Abstract
Schizophrenia may be associated with a fundamental disturbance in the temporal coordination of information processing in the brain, leading to classic symptoms of schizophrenia such as thought disorder and disorganized and contextually inappropriate behavior. Although a variety of behavioral studies have provided strong evidence for perceptual timing deficits in schizophrenia, no study to date has directly examined overt temporal performance in schizophrenia using a task that differentially engages perceptual and motor-based timing processes. The present study aimed to isolate perceptual and motor-based temporal performance in individuals diagnosed with schizophrenia using a repetitive finger-tapping task that has previously been shown to differentially engage brain regions associated with perceptual and motor-related timing behavior. Thirty-two individuals with schizophrenia and 31 non-psychiatric control participants completed the repetitive finger-tapping task, which required participants to first tap in time with computer-generated tones separated by a fixed intertone interval (tone-paced tapping), after which the tones were discontinued and participants were required to continue tapping at the established pace (self-paced tapping). Participants with schizophrenia displayed significantly faster tapping rates for both tone- and self-paced portions of the task compared to the non-psychiatric group. Individuals diagnosed with schizophrenia also displayed greater tapping variability during both tone- and self-paced portions of the task. The application of a mathematical timing model further indicated that group differences were primarily attributable to increased timing – as opposed to task implementation – difficulties in the schizophrenia group, which is noteworthy given the broad range of impairments typically associated with the disorder. These findings support the contention that schizophrenia is associated with a broad range of timing difficulties, including those associated with time perception as well as time production.
Keywords: schizophrenia, timing, temporal processing, finger tapping, variability
Attempts to understand the underlying pathophysiology of schizophrenia have increasingly emphasized disturbances in the temporal coordination of information processing in the brain, leading to perceptual, cognitive, and motor timing dysfunctions (Andreasen et al., 1998; Bressler, 2003; Paulus & Braff, 2003; Phillips & Silverstein, 2003; Tononi & Edelman, 2000). “Disconnection” models of schizophrenia posit that deficits in neural communications arise from disturbances in the regulation of time-dependent changes in synaptic connectivity, which manifest as dysfunctions in the timing or sequencing of mental activity and behavior (Friston, 1998; McGlashan & Hoffman, 2000). Accordingly, impairments of neural timing have been associated with many classic symptoms of schizophrenia, including hallucinations, delusions, and disorganized thinking and speech (Andreasen, 1999; McGlashan & Hoffman, 2000). Support for these conceptualizations is emerging with evidence that brain structures directly linked to neural timing processes are also impaired in schizophrenia (Andreasen, 1999; Andreasen et al., 1998; Rao et al., 1997, 2001; Volz et al., 2001).
The relationship between disturbances of neural timing and schizophrenia is further demonstrated by the fact that individuals with schizophrenia have consistently displayed temporal disturbances across a variety of timing tasks (Brown et al., 2005; Carroll et al., 2008; Densen, 1977; Johnson & Petzel, 1971; Tysk, 1983a,b, 1990; Volz et al., 2001; Wahl & Sieg, 1980). Specifically, individuals diagnosed with schizophrenia have consistently been found to experience time as lengthened relative to objective time, which has been interpreted to reflect an increase in speed of a hypothetical “internal clock” (Densen, 1977; Johnson & Petzel, 1971; Lhamon & Goldstone, 1956; Tracy et al., 1998; Tysk, 1983a, 1983b, 1990; Wahl & Sieg, 1990). For instance, numerous behavioral studies have reported overestimations and underproductions of temporal durations ranging from several seconds to one minute in schizophrenia (Densen, 1977; Johnson & Petzel, 1971; Tysk, 1983a, 1983b, 1990; Volz et al., 2001; Wahl & Sieg, 1980), which is consistent with patients’ subjective reports of an elongated experience of time (Freedman, 1974). Studies of eye-blink conditioning (EBC), in which performance is highly dependent upon acquisition of the temporal relationship between two stimuli, have also revealed abnormal patterns of temporal learning in schizophrenia and increased motor-timing variability (Brown et al., 2005; Hofer et al., 2001; Sears et al., 1999).
Of further interest is the link between brain regions that have been implicated in these timing tasks and those implicated in the pathophysiology of schizophrenia (Andreasen, 1999; Andreasen et al., 1998; Rao et al., 1997, 2001; Volz et al., 2001). Schizophrenia has been associated with deficits of neural communication within cortical-cerebellar (Andreasen, 1999; Andreasen et al., 1998) and cortical-striatal (Volz et al., 2001) brain circuits, which are also responsible for temporally coordinated motor activity (Rao et al., 1997, 2001) and the encoding and explicit representation of temporal information (Rao et al., 2001; Voltz et al., 2001), respectively. The parallels between the neural circuitry proposed to underlie internal timing functions and those which have been implicated in schizophrenia provide a strong impetus to further our understanding of temporal deficits associated with the disorder. Although behavioral-based studies of temporal perception have provided strong evidence for perceptual timing deficits in schizophrenia (Carroll et al., 2008; Densen, 1977; Elvevåg et al., 2003; Johnson & Petzel, 1971; Tysk, 1983a, b, 1990), no study to date has directly examined overt temporal performance in schizophrenia using a task that differentially engages perceptual and motor-based timing processes. Thus, the primary aim of the present study was to isolate perceptual and motor-based temporal performance in individuals diagnosed with schizophrenia using a well-established task of time production (Ivry, 1993; Ivry & Keele, 1989; Ivry et al., 1988; Rao et al., 1997).
The repetitive finger-tapping task requires participants to first tap in time with computer-generated tones separated by a fixed intertone interval (tone-paced tapping). After a series of tone-paced responses, the tones are discontinued and participants are required to continue tapping at the established pace (self-paced tapping). Using fMRI, the repetitive finger-tapping task has been shown to differentially activate striatal and cerebellar brain regions (Rao et al., 1997). Specifically, striatal regions were preferentially activated during self-paced tapping, which requires internal representations of time (Rao et al., 1997). In contrast, activation was restricted to the cerebellum during tone-paced tapping, during which participants were provided with the external pacing signal (Rao et al., 1997). Further evidence for the differential involvement of distinct timing networks in repetitive finger tapping comes from a study in which repetitive transcranial magnetic stimulation (rTMS) was applied over the cerebellum to investigate the effects of cerebellar interference on finger tapping performance (Del Olmo et al., 2007). Using a 500 ms intertone interval, rTMS of the cerebellum ipsilateral to the hand employed in the tapping procedure was found to interfere with tapping rates during the tone-paced portion of the task only; no effects of cerebellar interference were found for self-paced tapping (Del Olmo et al., 2007).
As indicated above, striatal regions of the basal ganglia have been largely implicated in perceptual timing processes, including the encoding and internal representation of temporal intervals (Harrington et al., 1998; Matell & Meck, 2000; Rao et al., 2001; Voltz et al., 2001). The involvement of the cerebellum in the tone-paced portion of the repetitive finger-tapping task (Del Olmo et al., 2007; Rao et al., 1997), in conjunction with evidence from other time-based tasks (Koch et al., 2007; Lee et al., 2007), suggests that the cerebellum may also be involved in the initial encoding of time. Despite this potential overlap between the striatum and cerebellum with regard to early temporal processing, it appears that the striatal and cerebellar timing circuits play unique roles in the repetitive finger tapping task. Whereas the timing functions of the cerebellum seem critical for temporal performance during tone-paced tapping which requires coordinated motor activity, striatal regions appear to play a critical role in maintaining an internal representation of time during the self-paced portion of the task (Maquet et al., 1996; Rao et al., 1997, 2001). Thus, the inclusion of a task that has been associated with the distinct activation of cerebellar and striatal regions may help delineate timing deficits in schizophrenia associated with a coordinated motor program from those related to the explicit representation of time, respectively.
It was hypothesized that individuals with schizophrenia would show distinct deficits in the tone- and self-paced segments of the repetitive finger-tapping task. Greater motor-timing deficits were expected for individuals with schizophrenia during tone-paced tapping, as reflected by increased tapping variability. Although greater variability in schizophrenia was also predicted for the self-paced portion of the task, the internal representations of time required for self-paced tapping should be dependent on internal clock speed, which were expected to further result in faster tapping rates in individuals with schizophrenia compared to non-psychiatric control participants.
In addition to the behavioral data, a mathematical model was applied to determine if any increases in tapping variability observed in schizophrenia could be attributed to motor implementation, rather than timing, deficits. The Wing-Kristofferson model is a two-process model of self-paced tapping that differentiates overall inter-response variance into clock and motor implementation components (Wing & Kristofferson, 1973a, 1973b, Wing, 1980). It was hypothesized that group differences in tapping variability would correspond to an increase in clock variance in the schizophrenia group, with no differences between individuals with schizophrenia and non-psychiatric participants on estimates of motor implementation variance.
Methods
Participants
Thirty-two (22 male: mean age = 37.3, SD = 11.4; 10 female: mean age = 43.2, SD = 8.3) individuals meeting DSM-IV (American Psychiatric Association, 1994) criteria for schizophrenia and 31 (9 male: mean age = 33.4, SD = 10.8; 22 female: mean age = 42.8, SD = 11.3) non-psychiatric participants volunteered for the present study. A subgroup of participants (23 individuals with schizophrenia, 22 non-psychiatric participants) also volunteered for a second timing study that was conducted concurrently with the present study as part of a larger body of research on temporal processing in schizophrenia (Carroll et al., 2008). Each of the participants diagnosed with schizophrenia was evaluated using the Structured Clinical Interview for the DSM-IV (Diagnostic and Statistical Manual IV) for Axis I disorders (SCID-I; First et al., 1995), supplemented by chart information. All non-psychiatric control participants were interviewed using the SCID-NP (Spitzer et al., 1990) to exclude individuals with Axis I disorders, antisocial personality disorder, and schizophrenia spectrum personality disorders. The patient sample was recruited through outpatient and inpatient units at community and state hospitals and comparison participants were recruited via newspaper advertisement. Exclusion criteria for all participants included self-reported neurological disease, head injury resulting in loss of consciousness for more than 5 minutes, and personal or family history of schizophrenia in the non-psychiatric group. All participants had normal or corrected-to-normal vision and hearing acuity. Although educational attainment was significantly greater in the non-psychiatric participants (mean years = 14.0, SD = 2.1) compared to the patient sample (mean years = 11.1, SD = 1.8), t(44) = 4.97, p < .001, Pearson correlations computed within each group revealed no significant relationships between years of education and any of the finger tapping variables. Indiana University’s Human Subjects Institutional Review Board approved of this study and written informed consent was obtained from all participants.
Current symptom levels in the patient group were assessed by trained diagnosticians using the Positive and Negative Syndrome Scale (PANSS; Kay, et al., 1987). Symptom ratings were categorized into a five-factor structure (Positive: M = 14.9, SD = 6.0; Negative: M = 16.3, SD = 5.9; Cognitive: M = 15.1, SD = 5.6; Hostility: M = 6.3, SD = 3.3; and Emotional Discomfort: M = 7.4, SD = 2.9) that has been suggested to provide a more sensitive subtype classification than the conventional Positive, Negative, and General subscale distribution (Bell et al., 1994a, 1994b; Kay & Sevy, 1990). In addition, the two-subtest form of the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999) was administered to obtain an estimate of general intellectual ability. The WAIS two-subtest format includes the Vocabulary subtest, which is used to assess crystallized intelligence, and the Matrix Reasoning subtest as a measure of nonverbal fluid abilities. A significant difference in estimated intellectual ability was found between the schizophrenia (M = 82.5, SD = 13.5) and non-psychiatric (M = 106.3, SD = 13.0) groups, t(52) = 6.59, p < .001. Finally, given that the repetitive finger-tapping task requires participants to maintain the pacing signal in memory during the self-paced portion of the task, the digit span (forward and backward) subtest of the Wechsler Adult Intelligence Scale - III (WAIS-III, Wechsler, 1997) was administered as a measure of working memory. Raw combined digit span scores were significantly lower for individuals with schizophrenia (M = 12.8, SD = 3.6) than non-psychiatric participants (M = 19.7, SD = 3.9), t(59) = 7.20, p < .001.
At the time of testing, 21 individuals with schizophrenia were taking antipsychotic medications (14 atypical, 7 typical), 1 individual was receiving psychotropic medications alone, 1 individual was not medicated, and the medication status of 9 patients could not be determined due to unavailable information. Chlorpromazine equivalents were computed for patients receiving antipsychotic medication, and the resulting dosages were correlated with all behavioral measures to assess for medication effects on timing performance. No significant relationships were noted.
Task Procedure
The repetitive tapping task was performed using the dominant index finger and alternating thumbs. For the dominant index finger, participants rested their dominant hand flat on a response box with the index finger positioned over a center push button. In the alternating thumbs condition, the response box was held in both hands, with the left and right thumbs placed over left and right push buttons, respectively. In both conditions, depression of the push button was used to calculate the inter-tap interval.
Each trial began with an auditory tone paced at 500 ms inter-tone intervals. Participants were instructed to tap the push button at the same rate as tone (tone-paced tapping). After 12 taps, the tone was discontinued and participants were required to continue tapping at the same rate as the previously presented signal (self-paced tapping). Trials were terminated following 30 self-paced button presses. Error taps were defined as inter-tap intervals 250 ms above or below the 500 ms pacing interval during either the tone or self-paced portion of the trial. A total of 6 error-free trials, or a maximum of 12 trials, signaled the completion of the task.
Behavioral Data and Analysis
Mean inter-tap intervals (ITIs) were computed across trials for the tone and self-paced portions of the task. Because total trials could range from 6 to 12, only the first six error-free trials were included in the analyses. If fewer than six error-free trials were available, the remaining trials were selected from the error trials with the exclusion of the first trial (to eliminate error due to unfamiliarity with task procedures). Tone and self-paced tapping variability were defined by the standard deviation of the ITI. In addition, the within-subject coefficients of variation CV were computed to examine differences in the relative dispersion of the ITIs between patients and non-psychiatric participants. Performance differences were assessed using repeated-measure ANOVAs, with a within-subjects factor of Condition (dominant index/alternating thumbs) and a between-subjects factor of Group (schizophrenia/non-psychiatric control).
Wing-Kristofferson Mathematical Model
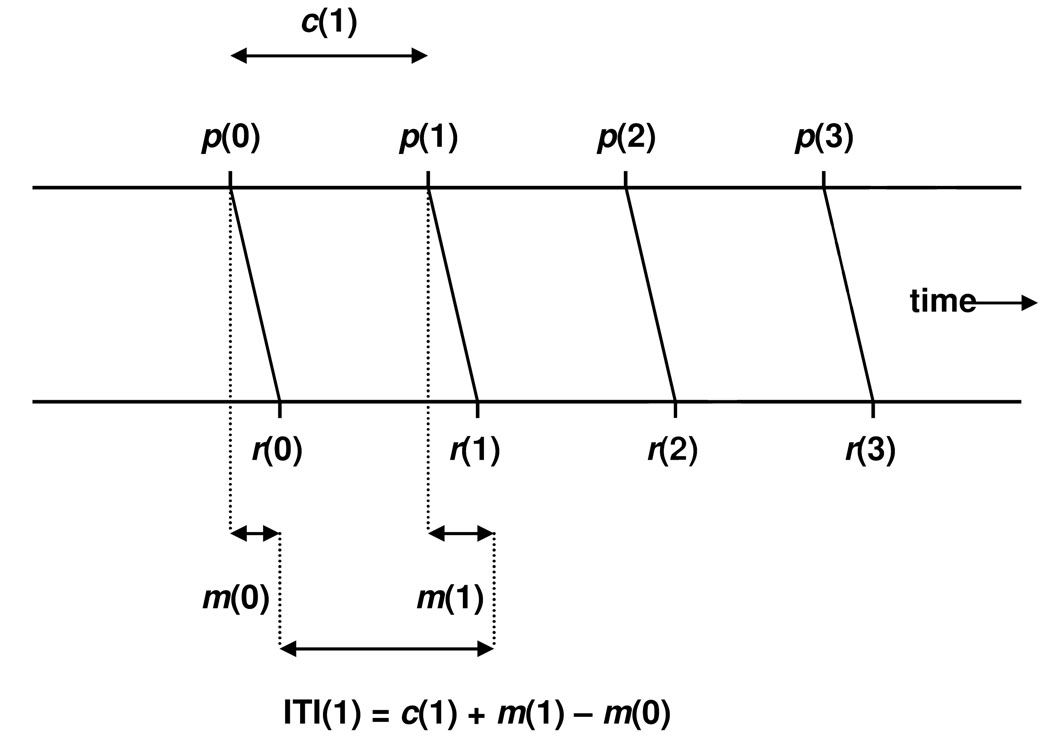
The Wing-Kristofferson model (1973a, 1973b) explains self-paced finger tapping variance with respect to Poisson-variable “ticks” of an internal timekeeper. Based on the observation that self-paced ITIs exhibit a clock-like periodic process, Wing and Kristofferson (1973a, 1973b) proposed a two-process model that predicts independent clock and motor sources of variance. A graphical depiction of this model is shown in Figure 1.
Figure 1.
Graphical depiction of the Wing-Kristofferson model (adapted from Wing & Kristofferson, 1973a, 1973b). p denotes a clock pulse, and c(1) is the clock interval between p(0) and p(1). r denotes a response, and m(0) and m(1) are motor delays between clock pulses and behavioral responses. ITI(1) represents the inter-tap interval between r(0) and r(1).
A pacemaker process emits a series of pulses, p(i), that delineate the clock intervals, c(i), which are encoded from the pacing signal during the tone-paced portion of the task. If the pacing interval is represented as a normal distribution of remembered time, the average value of the ci′s can be interpreted as the participant’s internal representation of the critical ITI. Each pulse initiates a tap, r(i), that is subject to a variable motor delay, m(i), prior to the occurrence of the observable response. Thus, the model computes time between two successive taps, ITI(i), as the clock interval adjusted to account for the preceding and current motor delays,
| (1) |
As can be noted in Equation 1, a given motor delay will have opposite effects on adjacent ITIs. The model therefore assumes statistical dependence between adjacent ITIs, and predicts that successive ITIs will be negatively correlated within a sequence of self-paced taps. Hence, a shorter-than-average ITI should be balanced by a longer-than-average ITI on the subsequent response, producing an oscillating sequence of ITIs about the average (Wing, 1980). Model assumptions predict correlational values between −0.05 and 0.0, where the magnitude of the correlation is a function of the relative contributions of clock and motor variance. Thus, correlations can range from perfect timekeeper periodicity (r = −.05; i.e., all ITI variability is accounted for by motor variance) to highly irregular clock pulses (r = 0.0).
The model further assumes independence between the clock intervals and motor delays, with ITI variability computed as the summation of timer and motor variance:
| (2) |
An estimate of Var(m) is obtained by calculating the lag-one autocorrelation between adjacent ITIs:
| (3) |
Estimating the ITI variance from the data, Var(c) can be obtained from Equation 2 and Equation 3.
Implementation and analysis
The Wing-Kristofferson model estimates clock and motor variance from the self-paced portion of continuation tapping, as tone-paced tapping is viewed only to provide participants with an internal representation of the response rate (Wing, 1980). Therefore, clock and motor variance were determined for each participant from the 30 self-paced trials in each block. Mean variance was then computed for clock and motor components across the six testing blocks and used in the subsequent analyses. This procedure was conducted separately for the dominant index and alternating thumb conditions. Repeated-measures ANOVAs were performed on the variance estimates, with Group (schizophrenia/non-psychiatric) as a between-participant factor and Condition (dominant index, alternating thumbs) as a within-participant factor. In addition, conformity of the data to model assumptions was assessed by computing the lag-one autocorrelation across adjacent ITIs for each participant and condition.
Planned Correlations
Theoretical considerations
Pearson product-moment correlations were performed between behavioral and model estimates of temporal precision to assess the correspondence between theoretical accounts of temporal production and actual timing behavior. Specifically, clock and motor variance estimates from the Wing-Kristofferson model were correlated with standard deviation (SD) and coefficient of variation (CV) measures of inter-response variability from self-paced tapping, with a Bonferroni correction applied for multiple comparisons.
Symptom and cognitive correlates
Pearson product-moment correlation coefficients were computed between PANSS symptom ratings and behavioral timing indices from the repetitive finger-taping task. Due to the significantly lower scores observed in the schizophrenia group on estimated intellectual functioning and digit span, patients’ scores from the WASI and digit span subtest of the WAIS were also correlated with behavioral timing indices to assess for any relationship between temporal processing and intellectual ability or working memory functioning, respectively. A Bonferroni correction for multiple comparisons was applied to all analyses.
Outlier Considerations
A within-group boxplot method of outlier identification was performed separately for each analysis to classify extreme cases, defined as data values more than six quartiles from the upper or lower ends of the interquartile range (Carroll et al., 2008). Extreme cases were identified for the following variables: tone-paced mean ITI (4 participants with schizophrenia, 3 non-psychiatric participants), tone-paced CV (1 non-psychiatric participant), self-paced standard deviation (1 non-psychiatric participant), and self-paced CV (1 non-psychiatric participant).
Results of the major dependent variables are reported with their corresponding partial eta2 [ηp2] effect sizes, where small effect sizes are less than .06, moderate effect sizes range from .06 to .14, and large effect sizes are greater than .14 (Cohen, 1973).
Results
Tone-Paced Tapping
Inter-tap interval
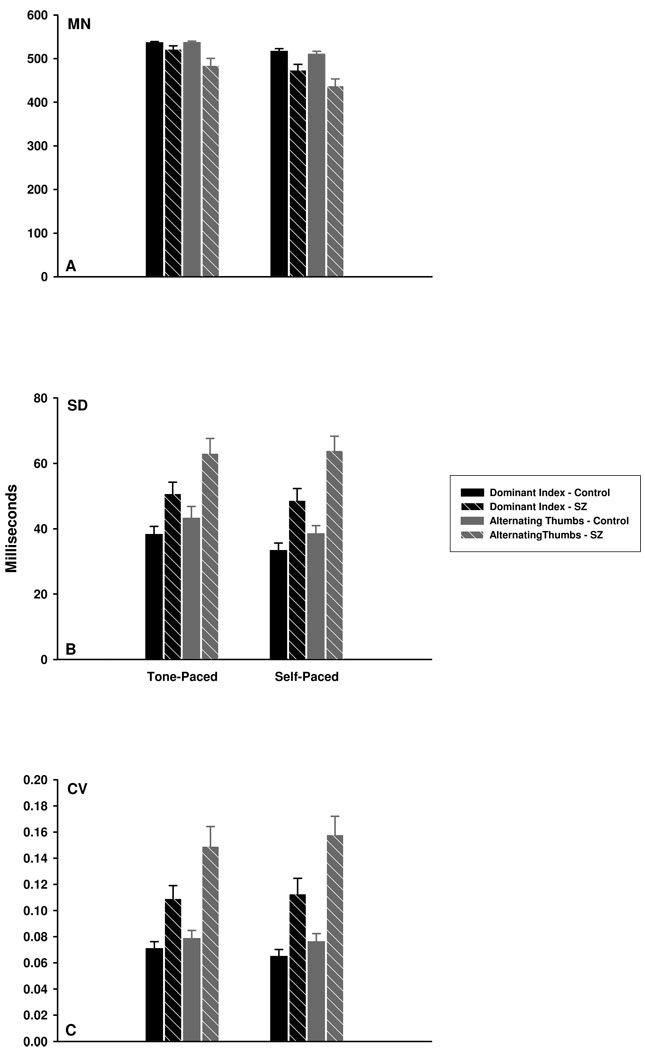
The mean inter-tap intervals (ITIs) produced by the schizophrenia and non-psychiatric groups are illustrated in Figure 2a. A main effect of group supports the observation that individuals diagnosed with schizophrenia produced significantly shorter ITIs than non-psychiatric participants across both index finger and alternating thumb conditions, F(1,54) = 9.99, ηp2 = 0.16, p = .003. The mode of tapping was also significant, with ITIs significantly shorter in the alternating thumb condition, F(1,54) = 7.54, ηp2 = 0.12, p = .008. In addition, a significant Group x Condition interaction revealed a larger group difference when tapping with alternating thumbs, F(1,54) = 7.89, ηp2 = 0.13, p = .007. This interaction was further explored post-hoc using independent-samples t-tests, which indicated significant group differences for alternating thumbs, t(54) = 3.25, p = .002, but only marginally significant differences for the index finger condition, t(54) = 1.99, p = .052.
Figure 2.
Mean (MN), standard deviation (SD), and coefficient of variation (CV) indices of tone-and self-paced tapping for the schizophrenia (SZ) and control groups. (A): For both tone- and self-paced tapping, individuals with schizophrenia produced significantly shorter mean ITIs than control participants under both dominant index and alternating thumb conditions. (B) and (C): For both tone- and self-paced tapping, individuals with schizophrenia showed greater tapping variability (as measured by SD and CV indices) than control participants. All participants demonstrated significantly greater tapping variability during the alternating thumbs compared to the dominant index finger condition.
Tapping variability
Variability of the ITIs was assessed using both standard deviation and coefficient of variation (CV) measures (see Figures 2b and 2c, respectively). For the standard deviation, main effects of group, F(1,61) = 12.21, ηp2 = 0.17, p = .001, and condition, F(1,61) = 16.35, ηp2 = 0.21, p < .001, were significant with no significant interaction. Specifically, individuals with schizophrenia showed greater tapping variability than non-psychiatric participants, and standard deviations were greater for the alternating thumb compared to the index finger condition.
This pattern of results was replicated for the CV, with significantly greater ITI variability found for individuals diagnosed with schizophrenia than non-psychiatric participants, F(1,60) = 16.92, ηp2 = 0.22, p < .001, and significantly larger CVs for alternating thumb versus index finger tapping, F(1,60) = 17.90, ηp2 = 0.23, p < .001. However, the Group x Condition interaction was also significant, F(1,60) = 8.30, ηp2 = 0.12, p = .005, indicating larger group differences using alternating thumbs (see Figure 2c). Self-Paced Tapping
Inter-tap interval
Similar to tone-paced tapping, significantly shorter ITIs were found for individuals with schizophrenia than non-psychiatric participants, F(1,61) = 15.99, ηp2 = 0.21, p < .001 (see Figure 2a). Inter-tap intervals were also significantly shorter when tapping with alternating thumbs compared to the index finger, F(1,61) = 11.13, ηp2 = 0.15, p = .001. Although a significant Group x Condition interaction indicated that group differences were larger in the alternating thumbs condition, F(1,61) = 5.29, ηp2 = 0.08, p = .025, post-hoc independent-samples t-tests revealed significant group differences for both index finger, t(61) = 2.97, p = .004, and alternating thumb, t(61) = 4.30, p < .001, tapping.
Tapping variability
Using the standard deviation of the ITI, tapping variability was significantly larger in the schizophrenia sample, F(1,60) = 23.52, ηp2 = 0.28, p < .001 (see Figure 2b). A main effect of condition further revealed greater variability when tapping with alternating thumbs than with the index finger, F(1,60) = 20.92, ηp2 = 0.26, p < .001, and a significant Group x Condition interaction indicated larger group differences in the alternating thumbs condition, F(1,60) = 5.13, partial η2 = 0.08, p = .027. Consistent with the standard deviation, examination of the CV revealed significant effects of group, F(1,60) = 23.36, ηp2 = 0.28, p < .001, condition, F(1,60) = 28.31, ηp2 = 0.32, p < .001, and a significant Group x Condition interaction, F(1,60) = 10.29, ηp2 = 0.15, p = .002 (see Figure 2c).
Wing-Kristofferson Mathematical Model
Inspection of the data distributions revealed differences between the schizophrenia and non-psychiatric groups, with individuals with schizophrenia displaying exceptionally large standard deviations. To stabilize the sample variance, a natural log transformation was applied to the clock and motor variance data. Table 1 displays the raw and transformed data corresponding to clock and motor implementation variance. The reported analyses were performed on the log-transformed scores.
Table 1.
Clock and Motor Implementation Variance Estimated from the Wing-Kristofferson Model for Non-Psychiatric Control Participants and Patients with Schizophrenia (SZ)
| Clock Variance | ||||||||
|---|---|---|---|---|---|---|---|---|
| Dominant Index Finger | Alternating Thumbs | |||||||
| Raw | Natural Log | Raw | Natural Log | |||||
| Group | M | SD | M | SD | M | SD | M | SD |
| Control | 1340.69 | 4531.41 | 6.14 | 1.15 | 1191.88 | 3294.11 | 6.14 | 1.27 |
| SZ | 1414.32 | 2101.47 | 6.79 | 1.03 | 3508.70 | 9232.12 | 7.32 | 1.79 |
| Motor Variance | ||||||||
| Dominant Index Finger | Alternating Thumbs | |||||||
| Raw | Natural Log | Raw | Natural Log | |||||
| Group | M | SD | M | SD | M | SD | M | SD |
| Control | 103.36 | 152.37 | 4.83 | 0.79 | 218.42 | 908.23 | 5.73 | 0.83 |
| SZ | 505.34 | 3122.39 | 4.91 | 1.83 | 1944.09 | 5002.05 | 6.68 | 1.44 |
Partitioning self-paced variability into clock and motor implementation variance revealed significantly greater clock variance in the schizophrenia compared to the non-psychiatric group, F(1,61) = 9.25, ηp 2 = 0.15, p = .004. No significant effect of condition or Group x Condition interaction was found. Analysis of motor implementation variance indicated no significant group differences, but a significant effect of condition, F(1,61) = 27.66, partial η2 = 0.44, p < .001, with motor variance greater for alternating thumbs. The Group x Condition interaction was not statistically significant.
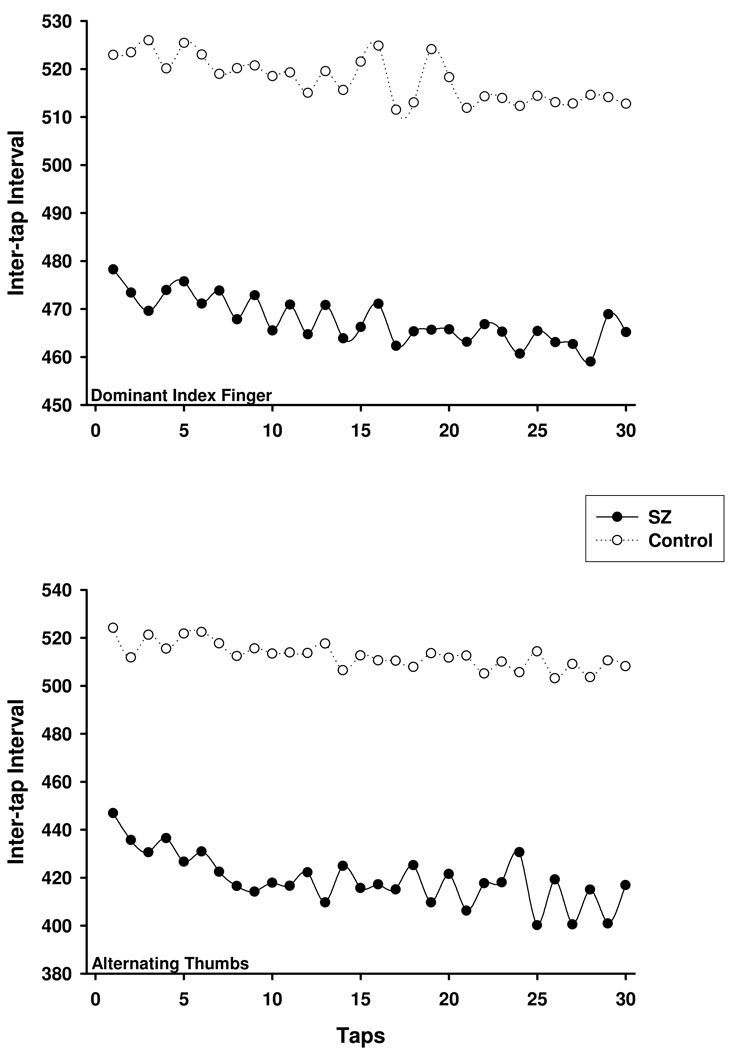
To test the Wing-Kristofferson prediction that adjacent ITIs are negatively correlated between zero and minus one-half, the lag-one autocorrelation was computed separately for the dominant index finger and alternating thumb conditions. As can be noted in Figure 3, the response intervals across the 30 self-paced taps do not appear as unstructured sequences. Rather, the series of ITIs appear to conform to the clock-like periodic process observed by Wing and Kristofferson (1973a, 1973b). In accord with this observation, mean autocorrelations for the non-psychiatric control group were −0.14 (SD = 0.15) and −0.24 (SD = 0.15) for the dominant index finger and alternating thumb conditions, respectively. These correlations correspond to the clock and motor variance estimates listed in Table 1, such that an autocorrelation of −0.14 implies less motor variance for dominant index finger tapping than tapping with alternating thumbs (M = −0.24).
Figure 3.
Mean sequence of inter-tap intervals (ITIs) across a sequence of 30 self-paced taps in the dominant index (top) and alternating thumb (bottom) conditions using a standard interstimulus interval equal to 500 ms. SZ = schizophrenia group.
The mean autocorrelation computed for dominant index finger tapping for the schizophrenia group indicated a predominance of clock-related variance (M = 0.01, SD = 0.22). Motor variability had a larger impact for alternating thumbs (M = −0.28, SD = 0.29), and is in accord with larger motor variance estimates for the schizophrenia group in the alternating thumbs, compared to the dominant index finger, condition.
Planned Correlations
Theoretical considerations
To assess the relationship between behavioral and model estimates of temporal precision, SD and CV indices of ITI variability from self-paced tapping were correlated with the log-transformed clock and motor variance estimates from the Wing-Kristofferson model (see Table 2). For dominant index finger tapping, clock variance significantly correlated with both SD and CV measures in the schizophrenia and non-psychiatric groups. Whereas motor variance was also found to correlate significantly with SD and CV measures for non-psychiatric patients, a similar relationship was not observed for individuals with schizophrenia. In the alternating thumbs condition, SD and CV measures of tapping variability for individuals with schizophrenia and non-psychiatric participants significantly correlated with both clock and motor variance estimates from the Wing-Kristofferson model.
Table 2.
Pearson Product-Moment Correlation Coefficients Between SD and CV Indices of Temporal Variance from the Self-Paced Tapping Condition and Clock and Motor Variance Estimates from the Wing-Kristofferson Model.
| Condition | Control | Schizophrenia | ||
|---|---|---|---|---|
| Dominant Index | Clock | Motor | Clock | Motor |
| Self-Paced SD | .596* | .686* | .787* | .045 |
| Self-Paced CV | .552* | .671* | .713* | −.151 |
| Alternating Thumbs | Clock | Motor | Clock | Motor |
| Self-Paced SD | .563* | .532* | .760* | .732* |
| Self-Paced CV | .499* | .521* | .691* | .783* |
p < .01
Symptom and cognitive correlates
No significant correlations were found between symptom ratings from the PANSS and any of the behavioral indices from the repetitive finger-tapping task. In addition, estimated intellectual functioning (WASI) scores and digit span scores did not significantly correlate with any of the behavioral timing variables, suggesting that the group differences observed in mean ITIs and tapping variability cannot be attributed to lower intellectual ability or poorer working memory functioning in the schizophrenia group.
Discussion
The primary aim of the present study was to help delineate timing deficits in schizophrenia related to the explicit representation of time from those associated with a coordinated motor activity using a repetitive tapping task. For both tone- and self-paced portions of the task, individuals diagnosed with schizophrenia demonstrated shorter ITIs, or faster tapping rates, compared to non-psychiatric participants. Inter-tap intervals were also significantly shorter when tapping with alternating thumbs than with the dominant index finger for both schizophrenia and non-psychiatric groups across the task. Individuals diagnosed with schizophrenia also displayed greater tapping variability during both tone- and self-paced portions of the task, with variance largest during the alternating thumbs condition. The application of the Wing-Kristofferson model to the self-paced tapping data indicated that group differences were primarily attributable to increased clock variability in the schizophrenia group. In contrast, individuals with schizophrenia and non-psychiatric participants did not differ on estimates of motor implementation variance, suggesting that faster tapping rates in the schizophrenia group were due to central as opposed to peripheral factors. These findings are discussed in detail below.
Repetitive tapping has been used to study temporal movement in a variety of motor disorders, as the single repetitive movement of tapping greatly minimizes processing demands imposed by spatial and quantitative aspects of motor control (Wing, 1980). However, the present research represents the first study to date to report on repetitive tapping in schizophrenia. In addition to single digit tapping, alternating thumb tapping was employed to assess the effects of increased motor coordination on the timing of movement.
During the tone-paced portion of the repetitive tapping task, individuals diagnosed with schizophrenia produced shorter ITIs during alternating thumb tapping than non-psychiatric participants. Shorter ITIs in the schizophrenia group were primarily confined to alternating thumbs, suggesting that the temporal aspects of tone-paced tapping are largely related to motor coordination and motor synchrony. For instance, bimanual tapping requires a more complicated motor plan than tapping with a single digit. It has also been suggested that bimanual tapping involves the coordination between a left and right timekeeping system (Wing, 2002), such that each thumb taps every 1000 ms (i.e., every other 500 ms interval). It is therefore possible that the increased temporal coordination required for bimanual tapping resulted in over-anticipation of the pacing signal in the schizophrenia group, such that motor processes were already initiated prior to the presentation of the tone.
The increased motor coordination and movement timing required for bimanual tapping was also reflected in greater tapping variability observed across all participants in the alternating thumbs condition. Group differences in tapping variance were also largest for alternating thumbs, though individuals with schizophrenia showed greater overall tapping variability compared to the non-psychiatric group across both thumb and index finger conditions. Taken together, results from the tone-paced portion of the continuation tapping task indicate deficits of temporal movement in schizophrenia, with impairments greatest for tasks requiring a coordinated motor program. The temporal role of the cerebellum in motor control implicates disturbances of the cerebellar circuitry in the schizophrenia group, which is consistent with findings of dysfunctional neural integration within the cortical-cerebellar circuit in schizophrenia (Andreasen, 1999).
In contrast to tone-paced tapping, the self-paced portion of the continuation task has been associated with activity in regions of the cerebellum and basal ganglia, capturing both movement and representational timing requirements of the task (Rao et al., 1997). Patients’ self-paced ITIs were significantly faster than those observed for non-psychiatric participants in both dominant index and alternating thumb conditions. Underproductions and overproductions on tapping tasks have commonly been interpreted to reflect increased and decreased clock speed, respectively (Pastor et al., 1992). Hence, the faster tapping rates in the schizophrenia group are consistent with evidence for a faster “internal clock” in schizophrenia (Densen, 1977; Johnson & Petzel, 1971; Lhamon & Goldstone, 1956; Tysk, 1983a, 1983b, 1990; Wahl & Sieg, 1990) and previous reports of underproductions found for individuals with schizophrenia using other time production tasks (Tysk, 1983a, 1983b).
Similar to the tone-paced task, greater tapping variability during self-paced tapping was observed for individuals with schizophrenia compared to the non-psychiatric participants, with group differences largest for alternating thumbs. The Wing-Kristofferson model was applied to the self-paced data to determine whether increased tapping variance in individuals diagnosed with schizophrenia should be attributed to clock or motor implementation factors. Consistent with the temporal representation demands of the self-paced task, clock variability was significantly higher in the schizophrenia group. Estimates of motor implementation variance did not differ between individuals with schizophrenia and non-psychiatric participants, though motor variability was significantly greater for alternating thumbs across all participants.
The partitioning of variance into clock and motor factors suggests that group differences in tapping variability were largely due to decreased temporal precision of the internal clock for individuals with schizophrenia. The absence of motor implementation differences between groups further indicates that variance due to the temporal representational aspects of self-paced tapping may have minimized the influence of the temporal motor differences observed for the tone-paced task. Given the role of the basal ganglia in temporal representations (Harrington et al., 1998; Matell & Meck, 2000; Rao et al., 2001; Voltz et al., 2001) and the preferential activation of striatal regions shown during the self-paced portion of the task (Rao et al., 1997), results from the Wing-Kristofferson model suggest that group differences in tapping variability may be primarily attributable to basal ganglion timing processes. Despite evidence for timing deficits specific to regions of the basal ganglia in schizophrenia during self-paced tapping, involvement of the cerebellum cannot be completely discounted. Increasing evidence points to the interconnectivity of the basal ganglian and cerebellar structures (Centonze et al., 2008; Hoshi et al., 2005; O’Reilly et al., 2008; Rossi et al., 2008), and suggests that striatal and cerebellar regions may work together during motor learning (Rossi et al., 2008). Thus, although the Wing-Kristofferson model points to increased clock as opposed to motor implementation variance in the schizophrenia group, the fact that the repetitive tapping task requires acquisition of a temporal motor program makes it impossible to rule out the influence of the cerebellum.
Consistent with results of the Wing-Kristofferson model, autocorrelational values for dominant index finger tapping in the schizophrenia group approached zero, indicating a predominance of clock-related variance. Accordingly, only estimates of clock variance were found to correlate significantly with dominant index variability for individuals with schizophrenia. In contrast, autocorrelational values for non-psychiatric participants implicated both clock and motor variance in dominant index variability, corresponding to the observed correlations between tapping variability and both clock and motor variance estimates. For alternating thumbs tapping, autocorrelations indicated a similar contribution of clock and motor implementation processes to tapping variability for both individuals with schizophrenia and non-psychiatric participants. Thus, although estimates of motor variance did not differ between groups, the contribution of motor implementation factors to overall tapping variability is consistent with the increased motor coordination and movement timing that is required for bimanual tapping.
Of interest is the absence of a relationship between chlorpromazine equivalents computed for individuals with schizophrenia and any of the timing variables. Antipsychotic medications typically have a high affinity for dopamine receptors, altering chemical activity in both cortical and striatal regions (Seeman, 1993). Accordingly, dopaminergic effects on temporal behavior are thought to be associated with changes in the effective level of striatal dopamine, as lesions of the substantia nigra pars compacta (SNPC) have been shown to disrupt performance on temporal discrimination tasks, which is restored following the administration of the dopamine metabolic precursor, L-dopa (Hinton & Meck, 1997). In addition, Parkinson’s disease, which produces neurodegeneration of dopamine cell bodies in the SNPC, has repeatedly been linked to the overestimation of time intervals (Pastor et al., 1992; Lange et al., 1995; Pastor & Artieda, 1996; Malapani et al., 1998) and increased variability on the repetitive finger tapping task (Harrington et al., 1998), deficits that have all been fully or partially alleviated by L-dopa medication.
The neuromodulatory role of dopamine on temporal processing has been further supported by findings from time estimation tasks with both animals and humans, where dopamine agonists have been linked to overestimations of temporal intervals, suggesting a “speeding up” the internal clock (Maricq et al., 1981; Meck 1983, 1996; Frederick & Allan, 1996). In contrast, dopamine antagonists have been found to produce underestimations of temporal durations, where decreased time estimation was proportional to the affinity of the dopamine antagonists for the dopamine D2 sub-receptor (Meck, 1986; Rammsayer, 1989, 1997; Frederick & Allan, 1996).
Given the modulatory influence that dopamine has been shown to have on temporal processes, it might be expected that greater antagonism of the dopamine receptor would be related to decreased tapping rates, or a “slowed” internal clock. This relationship was not evident in the present study, however, as no correlations were found between chlorpromazine equivalent doses of antipsychotic medications and tapping rates for the schizophrenia group. The lack of relationship could be due to a number of factors, including the fact that 14 of the 21 individuals with schizophrenia taking antipsychotic medications were receiving atypical medications, which have a larger affinity for multiple receptors and various neurotransmitter sites (Rogers & Goldsmith, 2009). Furthermore, the absence of a standardized method of antipsychotic drug comparison makes it difficult to reliably compare multiple medications across a patient sample (Nose et al., 2008; Rijcken et al., 2003). Thus, to better assess the relationship between dopamine and repetitive tapping indexes of timing in schizophrenia, it will be important for future studies to include larger samples of individuals receiving equivalent medications and dosages.
The present research represents the first study to date to apply the repetitive tapping procedure and the Wing-Kristofferson model to the study of timing in schizophrenia. Taken together, findings from the repetitive tapping task suggest that motor-related timing processes mediated group differences observed in tone-paced tapping, whereas perceptual timing functions may have largely contributed to many of the group differences found for the self-paced portion of the task. Though similar increases in tapping variability were found for individuals with schizophrenia during both tone-and self-paced tapping, the application of the Wing-Kristofferson model to self-paced tapping allowed for the differentiation of overall variance into estimates of clock and motor implementation processes. The application of quantitative models to the study of temporal behavior provides a necessary means of formally assessing different sources of variance on timing performance. The ability to isolate specific forms of variance is especially significant in the study of schizophrenia given the broad range of impairments associated with the disorder. Through the use of quantitative models, timing deficits across different tasks can be more clearly defined in terms of distinct processes, which may allow for the identification of task-specific impairments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: Author; 1994. [Google Scholar]
- Andreasen NC. A unitary model of schizophrenia. Bleuler’s “fragmented phrene” as schizencephaly. Archives of General Psychiatry. 1999;56:781–787. doi: 10.1001/archpsyc.56.9.781. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Paradiso S, O’Leary DS. “Cognitive dysmetria” as an integrative theory of schizophrenia: A dysfunction in cortical-subcortical-cerebellar circuitry? Schizophrenia Bulletin. 1998;24:203–218. doi: 10.1093/oxfordjournals.schbul.a033321. [DOI] [PubMed] [Google Scholar]
- Bell MD, Lysaker PH, Beam-Goulet JL, Milstein RM, Lindenmayer JP. Five-component model of schizophrenia: Assessing the factorial invariance of the positive and negative syndrome scale. Psychiatry Research. 1994a;52:295–303. doi: 10.1016/0165-1781(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Bell MD, Lysaker PH, Milstein RM, Beam-Goulet JL. Concurrent validity of the cognitive component of schizophrenia: relationship of PANSS scores to neuropsychological assessments. Psychiatry Research. 1994b;54:51–58. doi: 10.1016/0165-1781(94)90064-7. [DOI] [PubMed] [Google Scholar]
- Bressler SL. Cortical coordination dynamics and the disorganization syndrome in schizophrenia. Neuropsychopharmacology. 2003;28(Suppl 1):35–39. doi: 10.1038/sj.npp.1300145. [DOI] [PubMed] [Google Scholar]
- Brown SM, Kieffaber PD, Vohs JL, Carroll CA, Tracy JA, Shekhar A, O’Donnell BF, Steinmetz JE, Hetrick WP. Eye-blink conditioning deficits indicate timing and cerebellar abnormalities in schizophrenia. Brain and Cognition. 2005 doi: 10.1016/j.bandc.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Carroll CA, Boggs J, O’Donnell BF, Shekhar A, Hetrick WP. Temporal processing dysfunction in schizophrenia. Brain and Cognition. 2008;67:150–161. doi: 10.1016/j.bandc.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centonze D, Rossi S, De Bartolo P, De Chiara V, Foti F, Musella A, Mataluni G, Rossi S, Bernardi G, Koch G, Petrosini L. Adaptations of glutamatergic synapses in the striatum contribute to recovery from cerebellar damage. European Journal of Neuroscience. 2008;27:2188–2196. doi: 10.1111/j.1460-9568.2008.06182.x. [DOI] [PubMed] [Google Scholar]
- Cohen J. Eta-Squared and and Partial Eta-Squared in Communication Science. Human Communication Research. 1973;28:473–490. [Google Scholar]
- Del Olmo MF, Cheeran B, Koch G, Rothwell JC. Role of the cerebellum in externally paced rhythmic finger movements. Journal of Neurophysiology. 2007;98:145–152. doi: 10.1152/jn.01088.2006. [DOI] [PubMed] [Google Scholar]
- Densen ME. Time perception in schizophrenia. Perception and Motor Skills. 1977;44:436–438. doi: 10.2466/pms.1977.44.2.436. [DOI] [PubMed] [Google Scholar]
- Elvevåg B, McCormack T, Gilbert A, Brown GDA, Weinberger DR, Goldberg TE. Duration judgments in patients with schizophrenia. Psychological Medicine. 2003;33:1249–1261. doi: 10.1017/s0033291703008122. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for Axis I DSM-IV Disorders (SCID) Washington, DC: Psychiatry Press; 1994. [Google Scholar]
- Frederick DL, Allen JD. Effects of selective dopamine D1- and D2-agonists and antagonists on timing performance in rats. Pharmacology, Biochemistry, and Behavior. 1996;53:759–764. doi: 10.1016/0091-3057(95)02103-5. [DOI] [PubMed] [Google Scholar]
- Freedman BJ. The subjective experience of perceptual and cognitive disturbances in schizophrenia. Archives of General Psychiatry. 1974;30:333–340. doi: 10.1001/archpsyc.1974.01760090047008. [DOI] [PubMed] [Google Scholar]
- Friston KJ. The disconnection hypothesis. Schizophrenia Research. 1998;30:115–125. doi: 10.1016/s0920-9964(97)00140-0. [DOI] [PubMed] [Google Scholar]
- Harrington DL, Haaland KY, Hermanowicz N. Temporal processing in the basal ganglia. Neuropsychologia. 1998;12:3–12. doi: 10.1037//0894-4105.12.1.3. [DOI] [PubMed] [Google Scholar]
- Hinton SC, Meck WH. How time flies: Functional and Neural Mechanisms of Interval Timing. In: Bradshaw CM, Szabadi E, editors. Time and behavior: Psychological and neurobehavioral analyses. New York: Elsevier Science B.V; 1997. [Google Scholar]
- Hofer E, Doby D, Anderer P, Dantendorfer K. Impaired conditional discrimination learning in schizophrenia. Schizophrenia Research. 2001;51:127–136. doi: 10.1016/s0920-9964(00)00118-3. [DOI] [PubMed] [Google Scholar]
- Hoshi E, Tremblay L, Feger J, Carras PL, Strick PL. The cerebellum communicates with the basal ganglia. Nature Neuroscience. 2005;8:1491–1493. doi: 10.1038/nn1544. [DOI] [PubMed] [Google Scholar]
- Ivry RB. Cerebellar involvement in the explicit representation of temporal information. In: Tallal P, Galaburda AM, Linas RR, von Euler C, editors. Annals of the New York Academy of Sciences. Vol. 682. New York: New York Academy of Sciences; 1993. pp. 214–230. [DOI] [PubMed] [Google Scholar]
- Ivry RB, Keele SW. Timing of functions of the cerebellum. Journal of Cognitive Neuroscience. 1989;1:136–152. doi: 10.1162/jocn.1989.1.2.136. [DOI] [PubMed] [Google Scholar]
- Ivry RB, Keele SW, Diener HC. Dissociation of the lateral and medial cerebellum in movement timing and movement execution. Experimental Brain Research. 1988;73:167–180. doi: 10.1007/BF00279670. [DOI] [PubMed] [Google Scholar]
- Johnson JE, Petzel TP. Temporal orientation and time estimation in chronic schizophrenia. Journal of Clinical Psychology. 1971;27:194–196. doi: 10.1002/1097-4679(197104)27:2<194::aid-jclp2270270210>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophrenia Bulletin. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kay SR, Sevy S. Pyramidical model of schizophrenia. Schizophrenia Bulletin. 1990;16:537–545. doi: 10.1093/schbul/16.3.537. [DOI] [PubMed] [Google Scholar]
- Koch G, Oliveri M, Torriero S, Salerno S, Lo Gerfo M, Caltagirone C. Repetitive TMS of cerebellum interferes with millisecond time processing. Experimental Brain Research. 2007;179:291–299. doi: 10.1007/s00221-006-0791-1. [DOI] [PubMed] [Google Scholar]
- Lange KW, Tucha O, Steup A, Gsell W, Naumann M. Subjective time estimation in Parkinson’s disease. Journal of Neural Transmission. 1995;46 Suppl.:433–438. [PubMed] [Google Scholar]
- Lee K-H, Egleston PN, Brown WH, Gregory AN, Barker AT, Woodruff PWR. The role of the cerebellum in subsecond time perception: Evidence from repetitive transcranial magnetic stimulation. Journal of Cognitive Neuroscience. 2007;19:147–157. doi: 10.1162/jocn.2007.19.1.147. [DOI] [PubMed] [Google Scholar]
- Lhamon WT, Goldstone S. The time sense: Estimation of one second durations by schizophrenic patients. Archives of Neurological Psychiatry. 1956;76:625–629. [PubMed] [Google Scholar]
- Malapani C, Rakitin B, Levy R, Meck WH, Deweer B, Dubois B, Gibbon J. Coupled temporal memories in Parkinson’s disease: A dopamine-related dysfunction. Journal of Cognitive Neuroscience. 1998;10:316–331. doi: 10.1162/089892998562762. [DOI] [PubMed] [Google Scholar]
- Maquet P, Lejeune H, Pouthas V, Bonnet M, Casini L, Macar F, Timsit-Berthier M, Vidal F, Ferrara A, Degueldre C, Quaglia L, Delfiore G, Luxen A, Woods R, Mazziotta JC, Comar D. Brain activation induced by estimation of duration. A PET study. NeuroImage. 1996;3:119–126. doi: 10.1006/nimg.1996.0014. [DOI] [PubMed] [Google Scholar]
- Maricq AV, Roberts S, Church RM. Methamphetamine and time estimation. Journal of Experimental Psychology. 1981;7:18–30. doi: 10.1037//0097-7403.7.1.18. [DOI] [PubMed] [Google Scholar]
- Matell MS, Meck WH. Neuropsychological mechanisms of interval timing behavior. Bioessays. 2000;22:94–103. doi: 10.1002/(SICI)1521-1878(200001)22:1<94::AID-BIES14>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- McGlashan TH, Hoffman RE. Schizophrenia as a disorder of developmentally reduced synaptic connectivity. Archives of General Psychiatry. 2000;57:637–648. doi: 10.1001/archpsyc.57.7.637. [DOI] [PubMed] [Google Scholar]
- Meck WH. Selective adjustment of the speed of internal clock and memory processes. Journal of Experimental Psychology. 1983;9:171–201. [PubMed] [Google Scholar]
- Meck WH. Affinity of the dopamine D2 receptor predicts neuroleptic potency in decreasing the speed of an internal clock. Pharmacology, Biochemistry, & Behavior. 1986;25:1185–1189. doi: 10.1016/0091-3057(86)90109-7. [DOI] [PubMed] [Google Scholar]
- Meck WH. Neuropharmacology of timing and time perception. Cognitive Brain Research. 1996;3:227–242. doi: 10.1016/0926-6410(96)00009-2. [DOI] [PubMed] [Google Scholar]
- Nose M, Tansella M, Thornicroft G, Schene A, Becker T, Veronese A, Leese M, Koeter M, Angermeyer M, Barbui C. Is the Defined Daily Dose system a reliable tool for standardizing antipsychotic dosages? International Clinical Psychopharmacology. 2008;23:287–290. doi: 10.1097/YIC.0b013e328303ac75. [DOI] [PubMed] [Google Scholar]
- O’Reilly JX, Mesulam MM, Nobre AC. The cerebellum predicts the timing of perceptual events. Journal of Neuroscience. 2008;28:2252–2260. doi: 10.1523/JNEUROSCI.2742-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor MA, Artieda J. Involvement of the basal ganglia in timing perceptual and motor tasks. In: Pastor MA, Artieda J, editors. Time, internal clocks and movement. New York: Elsevier Sciences B.V; 1996. pp. 235–255. [Google Scholar]
- Pastor MA, Artieda J, Jahanshahi M, Obeso JA. Time estimation and reproduction is abnormal in Parkinson’s disease. Brain. 1992;115:211–225. doi: 10.1093/brain/115.1.211. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Braff DL. Chaos and schizophrenia : Does the method fit the madness ? Biological Psychiatry. 2003;53:3–11. doi: 10.1016/s0006-3223(02)01701-8. [DOI] [PubMed] [Google Scholar]
- Phillips WA, Silverstein SM. Convergence of biological and psychological perspectives on cognitive coordination in schizophrenia. Behavioral and Brain Sciences. 2003;26:63–135. doi: 10.1017/s0140525x03000025. [DOI] [PubMed] [Google Scholar]
- Rammsayer T. Is there a common dopaminergic basis of time perception and reaction times? Neuropsychobiology. 1989;21:37–42. doi: 10.1159/000118549. [DOI] [PubMed] [Google Scholar]
- Rammsayer TH. Are there dissociable roles of the mesostriatal and mesolimbocortical dopamine systems on temporal information processing in humans? Nuropsychobiology. 1997;35:36–45. doi: 10.1159/000119328. [DOI] [PubMed] [Google Scholar]
- Rao SM, Harrington DL, Haaland KY, Bobholz JA, Cox RW, Binder JR. Distributed neural systems underlying the timing of movements. Journal of Neuroscience. 1997;17:5528–5535. doi: 10.1523/JNEUROSCI.17-14-05528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SM, Mayer AR, Harrington DL. The evolution of brain activation during temporal processing. Neuroscience. 2001;4:317–323. doi: 10.1038/85191. [DOI] [PubMed] [Google Scholar]
- Rijcken C, Monster T, Brouwers J, de Jong-van den Berg L. Chlorpromazine equivalents versus defined daily doses: how to compare antipsychotic drug doses? Journal of Clinical Psychopharmacology. 2003;23:657–659. doi: 10.1097/01.jcp.0000096247.29231.3a. [DOI] [PubMed] [Google Scholar]
- Rogers DP, Goldsmith CA. Treatment of schizophrenia in the 21st century: Beyond the neurotransmitter hypothesis. Expert Review of Neurotherapeutics. 2009;9:47–54. doi: 10.1586/14737175.9.1.47. [DOI] [PubMed] [Google Scholar]
- Rossi S, Mataluni G, De Bartolo P, Prosperetti C, Foti F, De Chiara V, Musella A, Mandolesi L, Bernardi G, Centonze D, Petrosini L. Cerebellar control of cortico-striatal LTD. Restorative Neurology & Neuroscience. 2008;26:475–480. [PubMed] [Google Scholar]
- Sears LL, Andreasen NC, O’Leary DS. Cerebellar functional abnormalities in schizophrenia are suggested by classical eyeblink conditioning. Biological Psychiatry. 1999;48:204–209. doi: 10.1016/s0006-3223(00)00247-x. [DOI] [PubMed] [Google Scholar]
- Seeman P. Schizophrenia as a brain disease: The dopamine receptor story. Archives of Neurology. 1993;50:1093–1095. doi: 10.1001/archneur.1993.00540100078020. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M, First MB. Structured clinical interview for DSM-III-R - Nonpatient edition. Washington, DC: American Psychiatric Press; 1990. SCID-NP, Version 1.0. [Google Scholar]
- Tononi G, Edelman GM. Schizophrenia and the mechanisms of conscious integration. Brain Research – Brain Research Reviews. 2000;31:391–400. doi: 10.1016/s0165-0173(99)00056-9. [DOI] [PubMed] [Google Scholar]
- Tracy JI, Monaco C, McMichael H, Tyson K, Chambliss C, Christensen HL, Celenza MA. Information-processing characteristics of explicit time estimation by patients with schizophrenia and normal controls. Perceptual and Motor Skills. 1998;86:515–526. doi: 10.2466/pms.1998.86.2.515. [DOI] [PubMed] [Google Scholar]
- Tysk L. Time estimation by healthy subjects and schizophrenic patients: A methological study. Perceptual and Motor Skills. 1983a;56:983–988. doi: 10.2466/pms.1983.56.3.983. [DOI] [PubMed] [Google Scholar]
- Tysk L. Estimation of time and the subclassification of schizophrenic disorders. Perceptual and Motor Skills. 1983b;57:911–918. doi: 10.2466/pms.1983.57.3.911. [DOI] [PubMed] [Google Scholar]
- Tysk L. Estimation of time by patients with positive and negative schizophrenia. Perceptual and Motor Skills. 1990;71:826. doi: 10.2466/pms.1990.71.3.826. [DOI] [PubMed] [Google Scholar]
- Volz H-P, Nenadic I, Gaser C, Rammsayer T, Hager F, Sauer H. Time estimation in schizophrenia: An fMRI study at adjusted levels of difficulty. NeuroReport. 2001;12:313–316. doi: 10.1097/00001756-200102120-00026. [DOI] [PubMed] [Google Scholar]
- Wahl OF, Sieg D. Time estimation among schizophrenics. Perception and Motor Skills. 1980;50:535–541. doi: 10.1177/003151258005000232. [DOI] [PubMed] [Google Scholar]
- Wechsler D. New York: Psychological Corporation; 1997. WAIS-III: Administration and Scoring Manuel: Wechsler Adult Intelligence Scale. [Google Scholar]
- Wechsler D. New York: The Psychological Corporation; 1999. Abbreviated Scale of Intelligence. [Google Scholar]
- Wing AM. The long and short of timing in response sequences. In: Stelmach GE, Requin J, editors. Tutorials in motor behavior. North-Holland: Amsterdam; 1980. pp. 469–486. [Google Scholar]
- Wing AM. Voluntary timing and brain function: An information processing approach. Brain & Cognition. 2002;48:7–30. doi: 10.1006/brcg.2001.1301. [DOI] [PubMed] [Google Scholar]
- Wing AM, Kristofferson AB. Response delays and the timing of discrete motor responses. Perception and Psychophysics. 1973b;14:5–12. [Google Scholar]
- Wing AM, Kristofferson AB. The timing of inter-response intervals. Perception and Psychophysics. 1973b;13:455–460. [Google Scholar]