Abstract
The goal of time-resolved cryo-electron microscopy is to determine structural models for transient functional states of large macromolecular complexes such as ribosomes and viruses. The challenge of time-resolved cryo-electron microscopy is to rapidly mix reactants, and then, following a defined time interval, to rapidly deposit them as a thin film and freeze the sample to the vitreous state. Here we describe a methodology in which reaction components are mixed and allowed to react, and are then sprayed onto an EM grid as it is being plunged into cryogen. All steps are accomplished by a monolithic, microfabricated silicon device that incorporates a mixer, reaction channel, and pneumatic sprayer in a single chip. We have found that microdroplets produced by air atomization spread to sufficiently thin films on a millisecond time scale provided that the carbon supporting film is made suitably hydrophilic. The device incorporates two T-mixers flowing into a single channel of four butterfly-shaped mixing elements that ensure effective mixing, followed by a microfluidic reaction channel whose length can be varied to achieve the desired reaction time. The reaction channel is flanked by two ports connected to compressed humidified nitrogen gas (at 50 psi) to generate the spray. The monolithic mixer-sprayer is incorporated into a computer-controlled plunging apparatus. To test the mixing performance and the suitability of the device for preparation of biological macromolecules for cryo-EM, ribosomes and ferritin were mixed in the device and sprayed onto grids. Three-dimensional reconstructions of the ribosomes demonstrated retention of native structure, and 30S and 50S subunits were shown to be capable of reassociation into ribosomes after passage through the device.
Introduction
Cryo-electron microscopy has emerged as a powerful technique for structural characterization of isolated biomacromolecular complexes, such as viruses and ribosomes, in their native state. In principle, the submillisecond freezing rates that are routinely achieved in the preparation of macromolecules for cryo-EM should allow the trapping and characterization of transient structural states of functioning macromolecules that occur on time scales of about one millisecond. Indeed, several experimental methodologies for performing time-resolved cryo-EM have been described. A shortcoming of the methods described is that initiation of the kinetic process occurs while the macromolecular complex exists as a thin aqueous film in which some, or perhaps most, of the macromolecules are interacting with the carbon supporting surface or the air-water interface of the EM grid, a situation that could greatly alter the kinetics, in particular for second- or higher-order reactions in which free diffusion is an important parameter. Moreover, the existing methodologies (reviewed by Frederik and Sommerdijk (2005)) have not been widely applied, due to methodological encumbrances (Menetret et al., 1991; Subramaniam et al., 1993; Berriman and Unwin, 1994; White et al., 2003).
One existing method entails the spraying of the initiator reactant onto a grid bearing a thin film of the other reactants, as the grid is being transferred into cryogen (Berriman and Unwin, 1994). This method, which requires a nebulizer capable of reproducibly generating micrometer-sized droplets, unfortunately produces compositional heterogeneity in the sparsely generated regions where mixing of reactants occurs. Only a few applications of this method have been described since its introduction over 15 years ago (Fuller et al., 1995; Unwin, 1995; Walker et al., 1995). The other principal methodology for time-resolved cryo-EM requires that one of the reactants be photoactivatable, so that the reaction can be initiated by exposing the EM grid, as it is being transferred into cryogen, to a short pulse of light of the appropriate wavelength (Menetret et al., 1991; Subramaniam et al., 1993; Subramaniam and Henderson, 1999). Again, this method has not been widely used, most likely because of the limited availability of photoactive substrates, as well as other technical problems such as heating of the reaction mixture upon exposure to an intense flash of ultraviolet light. Recently, we implemented an improved methodology for time-resolved cryo-EM employing flash photolysis (Shaikh et al., 2009).
The challenges of time-resolved cryo-EM are (1) to rapidly mix microliter volumes of reactants, (2) allow them to react as freely diffusing species in aqueous solution for a specified time interval, (3) deposit as rapidly as possible the mixture as a thin aqueous film (<200 nm) on a carbon film supported by an EM grid, and (4) finally cool it to below the vitrification temperature. Previously we reported progress in the development and construction of rapid micromixers using conventional microfabrication techniques to obtain sub-millisecond mixing times, with sample consumption rates of several microliters per second (Lu et al., 2009). Here we present one solution to the problem of rapid deposition of microvolumes of a reaction mixture as a film sufficiently thin for use in cryo-EM.
Numerous potential methods could be envisioned to deposit the mixture on a TEM grid, including deposition-blotting, inkjet droplet printing, and micro spraying. Among these techniques, the extensively utilized spraying methods are the simplest, and they can generate droplets having diameters of several micrometers in a high velocity spray that can be deposited on a grid on a millisecond time scale. Although microdroplet spraying of specimens is not widely practiced in cryo-electron microscopy, this technique was described in the pioneering work by Dubochet and colleagues (Dubochet et al., 1982), and it has its origins in earlier electron microscopy studies of macromolecules by the negative staining technique (Oliver, 1973).
Liquid atomization and spray technology on the macro scale has been broadly applied in numerous industries (Bayvel and Orzechowski, 1993; Lefebvre, 1989; Liu, 2000; Nasr et al., 2002), e.g. fuel combustion, thin film coating, sprinkling, and powdering. Various nozzle types, based on spraying mechanism, have been developed, such as one phase (liquid) high pressure supersonic jets (Pianthong et al., 2003), ultrasonic spray (Rajan and Pandit, 2009), electrospray (Deng et al., 2007), and two phase (liquid/gas) internal or external atomization (Butler et al., 2002; Cavaliere et al., 2003; Jazayeri and Li, 2000; Kufferath et al., 1999; Lörcher and Mewes, 2001). The dimension of macro-scale spray nozzles usually ranges from several millimeters down to less than a millimeter, and the sizes of the generated droplets are in the range of tens of micrometers to greater than one millimeter. To obtain smaller droplet sizes and to minimize consumption of sample solutions, spray nozzles at the micro scale are a necessity. Micro-nozzles fabricated based on micro-electro-mechanical systems (MEMS) and microfabrication technologies have been studied in fields such as fuel injection (Baik et al., 2003; Deng et al., 2007; Satoha et al., 2005) and electrospray mass spectrometry (Griss et al., 2002; Kim et al., 2007). In those studies, nozzles with dimensions as low as several micrometers can be fabricated, the droplet size can be reduced to several micrometers, and sample consumption rate can be reduced to sub-microliters per minute. Most of the micro sprayers utilize high-voltage electro spraying techniques (Deng et al., 2006) or ultra high pressure (up to tens of MPa) atomization methods (Naik et al., 2007; Yang et al., 2004) to generate microdroplets, and these techniques could be damaging to biological macromolecular complexes.
Previously, our laboratory designed and tested a mechanically machined air-assisted sprayer that generated microdroplets suitable for rapid freezing and cryo-EM, and that consumed sample at a modest rate of several microliters per second (Barnard and Wagenknecht, 2005). However, it proved difficult to combine the sprayer with the micromixers that we subsequently designed (Lu et al., 2009) in a configuration that could achieve time resolutions of less than hundreds of milliseconds. Here we describe the first fabrication of a monolithic device integrating a rapid micromixer together with an air-assisted microsprayer to achieve processing times as short as a few milliseconds. We expect this device to allow routine cryo-EM studies of the dynamics of biological macromolecules on times scales of milliseconds or longer. Device construction uses standard microfabrication techniques adapted from the semiconductor industry. We used silicon and Pyrex glass wafers as substrates, but other materials can also be chosen, such as polydimethylsiloxane (PDMS), polymethyl methacrylate (PMMA), or another suitable biocompatible polymer. We tested the device’s performance in generation of micrometer-size droplets. Mixing was demonstrated for biomolecules using solutions of ribosomes and ferritin, and retention of biological structure and function was demonstrated for the ribosomes by several experimental criteria, including three-dimensional reconstruction from cryo-EM data.
Methods
Device Design and Fabrication
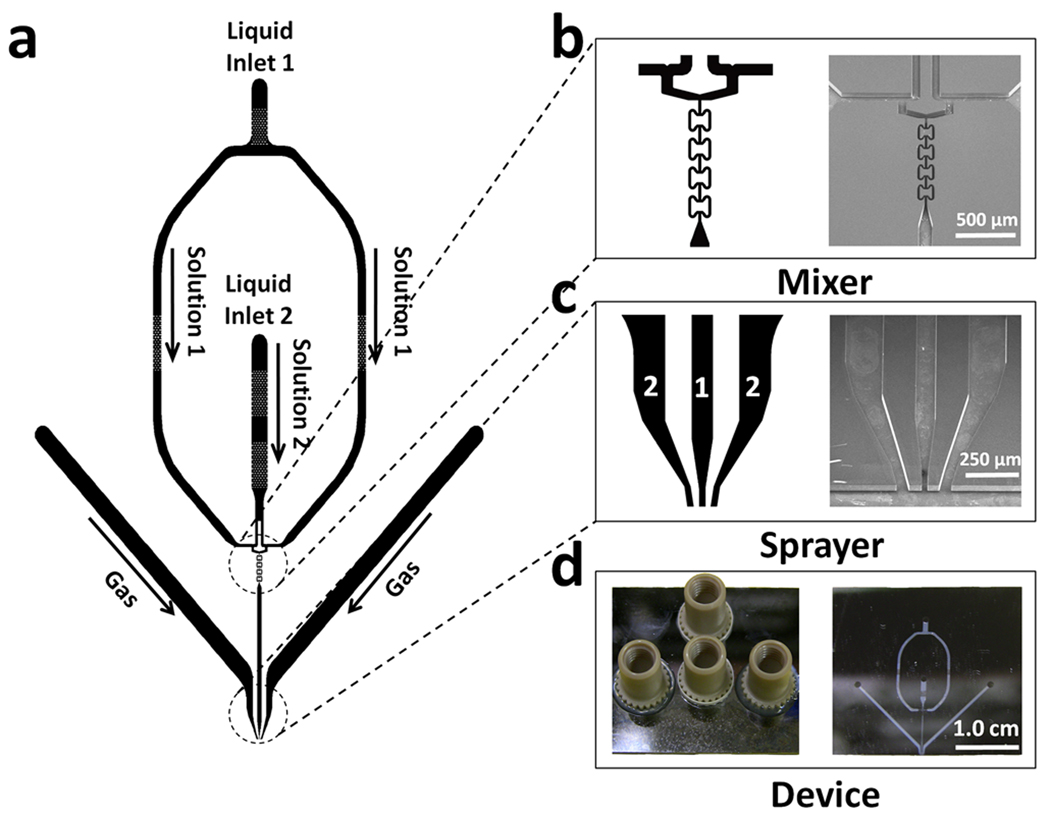
Configurations of devices that were constructed are shown in Figure 1. Each device includes one micromixer and one microsprayer. The mixer includes two T-type pre-mixers and four butterfly-shaped in-channel mixing elements; as we described previously (Lu et al., 2009), this mixer can attain complete mixing at total flow rates ≥6.0 µL/s in less than 0.5 ms. Two inlets in the device center serve as liquid inlets for reactants to feed in. Each reactant stream is split into two, and each sub-stream is directed to one of the two T-type premixers (in which the two reactant sub-streams meet each other at a right angle). The output from each T-pre-mixer then enters a channel leading to the butterfly mixing elements. The purpose of the two T-pre-mixers is to create additional boundaries (thereby increasing contact area) between the two fluids containing the reactants upon entering the butterfly mixing elements; this effectively reduces the diffusion distance for the reactants to be mixed, and, thus, reduces the mixing time. After mixing, the liquid mixture is fed to the micronozzle via a 100 µm wide, 40 µm deep, and 6000 µm long microfluidic “reaction channel.” For longer or shorter reaction times the channel length or geometry could be modified. Two gas-flow channels feed humidified nitrogen gas to the gas nozzles from both sides of the liquid nozzle. The gas channels are 400 µm wide and 40 µm deep, except near the nozzle itself, where they narrow as described below (Fig. 1). The micronozzles are basically planar, liquid-sheet spray nozzles with a gas-liquid-gas sandwich structure, a so-called prefilming type of atomizer (Liu, 2000). The gas and the liquid nozzles are rectangular in cross-section.
Figure 1. Monolithic micro mixing-spraying device.
(a) Device layout. (b) Detail of the micromixer configuration (left) and corresponding SEM image (right). (c) Detail of the fabricated nozzle (left) and SEM image (right). “1” indicates the liquid-filled reaction channel, and “2” denotes a channel carrying pressurized gas. (d) Photograph showing the top view of a finished device, with four nanoports attached for tubing connections (left) and a bottom view of the device, showing the transparent glass wafer cover through which the device’s micromixer and the microsprayer can be seen (right).
The external atomization nozzle has one liquid opening that is sandwiched by the openings from the two gas channels. Two devices of this configuration were fabricated with different dimensions: device number 1 has a liquid nozzle of 60-µm width and two gas nozzles each of 120-µm width and which bend to an angle of 7° with respect to the axis of the liquid-feeding channel. The gap between the liquid and gas nozzle is 120 µm. Device number 2 has a narrower liquid nozzle of 30-µm width and two gas nozzles of 60 µm width, and the gap between the liquid and gas nozzle is 60-µm. The devices have overall dimensions of 31.0 mm × 28.5 mm, and a thickness of 0.95 mm. The total volume of each device is ~1.2 microliters.
The device fabrication procedure was as follows (see supplementary Figure 1). First, a quartz photo mask was designed with all of the desired device patterns (mixer and nozzle), and was generated by Photo Sciences Inc. (Torrance, CA). A 4-inch-diameter silicon wafer of 475 µm thickness was cleaned and spin-coated on the front side with a 1.0-µm thick positive 1813 photoresist. Then, the device patterns were transferred from the mask to the resist layer, using an OAI mask aligner (OAI Inc., San Jose, CA). After the resist was developed to remove the exposed photoresist, the silicon wafer with the patterned resist was etched using DRIE (Deep Reactive-Ion Etching) in an Alcatel AMS100 DRIE etcher (Alcatel Vacuum Products, Tempe, AZ) to produce 40-µm deep channels. Then, the silicon wafer was aligned and patterned on the back side with a 20-µm negative SU-8 photoresist, and this surface was DRIE-etched through the whole wafer to produce the liquid/gas inlets. The SU-8 resist served as hard mask to protect the wafer from undesired etching. The wafer was then cleaned and anodically bonded to a 500-µm thick Pyrex glass wafer on the front side so as to seal the microchannels. The bonded wafer contained five devices, and was diced using a ThermoCarbon Tcar 864-1 dicing saw (Thermocarbon Inc., Casselberry, FL) to produce the individual devices. Four NanoPort assemblies (Upchurch Scientific, Oak Harbor, WA), which served as fittings for tubing connections, were attached to each device. The devices were then sonicated in isopropyl alcohol for 10 min to clean the interior of the microchannels, and were finally dried with a stream of nitrogen gas. SEM images of one micromixer and one microsprayer are shown in Figure 1.
Prior to use of the devices for experiments, they were cleaned by flushing with 4.0 mL double-distilled, deionized, autoclaved water, followed by 2.0 mL of buffer (for detailed buffer descriptions see "Ferritin-Ribosome Mixing" and "Preparation of Ribosomes for Kinetics Experiments" sections below) immediately before an experiment. After completion of the experiment the device was cleaned by flushing with 2.0 mL 1% Alconox detergent solution, followed by rinsing with 10 mL deionized water to remove the detergent. Finally, the device was dried by blowing nitrogen gas through the microchannels, and checked for cleanness of the microchannels by optical microscopy. The device was stored at room temperature until the next use.
Droplet Size Determination
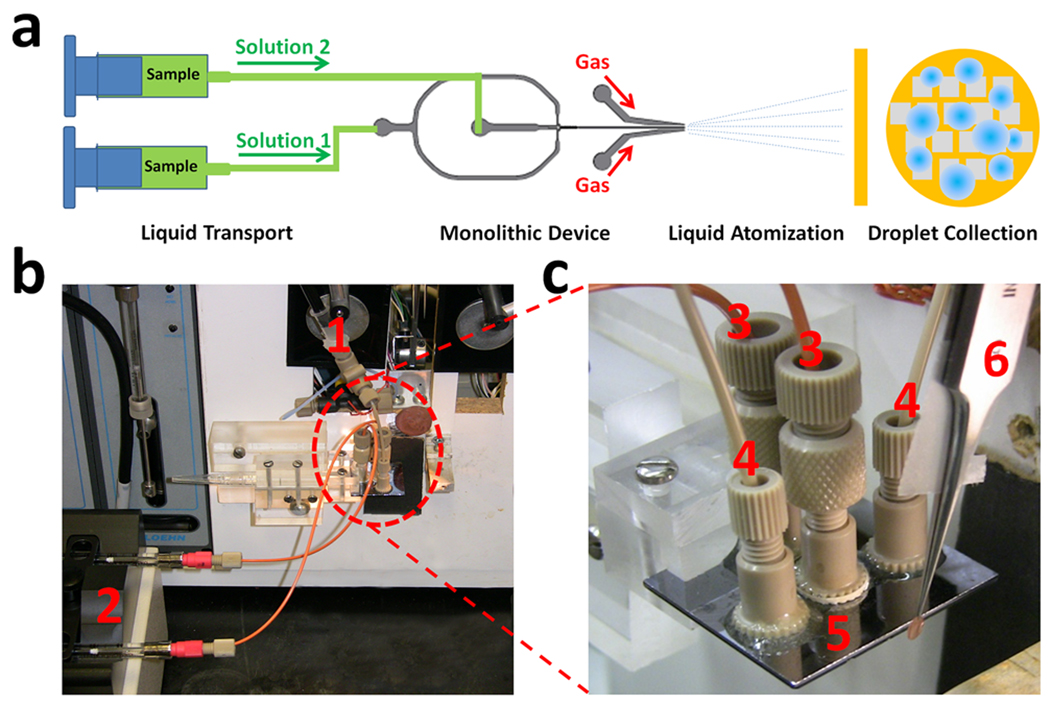
The performance of each device in generating microdroplets was determined by spraying solutions onto standard carbon-coated EM grids and then examining them in the transmission electron microscope (TEM). The testing system setup scheme is shown in Figure 2. Ferritin (5 mg/mL) suspensions were fed to the device through 1/16” polyetheretherketone (PEEK) capillary tubing (125 µm inner diameter) and 2.0-µm PEEK micro filters by a dual syringe pump (Cole Parmer Inc., Vernon Hills, IL). The pump produced equal flow rates from the two syringes in the range of 0.001 to 88.1 µL/s. The summed outputs from both syringes used for the testing were 3.0, 4.5, and 6.0 µL/s. Nitrogen gas was fed to the two gas nozzles by a nitrogen tank; the initial pressure was regulated to 50 psi for all tests.
Figure 2. Experimental design and setup.
(a) Diagram of experimental setup. (b) Photograph of a device mounted on a computer-controlled grid-making instrument (White et al., 2003). (c) Close-up photograph of the mounted monlithic device. The system components annotated in the photos are: (1) gas feed-in connection, (2) syringe pump with mounted 250-µL syringes, (3) orange-colored solution feed-in tubing, (4) grey-colored gas feed-in tubing, (5) monolithic device mounted on holder, and (6) tweezers mounted in plunger with an EM grid on the tip
Each grid to be tested (3-mm diameter copper TEM grid coated with a hydrophobic carbon thin film) was held in a sharp-tip tweezers, and was aligned and plunged through the spray; the plunge speed (1.0 m/s) was controlled by a computer-controlled grid freezing instrument (White et al., 2003). The grid containing the collected microdroplets was air-dried, placed in the TEM grid holder, and then examined using a FEI F20 TEM (FEI Company, Hillsboro OR) for evaluation of the droplet size.
Ferritin-Ribosome Mixing
To demonstrate mixing and preservation of macromolecular structures, we mixed ferritin (7 µM) and 70S E. coli ribosomes (7 µM), and sprayed the mixture (final concentrations 3.5 µM) at room temperature. Ferritin was from Sigma-Aldrich (St. Louis, MO). Ribosomes were purified as described below. Samples were prepared in polymix buffer (95mM KCl, 8mM putrescine, 5mM KH2PO4, 5mM NH4Cl, 5mM magnesium acetate, 1mM spermidine, 1mM dithiothreitol, 0.5mM CaCl2, (pH 7.3)). The selected micromixer was mounted onto the computer-controlled grid-plunging device. Ferritin and ribosomes were loaded in separate syringes, sprayed as described above (flow rate 6 µL/s) onto R2/4 Quantifoil grids (Micro Tools GmbH, Jena, Germany), and plunged into liquid ethane. Prior to use of the grids, a ~5-nm thick layer of continuous carbon was transferred from a mica substrate to the grids, and they were glow discharged for 30 s using a plasma cleaner (medium setting on Harrick model PKC-3xG) to render them hydrophilic (Grassucci et al., 2007).
Electron microscopy was performed on an FEI Tecnai F20 transmission electron microscope operating at 200kV, at nominal 50,000× magnification (50,360× calibrated), under low-dose conditions, with defocus ranging from 2.3 to 4.4 µm underfocus. Micrographs were digitized on a Zeiss scanner (Z/I Imaging) with a step size of 14 µm, corresponding to 2.78Å on the object scale. Image-processing was performed with the SPIDER software package (Frank et al., 1996). Particle images were aligned by projection-matching (Penczek et al., 1992; Shaikh et al., 2008b), using a previous reconstructed E. coli ribosome as a reference (Gabashvili et al., 2000). Particle images were screened by classification-based verification (Shaikh et al., 2008a).
Preparation of Ribosomes
Ribosomes were prepared according to Blaha and coworkers (Blaha et al., 2000) with slight modification. The S-100 pellet was dissolved in HEPES-K buffer (20 mM HEPES-KOH, 30 mM NH4Cl, 5 mM 2-mercaptoethanol) containing 4 mM MgCl2 to separate tight-coupled (TC 70S) from loose-coupled (LC 30S and 50S) ribosomes as described previously (Agrawal and Burma, 1996). The TC 30S and TC 50S ribosomal subunits were subsequently prepared from TC 70S ribosomes using HEPES-K buffer with 0.1 mM MgCl2.
Association experiments using the monolithic mixer-sprayer device
To study reasocciation of ribosomal subunits we used equal volumes of the 30S and 50S subunits, each at a concentration of 1.2 µM in HEPES-K buffer containing 12 mM MgCl2, for spraying onto hydrophilic EM grids or for collection into Eppendorf tubes for analysis by ultracentrifugation. The 30S and 50S subunits were pre-incubated at 37°C for 45 min before injection into the device (device #2), which was held at room temperature (20–22 °C). The flow rate was controlled at 6.0 µL/s.
Association experiments on sucrose gradients
The reaction mixture of 30S and 50S subunits was sprayed onto grids for cryo-EM, or, for some control experiments, the spray was collected into Eppendorf tubes for analysis by ultracentrifugation. The collected material (0.75 OD260 units) was diluted to 0.5 mL (concentration estimated at 0.03 µM, assuming complete formation of 70S ribosomes), and was loaded on a 5–30% sucrose gradient in HEPES-K buffer containing 12 mM MgCl2 to detect association of the ribosomal subunits to form 70S ribosomes. As a negative control, an equivalent amount of the mixture was loaded on a 5–30% sucrose gradient in HEPES-K buffer with 0.1 mM MgCl2. Separate gradients of each of the individual subunits (0.75 OD260 units) used in the spraying experiment were also loaded on to separate gradients as controls.
Results
Spraying Characteristics of the Devices
Two similar devices (differing from one another in the design of the spray nozzle) that are described above in “Device Design and Fabrication” were tested in this study. Figure 3 shows images of microsprays generated by the devices at the liquid flow rate of 6.0 µL/s; under this condition, the mean mixing time is less than 0.5 ms and the time interval from mixing to spray is 4 ms. Microsprays were also obtained for liquid flow rates of 3.0 and 4.5 µL/s (data not shown).
Figure 3. Photographs of micro-sprays generated by two of the devices.
(a) Device #1 mounted for spraying. (b) Spray plume for device #1. (c) Spray plume for device #2. The nitrogen gas pressure was fixed at 50 psi, and the liquid flow rate was 6.0 µL/s. The arrows in the photos indicate the spray boundary and span.
Generally, both devices successfully generated microsprays, and clogging of the nozzles, a potential problem, did not occur when the precautions for usage and cleaning described above were followed. Device #1 generated wider sprays than did device #2. The measured spray angle for device #1 was in the range of 30–35° for flow rates of 3.0–6.0 µL/s. Large droplets (>20 µm in diameter) were generated for all of the flow rates tested, and the droplet density for device # 1 was not as high as for device #2 (see below), as assessed visually by light scattering. The microsprays were sometimes unstable initially, displaying a change in spray angle during the test (i.e., occasional spray position shifting occurred).
Device #2 generated more stable and much denser microsprays at the higher flow rates that were tested. However, at the low flow rate of 3.0 µL/s, the droplets were sparse and few small droplets (< 25 µm diameter) were produced. When the flow rate was increased to 4.5 µL/s, a spray with smaller droplets was generated. The spray angle increased with increasing flow rates: from about 8–10° to 25–30°, as the flow rate increased from 3.0 µL/s to 6.0 µL/s.
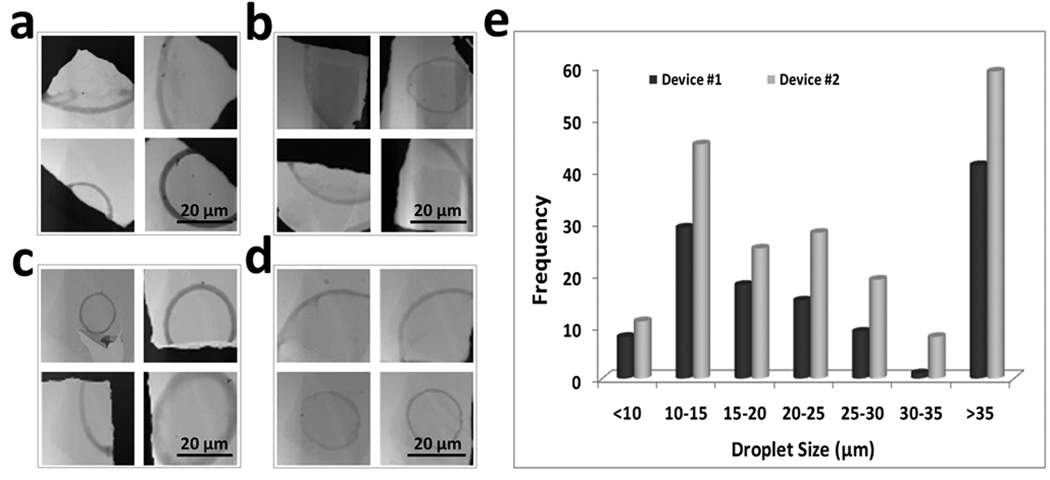
Figure 4 shows representative TEM images of air-dried droplets captured by grids, at flow rates of 4.5 and 6.0 µL/s for both of the devices. The ferritin inside the droplets formed a light brown boundary on the grids when it dried. Because the grid was hydrophobic, the droplets were resistant to spreading out when they hit the grid, as has been observed previously by Berriman and Unwin (1994). Thus, the ferritin accumulating at the edges of the droplets enabled us to estimate the distribution of droplet sizes. Data are not shown for the flowrate of 3.0 µL/s, because most droplets generated at this flow rate are larger than 25–30 µm, a scale not useful for cryo-EM. For device #1 operating at a flow rate of 4.5 µL/s, most of the droplets generated had diameters larger than 15–20 µm, and the grid coverage (fraction of area occupied by microdroplets) was less than 5%. The situation was similar for device #2 with respect to the droplet size distribution, but the grid coverage was more extensive, ~15%. When the flow rate was increased to 6.0 µL/s, the droplets generated by all the devices were significantly reduced in size. For both devices #1 and #2, the majority of droplets were less than 25 µm in diameter. The coverage of grids by droplets was slightly higher for device #2 (~20%) than for device #1 (~15%.). These results indicate that, at the flow rate of 6.0 µL/s, both devices can generate adequate numbers of microdroplets in the size range that is useful for cryo-TEM, as we show below. For atomizers of the pre-filming type (i.e., for which a thin film of solution forms on the interior surface of the nozzle prior to discharge), operated at low gas pressures, an increase in liquid flow rate generally corresponds to a decrease in the mean droplet size (Liu, 2000). The experimental results obtained here are consistent with that observation.
Figure 4. Electron microscopy of dried microdroplets resulting from a solution containing ferritin sprayed onto TEM grids.
Micrographs obtained with (a) device #1, flow rate, 4.5 µL/s. (b) device #2, flow rate, 4.5 µL/s. (c) device #1, flow rate, 6.0 µL/s; and (d) device #2, flow rate, 6.0 µL/s. (e) Droplet size distributions with liquid flow rate of 6.0 µL/s for devices #1 and #2.
Cryo-electron microscopy of ribosome-ferritin mixtures and 3D reconstruction of 70S ribosome
We found that when grids are rendered hydrophilic by glow discharge, most of the microdroplets generated by the devices spread sufficiently thinly in regions as to be useful for imaging by cryo-EM. Figure 5 shows a micrograph obtained using monolithic device #2 to mix ribosomes and ferritin. The liquid flow rate used was 6 µL/s and the elapsed time from mixing to freezing was ~9 ms (4 ms in the device and 5 ms from microspray to freezing). As anticipated, based upon the previous characterization of the mixer’s performance (Lu et al., 2009), the micrograph shows both ferritin and ribosomal particles within a microdroplet, confirming that mixing had occurred, and this was true of all microdroplets examined. The ribosomes appear to be structurally intact in that they appear identical to those observed when grids are prepared by the blotting methods (Grassucci et al., 2007). Most grids that had been treated by glow discharge within 30 min of use contained sufficient numbers of well-spread droplets to enable us to collect thousands of ribosomal images.
Figure 5. Cryo-EM of 70S ribosomes and ferritin that were mixed and sprayed by the monolithic device #2.

(a) Low magnification micrograph showing the edge of a droplet that has spread sufficiently thin for imaging. (b) High magnification field obtained from one of the holes at the perimeter of the droplet (“*” in (a)) and showing presence of both ferritin (smaller dense structures) and ribosomes (larger, less dense structures). (c) Surface representation of a three-dimensional reconstruction of the 70S ribosome, determined from images such as those shown in (b). Resolution is 18.9 Å
Sometimes the carbon film over an entire grid square was either absent or severely broken, and other grid squares were unsatisfactory due to the presence of large chunks of contaminating material. The extent to which these effects occur was highly variable, but even in the worst cases most of the grid was not affected. These two problems may have a common source, the liquid microspray (as opposed to the humid air stream), but the precise mechanism(s) have not been identified. Finally, the usual kinds of contamination (e.g., from ethane) that are commonly found in specimens prepared by the conventional blotting technique were also present. Nevertheless, when care was taken to clean the device thoroughly and to ensure that the grids were sufficiently hydrophilic, nearly all experiments resulted in grids that were suitable for cryo-EM; we are continuing our efforts to refine and improve both the device and experimental protocols.
To demonstrate structural integrity of ribosomes that had passed through the devices and were deposited onto EM grids, we computed reconstructions from particle images that were selected from electron micrographs. From an experiment using device #1, 4,391 particle images from 51 micrographs were selected, yielding a reconstruction with a resolution of 23.8Å, according to the 0.5 FSC criterion (Malhotra et al., 1998). From 83 micrographs from Device 2, 3,314 particle images were selected, yielding a reconstruction with a resolution of 22.7Å. When the images from these data sets were merged, and the particle orientations were refined iteratively (Penczek et al., 1994), we obtained a reconstruction with a resolution of 18.9Å (Table 1), a value which is comparable to the resolution achieved for ribosomes prepared by the standard blotting (non-time-resolved) technique when similar numbers of particles were used (Frank, 2001; Agrawal et al., 1998).
Table 1.
Summary of image processing of 70S ribosome particles.
| Device | No. Micrographs | No. Particles | Resolution* of 3D Reconstruction |
|---|---|---|---|
| 1 | 51 | 4391 | 23.8Å |
| 2 | 83 | 3314 | 22.7Å |
| Combined | 134 | 7705 | 18.9Å |
Nominal resolution was determined using the 0.5 FSC criterion.
Time-Resolved Cryo-EM of Reassociation of Ribosomal Subunits
To demonstrate the utility of the monolithic devices for characterizaation of the kinetics of macromolecular assembly reactions, we have begun an investigation of the reassociation of 30S and 50S ribosomal subunit in the early phases of the assembly reaction. Note that a bimolecular reaction involving macromolecules of this type could not be readily carried out on the grid, because of the necessity for free translational diffusion. The kinetics of ribosomal subunit association were investigated recently by light scattering (Hennelly et al., 2005) and were used as a guide in the design of our experiments. Ribosomal subunits (1.2 µM initial concentrations) were mixed and sprayed at room temperature onto grids using device #2 at a flow rate of 6 µL/s, yielding a net reaction time of 9 ms (4 ms in the device and 5 ms in transit to the cryogen); note that the time interval for the quenching of the reaction by freezing, once the grid is submerged in ethane, is a small fraction of a millisecond, and has therefore not been included in the total reaction time. Although initial studies show clear examples of isolated reassociated 70S ribosomes, the vast majority of the subunits are not expected to assemble in 4–9 ms (half-time for the reaction is estimated to be ~60 ms based upon the rate constant determined by Hennelly et al. (2005)); thus a more thorough study must be done using a monolithic mixer/sprayer in which the reaction channel is lengthened to permit use of longer reaction times. Such studies are in progress.
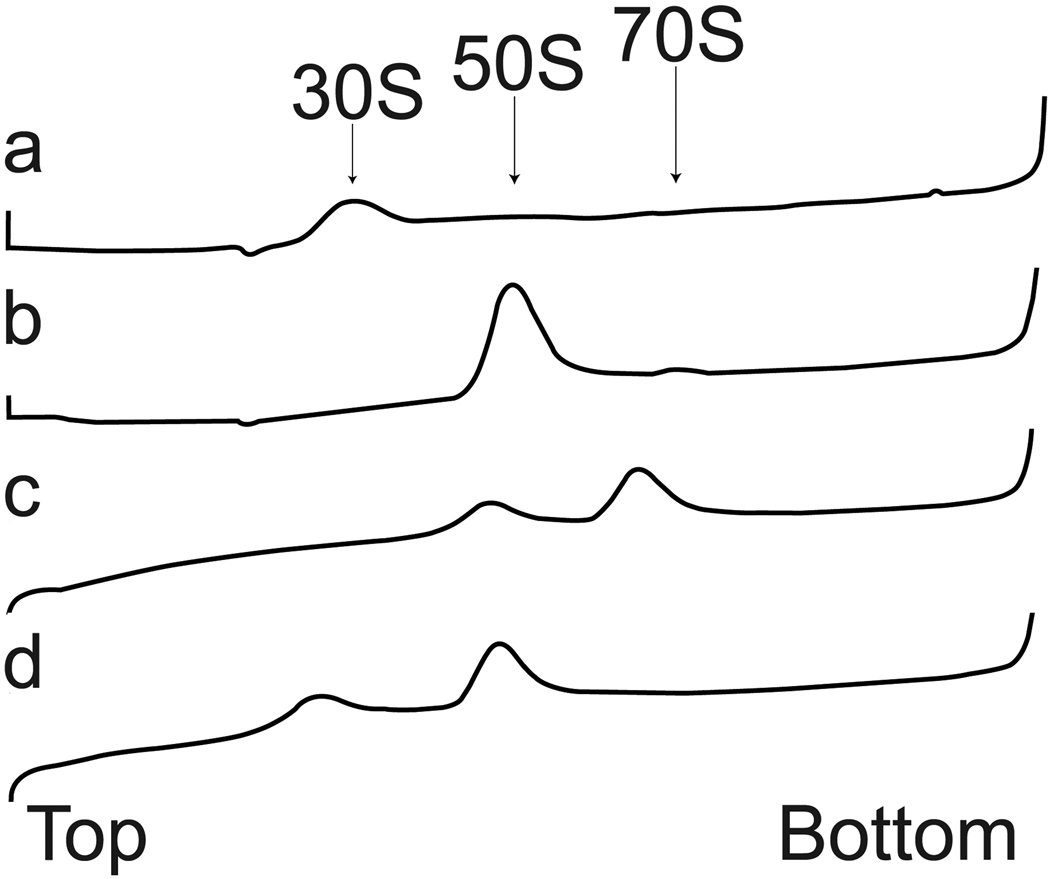
To confirm that subunits that have passed through the device are capable of reassociation into ribosomes, we collected sufficient reactant solution from the spray for analysis by sucrose gradient ultracentrifugation (Fig 6). The reactant solution showed a prominent peak at 70S and a minor peak at 50S when the Mg2+ was maintained at 12 mM, indicating that the majority of subunits were capable of assembly after passing through the monolithic device (Fig. 6C). Control experiments at reduced Mg2+ concentration showed dissociation of the 70S into 50S and 30S subunits as expected (Fig. 6D).
Figure 6. Sucrose density gradient profiles of dissociated ribosomal subunits and re-associated ribosomes.
(a) 30S subunit (control). (b) 50S subunit (control). (c) Collected microspray from a reassociation experiment with 12 mM Mg2+ present in reaction and in gradient buffer. (d) Same as (c) except that the gradient buffer contained 0.1 mM Mg2+. The arrows indicate the relative positions of the 30S, 50S and 70S peaks. The direction of fractionation is from top (on the left) to bottom (on the right).
Discussion
Assessment of Device Performance
A widely appreciated potential application of cryo-electron microscopy is the structural characterization of the intermediate structures that occur during the functioning or assembly/disassembly of large macromolecular complexes such as ribosomes, multi-enzyme complexes, and other subcellular complexes that can be isolated for in vitro characterization. Here we have described a micro-mixer and sprayer device that represents a significant step forward in the practical achievement of time-resolved cryo-electron microscopy of macromolecular specimens on a millisecond time-scale. An improvement over previous implementations is that the reaction of interest occurs in solution, that is, in a micro channel while the solution is being transported to the specimen grid for immediate freezing. This advantage is in contrast to other methods, in which the reaction occurs on the grid while the macromolecules are interacting with the carbon support film and/or air-water interfaces, with the implicit, and quite possibly flawed, assumption that these interfaces have no effect on the reaction. Actually, in our current implementation, the macromolecular specimen does reside for a few milliseconds on the grid prior to being frozen, so not all of the reaction necessarily occurs in the fluid channel prior to spray deposition (see discussion below).
Ribosomes were chosen as a model system on which to test the devices, because they have been well-characterized by cryo-EM, and because milligram quantities are easily purified. From approximately 7,700 ribosome images, a reconstruction with a resolution of 18.9Å was obtained. Such a resolution figure is typical for this number of images (Frank, 2001; Agrawal et al., 1998), indicating that structural integrity was preserved after spraying. While the application of specimens to EM grids by aerosol spraying has been used since the infancy of biological electron microscopy (Horne, 1965), applications of microdroplet spraying in cryo-EM have been rare (Dubochet et al., 1982). Most likely, the unpopularity of spraying is due to the convenience of the alternative, widely used procedure in which a few microliters of specimen are applied to the grid, blotted with filter paper, and frozen by plunging into liquid ethane or propane. In any case, we know of no published reports of damage to macromolecular specimens induced by microdroplet spraying.
For the reconstruction shown in Figure 5, we processed 7,700 ribosome images from 134 micrographs; such a yield is several-fold lower than is typical for grids prepared by conventional blotting methods. At least four reasons for this low yield can be envisaged. First, particle images in which a ferritin molecule obscured any part of the ribosome were excluded. Second, clumping of ribosomes sometimes occurred in this method, and particles too close together are excluded by the automated windowing procedure used (Shaikh et al., 2008b). Third, spray methods, in contrast to blotting methods, do not take advantage of the concentrating effects of the carbon layer. In conventional blotting, the 3–5 µL drop containing the macromolecules resides on the carbon for several seconds. Macromolecules during this time adsorb strongly to the carbon from the drop, which contains a vast excess of specimen, thereby resulting in a concentration effect. With the use of spray methods, however, there is no such effect; the density of macromolecules seen on the carbon equals that of the bulk suspension. For this reason, the concentrations required for spray experiments are 1–2 orders of magnitude higher than for conventional blotting experiments (e.g., 1–3.5 µM used here, vs. ~30 nM typically used for 70S ribosomes). Fourth, the ice thickness frequently varies across the area of spread microdroplets, and in regions where the ice is thicker, the contrast is reduced, and the number of particles that can be unambiguously identified as ribosomes is also reduced. We cannot exclude completely the possibility that some ribosomes are damaged during spraying, but if this were a serious problem, we would have expected to see a deleterious effect on the resolution of the 3D reconstructions, or on the reassociation potential of the subunits after spraying. Neither effect was observed.
Time resolution and reaction time
The ultimate time resolution attainable by the protocol that we have described is probably a few milliseconds. Although the mixing and transit time through the device could be reduced to less than 0.5 ms (Lu et al., 2009), and the actual time to freeze a 100-nm thick aqueous film in ethane has been estimated to be 0.1 ms, the time required to transport a 3-mm diameter EM grid to cryogen and to freeze it probably cannot be made less than about a millisecond (Heymann et al., 2004).
Longer reaction times can be achieved by reducing the flow rate or by increasing the length of the reaction-carrying channel between the mixer and the spray nozzle. Varying the flow rate is feasible only over a limited range, because the flow rate affects the mixing and spray characteristics. Therefore, reaction times are more easily adjusted by modifying the reaction channel configurations and dimensions. The range of times achievable by modifying the reaction channel geometry remains to be investigated, but a range from less than 10 ms to perhaps several hundred milliseconds is reasonable. However, as the channel is lengthened in order to increase the reaction time, the reaction time distribution broadens, due to laminar flow. Such broadening is undesirable if a precise time interval is required.
Microdroplet spreading
The distribution of microdroplet diameters shows many microdroplets that are larger than 20 µm in diameter, and we were surprised to find that on grids with sufficient hydrophilicity even microdroplets of this size spread to thicknesses that are useful for cryo-EM (less than 0.2 µm). This observation contrasts with the finding of Berriman and Unwin (1994) that microdroplets being deposited onto a second reactant already present in a thin water layer must be no larger than about 2 µm. Sometimes, on less hydrophilic grids, thin ice was located only at the perimeters of the microdroplets; on such grids regions with contrast suitable for high-resolution imaging were limited. Achievement of the highest resolution in 3D reconstructions is facilitated by having the macromolecule of interest embedded in the thinnest ice attainable. Currently, we are investigating alternative operating parameters and nozzle designs for the sprayer, in an effort to obtain greater numbers of small diameter (<20 µm) microdroplets. These modifications should improve the likelihood of obtaining thin ice.
Specimen consumption
As was mentioned above, deposition of macromolecules as microdroplets requires concentrations at least 10-fold higher than does the conventional blotting technique for preparation of grids for cryo-EM. Note, however, that similarly high concentrations are required for the blotting technique in those applications where it is desired to suspend macromolecules in ice over the holes in a holey carbon film. Our current experimental set-up also requires severalfold more volume of specimen per grid than does the conventional blotting technique; this is because of the dead volume of the system, and the need to re-establish a stable microdroplet spray before each grid is prepared. For example, to prepare four grids identically requires in total about 80 µL of specimen. It should be straightforward to reduce the dead volume of the system, and also the spray-time interval per grid, so that less than 10 µL of specimen is required to prepare a grid.
All of the experiments described used a type of grid that contained a continuous thin layer of carbon that was supported by a thicker “holey” carbon film. Some attempts were made to image microdroplets that had spread over holes that did not contain a thin layer of carbon, but, thus far, we have not found conditions under which the solutions spread sufficiently thin over the holes.
Conclusions
We have described a first-generation monolithic device that enables two reactant solutions, one or both of which can contain macromolecules, to be mixed and to react in a microfluidic channel, prior to being sprayed onto a conventional electron microscope grid as it is being plunged into cryogen to quench the reaction. Ribosomes that have been prepared for cryo-EM with the device are shown to be preserved equivalently to ribosomes prepared by conventional cryo methods. The devices are constructed using standard microfabrication technology, such that with minor modifications a wide range of time scales should be attainable.
Supplementary Material
Acknowledgments
This work is supported by the Wadsworth Center’s Resource for Visualization of Biological Complexity (NIH Biotechnology Resource Grant RR01219), and NIH grant GM61576 (R.K.A.). We acknowledge the Micro and Nano Fabrication Clean Room at Rensselaer Polytechnic Institute, and the Michigan Nanofabrication Facility at the University of Michigan for their help with device fabrication. We also thank Ingrid Hahn for preparation of 70S ribosomes in the early stages of this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Agrawal RK, Penczek P, Grassucci RA, Frank J. Visualization of elongation factor G on the Escherichia coli 70S ribosome: the mechanism of translocation. Proc. Natl. Acad. Sci. USA. 1998;95:6134–6138. doi: 10.1073/pnas.95.11.6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agrawal RK, Burma DP. Sites of Ribosomal RNAs Involved in the Subunit Association of Tight and Loose Couple Ribosomes. J. Biol. Chem. 1996;271:21285–21291. doi: 10.1074/jbc.271.35.21285. [DOI] [PubMed] [Google Scholar]
- 3.Baik S, Blanchard JP, Corradini ML. Development of micro-diesel injector nozzles via microelectromechanical systems technology and effects on spray characteristics. Journal of Engineering for Gas Turbines and Power. 2003;125:427–434. 2003. [Google Scholar]
- 4.Barnard D, Wagenknecht T. Pneumatic Micro-Sprayer for Millisecond Time Resolution in Cryo-Electron Microscopy. Microsc. Microanal. 2005;11:290–291. [Google Scholar]
- 5.Bayvel L, Orzechowski Z. Types of atomizers, in Liquid Atomization. Washington D.C.: Taylor and Francis; 1993. Chapter 4. [Google Scholar]
- 6.Berriman J, Unwin N. Analysis of transient structures by cryo-microscopy combined with rapid mixing of spray droplets. Ultramicroscopy. 1994;56:241–252. doi: 10.1016/0304-3991(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 7.Blaha G, Stelz U, Spahn CMT, Agrawal RK, Frank J, Nierhaus KH. Preparation of functional ribosomal complexes and effect of buffer conditions on tRNA positions observed by cryoelectron microscopy [Review] RNA-Ligand Interactions Pt A : Structural Biology Methods. 2000;317:292–309. doi: 10.1016/s0076-6879(00)17021-1. [DOI] [PubMed] [Google Scholar]
- 8.Butler MC, Ellis T, Swan T, Miller PCH, Waddelow A, Bradley A, Tuck CR. Design Factors affecting spray characteristics and drift performance of air induction nozzles. Biosyst. Eng. 2002;82:289–296. [Google Scholar]
- 9.Cavaliere A, Ragucci R, Noviello C. Bending and break-up of a liquid jet in a high pressure airflow. Exp. Therm. Fluid Sci. 2003;27:449–454. [Google Scholar]
- 10.Deng W, Klemic JF, Li X, Reed A, Gomez A. Increase of electrospray throughput using multiplexed microfabricated sources for the scalable generation of monodisperse droplets. Aerosol Sci. 2006;37:696–714. [Google Scholar]
- 11.Deng W, Klemic JF, Li X, Reed A, Gomez A. Liquid fuel microcombustor using microfabricated multiplexed electrospray sources. Proc. Combust. Inst. 2007;31:2239–2246. [Google Scholar]
- 12.Dubochet J, Lepault J, Freeman R, Berriman JA, Homo J-C. Electron microscopy of frozen water and aqueous solutions. J. Micros. 1982;128:219–237. [Google Scholar]
- 13.Frank J. Cryo-electron microscopy as an investigative tool: the ribosome as an example [Review] BioEssays. 2001;23:25–732. doi: 10.1002/bies.1102. [DOI] [PubMed] [Google Scholar]
- 14.Frank J, Radermacher M, Penczek P, Zhu J, Li Y, Ladjadj M, Leith A. SPIDER and WEB: Processing and visualization of images in 3D electron microscopy and related fields. J. Struct. Biol. 1996;116:190–199. doi: 10.1006/jsbi.1996.0030. [DOI] [PubMed] [Google Scholar]
- 15.Frederik PM, Sommerdijk N. Spatial and temporal. resolution in cryo-electron microscopy - A scope for nano-chemistry [Review] Curr. Opin. Colloid Interface Sci. 2005;10:245–249. [Google Scholar]
- 16.Fuller SD, Berriman JA, Butcher SJ, Gowen BE. Low pH induces swiveling of the glycoprotein heterodimers in the Semliki Forest virus spike complex. Cell. 1995;81:715–725. doi: 10.1016/0092-8674(95)90533-2. [DOI] [PubMed] [Google Scholar]
- 17.Gabashvili IS, Agrawal RK, Spahn CMT, Grassucci RA, Svergun DI, Frank J, Penczek P. Solution Structure of the E. coli 70S ribosome at 11.5 Å resolution. Cell. 2000;100:537–549. doi: 10.1016/s0092-8674(00)80690-x. [DOI] [PubMed] [Google Scholar]
- 18.Grassucci RA, Taylor DJ, Frank J. Preparation of macromolecular complexes for cryo-electron microscopy. Nature Protoc. 2007;2:3239–3246. doi: 10.1038/nprot.2007.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griss P, Melin J, Sjödahl J, Roeraade J, Stemme G. Development of micromachined hollow tips for protein analysis based on nanoelectrospray ionization mass spectrometry. J. Micromech. Microeng. 2002;12:682–687. [Google Scholar]
- 20.Hennelly SP, Antoun A, Ehrenberg M, Gualerzi CO, Knight W, Lodmell JS, Hill WE. A time-resolved investigation of ribosomal subunit association. J. Mol. Biol. 2005;346:1243–1258. doi: 10.1016/j.jmb.2004.12.054. [DOI] [PubMed] [Google Scholar]
- 21.Heymann JB, Conway JF, Steven AC. Molecular dynamics of protein complexes from four-dimensional cryo-electron microscopy. J. Struct. Biol. 2004;147:291–301. doi: 10.1016/j.jsb.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Horne RW. In: The examination of small particles, in Techniques for electron microscopy. Kay DH, editor. Oxford: Blackwell; 1965. p. 311. [Google Scholar]
- 23.Jazayeri SA, Li X. Structure of liquid-sheet sprays. Part. Part. Syst. Char. 2000;17:56–65. [Google Scholar]
- 24.Kim W, Guo M, Yang P, Wang D. Microfabricated monolithic multinozzle emitters for nanoelectrospray mass spectrometry. Anal. Chem. 2007;79:3703–3707. doi: 10.1021/ac070010j. [DOI] [PubMed] [Google Scholar]
- 25.Kufferath A, Wende B, Leuckel W. Influence of liquid flow conditions on spray characteristics of internal-mixing twin-fluid atomizers. Int. J. Heat Fluid Flow. 1999;20:513–519. [Google Scholar]
- 26.Lefebvre AH. Atomizers, in Atomization and Sprays. New York: Hemisphere Publishing Corp.; 1989. Chapter 4. [Google Scholar]
- 27.Liu H. Processes and techniques for droplet generation, in Science and Engineering of Droplets: Fundamentals and Applications. Park Ridge, NJ: Noyes Publications; 2000. Chapter 1. [Google Scholar]
- 28.Lörcher M, Mewes D. Atomization of liquids by two-phase gas-liquid flow through a plain-orifice nozzle: flow regimes inside the nozzle. Chem. Eng. Technol. 2001;24:167–172. [Google Scholar]
- 29.Lu Z, McMahon JJ, Mohamed H, Barnard D, Shaikh TR, Mannella CA, Wagenknecht T, Lu TM. Passive microfluidic device for sub-millisecond chaotic mixing. Sens. Actuators B. 2009 doi: 10.1016/j.snb.2009.10.036. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malhotra A, Penczek P, Agrawal RK, Gabashvilli IS, Grassucci RA, Jünemann R, Burkhardt N, Nierhaus KH, Frank J. Escherichia coli 70S ribosome at 15 Å resolution by cryo-electron microscopy: localization of fMet-tRNAMetf and fitting of L1 protein. J. Mol. Biol. 1998;280:103–116. doi: 10.1006/jmbi.1998.1859. [DOI] [PubMed] [Google Scholar]
- 31.Menetret JF, Hofmann W, Schroder RR, Rapp G, Goody RS. Time-resolved cryo-electron microscopic study of the dissociation of actomyosin induced by photolysis of photolabile nucleotides. J. Mol. Biol. 1991;219:139–144. doi: 10.1016/0022-2836(91)90554-j. [DOI] [PubMed] [Google Scholar]
- 32.Naik N, Courcimault C, Hunter H, Berg J, Lee J, Naeli K, Wright T, Allen M, Brand O, Glezer A, King W. Microfluidics for generation and characterization of liquid and gaseous micro- and nanojets. Sens. Actuators A. 2007;134:119–127. [Google Scholar]
- 33.Nasr GG, Yule AJ, Bendig L. Sprays in industrial production processes, in Industrial Sprays and Atomization: Design, Analysis, and Applications. New York: Springer-Verlag; 2002. Chapter 3. [Google Scholar]
- 34.Oliver RM. Negative stain electron microscopy of protein macromolecules. Method. Enzymol. 1973;27:616–672. doi: 10.1016/s0076-6879(73)27029-5. [DOI] [PubMed] [Google Scholar]
- 35.Penczek P, Radermacher M, Frank J. Three-dimensional reconstruction of single particles embedded in ice. Ultramicroscopy. 1992;40:33–53. [PubMed] [Google Scholar]
- 36.Penczek PA, Grassucci RA, Frank J. The Ribosome at Improved Resolution - New Techniques for Merging and Orientation Refinement in 3D Cryo-Electron Microscopy of Biological Particles. Ultramicroscopy. 1994;53:251–270. doi: 10.1016/0304-3991(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 37.Pianthong K, Zakrzewski S, Behnia M, Miltion BE. Exp. Therm. Fluid Sci. 2003;27:589–598. [Google Scholar]
- 38.Rajan R, Pandi AB. Correlations to predict droplet size in ultrasonic atomization. Ultrasonics. 2009;39:235–255. doi: 10.1016/s0041-624x(01)00054-3. [DOI] [PubMed] [Google Scholar]
- 39.Satoha D, Tanaka S, Yoshida K, Esashi M. Micro-ejector to supply fuel–air mixture to a micro-combustor. Sens. Actuators A. 2005;119:528–536. [Google Scholar]
- 40.Shaikh TR, Barnard D, Meng X, Wagenknecht T. Implementation of a flash-photolysis system for time-resolved cryo-electron microscopy. J. Struct. Biol. 2009;165:184–189. doi: 10.1016/j.jsb.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shaikh TR, Gao H, Baxter WT, Asturias FJ, Boisset N, Leith A, Frank J. SPIDER image processing for single-particle reconstruction of biological macromolecules from electron micrographs. Nat. Protocols. 2008a;3:1941–1974. doi: 10.1038/nprot.2008.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shaikh TR, Trujillo R, LeBarron JS, Baxter WT, Frank J. Particle-verification for single-particle, reference-based reconstruction using multivariate data analysis and classification. J. Struct. Biol. 2008b;164:41–48. doi: 10.1016/j.jsb.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Subramaniam S, Gerstein M, Oesterhelt D, Henderson R. Electron Diffraction Analysis of Structural Changes in the Photocycle of Bacteriorhodopsin. EMBO J. 1993;12:1–8. doi: 10.1002/j.1460-2075.1993.tb05625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Subramaniam S, Henderson R. Electron crystallography of bacteriorhodopsin with millisecond time resolution. J. Struct. Biol. 1999;128:19–25. doi: 10.1006/jsbi.1999.4178. [DOI] [PubMed] [Google Scholar]
- 45.Unwin N. Acetylcholine receptor channel imaged in the open state. Nature. 1995;373:37–43. doi: 10.1038/373037a0. [DOI] [PubMed] [Google Scholar]
- 46.Walker M, Trinick J, White H. Millisecond time resolution electron cryo-microscopy of the M-ATP transient kinetic state of the acto-myosin ATPase. Biophys. J. 1995;68:87s–91s. [PMC free article] [PubMed] [Google Scholar]
- 47.White HD, Thirumurugan K, Walker ML, Trinick J. A second generation apparatus for time-resolved electron cryo-microscopy using stepper motors and electrospray. J. Struct. Biol. 2003;144:246–252. doi: 10.1016/j.jsb.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 48.Yang JT, Huang KJ, Chen AC. Microfabrication and laser diagnosis of pressure-swirl atomizers. J. Microelectromech. Syst. 2004;13:843–850. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.