Abstract
Superoxide anion (O2−•) production was previously reported to be increased in celiac ganglia (CG) during DOCA-salt hypertension, possibly via activation of the reduced nicotinamide-adenine dinucleotide phosphate (NADPH) oxidase. This suggested a role for neuronal NADPH oxidase in autonomic neurovascular control. However, the expression and localization of NADPH oxidase in the peripheral neurons is not fully known. The purpose of this study was to examine the subcellular localization of NADPH oxidase in sympathetic and sensory ganglion neurons and perivascular nerve fibers. In rat CG, p22phox and neuropeptide Y (NPY) were colocalized in all neurons. P22phox was also localized to dorsal root ganglia (DRG) neurons that contain calcitonin gene related peptide (CGRP). In mesenteric arteries, p22phox and p47phox were colocalized with NPY or CGRP in perivascular nerve terminals. A similar pattern of nerve terminal staining of p22phox and p47phox was also found in cultured CG neurons and nerve growth factor (NGF)-differentiated PC12 cells. These data demonstrate a previously uncharacterized localization of NADPH oxidase in perivascular nerve fibers. The presence of a O2−• – generating enzyme in close vicinity to the sites of neurotransmitter handling in the nerve fibers suggests the possibility of novel redox-mediated mechanisms in peripheral neurovascular control.
Introduction
Reactive oxygen species (ROS), such as superoxide (O2−•) and hydrogen peroxide (H2O2) are signaling molecules which play important roles in regulating cardiovascular function (Griendling et al., 2000a; Griendling et al., 2000b). While first discovered in neutrophils, NADPH oxidase is now thought to be a significant source of ROS in many cell types, including smooth muscle cells (Griendling et al., 1994; Patterson et al., 1999; Ushio-Fukai et al., 1996), endothelial cells (Gorlach et al., 2000) and fibroblasts (Jones et al., 1994). NADPH oxidase has also been localized to the nervous system. For example, we previously showed that NADPH oxidase subunits were present in rat sympathetic and sensory ganglia (Cao et al., 2007; Dai et al., 2006).
Increased ROS production and NADPH oxidase activity are associated with cardiovascular dysfunction in hypertension (Beswick et al., 2001), diabetes (Gunes et al., 2005) and senescence (Chakravarti and Chakravarti, 2007). Studies of ROS in hypertension have focused primarily on vascular and endothelial ROS signaling (Griendling et al., 2000b). However, accumulating evidence indicates that peripheral neural components play a key role in regulating blood pressure. For example, mesenteric circulation is innervated by postganglionic sympathetic nerves and spinal sensory nerves. It can also be mobilized by peripheral reflex pathways that involve non-spinal peripheral sensory neurons (King and Szurszewski, 1989; Meehan and Kreulen, 1992). Abnormalities in the peripheral sympathetic and sensory neurons contribute to increased salt sensitivity and the development of hypertension (King et al., 2007; Mangiarua and Lee, 1990; Mathias, 1991; Wang and Li, 1999; Whitelaw and Smithwick, 1958). In particular, enhanced NADPH oxidase activity in peripheral sympathetic neurons is associated with the onset of cardiovascular disorders (Cao et al., 2007; Dai et al., 2004; Ma et al., 2006). This suggests a role of peripheral neuronal NADPH oxidase in the regulation of blood pressure.
Because of a short half life (1×10−6 sec), the direct actions of O2−• in the cell are confined to a limited region near the subcellular site of its production. In neurons the major functional compartments–the cell body, dendrites, axons, and terminals– are separated by considerable distances; therefore it is important to evaluate the localization of NADPH oxidase in these compartments in order to fully understand the physiological consequences of O2−• production. In particular, transmission at the neuro-vascular junctions modulates vascular tone, the O2−• produced in the cell body would not be expected to diffuse to the terminals; if O2−• were to influence neurotransmitter dynamics it would have to be produced locally. However, it is not known if NADPH oxidase is localized to perivascular nerve fibers.
To address this issue, a series of immunohistochemical experiments were designed to localize NADPH oxidase subunits, p22phox and p47phox, to the neuronal cell bodies in celiac ganglia (CG) and dorsal root ganglia (DRG) as well as perivascular nerve fibers on the mesenteric arteries. We found that NADPH oxidase localized to sympathetic and sensory neurons as well as periarterial nerve fibers and endings. The presence of NADPH oxidase subunits in fibers innervating the mesenteric circulation is novel and may have important implications in the role of NADPH oxidase in blood pressure regulation and hypertension.
Methods
Animals
Unless noted, all experiments were done using Sprague-Dawley rats from Charles River Laboratories (Portage, MI). Upon arrival at the animal care facility, animals were maintained according to standards approved by the Institutional Animal Care and Use Committee at Michigan State University. All experimental procedures were carried out in accordance with the “Guiding Principles in the Care and Use of Animals” of the American Physiological Society.
Drug Treatment and Surgeries
Capsaisin-treatment (cap-tx)
Briefly, on days 1 and 2 of life, neonatal Wistar rats received capsaicin (50 mg/kg) subcutaneously, as described previously (Wang et al., 1998). Control rats were treated with equal volumes of vehicle solution (5% ethanol, 5% Tween 80 in saline). All treatments were performed with rats under ether anesthesia (Wang and Li, 1999).
Celiac ganglionectomy (CGx)
CGx was performed by locating the celiac plexus in between the aorta, celiac artery, and cranial mesenteric artery; dissecting it free from surrounding tissue; and removing it. Any additional nerves along these vessels in the area of the CG were also dissected free and transected (King et al., 2007). Treatments were performed in anesthetized adult Sprague-Dawley rats.
Antibodies
All antibodies used in this study are listed in Table 1.
Table 1.
Antibodies for Immunohistochemical Staining
| Primary antibodies | |||
|---|---|---|---|
| Antigen | Host species | Dilution | Source |
| p47phox (R360) | Rabbit | 1:300 | (De Leo et al., 1996) |
| p22phox (R5554) | Rabbit | 1:300 | (Jesaitis et al., 1990) |
| SGII^ | Mouse | 1:1000 | Abcam Inc., Cambridge, MA |
| TH^ | Mouse | 1:150 | Calbiochem, La Jolla, CA |
| NPY | Goat | 1:300 | Santa Cruz Biotech., Inc., Santa Cruz, CA |
| CGRP | Sheep | 1:1000 | Abcam Inc., Cambridge, MA |
|
Secondary antibodies | |||
| Target species | Host species | Conjugated to: | Dilution |
| Mouse | Donkey | FITC | 1:40 |
| Rabbit | Donkey | Cy3 | 1:200 |
| Goat | Donkey | FITC | 1:200 |
| Mouse | Donkey | Cy3 | 1:200 |
| Rabbit | Goat | Alexa 488 | 1:500 |
| Sheep | Donkey | Rhodamine Red | 1:500 |
| Rabbit | Donkey | FITC | 1:500 |
SGII = secretogranin II; TH = tyrosine hydroxylase; NPY = Neuropeptide Y;
CGRP = calcitonin gene-related peptide; ^=monoclonal antibodies
All secondary antibodies were purchased from Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, except for goat anti-rabbit Alexa 488 was purchased from Invitrogen, Inc., Carlsbad, CA
Cell Culture
Unless noted, all cell culture reagents were GIBCO® brand (Invitrogen, Carlsbad, CA).
Primary ganglion neuron culture
CG and DRG from neonatal rats were harvested and enzymatically dissociated (2.5mg/ml collagenase for 10 minutes at 37°C followed by 2.5mg/ml trypsin for 45min at 37°C). Freshly dissociated neurons were plated as a monolayer on cover glass in culture dishes double-coated with poly-D-lysine (Sigma, St. Louis, MO) and collagen. Cells were maintained in N2 medium (49% DMEM, 49% F-12 nutrient mixture, 0.5mg/ml bovine serum albumin, 2mM L-glutamine, 1% N2 supplement, 100ng/ml nerve growth factor (NGF) 2.5S (Millipore, Billerica, MA), 0.7% B-27) supplemented with 1% fetal bovine serum at 37°C in a 5% CO2 humidified incubator. From the second day of culture, 0.24μg/ml Ara-C (Calbiochem, San Diego, CA) was added to the N2 medium to eliminate non-neuronal cell growth. Neurons were kept in culture for 7 days before immunostaining to ensure full neurite outgrowth.
PC12 Cell Culture
PC12 cells are derived from a rat catecholamine-secreting chromaffin tumor. They can differentiate into cells with a sympathetic neuronal phenotype after one week of NGF treatment (Greene et al., 1998). PC 12 cells were obtained from American Type Culture Collection (ATCC) and maintained at 37°C in a 5% CO2 humidified incubator in RPMI 1640 medium supplemented with 10% heat inactivated horse serum, 5% fetal bovine serum, 100U/ml penicillin, 100ug/ml streptomycin and 0.25ug/ml Fungizone. To differentiate PC12 cells, 50ng/ml NGF 2.5S was added to the medium for 7 days.
Immunocytochemistry of cultured ganglion neurons and PC12 cells
Cultured cells were cleaned from culture medium by three washes in Dulbecco’s phosphate-buffered saline (DPBS) and then placed into fixative (4% paraformaldehyde, 0.1% Triton X-100 in DPBS) for 30min. Cells were then incubated in DPBS with blocking solution (5% goat serum, 3% bovine serum albumin) for 1 hour at room temperature, followed by primary antibody incubation for overnight at 4°C. The next day, samples were washed in DPBS three times and then incubated with corresponding secondary antibodies in a dark chamber at room temperature for 1 hour, followed by three washes in DPBS. Samples were mounted onto glass slides using Prolong Gold anti-fade reagent (Invitrogen) for confocal microscopy using Pascal (Zeiss, Thornwood, NY) or Fluoview (Olympus, Center Valley, PA).
Immunostaining of Periarterial Nerve Fibers
Eight week old rats were euthanized with an intraperitoneal injection of sodium pentobarbital (50 mg/kg). The mesentery was surgically removed and maintained in 0.1M phosphate-buffered saline (PBS). Mesenteric arteries were cleaned of adipose and connective tissue and cleared of blood via an intravascular PBS bolus. Tertiary branches were excised, and isolated tissues were placed in Zamboni fixative (2% [vol/vol] formaldehyde and 0.2% [vol/vol] picric acid in 0.1M phosphate buffered saline, PBS) overnight (4 °C). The next day, the tissues were washed 3x with 0.1M PBS and then incubated in PBS with blocking serum diluted in Triton X-100 (1.0 %) for 1 hour. Tissues were then incubated with primary antibodies for 2 hours at 37°C. Next, tissues were washed 3 times in 0.1M PBS buffer and then incubated with secondary antibodies for 1 hour in a dark, humidified chamber at room temperature. Vessels were then washed 3 times with 0.1 M PBS at 5-minute intervals, and coverslips were mounted with Prolong Gold anti-fade reagent for fluorescence confocal microscopy. Tissues were examined using a Leica TSL laser confocal microscope (Leica Microsystems Inc., Bannockburn, IL).
Results
P22phox is localized in neuropeptide Y (NPY)-containing CG neurons and calcitonin gene related peptide (CGRP)-containing DRG neurons
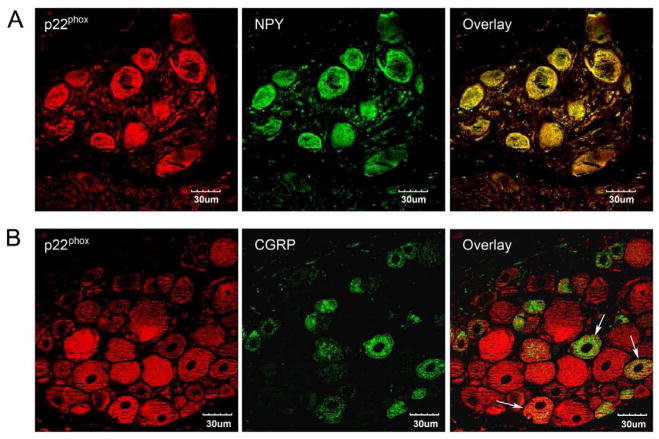
In guinea pig prevertebral sympathetic ganglia, approximately 20% of all neurons contain IR to NPY and have been speculated to be vasoconstrictor neurons (McLachlan and Llewellyn-Smith, 1986). On the other hand, 18.9% of neurons in the IMG that innervate the inferior mesenteric artery are NPY-positive (Browning et al., 1999). In the rat CG, we found that all neuron cell bodies examined were immunopositive for NPY and the NADPH oxidase subunit p22phox (Fig. 1A). The presence of both proteins was not limited to a subpopulation of neurons in rat CG. CGRP is a vasodilatory neuropeptide that is released from the sensory nerve fibers (Uddman et al., 1986). The synthesis of the peptide occurs in neuronal cell bodies of the DRG. CGRP immunostaining in rat DRG was primarily found in small-diameter neurons, while the distribution of p22phox immunoreactivity was more universal throughout the ganglia (Fig. 1B). Staining for both CGRP and p22phox was found in some cells, indicating colocalization of these two proteins in the same sensory neurons.
Fig. 1. Immunolocalization of p22phox in CG and DRG neurons.
CG and lumbar DRG from normal adult rats were cut to 5μm sections and immunostained with p22phox and NPY or p22phox and CGRP, respectively. A) All the neurons in CG that contain p22phox (red) immunoreactivity showed positive staining for NPY (green); B) In DRG, p22phox (red) and CGRP (green) colocalize to some neurons (arrows) but not all of them. Scale bar: 30μm. Single digital slice fluorescent images were taken under confocal microscopy.
P47phox and p22phox are present in the neurites of PC12 cells and cultured CG and DRG neurons
In order to examine the presence of NADPH oxidase in neuronal compartments outside of the cell bodies, we first immunostained p47phox in NGF-differentiated PC12 cells. P47phox was present in PC 12 cell bodies, as well as the neurites that extended from the somata. It was colocalized with secretogranin II (SGII), a darge dense core vesicle marker protein (Fischer-Colbrie et al., 1995), on the cell membrane and the neurites (Fig. 2A). In dissociated CG and DRG neurons, p47phox was colocalized with SGII in both cell bodies and neurites (Fig. 2B and 2C). p22phox was also found to be colocalized with SGII in CG and DRG cultured cells (data not shown). These findings suggest that in addition to cell bodies, NADPH oxidase is present in nerve fibers in cultured ganglion neurons.
Fig. 2. Immunolocalization of p47phox in PC12 cells and cultured CG and DRG neurons.
NGF-differentiated PC12 and dissociated CG and DRG neurons were immunostained with antibodies against p47phox and secretogranin II (SGII), a marker for large dense core vesicles in neuronal cell body and nerve endings. P47phox was co-localized with SGII in the cell bodies and the neurites in PC12 cells (A), CG neurons (B) and DRG neurons (C). Scale bar: 20μm. Single digital slice fluorescent images were taken under confocal microscopy.
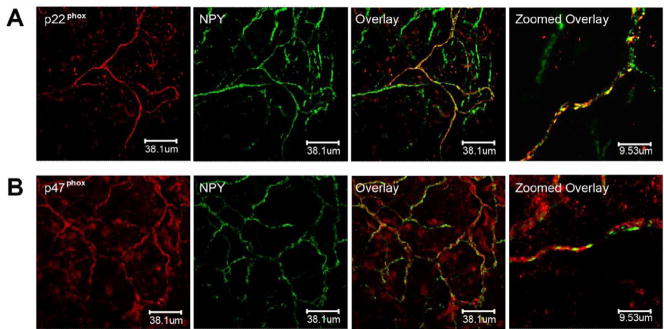
NADPH oxidase subunits p47phox and p22phox colocalize to NPY- immunoreactive periarterial nerve fibers
In order to determine if NADPH oxidase subunits colocalize to sympathetic nerve fibers and endings in tissue, tertiary branches of mesenteric arteries were fixed and labeled with NPY and either anti-p22phox (Fig. 3A) or anti-p47phox (Fig. 3B). P22phox or p47phox and NPY were found in the same nerve fiber bundles. The overlay images show that p22phox or p47phox and NPY co-localize to some, but not all periarterial nerve fibers, indicating that NADPH oxidase is also localized to non-sympathetic nerve fibers on the blood vessels. Anti- tyrosine hydroxylase (TH) showed similar results to NPY-stained fibers (data not shown).
Fig. 3. p22phox and p47phox colocalize with NPY in periarterial nerve fibers in mesenteric arteries.
Tertiary branches of mesenteric arteries from adult rats were fixed and labeled with anti-NPY, as a marker for sympathetic perivascular nerves, and either anti-p47phox or anti-p22phox. In each panel, three low power magnification images on the left show the meshwork pattern of nerve fibers innervating mesenteric arteries. The zoomed overlay images on the right are high power magnifications, showing colocalization in a single peri-vascular nerve bundle. A) Immunostaining for p22phox was found in some of the same nerve fibers as NPY. The overlay images show that p22phox and NPY co-localize to some, but not all periarterial nerve fibers. B) p47phox and NPY were found in the same nerve fiber bundles although the localization of the staining within the nerve fiber was variable. NPY staining was discontinuous, while p47phox staining distributed throughout the nerve fibers. Single digital slice fluorescent images were taken under confocal microscopy.
NAPDH oxidase subunits p47phox and p22phox colocalize to CGRP immunoreactive periarterial nerve fibers
CGRP was used as a marker for sensory nerves on tertiary mesenteric arteries. Fig. 4 shows that p22phox or p47phox was colocalized with CGRP in the same nerve fibers. The immunostaining for NADPH oxidase was not limited to CGRP positive fibers, indicating non-sensory source of NADPH oxidase on the nerve fibers. This is consistent with the findings of sympathetic localization of NADPH oxidase as was shown above. These results indicate that NADPH oxidase subunits are present in sensory periarterial nerve fibers.
Fig. 4. p22phox and p47phox colocalize with CGRP in periarterial nerve fibers in mesenteric arteries.
Tertiary branches of mesenteric arteries were fixed and labeled with anti-CGRP, as a marker for sensory perivascular nerves, and either anti-p22phox or anti-p47phox. In each panel, three low power magnification images on the left show the meshwork pattern of nerve fibers innervating mesenteric arteries. The zoomed overlay images on the right are high power magnifications, showing colocalization in a single peri-vascular nerve bundle. A) p22phox was found in some of the CGRP immunoreactive nerve fibers; B) p47phox and CGRP were found in the same nerve fiber bundles. Single digital slice fluorescent images were taken under confocal microscopy.
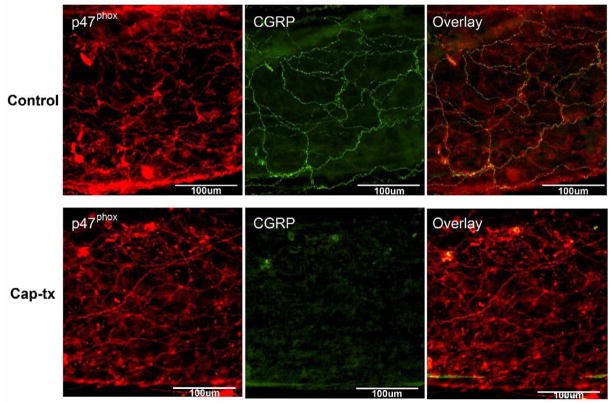
Perivascular NADPH oxidase immunostaining remains after capsaicin treatment (cap-tx)
Because sensory and sympathetic nerve fibers run close to each other, it is difficult to localize the precise labeling patterns of proteins to one or the other. Therefore, we chemically removed the sensory nerve fibers by treating animals with capsaicin to confirm the presence of NADPH oxidase in non-sensory fibers. Small mesenteric arteries removed from cap-tx animals were labeled with anti-p47phox and anti-CGRP (Fig. 5). The absence of anti-CGRP labeling in cap-tx rats indicated that treatment was effective in depleting sensory nerve fibers. However, significant p47phox immunostaining remained. The higher density of perivascular p47phox staining in cap-tx rats may be due to an increase in p47phox-containing sympathetic nerve sprouting, because long-term sensory denervation augments sympathetic neurotransmission (Ralevic et al., 1995). These results suggest that p47phox exists at least partly in non-sensory peri-arterial nerve fibers.
Fig. 5. Perivascular fiber p47phox staining remains after capsaicin treatment (cap-tx).
Small mesenteric arteries removed from cap-tx animals were labeled with anti-p47phox and anti-CGRP. Control animals were sacrificed and vessels were run at the same time as cap-tx animals. A) Vessels from control animals showed immunoreactivity for both p47phox and CGRP on the perivascular nerve fibers. Colocalization of p47phox and CGRP are present on some of the fibers but not all of them. B) The absence of anti-CGRP labeling in cap-tx rats indicates that treatment was effective in depleting sensory nerve fibers. However, p47phox immunostaining remains. This suggests that p47phox exists partly in non-sensory peri-arterial nerve fibers. Scale bar: 100μm. Images were taken using conventional fluorescence microscopy.
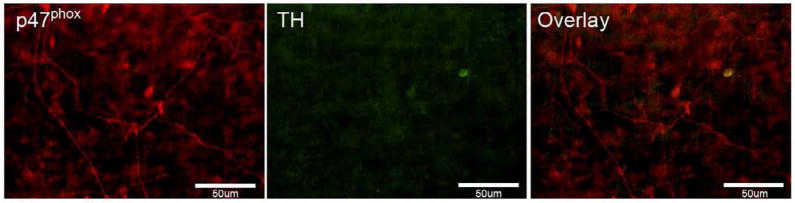
Celiac ganglionectomy (CGx) reduces peri-arterial p47phox immunostaining
Most sympathetic peri-arterial nerve fibers supplying mesenteric arteries originate in the CG, and spinal sensory nerves pass through the CG on their way to the DRG. Therefore, CGx was performed in adult rats to investigate possible presence of NADPH oxidase in perivascular nerve fibers that are neither sympathetic nor spinal sensory source. After CGx, TH staining on the mesenteric arteries was completely abolished (Fig. 6), so was CGRP staining (data not shown). These results indicate that the perivascular sympathetic and spinal sensory nerve fibers were successfully depleted by CGs. However, minor p47phox immunoreactivity was still detected in some superficially-located nerve fibers. These remaining p47phox-positive perivascular nerves may be either peripheral sensory fibers that were not abolished during the surgery or intestinofugal fibers, which originate in the myenteric plexus and terminate in prevertebral ganglia (Luckensmeyer and Keast, 1995).
Fig. 6. Periarterial fiber p47phox staining remains two weeks after celiac ganglionectomy (CGx).
After CGx, perivascular TH staining was completely abolished. This indicates that sympathetic nerve fibers were successfully depleted after CGx. However, few superficial p47phox-positive fibers remain. Scale bar: 50μm. Images were taken using conventional fluorescence microscopy.
Discussion
The primary finding of this study is that NADPH oxidase subunits are present in perivascular nerve fibers on mesenteric arteries. The localization of the same NADPH oxidase subunits in the neuronal cell bodies in CG and DRG indicates the sympathetic and sensory sources of these fibers. The presence of a local O2−•-producing enzyme at the neurovascular junction may indicate roles of O2−• in the modulation of neurotransmission at the nerve terminals.
NADPH oxidase is a multi-subunit enzyme complex; the proteins p22phox and p47phox are key catalytic and regulatory components, respectively and have been used in this study to localize NADPH oxidase. Enzymatic activity requires the assembly of all or some the subunits centered around a NOX protein for which various isoforms have been identified, including NOX1, NOX2 and NOX4 (Lambeth et al., 2000). All of the isoforms of NOX produce ROS when expressed with p22phox but only NOX2 requires p47phox (Sumimoto et al., 2005). The localization of p47phox in perivascular nerve fibers suggests that NOX2 is one of the isoforms present in these fibers. Although mRNAs for all of the NOX isoforms including NOX2 have been identified in sympathetic ganglia and in DRG, the NOX isoform composition of perivascular nerve fibers is not known.
The involvement of NADPH oxidase in the pathogenesis of cardiovascular diseases has been characterized in the vasculature. NADPH oxidase-mediated superoxide anion (O2−•) production is associated with hypertension or pro-hypertensive changes in different components of blood vessels. These include vascular smooth muscle cells (Griendling et al., 1994; Ushio-Fukai et al., 1996), endothelial cells (Gorlach et al., 2000), adventitial fibroblasts (Pagano et al., 1997) and perivascular adipose tissue (Gao et al., 2006). However, in spite of the presence of NADPH oxidase in sympathetic and sensory neurons that are known to innervate the vasculature (Cao et al., 2007), there has been no examination of its localization in perivascular nerves. We have previously shown that the activation and regulation of NADPH oxidase-derived O2−• in prevertebral sympathetic ganglia and primary sensory ganglia were associated with DOCA-salt hypertension (Cao et al., 2007; Dai et al., 2006). The two types of ganglia neurons both innervate the mesenteric vasculature. Axons of the neurons travel to the mesenteric arteries and veins in the paravascular nerves, which divide in the adventitia of blood vessels to form the perivascular nerve plexus (Kreulen, 2003; Meehan et al., 1991). In this study, we showed that the expression of NADPH oxidase in the peripheral sympathetic neurons and sensory neurons is not limited to the cell bodies in the ganglia, but is also found in the perivascular nerves, thus providing a possible additional source of O2−• on the blood vessels.
Changes in the activity of NADPH oxidase localized in the perivascular nerve terminals could influence neurovascular transmission. The prejunctional sympathetic nerve terminal is the site for synthesis, storage and release of the vasoconstrictor neurotransmitters norepinephrine (NE), NPY and ATP, while perivascular sensory nerves mediate vasodilatation by releasing CGRP, substance P and nitric oxide (NO) (Zheng et al., 1997). In DOCA-salt hypertension, NADPH oxidase activity and expression are upregulated in sympathetic ganglia and downregulated in spinal sensory ganglia (Cao et al., 2007). If the reciprocal changes of NADPH oxidase occur in the same manner in the terminals of these neurons, neurotransmission to the vasculature from sympathetic and sensory nerves may be differentially modulated by ROS in a manner that affects vascular tone in hypertension. For example, NO is a vasodilatory neurotransmitter released by perivascular sensory nerve fibers. In addition to being a direct vasodilatator, NO can also modulate neurotransmission at the sympathetic neurovascular junction by deactivating NE released from the sympathetic nerve terminals (Kolo et al., 2004). With the presence of NADPH oxidase at the nerve terminal, it can be predicted that the bioavailability of NO will be closely related to the activity of NADPH oxidase because O2−• rapidly inactivates NO (Gryglewski et al., 1986). In hypertension, increased sympathetic NADPH oxidase activity which would lead to higher O2−• production in the nerve terminals would diminish the effect from NO and thereby facilitate higher junctional NE levels and greater vasoconstriction. Sensory nerves, where NADPH oxidase activity is decreased, would play a compensatory role: the NO supply to the neurovascular junction would be elevated because of reduced NADPH oxidase activity.
Additional targets for nerve terminal O2−• are the neurotransmitter transporters norepinephrine transporter (NET) and vesicular monoamine transporter 2 (VMAT2), both of which are impaired by ROS (Elroy-Stein and Groner, 1988; Mao et al., 2004). When sympathetic NADPH oxidase activity is upregulated, as in hypertension, increased local O2−• at the nerve terminal may interfere with NE recycling and lead to increased junctional NE spillover and vasoconstriction. In DOCA-salt hypertensive rats, vesicular refilling of neurotransmitter ATP is impaired although it is not known if this is related to increased NADPH oxidase activity and elevated O2−• (Demel and Galligan, 2008). Furthermore, NET activity is present in perivascular sensory fibers (Zheng et al., 2000) and the activity of the transporter in these terminals may also be influenced by NADPH-generated O2−•.
The staining of NADPH oxidase was not limited to sympathetic and sensory nerve fibers. After both types of fibers were eliminated from the mesenteric circulation by CGx, there was still substantial amount of NADPH oxidase present in some nerve fibers. These could either be CGRP-negative sensory fibers that innervate the blood vessels without passing through the prevertebral ganglia, which therefore cannot be abolished by CGx, or intestinofugal fibers, which originate in the myenteric plexus and terminate in prevertebral ganglia (Luckensmeyer and Keast, 1995). Further studies are necessary to identify the sources of these nerve fibers.
In summary, we have demonstrated that NADPH oxidase is expressed in sympathetic and sensory neurons, as well as in their perivascular nerve fibers. The findings of NADPH oxidase in both neuronal cell bodies and prejunctional nerve terminals on blood vessels may indicate novel roles of locally-produced O2−• in the modulation of neurotransmission to blood vessels.
Acknowledgments
We would like to thank Sachin Kandlikar and Dr. Gregory D. Fink (Dept. of Pharmacology and Toxicology, Michigan State University) for helping with the celiac ganglionectomy surgeries and Dr. Donna H. Wang (Dept. of Medicine, Michigan State University) for helping with the capsaicin treatment. Special thanks to Dr. Fred S. Lamb (Dept. of Pediatrics, the University of Iowa) for helpful discussions.
Sources of funding: This work was supported by grant P01HL70687 to Dr. David L. Kreulen (Michigan State University), and NIH AR40426 and RR020185 to Dr. Mark T. Quinn (Montana State University).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beswick RA, Dorrance AM, Leite R, Webb RC. NADH/NADPH oxidase and enhanced superoxide production in the mineralocorticoid hypertensive rat. Hypertension. 2001;38:1107–1111. doi: 10.1161/hy1101.093423. [DOI] [PubMed] [Google Scholar]
- Browning KN, Zheng ZL, Kreulen DL, Travagli RA. Two populations of sympathetic neurons project selectively to mesenteric artery or vein. Am J Physiol. 1999;276:H1263–H1272. doi: 10.1152/ajpheart.1999.276.4.H1263. [DOI] [PubMed] [Google Scholar]
- Cao X, Dai X, Parker LM, Kreulen DL. Differential regulation of NADPH oxidase in sympathetic and sensory ganglia in deoxycorticosterone acetate salt hypertension. Hypertension. 2007;50:663–671. doi: 10.1161/HYPERTENSIONAHA.107.089748. [DOI] [PubMed] [Google Scholar]
- Chakravarti B, Chakravarti DN. Oxidative modification of proteins: age-related changes. Gerontology. 2007;53:128–139. doi: 10.1159/000097865. [DOI] [PubMed] [Google Scholar]
- Dai X, Cao X, Kreulen DL. Superoxide anion is elevated in sympathetic neurons in DOCA-salt hypertension via activation of NADPH oxidase. Am J Physiol Heart Circ Physiol. 2006;290:H1019–H1026. doi: 10.1152/ajpheart.00052.2005. [DOI] [PubMed] [Google Scholar]
- Dai X, Galligan JJ, Watts SW, Fink GD, Kreulen DL. Increased O2*-production and upregulation of ETB receptors by sympathetic neurons in DOCA-salt hypertensive rats. Hypertension. 2004;43:1048–1054. doi: 10.1161/01.HYP.0000126068.27125.42. [DOI] [PubMed] [Google Scholar]
- De Leo FR, Ulman KV, Davis AR, Jutila KL, Quinn MT. Assembly of the human neutrophil NADPH oxidase involves binding of p67phox and flavocytochrome b to a common functional domain in p47phox. Journal of Biological Chemistry. 1996;271:17013–17020. doi: 10.1074/jbc.271.29.17013. [DOI] [PubMed] [Google Scholar]
- Demel SL, Galligan JJ. Impaired purinergic neurotransmission to mesenteric arteries in deoxycorticosterone acetate-salt hypertensive rats. Hypertension. 2008;52:322–329. doi: 10.1161/HYPERTENSIONAHA.108.110353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elroy-Stein O, Groner Y. Impaired neurotransmitter uptake in PC12 cells overexpressing human Cu/Zn-superoxide dismutase--implication for gene dosage effects in Down syndrome. CELL. 1988;52:259–267. doi: 10.1016/0092-8674(88)90515-6. [DOI] [PubMed] [Google Scholar]
- Fischer-Colbrie R, Laslop A, Kirchmair R. Secretogranin II: molecular properties, regulation of biosynthesis and processing to the neuropeptide secretoneurin. Progress in Neurobiology. 1995;46:49–70. doi: 10.1016/0301-0082(94)00060-u. [DOI] [PubMed] [Google Scholar]
- Gao YJ, Takemori K, Su LY, An WS, Lu C, Sharma AM, Lee RM. Perivascular adipose tissue promotes vasoconstriction: the role of superoxide anion. Cardiovasc Res. 2006;71:363–373. doi: 10.1016/j.cardiores.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Gorlach A, Brandes RP, Nguyen K, Amidi M, Dehghani F, Busse R. A gp91phox containing NADPH oxidase selectively expressed in endothelial cells is a major source of oxygen radical generation in the arterial wall. Circ Res. 2000;87:26–32. doi: 10.1161/01.res.87.1.26. [DOI] [PubMed] [Google Scholar]
- Greene LA, Farinelli SE, Cunningham ME, Park DS. Culture and experimental use of the PC 12 rat pheochromacytoma cell line. second 1998 [Google Scholar]
- Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circulation Research. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- Griendling KK, Sorescu D, Lassegue B, Ushio-Fukai M. Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. Arterioscler Thromb Vasc Biol. 2000a;20:2175–2183. doi: 10.1161/01.atv.20.10.2175. [DOI] [PubMed] [Google Scholar]
- Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000b;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- Gryglewski RJ, Palmer RM, Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986;320:454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- Gunes A, Ceylan A, Sarioglu Y, Stefek M, Bauer V, Karasu C. Reactive oxygen species mediate abnormal contractile response to sympathetic nerve stimulation and noradrenaline in the vas deferens of chronically diabetic rats: effects of in vivo treatment with antioxidants. Fundam Clin Pharmacol. 2005;19:73–79. doi: 10.1111/j.1472-8206.2004.00312.x. [DOI] [PubMed] [Google Scholar]
- Jesaitis AJ, Buescher ES, Harrison D, Quinn MT, Parkos CA, Livesey S, Linner J. Ultrastructural localization of cytochrome b in the membranes of resting and phagocytosing human granulocytes. J Clin Invest. 1990;85:821–835. doi: 10.1172/JCI114509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SA, Wood JD, Coffey MJ, Jones OT. The functional expression of p47-phox and p67-phox may contribute to the generation of superoxide by an NADPH oxidase-like system in human fibroblasts. FEBS Lett. 1994;355:178–182. doi: 10.1016/0014-5793(94)01201-6. [DOI] [PubMed] [Google Scholar]
- King AJ, Osborn JW, Fink GD. Splanchnic circulation is a critical neural target in angiotensin II salt hypertension in rats. Hypertension. 2007;50:547–556. doi: 10.1161/HYPERTENSIONAHA.107.090696. [DOI] [PubMed] [Google Scholar]
- King BF, Szurszewski JH. Peripheral reflex pathways involving abdominal viscera: transmission of impulses through prevertebral ganglia. Am J Physiol. 1989;256:G581–G588. doi: 10.1152/ajpgi.1989.256.3.G581. [DOI] [PubMed] [Google Scholar]
- Kolo LL, Westfall TC, Macarthur H. Nitric oxide decreases the biological activity of norepinephrine resulting in altered vascular tone in the rat mesenteric arterial bed. Am J Physiol Heart Circ Physiol. 2004;286:H296–H303. doi: 10.1152/ajpheart.00668.2003. [DOI] [PubMed] [Google Scholar]
- Kreulen DL. Properties of the venous and arterial innervation in the mesentery. J Smooth Muscle Res. 2003;39:269–279. doi: 10.1540/jsmr.39.269. [DOI] [PubMed] [Google Scholar]
- Lambeth JD, Cheng G, Arnold RS, Edens WA. Novel homologs of gp91phox. Trends Biochem Sci. 2000;25:459–461. doi: 10.1016/s0968-0004(00)01658-3. [DOI] [PubMed] [Google Scholar]
- Luckensmeyer GB, Keast JR. Distribution and morphological characterization of viscerofugal projections from the large intestine to the inferior mesenteric and pelvic ganglia of the male rat. Neuroscience. 1995;66:663–671. doi: 10.1016/0306-4522(94)00599-z. [DOI] [PubMed] [Google Scholar]
- Ma X, Zhang HJ, Whiteis CA, Tian X, Davisson RL, Kregel KC, Abboud FM, Chapleau MW. NAD(P)H oxidase-induced oxidative stress in sympathetic ganglia of apolipoprotein E deficient mice. Auton Neurosci. 2006;126–127:285–291. doi: 10.1016/j.autneu.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Mangiarua EI, Lee RM. Increased sympathetic innervation in the cerebral and mesenteric arteries of hypertensive rats. Can J Physiol Pharmacol. 1990;68:492–499. doi: 10.1139/y90-070. [DOI] [PubMed] [Google Scholar]
- Mao W, Qin F, Iwai C, Vulapalli R, Keng PC, Liang CS. Extracellular norepinephrine reduces neuronal uptake of norepinephrine by oxidative stress in PC12 cells. Am J Physiol Heart Circ Physiol. 2004;287:H29–H39. doi: 10.1152/ajpheart.01168.2003. [DOI] [PubMed] [Google Scholar]
- Mathias CJ. Role of sympathetic efferent nerves in blood pressure regulation and in hypertension. Hypertension. 1991;18:III22–III30. doi: 10.1161/01.hyp.18.5_suppl.iii22. [DOI] [PubMed] [Google Scholar]
- McLachlan EM, Llewellyn-Smith IJ. The immunohistochemical distribution of neuropeptide Y in lumbar pre- and paravertebral sympathetic ganglia of the guinea pig. J Auton Nerv Syst. 1986;17:313–324. doi: 10.1016/0165-1838(86)90097-4. [DOI] [PubMed] [Google Scholar]
- Meehan AG, Hottenstein OD, Kreulen DL. Capsaicin-sensitive nerves mediate inhibitory junction potentials and dilatation in guinea-pig mesenteric artery. J Physiol (Lond) 1991;443:161–174. doi: 10.1113/jphysiol.1991.sp018828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan AG, Kreulen DL. A capsaicin-sensitive inhibitory reflex from the colon to mesenteric arteries in the guinea-pig. J Physiol (Lond) 1992;448:153–159. doi: 10.1113/jphysiol.1992.sp019034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano PJ, Clark JK, Cifuentes-Pagano ME, Clark SM, Callis GM, Quinn MT. Localization of a constitutively active, phagocyte-like NADPH oxidase in rabbit aortic adventitia: enhancement by angiotensin II. Proc Natl Acad Sci USA. 1997;94:14483–14488. doi: 10.1073/pnas.94.26.14483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson C, Ruef J, Madamanchi NR, Barry-Lane P, Hu Z, Horaist C, Ballinger CA, Brasier AR, Bode C, Runge MS. Stimulation of a vascular smooth muscle cell NAD(P)H oxidase by thrombin. Evidence that p47(phox) may participate in forming this oxidase in vitro and in vivo. Journal of Biological Chemistry. 1999;274:19814–19822. doi: 10.1074/jbc.274.28.19814. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Karoon P, Burnstock G. Long-term sensory denervation by neonatal capsaicin treatment augments sympathetic neurotransmission in rat mesenteric arteries by increasing levels of norepinephrine and selectively enhancing postjunctional actions. Journal of Pharmacology and Experimental Therapeutics. 1995;274:64–71. [PubMed] [Google Scholar]
- Sumimoto H, Miyano K, Takeya R. Molecular composition and regulation of the Nox family NAD(P)H oxidases. Biochem Biophys Res Commun. 2005;338:677–686. doi: 10.1016/j.bbrc.2005.08.210. [DOI] [PubMed] [Google Scholar]
- Uddman R, Edvinsson L, Ekblad E, Hakanson R, Sundler F. Calcitonin gene-related peptide (CGRP): perivascular distribution and vasodilatory effects. Regulatory Peptides. 1986;15:1–23. doi: 10.1016/0167-0115(86)90071-6. [DOI] [PubMed] [Google Scholar]
- Ushio-Fukai M, Zafari AM, Fukui T, Ishizaka N, Griendling KK. p22phox is a critical component of the superoxide-generating NADH/NADPH oxidase system and regulates angiotensin II-induced hypertrophy in vascular smooth muscle cells. J Biol Chem. 1996;271:23317–23321. doi: 10.1074/jbc.271.38.23317. [DOI] [PubMed] [Google Scholar]
- Wang DH, Li J. Antihypertensive mechanisms underlying a novel salt-sensitive hypertensive model induced by sensory denervation. Hypertension. 1999;33:499–503. doi: 10.1161/01.hyp.33.1.499. [DOI] [PubMed] [Google Scholar]
- Wang DH, Li J, Qiu J. Salt-sensitive hypertension induced by sensory denervation: introduction of a new model. Hypertension. 1998;32:649–653. doi: 10.1161/01.hyp.32.4.649. [DOI] [PubMed] [Google Scholar]
- Whitelaw GP, Smithwick RH. Lumbodorsal splanchnicectomy in the treatment of essential hypertension. J Med Assoc Ga. 1958;47:492–497. [PubMed] [Google Scholar]
- Zheng ZL, Shimamura K, Anthony TL, Kreulen DL. Guanethidine evokes vasodilatation in guinea pig mesenteric artery by acting on sensory nerves. Neuroscience Letters. 2000;288:231–235. doi: 10.1016/s0304-3940(00)01234-9. [DOI] [PubMed] [Google Scholar]
- Zheng ZL, Shimamura K, Anthony TL, Travagli RA, Kreulen DL. Nitric oxide is a sensory nerve neurotransmitter in the mesenteric artery of guinea pig. J Auton Nerv Syst. 1997;67:137–144. doi: 10.1016/s0165-1838(97)00100-8. [DOI] [PubMed] [Google Scholar]