Abstract
Coordinated interactions between helper and cytotoxic T-lymphocytes (HTL and CTL) are needed for optimal effector cell functions and the establishment of immunological memory. We, therefore, designed a mixed format vaccine based on the use of highly-conserved HIV-derived T-lymphocyte epitopes wherein the HTL epitopes were delivered as a recombinant protein and the CTL epitopes which were encoded in a DNA vaccine plasmid. Immunogenicity testing in HLA transgenic mice and GLP preclinical safety testing in rabbits and guinea pigs were used to document the utility of this approach and to support Phase 1 trial clinical testing. Both vaccine components were immunogenic and safely co-administered.
Introduction
The role of HIV-1-specific CD8+ cytotoxic T-lymphocytes (CTL) in controlling HIV replication was initially demonstrated in acute HIV-1 infection where an early expansion of CD8+ T-lymphocytes with CTL activity was observed and this rise was temporally associated with the initial in vivo decline of viremia [1, 2]. Data also indicate CTL to be involved in controlling longer term HIV-1 replication and in delaying disease progression in chronically infected individuals [3–7]. This relationship is most notable for individuals capable of controlling viral replication in vivo without anti-retroviral drug therapy where the breadth of epitope recognition, recognition of non-dominant epitopes, HLA restriction and the types and quantities of cytokines produced, may all be involved [8–11]. The potent, broadly-reactive HIV-1-specific CTL responses found in these individuals are likely attributable to the above [12–18]. Thus, it is highly likely that HIV vaccine efficacy will correlate, at least in part, with effectiveness of CTL induction.
The critical role of CD4+ helper T-lymphocytes (HTL) in effective HIV-specific immunity was documented using HIV-infected individuals where loss of CD4+ T-lymphocytes was noted as the hallmark symptom of disease. The observations that HIV-specific CD4+ T-lymphocytes proliferate and produce cytokines, specifically interleukin-2 (IL-2) and interferon-γ (IFN-γ), more effectively and frequently in individuals who control viral replication long-term also document their importance [19–23]. More recently, dysfunctional HTL that are unable to appropriately assist in the expansion, differentiation, and maintenance of HIV-specific CTL, were identified as the likely cause of ineffective CTL control over viral replication [24]. Thus, the combined function of CD4+ and CD8+ T-lymphocytes is required for optimal control of HIV replication [25].
The need for coordinated functioning of CTL and HTL for optimal long-term control of HIV thus appears similar to that characterized for many viral and intracellular pathogens. For example, the lack of HTL following chronic lymphocytic choriomeningitis virus infection severely limits the ability of the immune system to control viral replication and results in functional inactivation of CTL. Contributions of CD4+ HTL are similarly important for sustaining herpesvirus-specific CTL [26, 27]. The induction of CTL responses against Listeria and vaccinia viruses without the induction of HTL memory was also demonstrated to be of limited benefit following infection, indicating the contribution of HTL in the development of long-lived CTL memory T cell function [28–30]. Failure to induce both CD4+ and CD8+ memory cells may, in fact, be detrimental because CTL priming in the absence of HTL activity can result in CTL apoptosis upon re-exposure to antigen [29].
Prophylactic HIV vaccines are now the focus of multiple research and development programs with a common clinical goal being the induction of both CTL and HTL responses [31–33]. We have identified and characterized highly conserved CTL and HTL epitopes in numbers sufficient to support the production and clinical testing of experimental HIV vaccines based solely on the use of the CTL and HTL epitopes in combinations that theoretically can provide for high levels of predicted human population coverage while inducing immune responses to epitopes common to all of the major HIV subtypes. There are no well defined designs or formulation parameters to support the development of vaccines composed of large numbers of T cell epitopes. Thus, we developed a program to assess delivery of HIV-derived CTL or HTL epitopes alone or in combinations using DNA plasmids, viral vectors and recombinant proteins. We initially designed a DNA plasmid vaccine, designated EP HIV-1090, encoding 21 CTL epitopes that are restricted to allelic products of three common HLA supertypes, HLA-A2, -A3 and –B7, and the universal HTL epitope, termed PADRE [34]. This experimental vaccine was designed to induce HIV-specific CTL responses, but not HIV-specific HTL responses, as it was primarily intended for use as a post-infection HIV therapeutic product. The reason for this unique design is that activated CD4+ T-lymphocytes are the major targets of HIV infection and HIV-specific HTL appear to be preferentially infected and killed [35, 36].
The vaccine was successfully tested HLA transgenic mice and other animal species to document product safety and immunogenicity of both the CTL epitopes and the single PADRE HTL epitope. However, the immunogenicity of the CTL epitopes proved to be suboptimal in Phase 1 clinical trials involving either HIV-infected or uninfected volunteers and the PADRE HTL epitope was essentially non-immunogenic [34, 45]. These clinical data suggest the DNA vaccine format may be inefficient as a means to deliver a vaccine immunogen composed of individual T cell epitopes.
The lack of the PADRE HTL epitope immunogenicity associated with the use of the DNA vaccine format was a primary concern since lack of HTL responses will impact the effective induction of CTL responses. Efficient induction of both CTL and HTL responses using a single vaccine format can be difficult because the epitopes that provide the specificity are generated through different cellular pathways [38]. Thus, we assumed that a single vaccine format may not be optimal and we opted to produce and test vaccine components which differ in format and are, thus, better designed specifically to induce CTL or HTL responses. Specifically, we designed a recombinant protein composed of HIV-derived conserved HTL epitopes for use in combination with the DNA vaccine encoding the CTL epitopes. Herein, we describe the design, manufacture and preclinical animal testing completed to support Phase 1 clinical testing of this mixed format vaccine for HIV.
Materials and methods
T-lymphocyte epitope identification and characterization
CTL and HTL epitopes were previously identified from intact HIV-1 amino acid sequences (Los Alamos database) representing common subtypes and the two circulating recombinant forms (Tables 1 and 2) using peptide binding motifs characteristic for the HLA-A2, -A3, -B7 and HLA-DR1,4,7 supertypes, respectively. Synthetic peptides representing individual epitopes were synthesized using an Applied Biosystems (Foster City, CA) 430A peptide synthesizer and standard FMOC chemistry. Peptides were purified by reverse phase HPLC using a Gilson (Middleton, WI) preparative system and characterized using analytical HPLC (HP1090; Palo Alto, CA) and mass spectrometry analysis (API 100 electrospray; Applied Biosystems). Peptides representing individual epitopes were used to measure HLA binding [39, 40] and recognition by T-lymphocytes obtained from HIV-infected donors [34, 41, 42].
Table 1.
HIV-derived CTL epitopes used in vaccine design and synthesis
| HLA-A2 Epitopes | HLA-A3 Epitopes | HLA-B7 Epitopes | |||
|---|---|---|---|---|---|
| Protein Designation1 | Amino Acid Sequence1 | Protein Designation | Amino Acid Sequence | Protein Designation | Amino Acid Sequence |
| Pol 498 | ILKEPVHGV | Env 47 | VTVYYGVPVWK | Nef 94 | FPVRPQVPL |
| Gag 386 | VLAEAMSQV | Pol 929 | QMAVFIHNFK | Gag 545 | YPLASLRSLF |
| Pol 448 | KLVGKLNWA | Pol 98 | VTIKIGGQLK | Rev 75 | VPLQLPPL |
| Env 134 | KLTPLCVTL | Pol 971 | KIQNFRVYYR | Env 259 | IPIHYCAPA |
| Vpr 62 | RILQQLLFI | Pol 347 | AIFQSSMTK | Gag 237 | HPVHAGPIA |
| Nef 221 | LTFGWCFKL | Pol 722 | KVYLAWPAHK | Pol 893 | IPYNPQSQGW |
| Gag 271 | MTNNPPIPV | Env 61 | TTLFCASDAK | Env 250 | CPKVSFEPI |
Epitopes are designed based on their location within HIV viral gene products using gene name and the position of the first amino acid using an internal consensus sequence specific for the data base used. Sequences are shown using the single letter amino acid designations.
Table 2.
HIV-derived HTL epitopes used in vaccine design and synthesis
| Protein Designation1 | Amino Acid Sequence | HLA-DR Antigens Bound |
|---|---|---|
| Env 566 | IKQFINMWQEVGKAMY | 8 |
| Env 729 | QHLLQLTVWGIKQLQ | 9 |
| Gag1712 | QGQMVHQAISPRTLN | 9 |
| Gag 2942 | GEIYKRWIILGLNKI | 10 |
| Gag 2982 | KRWIILGLNKIVRMY | 13 |
| Pol 3032 | FRKYTAFTIPSINNE | 7 |
| Pol 3352 | SPAIFQSSMTKILEP | 9 |
| Pol 596 | WEFVNTPPLVKLWYQ | 11 |
| Pol 7112 | EKVYLAWVPAHKGIG | 10 |
| Pol 7122 | KVYLAWVPAHKGIGG | 10 |
| Pol 758 | HSNWRAMASDFNLPP | 8 |
| Pol 9152 | KTAVQMAVFIHNFKR | 8 |
| Pol 9562 | QKQITKIQNFRVYYR | 12 |
| Pol 6192 | AETFYVDGAANRETK | 2 |
| Pol 674 | EVNIVTDSQYALGII | 3 |
| Pol 874 | WAGIKQEFGIPYNPQ | 5 |
| Pol 989 | GAVVIQDNSDIKVVP | 5 |
| Vpu 312 | YRKILRQRKIDRLID | 7 |
Epitopes are designed based on their location within HIV viral gene products as noted in Table 1.
Immunogenicity of the individual peptides was established by immunizing H2bxd mice with peptide emulsified in CFA and measured as a function of peptide-specific IFN-γ production using an EPISPOT. Those indicated were immunogenic in mice and used for immunization controls and assays.
Design and production of a recombinant baculovirus for EP-1043 protein expression
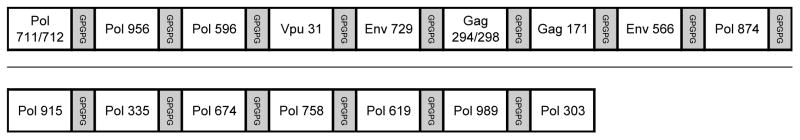
The EP-1043 protein was designed using the 18 HTL epitopes, organized as 16 individual peptide sequences separated by glycine-proline-based (GPGPG) spacers to enhance extracellular proteolytic cleavage between individual epitopes (Figure 1) [43]. The gene segment encoding the EP-1043 recombinant protein was produced synthetically using overlapping oligonucleotides in an overlap extension PCR-based synthesis [34] but with codon selection suitable for expression in drosophila insect cells using a baculovirus expression system. A synthetic peptide composed of 80 amino acids from region 109–182 of the EP-1043 protein was used as an immunogen to produce antisera in rabbits (Green Mountain Antibodies, Inc, Burlington, VT) to support the protein expression and purification.
Figure 1. Schematic representation of the epitope configuration in the EP-1043 recombinant protein.
Note that epitopes Gag 294 and Gag 298 and Pol 711 and 712 are combined and designed to be initially processed into larger peptides with nested epitopes.
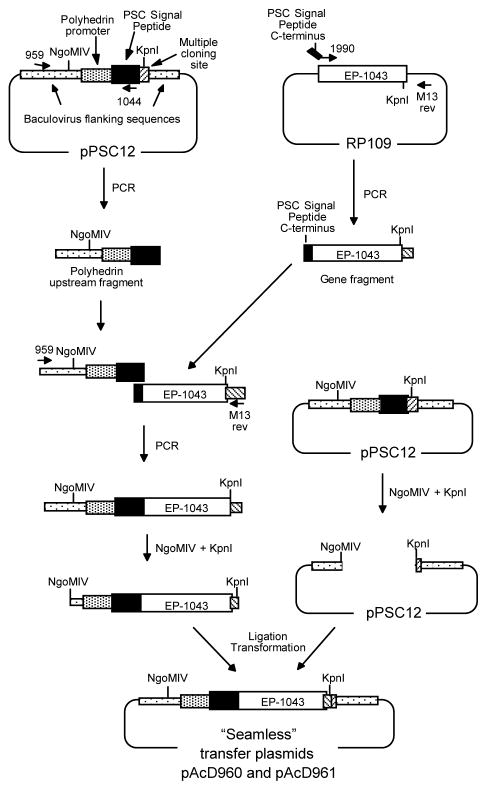
A two step overlap extension PCR based synthesis strategy was used to construct a recombinant baculovirus vector for expression of the EP-1043 protein in insect cells (Figure 2). First, the synthetic HTL epitope coding sequence was fused to a partial sequence of the signal peptide, derived from the chitinase gene of Autographa californica nuclear polyhedrosis virus (AcNPV), using the EP-1043 plasmid as the template. The resulting 1.1 kbp product was gel-purified and the second PCR reaction was performed to fuse the baculovirus polyhedron promoter and intact AcNPV signal peptide using the pPSC12 baculovirus transfer plasmid as the template. Following digestion of the resulting product with NgoMIV and KpnI, the 1.6 kbp fragment was gel-purified and ligated into NgoMIV-KpnI-cut pPSC12. After transformation of competent E. coli (DH5α) cells with the ligation mix, several positive clones were identified by restriction digest and sequence analyses of the plasmid DNA.
Figure 2. Overlap extension synthesis and cloning strategy for insertion of the EP-1043 HTL epitope coding sequence into the baculovirus transfer plasmid.
The EP-1043 gene product was synthesized using overlapping oligonucleotides, averaging 60 to 90 bp in length with overlaps of approximately 15–20 bp, (Operon Technologies, Alameda, CA). Constructs were assembled by extending the overlapping oligonucleotides using pfu polymerase (Stratagene, San Diego). The resulting full-length product was sequenced and sub-cloned into RP109 DNA plasmid vector. The EP-1043 DNA plasmid was used as a template to fuse a segment of the AcNPV signal peptide to the HTL epitope coding sequence using the O-1990 5′ primer (GGTCGCCGTTTCTAACGCG/GAAAAAGTCTACCTGGCATGG: the C-terminus of the signal peptide/N-terminus of the EP-1043 HTL epitope gene) and the M13rev 3′ primer (TCACACAGGAAACAGCTATGAC: pCR Blunt downstream). The resulting 1.1 kbp PCR product was purified. The pPSC12 plasmid consists of the AcNPV EcoRI “I” fragment inserted into pUC8, with the polyhedrin coding sequences 3′ of the ATG start codon replaced with the AcNPN signal peptide and a polylinker site. The pPSC12 plasmid was used as a PCR template with the O-959 5′ primer (CTGGTAGTTCTTCGGAGTGTG: polyhedron upstream region) which annealed upstream of the unique NgoMIV and EcoRV sites and the O-1044 3′ primer (CGCGTTAGAAACGGCGACC: C-terminus of the signal peptide) which annealed to the 3′ end to produce the PSC signal peptide. To fuse the PSC signal peptide sequence to EP-1043, another PCR reaction was run. Using primers O-959 and M13rev, 1.1kb EP-1043 was combined with PSC signal peptide annealed and extended by PCR. The resulting PCR product was digested with NgoMIV and KpnI, the 1.6 kbp fragment was purified and ligated into NgoMIV-KpnI-cut pPSC12.
Recombinant baculovirus expression vector, S. frugiperda (Sf9) insect cells were co-transfected with linearized parental AcNPV baculovirus DNA and the pAcD961 baculovirus transfer plasmid to transfer the HTL epitopes expression cassette from the pAcD961 plasmid into the baculovirus genome via homologous recombination. Recombinant baculovirus stocks were prepared using sequential in vitro culture based infections of Sf9 cells, adapted for growth in serum-free SF900 II-SFM media (Life Technologies, Inc., Grand Island, NY), supplemented with 0.2 μg/ml of recombinant human insulin (Sigma-Aldrich, St. Louis, MO); cells are designated express SF+ [44].
Production, purification and characterization of EP-1043 recombinant protein vaccine
Master Cell Bank (MCB) 081093 of the expresSF+ cell line was used for the manufacture of the EP-1043 recombinant protein; product was produced using methods and documentation consistent with Current Good Manufacturing Practices (cGMP) for the portion of the material used for animal safety testing. Cells were infected at the concentration of 1.5 – 3 × 106 cells/ml with a multiplicity of infection of 1.0 and cultured for 96 hr with the addition of 1.0μg/ml leupeptin at 48 hr to control protein degradation. Fermentation batches for EP-1043 were completed using 500 L culture volumes in a 600 L bioreactor (ABEC, Bethleham, PA). Cultures were maintained at 28 ± 1 °C with a stirring speed of 40 ± 2 rpm and a dissolved oxygen level of 60 ± 5% dissolved oxygen. Cells were harvested using continuous flow centrifugation and pellets were stored at −20 °C.
Thawed cells were washed and disrupted at 4 C in 30 mM Tris-base, 50 mM NaCl, 0.5% Tergitol, and 0.05% β-mercaptoethanol (pH 8.0) using a Polytron homogenizer (Brinkmann Instruments Inc., Westbury, NY). The insoluble EP-1043-containing fraction was separated from soluble protein contaminants by low-speed centrifugation (60 minutes, 5900 × g, at 2 – 8 °C) and solubilized with 6 M urea, 11 mM monosodium phosphate monohydrate (NaH2PO4•1H2O), 36 mM disodium phosphate decahydrate (Na2HPO4•12H2O), 10 mM glycine, 0.06% β-mercaptoethanol, 10 μg/mL leupeptin, pH 7.5; designated as solubilization buffer. The material was applied to tandem-linked Q-Sepharose Fast Flow and SP-Sepharose Fast Flow IEX columns (GE Healthcare Bio-Sciences, Piscataway, NJ) equilibrated with solubilization buffer supplemented with 30 mM NaCl. The EP-1043 protein flowed through the Q-Sepharose but bound to the SP-Sepharose and was eluted following the increase of the NaCl to 200 mM.
The eluted material was adjusted to contain 0.7 M ammonium sulfate and 0.01% Tween-20, pH 7.4, and was applied to a Phenyl Sepharose High Performance HIC column (GE Healthcare) equilibrated with 10 mM Tris base, 6.0 M urea, 0.7 M ammonium sulfate, 0.01% Tween-20, pH 8.0. The EP-1043 protein, which bound to the column, was washed with 10 mM Tris base, 6.0 M urea, 0.7 M ammonium sulfate, pH 8, to remove the Tween-20 and eluted with a 10 mM Tris base, 6.0 M urea, 0.2 M ammonium sulfate, pH 8.0. The eluted material was adjusted to contain 20% acetonitrile, the pH adjusted with trifluoroacetic acid (TFA) to pH 3.0 – 3.4, and applied to a POROS 10μM R1 RPC column equilibrated with 2% acetonitrile and 0.1% TFA. A step gradient, 15% – 50% acetonitrile in 0.1% TFA, was run followed by a wash step with 100% acetonitrile/0.1% TFA. Fractions containing the EP-1043 protein were pooled, diluted to 20% acetonitrile in 0.1% TFA and re-applied to the POROS column equilibrated with 20% acetonitrile in 0.1% TFA. For the final purification step, the eluted material was pooled, diluted using 20% acetonitrile in 0.1% TFA, applied to the POROS and eluted with 50% acetonitrile, 0.1% TFA. Eluted material was dialyzed into 1% acetic acid, filtered using a 0.2 μm filter and stored at −20 °C.
Protein purification was monitored using standard SDS-PAGE, gel scanning densitometry and Western blot analyses. Protein concentration was determined using the BCA assay (Pierce Chemical Co., Rockford IL). Release of the protein as a drug substance was dependent on tests to assess USP sterility, purity, molecular weight, identity, amount of contaminating host cell protein and DNA and endotoxin levels (Table 3). These release criteria and assays were also used to support product stability testing.
Table 3.
Release testing and specifications for EP-1043 bulk protein
| Test | Method | Acceptance Criteria |
|---|---|---|
| Protein Concentration | BCA assay | ≥ 0.4 mg/mL |
| Identity | SDS-PAGE/Western Blot | ±38 kDa apparent MW protein detected by peptide specific antisera produce in rabbits |
| Purity | SDS-PAGE/Scanning Densitometry | Not less than 80% pure |
| Residual Host Cell Protein Assay | Western Blot | Not more than 5% |
| Endotoxin | Limulus Amebocyte Lysate | Less than 30 EU per mg of protein |
| DNA Content | PCR | Less than 100 pg DNA per highest clinical dose |
| Sterility | 21 CFR 610.12 | No bacterial or fungal growth observed |
| Viruses | Co-cultivation, 3 cell types (MRC-5, Vero, BHK) | No viral contamination observed |
| Mycoplasma/spiroplasma | 21CFR 610.30 | No mycoplasma or spiroplasma detected |
Production of EP HIV-1090 DNA vaccine
The EP HIV-1090 plasmid DNA vaccine plasmid is 5075 nucleotide pairs in length with an approximate molecular weight of 3.3 MDa and encodes only two open reading frames, the kanamycin resistance gene and the synthetic gene encoding a 843 base pair sequence that includes a consensus Kozak sequence, 21 HIV-derived CTL epitopes and the PADRE HTL epitope [34, 45]. Bulk DNA plasmid was manufactured under cGMP regulations using fermentation growth in E. coli DH5α and purified using precipitations, chromatography, dialysis and sterile filtration. The plasmid was formulated in PBS containing 3.4% (w/w) polyvinylpirrolidone (PVP, Plasdone, International Specialty Products, Wayne, NJ) which is a ratio of 17 parts PVP to 1 part DNA [46]. Sterile filtration and fill operations were conducted in a Class 100 environment using Type 1 borosilicate glass vials filled with 1.2 ± 0.1 ml final product at the concentration of 2 mg/ml. Vials were sealed with gray, chlorobutyl, Teflon-faced stoppers and crimped aluminum seals and stored frozen at −30 ± 10 °C.
Preparation of research EP-1043 recombinant protein formulations
The EP-1043 protein aggregated at neutral pH and, for immunogenicity testing, the material was used in this form or adsorbed onto aluminum phosphate (Adju-Phos, Brenntag Biosector, DK-3600 Frederikssund, Denmark) or aluminum hydroxide (Alhydrogel, Brenntag Biosector). For each experimental dose, adsorption was completed by simple mixing of 25μg of EP-1043 in 1% acetic acid and 0.5 mg of the Adju-Phos or Alhydrogel at 2–8 °C for 30 minutes. Prior to use, the pH of the suspensions was raised to 7.0 by the addition of 1M sodium hydroxide and the concentration of unadsorbed protein measured using the BCA assay; adsorption efficiency was greater >90%.
Initial studies indicated the Alhydrogel to be a suitable base adjuvant so additional testing was completed using formulations containing 25 μg of adsorbed protein mixed with 10μg QS-21 [47] (Antigenics, Lexington, Massachusetts), 50μg CpG [48] (Coley Pharmaceutical Group, Wellesley, MA), 50μg of the synthetic phospholipid E6020 [49] (Eisai Inc., Research Triangle Park, North Carolina) or 50μg of the synthetic lipid A analog OM-174 [50] (OM Pharma, Geneva, Switzerland). Aggregated protein and a pool containing 10μg of each of the 11 selected HTL epitope peptides (Table 2) emulsified in Complete or Incomplete Freund’s Adjuvant (CFA and IFA) was prepared for use as the positive control and for comparison.
Preparation of GMP EP-1043 recombinant protein formulation
The Alhydrogel-adsorbed protein formulation without additional adjuvants was selected for initial Phase 1 clinical testing. The GMP vaccine product was produced by adsorption of the EP-1043 protein to the Alhydrogel using two protein concentrations, 50 and 200μg/1.0 mg Alhydrogel. Formulated vaccine product was aseptically dispensed into Type 1 borosilicate vials, 2 ml capacity, at 1.1 – 1.2 ml per vial (for a 1.0 ml dose). Vials were sealed using gray butyl siliconized stoppers and crimp aluminum seals. Each final container lot was subjected to visual inspection. Quality control testing performed on the vaccine included sterility, pyrogenicity, immunogenicity, concentration of polypeptide, general safety (USP), pH, fill volume and potency. Vaccine vials were stored at 2 – 8 °C until use.
Immune response testing
Immune response testing of vaccines composed of CTL epitopes restricted to HLA antigens requires the use of HLA transgenic mice. However, sufficient structural similarity of H2 and HLA Class II molecules exists to support measurement of HTL responses using common inbred mouse strains. Eleven of the HTL epitope peptides were immunogenic in C57BL/6 X BALB/c F1 mice (H2bxd). Thus, to measure HTL responses and CTL responses we used HLA-A2.1 transgenic mice bred using C57BL/6 X BALB/c F1 mice (H2bxd) [51]. Mice, 6–18 wks of age and of either sex, were immunized in groups of six with formulations based on 25μg EP-1043 protein adsorbed to Alhydrogel by subcutaneous injection at the base of the tail using two immunizations administered 14 days apart. To simultaneously test the EP HIV-1090 DNA vaccine and EP-1043 protein, a volume of 50μl DNA formulation (total of 0.1 mg per mouse) was administered intramuscularly into each tibialis anterior muscle by needle injection within one hour of the EP-1043 administration. The DNA and protein formulations were not administered in a single injection because of the formulation complexity; however, administration sites were selected with the belief that the injection sites were proximal enough to potentially invoke the same draining lymph nodes.
Animals were euthanized 11–14 days following the final immunization and CD8+ and CD4+ T-lymphocytes purified from splenocyte preparation using the MACS CD8a (Ly-2) or CD4 (L3T4) microbead system (Miltenyi Biotec, Auburn, CA). Responses were measured using an ELISPOT assay with a 18 hour incubation and the production of IFN-γ as the primary assay readout but also measuring IL-2 or IL-5 for a limited number of experiments [34]; appropriate antibody pairs were purchased commercially (BD Biosciences, San Jose, CA). Plates were read using an AID ELISPOT plate reader (Strassbourg, Germany) and data downloaded into Excel spreadsheets. Responses were adjusted for naturally occurring background by subtracting the responses observed using an irrelevant peptide included in each experiment. Data were reported as the number of Spot-Forming Cells (SFC)/106 CD8+ or CD4+ T-lymphocytes. A Type 1, one-tailed T test was performed to compare the data from immunized mice to those from naïve control mice. Responses of immunized mice were considered significant when they were greater than responses observed using naïve control mice (p ≤0.05). Comparisons between groups were tested using a standard T-test or the Mann-Whitney rank sum test using SigmaStat 3.0 software (Systat Software, San Jose, CA). The IFN-γ ELISPOT assays were used to monitor potency of the vaccine EP-1043 protein and EP-1090 DNA vaccines as part of the product stability program.
Additional analyses were completed using splenocyte preparations tested without purification of CD8+ or CD4+ T-lymphocytes and a flow cytometry-based intracellular cytokine staining (ICS). Antibodies used to detect cell surface antigens and cytokines were as follows: CD3 PerCP (145-2C11), CD4-PE (GK1.5), IFN-γ-FITC (XMG1.2), IL-2-APC (JES6-5H4), IL4-APC (11B11) and IL5-APC (TRFK5). Briefly, 1–5 million splenocytes were stimulated in the presence of GolgiStop (4 μl/6 ml) and the HTL epitope peptide pool, at the concentration of 10μg/ml for each peptide, or an irrelevant peptide at 37°C for 4 hr. Cells were surface-labeled for CD3 and CD4 and intracellularly labeled IFN-γ, IL-2, IL-4 and IL-5 using a Cytofix/Cytoperm kit (BD Biosciences, San Jose, CA) following manufacturer’s instructions. Flow cytometry was done using a FACS Canto and data was analyzed using FACS Diva software (BD Biosciences).
Cell proliferation was measured using the CFSE-based method [52]. Briefly, splenocytes were incubated at 37°C for 10 minutes at the concentration of 106/ml in PBS, 0.1% BSA and 5 μM CFSE (Invitrogen, Carlsbad, CA). Following a wash step with cold PBS and 0.1% BSA, the cells were cultured for five days at 37°C in 5% CO2 in RPMI medium with 10% FBS, 2 mM L-glutamine, 0.1 mM MEM non-essential amino acid, 1 mM MEM sodium pyruvate, 0.05 mM 2-ME, 100 U/ml penicillin, 100μg/ml streptomycin and the HTL peptide pool containing 1μg/ml of each peptide. Cells were recovered, washed, labeled with the CD4-PE antibody, and analyzed by flow cytometry.
Toxicity and safety testing
The GLP preclinical safety study was designed and conducted to evaluate the potential toxicity of the Alhydrogel-adsorbed EP-1043 protein when co-administered in combination with the EP HIV-1090 DNA vaccine. The focus on the combined delivery of the products was deemed appropriate because the safety of the DNA vaccine administered alone was established [45, 46]. The study was designed to incorporate the use of a dose that matched or exceeded the anticipated human dose levels of both products without adjustment for animal weight; 250 μg EP-1043 protein adsorbed to 1.0 mg of Alhydrogel and 4 mg of the EP HIV-1090 DNA vaccine. The immunization schedule was compressed with vaccine doses administered every 15 days, which was approximately half of the time between planned sequential administrations in humans and included an additional vaccine immunization to that proposed in the human clinical study.
The study was completed using New Zealand white rabbits with vaccine products administered intramuscularly by needle injection. Test group 1 was composed of 10 animals of each gender all of which received two 0.5 ml injections of EP-1043 absorbed to alhydrogel in the right leg and two 1.0 ml injections of EP HIV-1090 formulation in PVP in the left leg on study days 1, 15, 29, 43, and 57. The control group was immunized with identical volumes of PBS as the control. On study days 58 and 85, five animals of each gender in both groups were necropsied; providing the means to assess toxicity in the acute and recovery phases. Parameters evaluated during the study period included mortality, physical and cage-side observations, Draize observations, body weights, body weight changes, food consumption, organ weights, organ weight ratios, and ophthalmology examinations. Clinical pathology, gross pathology, bone marrow, and histopathology analyses were completed following necropsy. Immune response measurements were not completed as part of this study because it was not feasible to measure cellular immune responses specific for HLA-restricted epitopes using rabbits.
The general vaccine safety test was part of the Quality control testing of the clinical lot and was completed using Alhydrogel-adsorbed vaccine in BALB/c mice and guinea pigs according to 21CFR610.11.
Results
EP-1043 protein and formulation characterization
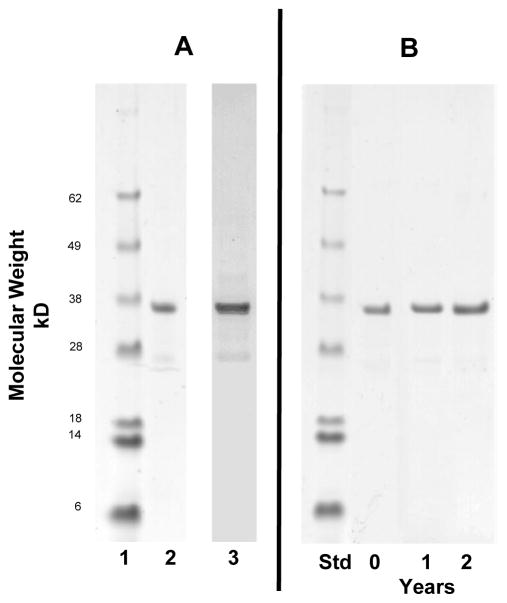
The initial yield of the polypeptide in the cell pellet following fermentation was approximately 25 mg/l and the yield of the purification was 2–4%. Intact EP-1043 protein, molecular weight of approximately 38kDa, was purified to 85% purity (Figure 3). Proteins of lower molecular weight were also detected in the Western Blot indicating a measurable level of degradation occurred during the purification process. The cGMP-produced drug product was stable with respect to protein integrity for at least two years stored at 2 – 8 °C as a suspension of Alhydrogel adsorbed protein (Figure 3). The product passed all specifications and was released for GLP animal safety testing (Table 3).
Figure 3. SDS-PAGE and Western Blot analysis of EP-1043 recombinant protein.
EP-1043 drug substance (0.75 μg) was separated by SDS-PAGE on a 12% gel (panel A, lane 2) and stained with Coomassie Blue; molecular weight standards are shown in panel A, lane 1. The protein bands were quantitated using scanning densitometry and the major band represents >85% of the total detected protein; product release specification was 80%. An equivalent sample was analyzed by Western Blot using rabbit antisera specific for the synthetic peptide derived from the EP-1043 protein to demonstrate the identity of the major band to EP-1043 protein (panel A, lane 3). EP-1043 drug product was analyzed using SDS-PAGE and Coomassie Blue staining following storage at 2–8° C for up to 2 years to demonstrate stability (panel B).
Immunogenicity testing
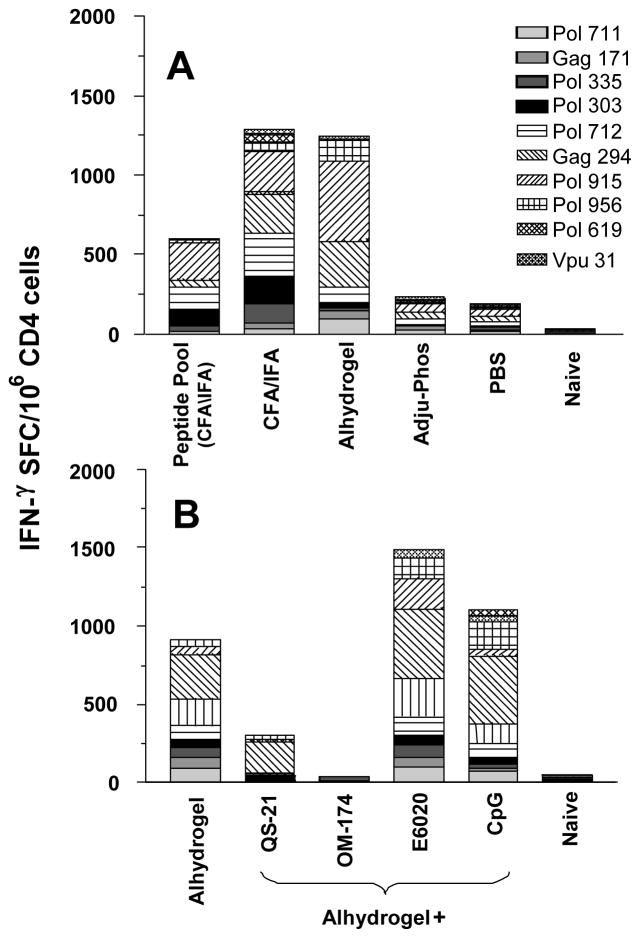
Initial testing was completed to compare the immunogenicity of the aggregated EP-1043 protein to Alhydrogel or Adju-Phos adsorbed protein. The Alhydrogel adsorbed protein was significantly more immunogenic with respect to magnitude than the aggregated protein delivered in PBS or the individual pooled peptides delivered using CFA and IFA (Figure 4A). The Adju-Phos adjuvant proved inferior in these studies and, thus, testing of other adjuvants was completed using the Alhydrogel adsorbed protein. However, none of the additional adjuvant products significantly increased breadth of epitope recognition or magnitude of responses but rather, responses were reduced in some instances (Figure 4B).
Figure 4. Effect of formulation on the immunogenicity of EP-1043.
EP-1043 protein was formulated with Alhydrogel and Adju-Phos and the immunogenicity compared to a peptide pool containing all the epitopes present in EP-1043 protein and formulated in CFA/IFA, EP-1043 protein formulated in CFA/IFA and the aggregated EP-1043 protein in PBS (panel A). The effect on immunogenicity of EP-1043 absorbed to Alhydrogel was compared to EP-1043 absorbed to Alhydrogel co-formulated with other adjuvants (panel B). Immune responses were measured using purified CD4 T-lymphocytes in an ELISPOT with IFN-γ production as the readout. Data are presented for each of the epitopes as the mean number of Spot Forming Cells (SFC) per million CD4+ T-lymphocytes.
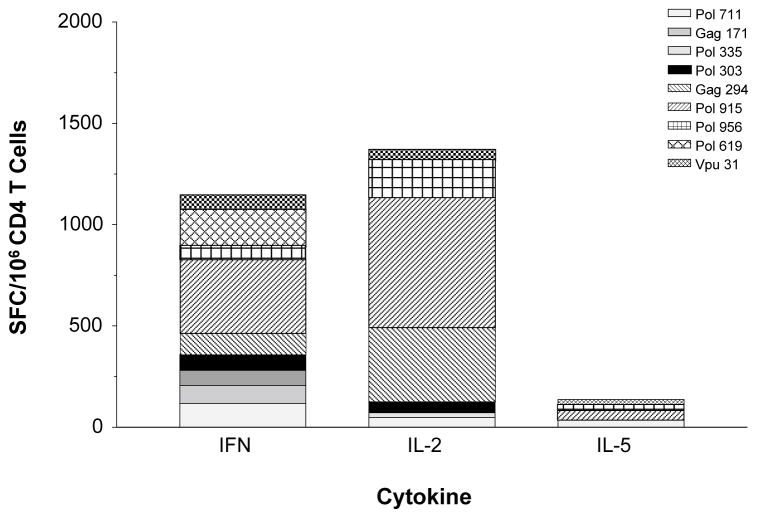
The use of Alhydrogel adjuvant raised the concern that Type 1 HTL responses might not be induced. The immunogenicity of the adsorbed protein was, therefore, measured by ELISPOT using IL-2 and IFN-γ production as representative cytokines for a Type 1 response and IL-5 production as representative of Type 2 responses. Significantly higher numbers of responding CD4+ splenocytes produced IL-2 and IFN-γ (Figure 5), indicating the desired Type 1 responses were induced.
Figure 5. Cytokine profile following immunization with EP-1043.
IFN-γ, IL-2 and IL-5 responses were measured for each epitope following immunization with EP-1043 formulated on Alydrogel. Immune responses were measured using purified CD4 T-lymphocytes in an ELISPOT with IFN-γ, IL-2 or IL-5 production as the readout. Data are presented for each of the epitopes as the mean number of Spot Forming Cells (SFC) per million CD4+ T-lymphocytes.
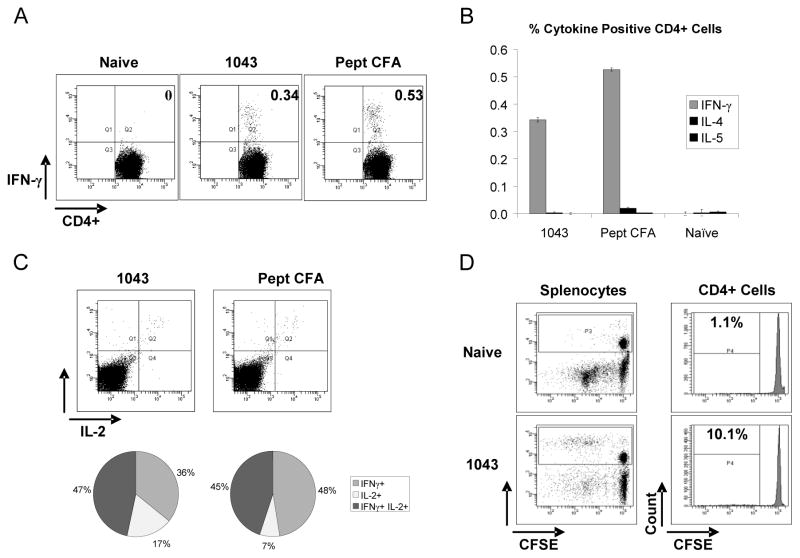
Immune responses to Alhydrogel-absorbed EP-1043 protein and the peptide pool in CFA/IFA were characterized using ICS and flow cytometry to assess the number and phenotype of multifunctional T-lymphocytes, with respect to cytokine production, using unfractionated splenocyte preparations. These analyses again showed the response to Alhydrogel-adsorbed protein to be of high magnitude and only slighter less than that induced using peptides in CFA/IFA; 0.34 % compared to 0.53% (Figure 6A). The responses were again classified as Type 1 with respect to cytokines produced as the numbers of cells producing IL-4 and IL-5 were very low in number (Figure 6B) and almost half of the responding CD4+ T-lymphocytes produced both IL-2 and IFN-γ (Figure 6C). Proliferation was also observed as approximately 10.1% of the CD4 T-lymphocytes were identified in the CFSE low population indicating one or more rounds of division (Figure 6D).
Figure 6. Flow cytometric analysis of unfractionated splenocytes to characterize multifunctional cellular immune responses.
Immune responses to Alhydrogel-absorbed EP-1043 protein and the peptide pool in CFA/IFA were characterized using ICS and flow cytometry to assess the number and phenotype of multifunctional T-lymphocytes with respect to cytokine production. The production of IFN-γ and IL-2 by CD4+ T-lymphocytes is shown in panels A and C, respectively. Data indicating a lack of IL-4 and IL-5 production is shown in panel C. Proliferation of CD4+ T-lymphocytes following epitope peptide stimulation is shown in panel D.
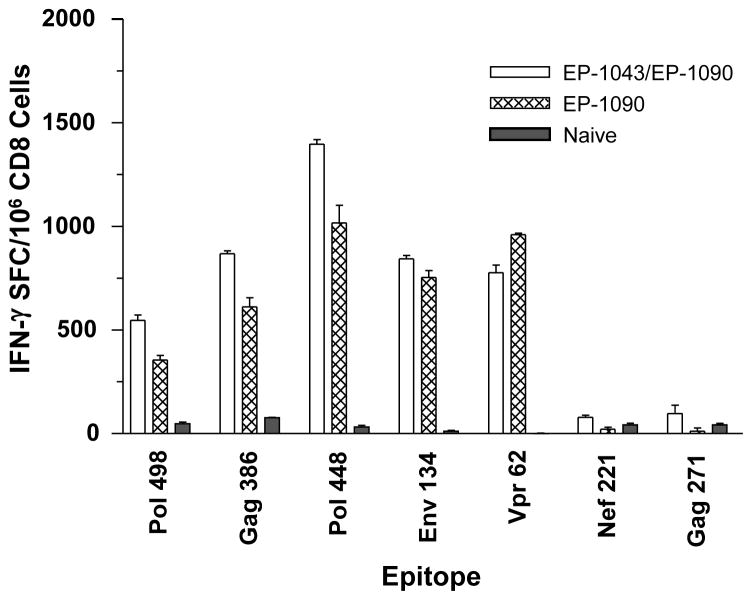
The DNA plasmid and protein vaccines were co-administered to HLA-A2.1 transgenic C57BL/6 X BALB/c F1 mice to document the induction of appropriate epitope-restricted responses in individual animals and to determine if the induction of HTL responses could augment the CTL responses despite being administered to separate sites. Data from these studies indicated modest but significant increases of the CTL responses for six of the seven HLA-A2.1-restricted epitopes (Figure 7); increased responses were observed for all bu the Vpr62 epitope. Also of importance is data to indicate interference between the two vaccine products was unlikely as HTL responses were not impacted negatively (data not shown).
Figure 7. Comparison of co-immunization of EP HIV-1090 DNA vaccine and EP-1043 protein vaccine with EP HIV-1090 vaccine alone.
The DNA plasmid was administered to HLA-A2.1 transgenic C57BL/6 X BALB/c F1 mice alone or in combination with EP-1043 protein vaccine. Immune responses were measured using purified CD8 T-lymphocytes in an ELISPOT with IFN-γ production as the readout. Data are presented for each of the epitopes as the mean number of Spot Forming Cells (SFC) per million CD8+ T-lymphocytes.
Toxicology and Safety testing
No test article-related changes in mortality, physical and cage-side observations, Draize observations, body weights, body weight changes, food consumption, organ weights, organ weight ratios, ophthalmology examinations, clinical pathology or bone marrow were observed in the GLP rabbit study. Gross pathology observations of injection sites revealed discoloration prevalent in the tissues obtained on study day 58 at sites used for injection of the Alhydrogel-adsorbed EP-1043, but similar reactions were not observed in analogous tissues obtained on study day 85 indicating that the site of injection effects were resolved. Histopathology analyses of these tissues were consistent with the interpretation that the lesions were a host inflammatory response to a foreign body, and no direct compound effect on muscle fibers was apparent. These localized host responses to the Alhydrogel-adsorbed EP-1043 material were more severe and at a higher incidence than that observed for the EP HIV-1090 formulation. There were, however, no obvious signs of systemic toxicity and the product passed general safety testing. The products also passed the General Animal Safety Test.
Discussion
The working goals of this project were to design, produce and test a recombinant protein composed of HTL epitopes in a manner that would support Phase 1 clinical testing in combination with the EP HIV-1090 DNA vaccine [34, 37, 45]. The design features of the EP-1043 protein included the use of HLA-DR supertype restricted epitopes separated by glycine-proline repeat spacers to target proteolytic cleavage between epitopes [53]. These features contributed to the design of a completely synthetic protein with a predicted pI of 9.91 that aggregates at neutral pH, likely due to the inclusion of the numerous epitopes which are individually hydrophobic, and a non-natural structure that was predicted to be highly prone to degradation during production. All of these issues were considered as significant obstacles for expression using E. coli-based systems. We, therefore, selected the baculovirus-insect cell expression system which has been used successfully to express proteins that cannot be produced readily using E coli systems, for example, intact influenza HA and HIV envelope proteins which have been safely tested in clinical trials [44, 54–56]. Although protein was not secreted and expressed only at a relatively low level of 25 mg/l, we were successful in producing sufficient material to support purification and characterization studies.
A unique feature of the protein is that it is designed to be cleaved into individual HTL epitope peptides; presumably by proteolytic enzymes resident in tissues and not through intracellular pathways or the proteosome system. Thus, there was no requirement to maintain any tertiary structure and this allowed for purification using organic solvents and standard column chromatography methods. Intact EP-1043 protein was purified to approximately 85% purity with only minimal degradation despite the likely presence of bacterial proteolytic enzymes in the crude material. Here again we believe the use of denaturing conditions during the purification likely provided a benefit thought the inactivation of these enzymes.
The protein was insoluble at neutral pH and this necessitated adsorption to a solid phase suitable for vaccine use. The high pI of the protein supported the use of aluminum phosphate (Adju-Phos); however, aluminum hydroxide (Alhydrogel) was included in our initial studies because of our familiarity with this product and our concern that very high affinity binding to Adju-Phos could limit the efficient release of the HTL epitope peptides following proteolytic cleavage in vivo. Formulation testing indicated the protein adsorbed efficiently to both products and immune responses were highest in magnitude and breadth using the Alhydrogel based formulation whereas aggregated protein was only marginally immunogenic with responses of limited breadth. We interpret these data to indicate the need for a solid phase support to optimally expose the cleavage sites between the epitopes. However, protein emulsified in CFA and IFA was similarly immunogenic indicating appropriate processing could be achieved with this potent emulsion-based formulation. The Alhydrogel formulation protein also proved to be stable to degradation when stored at 4 °C for at least 2 years and to retain immunogenicity. Thus, properties of this type of protein-based formulation appear compatible with standard vaccine distribution and storage practices.
It was interesting to note that the other adjuvants tested, which included Qs-21 saponin, CpG, the OM174 synthetic lipid A analog, and the synthetic phospholipid E6020, did not significantly increase the immunogenicity of the EP-1043 protein and, in some instances, the potency appears to be reduced in the more complex formulations. These data most likely indicate physical incompatibility between the different components of the formulations given the well known activity of these adjuvants. For example, we believe that the surfactant properties of Qs-21 could have stripped the EP-1043 protein from the Alhydrogel and in its soluble form the protein was not very immunogenic. These negative data did not impact the development effort though, as the safety of the Alhydrogel simplified and supported the limited scope of the animal safety testing.
Co-administration of the DNA and protein components could not be tested clinically using a single site because the Phase 1 trial needed to be designed primarily to document the safety of the two products. This limitation is reflected in the design of our animal studies. In mice, the EP-1043 protein was delivered subcutaneously whereas the EP-1090 DNA vaccine was delivered intramuscularly. For the GLP safety testing in rabbits, different legs were used for intramuscular injection of each vaccine and this approach mimicked the clinical safety testing plans wherein administration to different sites was required to accurately assess site of injection reactogenicity. The experimental data obtained from both animal species did document the feasibility of co-administration in terms of safety. CTL and HTL responses were also induced in individual mice indicating a lack of interference between the products.
Demonstration of synergy which would augment CTL responses was deemed to be highly desirable because the EP HIV-1090 DNA vaccine has proved to be only minimally immunogenic in clinical trials [37, 45]. In the current study using mice, co-administration was assessed and resulted in a modest but significant level of synergy resulting in augmentation of CTL responses to the majority of the epitopes. However, co-administration of the DNA and protein in the same formulation is likely to be required for optimal immunogenicity of both vaccine components. This will require additional formulations efforts to insure stability and potency of the two vaccine components and these studies are ongoing.
Defined CTL and HTL epitopes are, theoretically, amenable for use with numerous vaccine formats and delivery methods. We previously evaluated the DNA plasmid format to deliver CTL and HTL epitopes with only minimal success and this supported the development of a recombinant protein composed of HTL epitopes. Of note, further experimentation is ongoing and experimental HIV vaccines are being designed specifically to co-deliver the HTL and CTL epitopes using DNA plasmids and viral vector delivery formats. We assume that delivery of both CTL and HTL epitopes to the same site, and even the same cells in vivo, will be required for optimal immunogenicity.
Acknowledgments
These studies were supported in part by the Division of AIDS, NIAID, NIH Contract N01 AI-30031. Access to cGMP protein manufacturing and GLP animal safety testing was provided through Division of AIDS contracts with Protein Sciences Inc., and Bridge Laboratories. We gratefully acknowledge the advice and support of Chris Butler and Michael Pensiero, Division of AIDS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Borrow P, Lewicki H, Hahn BH, Shaw GM, Oldstone MBA. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68:6103–10. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koup RA, Safrit JT, Cao Y, et al. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–5. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang OO, Walker BD. CD8+ cells in human immunodeficiency virus type I pathogenesis: Cytolytic and noncytolytic inhibition of viral replication. Adv Immunol. 1997;66:273–311. doi: 10.1016/s0065-2776(08)60600-8. [DOI] [PubMed] [Google Scholar]
- 4.Klein MR, Van Baalen CA, Holwerda AM, et al. Kinetics of Gag-specific cytotoxic T lymphocyte responses during the clinical course of HIV-1 infection: a longitudinal analysis of rapid progressors and long-term asymptomatics. J Exp Med. 1995;181:1365–73. doi: 10.1084/jem.181.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rowland-Jones S, Dong T, Krausa P, et al. The role of cytotoxic T-cells in HIV infection. Dev Biol Stand. 1998;92:209–14. [PubMed] [Google Scholar]
- 6.Rowland-Jones S, Tan RS, McMichael A. Role of cellular immunity in protection against HIV infection. Adv Immunol. 1997;65:277–346. [PubMed] [Google Scholar]
- 7.Migueles SA, Laborico AC, Shupert WL, et al. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat Immunol. 2002;3:1061–8. doi: 10.1038/ni845. [DOI] [PubMed] [Google Scholar]
- 8.Frahm N, Adams S, Kiepiela P, et al. HLA-B63 presents HLA-B57/B58-restricted cytotoxic T-lymphocyte epitopes and is associated with low human immunodeficiency virus load. J Virol. 2005;79:10218–25. doi: 10.1128/JVI.79.16.10218-10225.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frahm N, Kiepiela P, Adams S, et al. Control of human immunodeficiency virus replication by cytotoxic T lymphocytes targeting subdominant epitopes. Nat Immunol. 2006;7:173–8. doi: 10.1038/ni1281. [DOI] [PubMed] [Google Scholar]
- 10.Zuniga R, Lucchetti A, Galvan P, et al. Relative dominance of Gag p24-specific cytotoxic T lymphocytes is associated with human immunodeficiency virus control. J Virol. 2006;80:3122–5. doi: 10.1128/JVI.80.6.3122-3125.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Betts MR, Nason MC, West SM, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–9. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao Y, Qin L, Zhang L, Safrit J, Ho DD. Virologic and immunologic characterization of long-term survivors of human immunodeficiency virus type 1 infection. N Engl J Med. 1995;332:201–8. doi: 10.1056/NEJM199501263320401. [DOI] [PubMed] [Google Scholar]
- 13.Pantaleo G, Menzo S, Vaccarezza M, et al. Studies in subjects with long-term nonprogressive human immunodeficiency virus infection. N Engl J Med. 1995;332:209–16. doi: 10.1056/NEJM199501263320402. [DOI] [PubMed] [Google Scholar]
- 14.Rinaldo C, Huang X-L, Fan Z, et al. High levels of anti-human immunodeficiency virus type 1 (HIV-1) memory cytotoxic T-lymphocyte activity and low viral load are associated with lack of disease in HIV-1-infected long-term nonprogressors. J Virol. 1995;69:5838–42. doi: 10.1128/jvi.69.9.5838-5842.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferbas J, Kaplan AH, Hausner MA, et al. Virus burden in long-term survivors of human immunodeficiency virus (HIV) infection is a determinant of anti-HIV CD8+ lymphocyte activity. J Infect Dis. 1995;172:329–39. doi: 10.1093/infdis/172.2.329. [DOI] [PubMed] [Google Scholar]
- 16.Harrer E, Harrer T, Barbosa P, et al. Recognition of the highly conserved YMDD region in the human immunodeficiency virus type 1 reverse transcriptase by HLA-A2- restricted cytotoxic T lymphocytes from an asymptomatic long-term nonprogressor. J Infect Dis. 1996;173:476–9. doi: 10.1093/infdis/173.2.476. [DOI] [PubMed] [Google Scholar]
- 17.Ogg GS, Jin X, Bonhoeffer S, et al. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103–6. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 18.Rowland-Jones S. Long-term non-progression in HIV infection: clinico pathological issues. J Infect. 1999;38:67–70. doi: 10.1016/s0163-4453(99)90070-1. [DOI] [PubMed] [Google Scholar]
- 19.Kalams SA, Buchbinder SP, Rosenberg ES, et al. Association between virus-specific cytotoxic T-lymphocyte and helper responses in human immunodeficiency virus type 1 infection. J Virol. 1999;73:6715–20. doi: 10.1128/jvi.73.8.6715-6720.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalams SA, Walker BD. The critical need for CD4 help in maintaining effective cytotoxic T lymphocyte responses. J Exp Med. 1998;188:2199–204. doi: 10.1084/jem.188.12.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pitcher CJ, Quittner C, Peterson DM, et al. HIV-1-specific CD4+ cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nature Med. 1999;5:518–25. doi: 10.1038/8400. [DOI] [PubMed] [Google Scholar]
- 22.Altfeld M, Rosenberg ES. The role of CD4+ T helper cells in the cytotoxic T lymphocyte response to HIV-1. Curr Opin Immunol. 2000;12:375–80. doi: 10.1016/s0952-7915(00)00103-5. [DOI] [PubMed] [Google Scholar]
- 23.Rosenberg ES, Billingsley JM, Caliendo AM, et al. Vigorous HIV-1-specific CD4+ Tcell respsonses associated with control of viremia. Science. 1997;278:1447–50. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 24.Boritz E, Palmer BE, Wilson CC. Human immunodeficiency virus type 1 (HIV-1)-specific CD4+ T cells that proliferate in vitro detected in samples from most viremic subjects and inversely associated with plasma HIV-1 levels. J Virol. 2004;78:12638–46. doi: 10.1128/JVI.78.22.12638-12646.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Migueles SA, Connors M. The Role of CD4(+) and CD8(+) T Cells in Controlling HIV Infection. Curr Infect Dis Rev. 2002;4:461–7. doi: 10.1007/s11908-002-0014-2. [DOI] [PubMed] [Google Scholar]
- 26.Matloubian M, Concepcion RJ, Ahmed R. Cd4(+) T-Cells Are Required to Sustain Cd8(+) Cytotoxic T-Cell Responses During Chronic Viral-Infection. JVirol. 1994;68:8056–63. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cardin RD, Brooks JW, Sarawar SR, Doherty PC. Progressive loss of CD8(+) T cell-mediated control of a gamma-herpesvirus in the absence of CD4(+) T cells. J Exp Med. 1996;184:863–71. doi: 10.1084/jem.184.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janssen EM, Lemmens EE, Wolfe T, Christen U, Von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–6. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 29.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–9. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 30.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–42. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Catanzaro AT, Koup RA, Roederer M, et al. Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 candidate vaccine delivered by a replication-defective recombinant adenovirus vector. J Infect Dis. 2006;194:1638–49. doi: 10.1086/509258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Catanzaro AT, Roederer M, Koup RA, et al. Phase I clinical evaluation of a six-plasmid multiclade HIV-1 DNA candidate vaccine. Vaccine. 2007;25:4085–92. doi: 10.1016/j.vaccine.2007.02.050. [DOI] [PubMed] [Google Scholar]
- 33.Tavel JA, Martin JE, Kelly GG, et al. Safety and immunogenicity of a Gag-Pol candidate HIV-1 DNA vaccine administered by a needle-free device in HIV-1-seronegative subjects. J Acquir Immune Defic Syndr. 2007;44:601–5. doi: 10.1097/QAI.0b013e3180417cb6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson CC, McKinney D, Anders M, et al. Development of a DNA vaccine designed to induce cytotoxic T lymphocyte responses to multiple conserved epitopes in HIV-1. J Immunol. 2003;171:5611–23. doi: 10.4049/jimmunol.171.10.5611. [DOI] [PubMed] [Google Scholar]
- 35.Douek DC, Brenchley JM, Betts MR, et al. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 2002;417:95–8. doi: 10.1038/417095a. [DOI] [PubMed] [Google Scholar]
- 36.Brenchley JM, Ruff LE, Casazza JP, Koup RA, Price DA, Douek DC. Preferential Infection Shortens the Life Span of Human Immunodeficiency Virus-Specific CD4+ T Cells In Vivo. J Virol. 2006;80:6801–9. doi: 10.1128/JVI.00070-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson CC, Newman MJ, Livingston BD, et al. Clinical phase 1 testing of the safety and immunogenicity of an epitope-based DNA vaccine in human immunodeficiency virus type 1-infected subjects receiving highly active antiretroviral therapy. Clin Vaccine Immunol. 2008;15:986–94. doi: 10.1128/CVI.00492-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol. 2005;23:975–1028. doi: 10.1146/annurev.immunol.22.012703.104538. [DOI] [PubMed] [Google Scholar]
- 39.Sidney J, Southwood S, Oseroff C, Del Guercio M-F, Grey HM, Sette A. Measurement of MHC/peptide interactions by gel filtration. Curr Prot Immunol. 1998;18:18.3.2–18.3.19. doi: 10.1002/0471142735.im1803s31. [DOI] [PubMed] [Google Scholar]
- 40.Sette A, Sidney J, del Guercio MF, et al. Peptide binding to the most frequent HLA-A class I alleles measured by quantitative molecular binding assays. Mol Immunol. 1994;31:813–22. doi: 10.1016/0161-5890(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 41.Wilson CC, Palmer B, Southwood S, et al. Identification and antigenicity of broadly cross-reactive and conserved human immunodeficiency virus type 1-derived helper T-lymphocyte epitopes. J Virol. 2001;75:4195–207. doi: 10.1128/JVI.75.9.4195-4207.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Altfeld MA, Livingston B, Reshamwala N, et al. Identification of novel HLA-A2-restricted human immunodeficiency virus type 1-specific cytotoxic T-lymphocyte epitopes predicted by the HLA-A2 supertype peptide-binding motif. J Virol. 2001;75:1301–11. doi: 10.1128/JVI.75.3.1301-1311.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Livingston B, Crimi C, Newman M, et al. A rational strategy to design multiepitope immunogens based on multiple Th lymphocyte epitopes. J Immunol. 2002;168:5499–506. doi: 10.4049/jimmunol.168.11.5499. [DOI] [PubMed] [Google Scholar]
- 44.McPherson CE. Development of a novel recombinant influenza vaccine in insect cells. Biologicals. 2008 doi: 10.1016/j.biologicals.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 45.Gorse GJ, Baden LR, Wecker M, et al. Safety and immunogenicity of cytotoxic T-lymphocyte poly-epitope, DNA plasmid (EP HIV-1090) vaccine in healthy, human immunodeficiency virus type 1 (HIV-1)-uninfected adults. Vaccine. 2008;26:215–23. doi: 10.1016/j.vaccine.2007.10.061. [DOI] [PubMed] [Google Scholar]
- 46.Nicolaou M, Chang P, Newman MJ. Use of Poly_N-vinyl pyrrolidone) with Noncondensed Plasmid DNA Formulations for Gene Therapy and Vaccines. In: Amiji MM, editor. Polymeric Gene Delivery Principles and Applications. 329–344. CRC Press; 2005. [Google Scholar]
- 47.Kensil CR, Patel U, Lennick M, Marciani D. Separation and characterization of saponins with adjuvant activity from Quillaja saponaria Molina cortex. J Immunol. 1991;146:431–7. [PubMed] [Google Scholar]
- 48.Krieg AM. Immune effects and mechanisms of action of CpG motifs. Vaccine. 2000;19:618–22. doi: 10.1016/s0264-410x(00)00249-8. [DOI] [PubMed] [Google Scholar]
- 49.Ishizaka ST, Hawkins LD. E6020: a synthetic Toll-like receptor 4 agonist as a vaccine adjuvant. Expert Rev Vaccines. 2007;6:773–84. doi: 10.1586/14760584.6.5.773. [DOI] [PubMed] [Google Scholar]
- 50.Brandenburg K, Lindner B, Schromm A, et al. Physicochemical characteristics of triacyl lipid A partial structure OM-174 in relation to biological activity. Eur J Biochem. 2000;267:3370–7. doi: 10.1046/j.1432-1327.2000.01370.x. [DOI] [PubMed] [Google Scholar]
- 51.Wentworth PA, Vitiello A, Sidney J, et al. Differences and similarities in the A2. 1-restricted cytotoxic T cell repertoire in humans and human leukocyte antigen-transgenic mice. Eur J Immunol. 1996;26:97–101. doi: 10.1002/eji.1830260115. [DOI] [PubMed] [Google Scholar]
- 52.Lyons AB, Parish CR. Determination of Lymphocyte Division by Flow-Cytometry. J Immunol Meth. 1994;171:131–7. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 53.Livingston B, Crimi C, Newman M, et al. A Rational Strategy to Design Multiepitope Immunognes Based on Multiple TH Lymphocyte Epitopes. J Immunol. 2002;168:5499–506. doi: 10.4049/jimmunol.168.11.5499. [DOI] [PubMed] [Google Scholar]
- 54.Dolin R, Graham BS, Greenberg SB, et al. The safety and immunogenicity of a human immunodeficiency virus type 1 (HIV-1) recombinant gp160 candidate vaccine in humans. Ann Intern Med. 1991;114:119–27. doi: 10.7326/0003-4819-114-2-119. [DOI] [PubMed] [Google Scholar]
- 55.Birx DL, Loomis-Price LD, Aronson N, et al. Efficacy testing of recombinant human immunodeficiency virus (HIV) gp160 as a therapeutic vaccine in early-stage HIV-1-infected volunteers. J Inect Dis. 2000;181:881–9. doi: 10.1086/315308. [DOI] [PubMed] [Google Scholar]
- 56.Dalakouras T, Smith BJ, Platis D, Cox MM, Labrou NE. Development of recombinant protein-based influenza vaccine. Expression and affinity purification of H1N1 influenza virus neuraminidase. J Chromatogr. 2006;1136:48–56. doi: 10.1016/j.chroma.2006.09.067. [DOI] [PubMed] [Google Scholar]