Abstract
4-vinylcyclohexene diepoxide (VCD) is an ovotoxicant that specifically destroys primordial and small primary follicles in the ovaries of mice and rats. In contrast, 7,12-dimethylbenz[a]anthracene (DMBA) is ovotoxic to all ovarian follicle classes. This study investigated phosphatidylinositol-3 kinase signaling involvement in VCD- and DMBA-induced ovotoxicity. Postnatal day (PND) 4 Fischer 344 (F344) rat whole ovaries were cultured for 2–12d in vehicle control, VCD (30 μM), or DMBA (1 μM), ± PI3 kinase inhibitor LY294002 (20 μM) or its inactive analog LY303511 (20 μM). Following culture, ovaries were histologically evaluated, and healthy follicles were classified and counted. PI3 kinase inhibition had no effect on primordial follicle number, but reduced (P < 0.05) small primary and larger follicles beginning on d4. VCD caused primordial and small primary follicle loss (P < 0.05) beginning on d6. With PI3 kinase inhibition, VCD did not affect primordial follicles (P > 0.05) at any time, but did cause loss (P < 0.05) of small primary follicles. DMBA exposure caused primordial and small primary follicle loss (P < 0.05) on d6. Further, DMBA-induced primordial and small primary follicle loss was greater with PI3 kinase inhibition (P < 0.05) than with DMBA alone. These results support that 1) PI3 kinase mediates primordial to small primary follicle recruitment, 2) VCD, but not DMBA, enhances ovotoxicity by increasing primordial to small primary follicle recruitment, and 3) in addition to xenobiotic-induced ovotoxicity, VCD is also a useful model chemical with which to elucidate signaling mechanisms involved in primordial follicle recruitment.
Keywords: Ovary, 4-vinylcyclohexene diepoxide, ovotoxicity, phosphatidylinositol-3 kinase, follicle activation
Introduction
The ovary is a heterogeneous organ composed of follicles at various stages of growth. At birth the ovary contains a finite number of primordial follicles (Hirshfield, 1991), which can be recruited to grow and mature toward ovulation. Following fetal development, primordial follicles cannot be regenerated, thus, chemical-induced depletion of this follicle pool can lead to premature ovarian failure. 4-vinylcyclohexene diepoxide (VCD) is an occupational chemical that is used as an industrial diluent for epoxides (IARC, 1976). VCD has been shown to selectively destroy small pre-antral follicles (primordial and primary; Springer et al., 1996a; 1996b; Kao et al., 1999; Smith et al., 1990) in mice and rats via acceleration of atresia (apoptosis; Springer et al., 1996a; 1996b; Hu et al., 2001a; 2001b; 2002).
Studies investigating mechanisms involved in VCD-induced ovotoxicity in Fischer 344 (F344) rats in vivo have demonstrated increased activity of pro-apoptotic members of the BCL-2 (Hu et al., 2001b; Springer et al., 1996b) and MAPK families (Hu et al., 2002). However, those effects were observed at late timepoints during VCD-induced follicle loss (days 10 and 15 of dosing) and may reflect downstream responses once ovotoxicity is underway rather than an initiating effect. Additionally, following in vitro exposure of neonatal rat ovaries (highly enriched in primordial and small primary follicles), VCD decreased the expression of mRNA encoding the pro-survival gene Kit, and increased expression of mRNA encoding its ligand, Kit Ligand (Kitl). A time course of VCD exposure demonstrated that the decrease in Kit mRNA (d4) preceded the increase in mRNA encoding Kitl (d6). Exogenous growth and differentiation factor 9 (GDF9) or bone morphogenic protein 4 (BMP4) had no effect on VCD-induced ovotoxicity, whereas KITL addition attenuated VCD-induced ovotoxicity (Fernandez et al., 2008). Thus, KIT is a possible signaling molecule through which VCD-induced ovotoxicity is initiated.
KIT is a receptor protein tyrosine kinase (RPTK) expressed by the oocyte (Horie et al., 1991; Manova et al., 1990; Orr-Utreger et al., 1990). The ligand for KIT, KITL (also known as Stem Cell factor, Steel Factor) is expressed by granulosa cells (Ismail et al., 1996). Once KIT is activated by KITL, signaling cascades such as the phosphatidyl inositol-3 (PI3) kinase pathway are activated (Castrillon et al., 2003; Reddy et al., 2005; John et al., 2008; Liu et al., 2006; Reddy et al., 2008). It is thought that KITL/KIT signaling in small pre-antral follicles is essential for oocyte viability and survival in a developmental stage when functional follicle stimulating hormone (FSH) receptors are not yet expressed by the ovary (Parrott and Skinner, 1999; Yoshida et al., 1997). Additionally, PI3 kinase signaling molecules have been demonstrated to play critical roles in primordial to small primary follicle activation/recruitment (Yoshida et al., 1997; Kissel et al., 2000; Castrillon et al., 2003; Reddy et al., 2005; Liu et al., 2006; John et al., 2008; Reddy et al., 2008).
Because expression of KIT, an activator of the PI3 kinase signaling pathway, is decreased by VCD exposure and exogenous KITL can attenuate VCD-induced follicle loss, it was of interest to investigate a role of PI3 kinase in VCD-induced ovotoxicity. Additionally, to investigate any common mechanism between ovotoxic chemicals on follicle recruitment and activation, a comparison was also made of the role of PI3 kinase signaling in 7,12-dimethylbenz[a]anthracene (DMBA)-induced ovotoxicity. DMBA is a polycyclic aromatic hydrocarbon that targets and destroys all follicle types in ovaries of rats and mice (Mattison and Schulman, 1980). DMBA can be bioactivated in the ovary to the ultimate ovotoxic form, DMBA-3,4-diol, 1,2-epoxide (Rajapaksa et al., 2007; Igawa et al., 2009). A comparison of ovotoxicity induced by VCD and DMBA has demonstrated that DMBA is approximately 20 times more potent than VCD (Borman et al., 2000). This is thought to be due, in part, to the ability of ovarian microsomal epoxide hydrolase (EPHX1) to metabolize VCD to an inactive tetrol metabolite but to bioactivate DMBA to a more ovotoxic metabolite (Rajapaksa et al., 2007; Igawa et al., 2009). Therefore, the present study was designed to investigate the effect of inhibition of PI3 kinase signaling on both VCD- and DMBA-induced ovotoxicity in PND4 F344 rat ovaries using an in vitro culture system.
Materials and Methods
Reagents
4-vinylcyclohexene diepoxide(VCD; CAS # 106-87-6; >99% purity), 7,12-dimethylbenz[a]anthracene (DMBA; CAS # 57-97-6; 95% purity), bovine serum albumin (BSA), ascorbic acid (Vitamin C), and transferrin were purchased from Sigma-Aldrich Inc. (St Louis, MO). Dulbecco’s Modified Eagle Medium: nutrient mixture F-12 (Ham) 1X (DMEM/Ham’s F12), Albumax, penicillin/streptomycin (5000U/ml, 5000μg/ml, respectively), Hanks’ Balanced Salt Solution (without CaCl2, MgCl2, or MgSO4) were obtained from Invitrogen Co. (Carlsbad, CA). Millicell-CM filter inserts were purchased from Millipore (Bedford, MA), and 48 well cell culture plates were obtained from Corning Inc. (Corning, NY). 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002; CAS # 154447-36-6) was purchased from A.G. Scientific, Inc. (San Diego, CA). 2-piperazinyl-8-phenyl-4H-1-benzopyran-4-one (LY303511: CAS # 154447-38-8) was purchased from EMD Chemicals (Gibbstown, NJ). Anti-PI3 kinase antibody was purchased from Upstate Biotechnology (Lake Placid, NY). Goat anti-rabbit secondary antibody was purchased from Pierce Biotechnology (Rockford, IL). Cy-5-streptavidin was obtained from Vector (Burlingame, CA). YOYO-1 was purchased from Molecular Probes (Eugene, OR).
Animals
A breedingcolony was established from Fischer 344 rats that were originally purchased from Harlan Laboratories (Indianapolis, IN) to use as a source of PND4 female rat pup ovaries for culture. All pregnant animals were housed singly in plastic cages, and maintained in a controlled environment (22 ± 2°C; 12h light/12h dark cycles). The animals were provided a standard diet with ad libidum access to food and water, and allowed to give birth. All animal experiments were approved by the University of Arizona’s Institutional Animal Care and Use Committee.
In vitro ovarian culture
Ovaries from PND4 F344 rats were cultured as described by Devine et al. (2002). Briefly, PND4 female F344 rats were euthanized by CO2 inhalation followed by decapitation. Each ovary was removed, the oviduct and excess tissue were trimmed, and it was placed on a piece of Millicell-CM membrane floating on 250 μl of DMEM/Ham’s F12 medium containing 1 mg/ml BSA, 1 mg/ml Albumax, 50 μg/ml ascorbic acid, 5 U/ml penicillin/5 μg/ml streptomycin, and 27.5 μg/ml transferrin per well in a 48 well plate previously equilibrated to 37°C. Using fine forceps, a drop of medium was placed to cover the top of the ovary to prevent drying. Plates containing ovaries were cultured at 37°C and 5% CO2 in air. For those cultures lasting more than 2d, media were removed and fresh media and treatment were replaced every 2d. Ovaries were treated with vehicle control medium (1% DMSO), VCD (30 μM) or DMBA (1 μM), ± LY294002 (20 μM) or ± LY303511 (20 μM) for variable times as described in individual figure legends. The concentrations and times of VCD and DMBA exposures used were previously determined to cause approximately 50% primordial and small primary follicle loss (Devine et al., 2002; Igawa et al., 2009). Note: a preliminary dose finding experiment determined that LY294002 at 20 μM was optimal for not inducing toxic effects in PND4 cultured ovaries.
Histological evaluation of follicle numbers
Following incubation, ovaries were placed in Bouin’s fixative for 1.5h, transferred to 70% ethanol, embedded in paraffin, serially sectioned (5 μM thick), and every 6th section was mounted. All ovarian sections were stained with hematoxylin and eosin. Healthy oocyte-containing follicles were classified and counted in every 12th section. Unhealthy follicles were distinguished from healthy follicles by their granulosa cell content of pyknotic bodies and intense eosinophilic staining of oocytes (Devine et al., 2002). Follicle population classification was according to the procedure of Flaws et al. (1994). Briefly, primordial follicles contained the oocyte surrounded by a single layer of squamous-shaped granulosa cells, primary follicles contained the oocyte surrounded by a single layer of cuboidal-shaped granulosa cells, and secondary follicles contained the oocyte surrounded by multiple layers of granulosa cells. In cultured neonatal rat ovaries, no follicle development beyond the secondary stage was observed (Flaws et al., 1994). Histological images were captured with an Olympus IX-70 inverted microscope.
Immunofluorescence staining and confocal microscopy
Following in vitro culture, ovaries treated with vehicle control were fixed in 4% buffered formalin for 2 h, transferred to 70% ethanol, embedded in paraffin, serially sectioned, and every 10th section was mounted. Sections were deparaffinized (approximately 10 sections/ovary) and incubated with primary antibody directed against PI3 kinase (1:200 dilution) 4°C overnight. Specificity for this antibody was determined by Western blotting (data not shown). Secondary biotinylated antibody was applied for 1 h, followed by CY-5-streptavidin (1 h; 1:100 dilution). Sections were treated with Ribonuclease A (100μg/ml) for 1 h, followed by staining with YOYO-1 (10 min; 5nM). Slides were repeatedly rinsed with phosphate buffer saline (PBS), cover-slipped, and stored in the dark (4°C) until visualization. Primary antibody was not added to immuno-negative ovarian sections. Immunofluorescence was visualized on a Zeiss (LSM 510 NLO-Meta) confocal microscope with an argon and helium-neon laser projected through the tissue into a photomultiplier at λ = 488 and 633 nm for YOYO-1 (green) and CY-5 (red), respectively. All images were captured using a 40 X objective lens. Multiple readings were taken throughout the sections.
Statistical analysis
Comparisons were made between treatments using Statview software analysis of variance (ANOVA) and Fisher’s protected least significant difference (PLSD) multiple range test. Statistical analysis comparing follicle loss between primordial and small primary follicles was carried out by converting values to a percentage of the control mean for each timepoint. The assigned level of significance for all tests was P < 0.05, with P < 0.1 considered as a trend for a difference.
Results
Time-course of VCD induced ovotoxicity in Fischer 344 rat ovaries
A previous study (Devine et al., 2002) demonstrated that VCD (30 μM) did not cause primordial and small primary follicle loss in neonatal rat ovaries cultured for 4d, but caused loss after 8d. In order to determine if VCD-induced follicle loss could be observed earlier than d8, PND4 rat ovaries were cultured in VCD (30 μM) for 6d. Relative to control-treated ovaries, significant loss (P < 0.05) of primordial and small primary follicles occurred after 6d of VCD exposure (Figure 1). Additionally, relative to small primary follicle loss, there was greater (P < 0.05) loss of primordial follicles by d15.
Figure 1. Time-course of VCD-induced ovotoxicity.
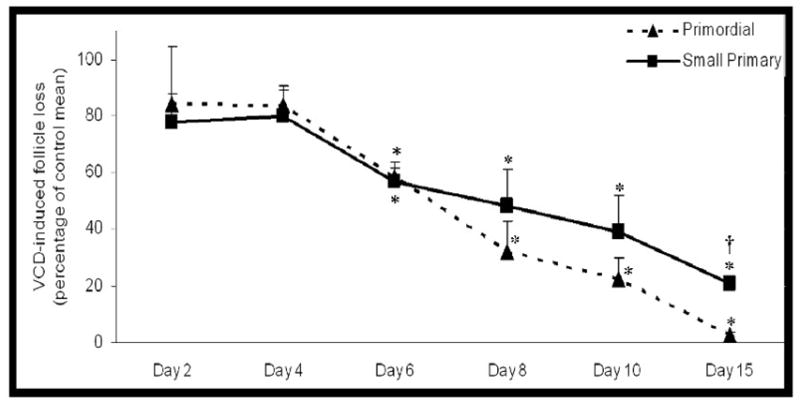
Ovaries from PND4 F344 rats were cultured with control medium or medium containing VCD (30 μM). Following incubation, ovaries were collected and processed for histological evaluation as described in materials and methods. Primordial (broken line) and small primary (solid line) follicles were classified and counted. Follicle numbers were expressed as percentage follicles remaining relative to the respective controls ± SE; n = 3–5 ovaries per timepoint. * P < 0.05, Different from control. †P < 0.05, Different from percentage primordial follicle loss. d2, 4, 8, 10, 15; Adapted from Devine et al., (2002).
Localization of PI3 kinase protein in neonatal rat ovaries in vitro
In order to determine localization of PI3 kinase protein expression in neonatal ovaries, PND4 F344 rat ovaries were cultured in control medium for 8 days. This timepoint was chosen to confirm PI3 kinase protein expression at the timepoint for the experiment presented in Figure 4. Immunofluorescence staining with confocal microscopy using an anti-PI3 kinase primary antibody determined that PI3 kinase protein is highly concentrated in the oocyte cytoplasm of primordial and small primary follicles (Figure 2).
Figure 4. Effect of PI3 kinase inhibition on VCD-induced ovotoxicity.
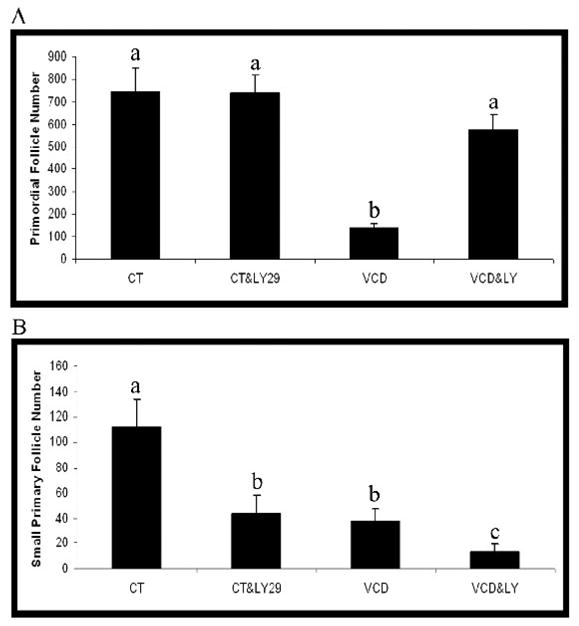
Ovaries from PND4 F344 rats were cultured with vehicle control medium (CT) or medium containing VCD (30 μM) in the presence or absence of LY294002 (LY29; 20 μM) for 8d. Following incubation, ovaries were collected and processed for histological evaluation as described in materials and methods. (A) Primordial and (B) small primary follicles were classified and counted. Values are mean ± SE total follicles counted/ovary, n=3; Different letters differ from one another within each group; P < 0.05.
Figure 2. Localization of PI3 kinase protein in vitro.
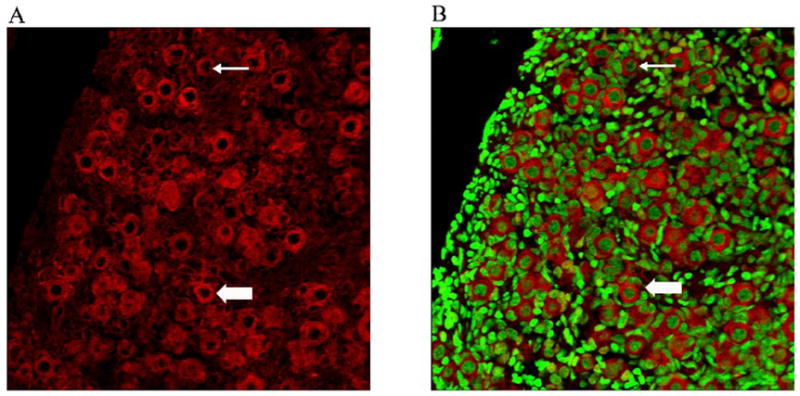
PND4 Fischer 344 rat ovaries were cultured with control medium for 8 days and processed for confocal microscopy as described in materials and methods. (A) PI3 kinase (Cy-5 red stain) and (B) genomic DNA (green YOYO1 stain) overlay at 40X magnification. Thin arrow = primordial follicle; Block arrow = Small Primary follicle; Scale-bar equal to 25μM
Effect of PI3 kinase inhibition on temporal pattern of follicle recruitment
PND4 F344 rat ovaries were cultured in control medium or medium containing a competitive inhibitor of PI3 kinase, LY294002 (20 μM), over a time course from d2 to d8 (Figure 3). Relative to control medium, there was no effect (P > 0.05) of LY294002 on primordial follicle number at any timepoint. However, incubation with LY294002 caused a reduction (P < 0.05) in small primary follicle number on d4–d8. In addition, there was a reduction (P < 0.05) in large follicles (large primary and secondary) on d4 and d6, with a non-significant trend (P = 0.12) for a reduction on d8.
Figure 3. Effect of PI3 kinase inhibition over a time course of exposure.
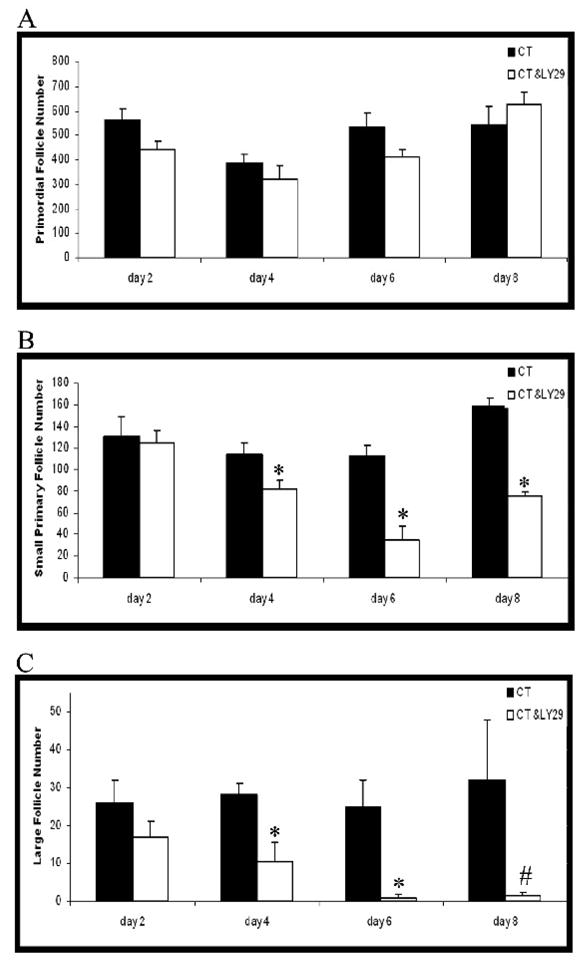
Ovaries from PND4 F344 rats were cultured with vehicle control medium (CT) or medium containing LY294002 (LY29; 20 μM) for 2–8d. Following incubation, ovaries were collected and processed for histological evaluation as described in materials and methods. (A) Primordial, (B) small primary and (C) large follicles (large primary + secondary) were classified and counted. Values are mean ± SE total follicles counted/ovary, n=3–5; Different from control; *P < 0.05; #P = 0.12.
Effect of PI3 kinase inhibition on VCD-induced ovotoxicity in Fischer 344 rat ovaries
To evaluate the effect of continuous PI3 kinase inhibition on VCD-induced ovotoxicity, PND4 rat ovaries were cultured for 8d in control medium or medium containing VCD (30 μM) in the absence or presence of LY294002 (20 μM; Figure 4). Relative to control, VCD caused a loss (P < 0.05) of primordial and small primary follicles. With VCD in the presence of LY294002, relative to VCD alone, there were increased (P < 0.05) primordial follicle numbers, whereas, small primary follicle numbers were reduced (P < 0.05) even further. VCD did not affect large primary or secondary follicle numbers and, furthermore, caused no added loss of large primary or secondary follicles in ovaries incubated with LY294002 (data not shown).
To investigate the effect of PI3 kinase inhibition on VCD-induced primordial follicle loss after the onset of VCD-induced ovotoxicity, ovaries were cultured in control medium for 6 days; control medium for 6 days followed by control medium ± LY294002 for an additional 6 days (d6–d12); VCD for 6 days; VCD for 12 days; or VCD for 12 days + LY294002 (d6–12). Ovaries treated with control medium for 6 or 12 days contained similar numbers of follicles (primordial follicle number – 661 ± 23 and 759 ± 50; small primary follicles – 156 ± 4 and 168 ± 11.5, on day 6 and 12, respectively; P > 0.05). Relative to the ovaries treated with control medium for 6 or 12 days, there was no difference in primordial follicle number (705 ± 45; P > 0.05), but small primary follicle numbers were reduced (85 ± 18; P < 0.05) when LY294002 was included from day 6–12 of culture. Relative to ovaries treated for 6d with VCD, the numbers of primordial and small primary follicles were further reduced (P < 0.05) by VCD when exposed for an additional 6d regardless of the inclusion of LY294002 (Figure 5). However, when LY294002 was added to the medium (d6–12), VCD-induced loss of primordial follicles was retarded (Figure 5A; P < 0.05), whereas, loss of small primary follicles was greater (Figure 5B; P < 0.05), relative to the effect of continuous exposure to VCD for 12d.
Figure 5. Effect of PI3 kinase inhibition after onset of VCD-induced ovotoxicity.
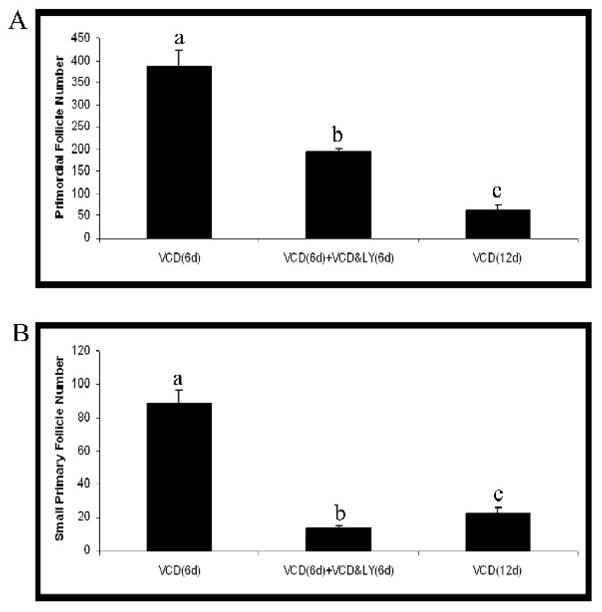
Ovaries from PND4 F344 rats were cultured with medium containing VCD (30 μM) for 6d. After the initial 6d of treatment, ovaries were removed from culture, or treated with VCD (30 μM) ± LY294002 (LY29; 20 μM) for an additional 6d. Following incubation, ovaries were collected and processed for histological evaluation as described in materials and methods. (A) Primordial and (B) small primary follicles were classified and counted. Values are mean ± SE total follicles counted/ovary, n=5; Different letters differ from one another within each group; P < 0.05.
Effect of negative control for LY294002, LY303511, on VCD-induced ovotoxicity
In order to confirm that PI3 kinase is a signaling protein targeted by VCD, a negative control for LY294002 was used. LY303511 is a compound with a single substitution of the oxygen with an amine in the morpholine ring of LY294002. It does not inhibit PI3 kinase activity and so acts as a negative control for the PI3 kinase inhibitory activity of LY294002 (Vlahos et al., 1994). PND4 ovaries were cultured for 8 days in control medium or medium containing VCD (30 μM) in the presence or absence of LY303511 (20 μM; Figure 6). Incubation with LY303511 alone caused a reduction (P < 0.05) in primordial and small primary follicle numbers. On the other hand, whereas VCD alone depleted (P < 0.05) primordial and small primary follicle numbers, this depletion was not prevented by co-incubation with LY303511 (Figure 6).
Figure 6. Effect of negative control for PI3 kinase inhibition on VCD-induced ovotoxicity.
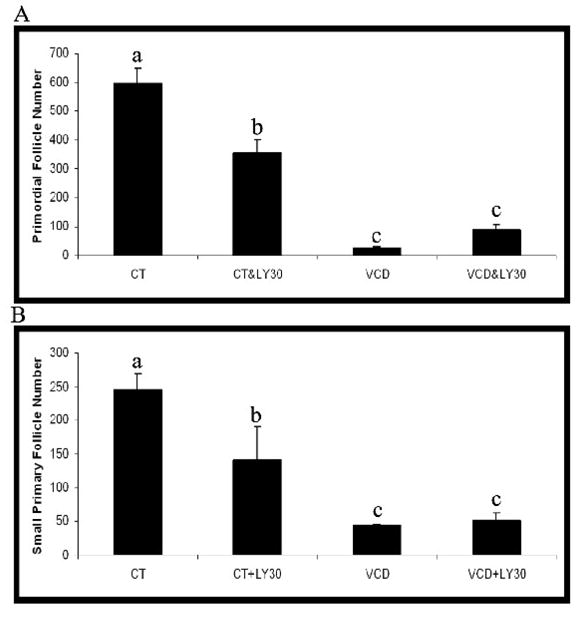
Ovaries from PND4 F344 rats were cultured with vehicle control medium (CT) or medium containing VCD (30 μM) in the presence or absence of LY303511 (LY30; 20 μM) for 8d. Following incubation, ovaries were collected and processed for histological evaluation as described in materials and methods. (A) Primordial and (B) small primary follicles were classified and counted. Values are mean ± SE total follicles counted/ovary, n=4–5; Different letters differ from one another within each group; P < 0.05.
Effect of PI3 kinase inhibition on DMBA-induced ovotoxicity in Fischer 344 rat ovaries
To determine if an interaction with follicle recruitment is a common mechanism between ovotoxic chemicals, PND4 F344 rat ovaries were cultured for 6d in the presence of vehicle control (1% DMSO) or DMBA (1 μM) in the presence or absence of LY294002 (20 μM; Figure 7). Incubation of ovaries with DMBA resulted in loss of both primordial and small primary follicles (P < 0.05). Furthermore, DMBA-induced loss of primordial and small primary follicles was greater (P < 0.05) when ovaries were incubated in the presence of LY294002 than with DMBA alone.
Figure 7. Effect of PI3 kinase inhibition on DMBA-induced ovotoxicity.
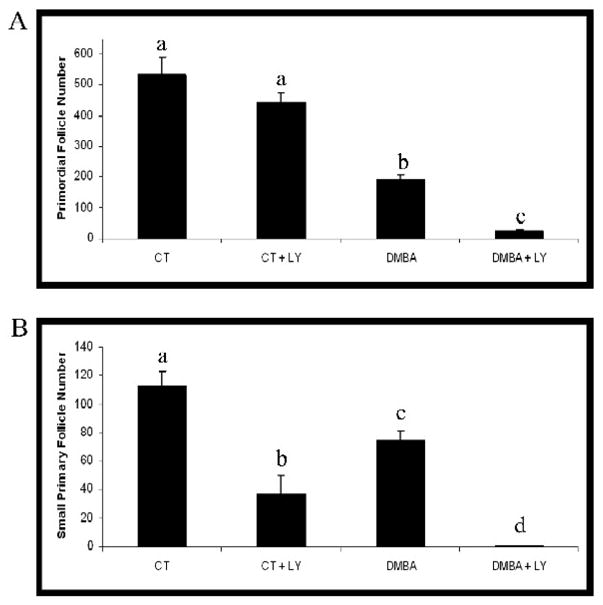
Ovaries from PND4 F344 rats were cultured with vehicle control medium (CT) or medium containing DMBA (1 μM) in the presence or absence of LY294002 (LY29; 20 μM) for 6d. Following incubation, ovaries were collected and processed for histological evaluation as described in materials and methods. (A) Primordial and (B) small primary follicles were classified and counted. Values are mean ± SE total follicles counted/ovary, n=4–5; Different letters differ from one another within each group; P < 0.05.
Ovarian Morphology following exposure to VCD or DMBA ± LY294002
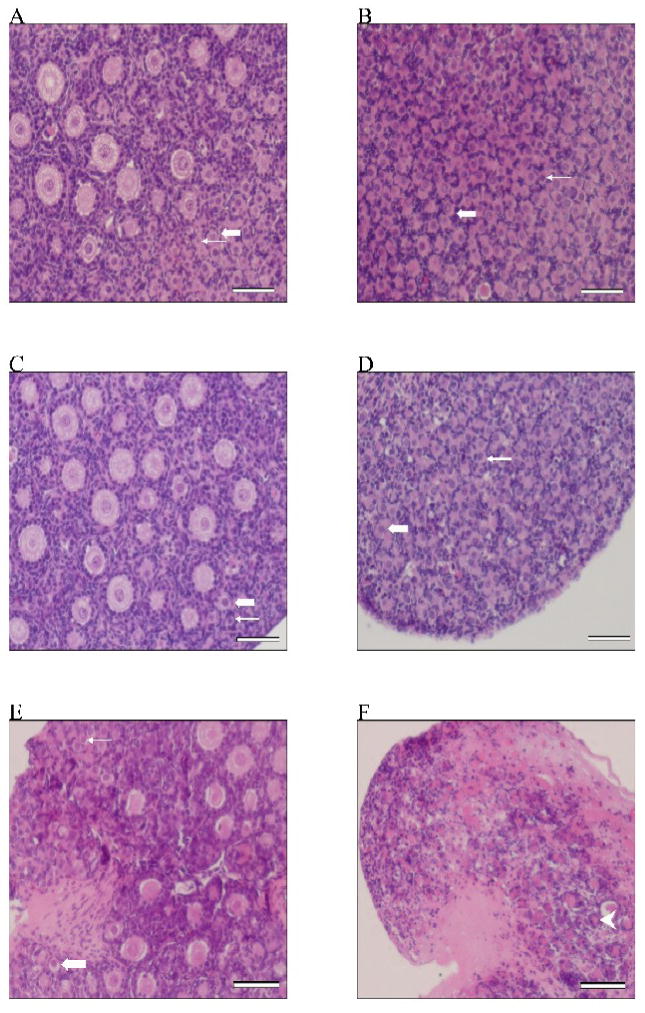
Incubation of ovaries with LY294002 resulted in dramatic VCD- and DMBA-induced morphological alterations. As expected, reduced numbers of large primary or secondary follicles were observed in ovaries treated with LY294002 alone (Figure 8B). Whereas, LY294002 prevented VCD-induced loss of primordial follicles, it did not prevent DMBA-induced primordial follicle loss (Figure 8C–F). Furthermore, in the presence of LY294002 (Figure 8F), DMBA caused loss of all follicles as well as onset of extensive necrosis and ovarian atrophy compared to DMBA alone (Figure 8E).
Figure 8. Effect of VCD, DMBA and PI3 kinase inhibition on ovarian morphology.
Ovaries from PND4 F344 rats were cultured with vehicle control medium (1% DMSO) or medium containing VCD (30 μM) ± LY294002 (20 μM) for 8d or DMBA ± LY294002 (20 μM) for 6d. Following incubation, ovaries were sectioned and stained with haemotoxylin and eosin. Images were collected using an Olympus IX-70 inverted microscope at 40X magnification. Treatments were (A) control, (B) control + LY294002, (C) VCD, (D) VCD + LY294002, (E) DMBA, and (F) DMBA + LY294002. Thin arrow, primordial follicle; Block arrow, small primary follicle; Arrowhead = follicle remnant; Scalebar = 25 μM.
Discussion
VCD is an occupational chemical that causes selective destruction of primordial and small primary follicles by accelerating the natural process of atresia (Springer et al., 1996a, 1996b; Hu et al., 2001a, 2001b, 2002). Ovaries from neonatal rats (PND4) are highly enriched in primordial and small primary follicles because active follicle development has not yet begun (Devine et al., 2002). Therefore, in vitro culture of PND4 rat ovaries exposed to VCD is a useful model by which to identify mechanisms involved in chemical-induced destruction of small pre-antral (primordial and primary) follicles.
The time course of VCD exposure reported in this study demonstrated a similar depletion of both primordial and small primary follicles occurring following 6d of exposure. However, the follicle loss became greater in primordial as compared with small primary follicles on d8–15. This observation provides initial support that primordial follicles may be more sensitive than small primary to VCD-induced ovotoxicity.
A previous study identified the KIT/KITL signaling pathway as a candidate by which VCD mediates its ovotoxic effects (Fernandez et al., 2008). That study suggested that a decrease in KIT may be an initiating signaling event in VCD-induced ovotoxicity. In addition to its pro-survival effects, the KIT/KITL signaling pathway has also been identified as a regulator of primordial to small primary follicle recruitment. Therefore, due to the previously reported effect of VCD on KIT signaling (Fernandez et al., 2008) and the involvement of the PI3 kinase signaling pathway in control of primordial to small primary follicle recruitment as well as follicle survival, the involvement of PI3 kinase in VCD-induced ovotoxicity was investigated using a competitive inhibitor of PI3 kinase, LY294002 (Vlahos et al., 1994). Immunofluoresence staining confirmed that PI3 kinase protein is highly expressed in the cytoplasm of oocytes contained in primordial and small primary follicles. Thus, the PI3 kinase pathway could be potentially involved in VCD effects.
If PI3 kinase solely maintains follicle survival, its inhibition should result in loss of both primordial and small primary follicles. Conversely, if PI3 kinase promotes primordial follicle activation and recruitment, its inhibition should result in no primordial follicle loss (back up of recruitment). PND4 rat ovaries cultured in the presence of LY294002 showed no effect on primordial follicle number at any time, however, numbers of small primary and larger follicles were reduced beginning on d4 in culture. Thus, transition of primordial to small primary follicles appears to have been inhibited by LY294002. This likely relates to the previously reported role of PI3 kinase in activation and recruitment of primordial follicles (Castrillon et al., 2003; Reddy et al., 2005; John et al., 2008; Liu et al., 2006; Reddy et al., 2008, Rajareddy et al., 2007).
Based on the ability of LY294002 to retard recruitment of primordial follicles, (follicle population reduced by VCD), the effect of VCD-induced ovotoxicity in the presence of LY294002 was investigated. If VCD has a direct ovotoxic effect on primordial follicles, inhibition of their recruitment into the primary follicle pool should not affect VCD-induced primordial follicle loss. However, with inhibition of PI3 kinase VCD-induced loss of primordial follicles was prevented, whereas small primary follicle loss was greater. This supports that primordial follicles are not directly targeted by VCD, and reductions in primordial follicle numbers during VCD-induced ovotoxicity are due to increased recruitment into the small primary pool.
In further support of this hypothesis, an experiment was conducted to determine if PI3 kinase inhibition could protect primordial follicles from further VCD-induced follicle loss after ovotoxicity was underway. VCD-induced primordial follicle loss (d1–12) was retarded whereas, small primary follicle loss was accelerated by PI3 kinase inhibition when compared with continuous exposure to VCD alone (d1–12). However, primordial follicle loss was not completely prevented, when compared to that which had occurred by d6. The reason for this incomplete effect could be due to the existence of a sub-groupof primordial follicles that were activated by VCD prior to PI3 kinase inhibition, such that events downstream of PI3 kinase may have already been initiated in the recruitment pathway. Alternatively, because PI3 kinase inhibition did not prevent recruitment of primordial to primary follicles prior to 4 days in culture, VCD-induced ovotoxicity may have continued beyond that observed on d6 until the time that LY294002 had its effect. As with continuous PI3 kinase inhibition, these results also support that primordial follicles are not directly targeted by VCD. Rather, recruitment into the small primary follicle pool is enhanced.
In an experiment to confirm that the effects on VCD-induced ovotoxicity were a direct consequence of PI3 kinase inhibition, the negative control for LY294002, LY303511, was used (Vlahos et al., 1994). Curiously, by itself, LY303511 caused loss of primordial and primary follicles. Because this effect was not seen with LY294002, the cause of this follicle loss did not likely involve PI3 kinase suggesting that, unlike LY294002, this chemical interacts with viability pathways distinct from PI3 kinase in those follicles. However, there was no effect of LY303511 on VCD-induced loss of primordial or small primary follicles. Therefore, these results confirmed that PI3 kinase inhibition was the reason for primordial follicle protection from VCD-induced ovotoxicity. The events downstream of PI3 kinase that are affected by VCD are currently unknown, however, the previously reported activation of pro-apoptotic MAPK and BCL-2 family members during VCD-induced ovotoxicity (Hu et al., 2001b; 2002; Springer et al., 1996b) may also be related to a decrease in PI3 kinase activity induced by VCD.
Previous observations have shown that 1) primordial follicle numbers are reduced at a lower VCD concentration (20 μM) than that required to cause a reduction in small primary follicles (30 μM; Fernandez et al., 2008), and 2) the rate of primordial as compared with small primary follicle loss is greater as the time-course progresses (Figure 1). Whereas, these observations suggest that primordial follicles may be more sensitive than primary to the ovotoxic effects of VCD, the results presented here suggest an alternative mechanism. In the face of PI3 kinase inhibition, VCD does not directly target primordial follicles. In fact, under those conditions, small primary are those follicles sensitive to VCD.
Collectively, the results presented here support that, in part, VCD directly targets and destroys small primary follicles resulting in increased recruitment from the primordial pool to replace depleted small primary follicles (Figure 9A). Thus, with continued exposure to VCD, small primary follicle numbers may be maintained by replacement from the primordial follicle pool, until such a time that the rate of small primary follicle destruction becomes greater than its rate of replacement and there is also a reduction in that pool. As a result of the reduced pool for recruitment, larger follicle populations subsequently also become reduced (Springer et al., 1996a; Flaws et al., 1994).
Figure 9. Model of mechanism of VCD- and DMBA-induced ovotoxicity.
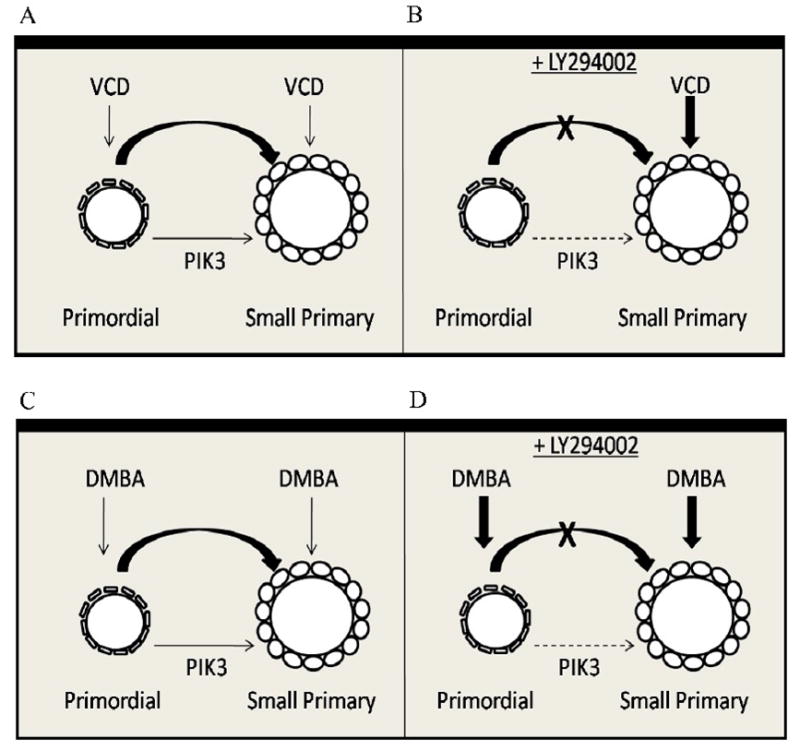
Under basal conditions, PI3 kinase signaling (thin arrow) regulates recruitment from the primordial to small primary follicle stage. (A) In the presence of VCD, primordial and small primary follicles are lost. (B) With PI3 kinase signaling inhibition (LY294002), VCD causes loss of small primary (direct targeting, ovotoxicity) but not primordial follicles because the ability to recruit from the primordial pool is lost. (C) In the presence of DMBA, primordial and small primary follicles are lost. (D) With inhibited PI3 kinase signaling, DMBA causes loss of primordial and small primary follicles due to direct targeting (ovotoxicity).
The reduced susceptibility of primordial follicles to PI3 kinase inhibition and VCD-induced ovotoxicity suggests that primordial follicles may be resistant to cell death and are only destroyed once a commitment to activation/recruitment has been made. Therefore, it was of interest to determine if this phenomenon was also involved in follicle destruction caused by another known ovotoxic chemical. Unlike VCD, the polycyclic aromatic hydrocarbon DMBA acts as an ovotoxicant by targeting follicles of all types (Matikainen et al., 2001). A previous study in PND4 mouse ovaries demonstrated that after 6h in culture, DMBA (1 μM) caused follicle loss by apoptosis (Rajapaksa et al., 2007). In contrast to VCD, in the present study, DMBA caused loss of primordial as well as small primary follicles in the presence of PI3 kinase inhibition. Also, unlike VCD, there was clearly an additive effect of DMBA on PI3 kinase inhibition. This suggests that DMBA does not cause ovotoxicity via increased primordial follicle recruitment, but directly targets primordial and primary follicles. Furthermore, ovaries incubated with DMBA plus LY294002 also showed signs of extensive necrosis and ovarian atrophy (not seen with DMBA alone). Therefore, in addition to targeting different follicle types, VCD and DMBA appear to also use different mechanisms to cause ovotoxicity. The DMBA findings also demonstrate that primordial follicles can be directly susceptible to destruction by some ovotoxic chemicals (DMBA; Figure 9C,D), but are targeted differently by others (VCD: Figure 9A,B).
In summary, the studies reported here support an involvement of PI3 kinase as well as VCD in primordial follicle activation and recruitment. These results also provide evidence that ovotoxic chemicals may use different mechanisms to destroy primordial follicles as shown by the differing effects of PI3 kinase inhibition on VCD- and DMBA-induced ovotoxicity. It is clear that primordial follicle activation is a fine tuned balancing act, which requires further investigation. Currently, studies are underway to identify events downstream of PI3 kinase signaling that are initiated by VCD. These studies also support that, in addition to mechanisms of xenobiotic-induced ovotoxicity, VCD is a useful tool for dissecting dynamics of follicular recruitment and survival.
Acknowledgments
This work was supported by National Institutes of Health grant ES09246, Center Grant 06694, and K99 ES016818 (to AFK).
Footnotes
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Borman SM, Christian PJ, Sipes IG, Hoyer PB. Ovotoxicity in female fischer rats and B6 mice induced by low-dose exposure to three polycyclic aromatic hydrocarbons: comparison through calculation of an ovotoxic index. Toxicol Appl Pharmacol. 2000;167:191–198. doi: 10.1006/taap.2000.9006. [DOI] [PubMed] [Google Scholar]
- Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 2003;301:215–218. doi: 10.1126/science.1086336. [DOI] [PubMed] [Google Scholar]
- Devine PJ, Sipes IG, Skinner MK, Hoyer PB. Characterization of a rat in vitro ovarian culture system to study the ovarian toxicant 4-vinylcyclohexene diepoxide. Toxicol Appl Pharmacol. 2002;184:107–115. [PubMed] [Google Scholar]
- Fernandez SM, Keating AF, Christian PJ, Sen N, Hoying JB, Brooks HL, Hoyer PB. Involvement of the Kit/kit ligand signaling pathway in 4-vinylcyclohexene diepoxide-induced ovarian follicle loss in rats. Biol Reprod. 2008;79:318–327. doi: 10.1095/biolreprod.108.067744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaws JA, Doerr JK, Sipes IG, Hoyer PB. Destruction of preantral follicles in adult rats by 4-vinyl-1-cyclohexene diepoxide. Reprod Toxicol. 1994;8:509–514. doi: 10.1016/0890-6238(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Hirshfield AN. Development of follicles in the mammalian ovary. Int Rev Cytol. 1991;124:43–101. doi: 10.1016/s0074-7696(08)61524-7. [DOI] [PubMed] [Google Scholar]
- Horie K, Takakura K, Taii S, Narimoto K, Noda Y, Nishikawa S, Nakayama H, Fujita J, Mori T. The expression of Kit protein during oogenesis and early embryonic development. Biol Reprod. 1991;45:547–552. doi: 10.1095/biolreprod45.4.547. [DOI] [PubMed] [Google Scholar]
- Hu X, Christian PJ, Thompson KE, Sipes IG, Hoyer PB. Apoptosis induced in rats by 4-vinylcyclohexene diepoxide is associated with activation of the caspase cascades. Biol Reprod. 2001a;65:87–93. doi: 10.1095/biolreprod65.1.87. [DOI] [PubMed] [Google Scholar]
- Hu X, Christian PJ, Sipes IG, Hoyer PB. Expression and redistribution of cellular Bad, Bax, and Bcl-X(L) protein is associated with VCD-induced ovotoxicity in rats. Biol Reprod. 2001b;65:1489–1495. doi: 10.1095/biolreprod65.5.1489. [DOI] [PubMed] [Google Scholar]
- Hu X, Flaws JA, Sipes IG, Hoyer PB. Activation of mitogen-activated protein kinases and AP-1 transcription factor in ovotoxicity induced by 4-vinylcyclohexene diepoxide in rats. Biol Reprod. 2002;67:718–724. doi: 10.1095/biolreprod.102.004259. [DOI] [PubMed] [Google Scholar]
- Igawa Y, Keating AF, Rajapaksa KS, Hoyer PB, Sipes IG. Involvement of microsomal epoxide hydrolase in 9,10-dimethylbenz[a]anthracene-induced ovotoxicity in rats. Toxicol Appl Pharmacol. 2009;234:361–369. doi: 10.1016/j.taap.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Agency for Research on Cancer (IARC) IARC Monograph on the evaluation of carcinogenic risk of chemicals to humans. International Agency for Research on Cancer; Lyon, France: 1976. Cadmium, nickel, some epoxides, miscellaneous industrial chemicals and general considerations on volatile anaesthetics; pp. 141–145. [Google Scholar]
- Ismail RS, Okawara Y, Fryer JN, Vanderhyden BC. Hormonal regulation of the ligand for c-kit in the ovary and its effects on spontaneous oocyte meiotic maturation. Mol Reprod Dev. 1996;43:458–469. doi: 10.1002/(SICI)1098-2795(199604)43:4<458::AID-MRD8>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- John GB, Gallardo TD, Shirley LJ, Castrillon DH. Foxo3 is a PI3 kinase-dependent molecular switch controlling the initiation of oocyte growth. Dev Biol. 2008;321:197–204. doi: 10.1016/j.ydbio.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao SW, Sipes IG, Hoyer PB. Early effects induced by 4-vinylcyclohexene diepoxide in rats and mice. Reprod Toxicol. 1999;13:67–75. doi: 10.1016/s0890-6238(98)00061-6. [DOI] [PubMed] [Google Scholar]
- Kissel H, Timokhina I, Hardy MP, Rothschild G, Tajima Y, Soares V, Angeles M, Whitlow MR, Manova K, Besmer P. Point mutation in Kit receptor tyrosine kinase reveals essential roles for Kit signaling in spermatogenesis and oogenesis without affecting other Kit responses. EMBO J. 2000;19:1312–1326. doi: 10.1093/emboj/19.6.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Rajareddy S, Liu L, Jagarlamundi K, Boman K, Selstam G, Reddy P. Control of mammalian oocyte growth and early follicular development by the oocyte PI3 kinase pathway: New roles for an old timer. Dev Biol. 2006;299:1–11. doi: 10.1016/j.ydbio.2006.07.038. [DOI] [PubMed] [Google Scholar]
- Manova K, Nocka K, Besmer P, Bacharova RF. Gonadal expression of Kit encoded at the W locus of the mouse. Development. 1990;110:1057–1069. doi: 10.1242/dev.110.4.1057. [DOI] [PubMed] [Google Scholar]
- Matikainen T, Perez GI, Jurisicova A, Pru JK, Schlezinger JJ, Ryu H, Laine J, Sakai T, Korsmeyer S, Casper R, Sherr DH, Tilly JL. Aromatic hydrocarbon receptor-driven Bax gene expression is required for premature ovarian failure caused by biohazard environmental chemicals. Nat Genet. 2001;28:355–360. doi: 10.1038/ng575. [DOI] [PubMed] [Google Scholar]
- Mattison DR, Schulman JD. How xenobiotic chemicals can destroy oocytes. Contemp Obstet Gynecol. 1980;15:157–169. [Google Scholar]
- Orr-Utreger A, Aviva A, Zimmer Y, Givol D, Yarden Y, Lonai P. Developmental expression of Kit, a proto-oncogene encoded by the W locus. Development. 1990;109:911–923. doi: 10.1242/dev.109.4.911. [DOI] [PubMed] [Google Scholar]
- Parrott JA, Skinner MK. Kit-ligand/stem cell factor induces primordial follicle development and initiates folliculogenesis. Endocrinol. 1999;140:4262–4271. doi: 10.1210/endo.140.9.6994. [DOI] [PubMed] [Google Scholar]
- Rajapaksa KS, Sipes IG, Hoyer PB. Involvement of microsomal epoxides hydrolase (mEH) enzyme in ovotoxicity caused by 7,12-Dimethylbenz[a]anthracene (DMBA) Toxicol Sci. 2007;96:327–334. doi: 10.1093/toxsci/kfl202. [DOI] [PubMed] [Google Scholar]
- Rajareddy S, Reddy P, Du C, Liu L, Jagarlamundi K, Tang W, Shen Y, Berthet C, Peng SL, Kaldis P, Liu K. p27kip1 (cyclin-dependent kinase inhibitor 1B) controls ovarian development by suppressing follicle endowment and activation and promoting follicle atresia in mice. Mol Endocrinol. 2007;21:2189–2202. doi: 10.1210/me.2007-0172. [DOI] [PubMed] [Google Scholar]
- Reddy P, Shen L, Ren C, Boman K, Lundin E, Ottander U, Lindgren P, Liu YX, Sun QY, Liu K. Activation of Akt (PKB) and suppression of FKHRL1 in mouse and rat oocytes by stem cell factor during follicular activation and development. Dev Biol. 2005;281:160–170. doi: 10.1016/j.ydbio.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Reddy P, Liu L, Adhikary D, Jagarlamundi K, Rajareddy S, Shen Y, Du C, Tang W, Hämäläinen T, Peng SL, Lan ZJ, Cooney AJ, Huhtaniemi I, Liu K. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science. 2008;319:611–613. doi: 10.1126/science.1152257. [DOI] [PubMed] [Google Scholar]
- Smith BJ, Mattison DR, Sipes IG. The role of epoxidation in 4-vinylcyclohexene-induced ovarian toxicity. Toxicol Appl Pharmacol. 1990;105:372–381. doi: 10.1016/0041-008x(90)90141-g. [DOI] [PubMed] [Google Scholar]
- Springer LN, McAsey ME, Flaws JA, Tilly JL, Sipes IG, Hoyer PB. Involvement of apoptosis in 4-vinylcyclohexene diepoxide-induced ovotoxicity in rats. Toxicol Appl Pharmacol. 1996a;139:394–401. doi: 10.1006/taap.1996.0180. [DOI] [PubMed] [Google Scholar]
- Springer LN, Tilly JL, Sipes IG, Hoyer PB. Enhanced expression of bax in small preantral follicles during 4-vinylcyclohexene diepoxide-induced ovotoxicity in the rat. Toxicol Appl Pharmacol. 1996b;139:402–410. doi: 10.1006/taap.1996.0181. [DOI] [PubMed] [Google Scholar]
- Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;18:5241–5248. [PubMed] [Google Scholar]
- Yoshida H, Takakura N, Kataoka H, Kunisada T, Okamura H, Nishikawa SI. Stepwise requirement of Kit tyrosine kinase in mouse ovarian follicle development. Dev Biol. 1997;184:122–137. doi: 10.1006/dbio.1997.8503. [DOI] [PubMed] [Google Scholar]