue factor-bearing microparticles was 1.02 × 106/μl. These microparticles were undetectable 26 days postoperatively. In a third patient, the preoperative concentration of tissue factor-bearing microparticles was 7.06 × 105/μl, and similarly these microparticles were undetectable 27 days postoperatively. These results are consistent with the concept that the tissue factor-bearing microparticles in these patients are tumor-derived.
Figure 7.
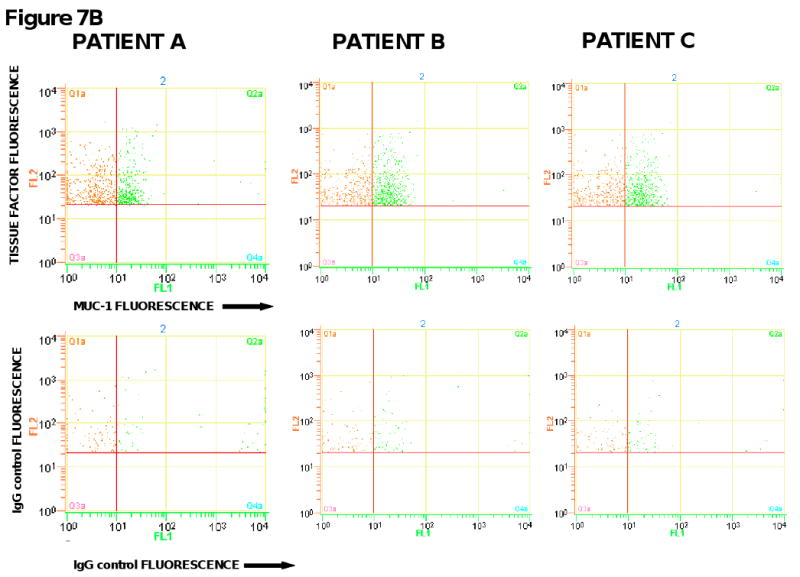
Concentration of tissue factor-bearing microparticles and MUC-1 expression in patients with pancreatic carcinoma undergoing surgery with curative intent. (6A) Patient A was evaluated on postoperative Day 44; Patient B was Day 26; Patient C was evaluated on postoperative Day 27. Pre, preoperative measurements (black); Post, postoperative measurements (white). (6B). The preoperative plasma samples from three pancreatic cancer patients were analyzed by flow cytometry for the expression of tissue factor and MUC-1 on microparticles. Samples were labeled with either anti-tissue factor and anti-MUC-1 antibodies or IgG isotype-matched controls. FL1 represents the fluorescence associated with anti-MUC1 antibody conjugated with Alexa 488 (x-axis) and FL2 represents the fluorescence associated with anti-tissue factor conjugated with phycoerythrin (y-axis).
To further explore this question, we examined the tissue factor-bearing microparticles from these patients with pancreatic cancer for the presence of the tumor marker, MUC-1. The MUC-1 antigen is a transmembrane glycoprotein overexpressed in epithelial malignancies such as pancreatic cancer but is not found on circulating hematopoietic cells (35, 36). The pre-operative samples were labeled with the anti-tissue factor antibody and a monoclonal antibody directed against the MUC-1 antigen. In the three patients tested, approximately 50% of the circulating tissue factor-bearing microparticles in these patients were also positive for the MUC-1 antigen (Figure 7B). These results support the premise that a population of microparticles is derived from the underlying malignancy. Whether the tissue factor-positive, MUC-1-negative microparticles are derived from the underlying tumor where the MUC-1 signal may be below the level of sensitivity or from hematopoietic cells has not been established.
Discussion
In this study, we have examined the hypothesis that the pathogenesis of thrombosis that complicates many forms of cancer is due to high levels of tissue factor associated with circulating tumor-derived microparticles. To test this hypothesis, we had four requirements: (1) to develop a method for reliably detecting, sizing and quantitating tissue factor antigen-bearing microparticles in platelet-poor plasma, without isolation and manipulation of microparticles; (2) to survey patients with various forms of cancer to approximate the prevalence of tissue factor-bearing microparticles in their plasma; (3) to design and execute a case-control study to determine whether the detection of tissue factor-bearing microparticles is more closely associated with cancer patients with acute thrombosis than with cancer patients without thrombosis; (4) to determine whether tissue factor-bearing microparticles associated with malignancy are tumor-derived.
In order to address the analytical issues of identifying and quantitating microparticles in platelet-poor plasma, we adapted a flow cytometric methodology capable of particle size measurements based upon impedance. Triggering on fluorescence, the diameter, in micrometers, of each fluorescent particle was determined. We demonstrated that this system was capable of reproducibly determining both the size and number of fluorescent microspheres to an accuracy of ± 5%. This method demonstrates marked improvement over light scattering-based flow cytometry, a non-optimal technique for estimating microparticle size and number.
In the current work, we have shown a significant association between the presence of tissue factor-bearing microparticles and acute cancer-associated thrombosis. In contrast, acute thromboembolic disease in the absence of malignant disease was not characterized by a similar increase in levels of tissue factor-bearing microparticles. The cancer patients who presented with an acute VTE were similar to a cancer-no VTE population for multiple factors including age, sex, cancer diagnosis, current cancer stage, diabetes, and smoking. However, the cancer patients with VTE tended to have lower platelet counts or hemoglobin and were less likely to be receiving chemotherapy. The basis for the differences in blood counts and active therapy between the cancer VTE and cancer no VTE groups is likely due to differences in timing of study enrollment. The acute VTE group was identified at any point during the treatment cycle whereas control patients were enrolled at outpatient visits and were more likely to have recovered from chemotherapy-associated cytopenias. As both higher platelet counts and chemotherapy are considered contributing risk factors for cancer-associated VTE (9, 37, 38), the risk of thrombosis attributed to tissue factor-bearing microparticles may in fact be greater than observed. In this case-control study, we established that tissue factor-bearing microparticles are four-fold more likely to be found in cancer patients with an acute thrombotic event than in cancer patients without an acute thrombotic event. These results extend previous observations that tissue factor-bearing microparticles can be found in the plasma of some patients with cancer, and demonstrate that tissue factor-bearing microparticles are a risk factor for the development of thrombosis.
To further explore the association of circulating tissue factor-bearing microparticles and VTE, we performed a review of all radiographic studies obtained following enrollment to assess for the development of proximal deep vein thrombosis or pulmonary emboli in cancer patients who had not incurred VTE at time of enrollment. Although this retrospective review was performed in a blinded manner, the patients were not systematically assessed for the development of VTE. However, the potential for systematic bias is low as this was a non-intervention trial and enrollment did not impact the subsequent management of these patients. Only those cancer patients with detectable tissue factor-bearing microparticles subsequently developed a thrombotic event in the year following enrollment. This analysis corroborated the association between tissue factor-bearing microparticles and cancer-associated thrombosis identified in the case-control study and suggests that these microparticles may be central to the pathogenesis of thromboembolic disorders associated with malignant disease. Due to the inherent limitations of small case control studies and retrospective cohort analysis, larger prospective studies with systematic monitoring for the development of VTE is required to confirm these findings.
Other groups have also observed that tissue factor-bearing microparticles can be measured in patients with advanced cancer (29, 30, 39). Tesselaar and colleagues demonstrated an increase in tissue factor activity in platelet-poor plasma from patients with cancer compared with platelet-poor plasma from healthy controls (29). Tissue factor activity associated with microparticles was measured in all seven metastatic cancer patients with a recent history of venous thromboembolic disease. However, the absolute number of tissue factor-bearing microparticles measured by flow cytometry was not significantly different in cancer patients compared with healthy controls (29). Tesselaar et al using light scattering-based flow cytometry reported a median of 460 tissue factor-bearing microparticles per μl of platelet-poor plasma in pancreatic cancer patients (range: 240-1550/μl)(29), or less than one tissue factor-bearing microparticle per leukocyte, whereas the tissue factor-bearing microparticle concentrations in our cancer patients varied from 70,000 per μl to 3,200,000 per μl. This is a discrepancy of from three to four orders of magnitude, and raises issues of the sensitivity for the identification of tissue factor-bearing microparticles by light scattering. Hron et al reported that the median number of tissue factor-bearing microparticles was significantly greater in twenty cancer patients with colorectal cancer compared with controls while the individuals' history of VTE was not specified (30). More recently, Khorana and colleagues observed that two patients with pancreatic cancer with increased levels of plasma tissue factor subsequently developed a venous thromboembolic event.(39)
We present two independent arguments that the tissue factor-bearing microparticles are largely derived from the tumor itself rather than from inflammatory cells. First, in three patients with pancreatic carcinoma undergoing pancreatectomy with curative intent, the microparticle concentration before surgery was reduced to very low or unmeasurable levels about a month after surgery. Second, in three patients with pancreatic carcinoma, we have demonstrated that about 50% of the tissue factor-bearing microparticles co-express MUC-1, a tumor marker for pancreatic carcinoma. Tumor cells express tissue factor (12), the expression of tissue factor activity on tumor cells correlates with thrombotic risk in pancreatic cancer patients (40), cancer cells in culture shed procoagulant vesicular structures (34), and circulating tumor-derived tissue factor-bearing microparticles can be detected in mice xenografted with human pancreatic tumors (41). These results provide compelling evidence that tissue factor-bearing microparticles are derived from certain tumors. Indeed, we suspect that the variability of microparticle generation from different tumors, as well as the total tumor burden, is the basis for the variability of thrombotic events in cancer-associated thrombosis. However, the presence of circulating tissue factor bearing microparticles is not unique to cancer patients as high levels were identified in a subset of non-cancer controls as well as individuals with an idiopathic VTE. The clinical significance of these elevations in non-cancer populations is not known.
Venous thromboembolic disease is a leading cause of death in patients with malignant disease (42) but recent randomized studies failed to demonstrate the benefit of prophylactic anticoagulation in cancer patients even in the presence of indwelling central catheters (43-45). The methodologies and conclusions of this work provide a rationale basis to determine whether the detection of tissue factor-bearing microparticles in cancer patients and their use as a biomarker predict an increased risk of a thromboembolic event. If so, it is critical to determine whether patients identified with this biomarker will benefit from primary thromboprophylaxis.
Acknowledgments
We thank Cynthia Kos, Koryn Johnson, and Thomas LaRocca for excellent technical assistance and Marie Mahony RN for clinical support. We also thank Michael Brochu Sr, Rick Thomas, Michael Brochu Jr, and Raquel Cabana at NPE Systems and Gus Alban at Beckman-Coulter for their collaboration in flow cytometer development for this application and Vasilis Toxavidis at the BIDMC Flow Core Facility. We thank Dr. Hing Wong at Altor Biosciences for the gift of humanized tissue factor antibody and Dr. Donald Kufe for the monoclonal antibody to MUC1. This work was supported by grants from the National Institutes of Health (JZ, BCF, BF).
Footnotes
Translational Relevance: There is a clear association between cancer and venous thromboembolic events but prognostic risk factors and underlying mechanisms of this association are poorly understood. Procoagulant vesicles expressing tissue factor, known as microparticles based on their submicron diameter, are known to propagate a fibrin clot in vivo and may be qualitatively or quantatively altered in various disease states. We describe the initial application of an impedance based flow cytometer to accurately characterize these microparticles and demonstrate that tumor-derived tissue factor bearing microparticles are associated with venous thromboembolic events in cancer patients. Based on our observations, a randomized clinical trial has been initiated to investigate the benefit of primary thromboprophylaxis in cancer patients with high levels of circulating tissue factor bearing microparticles.
References
- 1.Rickles FR, Edwards RL. Activation of blood coagulation in cancer: Trousseau's syndrome revisited. Blood. 1983;62:14–31. [PubMed] [Google Scholar]
- 2.Donati MB. Cancer and thrombosis: from Phlegmasia alba dolens to transgenic mice. Thromb Haemost. 1995;74:278–81. [PubMed] [Google Scholar]
- 3.Francis JL, Biggerstaff J, Amirkhosravi A. Hemostasis and malignancy. Semin Thromb Hemost. 1998;24:93–109. doi: 10.1055/s-2007-995829. [DOI] [PubMed] [Google Scholar]
- 4.Arkel YS. Thrombosis and cancer. Semin Oncol. 2000;27:362–74. [PubMed] [Google Scholar]
- 5.Rickles FR, Falanga A. Molecular basis for the relationship between thrombosis and cancer. Thromb Res. 2001;102:V215–24. doi: 10.1016/s0049-3848(01)00285-7. [DOI] [PubMed] [Google Scholar]
- 6.Rickles FR, Patierno S, Fernandez PM. Tissue factor, thrombin, and cancer. Chest. 2003;124:58S–68S. doi: 10.1378/chest.124.3_suppl.58s. [DOI] [PubMed] [Google Scholar]
- 7.Khorana AA, Fine RL. Pancreatic cancer and thromboembolic disease. Lancet Oncol. 2004;5:655–63. doi: 10.1016/S1470-2045(04)01606-7. [DOI] [PubMed] [Google Scholar]
- 8.Varki A. Trousseau's syndrome: multiple definitions and multiple mechanisms. Blood. 2007;110:1723–9. doi: 10.1182/blood-2006-10-053736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heit JA, Silverstein MD, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ., 3rd Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med. 2000;160:809–15. doi: 10.1001/archinte.160.6.809. [DOI] [PubMed] [Google Scholar]
- 10.Blom JW, Vanderschoot JP, Oostindier MJ, Osanto S, van der Meer FJ, Rosendaal FR. Incidence of venous thrombosis in a large cohort of 66,329 cancer patients: results of a record linkage study. J Thromb Haemost. 2006;4:529–35. doi: 10.1111/j.1538-7836.2006.01804.x. [DOI] [PubMed] [Google Scholar]
- 11.Levitan N, Dowlati A, Remick SC, et al. Rates of initial and recurrent thromboembolic disease among patients with malignancy versus those without malignancy. Risk analysis using Medicare claims data. Medicine (Baltimore) 1999;78:285–91. doi: 10.1097/00005792-199909000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Callander NS, Varki N, Rao LV. Immunohistochemical identification of tissue factor in solid tumors. Cancer. 1992;70:1194–201. doi: 10.1002/1097-0142(19920901)70:5<1194::aid-cncr2820700528>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 13.Gordon SG, Cross BA. A factor X-activating cysteine protease from malignant tissue. J Clin Invest. 1981;67:1665–71. doi: 10.1172/JCI110203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boccaccio C, Sabatino G, Medico E, et al. The MET oncogene drives a genetic programme linking cancer to haemostasis. Nature. 2005;434:396–400. doi: 10.1038/nature03357. [DOI] [PubMed] [Google Scholar]
- 15.Sun NC, McAfee WM, Hum GJ, Weiner JM. Hemostatic abnormalities in malignancy, a prospective study of one hundred eight patients. Part I. Coagulation studies. Am J Clin Pathol. 1979;71:10–6. doi: 10.1093/ajcp/71.1.10. [DOI] [PubMed] [Google Scholar]
- 16.Nand S, Fisher SG, Salgia R, Fisher RI. Hemostatic abnormalities in untreated cancer: incidence and correlation with thrombotic and hemorrhagic complications. J Clin Oncol. 1987;5:1998–2003. doi: 10.1200/JCO.1987.5.12.1998. [DOI] [PubMed] [Google Scholar]
- 17.Buccheri G, Ferrigno D, Ginardi C, Zuliani C. Haemostatic abnormalities in lung cancer: prognostic implications. Eur J Cancer. 1997;33:50–5. doi: 10.1016/s0959-8049(96)00310-3. [DOI] [PubMed] [Google Scholar]
- 18.Johnson MJ, Walker ID, Sproule MW, Conkie J. Abnormal coagulation and deep venous thrombosis in patients with advanced cancer. Clin Lab Haematol. 1999;21:51–4. doi: 10.1046/j.1365-2257.1999.00163.x. [DOI] [PubMed] [Google Scholar]
- 19.Sallah S, Wan JY, Nguyen NP, Hanrahan LR, Sigounas G. Disseminated intravascular coagulation in solid tumors: clinical and pathologic study. Thromb Haemost. 2001;86:828–33. [PubMed] [Google Scholar]
- 20.Sallah S, Husain A, Sigounas V, et al. Plasma coagulation markers in patients with solid tumors and venous thromboembolic disease receiving oral anticoagulation therapy. Clin Cancer Res. 2004;10:7238–43. doi: 10.1158/1078-0432.CCR-04-0445. [DOI] [PubMed] [Google Scholar]
- 21.Miller GJ, Bauer KA, Howarth DJ, Cooper JA, Humphries SE, Rosenberg RD. Increased incidence of neoplasia of the digestive tract in men with persistent activation of the coagulant pathway. J Thromb Haemost. 2004;2:2107–14. doi: 10.1111/j.1538-7836.2004.01011.x. [DOI] [PubMed] [Google Scholar]
- 22.Furie B, Furie BC. Mechanisms of thrombus formation. N Engl J Med. 2008;359:938–49. doi: 10.1056/NEJMra0801082. [DOI] [PubMed] [Google Scholar]
- 23.Giesen PL, Rauch U, Bohrmann B, et al. Blood-borne tissue factor: another view of thrombosis. Proc Natl Acad Sci U S A. 1999;96:2311–5. doi: 10.1073/pnas.96.5.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Falati S, Liu Q, Gross P, et al. Accumulation of Tissue Factor into Developing Thrombi In Vivo Is Dependent upon Microparticle P-Selectin Glycoprotein Ligand 1 and Platelet P-Selectin. J Exp Med. 2003;197:1585–98. doi: 10.1084/jem.20021868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jimenez JJ, Jy W, Mauro LM, Horstman LL, Ahn YS. Elevated endothelial microparticles in thrombotic thrombocytopenic purpura: findings from brain and renal microvascular cell culture and patients with active disease. Br J Haematol. 2001;112:81–90. doi: 10.1046/j.1365-2141.2001.02516.x. [DOI] [PubMed] [Google Scholar]
- 26.Shet AS, Aras O, Gupta K, et al. Sickle blood contains tissue factor positive microparticles derived from endothelial cells and monocytes. Blood. 2003 doi: 10.1182/blood-2003-03-0693. [DOI] [PubMed] [Google Scholar]
- 27.Combes V, Simon AC, Grau GE, et al. In vitro generation of endothelial microparticles and possible prothrombotic activity in patients with lupus anticoagulant. J Clin Invest. 1999;104:93–102. doi: 10.1172/JCI4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chironi G, Simon A, Hugel B, et al. Circulating leukocyte-derived microparticles predict subclinical atherosclerosis burden in asymptomatic subjects. Arterioscler Thromb Vasc Biol. 2006;26:2775–80. doi: 10.1161/01.ATV.0000249639.36915.04. [DOI] [PubMed] [Google Scholar]
- 29.Tesselaar ME, Romijn FP, Van Der Linden IK, Prins FA, Bertina RM, Osanto S. Microparticle-associated tissue factor activity: a link between cancer and thrombosis? J Thromb Haemost. 2007;5:520–7. doi: 10.1111/j.1538-7836.2007.02369.x. [DOI] [PubMed] [Google Scholar]
- 30.Hron G, Kollars M, Weber H, et al. Tissue factor-positive microparticles: cellular origin and association with coagulation activation in patients with colorectal cancer. Thromb Haemost. 2007;97:119–23. [PubMed] [Google Scholar]
- 31.Zwicker JI, Furie BC, Kos C, LaRocca T, Bauer KA, Furie B. Trousseau's syndrome revisited: tissue factor-bearing microparticles in pancreatic cancer. Blood. 2005:106. Abstract 259. [Google Scholar]
- 32.Hercher M, Mueller W, Shapiro HM. Detection and discrimination of individual viruses by flow cytometry. J Histochem Cytochem. 1979;27:350–2. doi: 10.1177/27.1.374599. [DOI] [PubMed] [Google Scholar]
- 33.Shapiro HM. Practical Flow Cytometry. 4th. New York: Wiley-Liss; 2003. [Google Scholar]
- 34.Dvorak HF, Quay SC, Orenstein NS, et al. Tumor shedding and coagulation. Science. 1981;212:923–4. doi: 10.1126/science.7195067. [DOI] [PubMed] [Google Scholar]
- 35.Ho SB, Niehans GA, Lyftogt C, et al. Heterogeneity of mucin gene expression in normal and neoplastic tissues. Cancer Res. 1993;53:641–51. [PubMed] [Google Scholar]
- 36.Levi E, Klimstra DS, Andea A, Basturk O, Adsay NV. MUC1 and MUC2 in pancreatic neoplasia. J Clin Pathol. 2004;57:456–62. doi: 10.1136/jcp.2003.013292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Frequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patients. Cancer. 2007;110:2339–46. doi: 10.1002/cncr.23062. [DOI] [PubMed] [Google Scholar]
- 38.Khorana AA, Francis CW, Culakova E, Lyman GH. Risk factors for chemotherapy-associated venous thromboembolism in a prospective observational study. Cancer. 2005;104:2822–9. doi: 10.1002/cncr.21496. [DOI] [PubMed] [Google Scholar]
- 39.Khorana AA, Francis CW, Menzies KE, et al. Plasma tissue factor may be predictive of venous thromboembolism in pancreatic cancer. J Thromb Haemost. 2008;6:1983–5. doi: 10.1111/j.1538-7836.2008.03156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khorana AA, Ahrendt SA, Ryan CK, et al. Tissue factor expression, angiogenesis, and thrombosis in pancreatic cancer. Clin Cancer Res. 2007;13:2870–5. doi: 10.1158/1078-0432.CCR-06-2351. [DOI] [PubMed] [Google Scholar]
- 41.Davila M, Amirkhosravi A, Coll E, et al. Tissue factor-bearing microparticles derived from tumor cells: impact on coagulation activation. J Thromb Haemost. 2008;6:1517–24. doi: 10.1111/j.1538-7836.2008.02987.x. [DOI] [PubMed] [Google Scholar]
- 42.Donati MB. Cancer and thrombosis. Haemostasis. 1994;24:128–31. doi: 10.1159/000217092. [DOI] [PubMed] [Google Scholar]
- 43.Couban S, Goodyear M, Burnell M, et al. Randomized placebo-controlled study of low-dose warfarin for the prevention of central venous catheter-associated thrombosis in patients with cancer. J Clin Oncol. 2005;23:4063–9. doi: 10.1200/JCO.2005.10.192. [DOI] [PubMed] [Google Scholar]
- 44.Karthaus M, Kretzschmar A, Kroning H, et al. Dalteparin for prevention of catheter-related complications in cancer patients with central venous catheters: final results of a double-blind, placebo-controlled phase III trial. Ann Oncol. 2006;17:289–96. doi: 10.1093/annonc/mdj059. [DOI] [PubMed] [Google Scholar]
- 45.Verso M, Agnelli G, Bertoglio S, et al. Enoxaparin for the prevention of venous thromboembolism associated with central vein catheter: a double-blind, placebo-controlled, randomized study in cancer patients. J Clin Oncol. 2005;23:4057–62. doi: 10.1200/JCO.2005.06.084. [DOI] [PubMed] [Google Scholar]