Abstract
Studies of the effects of estrogen replacement therapy on coronary heart disease risk have produced conflicting results. We hypothesize that this may be explained by differences in the length of estrogen deficiency prior to initiation of treatment and associated variation in plaque inflammation or stage of progression. The goal of this study was to determine whether estrogen administered after a period of deficiency affects plaque progression and leukocyte populations. Ovariectomized ApoE−/− mice were treated as follows: Group 1: continuous estrogen for 90 days (E+/+); group 2: placebo for 45 days followed by estrogen for 45 days (E−/+); group 3: estrogen for 45 days followed by placebo for 45 days (E+/−); and group 4: placebo for 90 days (E−/−). Serum lipoprotein concentrations, plaque size and inflammatory cell (macrophage, CD3+, CD4+, CD8+, dendritic cell, and NK cell) densities were quantified. Plaque size was smaller in groups receiving early estrogen therapy. CD3+ and total inflammatory cell densities were lower in late estrogen therapy groups. The CD8 to dendritic cell ratio was significantly lower in the E−/+ group only. These results suggest that a period of estrogen deficiency followed by reintroduction alters the immunologic environment of atherosclerotic lesions as well as plaque progression.
Keywords: atherosclerosis, estrogen, timing, inflammation, immunity
1. Introduction
Observational studies, clinical trials, and animal model studies have produced conflicting results regarding effects of estrogen replacement on risk of coronary heart disease and progression of coronary artery atherosclerosis.1-8 These divergent results may be explained by the presence or absence of a lengthy period of estrogen deficiency preceding the initiation of hormone replacement therapy.9-10 Specifically, there seems to be a beneficial effect of estrogen replacement on risk of coronary events and the progression of atherosclerosis when initiated early in the postmenopausal period and a lack of beneficial effect when initiated in subjects with preexisting coronary artery atherosclerosis who are several years beyond the onset of menopause.1-8, 11 However, the pathobiological pathways or processes responsible for this divergence have not been determined. Given that chronic inflammation is a hallmark of atherosclerosis,12-13 it seems possible that the divergent effects of estrogen related to the timing of onset of treatment are related to differences in the inflammatory environment of the plaque. We hypothesized that estrogen administered to mice after a period of estrogen deficiency results in alterations in intraplaque inflammation relative to plaques of mice treated continuously with placebo or estrogen.
2. Materials and Methods
2.1. Animals and Estradiol Delivery
All procedures involving animals were conducted in compliance with state and federal laws of the US Department of Health and Human Services, and guidelines established by the Wake Forest University Animal Care and Use Committee. The animal facilities and procedures of Wake Forest University Health Sciences are AAALAC accredited.
Fifty female ApoE null mice were obtained from the breeding colony at Wake Forest University's Comparative Medicine Clinical Research Center with original breeding pairs, backcrossed >99% to C57BL6/J, provided by Dr. Nobuyo Maeda at UNC Chapel Hill. All mice were fed a casein/lactalbumin-based diet and had access to water ad libitum. At 20 weeks of age, ten mice were sacrificed for use as a baseline control group, and the remaining forty mice were ovariectomized and randomly assigned to four treatment groups (n=10/group) in a 2×2 factorial design. 17β- estradiol or placebo were administered at a dose of 1.1ug/d for either 90-day or 45-day treatment periods via time release pellets (Innovative Research of America, Sarasota, FL) surgically implanted in the interscapular subcutis. Group 1 (E+/+) received 17β -estradiol continuously for 90 days; group 2 (E−/+) received placebo for 45 days followed by 17β -estradiol for 45 days; group 3 (E+/−) received 17β -estradiol for 45 days followed by placebo for 45 days; and group 4 (E−/−) received placebo continuously for 90 days. At the end of the 90 day treatment period, all mice were anesthetized (ketamine 80mg/kg and xylazine 8mg/kg, IP), blood was collected via cardiac puncture, and the arterial system was perfused with normal saline at 100mmHg for 10 minutes.
2.2. Tissue Collection and Processing
After perfusion, the innominate artery, including its bifurcation from the aorta, was collected, embedded in OCT medium with the lumen of the artery oriented perpendicular to the bottom of the mold, and frozen at −80°C. Nine (5um) cryosections were serially obtained from each artery with the first section defined as the point at which the aorta no longer appeared in the section. Sections were mounted on Superfrost Plus slides for routine histology or microcapillary gap slides for immunohistochemistry, fixed in cold acetone for 5 minutes, and frozen at −80°C until use. In addition, serum was collected and frozen at −80°C until use, and the uterus was removed, blotted dry, and weighed.
2.3. Histology and Immunohistochemistry
The first and second sections from each artery were stained with Verhoeff van Geison and hematoxylin and eosin, respectively. Sections 4, 6, 7, and 8 were immunostained for CD3+ cells, CD4+ cells, CD8+ cells, and macrophages, respectively. Section 9 was double immunostained for CD11c+ dendritic cells and Ly49G2+ natural killer (NK) cells. For the single immunostains the slides were incubated with rat anti-mouse CD3 (1:100; Serotec, Raleigh, NC), goat anti-mouse CD4 (1:50; R&D Systems, Minneapolis, MN), rat anti-mouse CD8 (1:100; Serotec, Raleigh, NC), or rat anti-mouse F4/80 (1:100; Serotec, Raleigh, NC) for 1.5 hours at 25ºC. Thymus and lymph node were used as positive and negative control tissue and the negative controls were incubated with non-immune sera instead of primary antibody for 1.5 hours at 25ºC. Primary antibodies were localized with appropriate biotinylated secondary antibodies, streptavidin-alkaline phosphatase (Biogenex, San Ramon, CA) and Vector Red (Vector Labs, Burlingame, CA) substrate. Sections were counterstained with Mayer's Hematoxylin and examined by light microscopy. For the double immunostain, slides were incubated with hamster anti-mouse CD11c (1:100; Biolegend, San Diego, CA) for dendritic cells or rat anti-mouse Ly49G2 (1:100; BDPharmingen, San Diego, CA) for NK cells for 1.5 hours at 25ºC. Uterus was used as positive and negative control tissue and the negative control was incubated with non-immune serum instead of primary antibody for 1.5 hours at 25ºC. Primary antibodies were localized with appropriate biotinylated secondary antibodies followed by either streptavidin-alkaline phosphatase (Biogenex, San Ramon, CA) with Vector Red (Vector Labs, Burlingame, CA) substrate for NK cells, or ABC-horseradish peroxidase (Vector Labs, Burlingame, CA) with DAB substrate (Biogenex, San Ramon, CA) for dendritic cells. Sections were counterstained with Mayer's Hematoxylin and examined by light microscopy.
2.4. Histomorphometry
Plaque size, areas of mineralization and necrosis, fibrous cap presence, and inflammatory cell densities were determined using computer assisted morphometric analysis (Image ProPlus 5.1). The extent of atherosclerosis was determined as the cross-sectional area of plaque in each of five sections per artery (sections 1, 2, 4, 6, and 7) and plaque area was calculated for each animal as the mean of the five sections examined. H&E stained sections were evaluated for foci of mineralization, hemorrhage, and necrosis within the tunica intima and the cross-sectional areas of such foci were measured. Cell density for macrophages, CD3+ cells, CD4+ cells, CD8+ cells, dendritic cells, and NK cells was determined by overlay of a 102 pixel grid with evaluation of each crosshatch for positive or negative staining by a trained observer blinded to treatment. Cell densities are expressed as the percentage of intima occupied by positive staining.
2.5. Serum Estradiol
For estradiol determinations, only small volumes of serum samples (0.05 ml) were available for analysis which was performed with a modified E2 assay procedure in an attempt to validate E2 delivery by a second method other than uterine weight. Prior to assay, samples were extracted with ethyl ether (2 mls), the organic phase was decanted, dried, and reconstituted with the same volume of zero standard from the radioimmunoassay kit (DSL 4800, Ultrasensitive Estradiol, Diagnostic Systems Laboratories; Webster, TX). Signal to noise ratio was low and results were inconclusive relative to circulating E2 concentrations. However, the time release pellets are a well-established method of hormone delivery and the 1.1ug/d dose has been shown to produce physiologic concentrations of estradiol and reduction of atherosclerotic extent in ApoE null mice.14
2.6. Serum Lipoproteins
Serum lipoproteins were separated by high pressure liquid chromatography,15 and aliquots of isolated lipoprotein fractions were used for enzymatic determination of cholesterol.16
2.7. Statistical Analysis
Innominate artery plaque area was calculated for each animal as the mean of the five sections examined and differences between treatment group means were analyzed by two-way analysis of variance (ANOVA) to assess the impact of early and late hormone therapy on plaque size. Oneway ANOVA was used to determine effects of treatment on intraplaque cellular densities, serum lipoprotein concentrations, serum estradiol concentrations, and uterine weight to body weight ratios with posthoc analysis using Fisher's LSD. The means of measures from animals sacrificed at 20 weeks (baseline) are also reported and used to estimate changes from baseline among each of the four treatment groups. Within each treatment group differences between CD8+ cell and dendritic cell densities were assessed by paired t-tests. Pearson's correlations were determined for serum lipoproteins and morphologic measures. Differences in the presence of fibrous caps among treatment groups were evaluated by chi-squared analysis. Data are expressed as mean +/−SEM.
3. Results
3.1. Plaque size
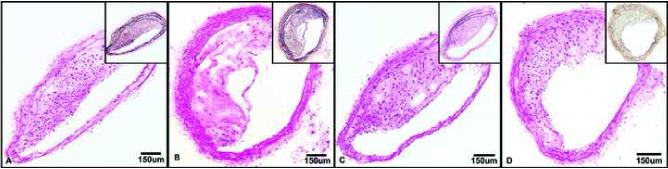
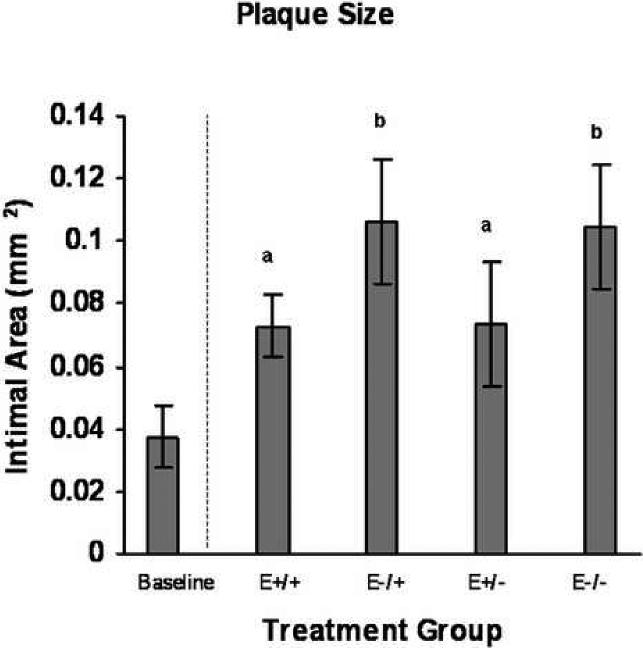
Examination of hematoxylin and eosin stained sections revealed moderate eccentric expansion of the tunica intima by moderate to large numbers of mononuclear cells and extracellular matrix in all four treatment groups (Figure 1). Intimal areas were measured in five sections per animal and no significant differences in plaque size between the five sections were found (p≥0.58). Mean plaque area (Figure 2) was significantly smaller in the groups receiving early hormone therapy (E+/+ and E+/−) compared to those receiving early placebo therapy (E−/+ and E−/−) [0.07 mm2+/−0.01 vs 0.11mm2+/−0.01 (p=0.03)], regardless of late treatment. Compared to baseline values, plaque size was 98.6% larger in groups that received early hormone therapy and 185% larger in groups which did not.
Figure 1.
Representative hematoxylin and eosin and Verhoeff vanGeison (insets) stained innominate arteries from each treatment group showing moderate eccentric expansion of the intima by extracellular matrix containing moderate to large numbers of mononuclear cells. A. E+/+ group; 10X. B. E−/+ group; 10X. C. E+/− group; 10X. D. E−/− group; 10X.
Figure 2.
Treatment group comparisons of plaque size. Data are expressed as mean +/−SEM; different letters indicate statistically significant differences by 2-way ANOVA, p=0.03.
3.2. Cellular Changes
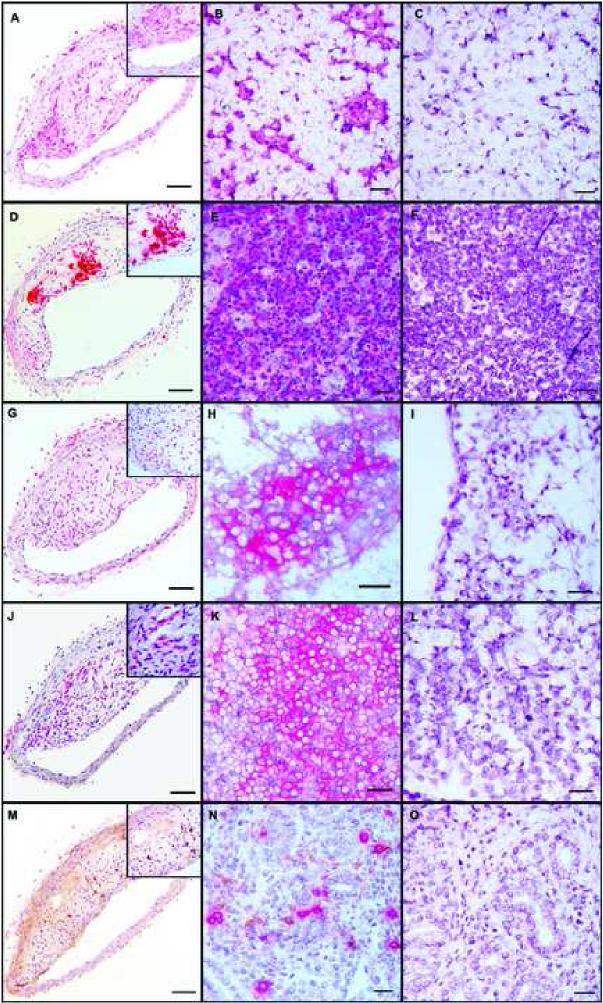
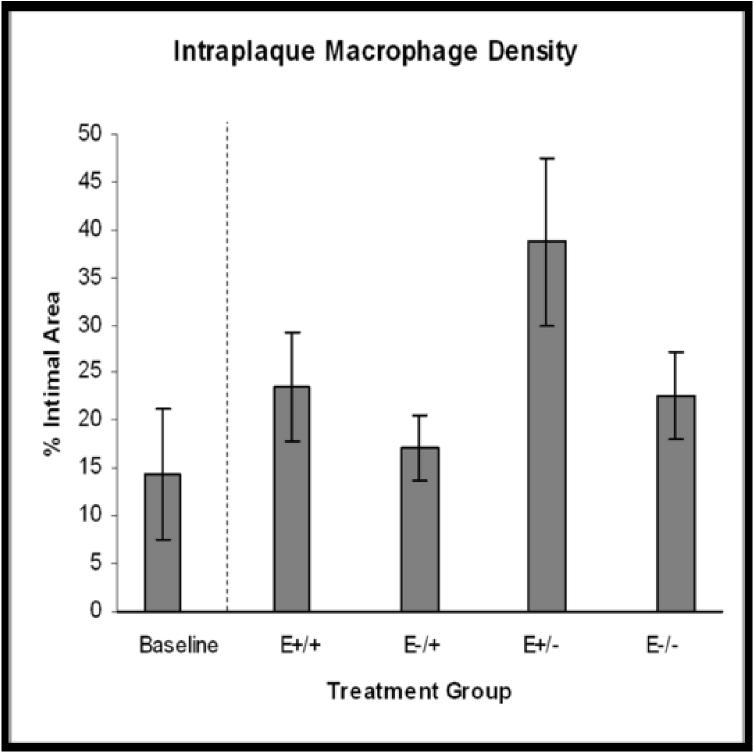
Strong positive cytoplasmic staining for the F4/80 marker was seen in 92% of arteries. Positively stained cells were generally randomly distributed throughout the plaque, however, in some arteries moderate numbers of macrophages accumulated in the lateral shoulder regions of the plaque (Figure 3A). Mean intraplaque macrophage density tended to be higher in animals that received early hormone therapy versus those that did not, however, statistically significant differences among the four treatment groups were not found (E+/+: 23.5%+/−5.8; E−/+: 17.0%+/−3.5; E+/−: 38.8%+/−8.9; E−/−: 22.6%+/− 4.5; p=0.08) (Figure 4A). Interestingly, the estimated change in macrophage density from baseline revealed a marked increase in the E+/− group (169%) versus the E+/+ (63%), E−/+ (19%), and E−/− (57%) groups.
Figure 3.
Representative immunohistochemical staining of innominate arteries (left column), positive control tissue (center column) and negative control tissue (right column); 10X magnification, insets 40X magnification. A-C: anti-F4/80 monoclonal antibody staining for macrophages; positive and negative control tissue is lymph node. D-F: Anti-CD3 monoclonal antibody staining for CD3+ cells; positive and negative control tissue is thymus. G-I: Anti-CD4 polyclonal antibody staining for CD4+ cells; positive and negative control tissue is lymph node. J-L: Anti-CD8 monoclonal antibody staining for CD8+ cells; positive and negative control tissue is lymph node. M-O: Double immunostaining for NK cells (red) using anti-Ly49G2 monoclonal antibody and for dendritic cells (brown) using anti-CD11c monoclonal antibody; positive and negative control tissue is uterus.
Figure 4.
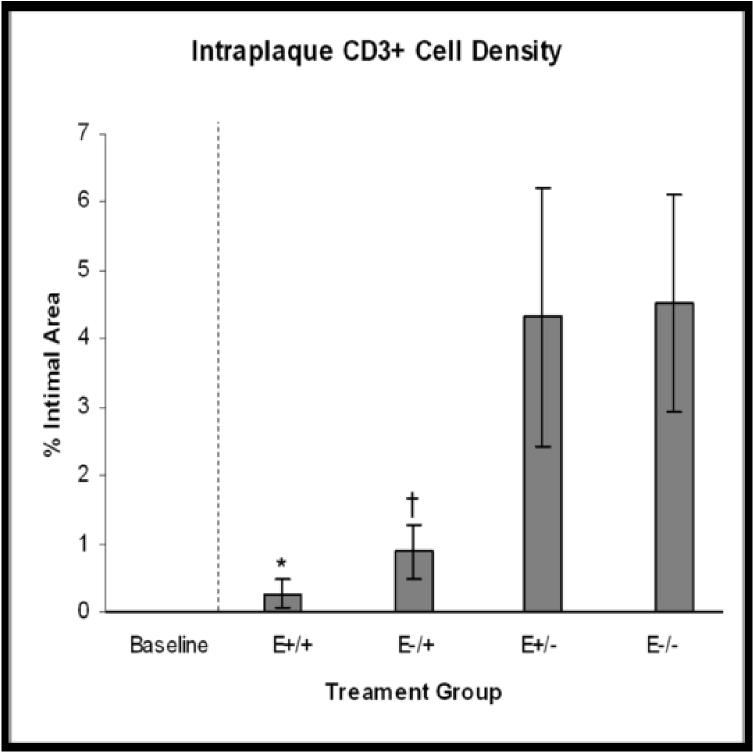
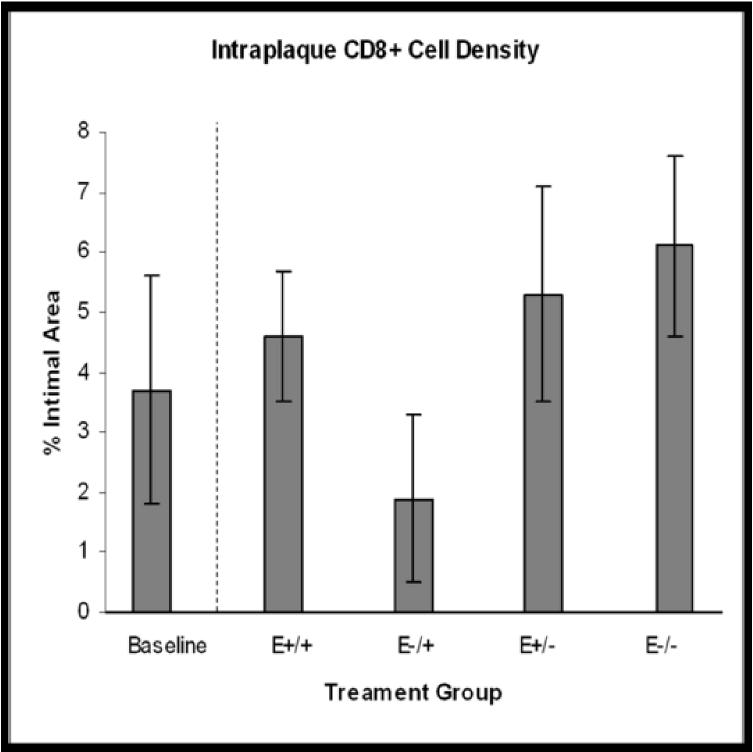
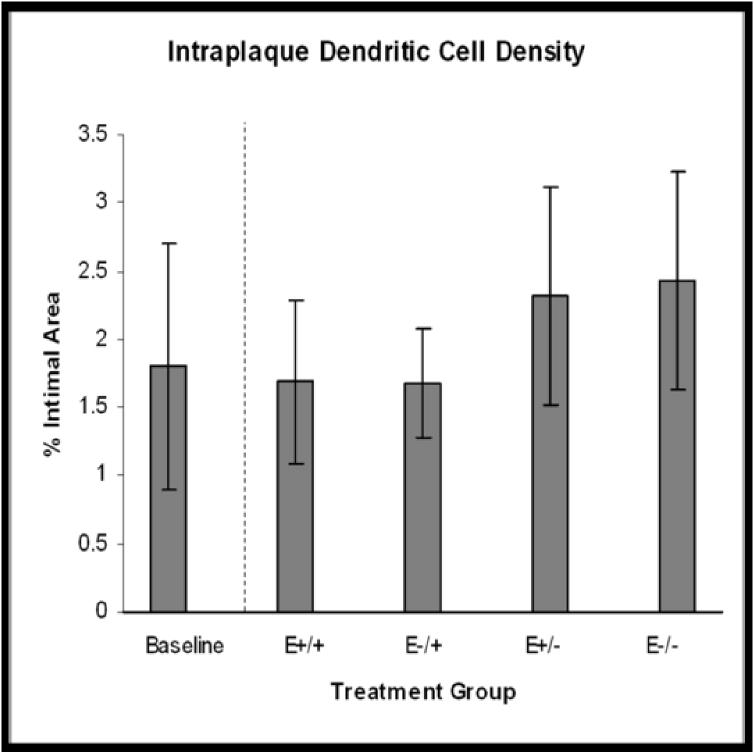
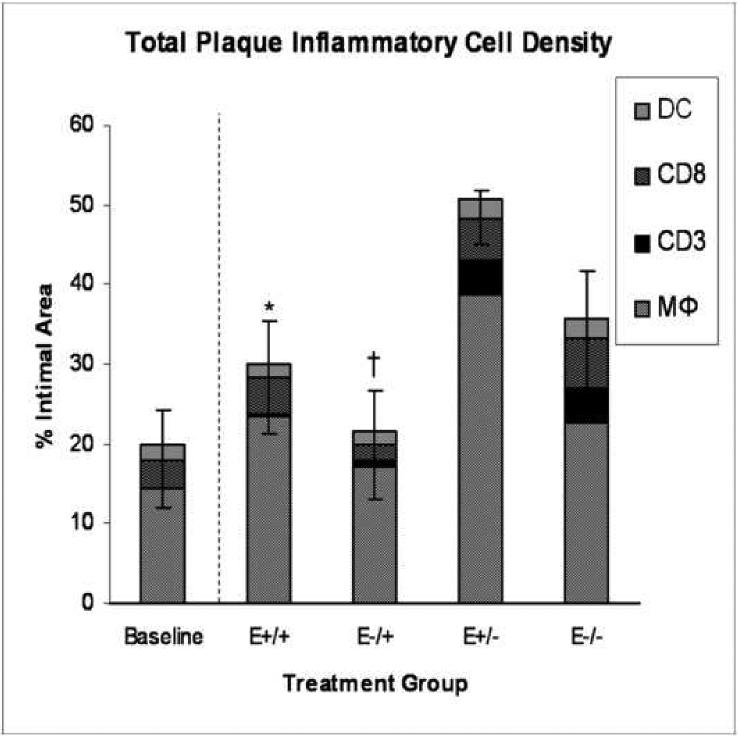
Treatment group comparisons of cellular outcomes. Data are expressed as mean +/−SEM; p-values ≤0.05 are significant. A. Macrophage density, p=0.08. B. CD3+ cell density, p=0.04; *E+/+ vs E+/− and E−/−, p≤0.03; †E−/+ vs E+/− and E−/−, p≤0.05. C. CD8+ cell density, p=0.14. D. Dendritic cell density, p=0.8. E. Total cellularity, p=0.02; *E+/+ vs E+/−, p=0.03; †E−/+ vs E+/−, p=0.002.
Strong positive cytoplasmic staining for CD3 was seen in 50% of arteries and positively stained cells were randomly distributed throughout the plaque (Figure 3D). Mean intraplaque CD3+ cell density was significantly lower in groups that received late estrogen therapy with both the E+/+ and E−/+ groups being significantly lower than the E+/− and E−/− groups (E+/+: 0.26%+/−0.2; E−/+: 0.88%+/−3.7; E+/−: 4.32%+/−1.9; E−/−: 4.52%+/− 1.6; p=0.04) (Figure 4B).
Immunostaining for CD4+ T lymphocytes using a polyclonal antibody was minimal to negative within the intima of all four treatment groups, with occasional positive staining of cells within the adventitia, and abundant positive staining of cells within lymph node control tissue (Figure 3G-H). Additional application of an anti-CD4 monoclonal antibody (rat anti-mouse CD4; 1:100; Serotec, Raleigh, NC) to innominate arteries from a separate population of mice which were treated similarly (ovariectomy and initiation of treatment at same age) but euthanized at a younger age, 14 weeks versus 20 weeks, also resulted in minimal to no positive staining within the intima (data not shown).
Strong positive cytoplasmic staining for CD8 was seen in 95% of arteries and positively stained cells were randomly distributed throughout the plaque (Figure 3J). Mean intraplaque CD8+ cell did not differ among the four treatment groups (E+/+: 4.66%+/−1.10; E−/+: 1.93%+/−0.4; E+/−: 5.35%+/−1.8; E−/−: 6.09%+/− 1.5; p=0.14) (Figure 4C). However, estimation of the percent change from baseline revealed a decrease in CD8+ cell density in the E−/+ group, -48%, compared with increases of 26%, 44%, and 65% in the E+/+, E+/−, E−/− groups, respectively. We also found that in the continuous estrogen group only there were significantly greater numbers of CD8+ cells than CD3+ cells (CD8+: 4.6%+/−1.1; CD3+: 0.264%+/−0.20 p=0.001).
Ly49G2+ NK cells were found only in the E+/+ group and at a very low density (0.1%) (data not shown).
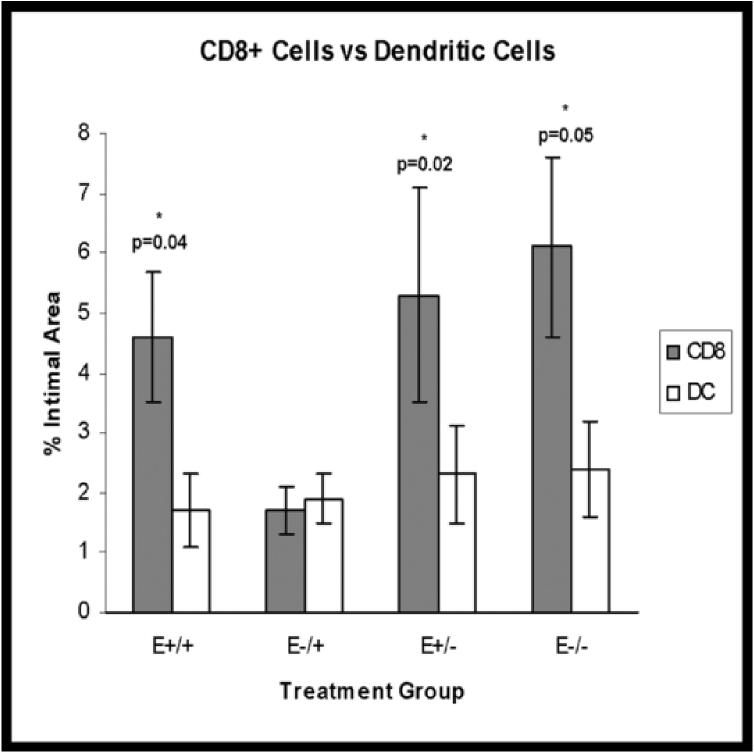
Strong positive cytoplasmic staining for CD11c (dendritic cells) was seen in 82% of arteries and positively stained cells were randomly distributed throughout the plaque (Figure 3M). No significant treatment effects were found (E+/+: 12.7%+/−11.0; E−/+: 11.6%+/−9.9; E+/−: 2.32%+/−0.8; E−/−: 13.4%+/− 11.0; p=0.8) (Figure 4D), however, relative to baseline levels, dendritic cell densities in the groups receiving estrogen in the late treatment period tended to be lower than at baseline (−8%) while densities in the groups receiving placebo in the late treatment period tended to be higher than at baseline (27-33%). In addition, the E+/+, E+/−, and E−/− groups had significantly greater numbers of CD8+ cells than dendritic cells (p=0.04, 0.02, 0.05, respectively) (Figure 5).
Figure 5.
Treatment group comparisons of CD8+ cells versus dendritic cells. Data are expressed as mean +/− SEM; p-values <0.05 are significant.
Total intraplaque density of the inflammatory cells types measured, including macrophages, CD3+ cells, CD8+ cells, and dendritic cells, were highest in the E+/− group and lowest in the E−/+ group. Both groups that received estrogen in the late treatment period, the E+/+ and E−/+ groups, contained significantly fewer inflammatory cells than the E+/− group (E+/+: 30.1%+/−6.9; E−/+: 21.4%+/−3.4; E+/−: 50.8%+/−8.4; E−/−: 35.3%+/− 6.2; p=0.02). (Figure 4E).
3.3. Extracellular Changes
Extracellular endpoints included intraplaque mineralization, fibrous cap development, necrosis, hemorrhage, and plaque rupture. Intraplaque mineralization was present in the E−/+, E+/−, and E−/− groups (E−/+: 0.007042mm2+/−0.003; E+/−: 0.006087mm2+/−0.005; E−/−: 0.006276mm2+/−0.004), and the percentage of intimal area mineralized was not significantly different between treatment groups (E+/+: 0; E−/+: 6.45%+/−3.6; E+/−: 3.46%+/−2.48; E−/−: 4.61%+/−3.23; p=0.4). However, plaques containing mineral were significantly larger than plaques not containing mineral (0.127mm2+/−0.014 vs 0.088mm2+/−0.009; p=0.04). Fibrous cap formation was found in all four treatment groups but not in the baseline group. There was no statistically significant difference in fibrous cap frequency between treatment groups (E+/+: 22%; E−/+: 60%; E+/−: 50%; E−/−: 44%; χ2=0.41), and there was no association between plaque size and fibrous cap development. Other extracellular measures of advanced plaque complication including necrosis, hemorrhage, and plaque rupture were not found.
3.4. Serum Lipoproteins
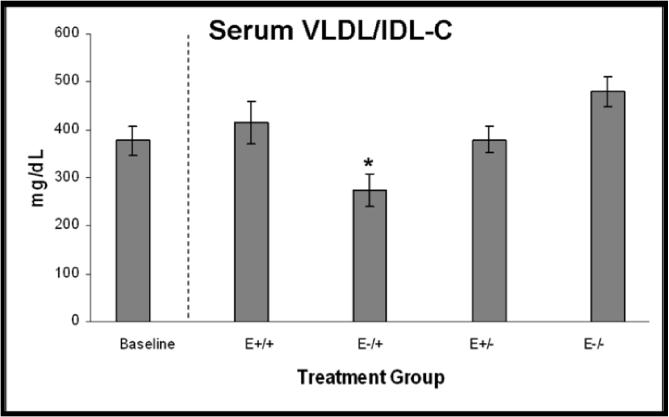
Determination of serum lipoprotein concentrations by HPLC revealed significantly lower concentrations of the very low density lipoprotein/intermediate density lipoprotein fraction (VLDL/IDL-C) in the E−/+ group as compared to the other three treatment groups (p<0.001) (Figure 6A). Low density lipoprotein (LDL) and high density lipoprotein (HDL) concentrations did not differ among the treatment groups (data not shown). VLDL/IDL-C positively correlated with intimal area (r=0.37, p=0.03) and CD8+ cell density (r=0.37, p=0.03), however covariate adjustment for VLDL/IDL-C did not alter significant differences in plaque size or CD8+ cell density among treatment groups.
Figure 6.
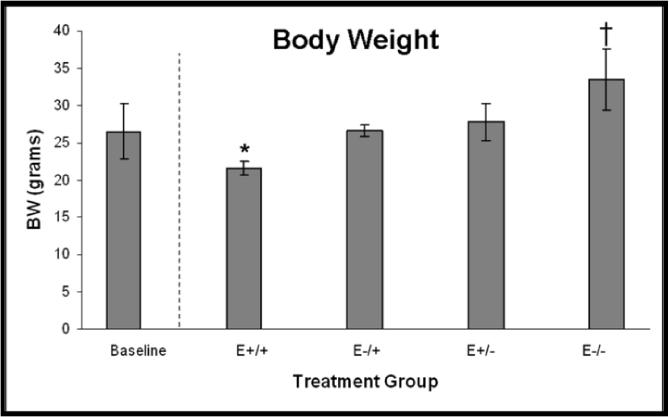
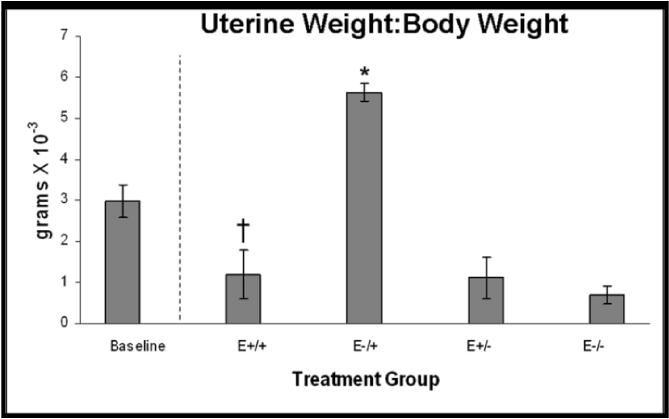
A. VLDL/IDL-C concentrations, p<0.001; *E−/+ vs E+/+, E+/−, E−/−, p≤0.05. B. Body weights at necropsy, p<0.001; *E+/+ vs E−/+, E+/−, E−/−, p≤0.05; †E−/− vs E+/+, E−/+, E+/−, p≤0.006. C. Uterine weight to body weight ratios, p<0.001; *E−/+ vs E+/+, E+/−, E−/−, p≤0.02; †E+/+ vs E−/−, p=0.05. Data are expressed as mean +/− SEM; p-values p≤0.05 are significant.
3.5. Body Weight and Uterine Weight
Body and uterine weights were determined as physical measures of hormone bioactivity. Consistent with physiologic effects of estrogen, mean body weights at necropsy were significantly lower in animals receiving continuous estrogen therapy and significantly higher in animals receiving only placebo; body weights of animals receiving both estrogen and placebo, regardless of timing, were intermediate (Figure 6B). Also consistent with estrogen exposure, mean UW:BW in the E−/+ group was significantly higher than the other three treatment groups, and UW:BW in the E+/+ group was higher than the E−/− group but not the E+/− group, while no difference was found between the E+/− and E−/− groups (Figure 6C).
4. Discussion
The results of this study indicate that timing of estrogen administration alters plaque size, T lymphocyte density, CD8 to dendritic cell ratios, and lipoprotein concentrations in ApoE−/− mice. Specifically, we demonstrated that animals receiving estrogen immediately following ovariectomy had significantly smaller lesions than those undergoing delayed or no estrogen exposure. This is in agreement with human and animal model data which suggests that estrogen is effective in primary prevention of atherosclerosis.17 Interestingly, this effect persisted even when estrogen was withdrawn later in the postovariectomy period. This is consistent with results of the Nurses' Health Study in which the relative risk of coronary disease among current hormone replacement therapy (HRT) users was 0.3, and the risk among participants who had ever received HRT was 0.5.1 Although loss of atheroprotection with estrogen withdrawal is well-documented,6,7,18 our findings support the notion that early estrogen replacement inhibits intimal expansion and that the effect is substantial enough to extend beyond the treatment period. Conversely, estrogen replacement after a period of deficiency had no suppressive effect on intimal expansion.
Another major finding of this study is that timing of estrogen administration altered the type and density of inflammatory cells within atherosclerotic plaques. Similar to other reports,13, 19-23 these lesions contained macrophages, T cells, NK cells, and dendritic cells. The macrophage was the predominant inflammatory cell type present, followed by T cells and dendritic cells with lesser numbers of NK cells. Among T cells, CD3+ T cell content was differentially regulated by estrogen, with late estrogen treatment resulting in lower concentrations of CD3+ cells. This is in agreement with studies showing induction of thymic atrophy24 and thymocyte apoptosis by estradiol.25 Likewise, estrogen treatment in mice decreases lymphocyte proliferation,26 and chronic estrogen deprivation in monkeys has been associated with a non-significant (p=0.15) increase in activated circulating CD3+ cells.27
Interestingly, CD8+ cell density was decreased in the E−/+ group but not in the E+/+ group. This suggests estrogen temporally modulated the immune response. Similarly, bilaterally oophorectomized women experienced a rise in circulating CD8+ cells in the thirty days following surgery prior to estrogen treatment, followed by a decrease in CD8+ cells after 30 days of estrogen replacement therapy.28 In addition, significantly greater numbers of CD8+ cells than CD3+ cells in the E+/+ group suggests the presence of a CD3−/CD8+ cell population such as NK cells or dendritic cells. Indeed, NK cells and dendritic cells were identified in these lesions and account for some of the suggested CD3−/CD8+ cell population.
With regard to dendritic cells, the CD8 to dendritic cell ratio was significantly lower in the E−/+ group. This decrease in CD8+ T cell density without significant change in dendritic cell density may be explained by induction of anergic and regulatory T cells by dendritic cells.29-31 Persistent presence of antigen has been associated with T cell anergy, deletion, or suppression as well as induction of tolerance in (or deletion of) activated CD8+ T cells.32-33 Such an effect could indicate lesion chronicity and is in agreement with other measures of plaque progression such as increased plaque size seen in the E−/+ group as compared to the E+/+ and E+/− groups. In addition, known modulatory effects of estrogen on dendritic cells include decreased antigen presentation and lymphocyte activation.34 In this case, the suppressive effect on CD8+ cell density was seen only in the E−/+ group, again suggesting temporal modulation of the immune response by estrogen.
The majority of T cells identified in this study were CD8+ and CD4-. This is in contrast to many published studies in which CD4+ cells are the predominant T cell present.21,35-36 In this study we used two different anti-CD4 antibodies, a monoclonal and a polyclonal, both of which consistently stained cells with morphologic characteristics of lymphocytes when applied to positive control lymph node sections. The lack of CD4+ cells in these lesions may represent differences in cellular infiltrate associated with stage of lesion progression. Most ApoE−/− mouse studies evaluating plaque T cell content have been performed on younger mice (8-18 weeks) at earlier stages of progression,21,35-36 and those sacrificing older mice (27-52 weeks) have not evaluated CD8+ cell density.37-38 Given the dynamic nature of the atherosclerotic process it seems likely that cell populations change as lesions progress. In addition, Elhage et al have shown that specific deletion of CD4+ or CD8+ cells does not change lesion size, suggesting that compensatory proatherogenic mechanisms are in place.38 The lack of Ly49G2 immunoreactivity (NK cells) in these lesions is also somewhat surprising, however, given the differential expression of NK cell markers in response to various differentiation, maturation, and activation states, the lack of positively staining NK cells in our lesions may not necessarily represent a lack of NK cells themselves but rather a lack of expression of the Ly49G2 marker by those NK cells present.
We also documented changes in extracellular plaque characteristics. Although we did not find an association between treatment and extent of intraplaque mineralization, we did find a positive association between mineralization and plaque size. This is in agreement with other studies which interpret mineralization and larger plaque size as indicators of lesion chronicity.22 Progression of atherosclerosis initially is associated with high plaque cellularity but at some point in their progression, atherosclerotic lesions become less cellular as extracellular changes such as matrix accumulation, fibrosis, proteolysis, necrosis, and mineralization predominate.23,39-40 The increased plaque size in the E−/+ group, combined with the suggestion that dendritic cell mediated suppression of the T cell response may be present in this group could indicate that these lesions may be more advanced than the more cellular and immunologically active lesions of the other three groups. On the other hand, lipid lowering and plaque regression has been associated with reductions in plaque foam cell and lipid content and an increase in matrix which also results in decreased cell density and presumably more stable lesions.41 So one might interpret the low cellularity of the lesions in the E−/+ group as being less advanced due to replacement of lipid with extracellular matrix in response to the lipid lowering effects of E2. Additional studies are required to test these hypotheses.
With regard to serum lipoproteins, there was a significant reduction in VLDL/IDL-C concentrations in the E−/+ group but not the E+/+ group. The lipid lowering effects of estrogen are well documented in the ApoE−/− mouse model, thus the lack of an effect in the E+/+ group was surprising. Other measures of acute estrogen exposure in this group, such as uterine weight to body weight ratios, suggest that estrogen delivery had diminished at the time of necropsy when lipoprotein concentrations were measured. By contrast, chronic measures of estrogen exposure such as decreased body weight and smaller plaque size suggest that estrogen delivery had occurred throughout the majority of the study. Thus, the most likely explanation for this discrepancy between acute and chronic measures of estrogen exposure is that delivery of estrogen from the time release subcutaneous pellet waned in the E+/+ group at the end of the 90-day treatment period, but was still releasing estrogen in the E−/+ group.
In conclusion, our results indicate that a period of estrogen deficiency followed by reintroduction alters the immunologic environment of atherosclerotic lesions and subsequent plaque progression. This suggests a potential pathophysiologic explanation for epidemiologic findings in which women undergoing a period of estrogen deficiency followed by reintroduction are more likely to experience a clinical event.
Funding Sources
This work was funded by the NIH (R01 AG17864). Dr. Cann is supported by National Center for Research Resources grant T32 RR 07009.
Footnotes
Conflicts of Interest There are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stampfer MJ, Willett WC, Colditz GA, Rosner B, Speizer FE, Hennekens CH. A prospective study of postmenopausal estrogen therapy and coronary heart disease. N Engl J Med. 1985;313:1044–1049. doi: 10.1056/NEJM198510243131703. [DOI] [PubMed] [Google Scholar]
- 2.Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Lasser NL, Trevisan M, Black HR, Heckbert SR, Detrano R, Strickland OL, Wong ND, Crouse JR, Stein E, Cushman M. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349:523–534. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- 3.Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, Vittinghoff E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 4.Herrington DM, Reboussin DM, Brosnihan KB, Sharp PC, Shumaker SA, Snyder TE, Furberg CD, Kowalchuk GJ, Stuckey TD, Rogers WJ, Givens DH, Waters D. Effects of estrogen replacement on the progression of coronary-artery atherosclerosis. N Engl J Med. 2000;343:522–529. doi: 10.1056/NEJM200008243430801. [DOI] [PubMed] [Google Scholar]
- 5.Hodis HN, Mack WJ, Lobo RA, Shoupe D, Sevanian A, Mahrer PR, Selzer RH, Liu CR, Liu CH, Azen SP. Estrogen in the prevention of atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2001;135:939–953. doi: 10.7326/0003-4819-135-11-200112040-00005. [DOI] [PubMed] [Google Scholar]
- 6.Adams MR, Kaplan JR, Manuck SB, Koritnik DR, Parks JS, Wolfe MS, Clarkson TB. Inhibition of coronary artery atherosclerosis by 17-beta estradiol in ovariectomized monkeys. Lack of an effect of added progesterone. Arteriosclerosis. 1990;10:1051–1057. doi: 10.1161/01.atv.10.6.1051. [DOI] [PubMed] [Google Scholar]
- 7.Adams MR, Register TC, Golden DL, Wagner JD, Williams JK. Medroxyprogesterone acetate antagonizes inhibitory effects of conjugated equine estrogens on coronary artery atherosclerosis. Arterioscler Thromb Vasc Biol. 1997;17:217–221. doi: 10.1161/01.atv.17.1.217. [DOI] [PubMed] [Google Scholar]
- 8.Clarkson TB, Anthony MS, Wagner JD. A comparison of tibolone and conjugated equine estrogens effects on coronary artery atherosclerosis and bone density of postmenopausal monkeys. J Clin Endocrinol Metab. 2001;86:5396–5404. doi: 10.1210/jcem.86.11.8021. [DOI] [PubMed] [Google Scholar]
- 9.Manson JE, Bassuk SS. Invited commentary: hormone therapy and risk of coronary heart disease why renew the focus on the early years of menopause? Am J Epidemiol. 2007;166(5):511–7. doi: 10.1093/aje/kwm213. [DOI] [PubMed] [Google Scholar]
- 10.Barrett-Connor E. Am J Epidemiol. Hormones and heart disease in women: the timing hypothesis. Am J Epidemiol. 2007;166(5):506–510. doi: 10.1093/aje/kwm214. [DOI] [PubMed] [Google Scholar]
- 11.Anthony MS, Clarkson TB. Does extent of pretreatment atherosclerosis influence the effects of conjugated equine estrogens on atherosclerosis progression? J Am Coll Cardiol. 2002;39:248A. [Google Scholar]
- 12.Kuiper J, van Puijvelde G, van Wanrooij E, van Es T, Habets K, Hauer A, van den Berkel T. Immunomodulation of the inflammatory response in atherosclerosis. Curt Opin Lipidol. 2007;18(5):521–6. doi: 10.1097/MOL.0b013e3282efd0d4. [DOI] [PubMed] [Google Scholar]
- 13.Vanderlaan PA, Reardon CA. Thematic review series: the immune system and atherogenesis. The unusual suspects:an overview of the minor leukocyte populations in atherosclerosis. J Lipid Res. 2005;46:829–838. doi: 10.1194/jlr.R500003-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Hodgin JB, Maeda N. Minireview: estrogen and mouse models of atherosclerosis. Endocrinology. 2002;143:4495–4501. doi: 10.1210/en.2002-220844. [DOI] [PubMed] [Google Scholar]
- 15.Carroll RM, Rudel LL. Lipoprotein separation and low density lipoprotein molecular weight determination using high performance gel-filtration chromatography. J Lipid Res. 1983;24:200–207. [PubMed] [Google Scholar]
- 16.Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–475. [PubMed] [Google Scholar]
- 17.Kaplan JR, Manuck SB, Anthony MS, Clarkson TB. Premenopausal social status and hormone exposure predict postmenopausal atherosclerosis in female monkeys. Obstet Gynecol. 2002;99:381–388. doi: 10.1016/s0029-7844(01)01659-3. [DOI] [PubMed] [Google Scholar]
- 18.Adams MR, Kaplan JR, Clarkson TB, Koritnik DR. Ovariectomy, social status, and atherosclerosis in cynomolgus monkeys. Arteriosclerosis. 1985;5:192–200. doi: 10.1161/01.atv.5.2.192. [DOI] [PubMed] [Google Scholar]
- 19.Yilmaz A, Lochno M, Traeg F, Cicha I, Reiss C, Stumpf C, Raaz D, Anger T, Amann K, Probst T, Ludwig J, Daniel WG, Garlichs CD. Emergence of dendritic cells in rupture-prone regions of vulnerable carotid plaques. Atherosclerosis. 2004;176:101–110. doi: 10.1016/j.atherosclerosis.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 20.Bobryshev YV, Taksir T, Lord RS, Freeman MW. Evidence that dendritic cells infiltrate atherosclerotic lesions in apolipoprotein E-deficient mice. Histol Histopathol. 2001;16:801–808. doi: 10.14670/HH-16.801. [DOI] [PubMed] [Google Scholar]
- 21.Zhou X, Nicoletti A, Elhage R, Hansson GK. Transfer of CD4(+) T cells aggravates atherosclerosis in immunodeficient apolipoprotein E knockout mice. Circulation. 2000;102:2919–2922. doi: 10.1161/01.cir.102.24.2919. [DOI] [PubMed] [Google Scholar]
- 22.Elhage R, Gourdy P, Jawien J, Brouchet L, Castano C, Fievet C, Hansson GK, Arnal JF, Bayard F. The atheroprotective effect of 17beta-estradiol depends on complex interactions in adaptive immunity. Am J Pathol. 2005;167:267–274. doi: 10.1016/s0002-9440(10)62971-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roselaar SE, Kakkanathu PX, Daugherty A. Lymphocyte populations in atherosclerotic lesions of apoE −/− and LDL receptor −/− mice. Decreasing density with disease progression. Arterioscler Thromb Vasc Biol. 1996;16:1013–1018. doi: 10.1161/01.atv.16.8.1013. [DOI] [PubMed] [Google Scholar]
- 24.Zoller AL, Kersh GJ. Estrogen induces thymic atrophy by eliminating early thymic progenitors and inhibiting proliferation of beta-selected thymocytes. J Immunol. 2006;176:7371–7378. doi: 10.4049/jimmunol.176.12.7371. [DOI] [PubMed] [Google Scholar]
- 25.Hoffman-Goetz L, Fietsch CL, McCutcheon D, Duerrstein L. Effect of 17beta-estradiol and voluntary exercise on lymphocyte apoptosis in mice. Physiol Behav. 2001;74:653–658. doi: 10.1016/s0031-9384(01)00622-9. [DOI] [PubMed] [Google Scholar]
- 26.Hoffman-Goetz L. Effect of estradiol and exercise on lymphocyte proliferation responses in female mice. Physiol Behav. 1999;68:169–174. doi: 10.1016/s0031-9384(99)00171-7. [DOI] [PubMed] [Google Scholar]
- 27.Keller ET, Zhang J, Yao Z, Qi Y. The impact of chronic estrogen deprivation on immunologic parameters in the ovariectomized rhesus monkey (Macaca mulatta) model of menopause. J Reprod Immunol. 2001;50:41–55. doi: 10.1016/s0165-0378(00)00087-5. [DOI] [PubMed] [Google Scholar]
- 28.Kumru S, Godekmerdan A, Yilmaz B. Immune effects of surgical menopause and estrogen replacement therapy in peri-menopausal women. J Reprod Immunol. 2004;63:31–38. doi: 10.1016/j.jri.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Kronin V, Wu L, Gong S, Nussenzweig MC, Shortman K. DEC-205 as a marker of dendritic cells with regulatory effects on CD8 T cell responses. Int Immunol. 2000;12:731–735. doi: 10.1093/intimm/12.5.731. [DOI] [PubMed] [Google Scholar]
- 30.Gilliet M, Liu YJ. Generation of human CD8 T regulatory cells by CD40 ligand-activated plasmacytoid dendritic cells. J Exp Med. 2002;195:695–704. doi: 10.1084/jem.20011603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steinman RM, Turley S, Mellman I, Inaba K. The induction of tolerance by dendritic cells that have captured apoptotic cells. J Exp Med. 2000;191:411–416. doi: 10.1084/jem.191.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rocha B, Grandien A, Freitas AA. Anergy and exhaustion are independent mechanisms of peripheral T cell tolerance. J Exp Med. 1995;181:993–1003. doi: 10.1084/jem.181.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang QH, Hu YZ, Cao J, Zhong YQ, Zhao YF, Mei QB. Estrogen influences the differentiation, maturation and function of dendritic cells in rats with experimental autoimmune encephalomyelitis. Acta Pharmacol Sin. 2004;25:508–513. [PubMed] [Google Scholar]
- 35.Zhou X, Robertson AK, Rudling M, Parini P, Hansson GK. Lesion development and response to immunization reveal a complex role for CD4 in atherosclerosis. Circ Res. 2005;96:427–434. doi: 10.1161/01.RES.0000156889.22364.f1. [DOI] [PubMed] [Google Scholar]
- 36.Zhou X, Robertson AK, Hjerpe C, Hansson GK. Adoptive transfer of CD4+ T cells reactive to modified low-density lipoprotein aggravates atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:864–870. doi: 10.1161/01.ATV.0000206122.61591.ff. [DOI] [PubMed] [Google Scholar]
- 37.Reardon CA, Blachowicz L, White T, Cabana V, Wang Y, Lukens J, Bluestone J, Getz GS. Effect of immune deficiency on lipoproteins and atherosclerosis in male apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2001;21:1011–1016. doi: 10.1161/01.atv.21.6.1011. [DOI] [PubMed] [Google Scholar]
- 38.Elhage R, Gourdy P, Brouchet L, Jawien J, Fouque MJ, Fievet C, Huc X, Barreira Y, Couloumiers JC, Arnal JF, Bayard F. Deleting TCR alpha beta+ or CD4+ T lymphocytes leads to opposite effects on site-specific atherosclerosis in female apolipoprotein E-deficient mice. Am J Pathol. 2004;165:2013–2018. doi: 10.1016/s0002-9440(10)63252-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rattazzi M, Bennett BJ, Bea F, Kirk EA, Ricks JL, Speer M, Schwartz SM, Giachelli CM, Rosenfeld ME. Calcification of advanced atherosclerotic lesions in the innominate arteries of ApoE-deficient mice: potential role of chondrocyte-like cells. Arterioscler Thromb Vasc Biol. 2005;25:1420–1425. doi: 10.1161/01.ATV.0000166600.58468.1b. [DOI] [PubMed] [Google Scholar]
- 40.Rosenfeld ME, Polinsky P, Virmani R, Kauser K, Rubanyi G, Schwartz SM. Advanced atherosclerotic lesions in the innominate artery of the ApoE knockout mouse. Arterioscler Thromb Vasc Biol. 2000;20:2587–2592. doi: 10.1161/01.atv.20.12.2587. [DOI] [PubMed] [Google Scholar]
- 41.Register TC, Adams MR, Golden DL, Clarkson TB. Conjugated equine estrogens alone, but not in combination with medroxyprogresterone acetate, inhibit aortic connective tissue remodeling after plama lipid lowering in female monkeys. Arterioscler Thromb Vasc Biol. 1998;18:1164–1171. doi: 10.1161/01.atv.18.7.1164. [DOI] [PubMed] [Google Scholar]