Abstract
We investigated the relative involvement of cortical regions supporting attentional control in older and younger adults during performance on a modified version of the Stroop task. Participants were exposed to two different types of incongruent trials. One of these, an incongruent-ineligible condition, produces conflict at the non-response level, while the second, an incongruent-eligible condition, produces conflict at both non-response and response levels of information processing. Greater attentional control is needed to perform the incongruent-eligible condition compared to other conditions. We examined the cortical recruitment associated with this task in an event-related functional magnetic resonance imaging paradigm in twenty-five older and twenty-five younger adults. Our results indicated that while younger adults demonstrated an increase in the activation of cortical regions responsible for maintaining attentional control in response to increased levels of conflict, such sensitivity and flexibility of the cortical regions to increased attentional control demands was absent in older adults. These results suggest a limitation in older adults’ capabilities for flexibly recruiting the attentional network in response to increasing attentional demands.
Keywords: attentional control, aging, fMRI, flexibility, Stroop task, interference, inhibition
Introduction
Representations arising from distracting stimuli interfere with our ability to selectively attend to task-relevant information. The effect of such representations is heightened when the tendency to respond to distracting information is habitual or reflexive, resulting in the need for greater attentional control to perform important tasks. The classic color-word Stroop task has been extensively used in both the behavioral and neuroimaging literatures to study the mechanisms of attentional control (Bench et al., 1993, Desimone and Duncan, 1995, Banich et al., 2000, Banich et al., 2001). In the Stroop task, participants are asked to inhibit information from the pre-potent word representations and attend to the color in which the words are printed. Heightened attentional control is needed to resolve the interference on trials in which the color and word information are incongruent (e.g. the word ‘Blue’ printed in red ink) compared to conditions in which the color and word information are not conflicting. Young adults during performance of the incongruent trials activate a network of regions including the bilateral middle frontal gyrus, bilateral inferior frontal gyrus, anterior cingulate cortex (ACC) and the parietal cortex (Bench et al., 1993, Banich et al, 2001) due to increased attentional control required to overcome the task-irrelevant dimensions of the incongruent condition.
Older adults, in comparison to young adults, are thought to have deficits in their ability to selectively attend to and ignore irrelevant information (see Hasher & Zacks, 1988; Kramer et al., 1994). For example, older adults often show performance deficits in the Stroop task, which is considered to be indicative of an age-related inability to effectively filter the task-irrelevant (word) information. Neuroimaging research has demonstrated increased recruitment of brain regions by older adults to perform cognitive tasks in which younger adults more selectively recruit neural resources (Cabeza et al., 2002, Langnecker et al., 2004, Colcombe et al., 2005). This increased recruitment of brain regions has been interpreted in a number of different ways with some researchers suggesting a compensatory role performed by the additional activation to counter the declines associated with advancing age. For example, Langnecker et al. (2004), using the Stroop task found that older adults showed more activation than younger adults in the premotor, dorsolateral, ventrolateral, and medial frontal areas. Particularly, the study reported greater activation in the left inferior frontal regions by the older participants, thus arguing for the task-specific and supportive role of this region to overcome the greater interference experienced by the older adults. Though this study did not report correlations between additional activation and behavioral performance, other studies (Cabeza et al., 2002 Reuter-Lorenz et al. 2000) using a variety of cognitive tasks have reported positive correlations between increased activation and behavioral performance in the older adults. The additional recruitment of cortical resources by the better performing subgroup has been interpreted as compensatory processing, serving to deal with, at least in part, age-related inefficiencies in processing associated with different tasks.
Though a compensatory interpretation of additional activation in the presence of declining neural efficiency has some support, the recruitment of more cortical resources on relatively easy tasks might have hidden costs (Reuter-Lorenz, 1999, 2005, 2006). Reuter-Lorenz and colleagues (2006) proposed that as a result of the need to allocate substantial cortical resources to tasks that younger adults find relatively easy, older adults have few resources available for more challenging tasks. This pattern of results has been termed the CRUNCH model or the compensation-related utilization of neural circuits hypothesis. According to this hypothesis, older adults show less of a difference compared with younger adults in the activation between two tasks or conditions that differ in difficulty. It is possible that such a finding would represent an age-related failure to flexibly allocate cortical resources with increasing task demands (see DiGirolamo et al., 2001 for a similar hypothesis).
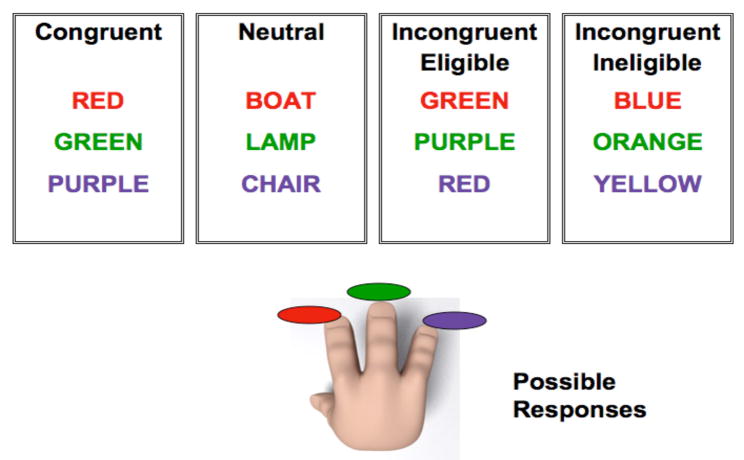
In this study, we manipulated the level of conflict in the incongruent conditions of the Stroop task (Milham et al., 2001; Liu et al., 2006) to examine differential recruitment of brain regions in both young and old participants. We presented a modified version of the Stroop task to our participants: older adults between 58 and 75 years of age, and younger adults between 18 and 35 years of age. In addition to the neutral (the word LAMP printed in red ink) and the congruent (the word RED printed in red ink) conditions we had two types of incongruent conditions (incongruent-eligible and incongruent-ineligible) enabling us to further study the effect of increases in conflict on behavioral performance and brain processes in younger and older adults. The paradigm was a three-choice manual response task, in which participants were asked to respond to the three ink colors (red, green or purple) using their right hand. An incongruent-eligible stimulus was one of the words from the set of colors that the participant could respond with (red, green or purple) printed in an incongruent ink-color (eg. the word RED printed in green ink). An incongruent-ineligible stimulus, in contrast, was any color-word other than red, green or purple printed in an incongruent color (e.g. the word BLUE printed in green ink). For both these trials, the participants were asked to make responses. (See Figure 1 for a pictorial representation of the task). The main difference between the two incongruent conditions was that in the eligible condition the actual color-word was a part of the response set with which the participant could respond and hence greater response conflict. In the ineligible trials, the color-word was not a part of the response set.
Figure I.
Pictorial representation of the modified version of the Stroop task used in the study. Participants could only respond to three ink colors: red, green or purple. For the incongruent-eligible condition (third box) the word was one of the ink colors that the participant could respond with (red, green or purple) but printed in an incongruent ink-color (such as GREEN printed in red ink). For the incongruent-ineligible condition (fourth box), the word could be any color word other than red, green or purple printed in an incongruent ink-color (such as the word BLUE printed in red ink).
Milham et al. (2001) employed this paradigm to investigate attentional control in young adults. They reported that young adults activated primarily the left prefrontal cortex during the incongruent-ineligible condition relative to the neutral condition. Performance on the incongruent-eligible trials relative to neutral trials resulted in greater activation in the left PFC along with additional activation in the right PFC and anterior cingulate cortex. The authors conceptualized the additional activation during the incongruent-eligible condition as being reflective of the differences in the amount of conflict experienced in the two tasks. That is, the incongruent-ineligible condition was thought to involve non-response conflict given that the word was not a part of the response set, while the incongruent-eligible condition was hypothesized to involve both response and non-response conflict.
Through this manipulation, we were interested in examining whether older adults have a reduced capability to flexibly allocate attentional resources. We used an event-related functional magnetic resonance imaging (fMRI) paradigm to investigate the recruitment of the prefrontal and parietal cortices in older adults in response to increased attentional demands in this version of the Stroop task. Previous studies using the traditional Stroop task have examined age-related differences in attentional control (see Milham et al., 2001; Langnecker et al., 2005) and in this study, in addition to examining cortical recruitment during the incongruent condition relative to the neutral condition, we examined the neural circuitry recruited by the older adults compared with the younger adults during the two incongruent conditions. This enables a unique comparison of the neural resources utilized by the two groups in response to increasing cognitive demands. We predicted that the older adults would demonstrate increased activation of the cortical resources in response to all conditions of the Stroop task, such that cortical resources recruited by the younger adults during the more challenging incongruent conditions would be recruited by older adults in response to the congruent and the neutral condition. In addition, we also predicted that older adults would recruit bilateral areas of the dorsolateral prefrontal cortex in response to the easier task condition (incongruent-ineligible), thereby leaving no additional resources for the more challenging condition (i.e. incongruent-eligible). In other words, as opposed to younger participants who would demonstrate an increase in activation in response to increases in conflict between the incompatible conditions, older adults would recruit similar brain regions in response to easier task conditions, supporting the claim that older adults have more difficulty flexibly and adaptively recruiting neural resources to assist task performance.
Methods
Participants
Twenty-five right-handed older adults (age range = 58–75, Mean = 65.5 years) and twenty-five younger adults (age range = 18–35, Mean = 23.6 years) were recruited for the study. All participants were screened for any contraindications for participating in an MR environment. All of the older participants scored 51 or higher on the Modified Mini Mental Status Examination (maximum possible score=57; Stern et al., 1987). The visual acuity of all participants was assessed and corrective lenses were provided in order to achieve visual acuity of at least 20/30. The University of Illinois Institutional Review Board approved the study, and all participants provided informed consent.
Neurocognitive Task and fMRI parameters
In order to investigate the differential patterns of activation in response to the modulation of conflict in older and younger adults, we employed a modified version of the color-word Stroop task (Milham et al, 2001; Erickson et al., in press). We employed two different color sets for the task, which were counterbalanced across participants. For the first color set, the eligible ink colors included red, green and purple and the ineligible words included blue, orange and yellow. Thus all words were printed in either of the three eligible responses such that the congruent condition was one in which the information conveyed by the word was consistent with the ink in which it was printed (e.g., the word RED printed in red ink); in the neutral condition a non-color word was used that matched the frequency and length of the color words (e.g. the word LAMP printed in red ink). There were two incongruent conditions: in the incongruent-eligible condition the word matched one of the potential responses, but the ink-color was incongruent with the meaning of the word (e.g., the word GREEN printed in red ink), and in the incongruent-ineligible condition the word did not match any of the potential responses, but the ink-color was incongruent with the meaning of the word (e.g. the word YELLOW printed in red ink). The order of the eligible color sets was counterbalanced across subjects. All four conditions required the participants to make a response (See Figure 1).
A total of 144 stimuli (36 of each type) were presented to each participant for a period of 1 second per trial with a 1.5 second response window and a 3 second stimulus-onset asynchrony (SOA). A crosshair (+) was presented on the screen during all interstimulus intervals. We employed an event-related stimulus design with a 40% jitter, such that the timing between trials varied, in order to optimize the stimulus sequence and timing. Each stimulus type was first-order counterbalanced across the entire run, which lasted for 9 minutes. Stimulus sequence and timing were generated with optseq2 (http://surfer.nmr.mgh.harvard.edu/optseq ; see also Dale & Greve, 1999; Dale, 1999). Participants were scanned in a 3T Siemens Allegra head-only scanner with a fast echo-planar imaging (EPI) sequence protocol (28 horizontal slices; TR = 1500ms; TE = 25ms; ascending slice acquisition; 60 degree flip angle; 4 mm isotropic thickness). High-resolution structural images were also collected for each participant using a spoiled gradient sequence (256×256mm FOV; 1.3mm thick slices, with a 1.3×1.3mm in-plane resolution) for registration.
Data Analysis
Behavioral data (reaction time and accuracy) were analyzed using a repeated measures analysis of variance with condition (congruent, neutral, incongruent-eligible and incongruent-ineligible) as a within-subjects factor and age as a between-subjects factor. We then carried out planned comparisons using paired t-tests across the two age groups to investigate differences between the experimental conditions. All behavioral data were analyzed using SPSS 11.0.3 for Mac.
The neuroimaging data were analyzed using a statistical parametric approach using FSL version 3.2 (http://www.fmrib.ox.ac.uk/fsl) and FEAT (fMRI Expert Analysis Tool) Version 5.43. The 380 images that were collected while the participants performed the task, were slice-time corrected, motion corrected using a rigid-body algorithm in MCFLIRT (Jenkinson, 2003), and temporally smoothed with a Gaussian low pass (1.5 second cut-off) and high pass (100 second cut-off) filter. Spatial smoothing was done with a 8-mm (Full Width at Half Maximum: FWHM) 3-dimensional Gaussian kernel. Following this, all high-resolution T1 images were skull stripped using a robust deformable brain extraction technique (BET) (Smith et al., 2002). These skull-stripped images for each participant were spatially registered using a 12-parameter affine transformation to a study-specific template in stereotaxic space that was specifically created for the study in order to avoid systematic registration error between older and younger adults. This was done by: (a) warping each participant’s high-resolution scan to MNI space, (b) creating an average of these registered images; and (c) spatially smoothing the average image with a 10-mm (FWHM) Gaussian kernel. This study-specific template was subsequently used for spatial registration of the fMRI data.
The resulting time series was then convolved using a double-gamma function with error trials as covariates of no interest in the model. Only correct trials were included in the model. We used a single canonical HRF across all our participants since previous research has shown that the basic temporal and amplitude characteristics of the HRF does not change significantly with advancing age (D’Esposito, Zarahan, Aguirre and Rypma, 1999). The parameter estimate maps and variance maps for each variable and each contrast for every participant were then forwarded to a whole-head second level analysis whereby inter-participant variability was treated as a random variable. Mixed-effects analysis was performed using FLAME (FMRIB’s Local Analysis of Mixed Effects) (Beckmann et al., 2003; Woolrich et al., 2004) to locate regions of cortex that were significantly active in the four conditions (congruent, neutral, incongruent-eligible, and incongruent-ineligible) and in the incongruent>neutral contrast for each participant. The two incongruent conditions were merged for each of the participant in order to create an incongruent condition that was then contrasted with the neutral condition to create the incongruent>neutral contrast. This contrast was then used to isolate attention-specific processes for the two groups of participants. Specifically, in order to examine age-related differences in the recruitment of cortical resources as a function of task difficulty, we collapsed across the two age groups to create a mask for the incongruent>neutral condition. The resulting map was thresholded at a voxel-wise z-score of 2.33 (p<0.01) and a cluster-wise threshold of p<0.05. Clusters identified through this contrast were then used to examine age-related differences in modulation of the prefrontal and the parietal regions as a function of the different Stroop conditions.
Results
Behavioral Results
Reaction Time
Reaction time (RT) and accuracy rates of all participants were recorded while they performed the Stroop Task in the scanner (Table I). For reaction time analyses, only RT’s for correct trials were included. The results from the repeated measures analysis of variance with condition (congruent, neutral, incongruent-eligible, and incongruent-ineligible) as a within subjects factor and age as a between subjects factor revealed a main effect of age (F(1,48)=47.81, p<0.001). These effects indicate that older adults were slower than younger adults. The main effect of condition (F(1, 48)=43.98, p < 0.0001) and the interaction of age X condition were also significant (F(1,48)=2.70, p<0.05), such that older adults showed a greater increase in RT for incongruent-eligible trials relative to the neutral and the incongruent-ineligible trials than younger adults. Post-hoc paired t-tests between different conditions indicated that there was a significant difference between the incongruent-eligible condition and the neutral condition, congruent condition and the incongruent-ineligible condition for both the young and the older groups of participants; the difference, however was much more pronounced for the older adults. These results thus suggest that the incongruent-eligible condition resulted in significant conflict for both groups of participants, relative to the other conditions.
Table I.
Reaction Time and Error Rates (expressed as %) for old and young adults. Standard Errors are included in parentheses.
| Congruent | Neutral | Incongruent-Eligible | Incongruent-Ineligible | ||
|---|---|---|---|---|---|
| Old | Reaction Time | 822.56 (15.3) | 825.48 (17.6) | 913.20 (21.03) | 883.08 (19.67) |
| Error Rates | 2.56 (0.59) | 2.23 (0.55) | 6.44 (1.36) | 3.34 (0.76) | |
| Young | Reaction Time | 656.57 (16.03) | 676.08 (18.86) | 727.16 (21.90) | 706.02 (18.05) |
| Error Rates | 2.33 (1.38) | 4.00 (1.87) | 4.89 (1.60) | 4.11 (1.15) |
Accuracy
Similar to the RT data, accuracy data was analyzed using a repeated measures ANOVA. We found a main effect of condition (F(1,48)=6.33, p<0.001), however the main effect of age and the age X condition interaction were not significant. Consistent with the RT data, paired t-tests indicated a significant difference between the error rates on the incongruent-eligible condition and the congruent, neutral and the incongruent-ineligible condition for the older adults. For younger adults, we found a significant difference between error rates of the incongruent-eligible condition and the congruent condition.
Neuroimaging Results
Main Stroop Effect
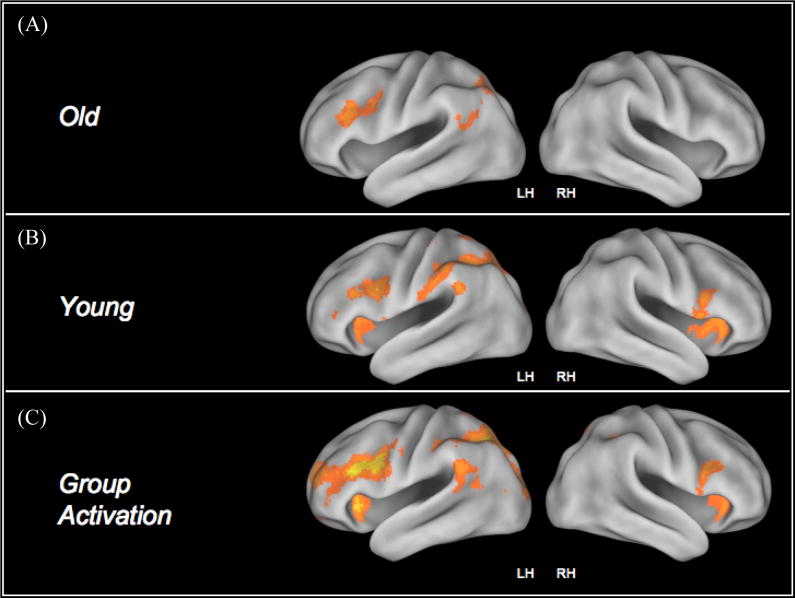
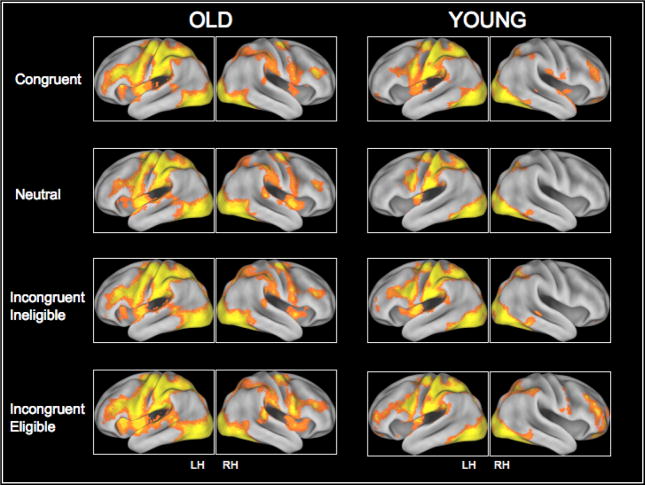
Collapsing across the two incongruent conditions, we found that the younger adults activated bilateral areas of the prefrontal cortex, left superior parietal lobules as well as the bilateral occipital cortices during the incongruent trials relative to the neutral trials (Figure II (B) and Table II(B)). Activation in the neutral condition was restricted to the parietal and occipital lobules, however with an increase in the level of conflict, younger adults demonstrated a greater recruitment of the prefrontal regions (Figure III). In contrast, we found that the older adults primarily recruited the left middle frontal gyrus, and the left superior parietal lobule in response to the incongruent>neutral contrast (Figure II (A) and Table II(A)). Figure III also shows activation in the congruent, neutral and the two incongruent conditions for the older and younger adults. As can be seen from the figure, for both the congruent and the neutral trials, older adults demonstrated significant activation in the prefrontal cortices.
Figure II.
Represents activation in the incongruent > neutral contrast for both groups of participants. The last row represents the inclusive mask generated for investigating differential recruitment patterns in the two groups as a function of task difficulty.
Table II.
Local maxima in the incongruent>neutral contrast for (A) older adults, and (B) younger adults.
| A | Region | Peak z-stat | MNI (X) | MNI (Y) | MNI (Z) |
|---|---|---|---|---|---|
| Cuneus (R) | 2.80 | 12 | −96 | 22 | |
| Inferior Frontal Gyrus (L) | 3.39 | −42 | 3 | 35 | |
| Inferior Parietal Lobule (L) | 3.38 | −36 | −68 | 43 | |
| Middle Frontal Gyrus (L) | 3.48 | −55 | 19 | 34 | |
| Precuneus (L) | 3.94 | −6 | −78 | 42 | |
| Supramarginal Gyrus (L) | 3.22 | −48 | −61 | 32 | |
| B | Region | Peak z-stat | MNI (X) | MNI(Y) | MNI(Z) |
|---|---|---|---|---|---|
| Fusiform Gyrus (L) | 3.28 | −22 | −72 | −19 | |
| Inferior Frontal Gyrus (L) | 3.68 | −48 | 7 | 29 | |
| Inferior Frontal Gyrus (R) | 3.43 | 48 | 11 | 20 | |
| Inferior Parietal Lobule (L) | 3.92 | −51 | −37 | 42 | |
| Insula (L) | 3.21 | −32 | 20 | 5 | |
| Insula (R) | 3.81 | 40 | 14 | 3 | |
| Lingual Gyrus (L) | 4.00 | −8 | −87 | −9 | |
| Lingual Gyrus (R) | 3.20 | 12 | −82 | −18 | |
| Precuneus (L) | 3.52 | −26 | −72 | 42 | |
| Posterior Cingulate (R) | 3.08 | 14 | −71 | 7 | |
| Superior Parietal Lobule (L) | 4.09 | −14 | −75 | 62 | |
| Supramarginal Gyrus (L) | 3.37 | −65 | −51 | 32 | |
Figure III.
Cortical recruitment in the four conditions of the Stroop task.
Eligibility Manipulation
In order to examine age differences in the recruitment of cortical resources and their subsequent manipulation as a function of task difficulty, we created an inclusive mask for the incongruent>neutral contrast by collapsing across age groups (Figure II (C)). We then created ROI’s based on the clusters identified in the collapsed incongruent>neutral contrast to examine modulation of the prefrontal and the parietal regions in response to increasing task demands for both older and younger participants.
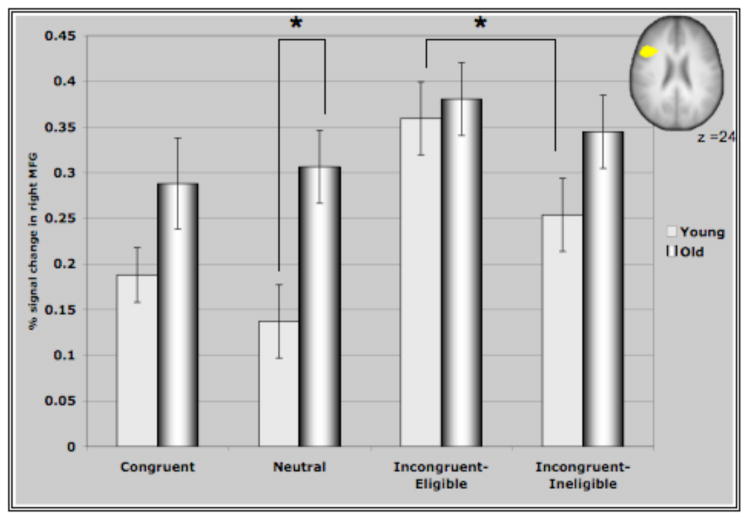
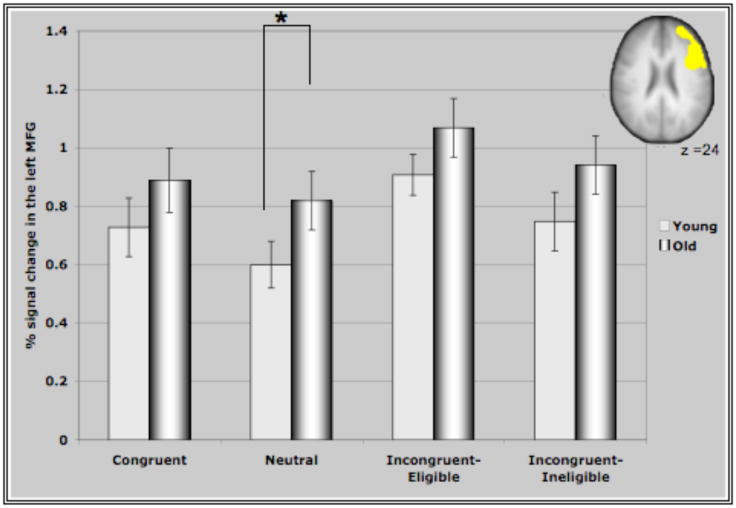
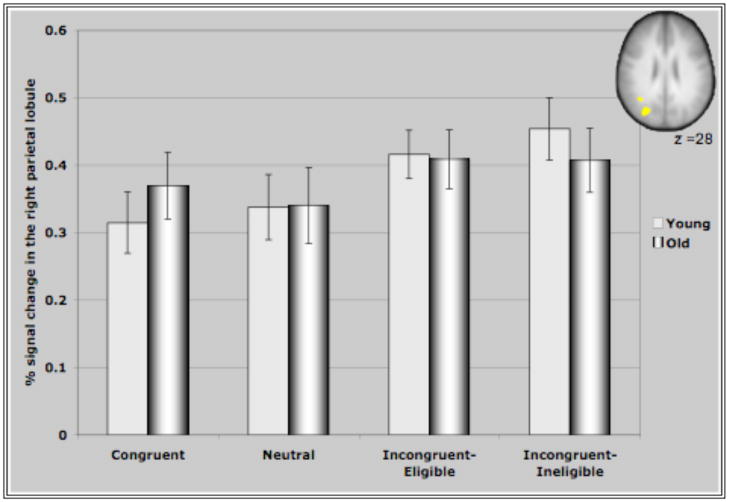
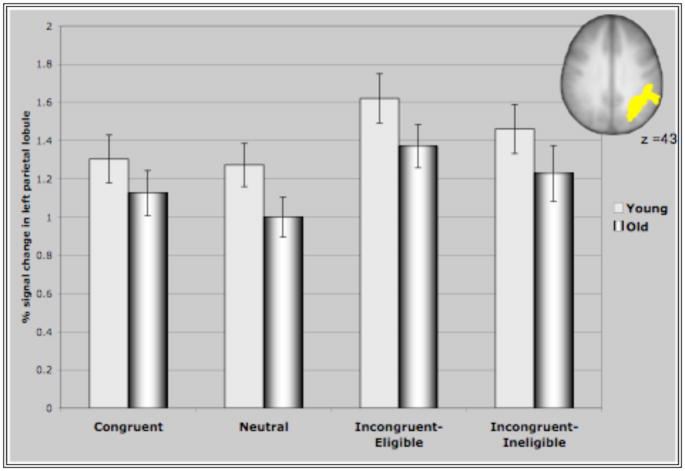
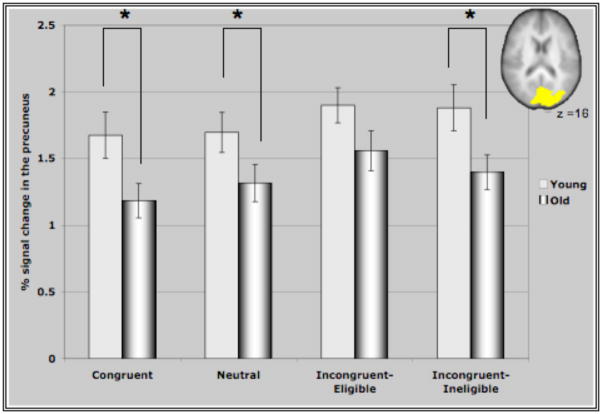
Figure IV represents the average percent signal change in the ROI’s identified through the incongruent>neutral contrast for all conditions of the Stroop task. These ROI’s comprising of bilateral MFG, bilateral areas of the parietal lobules and the precuneus demonstrated interesting patterns of recruitment for the two age groups. For both clusters in the prefrontal cortex (Figure IV (A &B)), older adults demonstrated either significantly greater activation than younger adults or a trend towards greater activation in the different conditions (with the exception of the incongruent-eligible condition). This is consistent with previous research reporting increased activation in older adults in response to different cognitive tasks (Cabeza et al., 2002, Colcombe et al., 2005). Consistent with our hypothesis, we found that younger adults demonstrated an increase in the recruitment of the right MFG in response to increasing task demands, reflected by increased activation in the incongruent-eligible condition relative to incongruent-ineligible condition (t=2.035, p < 0.05). In comparison, older adults failed to show such a modulation (t=1.26, ns) of the right PFC in response to increasing task demands. Though older adults did demonstrate an increase in the recruitment of right MFG for the incongruent-eligible and the ineligible condition relative to the neutral and congruent conditions, the increase was more pronounced for younger adults (Figure IV). Similarly for the left MFG, older adults demonstrated no difference between the two incongruent conditions, while younger adults showed a marginally significant difference (t=1.92, p=0.06) between the two incongruent conditions. The difference between the incongruent conditions and the congruent and neutral conditions was again much more pronounced for younger adults relative to older adults (Figure IV). These results thus suggest that older adults do have difficulty in flexibly recruiting of the prefrontal cortices in response to increases in task demands.
Figure IV. Represents percent signal change in the ROI’s identified through the incongruent>neutral contrast in the four conditions of the Stroop task.
(A) Activation in the right MFG for older and younger adults. As predicted, younger adults show a greater recruitment of the right MFG in response to the incongruent-eligible condition relative to the incongruent-ineligible condition. Older adults, failed to show such a modulation of the right MFG. * represents significance at p<0.05.
(B) Activation in the left MFG for older and young participants. There was a marginally significant different (p=0.06) between the two incongruent conditions for the younger adults. The recruitment of the left MFG was not significantly different between the two incongruent conditions for the older adults. * represents significance at p<0.05.
(C) Activation in the right parietal for both groups of participants. Both groups of participants showed a greater activation of the right parietal lobule during the incongruent trials as compared to the congruent and the neutral trials.
(D) Activation in the left parietal for both groups. Difference between the two incongruent conditions was not significant for either group of participants.
(E) Activation in the precuneus/lateral occipital cortex for both groups of participants. Cortical recruitment in this area for both groups did not differ for the two incongruent conditions. * represents significance at p<0.05.
In contrast, for the parietal regions and the precuneus, both older adults and younger adults failed to show a difference in activation between the two incongruent conditions of the Stroop task. Younger adults did show a significant increase in the recruitment of the bilateral parietal cortices and the precuneus during the incongruent-eligible condition relative to the congruent and the neutral conditions (p<0.05 for all paired t-tests conducted for the three cortical regions). During the incongruent-ineligible condition, they demonstrated a similar increase in the recruitment of the right parietal and the precuneus relative to the congruent and the neutral conditions (p <0.05), however the increase in the left parietal cortex during the incongruent-ineligible condition was marginally significant (p=0.09 relative to the neutral condition). Older adults, also demonstrated an increase in the recruitment of the left parietal and the precuneus during the incongruent-eligible relative to the neutral and the congruent conditions (p < 0.05), however for the right parietal this difference was only marginally significant (p=0.09). For the incongruent-ineligible condition, they demonstrated an increase in the left and the right parietal cortices (p<0.05) relative to the neutral condition, however the difference was not significant for the precuneus (Figure IV C, D & E).
Discussion
The results of this study support the hypothesis that older adults have more difficulty flexibly allocating prefrontal neural resources to increasing cognitive demands manifest in the Stroop task. In particular, older adults did not show differences in cortical activation under varying levels of conflict. However, they showed greater recruitment of neural resources, specifically the prefrontal and to some extent the parietal regions, at lower levels of conflict than younger adults. The consequence of this greater utilization of neural resources at lower levels of conflict represents an unavailability of resources to meet the demands of a more challenging condition (DiGirolamo et al., 2001; Reuter-Lorenz & Mikels, 2006). Regions of the prefrontal and the parietal cortices in older adults were less responsive to the heightened demands of attentional control, thereby suggesting a decrease in the efficiency and flexibility of such cortical regions responsible for imposing an attentional set. Our observation of a decreased responsiveness of prefrontal cortex to increased conflict extends a similar observation by Milham et al (2002) who compared age-related differences in DLPFC activation in a contrast between congruent and incongruent Stroop trials. In their blocked fMRI design with a relatively small number of participants younger adults showed increased activation in DLPFC between these contrasts while older adults did not. This effect was not due to less activity for the older adults in the more difficult condition, but rather greater activity in these regions for the less difficult (congruent) condition. All of these effects suggest that older adults have a clear deficit in the capabilities to flexibly allocate attentional control and cortical resources.
In contrast to the older participants, younger adults demonstrated enhanced sensitivity of the DLPFC in conditions of greater conflict. Increases in utilization of cortical resources by younger adults in response to increases in task complexity or in tasks that have high processing demands, like the one employed in the current study, has been demonstrated in a number of other studies (D’Esposito et al., 1998, Braver et al., 1997, Owen et al., 1997, Smith and Jonides et al., 1999). Interestingly, younger adults show an increase in activation of cortical areas that are known to be involved in imposing and maintaining an attentional set ensuing amplification of task-relevant attributes and a suppression of task-irrelevant representations (Milham et al., 2002, Banich et al., 2000). Specifically, models of attentional control posit the involvement of the dorsal and ventral prefrontal cortices in creating and maintaining an attentional set. This in turn is achieved by modulating activity in the posterior processing regions, such that regions involved in processing color attributes are amplified and regions specialized for identifying word or letter representations are suppressed. A recent study in our laboratory (Erickson et al., in press) with younger adults demonstrated such amplification of color-sensitive regions in the Stroop task. Older adults’ inflexible allocation of resources may also be apparent by assessing the capabilities of the prefrontal and parietal regions to modulate the activity in color and word sensitive regions of ventral visual cortex.
This study provides evidence for an age-induced inflexibility in the allocation of attentional resources. During performance on less demanding conditions, older adults utilized areas of the brain that were only recruited by younger adults in more demanding conditions. These results clearly indicate an age-related reduction in the capabilities for the neural substrates to flexibly react to dynamic and on-going attentional control demands. Such an inflexible allocation of resources may also underlie other age-related performance deficits in memory and attention processes. Such results have important implications for intervention studies that focus on reducing cognitive deficits in older adults. Cognitive training studies that partition complex tasks into component tasks and focus on training task components may be more beneficial to older adults. Cognitive training and aerobic exercise may benefit the aging brain by affecting the flexibility of the system to handle easier task conditions, thereby freeing resources to be devoted towards the more difficult task condition (Erickson et al., 2007; Dishman et al., 2006; Kramer & Willis, 2002). Automaticity of component tasks in older adults (as a result of training) could thus eventually aid in the performance of more complex, higher-level tasks.
Acknowledgments
We would like to thank the National Institute on Aging (RO1 AG25667 and RO1 AG25032) and the Institute for the Study of Aging for supporting this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Banich MT, Milhalm MP, Atchley R, Cohen NJ, Webb A, Wszalek T, Kramer AF, Liang Z, Wright A, Shenker J, Magin J. fMRI studies of Stroop tasks reveal unique roles of anterior and posterior brain systems in attentional selection. Journal of Cognitive Neuroscience. 2000;12:988–1000. doi: 10.1162/08989290051137521. [DOI] [PubMed] [Google Scholar]
- Banich MT, Milhalm MP, Jacobson BL, Webb A, Wszalek T, Cohen NJ. Attentional selection and the processing of task-irrelevant information: insights from fMRI examinations of the Stroop task. Progress in Brain Research. 2001;134:450–470. doi: 10.1016/s0079-6123(01)34030-x. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. General multi-level linear modeling for group analysis in FMRI. NeuroImage. 2003;20:1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Blasi G, Goldberg TE, Weickert T, Das S, Kohn P, Zoltick B, Bertolino A, Callicott JH, Weinberger DR, Mattay VS. Brain regions underlying response inhibition and interference monitoring and suppression. The European Journal of Neuroscience. 2006;23:1658–1664. doi: 10.1111/j.1460-9568.2006.04680.x. [DOI] [PubMed] [Google Scholar]
- Bench CJ, Frith CD, Grasby PM, Friston KJ, Paulesu E, Frackowiak RSJ, Dolan RJ. Investigations of the functional anatomy of attention using the Stroop test. Neuropsychologia. 1993;31:907–922. doi: 10.1016/0028-3932(93)90147-r. [DOI] [PubMed] [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. NeuroImage. 1997;5:49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. Journal of Cognitive Neuroscience. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Culham JC, Kanwisher NG. Neuroimaging of cognitive functions in human parietal cortex. Current Opinions in Neurobiology. 2001;11:157–163. doi: 10.1016/s0959-4388(00)00191-4. [DOI] [PubMed] [Google Scholar]
- Dale AM, Greve DN, Burock MA. Optimal Stimulus sequences for Event-Related fMRI. 5th International Conference on Functional Mapping of the Human Brain; Duesseldorf, Germany. June 11–16.1999. [Google Scholar]
- Dale AM. Optimal experimental design for event-related fMRI. Human Brain Mapping. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annual Review of Neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Aguirre GK, Zarahn E, Ballard D, Shin RK, Lease J. Functional MRI studies of spatial and nonspatial working memory. Cognitive Brain Research. 1998;7:1–13. doi: 10.1016/s0926-6410(98)00004-4. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Zarahan E, Aguirre GK, Rypma B. The effect of normal aging on the coupling of neural activity to the bold hemodynamic response. NeuroImage. 1999;10:6–14. doi: 10.1006/nimg.1999.0444. [DOI] [PubMed] [Google Scholar]
- DiGirolamo GJ, Kramer AF, Barad V, Cepeda N, Weissman DH, Wszalek TM, Cohen NJ, Banich M, Webb A, Beloposky A. General and task-specific frontal lobe recruitment in older adults during executive processes: A fMRI investigation of task switching. Neuroreport. 2001;12(9):2065–2072. doi: 10.1097/00001756-200107030-00054. [DOI] [PubMed] [Google Scholar]
- Dishman RK, Berthoud HR, Boot FW, Cotman CW, Edgerton VR, Fleshner MR, Gandevia SC, Gomez-Pinilla F, Greenwood BN, Hillman CH, Kramer AF, Levin BE, Toran TH, Russo-Neustadt AA, Salamone JD, Van Hoomissen JD, Wade CE, York DA, Zigmound MJ. The neurobiology of exercise. Obesity Research. 2006;14(3):345–356. doi: 10.1038/oby.2006.46. [DOI] [PubMed] [Google Scholar]
- Durston S, Davidson MC, Thomas KM, Worden MS, Tottenham N, Martinez A, Watts R, Ulug AM, Casey BJ. Parametric manipulation of conflict and response competition using rapid mixed-trial event-related fMRI. NeuroImage. 2003;20:2135–2141. doi: 10.1016/j.neuroimage.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Colcombe SJ, Wadhwa R, Bherer L, Peterson MS, Scalf PE, Kim JS, Alvarado M, Kramer AF. Training-induced plasticity in older adults: effects of training on hemispheric asymmetry. Neurobiology of Aging. 2007;28:272–283. doi: 10.1016/j.neurobiolaging.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Prakash RS, Colcombe SJ, Kramer AF. Top-down attentional control in the Stroop task enhances activity in color-sensitive regions of visual cortex. Behavioral Brain Research. doi: 10.1016/j.bbr.2008.08.028. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M. A fast, automated, n-dimensional phase unwarping algorithm. Magnetic Resonance in Medicine. 2003;49:193–197. doi: 10.1002/mrm.10354. [DOI] [PubMed] [Google Scholar]
- Kramer A, Humphrey D, Larish J, Logan G, Strayer D. Aging and inhibition: Beyond a unitary view of inhibitory processing in attention. Psychology and Aging. 1994;9:491–512. [PubMed] [Google Scholar]
- Kramer AF, Willis SL. Enhancing the cognitive vitality of older adults. Current Directions in Psychological Science. 2002;11:173–176. [Google Scholar]
- Liu X, Banich MT, Jacobson BL, Tanabe JL. Functional dissociation of attentional selection within PFC: response and non-response related aspects of attentional selection as ascertained by fMRI. Cerebral Cortex. 2006;16:827–834. doi: 10.1093/cercor/bhj026. [DOI] [PubMed] [Google Scholar]
- Milhalm MP, Banich MT, Webb A, Barad V, Cohen NJ, Wszalek T. The relative involvement of anterior cingulate and prefrontal cortex in attentional control depends on nature of conflict. Brain Research and Cognition. 2001;12:1325–1346. doi: 10.1016/s0926-6410(01)00076-3. [DOI] [PubMed] [Google Scholar]
- Milham MP, Erickson KI, Banich MT, Kramer AF, Webb A, Wszalek T, Cohen NJ. Attentional control in the aging brain: Insights from an fMRI study of the Stroop task. Brain & Cognition. 2002;49:467–73. doi: 10.1006/brcg.2001.1501. [DOI] [PubMed] [Google Scholar]
- Owen AM. The functional organization of working memory processes within human lateral frontal cortex: The contribution of functional neuroimaging. The European Journal of Neuroscience. 1997;9:1329–1339. doi: 10.1111/j.1460-9568.1997.tb01487.x. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Mikels J. The aging brain: implications of enduring plasticity for behavioral and cultural change. In: Baltes P, Reuter-Lorenz PA, Roesler F, editors. Lifespan Development and the brain: The perspective of biocultural co-constructivism. Cambridge University Press; Cambridge, UK: 2006. [Google Scholar]
- Smith SM, Zhang Y, Jenkinson M, Chen J, Mathews PM, Federico A, De Stefano N. Accurate, robust and automated longitudinal and cross-sectional brain change analysis. NeuroImage. 2002;17:479–489. doi: 10.1006/nimg.2002.1040. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Stern Y, Sano M, Paulsen J, Mayeux R. Modified Mini-Mental State Examination: Validity and Reliability. Neurology. 1987;37:179. [Google Scholar]
- vanVeen V, Carter JS. Separating semantic and response conflict in the Stroop task: A functional MRI study. NeuroImage. 2005;27:297–504. doi: 10.1016/j.neuroimage.2005.04.042. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM. Multi-level linear modelling for FMRI group analysis using Bayesian inference. NeuroImage. 2004;21:1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]