Abstract
Tissue factor (TF) is an integral membrane protein, normally separated from the blood by the vascular endothelium, which plays a key role in the initiation of blood coagulation. With a perforating vascular injury, TF becomes exposed to blood and binds plasma factor VIIa. The resulting complex initiates a series of enzymatic reactions leading to clot formation and vascular sealing. In some pathologic states, circulating blood cells express TF as a result of exposure to an inflammatory stimulus leading to intravascular clotting, vessel occlusion and thrombotic pathology. Numerous controversies have arisen related to the influence of structural features of TF, its presentation and its function. There are contradictory reports about the synthesis and presentation of TF on blood cells and the presence (or absence) of functionally active TF circulating in normal blood either on microparticles or as a soluble protein. In this review we discuss TF structure-function relationships and the role of TF during various phases of the blood coagulation process. We also highlight controversies concerning the expression/presence of TF on various cells and in blood in normal and pathologic states.
Keywords: tissue factor, monocytes, posttranslational modifications, platelets, thrombin generation
Tissue factor (TF) is an integral membrane protein that is the essential cofactor component of the TF-factor VIIa complex enzyme. TF is expressed in the vascular adventitia, in astroglial cells, in organ capsules and is found in the central nervous system, lungs, and placenta at relatively high concentrations.1–3 Many cells produce detectable amounts of TF when they are stimulated in vitro by various agents.4,5 Monocytes and macrophages are known to express TF after stimulation, primarily by inflammatory cytokines.6–8 In addition to its expression by normal tissues and cells, it is also known to be present on tumor cells, where its expression appears related to the metastatic potential of those cells.9–11 Furthermore, it has been identified in atherosclerotic plaques, which has suggested a role for TF in the progression of cardiovascular disease.12,13 Under normal circumstances, however, cells in contact with blood do not express physiologically active TF.14 When mechanical or chemical damage of the vascular wall occurs, subendothelial TF is expressed/exposed to blood and binds plasma factor VIIa, which circulates as an operationally inactive enzyme at a concentration of approximately 0.1 nM (1% of plasma factor VII)15 and escapes the inhibitors of coagulation proteases because of its poor enzymatic qualities. The TF-factor VIIa complex initiates blood coagulation by activating the zymogens factor IX and factor X to their respective serine proteases, factor IXa and factor Xa.
Structure-Function Relationships (Which?)
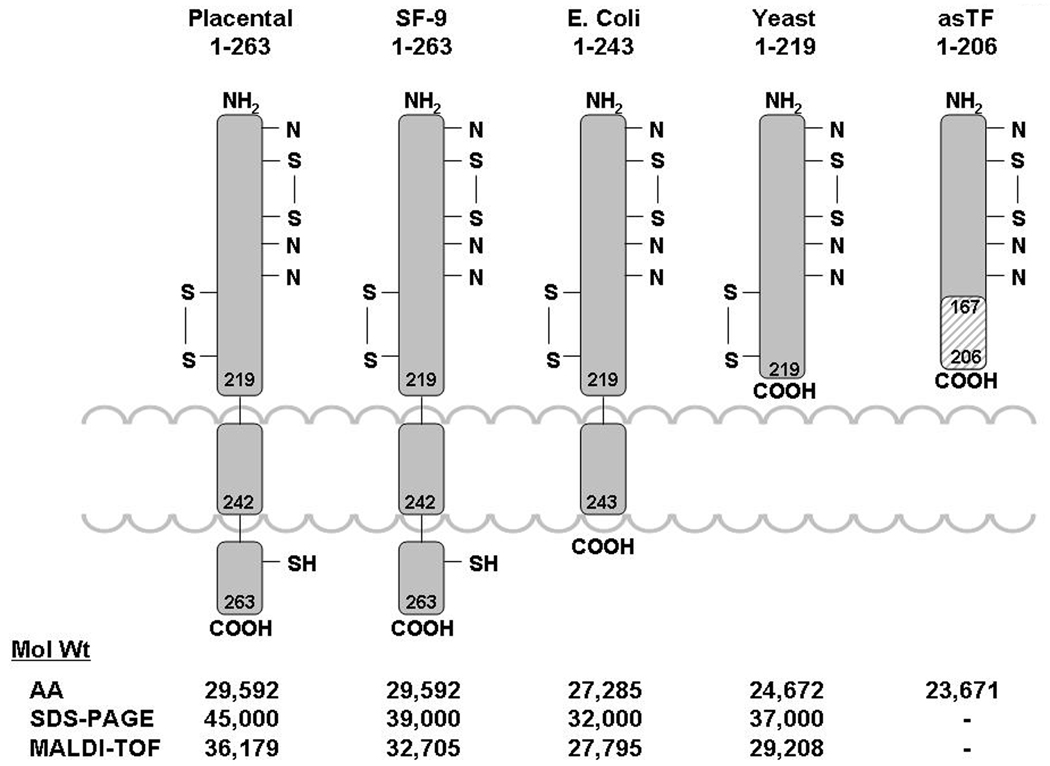
TF is a 263/261 amino acid transmembrane protein containing three domains (Figure 1): 1) an extracellular domain representing the NH2-terminal part of the molecule (residues 1–219) and composed of two fibronectin type III domains; 2) a transmembrane domain, which anchors TF to the membrane (residues 220–242); and 3) a cytoplasmic COOH-terminal domain (residues 243–263).16 The extracellular domain of TF is involved in complex formation with factor VIIa increasing, in a membrane dependent fashion, the activity of the protease toward its natural substrates factor IX, factor X, and factor VII by several orders of magnitude.17,18 Thus, two of the three domains of TF (extracellular and transmembrane) play distinct roles in the blood coagulation process. The major role of the cytoplasmic domain is related to signal transduction.19 As a consequence, it has been generally accepted that TF lacking the cytoplasmic domain is functionally identical to the full-length protein in the initiation of thrombin generation. On the other hand, recombinant TF lacking both the cytoplasmic and transmembrane domains cannot bind to the membrane, and therefore, while forming a complex with factor VIIa, does not activate factor VII and has decreased catalytic efficiency towards factor IX and factor X.17,18
Figure 1.
The structure of various TF species. Indicated molecular weights were determined from the amino acid composition (AA), gel electrophoresis (SDS) and mass-spectroscopy (MALDI-TOF). (This figure was originally published in Surgery108).
Over 20 years ago, sufficient natural TF was isolated to identify, clone and express the recombinant protein (rTF) in human kidney 293 cells and in E. coli.16,20,21 Subsequently, various forms of rTF ranging from the full-length protein to the extracellular domain of TF with different levels of posttranslational modifications have been expressed in a variety of vectors including yeast and insect cells (Figure 1). Mutational studies22 have been performed, and an x-ray structure23 has been derived using these rTFs. Although these rTFs have been used extensively as surrogates for the natural protein, the limited availability of purified natural TF has not allowed certification of results obtained with rTF.
The contributions of various regions of the primary structure of TF on its activity are relatively well established. However, data related to the influence of posttranslational modifications on the TF function are scarce, if available at all. The amino acid sequence data related to the structure of rTF indicate that the extracellular domain of protein has potential glycosylation sites at Asn11, Asn124 and Asn137.16,24 There are also two disulfide bonds (Cys49-Cys57 and Cys186-Cys209) located in this domain.25 The carboxyterminal cytoplasmic domain of TF contains a single Cys245 residue and three Ser residues. The Cys245 residue is linked to a palmitate or stearate fatty acyl-chain,25 while one of the Ser residues can be phosphorylated by a protein kinase C-dependent mechanism.26 Although the sites of glycosylation of the extracellular domain are established and a partial identification of carbohydrates attached to those sites has been accomplished,24 a complete analysis of the carbohydrate side chain structure is lacking. In addition, no systematic analyses have been reported which examine the influence of glycosylation on TF affinity for factor VIIa, or on the affinity of the TF-factor VIIa complex for its natural substrates factor IX and factor X, or on its effects on TF-factor VIIa catalytic efficiency. The apparent lack of interest related to TF glycosylation may have been caused by two early publications addressing the subject. In the only reported activity comparison for glycosylated and non-glycosylated rTFs by Paborsky and coworkers,21 it was suggested that TF glycosylation is not required for procoagulant activity. However, since no data were provided in the report, it is not established whether glycosylation influences TF activity. Waxman et al. reported that the activity of rTF1–263 is identical with that of natural TF from brain,27 however this also was not supported by data included in the publication. Similarly, studies suggesting that glycosylation could be essential for TF activity do not provide experimental evidence to support this hypothesis. 28,29 Thus the question whether the glycosylation of TF has an effect on its function remains open due to the absence of relevant data.
A controversial issue associated with the activity of TF is related to a hypothetical “encryption-decryption” process associated with TF activity presentation. It has been suggested that the majority of TF molecules located on the cell surface have low activity (are “encrypted”) and that “decryption” is essential for the expression of TF function.30 Several contradictory mechanisms have been hypothesized in attempts to explain the “encryption-decryption” and presentation of TF activity.
One established method for inducing TF activity on the cell surface consists of the treatment of quiescent TF-bearing cells with calcium ionophore.31–34 Ionophore treatment increases TF activity by 2 to 10-fold. While some authors assign this increased TF activity to increased expression of TF protein,35 others suggest this arises from changes in the cell membrane environment, particularly in an increased expression of acidic phospholipids,31,34,36 sometimes related to cell death.32,37 Several studies hypothesize a role for cholesterol in cell lipid rafts contributing to the “encryption-decryption” of TF activity,38–40 although there is little agreement between the proposed mechanisms for this process. An increase in TF activity has been reported when lipopolysaccharide (LPS)-stimulated monocytes are treated with platelets.41–43 However the observed increase in activity was quite limited (2 to 3-fold) and could be (in part) assigned to an increase in TF antigen expression by monocytes.43
It has been suggested44 that an “encryption” of TF preexisting and residing on the cell membrane is related to the reduction status of the Cys186-Cys209 bond, which leads to impaired TF activity. The presumed re-formation of this bond using an oxidizing agent (HgCl2) appears to restore TF activity. Unfortunately, structural data have not been provided in support of the re-formation of the disulfide bridge from a hypothesized reduced state. In addition the proposed mechanism is not supported by relevant studies that conclude that in general HgCl2 will oxidize only a single thiol group.45,46 Moreover, an increase in TF activity on cell surfaces similar to that caused by HgCl2 can be achieved by treating TF-bearing cells with other metal compounds, such as AgNO3 and phenylmercuric acetate,47 with the authors concluding that this increase is related to the elevated exposure of phosphatidylserine.48,49 Similar controversy surrounds publications related to the putative role for protein disulfide isomerase (PDI) in TF activity. Ahamed et al. 49 postulate that PDI disrupts the Cys186-Cys209 bond and, as a consequence, suppresses TF procoagulant activity. Reinhardt and co-workers, however, suggest in their study50 that PDI promotes TF activity, whereas Pendurthi et al.51 reports that PDI plays no role in TF activity, and that the observed increase in TF activity is related to the contamination of PDI with phospholipids.52
A soluble form of TF circulating in blood (alternatively spliced TF) was identified several years ago.53 It has been suggested that this form of TF is procoagulant54 and stimulates clot growth.53 However subsequent studies showed that this form of TF has no procoagulant activity55,56 but could promote tumor growth and angiogenesis.56 The potential origin of this discrepancy could be assigned to the physiologically-irrelevant conditions used for the detection of alternatively spliced TF activity and the lack of validated commercial assays for the detection of TF activity at its physiologic concentrations.57–59 The role of soluble TF remains problematic.
The Controversy Regarding Blood-Borne TF (Where?)
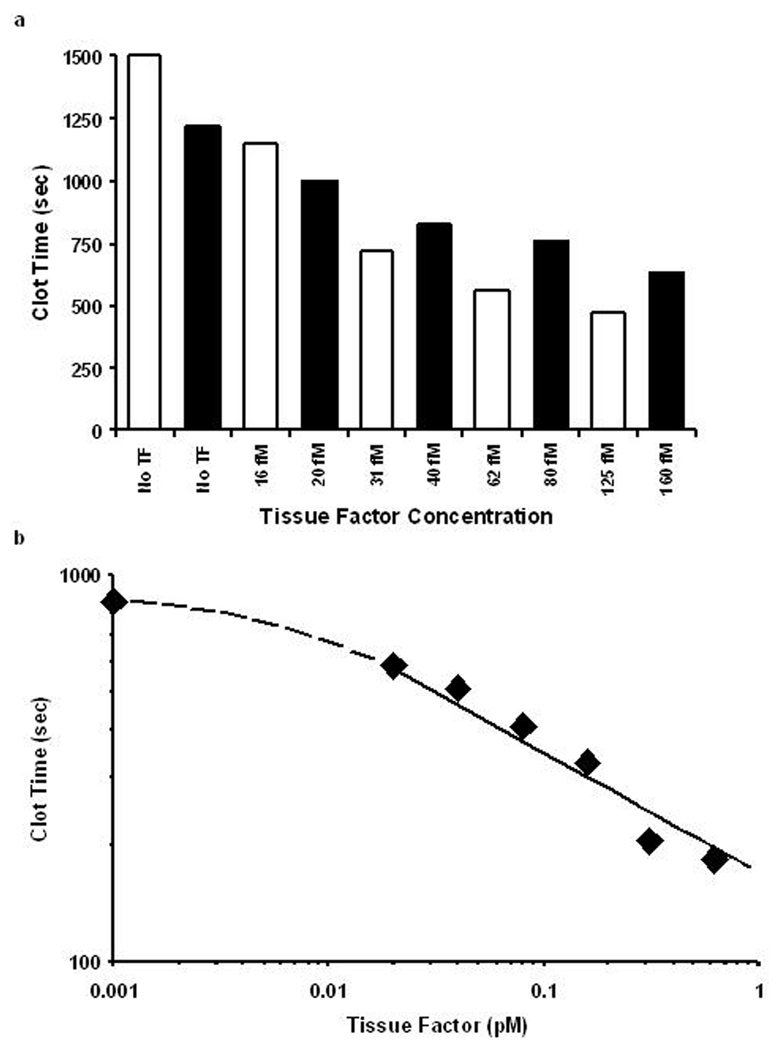
During the last several years, numerous conflicting studies related to the presence, concentration and functional activity of TF circulating in blood as a soluble protein and on/in various blood cells and platelets have been published. Several groups of investigators reported the presence of TF antigen circulating in blood at the concentrations as high as 5–10 nM60 and those of active protein reaching (sub)nanomolar concentrations.61 It has been reported that this blood-borne TF is located on blood cells, platelets and microparticles or that it circulates as a soluble protein. Frequently these reports have been developed using non-validated commercial assays. In contrast, data published by several other groups indicate that if there is TF-related activity either in blood or plasma from healthy humans, the concentration of active TF does not exceed 20 fM62–64 (Figure 2). Additionally, based upon the experience accumulated in our laboratory as well as on reports from other laboratories, blood or plasma activated with (sub)picomolar concentrations of functional TF clots within several minutes.65–69
Figure 2.
TF titrations in contact pathway inhibited whole blood (A) and plasma (B) from healthy individuals. Black and white bars represent two healthy donors. (This figure was originally published in Blood64).
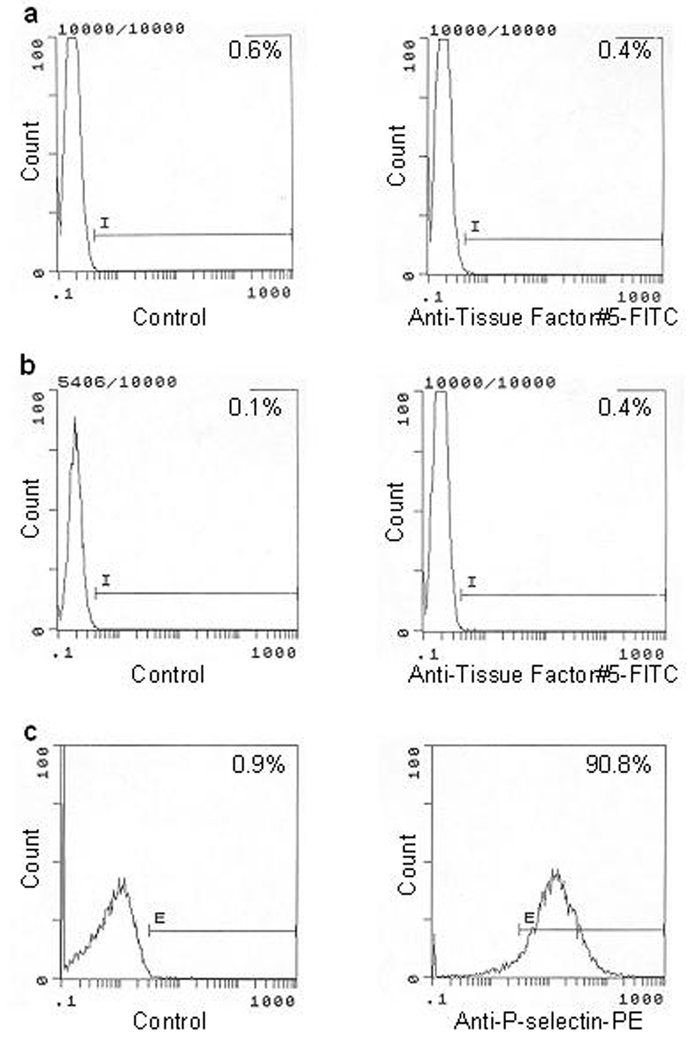
Another subject of controversy related to blood-borne TF is the location of this protein. It is generally agreed that TF can be expressed/exposed by monocytes upon cytokine stimulation. It has been also in general been accepted that the source of circulating TF in pathologic conditions could be cell-derived microparticles.70–74 More controversial is a reported presence of TF in/on platelets.75–78 In some of those publications it has been suggested that TF is transferred to platelets from the cells,75,78 whereas others suggest that TF is synthesized by platelets.76,77,79 In contrast to these publications, it has been reported that neither TF activity nor antigen were detected on resting and calcium ionophore stimulated platelets.64 In that study, no TF antigen-related signal was observed in resting or ionophore treated platelets using flow cytometry (Figures 3a and 3b) although 91% of platelets were activated upon treatment with the calcium ionophore (Figure 3c). Similarly, there is little agreement related to the presence of TF on granulocytes. Maugeri et al. suggested in their publication that granulocytes produce TF upon stimulation80 while other authors have reported the expression of TF in neutrophils81 and eosinophils.82 However, data from Osterud’s laboratory show no evidence of TF expression in any granulocytic cells.43,83,84
Figure 3.
Flow cytometric analyses of resting and calcium ionophore A23187-treated platelets. Resting (A) or A23187-treated platelets (B) were immunostained with anti-TF-5 monoclonal antibody. A23187-treated platelets were also treated with an anti-P-selectin antibody (C). An irrelevant, isotype-matched mouse IgG was used in the control experiments. (This figure was originally published in Blood64).
The major causes for the discrepancies related to the presence and concentration of TF are, most likely, the lack of validated and reliable assays for TF antigen and activity.57–59 The majority of studies reporting high concentrations of TF in plasma and the presence of TF in platelets and blood cells use commercial assays. We developed and validated in-house assays for the quantitation of TF antigen58 and activity.85 Using our assays, we have reported that the TF antigen concentrations in plasmas from patients with acute coronary syndrome are at low picomolar levels, with an average functional concentration less than 0.4 pM.85 In contrast, in a study by Bis et al., which used a commercial TF assay, nanomolar concentrations of TF in plasma from patients with a similar diagnosis were reported.86 Until there is agreement in the scientific community concerning the validity of the assays used by various laboratories incongruent reports will continue to accumulate in the literature.
TF Requirement Throughout the Process of Blood Coagulation (When?)
Although there is consensus on the requirement for TF for the initiation of the coagulation process and on the proteolytic coagulation complexes that emerge in response to TF,87 there is less agreement on the overall mechanism by which TF functions. In one construct of normal hemostasis, TF is found outside of blood vessels,1,2 requiring the disruption of blood vessel integrity to exert its effects, and within circulating blood cells, requiring specific signaling events to promote its intravascular expression.84,88 When an adequate TF challenge is presented, a full coagulant response follows; if the TF challenge is insufficient, the procoagulant response is arrested, primarily by the synergistic activities of the TF pathway inhibitor (TFPI), antithrombin and the protein C pathway.89,90 A competing hypothesis of TF biology has been advanced in which the initiating TF stimulus requires constant supplementation to the ongoing reaction with newly available TF, providing a mechanistic rationale for blood-borne TF in normal hemostasis.
Eliminating one of these hypotheses requires resolving two basic areas of dispute: the constitutive presence of TF in blood; and the identity of the procoagulant catalysts required to propagate clot growth. As has been noted, the controversy concerning the presence and activity of TF species in blood and on blood cells continues, and ultimately has become an important debate about the rigor of the quantitative methods used. The mechanistic argument for a requirement for ongoing supplementation of coagulation reactions with TF depends on three interdependent contentions: 1) that the maintenance of the coagulation process requires a continual contribution from additional TF cofactor activity (extrinsic factor Xase complex). 2) that the developing platelet/fibrin plug isolates the procoagulant complexes initially formed at the site of vascular injury from further supply of fresh reactants, thus eliminating participation of the triggering TF supply as the reaction proceeds;91 and 3) that TF is present in blood at levels below the threshold to support a coagulant response92 or in some cryptic state, but accumulates to an effective level on the vascular face of a forming thrombus.53,93 In this regard Panes et al.77 recently reported that activation of platelets leads to rapid de novo synthesis of TF and its expression. In this model, thrombus growth is viewed as self-limiting in the absence of an ongoing supply of TF to the outer face of the thrombus.
Other data consistent with this overall view of how a coagulant response is propagated include immunochemical dependent demonstrations of TF embedded in human94 and mouse thrombi,95,96 suggesting that some type of circulating TF species contributes to in vivo thrombus formation. The in vitro observation that supplementation of blood with a concentration of lipidated TF that is subthreshold in a static blood context but that results in increased fibrin formation when blood is flowed over immobilized TF97 also supports a role for circulating TF in the growth of thrombi.
On the other hand, substantial evidence supports the view that in normal hemostasis TF functions primarily in the initial phase of the clotting process and that other catalysts are involved in the propagation and maintenance of fibrin platelet clots. Our laboratory has explored the time dependence of the requirement for TF during the progress of a blood coagulation reaction using mathematical, synthetic coagulation proteome and whole blood models.98 When TF activity was eliminated either using inhibitory antibodies for factor VII and TF or mathematically at various times during the initiation phase, the results in all three models indicated that the progress of the reaction rapidly loses an absolute dependence on the presence of a functioning TF-factor VIIa complex and becomes fully independent of TF by the onset of the propagation phase of thrombin generation. In addition these studies indicated that the catalysts generated by transient expression of TF cofactor activity were sufficient to maintain a TF-independent procoagulant response as long as reactants were available and that this catalyst pool could reinitiate coagulation without input from the TF-factor VIIa complex.
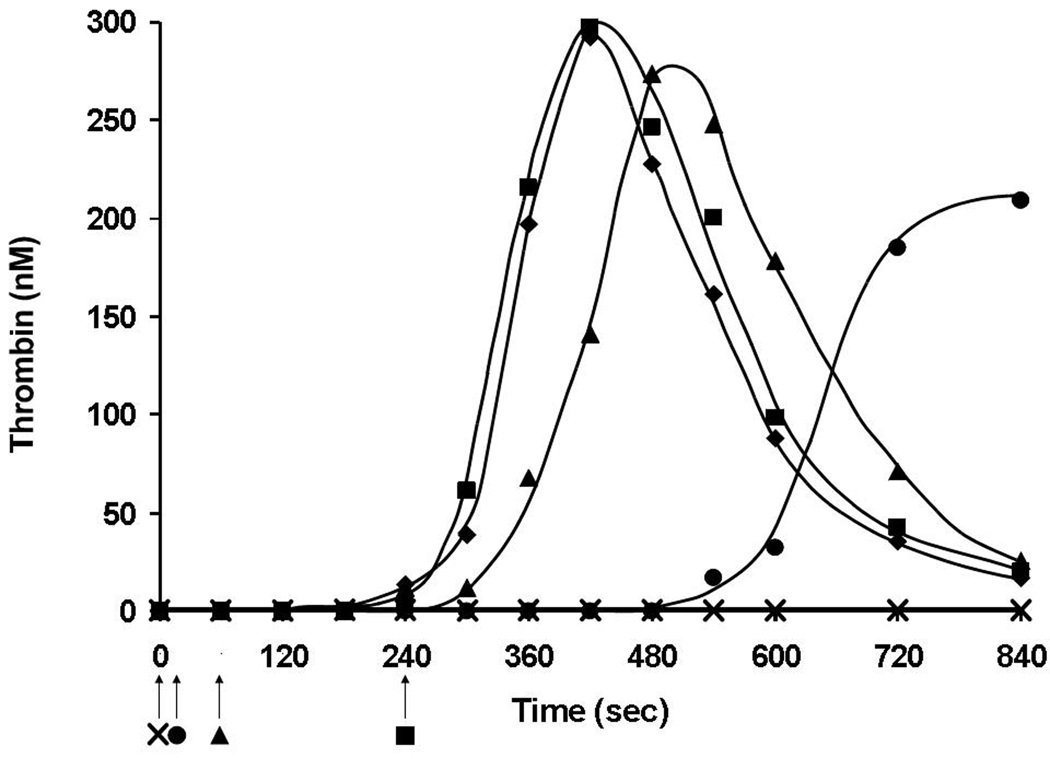
Figure 4 shows an example of this type of experiment using the synthetic coagulation proteome where inhibitory antibodies to TF and factor VIIa were added at the onset of the reaction or 10, 60 or 240 s post initiation. No thrombin generation is seen when antibodies are present at the beginning of the reaction. Conversely, addition of inhibitory antibodies at the onset of the propagation phase had no effect. However, when added 60 s after the start of the reaction, there is a slight prolongation of the initiation phase and almost no effect on other parameters of thrombin generation. Thus in several in vitro models of TF- initiated coagulation, the procoagulant response becomes independent of TF cofactor activity prior to the onset of clot formation, reflecting the emergence of the more efficient intrinsic factor Xase complex,98 and suggesting that transient expression of TF is sufficient to successfully achieve the first phase of hemorrhage control, formation of an impermeable platelet fibrin barrier.
Figure 4.
Termination of TF and factor VIIa activity in TF (5 pM) initiated thrombin generation in Synthetic Coagulation Proteome. Anti-TF and anti-factor VIIa inhibitory antibodies were added at 0 s (✖), 10 s (●), 60 s (▲) and 240 s (■) after the initiation of the reaction or not added at all (◆). Arrows indicate antibody addition time-points. (This figure was originally published in J Biol Chem98).
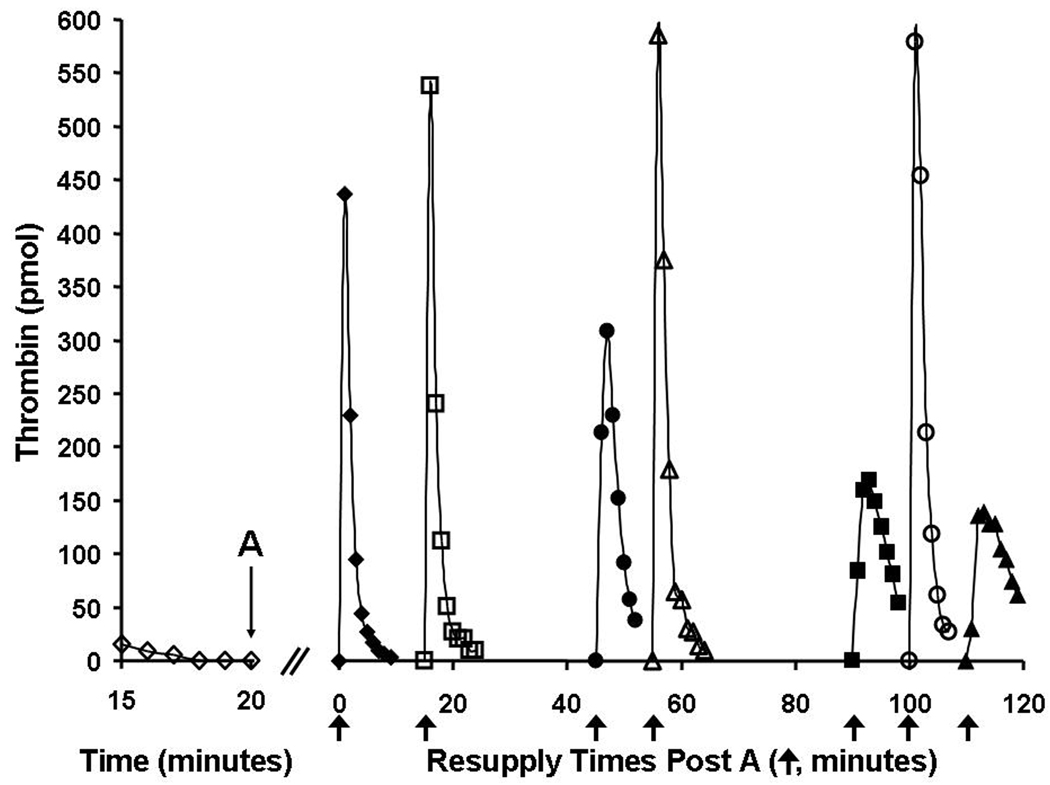
Figure 5 shows a synthetic coagulation proteome experiment testing the stability of the procoagulant catalysts generated by an episode of TF-initiated thrombin generation. A TF-initiated reaction in which thrombin production had ceased and no detectable thrombin remained (due to inhibition by antithrombin) was subdivided after 20 minutes, with individual aliquots then resupplied at various later times with mixtures containing prothrombin, antithrombin, phospholipid with or without factor VIII. In the absence of FVIII (closed symbols) thrombin generation by resupplied reactions was observed to decline slowly as the time period before resupply increased, reflecting a slow decline in the prothrombinase concentration. However inclusion of factor VIII (open symbols) into the resupply mixture yielded time courses of thrombin generation that appeared unaltered even after an additional 100 min of incubation prior to resupply.
Figure 5.
Resupply of the synthetic coagulation proteome—the effect of factor VIII on the stability of the response. A 5 pM TF-initiated reaction mixture was subdivided after 20 min (A), and the eight separate aliquots subsequently resupplied at different times with an equal volume of a mixture containing 1.4 µM prothrombin/3.4 µM antithrombin/2 µM phospholipids either without factor VIII (closed symbols) or with 0.7 nM factor VIII (open symbols). The resulting time courses of thrombin generation are presented. Resupply with the mixture without factor VIII was conducted immediately (20 min → t=0, (◆) and 45 (●), 90 (■) and 110 (▲) min later. Resupply with the mixture supplemented with factor VIII was conducted at 15 (□), 55 (△) and 100 (○) min after the subdivision of the TF-initiated reaction. Thrombin levels for the final 5 min of the TF-initiated episode are also shown (◊). Thrombin levels are expressed as total picomoles of active thrombin to normalize for the volume change. An arrow indicates the resupply time for each aliquot. Reproduced with permission from Orfeo et al.99
These studies indicate that prothrombinase and factor IXa formed during an episode of TF-initiated coagulation persist and also that they can function to restart thrombin generation. Complementary studies using our whole blood model have verified the importance of the prothrombinase complex in reinitiating coagulation.99 Numerous other studies have implicated fibrin bound thrombin as a relatively stable, localized, procoagulant product of TF-initiated coagulation, capable of activating procofactors, cleaving fibrinogen and activating platelets, and thus functioning to propagate thrombus growth.100–107
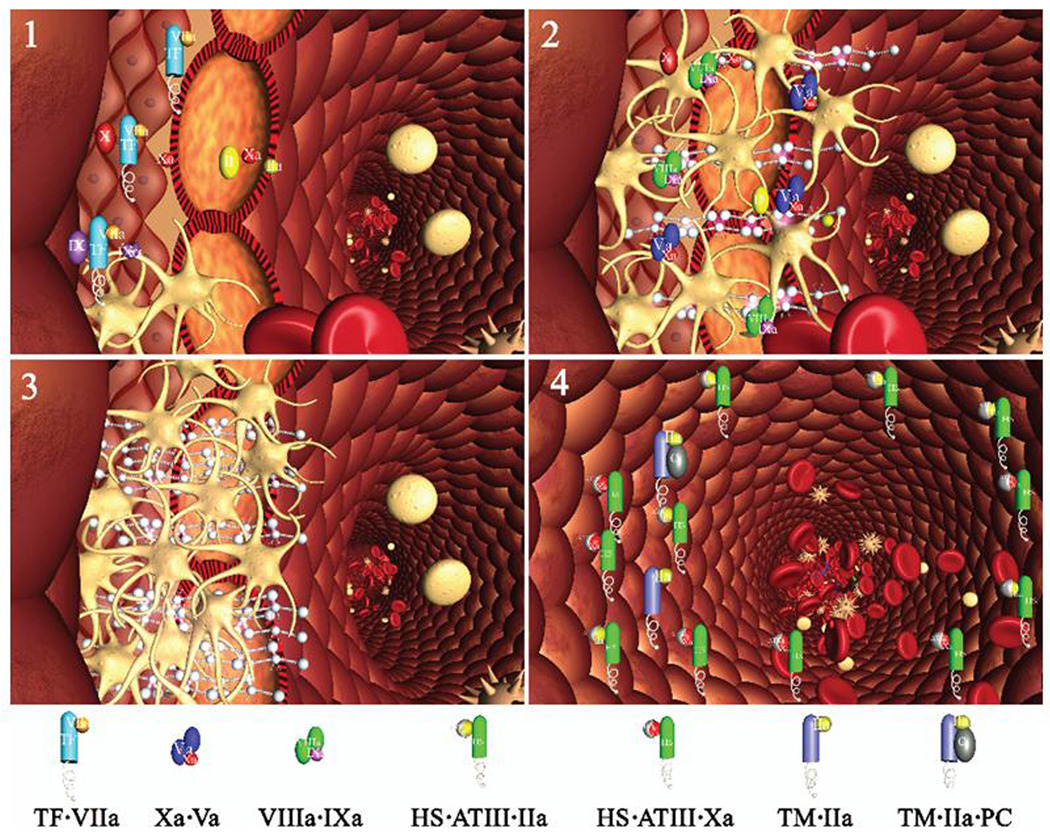
Thus, work from our laboratory and others100,101,103,104,106,107 has led us to propose a model of hemorrhage control (Figure 6),98,99 which contrasts with models requiring constant infusions of TF. In this model, two procoagulant compartments emerge as a consequence of the impermeable barrier formed by platelets and fibrin: an extravascular one, isolated from the blood, with quiescent (reactant starved) procoagulant catalysts that can respond immediately if the barrier fails; and a vascular side where the accumulated ensemble of procoagulant catalysts, exposed to flowing blood, continue the process of clot growth. On this side, however, these catalysts are exposed to the active anticoagulant properties of the vasculature that eventually neutralize them, rendering the vascular face of the clot inert. Thus, in this model, hemorrhage control in a healthy vasculature involves not only the formation of an effective barrier and appropriate control of clot growth on the vascular side but also involves the presence of a persisting, TF-independent procoagulant potential on the extravascular side including clot bound thrombin100,106,107 and the prothrombinase complex.98,99
Figure 6.
Schema of a two compartment model of the regulation of TF-initiated blood coagulation. A cross section of a blood vessel showing the luminal space, endothelial cell layer and extravascular region is presented at the site of a perforation. The blood coagulation process in response is depicted in four stages. Tissue factor-factor VIIa complex, TF•VIIa; prothrombinase complex, Xa•Va; intrinsic factor Xase, VIIIa•IXa; ATIII-endothelial cell heparan sulfate proteoglycan complex bound to thrombin or factor Xa, HS•ATIII•(IIa or Xa); protein C bound to thrombomodulin-thrombin, TM•IIa•PC.
Stage 1. Perforation results in delivery of blood, and with it circulating factor VIIa and platelets, to an extravascular space rich in membrane bound TF. Platelets adhere to collagen and von Willebrand factor associated with the extravascular tissue, and TF binds factor VIIa , initiating the process of factor IX and factor X activation. Factor Xa activates small amounts of prothrombin to thrombin that activates more platelets and converts factor V and factor VIII to factor Va and factor VIIIa.
Stage 2. The reaction is propagated by platelet-bound intrinsic factor Xase and prothrombinase with the former being the principle factor Xa generator. Initial clotting occurs and fibrin begins to fill in the void in cooperation with activated platelets.
Stage 3. A barrier composed of activated platelets ladened with procoagualant complexes and enmeshed in fibrin scaffolding is formed. The reaction in the now filled perforation is terminated by reagent consumption attenuating further thrombin generation but functional procoagulant enzyme complexes persist because they are protected from the dynamic inhibitory processes found on the intravascular face.
Stage 4. View downstream of the perforation. Enzymes escaping from the plugged perforation are captured by antithrombin-heparan complexes and the protein C system is activated by residual thrombin binding to endothelial cell thrombomodulin, initiating the dynamic anticoagulant system. These intravascular processes work against occlusion of the vessel despite the continuous resupply of reactants across the intravascular face of the thrombus. (This figure was originally published in J Biol Chem98).
Acknowledgments
Sources of Funding
This work was supported by P01 HL46703 grant from the National Institutes of Health.
Footnotes
Disclosures
None.
References
- 1.Drake TA, Morrissey JH, Edgington TS. Selective cellular expression of tissue factor in human tissues. Implications for disorders of hemostasis and thrombosis. Am J Pathol. 1989;134:1087–1097. [PMC free article] [PubMed] [Google Scholar]
- 2.Fleck RA, Rao LV, Rapaport SI, Varki N. Localization of human tissue factor antigen by immunostaining with monospecific, polyclonal anti-human tissue factor antibody [corrected and republished article originally printed in Thromb Res 1990 Mar 1;57(5):765–81] Thromb Res. 1990;59:421–437. doi: 10.1016/0049-3848(90)90148-6. [DOI] [PubMed] [Google Scholar]
- 3.Eddleston M, de la Torre JC, Oldstone MB, Loskutoff DJ, Edgington TS, Mackman N. Astrocytes are the primary source of tissue factor in the murine central nervous system. A role for astrocytes in cerebral hemostasis. J Clin Invest. 1993;92:349–358. doi: 10.1172/JCI116573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bloem LJ, Chen L, Konigsberg WH, Bach R. Serum stimulation of quiescent human fibroblasts induces the synthesis of tissue factor mRNA followed by the appearance of tissue factor antigen and procoagulant activity. J Cell Physiol. 1989;139:418–423. doi: 10.1002/jcp.1041390226. [DOI] [PubMed] [Google Scholar]
- 5.Levi M, van der Poll T, ten Cate H. Tissue factor in infection and severe inflammation. Seminars in Thrombosis and Hemostasis. 2006;32:33–39. doi: 10.1055/s-2006-933338. [DOI] [PubMed] [Google Scholar]
- 6.Bouchard BA, Tracy PB. The participation of leukocytes in coagulant reactions. J Thromb Haemost. 2003;1:464–469. doi: 10.1046/j.1538-7836.2003.00089.x. [DOI] [PubMed] [Google Scholar]
- 7.Nilziel M, van Oerle R, van't Veer C, Pampus E, Lindhout T, Hamulyak K. Tissue factor activity in human monocytes is regulated by plasma: implications for the high and low responder phenomenon. Br J Haematol. 2001;112:98–104. doi: 10.1046/j.1365-2141.2001.02545.x. [DOI] [PubMed] [Google Scholar]
- 8.Broussas M, Cornillet-Lefebvre P, Potron G, Nguyen P. Adenosine inhibits tissue factor expression by LPS-stimulated human monocytes: involvement of the A3 adenosine receptor. Thromb Haemost. 2002;88:123–130. [PubMed] [Google Scholar]
- 9.Edwards RL, Rickles FR, Cronlund M. Abnormalities of blood coagulation in individuals with cancer. J Lab Clin Med. 1981;98:917–928. [PubMed] [Google Scholar]
- 10.Ruf W, Mueller BM. Thrombin generation and the pathogenesis of cancer. Semin Thromb Hemost. 2006;32 Suppl 1:61–68. doi: 10.1055/s-2006-939555. [DOI] [PubMed] [Google Scholar]
- 11.Lopez-Pedrera C, Barbarroja N, Dorado G, Siendones E, Velasco F. Tissue factor as an effector of angiogenesis and tumor progression in hematological malignancies. Leukemia. 2006;20:1331–1340. doi: 10.1038/sj.leu.2404264. [DOI] [PubMed] [Google Scholar]
- 12.Jude B, Zawadzki C, Susen S, Corseaux D. Relevance of tissue factor in cardiovascular disease. Arch Mal Coeur Vaiss. 2005;98:667–671. [PubMed] [Google Scholar]
- 13.Mumford AD, McVey JH. Tissue factor in the myocardium: evidence of roles in haemostasis and inflammation. Dis Markers. 2004;20:353–358. doi: 10.1155/2004/963402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butenas S, Mann KG. Active tissue factor in blood? Nat Med. 2004;10:1155–1156. doi: 10.1038/nm1104-1155b. [DOI] [PubMed] [Google Scholar]
- 15.Morrissey JH, Macik BG, Neuenschwander PF, Comp PC. Quantitation of activated factor VII levels in plasma using a tissue factor mutant selectively deficient in promoting factor VII activation. Blood. 1993;81:734–744. [PubMed] [Google Scholar]
- 16.Spicer EK, Horton R, Bloem L, Bach R, Williams KR, Guha A, Kraus J, Lin TC, Nemerson Y, Konigsberg WH. Isolation of cDNA clones coding for human tissue factor: primary structure of the protein and cDNA. Proc Natl Acad Sci U S A. 1987;84:5148–5152. doi: 10.1073/pnas.84.15.5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruf W, Rehemtulla A, Morrissey JH, Edgington TS. Phospholipid-independent and -dependent interactions required for tissue factor receptor and cofactor function. J Biol Chem. 1991;266:2158–2166. [PubMed] [Google Scholar]
- 18.Fiore MM, Neuenschwander PF, Morrissey JH. The biochemical basis for the apparent defect of soluble mutant tissue factor in enhancing the proteolytic activities of factor VIIa. J Biol Chem. 1994;269:143–149. [PubMed] [Google Scholar]
- 19.Ahamed J, Ruf W. Protease-activated receptor 2-dependent phosphorylation of the tissue factor cytoplasmic domain. J Biol Chem. 2004;279:23038–23044. doi: 10.1074/jbc.M401376200. [DOI] [PubMed] [Google Scholar]
- 20.Bach R, Nemerson Y, Konigsberg W. Purification and characterization of bovine tissue factor. J Biol Chem. 1981;256:8324–8331. [PubMed] [Google Scholar]
- 21.Paborsky LR, Tate KM, Harris RJ, Yansura DG, Band L, McCray G, Gorman CM, O'Brien DP, Chang JY, Swartz JR. Purification of recombinant human tissue factor. Biochemistry. 1989;28:8072–8077. doi: 10.1021/bi00446a016. [DOI] [PubMed] [Google Scholar]
- 22.Kittur FS, Manithody C, Morrissey JH, Rezaie AR. The cofactor function of the N-terminal domain of tissue factor. J Biol Chem. 2004;279:39745–39749. doi: 10.1074/jbc.M406628200. [DOI] [PubMed] [Google Scholar]
- 23.Harlos K, Martin DM, O'Brien DP, Jones EY, Stuart DI, Polikarpov I, Miller A, Tuddenham EG, Boys CW. Crystal structure of the extracellular region of human tissue factor [published erratum appears in Nature 1994 Oct 20;371(6499):720] Nature. 1994;370:662–666. doi: 10.1038/370662a0. [DOI] [PubMed] [Google Scholar]
- 24.Paborsky LR, Harris RJ. Post-translational modifications of recombinant human tissue factor. Thromb Res. 1990;60:367–376. doi: 10.1016/0049-3848(90)90219-3. [DOI] [PubMed] [Google Scholar]
- 25.Bach R, Konigsberg WH, Nemerson Y. Human tissue factor contains thioester-linked palmitate and stearate on the cytoplasmic half-cystine. Biochemistry. 1988;27:4227–4231. doi: 10.1021/bi00412a004. [DOI] [PubMed] [Google Scholar]
- 26.Zioncheck TF, Roy S, Vehar GA. The cytoplasmic domain of tissue factor is phosphorylated by a protein kinase C-dependent mechanism. J Biol Chem. 1992;267:3561–3564. [PubMed] [Google Scholar]
- 27.Waxman E, Ross JB, Laue TM, Guha A, Thiruvikraman SV, Lin TC, Konigsberg WH, Nemerson Y. Tissue factor and its extracellular soluble domain: the relationship between intermolecular association with factor VIIa and enzymatic activity of the complex. Biochemistry. 1992;31:3998–4003. doi: 10.1021/bi00131a015. [DOI] [PubMed] [Google Scholar]
- 28.Pitlick FA. Concanavalin A inhibits tissue factor coagulant activity. J Clin Invest. 1975;55:175–179. doi: 10.1172/JCI107908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shands JW., Jr Macrophage factor X activator formation: metabolic requirements for synthesis of components. Blood. 1985;65:169–175. [PubMed] [Google Scholar]
- 30.Bach RR. Tissue factor encryption. Arterioscler Thromb Vasc Biol. 2006;26:456–461. doi: 10.1161/01.ATV.0000202656.53964.04. [DOI] [PubMed] [Google Scholar]
- 31.Bach R, Rifkin DB. Expression of tissue factor procoagulant activity: regulation by cytosolic calcium. Proc Natl Acad Sci U S A. 1990;87:6995–6999. doi: 10.1073/pnas.87.18.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henriksson CE, Klingenberg O, Hellum M, Landsverk KS, Joo GB, Westvik AB, Kierulf P. Calcium ionophore-induced de-encryption of tissue factor in monocytes is associated with extensive cell death. Thromb Res. 2007;119:621–630. doi: 10.1016/j.thromres.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 33.Carson SD, Perry GA, Pirruccello SJ. Fibroblast tissue factor: calcium and ionophore induce shape changes, release of membrane vesicles, and redistribution of tissue factor antigen in addition to increased procoagulant activity. Blood. 1994;84:526–534. [PubMed] [Google Scholar]
- 34.Rand ML, Wang H, Bang KW, Packham MA, Freedman J. Persistence of phosphatidylserine exposure on activated platelets in vivo in rabbits. Thromb Haemost. 2007;98:477–478. [PubMed] [Google Scholar]
- 35.Wakita K, Stearns-Kurosawa DJ, Marumoto Y. The effect of calcium ionophore A23187 on tissue factor activity and mRNA in endothelial cells. Thromb Res. 1994;74:95–103. doi: 10.1016/0049-3848(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 36.Stampfuss JJ, Censarek P, Bein D, Schror K, Grandoch M, Naber C, Weber AA. Membrane environment rather than tissue factor expression determines thrombin formation triggered by monocytic cells undergoing apoptosis. J Leukoc Biol. 2008;83:1379–1381. doi: 10.1189/jlb.1207843. [DOI] [PubMed] [Google Scholar]
- 37.Henriksson CE, Hellum M, Landsverk KS, Klingenberg O, Joo GB, Kierulf P. Flow cytometry-sorted non-viable endotoxin-treated human monocytes are strongly procoagulant. Thromb Haemost. 2006;96:29–37. doi: 10.1160/TH06-01-0052. [DOI] [PubMed] [Google Scholar]
- 38.Dietzen DJ, Page KL, Tetzloff TA. Lipid rafts are necessary for tonic inhibition of cellular tissue factor procoagulant activity. Blood. 2004;103:3038–3044. doi: 10.1182/blood-2003-07-2399. [DOI] [PubMed] [Google Scholar]
- 39.Liu ML, Reilly MP, Casasanto P, McKenzie SE, Williams KJ. Cholesterol enrichment of human monocyte/macrophages induces surface exposure of phosphatidylserine and the release of biologically-active tissue factor-positive microvesicles. Arterioscler Thromb Vasc Biol. 2007;27:430–435. doi: 10.1161/01.ATV.0000254674.47693.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mandal SK, Iakhiaev A, Pendurthi UR, Rao LV. Acute cholesterol depletion impairs functional expression of tissue factor in fibroblasts: modulation of tissue factor activity by membrane cholesterol. Blood. 2005;105:153–160. doi: 10.1182/blood-2004-03-0990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lorenzet R, Niemetz J, Marcus AJ, Broekman MJ. Enhancement of mononuclear procoagulant activity by platelet 12-hydroxyeicosatetraenoic acid. J Clin Invest. 1986;78:418–423. doi: 10.1172/JCI112592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Halvorsen H, Olsen JO, Osterud B. Granulocytes enhance LPS-induced tissue factor activity in monocytes via an interaction with platelets. J Leukoc Biol. 1993;54:275–282. doi: 10.1002/jlb.54.4.275. [DOI] [PubMed] [Google Scholar]
- 43.Osterud B, Rao LV, Olsen JO. Induction of tissue factor expression in whole blood: lack of evidence for the presence of tissue factor expression in granulocytes. Thromb Haemost. 2000;83:861–867. [PubMed] [Google Scholar]
- 44.Chen VM, Ahamed J, Versteeg HH, Berndt MC, Ruf W, Hogg PJ. Evidence for activation of tissue factor by an allosteric disulfide bond. Biochemistry. 2006;45:12020–12028. doi: 10.1021/bi061271a. [DOI] [PubMed] [Google Scholar]
- 45.Bednar RA, Fried WB, Lock YW, Pramanik B. Chemical modification of chalcone isomerase by mercurials and tetrathionate. Evidence for a single cysteine residue in the active site. J Biol Chem. 1989;264:14272–14276. [PubMed] [Google Scholar]
- 46.Weber GJ, Mehr AP, Sirota JC, Aller SG, Decker SE, Dawson DC, Forrest JN., Jr Mercury and zinc differentially inhibit shark and human CFTR orthologues: involvement of shark cysteine 102. Am J Physiol Cell Physiol. 2006;290:C793–C801. doi: 10.1152/ajpcell.00203.2005. [DOI] [PubMed] [Google Scholar]
- 47.Kaneko H, Kakkar VV, Scully MF. Mercury compounds induce a rapid increase in procoagulant activity of monocyte-like U937 cells. Br J Haematol. 1994;87:87–93. doi: 10.1111/j.1365-2141.1994.tb04875.x. [DOI] [PubMed] [Google Scholar]
- 48.Pendurthi UR, Rao LV. Response: Tissue factor de-encryption: the cell model system. Blood. 2008;112:913. [Google Scholar]
- 49.Ahamed J, Versteeg HH, Kerver M, Chen VM, Mueller BM, Hogg PJ, Ruf W. Disulfide isomerization switches tissue factor from coagulation to cell signaling. Proc Natl Acad Sci U S A. 2006;103:13932–13937. doi: 10.1073/pnas.0606411103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reinhardt C, von Bruhl ML, Manukyan D, Grahl L, Lorenz M, Altmann B, Dlugai S, Hess S, Konrad I, Orschiedt L, Mackman N, Ruddock L, Massberg S, Engelmann B. Protein disulfide isomerase acts as an injury response signal that enhances fibrin generation via tissue factor activation. J Clin Invest. 2008;118:1110–1122. doi: 10.1172/JCI32376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pendurthi UR, Ghosh S, Mandal SK, Rao LV. Tissue factor activation: is disulfide bond switching a regulatory mechanism? Blood. 2007;110:3900–3908. doi: 10.1182/blood-2007-07-101469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kothari H, Sen P, Pendurthi UR, Rao LV. Bovine protein disulfide isomerase-enhanced tissue factor coagulant function: is phospholipid contaminant in it the real culprit? Blood. 2008;111:3295–3296. doi: 10.1182/blood-2007-12-129825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bogdanov VY, Balasubramanian V, Hathcock J, Vele O, Lieb M, Nemerson Y. Alternatively spliced human tissue factor: a circulating, soluble, thrombogenic protein. Nat Med. 2003;9:458–462. doi: 10.1038/nm841. [DOI] [PubMed] [Google Scholar]
- 54.Szotowski B, Antoniak S, Poller W, Schultheiss HP, Rauch U. Procoagulant soluble tissue factor is released from endothelial cells in response to inflammatory cytokines. Circ Res. 2005;96:1233–1239. doi: 10.1161/01.RES.0000171805.24799.fa. [DOI] [PubMed] [Google Scholar]
- 55.Censarek P, Bobbe A, Grandoch M, Schror K, Weber AA. Alternatively spliced human tissue factor (asHTF) is not pro-coagulant. Thromb Haemost. 2007;97:11–14. [PubMed] [Google Scholar]
- 56.Hobbs JE, Zakarija A, Cundiff DL, Doll JA, Hymen E, Cornwell M, Crawford SE, Liu N, Signaevsky M, Soff GA. Alternatively spliced human tissue factor promotes tumor growth and angiogenesis in a pancreatic cancer tumor model. Thromb Res. 2007;120 Suppl 2:S13–S21. doi: 10.1016/S0049-3848(07)70126-3. [DOI] [PubMed] [Google Scholar]
- 57.Osterud B, Breimo ES, Olsen JO. Blood borne tissue factor revisited. Thromb Res. 2008;122:432–434. doi: 10.1016/j.thromres.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 58.Parhami-Seren B, Butenas S, Krudysz-Amblo J, Mann KG. Immunologic quantitation of tissue factors. J Thromb Haemost. 2006;4:1747–1755. doi: 10.1111/j.1538-7836.2006.02000.x. [DOI] [PubMed] [Google Scholar]
- 59.Bogdanov VY, Cimmino G, Tardos JG, Tunstead JR, Badimon JJ. Assessment of plasma tissue factor activity in patients presenting with coronary artery disease: limitations of a commercial assay. J Thromb Haemost. 2009 doi: 10.1111/j.1538-7836.2009.03315.x. [DOI] [PubMed] [Google Scholar]
- 60.Ray B, Chetter IC, Lee HL, Ettelaie C, McCollum PT. Plasma tissue factor is a predictor for restenosis after femoropopliteal angioplasty. Br J Surg. 2007;94:1092–1095. doi: 10.1002/bjs.5759. [DOI] [PubMed] [Google Scholar]
- 61.So AK, Varisco PA, Kemkes-Matthes B, Herkenne-Morard C, Chobaz-Peclat V, Gerster JC, Busso N. Arthritis is linked to local and systemic activation of coagulation and fibrinolysis pathways. J Thromb Haemost. 2003;1:2510–2515. doi: 10.1111/j.1538-7836.2003.00462.x. [DOI] [PubMed] [Google Scholar]
- 62.Santucci RA, Erlich J, Labriola J, Wilson M, Kao KJ, Kickler TS, Spillert C, Mackman N. Measurement of tissue factor activity in whole blood. Thromb Haemost. 2000;83:445–454. [PubMed] [Google Scholar]
- 63.Berckmans RJ, Neiuwland R, Boing AN, Romijn FP, Hack CE, Sturk A. Cell-derived microparticles circulate in healthy humans and support low grade thrombin generation. Thromb Haemost. 2001;85:639–646. [PubMed] [Google Scholar]
- 64.Butenas S, Bouchard BA, Brummel-Ziedins KE, Parhami-Seren B, Mann KG. Tissue factor activity in whole blood. Blood. 2005;105:2764–2770. doi: 10.1182/blood-2004-09-3567. [DOI] [PubMed] [Google Scholar]
- 65.Rand MD, Lock JB, van 't Veer C, Gaffney DP, Mann KG. Blood clotting in minimally altered whole blood. Blood. 1996;88:3432–3445. [PubMed] [Google Scholar]
- 66.Cawthern KM, van 't Veer C, Lock JB, DiLorenzo ME, Branda RF, Mann KG. Blood coagulation in hemophilia A and hemophilia C. Blood. 1998;91:4581–4592. [PubMed] [Google Scholar]
- 67.Peyrou V, Lormeau JC, Herault JP, Gaich C, Pfliegger AM, Herbert JM. Contribution of erythrocytes to thrombin generation in whole blood. Thrombosis and Haemostasis. 1999;81:400–406. [PubMed] [Google Scholar]
- 68.He R, Xiong SL, He XF, Liu FY, Han JZ, Li JC, He SL. The role of factor XI in a dilute thromboplastin assay of extrinsic coagulation pathway. Thrombosis and Haemostasis. 2001;85:1055–1059. [PubMed] [Google Scholar]
- 69.Keularts IM, Zivelin A, Seligsohn U, Hemker HC, Beguin S. The role of factor XI in thrombin generation induced by low concentrations of tissue factor. Thromb Haemost. 2001;85:1060–1065. [PubMed] [Google Scholar]
- 70.del Conde I, Shrimpton CN, Thiagarajan P, Lopez JA. Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood. 2005;106:1604–1611. doi: 10.1182/blood-2004-03-1095. [DOI] [PubMed] [Google Scholar]
- 71.Antoniak S, Boltzen U, Eisenreich A, Stellbaum C, Poller W, Schultheiss HP, Rauch U. Regulation of cardiomyocyte full-length Tissue Factor expression and microparticle release under inflammatory conditions in vitro. J Thromb Haemost. 2009 doi: 10.1111/j.1538-7836.2009.03323.x. [DOI] [PubMed] [Google Scholar]
- 72.Langer F, Spath B, Haubold K, Holstein K, Marx G, Wierecky J, Brummendorf TH, Dierlamm J, Bokemeyer C, Eifrig B. Tissue factor procoagulant activity of plasma microparticles in patients with cancer-associated disseminated intravascular coagulation. Ann Hematol. 2008;87:451–457. doi: 10.1007/s00277-008-0446-3. [DOI] [PubMed] [Google Scholar]
- 73.Morel O, Pereira B, Averous G, Faure A, Jesel L, Germain P, Grunebaum L, Ohlmann P, Freyssinet JM, Bareiss P, Toti F. Increased levels of procoagulant tissue factor-bearing microparticles within the occluded coronary artery of patients with ST-segment elevation myocardial infarction: Role of endothelial damage and leukocyte activation. Atherosclerosis. 2008 doi: 10.1016/j.atherosclerosis.2008.10.039. [DOI] [PubMed] [Google Scholar]
- 74.Huisse MG, Ajzenberg N, Feldman L, Guillin MC, Steg PG. Microparticle-linked tissue factor activity and increased thrombin activity play a potential role in fibrinolysis failure in ST-segment elevation myocardial infarction. Thromb Haemost. 2009;101:734–740. [PubMed] [Google Scholar]
- 75.Zillmann A, Luther T, Muller I, Kotzsch M, Spannagl M, Kauke T, Oelschlagel v, Zahler S, Engelmann B. Platelet-associated tissue factor contributes to the collagen-triggered activation of blood coagulation. Biochem Biophys Res Commun. 2001;281:603–609. doi: 10.1006/bbrc.2001.4399. [DOI] [PubMed] [Google Scholar]
- 76.Brambilla M, Camera M, Colnago D, Marenzi G, De Metrio M, Giesen PL, Balduini A, Veglia F, Gertow K, Biglioli P, Tremoli E. Tissue factor in patients with acute coronary syndromes: expression in platelets, leukocytes, and platelet-leukocyte aggregates. Arterioscler Thromb Vasc Biol. 2008;28:947–953. doi: 10.1161/ATVBAHA.107.161471. [DOI] [PubMed] [Google Scholar]
- 77.Panes O, Matus V, Saez CG, Quiroga T, Pereira J, Mezzano D. Human platelets synthesize and express functional tissue factor. Blood. 2007;109:5242–5250. doi: 10.1182/blood-2006-06-030619. [DOI] [PubMed] [Google Scholar]
- 78.Lopez-Vilchez I, Escolar G, Diaz-Ricart M, Fuste B, Galan AM, White JG. Tissue factor-enriched vesicles are taken up by platelets and induce platelet aggregation in the presence of factor VIIa. Thromb Haemost. 2007;97:202–211. [PubMed] [Google Scholar]
- 79.Schwertz H, Tolley ND, Foulks JM, Denis MM, Risenmay BW, Buerke M, Tilley RE, Rondina MT, Harris EM, Kraiss LW, Mackman N, Zimmerman GA, Weyrich AS. Signal-dependent splicing of tissue factor pre-mRNA modulates the thrombogenicity of human platelets. J Exp Med. 2006;203:2433–2440. doi: 10.1084/jem.20061302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Maugeri N, Brambilla M, Camera M, Carbone A, Tremoli E, Donati MB, de Gaetano G, Cerletti C. Human polymorphonuclear leukocytes produce and express functional tissue factor upon stimulation. J Thromb Haemost. 2006;4:1323–1330. doi: 10.1111/j.1538-7836.2006.01968.x. [DOI] [PubMed] [Google Scholar]
- 81.Ritis K, Doumas M, Mastellos D, Micheli A, Giaglis S, Magotti P, Rafail S, Kartalis G, Sideras P, Lambris JD. A novel C5a receptor-tissue factor cross-talk in neutrophils links innate immunity to coagulation pathways. J Immunol. 2006;177:4794–4802. doi: 10.4049/jimmunol.177.7.4794. [DOI] [PubMed] [Google Scholar]
- 82.Moosbauer C, Morgenstern E, Cuvelier SL, Manukyan D, Bidzhekov K, Albrecht S, Lohse P, Patel KD, Engelmann B. Eosinophils are a major intravascular location for tissue factor storage and exposure. Blood. 2007;109:995–1002. doi: 10.1182/blood-2006-02-004945. [DOI] [PubMed] [Google Scholar]
- 83.Sovershaev MA, Lind KF, Devold H, Jorgensen TO, Hansen JB, Osterud B, Egorina EM. No evidence for the presence of tissue factor in high-purity preparations of immunologically isolated eosinophils. J Thromb Haemost. 2008;6:1742–1749. doi: 10.1111/j.1538-7836.2008.03105.x. [DOI] [PubMed] [Google Scholar]
- 84.Osterud B. The role of platelets in decrypting monocyte tissue factor. Semin Hematol. 2001;38:2–5. doi: 10.1016/s0037-1963(01)90139-8. [DOI] [PubMed] [Google Scholar]
- 85.Butenas S, Undas A, Gissel MT, Szuldrzynski K, Zmudka K, Mann KG. Factor XIa and tissue factor activity in patients with coronary artery disease. Thromb Haemost. 2008;99:142–149. doi: 10.1160/TH07-08-0499. [DOI] [PubMed] [Google Scholar]
- 86.Bis J, Vojacek J, Dusek J, Pecka M, Palicka V, Stasek J, Maly J. Time - course of tissue factor plasma level in patients with acute coronary syndrome. Physiol Res. 2008 doi: 10.33549/physiolres.931521. [DOI] [PubMed] [Google Scholar]
- 87.Mann KG. Thrombin formation. Chest. 2003;124:4S–10S. doi: 10.1378/chest.124.3_suppl.4s. [DOI] [PubMed] [Google Scholar]
- 88.Brand K, Fowler BJ, Edgington TS, Mackman N. Tissue factor mRNA in THP-1 monocytic cells is regulated at both transcriptional and posttranscriptional levels in response to lipopolysaccharide. Mol Cell Biol. 1991;11:4732–4738. doi: 10.1128/mcb.11.9.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.van 't Veer C, Mann KG. Regulation of tissue factor initiated thrombin generation by the stoichiometric inhibitors tissue factor pathway inhibitor, antithrombin- III, and heparin cofactor-II. J Biol Chem. 1997;272:4367–4377. doi: 10.1074/jbc.272.7.4367. [DOI] [PubMed] [Google Scholar]
- 90.van 't Veer C, Golden NJ, Kalafatis M, Mann KG. Inhibitory mechanism of the protein C pathway on tissue factor-induced thrombin generation. Synergistic effect in combination with tissue factor pathway inhibitor. J Biol Chem. 1997;272:7983–7994. doi: 10.1074/jbc.272.12.7983. [DOI] [PubMed] [Google Scholar]
- 91.Hathcock JJ, Nemerson Y. Platelet deposition inhibits tissue factor activity: in vitro clots are impermeable to factor Xa. Blood. 2004;104:123–127. doi: 10.1182/blood-2003-12-4352. [DOI] [PubMed] [Google Scholar]
- 92.Jesty J, Beltrami E. Positive feedbacks of coagulation: their role in threshold regulation. Arterioscler Thromb Vasc Biol. 2005;25:2463–2469. doi: 10.1161/01.ATV.0000187463.91403.b2. [DOI] [PubMed] [Google Scholar]
- 93.Chou J, Mackman N, Merrill-Skoloff G, Pedersen B, Furie BC, Furie B. Hematopoietic cell-derived microparticle tissue factor contributes to fibrin formation during thrombus propagation. Blood. 2004;104:3190–3197. doi: 10.1182/blood-2004-03-0935. [DOI] [PubMed] [Google Scholar]
- 94.Wysokinski WE, Owen WG, Fass DN, Patrzalek DD, Murphy L, McBane RD. Atrial fibrillation and thrombosis: immunohistochemical differences between in situ and embolized thrombi. J Thromb Haemost. 2004;2:1637–1644. doi: 10.1111/j.1538-7836.2004.00899.x. [DOI] [PubMed] [Google Scholar]
- 95.Day SM, Reeve JL, Pedersen B, Farris DM, Myers DD, Im M, Wakefield TW, Mackman N, Fay WP. Macrovascular thrombosis is driven by tissue factor derived primarily from the blood vessel wall. Blood. 2005;105:192–198. doi: 10.1182/blood-2004-06-2225. [DOI] [PubMed] [Google Scholar]
- 96.Hoffman M, Whinna HC, Monroe DM. Circulating tissue factor accumulates in thrombi, but not in hemostatic plugs. J Thromb Haemost. 2006;4:2092–2093. doi: 10.1111/j.1538-7836.2006.02085.x. [DOI] [PubMed] [Google Scholar]
- 97.Okorie UM, Denney WS, Chatterjee MS, Neeves KB, Diamond SL. Determination of surface tissue factor thresholds that trigger coagulation at venous and arterial shear rates: amplification of 100 fM circulating tissue factor requires flow. Blood. 2008;111:3507–3513. doi: 10.1182/blood-2007-08-106229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Orfeo T, Butenas S, Brummel-Ziedins KE, Mann KG. The tissue factor requirement in blood coagulation. J Biol Chem. 2005;280:42887–42896. doi: 10.1074/jbc.M505506200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Orfeo T, Brummel-Ziedins KE, Gissel M, Butenas S, Mann KG. The nature of the stable blood clot procoagulant activities. J Biol Chem. 2008;283:9776–9786. doi: 10.1074/jbc.M707435200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Francis CW, Markham RE, Jr, Barlow GH, Florack TM, Dobrzynski DM, Marder VJ. Thrombin activity of fibrin thrombi and soluble plasmic derivatives. J Lab Clin Med. 1983;102:220–230. [PubMed] [Google Scholar]
- 101.Hogg PJ, Jackson CM. Fibrin monomer protects thrombin from inactivation by heparin-antithrombin III: implications for heparin efficacy. Proc Natl Acad Sci U S A. 1989;86:3619–3623. doi: 10.1073/pnas.86.10.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Naski MC, Shafer JA. Alpha-thrombin-catalyzed hydrolysis of fibrin I. Alternative binding modes and the accessibility of the active site in fibrin I-bound alpha-thrombin. J Biol Chem. 1990;265:1401–1407. [PubMed] [Google Scholar]
- 103.Weitz JI, Hudoba M, Massel D, Maraganore J, Hirsh J. Clot-bound thrombin is protected from inhibition by heparin- antithrombin III but is susceptible to inactivation by antithrombin III- independent inhibitors. J Clin Invest. 1990;86:385–391. doi: 10.1172/JCI114723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bendayan P, Boccalon H, Dupouy D, Boneu B. Dermatan sulfate is a more potent inhibitor of clot-bound thrombin than unfractionated and low molecular weight heparins. Thromb Haemost. 1994;71:576–580. [PubMed] [Google Scholar]
- 105.Fredenburgh JC, Stafford AR, Pospisil CH, Weitz JI. Modes and consequences of thrombin's interaction with fibrin. Biophys Chem. 2004;112:277–284. doi: 10.1016/j.bpc.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 106.Kumar R, Beguin S, Hemker HC. The influence of fibrinogen and fibrin on thrombin generation--evidence for feedback activation of the clotting system by clot bound thrombin. Thromb Haemost. 1994;72:713–721. [PubMed] [Google Scholar]
- 107.Kumar R, Beguin S, Hemker HC. The effect of fibrin clots and clot-bound thrombin on the development of platelet procoagulant activity. Thromb Haemost. 1995;74:962–968. [PubMed] [Google Scholar]
- 108.Butenas S, Orfeo T, Brummel-Ziedins KE, Mann KG. Tissue factor in thrombosis and hemorrhage. Surgery. 2007;142:S2–S14. doi: 10.1016/j.surg.2007.06.032. [DOI] [PubMed] [Google Scholar]