Abstract
One of the initial steps in the inflammatory process involves the adherence and transmigration of circulating polymorphonuclear leukocytes (PMN) across the endothelial cell monolayer. One of the main constituents of the neutrophil phagosome that contributes to bacterial killing is myeloperoxidase (MPO) which can be measured spectrophotometrically, using hydrogen peroxide as a substrate, and hence can be used as an index to quantify neutrophil adherence. To evaluate whether PMN isolated from umbilical cord blood could be used for in vitro experiments to monitor neutrophil adherence, we compared adherence to confluent endothelial and epithelial cell monolayers using PMN isolated from umbilical cord and adult peripheral blood. The extent of PMN adherence was assessed by measuring MPO activity. In initial experiments, we isolated PMN from umbilical cord and adult peripheral blood and measured MPO activity with respect to cell number and assay incubation times. Our data demonstrate that PMN obtained from either source had similar MPO activity and similar adherence to endothelial or epithelial cells. In conclusion, our data suggest that umbilical cord blood is a suitable source of leukocytes to examine PMN adherence in the setting of inflammation in a variety of disease processes.
Keywords: neutrophil adherence, myeloperoxidase, inflammation
INTRODUCTION
Neutrophils are among the first line of host defense mechanisms and are responsible for the destruction of microorganisms and damaged cells and tissues (Nathan, 2006). They target bacteria, fungi, protozoa, viruses, virally infected cells and tumor cells. Circulating neutrophils are closely apposed to the endothelial lining of capillaries. In response to endothelial cell activation, neutrophils adhere to the endothelium and transmigrate to the site of infection and inflammation (Rinaldo and Basford, 1987). On activation, neutrophils release their granules which contain mediators that contribute to host defense (Nauseef, 2007). Neutrophil adherence to cell monolayers is employed routinely in the laboratory to study endothelial inflammatory responses and involves isolation from adult peripheral blood and quantitation of myeloperoxidase (MPO) activity. MPO belongs to the haem peroxidase-cyclooxygenase superfamily and is abundantly expressed in neutrophils where it makes up 2–5% of the total cellular proteins (2 to 4 μg per 106 cells) (Malle et al., 2007). MPO utilizes hydrogen peroxide to form hypochlorous acid, a strong oxidant. This system is known to kill many species of bacteria by damaging the cell membrane, proteins and nucleic acids within the invading organism (Goldstein et al., 2007).
The importance of neutrophils to fight infections is well recognized. Adherence of neutrophils to endothelial cell monolayers in culture is a useful research tool to study the inflammatory process in vitro. Typically, neutrophils are isolated from the peripheral blood of adult human volunteers. Isolation of neutrophils from adult peripheral blood usually involves separation on a density gradient followed by further purification. We have used Polymorphprep™ (Axis-Shield, Oslo, Norway), a solution containing 13.8% (w/v) sodium diatrizoate and 8.0% (w/v) polysaccharide to isolate PMN from adult peripheral blood. The isolation procedure involves collection of blood into 1.5–2.0 mM EDTA, followed by centrifugation over Polymorphprep™ to isolate PMN which collect as a band below the interface. When applying this method to umbilical cord blood units, our attempts to isolate PMN failed repeatedly. Accordingly, we developed a modified technique to isolate PMN from umbilical cord blood units that resulted in a preparation of comparable quality to that from adult peripheral blood.
Umbilical cord blood could serve as a useful source of PMN for research purposes since the method of collection is non invasive and there is a greater population size and variation of donors (Lewis, 2002). However, details concerning a protocol for umbilical cord blood-derived PMN isolation, evaluation of PMN adherence and MPO activity are not readily available. We present data to show the PMN from adult and umbilical cord blood demonstrated similar MPO activity and adherence properties under experimental conditions. Thus we demonstrate that human umbilical cord blood can be used as a source of PMN for research studies in the investigation of inflammatory cell adherence to endothelial or epithelial cell monolayers.
MATERIALS AND METHODS
Culture of endothelial and epithelial cells
Human coronary artery endothelial cells (HCAECs) or human small airways epithelial cells (HSAEC) were obtained from Lonza (Walkersville, MD). Cells were grown in growth medium-2 (Lonza, Walkersville, MD) until they reached ~90% confluence. Cells were passaged at a 1:3 ratio using the Reagentpack™ subculture reagents (Lonza, Walkersville, MD). Cells from passages 3–4 were used for experiments.
Isolation of PMN from umbilical cord blood
Human umbilical cord blood units were obtained from the Saint Louis University Cord Blood Bank. Umbilical cord blood was layered over an equal volume of 87% Polymorphprep™ (Axis Shield, Oslo, Norway), diluted with phosphate buffered saline (PBS, 21-040-CM, Sigma Chemical Co., St. Louis, MO) pH=7.4, in a polypropylene 15ml conical tube (Corning Inc., Corning, NY). Tubes were centrifuged for 30 minutes at 450 × g, at 20°C with no brake. The topmost band at the sample-medium interface containing mononuclear cells was discarded and the lower band containing PMN was placed into a clean 50ml polypropylene conical tube (Corning Inc., Corning, NY). An equal amount of 0.5N Hanks’ buffered salt solution (HBSS, H6648, Sigma Chemical Co., St. Louis, MO) pH=7.4, was added to the cell suspension, and HBSS was used to bring the total volume up to 50ml. Tubes were centrifuged at 400 × g for 10 min at 4°C. Supernatant was discarded and the cell pellet was resuspended in 3ml of room temperature 0.2% NaCl, pH=7.4 and incubated for 3min at room temperature to lyse any residual contaminating erythrocytes. 3ml of ice cold 1.6 % NaCl was added and the solution transferred to a 15ml conical tube on ice. Cells were then centrifuged at 200 × g for 10min at 4°C. Supernatant was discarded and the PMN resuspended (4 × 106 PMN/ml) in minimum essential medium Eagle (MEM, M8042, Sigma Chemical Co., St. Louis, MO) and 10% fetal bovine serum (FBS, F4135, Sigma Chemical Co., St. Louis, MO). Care was taken to utilize cells within one hour of isolation.
Isolation of PMN from adult peripheral blood
Adult peripheral blood (20–40 ml) was collected from volunteers in vials containing EDTA as the anti-coagulant (BD Vacutainer®, 10.8 mg spray dried K2EDTA/tube, 6 ml blood draw, BD, Mississauga, Ontario, Canada). The protocol was approved by the Institutional Review Board of Saint Louis University School of Medicine, and all volunteers gave informed consent. Adult peripheral blood was layered over 100% Polymorphprep™, followed by centrifuging at 500 × g at 20°C with no brake. The remainder of the isolation protocol was identical to that used for isolating PMN from umbilical cord blood.
PMN Adherence Assay
Human coronary artery endothelial cells (HCAEC) or human small airways epithelial cells (HSAEC) were grown to confluence in 12-well plates and washed twice with HBSS. Following stimulation, 2 × 106 PMN, in 500 μl were added to each well and incubated for 20 min at room temperature. Media and unbound neutrophils were then discarded and plates were washed twice with phosphate buffered saline (PBS) at 37°C. To lyse the adherent cells and endothelial cells 1ml of 0.2% Triton X-100 was added to each well. Cell lysates were scraped from the plate and transferred to an eppendorf tube. A 500 μl (2 × 106 PMN) aliquot of neutrophil suspension was added to 500μl of 0.2% Triton X-100 and used as the theoretical maximal binding sample. Samples were sonicated (550 Sonic Dismembrator; Fisher Scientific, Pittsburgh, PA) for 10sec.
Myeloperoxidase Assay
400 μl of cell lysate was transferred to a glass tube containing 1ml of PBS, 1.2ml of HBSS + bovine serum albumin (BSA, fraction V, Research Organics, Cleveland, OH), 200μl of 0.125% 3,3′-dimethoxybenzidine dihydrochloride (Sigma Chemical Co., St. Louis, MO), and 200μl of 0.05% H2O2 (Sigma Chemical Co., St. Louis, MO). The reaction mixture was incubated for 15min at room temperature and the reaction terminated by the addition of 200μl of 1% sodium azide (Sigma Chemical Co., St. Louis, MO). The absorbance of each tube was then measured at 460 nm using a 4050 UV-Visible Spectrophotometer (Biochrom, Cambridge, England). A blank sample was made by adding 500μl of 0.2% Triton X-100 to 500μl of distilled water followed by addition of PBS, 1.2ml of HBSS + BSA, 200μl of 0.125% 3,3′-dimethoxybenzidine dihydrochloride, and 200μl of 0.05% H2O2. The absorbance of the blank was subtracted from the measured absorbance of the samples.
Annexin V staining
Apoptosis in freshly isolated PMN was assessed using an Annexin V-FITC apoptosis detection kit (BioVision Research Products, Mountain View, CA). Cells were incubated with Annexin V-FITC at room temperature in the dark for 5 minutes. The cell suspension was placed on a glass slide and cells were observed under a fluorescent microscope using a dual filter set for FITC and rhodamine. The number of Annexin V cells was counted and expressed as a percentage of the total number of cells that were counted under bright field.
Statistical analysis
Data were analyzed for significance using Students’ t-test. For all studies in which the experimental protocol involved three or more groups, data were analyzed using one-way ANOVA. Values are presented as mean ± SEM. P-values of <0.05 were considered statistically significant. Data were analyzed using StatMost software (Dataxiom Software Inc. Los Angeles, CA). Linear regression analysis was performed using PSIplot software (Poly Software International, Pearl River, NY).
RESULTS
To evaluate PMN obtained from cord blood we assessed MPO activity and adherence to endothelial cells and compared our data to that obtained using PMN from adult peripheral blood.
PMN Isolation
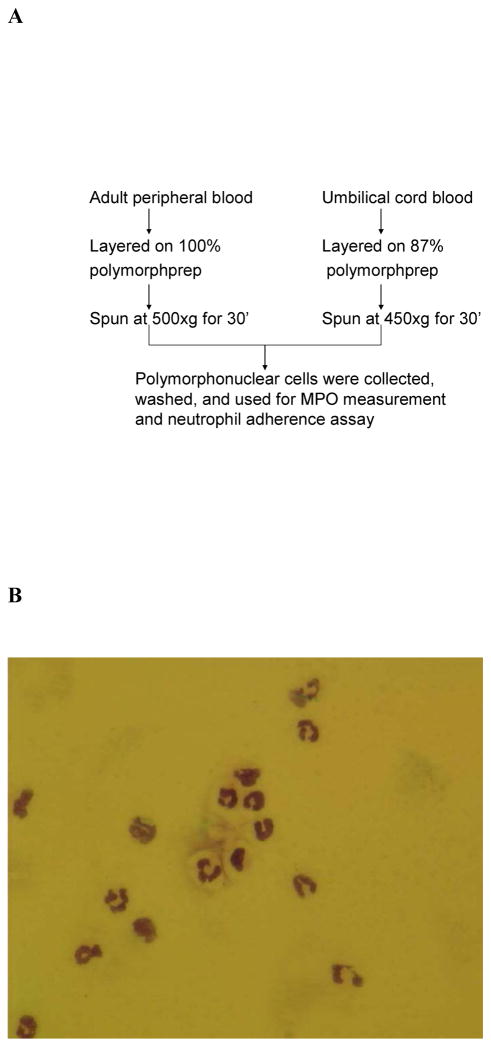
Isolation of PMN from umbilical cord blood was unsuccessful when using the protocol for isolation from adult peripheral blood. Since umbilical cord blood is collected into bags containing 35 ml of citrate phosphate dextrose as the anticoagulant, we diluted Polymorphprep™ from 100% to 80% at 1% intervals and centrifuged samples at 500, 450 and 400 × g. In preliminary studies, we consistently observed clean separations when using Polymorphprep™ diluted to 87% and centrifuging at 450 × g for 30 minutes (Figure 1A). Preparations obtained using our modified protocol consistently yielded products with greater than 90% PMN purity (Figure 1B, Table 1), similar to that obtained with adult peripheral blood. Isolated preparations from umbilical cord blood and adult peripheral blood contained greater than 90% cells that excluded trypan blue (Table 1). Annexin V staining revealed similar numbers of apoptotic cells in umbilical cord and adult peripheral blood (Table 1).
FIGURE 1.
Neutrophil isolation from adult peripheral blood and umbilical cord blood. (a) protocol showing modifications in neutrophil isolation protocol. (b) morphology of neutrophils isolated from umbilical cord blood.
Table 1.
Isolation purity and cell viability in PMN isolated from umbilical cord and adult peripheral blood. Data are expressed as mean ± SEM and were obtained from 10 separate isolations
| Umbilical Cord Blood | Adult Peripheral Blood | |
|---|---|---|
| PMN purity (% of total) | 92.3 ± 3.2 | 96.5 ± 2.1 |
| Trypan blue exclusion | 94.8 ± 1.6 | 98.7 ± 2.6 |
| Annexin V positive cells | 11.4 ± 3.5 | 7.6 ± 1.9 |
Myeloperoxidase release from neutrophils isolated from adult blood and cord blood
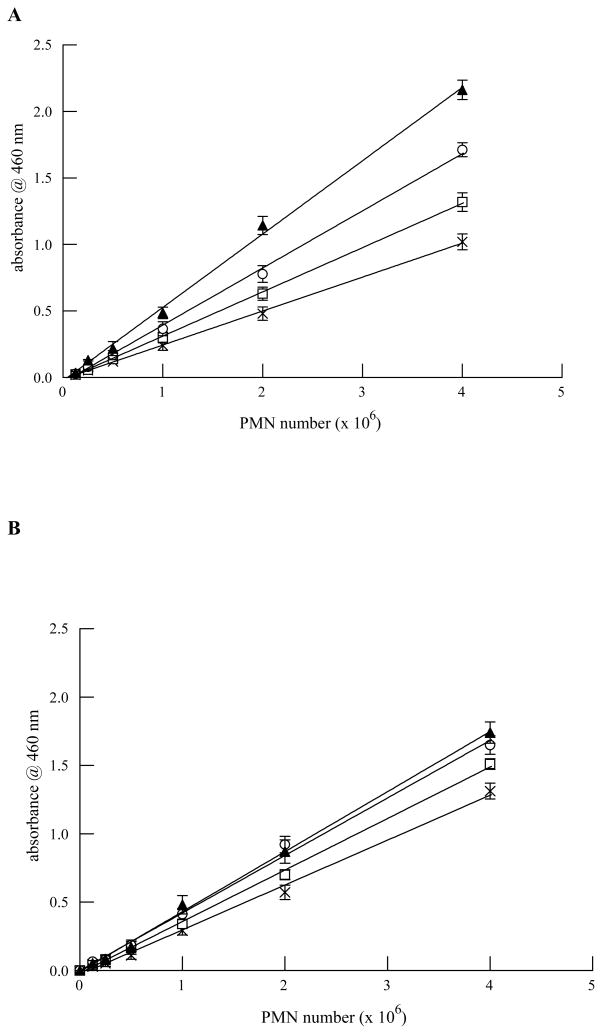
Since the time of collection between umbilical cord blood units and adult peripheral blood are different, we evaluated MPO activity in PMN isolated from either source to determine whether MPO activity was similar and that the assay system we were using would accurately determine MPO activity. After isolation from either source, PMN were used within 1 hour. All solutions for MPO activity assays were made fresh each day. This ensured optimal conditions to measure MPO activity. To be able to accurately assess PMN adherence to cells, the MPO assay must be linear over a wide range of PMN cell number. To ensure that our MPO assay conditions resulted in linear reaction velocities for each source of PMN, we incubated increasing numbers of PMN for up to 30 minutes and measured MPO activity. As shown in Figure 2, MPO activity was similar between PMN isolated from adult (Figure 2, Panel A) or umbilical cord blood (Figure 2, Panel B) with respect to number of neutrophils and time of incubation. Thus, the MPO assay conditions should yield reliable estimations of the extent of PMN adherence.
FIGURE 2.
MPO activity (measured as absorbance @ 460 nm) is linear with respect to PMN number (0.125–4 × 106) and incubation time (5–30 mins) for PMN isolated from adult peripheral blood (Panel A) and umbilical cord blood (Panel B). MPO activity was measured at 5 (X), 10 (open squares), 15 (open circles) and 30 minutes (filled triangles) following addition of all assay reagents. Data shown are mean ± SEM for 4 separate isolations.
Neutrophil adherence to endothelial cells
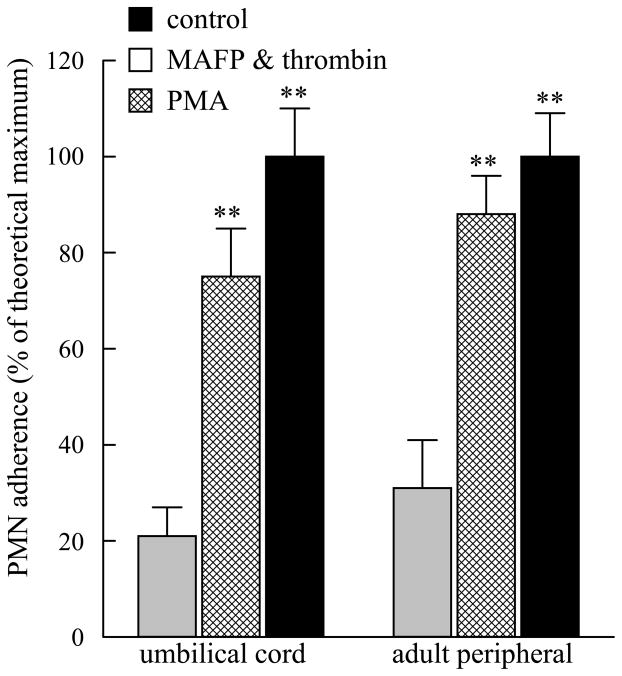
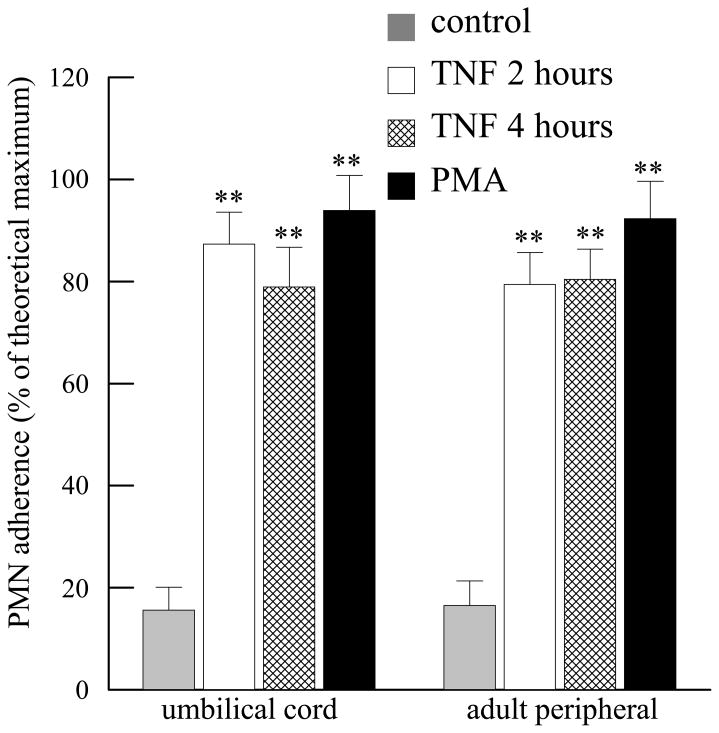
Human coronary artery endothelial cells (HCAEC) were grown to confluence in 12 well tissue culture plates and stimulated with phorobol 12-myristate 13-acetate (PMA, 100 nM, 10 mins). Medium was replaced and PMN isolated from umbilical cord or adult peripheral blood were added for 10 mins. As shown in Figure 3, incubation with PMA resulted in 100% adherence of PMN isolated from either source. When HCAEC were incubated with methyl arachidonyl fluorophosphonate (MAFP, 5 μM, 10 mins) to inhibit PAF acetylhydrolase activity (Kell et al., 2003; Vinson et al., 2005), followed by thrombin (1 IU/ml, 10 mins), PMN isolated from umbilical cord or adult peripheral blood demonstrated similar adherence (Figure 3). There was no statistical difference between adherence of umbilical cord or adult peripheral blood under any of the experimental conditions studied. HCAEC were stimulated with TNF-α (10 ng/ml) for 2 or 4 hours and PMN added for the last 20 minutes of incubation. TNF-α stimulation resulted in significant PMN adherence after 2 and 4 hours of incubation (Figure 4). PMN adherence was similar between cells isolated from adult peripheral or umbilical cord blood at each time point (Figure 4).
FIGURE 3.
Adherence of umbilical cord and adult peripheral blood PMN to human coronary artery endothelial cells incubated with methyl arachidonyl fluorophosphonate (MAFP, 5 μM, 10 mins) followed by thrombin (1U/ml, 10 mins) or phorbol 12-myristate 13-acetate (PMA, 100nM, 10 mins). **p<0.01 when compared to control. No statistical difference was detected between umbilical cord and adult peripheral blood under each experimental condition. Data shown are mean + SEM for 6 separate isolations
FIGURE 4.
Adherence of umbilical cord and adult peripheral blood PMN to human coronary artery endothelial cells incubated with TNFα (10 ng/ml) for 2 or 4 hours or with phorbol 12-myristate 13-acetate (PMA, 100nM, 10 mins). **p<0.01 when compared to control. No statistical difference was detected between umbilical cord and adult peripheral blood under each experimental condition. Data shown are mean + SEM for 6 separate isolations
Neutrophil adherence to epithelial cells
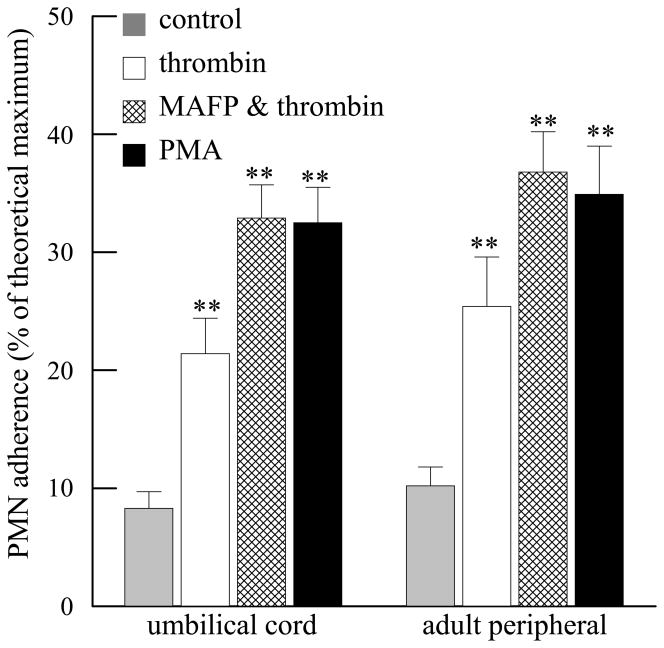
Human small airways epithelial cells (HSAEC) were grown to confluence in 12 well tissue culture plates and stimulated with thrombin (1IU/ml, 10 mins). Similar PMN adherence was observed with umbilical cord PMN and adult peripheral blood PMN (Figure 5). Inhibition of PAF acetylhydrolase by MAFP pretreatment prior to thrombin stimulation resulted in a similar degree of PMN adherence to that obtained with PMA treatment (Figure 5). There was no statistical difference in PMN adherence under any experimental condition when comparing PMN isolated from either source used (Figure 5).
FIGURE 5.
Adherence of umbilical cord and adult peripheral blood PMN to human small airways epithelial cells incubated with thrombin (1IU/ml, 10 mins), MAFP (5 μM, 10 mins) followed by thrombin (1IU/ml, 10 mins) or phorbol 12-myristate 13-acetate (PMA, 100nM, 10 mins). **p<0.01 when compared to control. No statistical difference was detected between umbilical cord and adult peripheral blood under each experimental condition. Data shown are mean + SEM for 4 separate isolations
Taken together, these data suggest that PMN isolated from umbilical cord blood demonstrated similar MPO activity and adherence properties to PMN isolated from adult peripheral blood and thus represent a suitable source of PMN for research.
DISCUSSION
We have modified the standard procedure for PMN isolation from adult peripheral blood to support the use of human umbilical cord blood, an established source of hematopoeitic stem cells (Goldstein et al., 2006; Goldstein et al. 2007). Cord blood is simple to collect and is without risk to the mother or newborn. Cord blood is collected before expulsion of the placenta, from the umbilical vein, in the delivery room (Goldstein et al., 2007). A typical collection volume ranges from 20 to 200 ml and blood is collected directly into bags containing 35 ml citrate, phosphate, dextrose solution. All products that have less than 45 ml of collected blood are not used for clinical purposes due to low total cell count. Additionally, approximately 30% of units that contain between 45 and 55 ml of cord blood are unsuitable for transplant purposes due to an insufficient number of cells. These units are available for research purposes and can be used to isolate several cell types. Currently, there is little data comparing the applicability of PMN isolated from umbilical cord blood for research.
In order to obtain a purified PMN product from cord blood units, we isolated PMN using a method modified to accommodate the use of diluted umbilical cord blood. Polymorphprep™, a solution of sodium diatrizoate and dextran 500, was diluted to 87% with phosphate buffered saline and an equal volume of citrate anticoagulated umbilical cord blood was layered on top. The dilution of Polymorphprep and decrease in centrifuge speed to 450 × g resulted in separation of PMN in a clear band, essentially free of mononuclear cells and erythrocytes. We used 15 ml conical centrifuge tubes to obtain a sharper band of PMN isolations. Modification of the isolation procedure resulted in a PMN preparation comprising of 90–95% PMN as determined by light microscopy (Figure 1B).
Myeloperoxidase activity in PMN isolated from umbilical cord blood or adult peripheral blood demonstrated similar characteristics. There was no significant difference in MPO activity between PMN isolated from umbilical cord or adult peripheral blood. These results agree with Nupponen and coworkers who found comparable MPO activity in preterm, term and adult blood samples (Nupponen et al., 2002).
In our MPO assays, we determined that solutions should be made fresh each day and assays should be carried out within 1 hour of PMN isolation to ensure optimal conditions. We found that MPO activity was linear with assay incubation time and total number of PMN isolated from both umbilical cord and adult peripheral blood, thus demonstrating their applicability to adherence assays.
The first major step in the migration of leukocytes from the bloodstream to inflamed tissues involves cell surface expression of P-selectin on activated endothelial cells (Burns et al., 1999; Geng et al., 1990; Gimbrone et al., 1990). In vivo, P-selectin binds to the P-selectin glycoprotein ligand on the leukocyte surface and results in rolling of the leukocyte along the vessel wall (Setiadi et al., 1998). Production of platelet-activating factor (PAF), which is also expressed on the endothelial cell surface, stimulates neutrophils leading to activation of β2 integrins (Carveth et al., 1992; Zimmerman et al., 1992; Hynes and Lander, 1992; Hynes, 1992). Leukocytes express a PAF receptor that enhances their endothelial cell adhesion and induces their β-integrin-mediated transmigration (Prescott et al., 2002). In previous studies, we have shown that thombin- or tryptase-stimulated endothelial cells demonstrate increased calcium-independent phospholipase A2 activity, resulting in increased PAF production and PMN adherence (Kell et al., 2003; Vinson et al., 2005; McHowat et al., 2001; Rickard et al., 2005; Meyer et al., 2005; White and McHowat, 2007a; White et al., 2007b). We have shown that methyl arachidonyl fluorophosphonate (MAFP) inhibits PAF acetylhydrolase (PAF-AH) the enzyme responsible for PAF degradation, and increases PAF production and PMN adherence to endothelial cell monolayers (Kell et al., 2003; Vinson et al., 2005). In this study, we demonstrate that PMN isolated from adult peripheral and umbilical cord blood adhere to HCAEC monolayers to a similar extent, suggesting that the PAF-PAF receptor interaction is the same in PMN isolated from either source. In fact, pretreatment of PMN from adult peripheral or umbilical cord blood with CV3988, a PAF receptor antagonist, completely inhibited adherence to HCAEC incubated with MAFP and thrombin (data not shown). We also demonstrated similar PMN adherence when HCAEC were stimulated with TNF-α for 2 and 4 hours. Incubation of HSAEC resulted in a similar pattern of PMN adherence as observed in HCAEC, but the amount of adherence was much lower under each experimental condition. PMA is routinely used as a positive control for maximal PMN adherence. PMN adherence in response to MAFP and thrombin was comparable to that for PMA in both endothelial and epithelial cells. No statistical difference was observed for adherence of PMN from umbilical cord or adult peripheral blood under any of the experimental conditions described, suggesting that umbilical cord blood can be used as a research tool for cell adherence assays.
We chose to use cord blood units that were less than 12 hours old, and freshly isolated adult peripheral blood for these studies in order to minimize PMN cell death by the time of use. We detected very few apoptotic PMN by Annexin V staining (data not shown) in either preparation following separation using Polymorphprep™, suggesting that apoptotic PMN are removed during the isolation procedure.
In summary, we have developed a modified isolation procedure for umbilical cord blood PMN that accounts for the variability in volume, dilution factor and age of samples. This procedure results in PMN preparations with 90–95% purity. Assay of PMN adherence to cell monolayers using MPO activity is comparable between umbilical cord and adult PMN over a wide range of cell numbers. Although we have not compared any functional assays between the two sources of PMN, we propose that umbilical cord blood represents a useful research tool to study PMN adherence to endothelial or epithelial cells when MPO activity assays are performed.
Acknowledgments
This work was supported by National Institutes of Health Grant DK66119. We thank the St. Louis University Umbilical Cord Blood Bank for cord blood supplies, assistance and advice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Burns AR, Bowden RA, Abe Y, Walker DC, Simon SI, Entman ML, Smith CW. P-selectin mediates neutrophil adhesion to endothelial cell borders. J Leukoc Biol. 1999 Mar;65(3):299–306. doi: 10.1002/jlb.65.3.299. [DOI] [PubMed] [Google Scholar]
- Carveth HJ, Shaddy RE, Whatley RE, McIntyre TM, Prescott SM, Zimmerman GA. Regulation of platelet-activating factor (PAF) synthesis and PAF-mediated neutrophil adhesion to endothelial cells activated by thrombin. Semin Thromb Hemost. 1992 Jan;18(1):126–34. doi: 10.1055/s-2007-1002417. [DOI] [PubMed] [Google Scholar]
- Geng JG, Bevilacqua MP, Moore KL, McIntyre TM, Prescott SM, Kim JM, Bliss GA, Zimmerman GA, McEver RP. Rapid neutrophil adhesion to activated endothelium mediated by GMP-140. Nature. 1990 Feb;22;343(6260):757–60. doi: 10.1038/343757a0. [DOI] [PubMed] [Google Scholar]
- Gimbrone MA, Jr, Bevilacqua MP, Cybulsky MI. Endothelial-dependent mechanisms of leukocyte adhesion in inflammation and atherosclerosis. Ann N Y Acad Sci. 1990;598:77–85. doi: 10.1111/j.1749-6632.1990.tb42279.x. [DOI] [PubMed] [Google Scholar]
- Goldstein G, Toren A, Nagler A. Human Umbilical Cord Blood Biology, Transplantation and Plasticity. Curr Med Chem. 2006;13(11):1249–59. doi: 10.2174/092986706776872998. [DOI] [PubMed] [Google Scholar]
- Goldstein G, Toren A, Nagler A. Transplantation and Other Uses of Human Umbilical Cord Blood and Stem Cells. Curr Pharm Des. 2007;13(13):1363–73. doi: 10.2174/138161207780618759. [DOI] [PubMed] [Google Scholar]
- Hynes RO, Lander AD. Contact and adhesive specificities in the associations, migrations, and targeting of cells and axons. Cell. 1992 Jan;24;68(2):303–22. doi: 10.1016/0092-8674(92)90472-o. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr;3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Kell PJ, Creer MH, Crown KN, Wirsig K, McHowat J. Inhibition of platelet-activating factor (PAF) acetylhydrolase by methyl arachidonyl fluorophosphonate potentiates PAF synthesis in thrombin-stimulated human coronary artery endothelial cells. J Pharmacol Exp Ther. 2003 Dec;307(3):1163–70. doi: 10.1124/jpet.103.055392. Epub 2003 Oct 14. [DOI] [PubMed] [Google Scholar]
- Lewis ID. Clinical and experimental uses of umbilical cord blood. Intern Med J. 2002 Dec;32(12):601–9. doi: 10.1046/j.1445-5994.2002.00276.x. [DOI] [PubMed] [Google Scholar]
- Malle E, Furtmüller PG, Sattler W, Obinger C. Myeloperoxidase: a target for new drug development? Br J Pharmacol. 2007 Nov;152(6):838–54. doi: 10.1038/sj.bjp.0707358. Epub 2007 Jun 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHowat J, Kell PJ, O’Neill HB, Creer MH. Endothelial cell PAF synthesis following thrombin stimulation utilizes Ca(2+)-independent phospholipase A(2) Biochemistry. 2001 Dec;11;40(49):14921–31. doi: 10.1021/bi0156153. [DOI] [PubMed] [Google Scholar]
- Meyer MC, Creer MH, McHowat J. Potential role for mast cell tryptase in recruitment of inflammatory cells to endothelium. Am J Physiol Cell Physiol. 2005 Dec;289(6):C1485–91. doi: 10.1152/ajpcell.00215.2005. Epub 2005 Aug 3. [DOI] [PubMed] [Google Scholar]
- Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006 Mar;6(3):173–82. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- Nauseef WM. How human neutrophils kill and degrade microbes: an integrated view. Immunol Rev. 2007 Oct;219:88–102. doi: 10.1111/j.1600-065X.2007.00550.x. [DOI] [PubMed] [Google Scholar]
- Nupponen I, Turunen R, Nevalainen T, Peuravuori H, Pohjavuori M, Repo H, Andersson S. Extracellular release of bactiricidal/permeability-increasing proteinin newborn infants. Pediatr Res. 2002 Jun;51(6):667–9. doi: 10.1203/00006450-200206000-00002. [DOI] [PubMed] [Google Scholar]
- Prescott SM, McIntyre TM, Zimmerman GA, Stafforini DM. Sol Sherry lecture in thrombosis: molecular events in acute inflammation. Arterioscler Thromb Vasc Biol. 2002 May;1;22(5):727–33. doi: 10.1161/01.atv.0000016153.47693.b2. [DOI] [PubMed] [Google Scholar]
- Rickard A, Portell C, Kell PJ, Vinson SM, McHowat J. Protease-activated receptor stimulation activates a Ca2+-independent phospholipase A2 in bladder microvascular endothelial cells. Am J Physiol Renal Physiol. 2005 Apr;288(4):F714–21. doi: 10.1152/ajprenal.00288.2004. Epub 2004 Nov 23. [DOI] [PubMed] [Google Scholar]
- Rinaldo JE, Basford RE. Neutrophil-endothelial interactions: Modulation of neutrophil activation responses by endothelial cells. Tissue Cell. 1987;19(5):599–606. [PubMed] [Google Scholar]
- Setiadi H, Sedgewick G, Erlandsen SL, McEver RP. Interactions of the cytoplasmic domain of P-selectin with clathrin-coated pits enhance leukocyte adhesion under flow. J Cell Biol. 1998 Aug;10;142(3):859–71. doi: 10.1083/jcb.142.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinson SM, Rickard A, Ryerse JS, McHowat J. Neutrophil adherence to bladder microvascular endothelial cells following platelet-activating factor acetylhydrolase inhibition. J Pharmacol Exp Ther. 2005 Sep;314(3):1241–7. doi: 10.1124/jpet.105.085365. Epub 2005 Jun 3. [DOI] [PubMed] [Google Scholar]
- White MC, McHowat J. Protease activation of calcium-independent phospholipase A2 leads to neutrophil recruitment to coronary artery endothelial cells. Thromb Res. 2007;120(4):597–605. doi: 10.1016/j.thromres.2006.11.007. Epub 2006 Dec 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MC, Rastogi P, McHowat J. Lysoplasmenylcholine increases neutrophil adherence to human coronary artery endothelial cells. Am J Physiol Cell Physiol. 2007 Nov;293(5):C1467–71. doi: 10.1152/ajpcell.00290.2007. Epub 2007 Aug 29. [DOI] [PubMed] [Google Scholar]
- Zimmerman GA, Prescott SM, McIntyre TM. Endothelial cell interactions with granulocytes: tethering and signaling molecules. Immunol Today. 1992 Mar;13(3):93–100. doi: 10.1016/0167-5699(92)90149-2. [DOI] [PubMed] [Google Scholar]