Abstract
The visual pigment rhodopsin is unique among the G protein-coupled receptors in having an 11-cis retinal chromophore covalently bound to the protein through a protonated Schiff base linkage. The chromophore locks the visual receptor in an inactive conformation through specific steric and electrostatic interactions. This efficient inverse agonist is rapidly converted to an agonist, the unprotonated Schiff base of all-trans retinal, upon light activation. Here, we use magic angle spinning NMR spectroscopy to obtain the 13C chemical shifts (C5-C20) of the all-trans retinylidene chromophore and the 15N chemical shift of the Schiff base nitrogen in the active metarhodopsin II intermediate. The retinal chemical shifts are sensitive to the conformation of the chromophore and its molecular interactions within the protein-binding site. Comparison of the retinal chemical shifts in metarhodopsin II with those of retinal model compounds reveals that the Schiff base environment is polar. In particular, the 13C15 and 15Nε chemical shifts indicate that the C=N bond is highly polarized in a manner that would facilitate Schiff base hydrolysis. We show that a strong perturbation of the retinal 13C12 chemical shift observed in rhodopsin is reduced in wild-type metarhodopsin II and in the E181Q mutant of rhodopsin. On the basis of the T1 relaxation time of the retinal 13C18 methyl group and the conjugated retinal 13C5 and 13C8 chemical shifts, we have determined that the conformation of the retinal C6-C7 single bond connecting the β-ionone ring and the retinylidene chain is 6-s-cis in both the inactive and the active states of rhodopsin. These results are discussed within the general framework of ligand-activated G protein-coupled receptors.
1. Introduction
One of the most striking features of the G protein-coupled receptor (GPCR) superfamily is that a relatively simple architecture of seven transmembrane (TM) helices can be adapted to specifically recognize over a thousand different signaling ligands. The visual receptors are unique among GPCRs in that they are activated by the photoisomerization of a covalently bound retinal chromophore rather than by binding of a diffusible ligand.1,2 Nevertheless, the retinal chromophores in the visual pigments can be analyzed in much the same way as the receptor bound ligands in the ligand-activated GPCRs. For example, in the visual receptor rhodopsin, the 11-cis isomer of retinal effectively functions as an inverse agonist by lowering the basal activity of the receptor to undetectable levels3,4 (Figure 1). The 11-cis retinal chromophore is bound as a protonated Schiff base (PSB) in the interior of rhodopsin and locks the receptor off through electrostatic interactions with Glu113, a protein counterion to the PSB,5,6 and through steric interactions with amino acids in the retinal binding pocket. Photoisomerization to the all-trans configuration and deprotonation of the Schiff base (SB) rapidly converts the chromophore from an inverse agonist to a full agonist. Using solid-state 13C and 15N NMR, we report on the structure and environment of the all-trans retinal unprotonated SB chromophore in metarhodopsin II (Meta II), the photoactivated state of the rhodopsin, and describe how the all-trans retinal molecule functions as the agonist for rhodopsin activation.
Figure 1.
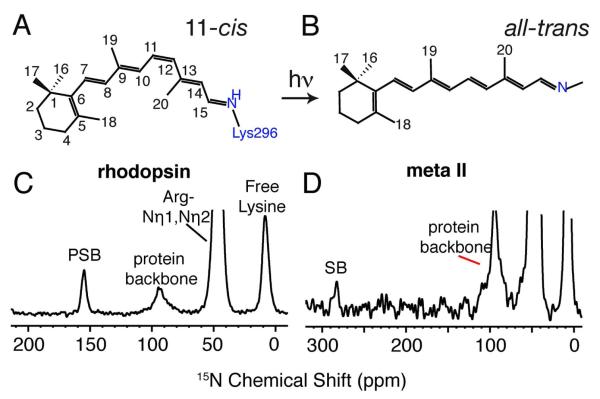
Molecular structures of the 11-cis retinal PSB chromophore in rhodopsin (A) and the all-trans retinal unprotonated SB chromophore in Meta II (B). 15N CP MAS spectra of rhodopsin (C) and Meta II (D) labeled with 15Nε-lysine. The 15Nε-Lys296 is observed as a distinct narrow peak at 155.4 ppm in rhodopsin and shifts ~127 ppm downfield to 282.8 ppm in Meta II. The 15Nε resonances of the free lysines in rhodopsin are observed as a broad peak around 8.7 ppm.
The retinal chromophore in the dark-state of rhodopsin can be conceptually divided into three distinct planes broken by the C6-C7 and the C11=C12-C13 bonds. These planes are twisted relative to one another in order to fit the retinal PSB chromophore into a tight receptor binding site.7 Specific packing interactions between the retinal and protein have two consequences. First, binding of the 11-cis retinal PSB is responsible for lowering the basal activity of the apo-protein opsin. For example, the bound 11-cis retinal restricts the motion of Trp265, a highly conserved aromatic amino acid on TM helix H6 that rotates toward the extracellular surface upon receptor activation.8,9 Second, NMR,7,10 crystallographic11 and computational12 studies argue that the protein binding site imparts a pre-twist about the C11=C12 bond. The ground state twist about the C11=C12 bond is thought to be required for the extremely fast and selective photoreaction to the all-trans conformation of the chromophore.13
Protein-retinal interactions are also responsible for the high quantum yield of the 11-cis to all-trans photoreaction. The quantum yield is controlled in part by Glu113 on TM helix H3, the counterion for the retinal PSB. Interestingly, Glu113 has also recently been shown to be responsible for the high quantum yield in UV-absorbing pigments where the retinal SB is unprotonated,14 suggesting that Schiff base protonation and associated π-electron delocalization are not necessary for maintaining a high quantum yield. A second glutamic acid residue, Glu181 on the second extracellular loop (EL2) is in close proximity to the retinal and may also contribute to the rapid and selective photoisomerization of the C11=C12 double bond. The sidechain of Glu181 is oriented into the retinal binding site with the glutamate carboxyl group near C12 of the retinal. The occurrence of a negative charge directed at C12 was first proposed by absorption measurements using dihydro derivatives of retinal15 and later confirmed by measurement of the retinal 13C NMR chemical shifts.16
The C6-C7 bond divides the plane containing the β-ionone ring from the two planes containing the retinylidene chain. In bacteriorhodopsin, Harbison et al.17 used three independent NMR measurements (the 13C5 chemical shift, the 13C8 chemical shift and the 13C18 T1 relaxation time) to establish a 6-s-trans conformation of the β-ionone ring about the C6-C7 single bond. On the basis of a number of retinal derivatives,17 the T1 relaxation times were observed to be on the order of seconds for C18 methyl groups in a planar 6-s-trans conformation due primarily to the steric interaction between the C18 methyl group and the proton on C7. On the other hand, the T1 relaxation time for the C18 methyl group in a skewed 6-s-cis conformation was found to be much shorter, on the order of milliseconds.
On the basis of 13C chemical shift measurements, we originally assigned the retinal C6-C7 conformation in rhodopsin as 6-s-cis.18 However, the assignment was subsequently challenged by experimental19 and computational20 studies. More recently, several studies have been used to address the C6-C7 single bond conformation.21-24 However, to date the distinctive relaxation measurements described for bacteriorhodopsin have not been repeated on rhodopsin or its photoreaction intermediates.
Much less is known about the conformation of the retinal in the active Meta II state of rhodopsin than in the inactive, dark state of rhodopsin. The crystal structure of a photoactivated intermediate of rhodopsin having an unprotonated Schiff base has been reported.25 However, the diffraction data are of low resolution for both the dark state and the photointermediate making it difficult to establish how the chromophore or the receptor changes structure upon illumination. In contrast, solid-state NMR spectroscopy is well suited for structural studies that target specific regions of membrane proteins in native membrane environments. High-resolution magic angle spinning (MAS) NMR methods have been used to investigate ligand conformation in ligand-activated GPCRs, such as the histamine26 and the neurotensin receptors27, as well as the structure of the retinal-containing membrane proteins.28,29
In this paper, we characterize the retinal 13C chemical shifts in the active Meta II intermediate to address how the all-trans retinal SB functions as a full agonist for receptor activation. Comparison of the Meta II chemical shifts with those of the 11-cis retinal PSB chromophore in rhodopsin and with retinal model compounds suggests that the retinal chromophore is in a polar environment in Meta II. Interestingly, the largest chemical shift differences between the all-trans retinal SB in Meta II and in solution exist at the Schiff base and β-ionone ring ends of the chromophore. The 15Nε resonance of the unprotonated SB in Meta II is further upfield (shielded) and the 13C15 chemical shift is further downfield (deshielded) than in all-trans SB model compounds reflecting a highly polarized C=N bond. Similarly, the 13C5 resonance within the retinal β-ionone ring of Meta II has an unusual upfield chemical shift despite our determination that the C6-C7 bond is in the 6-s-cis conformation in both rhodopsin and Meta II. The 13C NMR chemical shifts of the central portion of the all-trans retinal SB chromophore in Meta II are not dramatically different from those in the retinal model systems. For example, the 13C chemical shifts of the C19 and C20 methyl groups in the dark state of rhodopsin are unique due to the conformation and environment of the protein within a tight retinal-binding cavity. However, in Meta II they are roughly the same as in all-trans SB model compounds in solution. Together these results indicate that protein interactions at the two ends of the all-trans retinal SB chromophore play a role in the activation mechanism of rhodopsin.
2. Methods and Materials
2.1. Expression and purification of 13C-labeled rhodopsin
Opsin was expressed and isotope labeled using stable tetracycline-inducible HEK293S cells30 containing the wild-type opsin gene31 as previously described32. Rhodopsin was regenerated in harvested cells by the addition of 14 mM 11-cis retinal in ethanol.
Rhodopsin was solubilized in 1.0% n-dodecyl-β-D-maltoside (DDM) in phosphate buffered saline at pH 6.0 and purified by affinity chromatography using the 1D4 antibody (National Cell Culture Center, Minneapolis, MN).31 A peptide corresponding to the 9 C-terminal amino acids of rhodopsin in 0.02% DDM and sodium phosphate buffer at pH 6.0 was used as the antibody epitope to elute rhodopsin.
2.2. Synthesis of retinal model compounds
All-trans-N-retinylidene-n-butylimine was prepared by reacting all-trans retinal powder (Sigma-Aldrich) in methanol with an excess of butylimine over 3.0 Å molecular sieves as described by Harbison et al.17 We obtained the natural abundance 13C chemical shifts for all-trans-n-retinylidene-n-butylimine in polar (CD3OD) and non-polar (CDCl3) solvents at 25 °C. These NMR spectra were obtained on a Bruker Avance NMR spectrometer operating at a 1H frequency of 700 MHz.
All-trans retinoic acid crystals in monoclinic and triclinic form were prepared as described by Harbison et al.17 The 13C chemical shifts for the retinoic acid crystals were obtained using a 4 mm MAS probe at a 1H frequency of 600 MHz (see below) and referenced externally to the carbonyl 13C chemical shift for powdered glycine at 176.46 ppm.
2.3. Synthesis of 13C-labeled retinals and regeneration of rhodopsin
Retinals with specific 13C-labels were synthesized by standard methods.33,34 Rhodopsin pigments in DDM micelles were regenerated with HPLC purified 11-cis 13C-labeled retinal by illumination of concentrated samples in the presence of a 2:1 molar ratio of retinal-to-protein.
2.4. Solid-State NMR spectroscopy
Solid-state NMR experiments were performed at a 1H frequency of either 360 or 600 MHz on Bruker AVANCE spectrometers using 4 mm MAS probes containing 1.6 - 4.0 mM rhodopsin. Variable amplitude cross polarization35 was used with a 2 ms contact time and two-pulse phase-modulated36 or SPINAL6437 was used for proton decoupling during the acquisition periods with 85-90 kHz field strengths. The MAS spinning rate was maintained between 8-12 kHz to within ± 5 Hz. The sample temperature was maintained at 190 K for the duration of the experiment.
T1 relaxation measurements for the 13C18 methyl group on the β-ionone ring were performed on retinoic acid crystals (monoclinic and triclinic forms) and retinal in rhodopsin at 190 K using a standard cross-polarization inversion-recovery sequence.17
NMR measurements were first made on rhodopsin in the dark. Subsequently, the samples were illuminated for 45-60 secs at room temperature using a 400 W lamp with a >495 nm cutoff long pass filter. The rotor was then recapped and placed in the NMR probe with the probe stator warmed to 5°C. Under slow spinning (2 kHz), the heater was turned off and the sample was frozen within 3 min using N2 gas cooled to 190 K.
2.5. Chemical shift referencing
All 13C high-resolution NMR and solid-state MAS NMR spectra were externally referenced to the 13C resonance of neat TMS at 0 ppm at room temperature. Using TMS as the external reference, we calibrated the carbonyl resonance of solid glycine at 176.46 ppm. Our chosen reference compound is different from 1% TMS in CDCl3, which is the IUPAC recommended standard.38 This choice of reference compound was based on the fact that appreciable solvent shifts were observed for the 13C resonance of TMS in CDCl3 (+0.68 ppm) and CD3OH (−0.84 ppm). Zilm and coworkers39 have reported similar solvent shifts earlier for TMS in deuterated chloroform and methanol. Additionally, we compare our 13C chemical shifts of the retinal chromophore in rhodopsin and Meta II with previous NMR studies conducted on retinal model compounds40 where the 13C chemical shifts have been referenced externally to neat TMS.
The 15N solid-state MAS NMR spectra collected on rhodopsin and Meta II were referenced to the 15N resonance of 5.6 M aqueous NH4Cl at 0.0 ppm at room temperature by using 15N-labeled glycine as an external reference and the reported value of glycine at 8.1 ppm relative to 5.6 M aqueous NH4Cl.41
3. Results and Discussion
3.1. Characterization of Meta II trapped at low temperature
Meta II is the only intermediate in the rhodopsin photoreaction cascade with an unprotonated Schiff base nitrogen. We first confirmed that in our experiments the all-trans retinal chromophore in Meta II trapped in DDM detergent is bound to Lys296 through an unprotonated SB linkage, and that only a single intermediate is present in the NMR sample. 15Nε-lysine was incorporated into rhodopsin and the 15Nε chemical shifts were obtained of the 11-cis retinal PSB in rhodopsin and the all-trans retinal SB in Meta II using solid-state MAS NMR. In Figure 1C, the 15Nε resonance for the PSB nitrogen in rhodopsin solubilized in DDM is observed at 155.4 ppm, similar to the chemical shift for rhodopsin reconstituted in DOPC lipids.32 In Meta II (Figure 1D), the 15Nε resonance shifts to 282.8 ppm. The resonances at ~8.7 ppm correspond to the other 10 free lysines in the protein. The natural abundance 15N signal from the amide nitrogens is observed as a broad resonance at 95.3 ppm, and the intense peak at ~ 47 ppm results from 15N-labeling of arginine in the same sample.
The large change in the 15N chemical shift of the SB in Meta II is attributed to deprotonation of the PSB upon activation. In bacteriorhodopsin, two resolved M intermediates are observed with 15Nε chemical shifts of 288.6 ppm (Mn) and 296.4 ppm (Mo)42. In contrast, the all-trans-N-retinylidene-n-butylimine SB is observed at 315.3 ppm.40 The upfield chemical shifts of the M states relative to the SB model compound, as well as the upfield shift of the Mn-state of bacteriorhodopsin relative to the Mo-state, were attributed to stronger basicity.42 The 15Nε chemical shift in Meta II is unusual in being even further upfield than in the Mn-state of bacteriorhodopsin. One of the major differences between the rhodopsin and bacteriorhodopsin photoreactions is that the Schiff base linkage in rhodopsin is hydrolyzed in the transition of Meta II to opsin, while in bacteriorhodopsin the Schiff base nitrogen is reprotonated between the M and N photocycle intermediates. As we will discuss below, the chemical shift of the Schiff base nitrogen in Meta II may reflect the polarization of the C=N bond and facilitate hydrolysis, thereby shifting the receptor to the inactive opsin conformation. Hence, the Schiff base 15N chemical shift may reflect a key difference between the linear photoreaction in the visual receptors and the cyclic proton-pumping reaction in bacteriorhodopsin.
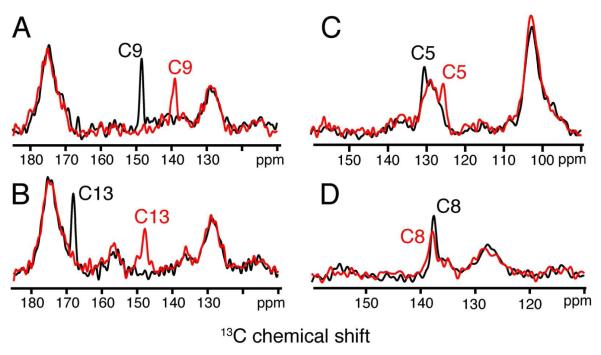
The 13C chemical shifts of the retinal carbons along the polyene chain also provide a way to characterize the ability to trap a homogeneous Meta II intermediate at low temperature. In this case, rhodopsin solubilized in DDM was regenerated with 11-cis retinal selectively 13C labeled within the β-ionone ring and along the conjugated polyene chain. One-dimensional 1H-13C cross polarization (CP) MAS NMR experiments were performed to obtain the chemical shifts of the retinal carbons in rhodopsin and Meta II. Figure 2 presents regions of the 13C CP-MAS spectra for rhodopsin regenerated with retinal 13C labeled at the C5, C8, C9 and C13 carbons along the polyene chain. The 13C MAS spectra of rhodopsin (black) have been overlaid with the MAS spectra of Meta II (red). The quaternary odd numbered carbons (C5, C9 and C13) with attached methyl groups exhibit the largest chemical shift changes of the retinal carbons among those shown in Figure 2.
Figure 2.
Representative one-dimensional 13C MAS NMR spectra of the dark state of rhodopsin (black) and the active Meta II intermediate (red) containing 13C-labeled retinal chromophores. Regions of the 13C spectra from ~110-180 ppm are shown for rhodopsin and Meta II containing retinal 13C-labeled at the C9 (A), C13 (B), C5 (C) and C8 (D) positions. The C5 resonance overlaps the natural abundance signal from the aromatics in the protein.
The retinal C5 and C9 resonances are observed in rhodopsin at 131.0 ppm and 148.9 ppm, respectively, and shift to 126.0 and 139.6 ppm in the Meta II intermediate. The broad resonance at 127.0 ppm is due to the natural abundance 13C signal from the aromatic carbons in the protein. The 13C resonance of C13 at 168.5 ppm in rhodopsin overlaps the broad natural abundance 13C signal from the protein carbonyls centered at ~175 ppm. A 20 ppm change in the 13C13 chemical shift to 148.2 ppm is observed in Meta II. In contrast, the C8 resonance does not shift significantly between rhodopsin and Meta II.
Integration of the 13C resonances shows that we can convert > 85% of rhodopsin to Meta II. The retinal 13C resonances are narrow (1-2 ppm) in both rhodopsin and Meta II, exhibiting no signs of splitting or differential broadening. The chemical shifts are reproducible to within 0.3 ppm. For comparison, the histamine ligand bound to the H1 receptor exhibits broad 13C resonances (4-8 ppm) suggesting conformational heterogeneity.27
Following the thermal decay of Meta II to opsin and free retinal, the 13C retinal resonances broaden considerably and are not observed. The origin of the broadening most likely results from heterogeneous interactions of the retinal with the protein and detergent after hydrolysis of the Schiff base linkage. Together these observations indicate that the conformational heterogeneity of the retinal in rhodopsin and Meta II is similar and limited.
Table 1 lists the 13C chemical shifts for carbons C5-C20 of the 11-cis retinal PSB in rhodopsin solubilized in DDM detergent. The 13C chemical shifts are in agreement within experimental error (0.3-0.4 ppm) with those reported earlier for rhodopsin reconstituted in lipids by de Groot and co-workers.43 The similar chemical shifts (and absorption spectra) indicate that the conformation of the 11-cis retinal PSB in rhodopsin solubilized in DDM is the same as for rhodopsin reconstituted into a lipid environment. In a comprehensive comparison of spin labeled rhodopsin, Hubbell and coworkers44 found that the most notable difference between the DDM and lipid environments is that the receptor has increased flexibility in detergent, which facilitates the conversion of rhodopsin to Meta II.
Table 1.
13C chemical shifts of the retinal carbons in wild-type rhodopsin and comparison with the retinal PSB model compound, N-(11-cis-retinylidene)-n-propyliminium trifluoroacetateA in CDCl3.
| Carbon | Rho (ppm) |
PSBA (ppm) |
△Rho (ppm) |
|---|---|---|---|
| 5 | 131.0 | 132.1 | −1.1 |
| 6 | 137.1 | 137.2 | −0.1 |
| 7 | 132.8 | 132.3 | 0.5 |
| 8 | 139.3 | 137.2 | 2.1 |
| 9 | 148.9 | 147.8 | 1.1 |
| 10 | 128.0 | 126.4 | 1.6 |
| 11 | 141.6 | 138.7 | 2.9 |
| 12 | 132.2 | 128.7 | 3.5 |
| 13 | 168.5 | 165.8 | 2.7 |
| 14 | 122.1 | 120.5 | 1.6 |
| 15 | 165.7 | 163.3 | 2.4 |
| 16 | 30.6 | 28.9 | 1.7 |
| 17 | 26.1 | 28.9 | −2.8 |
| 18 | 21.6 | 22.1 | −0.5 |
| 19 | 14.7 | 12.6 | 2.1 |
| 20 | 16.4 | 18.8 | −2.4 |
Data from Shriver et al.45
3.2. Charge delocalization along the retinal polyene chain in rhodopsin
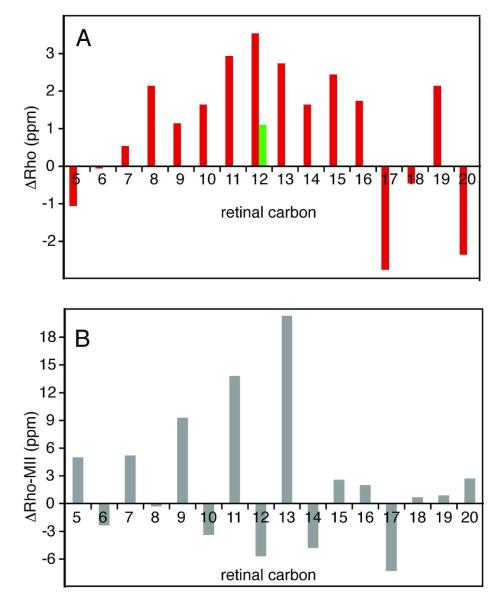
Table 1 compares the 13C chemical shifts of rhodopsin with those of the 11-cis retinal PSB model compound in CDCl3 having a trifluoroacetate counterion (N-(11-cis-retinylidene)propyliminium trifluoroacetate).45 The differences in chemical shifts ( △Rho) listed in Table 1 are shown as a histogram in Figure 3A. △Rho represents the effect of the protein on the conformation and environment of the 11-cis retinal PSB. As noted previously,46 the positive △Rho values observed for the C8-C15 carbons along the retinylidene chain are an unusual feature of the histogram. The most unusual chemical shift in Figure 3A is that observed for C12 since the partial charges on the retinal carbons typically alternate between partial negative on the even number carbons and partial positive on the odd numbered carbons. We previously attributed the large downfield chemical shift at C12 to a specific interaction with a negative charge in the retinal binding site.47 This observation was confirmed by the crystal structure of rhodopsin,48 which revealed that Glu181 on EL2 is oriented toward the retinal with the side chain carboxyl group ~4 Å from C12. Glu181 is one of the residues that distinguish the high-sensitivity rod-cell receptors associated with vision under low light conditions from the human green and red cone pigments where the corresponding residue is a histidine, which forms part of a chloride ion binding site.
Figure 3.
Comparison of retinal 13C chemical shifts in rhodopsin with an 11-cis retinal PSB model compound and with Meta II. (A) Differences △(Rho) are plotted between the retinal chemical shifts for C5-C20 carbons in rhodopsin and in the N-(11-cis-retinylidene)-n-propyliminium trifluoroacetate model compound in solution (red).43 The measurement of the 13C12 chemical shift in the E181Q mutant of rhodopsin is also shown (green bar). (B) Differences △(Rho-MII) are plotted between the retinal chemical shifts for C5-C20 plotted in rhodopsin and Meta II. All chemical shifts are externally referenced to neat TMS.
In order to test whether the unusual chemical shift at C12 is due to a charged Glu181 carboxyl group, we obtained NMR spectra of the E181Q rhodopsin mutant regenerated with 11-cis retinal 13C-labeled at C12. The E181Q mutation results in a reduction of the anomalous △Rho shift from +3.5 ppm to +1.1 ppm (Table 2). The reduction in △Rho confirms that Glu181 is responsible for the unusual shift at C12, which implies that Glu181 is also responsible for the accumulation of excess positive charge along the polyene chain and suggests that Glu181 is charged in rhodopsin. The large change in the C12 chemical shift is consistent with FTIR data that Glu181 is deprotonated in rhodopsin,49 but is in contrast to the lack of a change in the absorption spectra between wild-type rhodopsin and the E181Q mutant.50 The lack of a change of the λmax may reflect the fact that the residue at position 181 is interacting with the middle of the retinal polyene chain and could potentially affect the energy levels of both the ground and the excited electronic states of the retinal in a similar fashion leaving the λmax unperturbed. Hall et al.51 have shown computationally that the S1→ S2 energy level splitting is almost identical for model systems differing in the protonation state of Glu181. The E181Q mutant does not appear to affect the ground state C11=C12-C13 twists in rhodopsin since the hydrogen out-of-plane and fingerprint vibrations in resonance Raman spectra do not change compared with the E181Q mutant. 52
Table 2.
13C chemical shift of the retinal C12 carbon in wild-type rhodopsin and the E181Q mutant of rhodopsin and Meta II.
| Compound | Wild Type (ppm) |
E181Q Mutant (ppm) |
|---|---|---|
| Rhodopsin | 132.2 | 129.8 |
| Meta II | 137.9 | 137.4 |
In Meta II spectra of the E181Q mutant, the 13C12 chemical shift does not exhibit a significant change from its chemical shift in wild-type Meta II (Table 2). While the influence of protein charges on retinal chemical shifts should be much weaker for the unprotonated retinal SB compared to the PSB, the absence of an influence on the C12 chemical shift would be consistent with lack of interaction between C12 and Glu181 in Meta II. Nevertheless, mutation of Glu181 does have a pronounced effect on the visible absorption maximum of Meta II.50 The λmax shifts from 375 nm in the E181F mutant of Meta II to 392 nm in E181Y mutant. Yan et al.50 concluded that the Glu181 — retinal interactions may in fact be more dramatic in Meta II than in the dark state of rhodopsin. In this regard, it is important to note that FTIR studies do not find a difference band associated with Glu181 indicating that the protonation state of the carboxyl side chain does not change relative to rhodopsin.49 That is, the influence of Glu181 on the C12 chemical shift or on the λmax is not due to a change in protonation state. A shift of Glu181 away from C12 would be consistent with the lack of a change in the C12 chemical shift when comparing wild-type Meta II and Meta II in the E181Q mutant. An increase in the distance between Glu181 and the retinal C12 carbon might result from retinal isomerization, translation of the retinal within the binding site or displacement of EL2 from the retinal binding site upon rhodopsin activation.28 Different mutants of Glu181 may influence the position of the retinal and/or EL2 and thereby modulate the electrostatic interactions that regulate the λmax of the retinal SB possibly through alteration of the hydrogen bonding network to the Schiff base nitrogen.53
3.3. Retinal 13C chemical shifts suggest a polar retinal binding site in metarhodopsin II
The isotropic 13C chemical shifts of the retinal chromophore are sensitive to their environment and have been used in the past to map the polarity of the retinal binding site in rhodopsin.43,46 Table 3 lists the 13C chemical shifts of the C5-C20 carbons of the all-trans retinal in Meta II. The plot of the chemical shift differences in Figure 3B between rhodopsin and Meta II indicates a strong alternation of chemical shift from C5 to C15 due to a difference in electron delocalization along the polyene chain.47 The odd numbered carbon resonances are shifted upfield and the even numbered carbon resonances are shifted downfield in Meta II, with the most pronounced shifts being associated with the odd numbered carbons. This pattern reflects the dominant influence that the protonation state of the Schiff base has on electron delocalization. The retinal C16-C20 carbons do not exhibit significant shifts, indicating that they are not affected by charge delocalization along the retinal polyene chain. The major exception is C17, discussed below.
Table 3.
13C chemical shifts of the all-trans retinal SB in Meta II and comparison with the model compound N-all-trans-retinylidene-tert-butylimine in a nonpolar (MNP) and in a polar solvent (MP).
| Carbon | Meta II (ppm) |
SBA CDCl3 (ppm) |
MNP CDCl3 (ppm) |
MP CD3OH (ppm) |
△MIINP (ppm) |
△MIIP (ppm) |
|---|---|---|---|---|---|---|
| 5 | 126.0 | 129.9 | 130.4 | 129.3 | −4.3 | −3.3 |
| 6 | 139.5 | 137.4 | 138.5 | 138.2 | 1.0 | 1.3 |
| 7 | 127.6 | 127.4 | 128.5 | 128.2 | −0.8 | −0.6 |
| 8 | 139.6 | 137.3 | 138.2 | 138.2 | 1.5 | 1.4 |
| 9 | 139.6 | 138.0 | 138.6 | 138.2 | 1.0 | 1.4 |
| 10 | 131.4 | 129.8 | 130.7 | 130.5 | 0.8 | 1.0 |
| 11 | 127.8 | 127.8 | 128.5 | 128.2 | −0.6 | −0.4 |
| 12 | 137.9 | 135.8 | 136.8 | 136.1 | 1.2 | 1.9 |
| 13 | 148.2 | 144.4 | 144.6 | 146.9 | 3.7 | 1.4 |
| 14 | 126.9 | 129.0 | 130.2 | 129.3 | −3.2 | −2.4 |
| 15 | 163.1 | 159.7 | 160.0 | 161.5 | 3.2 | 1.7 |
| 16 | 28.6 | 28.8 | 29.7 | 28.7 | −1.0 | 0.0 |
| 17 | 33.4 | 28.8 | 29.7 | 28.7 | 3.8 | 4.8 |
| 18 | 20.9 | 21.9 | 22.4 | 21.2 | −1.4 | −0.2 |
| 19 | 13.8 | 12.9 | 13.5 | 12.0 | 0.4 | 1.9 |
| 20 | 13.7 | 13.1 | 13.7 | 12.2 | 0.1 | 1.5 |
Data from Shriver et al.45
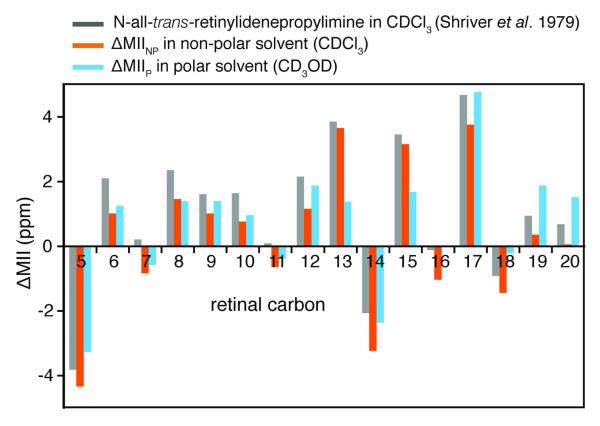
Table 3 and Figure 4 (orange) present the chemical shift differences ( △MIINP) between Meta II and the all-trans-N-retinylidene-n-butylimine model compound in a nonpolar solvent (CDCl3). Since the retinal SB is unprotonated in both Meta II and the all-trans retinal butylimine model compound, one would expect smaller differences than for △Rho. However, significant differences are seen at retinal carbons C5, C13, C14, C15 and C17. In order to account for the effect of the choice of solvent on the model compound spectra, we compared the chemical shifts of Meta II with an all-trans-N-retinylidene-n-butylimine model compound in a polar solvent (CD3OD) (Figure 4, blue). The downfield shifts for the retinal resonances along the polyene chain (C13, C14 and C15) are reduced, suggesting that the binding cavity near the SB end of the retinal is polar in Meta II. This observation is consistent with experiments showing that the retinal binding cavity becomes accessible to water (and hydroxylamine) in Meta II. 54,55 The observation that the C15 carbon is strongly deshielded (large partial positive charge) and the SB nitrogen is strongly shielded (large partial negative charge, see discussion above) shows that the C=N bond is highly polarized in Meta II.
Figure 4.
Comparison of retinal 13C chemical shifts between Meta II and all-trans retinal model compounds. Differences are shown between Meta II and the N-all-trans-retinylidene-tert-butylimine model compounds in a non-polar solvent (CDCl3) (△MII, grey73, △MIINP, orange74) and in a polar solvent (△MIIP, CD3OD, blue74). The smaller differences between retinal 13C13, 13C14 and 13C15 in Meta II and the butylimine model compound in a polar solvent suggest that the binding cavity is more polar near the SB end of the retinal in Meta II. All chemical shifts are externally referenced to neat TMS.
The accessibility to water and hydroxylamine is likely associated with motion of EL2 away from the retinal SB in Meta II.28 Weaker interactions between the retinal and EL2 in Meta II are also reflected in chemical shift changes of the retinal carbons. First, the lack of chemical shift changes at the C12 position in Meta II as observed for the E181Q mutation are consistent with the loss of a strong Glu181 interaction with the retinal chain. Second, the chemical shifts of the C19 and C20 methyl groups in rhodopsin are unique (Table 1, Figure 3) due to the conformation and environment of the protein within a tight retinal-binding cavity. However, in Meta II they are roughly the same as in all-trans SB model compounds in solution suggesting a reduced interaction with the protein upon isomerization.
The retinal 13C14 chemical shift is sensitive to isomerization about the C=N bond of the SB.56 In rhodopsin, the PSB linkage between the retinal and Lys296 on H7 is characterized by a 15-anti C=N geometry.48,57-59 When the C=N bond is in a syn configuration, steric interaction between the proton attached to C14 and the protons on the ε-carbon of Lys296 leads to an upfield shift of the isotropic resonances at both positions, referred to as a γ-effect.56,60 In our case, the ~ 3.0 ppm upfield shift of the 13C14 resonance is not associated with a corresponding upfield shift in the Lys296(Cε) resonance, which is observed at 60.8 ppm in Meta II.28 Together, the 13C14 and Lys296(Cε) chemical shifts are in agreement with FTIR studies that the geometry of the C=N double bond remains 15-anti in Meta II.61
Despite the polar nature of the binding site in Meta II, it is known that the unprotonated Schiff base in Meta II is not titratable.62,63 FTIR studies have shown that proton uptake in Meta II results from protonation of Glu134; in wild-type rhodopsin reconstituted into membranes there is a single titration with an apparent pKa of 6.3 that is abolished in the E134Q mutant.64,65 Deprotonation of the Schiff base in the Meta I — II transition results from an internal proton transfer to Glu113. The high upfield chemical shift of the 15N SB nitrogen and the downfield shift of the 13C15 carbon indicate that the SB is highly polarized in Meta II. NMR studies show that in the rhodopsin - Meta II transition the C20 methyl group rotates toward the extracellular surface suggesting that the NH proton rotates toward the hydrophobic receptor interior.28,29 In Meta II, such a rotation would orient the free electron pair on the unprotonated SB nitrogen toward Cys264/Ser298, while the C15H proton would be oriented toward Glu113. The orientation of the C15H proton would agree with the fact that the Schiff base is hydrolyzed in the decay of Meta II to opsin, and that the Meta II half-life (which reflects that the rate of hydrolysis) is increased from 12.5 min in wild-type rhodopsin to 153 min in the E113Q mutant.50 Hydrolysis likely involves nucleophilic attack at the retinal C15 carbon, a reaction that would be facilitated by a strongly polarized C=N bond. Interestingly, the SB is not hydrolyzed in squid rhodopsin where the “counterion” at the position equivalent to 113 is a tyrosine. These results may also provide an explanation for the conservation of Glu113 in the vertebrate UV-absorbing pigments where the SB is unprotonated and apparently not in need of a counterion. In these pigments, Glu113 (or its equivalent) may have multiple roles 66 including the regulation of the Meta II lifetime and SB hydrolysis. This suggestion is supported by measurements in the mouse UV-absorbing pigment where the Meta II half-life was increased from ~30 sec in the wild-type pigment to 6 min in the E108Q mutant 67.
3.4. 6-s-cis conformation of the β-ionone ring in rhodopsin and metarhodopsin II
T1 relaxation time measurements of the C18 methyl group have previously been used to assess the conformation of the C6-C7 bond of the retinal chromophore in bacteriorhodopsin.17 The C18 T1 relaxation times are much longer (of the order of tens of seconds) for a planar 6-s-trans conformation than for the skewed 6-s-cis conformation (of the order of milliseconds). The difference is mainly due to the strong steric interaction between the retinal C18 methyl group and the proton on C7 in the 6-s-trans geometry. Table 4 summarizes the T1 relaxation times for the C18 methyl group in rhodopsin, as well as in 6-s-cis and 6-s-trans retinal model compounds. T1 relaxation measurements of 6-s-cis and 6-s-trans retinoic acid obtained at the same external field strength (600 MHz, 1H field) and temperature (190 K) as rhodopsin fall outside of the range of the 6-s-cis and 6-s-trans model compounds reported by Harbison et al.,17 but still differ by roughly an order of magnitude and are characteristic of the C6-C7 geometry. Figure 5 presents the T1 relaxation time measurements of the C18 methyl group in rhodopsin (black) and Meta II (red) at 190 K. In rhodopsin, the T1 relaxation time is 343 ms, which is close to the value of 6-s-cis retinoic acid and well outside the range of the 6-s-trans model compounds. In Meta II, the T1 relaxation time is 561 ms, within the range of 6-cis model compounds.
Table 4.
Comparison of the 13C chemical shifts of the retinal C5 and C8 carbons along with the T1 relaxation measurement for the C18 methyl group in wild-type rhodopsin, Meta II and retinal model compounds
| Compound | Temperature (K) |
13C5 (ppm) |
13C8 (ppm) |
13C18 (T1) (sec) |
|---|---|---|---|---|
| 6-s-transB | 296 | 134.6-144.8 | 131.6-133.4 | 25.6-31.7 |
| 6-s-cisB | 296 | 126.7-129.4 | 138.2-140.6 | 0.41-3.69 |
| 6-s-trans retinoic acid (monoclinic) |
190 | 136.5 | 131.3 | 12.3 |
| 6-s-cis retinoic acid (triclinic) |
190 | 129.6 | 139.7 | 0.14 |
| Rhodopsin | 190 | 131.0 | 139.3 | 0.34 |
| Meta II | 190 | 126.0 | 139.6 | 0.56 |
Data from Harbison et al.17
Figure 5.
T1 relaxation curves for the retinal C18 methyl group in wild-type rhodopsin (black) and Meta II (red). The T1 measurements were made a sample temperature of 190 K and external 1H field strength of 600 MHz.
The 13C chemical shifts of the retinal C5 and C8 resonances are also sensitive to the conformation of the β-ionone ring about the C6-C7 single bond. Analysis of the chemical shift tensors of retinal model compounds has shown that the C5 resonance is not influenced by steric interactions, but by charge delocalization along the polyene chain.17 The C5=C6 double bond is within the ionone ring and conjugation with the rest of the chain is reduced in the 6-s-cis conformation. Comparison of the chemical shifts of crystalline retinal derivatives shows that the C5 chemical shift for 6-s-cis model compounds is ~7-9 ppm upfield of the 6-s-trans model compounds (Table 4). In contrast, the C8 chemical shift appears to be predominantly influenced by steric interactions.17 In the 6-s-trans conformation, the C8 proton is located between the C16 and C17 methyl groups, and the C8 chemical shift for 6-s-trans model compounds is ~5-8 ppm upfield compared with the 6-s-cis model compounds. In dark rhodopsin, we previously observed the C5 resonance at 131.0 ppm and the C8 resonance at 139.3 ppm indicating a 6-s-cis conformation of the β-ionone ring.46
In the Meta II intermediate, the 13C5 and 13C8 chemical shifts are observed at 126.0 ppm and 139.6 ppm, respectively, in agreement with a 6-s-cis conformation of the ring. The retinal C5 chemical shift is shifted markedly upfield as compared to SB model compound in both polar and non-polar solvents (see Figure 4). Although charge delocalization is reduced in unprotonated SB chromophores, as compared to their protonated forms, the upfield shift suggests that the ring is appreciably more non-planar with respect to the retinal polyene chain in Meta II than in the retinal model compounds, and charge delocalization does not extend into the C5=C6 bond of the β-ionone ring.
Interestingly, one might have anticipated a larger red-shift in the λmax of Meta II on the basis of two observations. First, we have shown that the 15N resonance for the Schiff base nitrogen in Meta II is shifted further upfield than that observed for the Mn and Mo states of bacteriorhodopsin. The upfield shift is in the direction of protonation and is associated with stronger basicity/hydrogen-bonding.42 Second, Gat and Sheves53 have shown using a series of retinal model compounds that the red-shifted absorption maximum in the M state of bacteriorhodopsin is correlated with the environment of the SB rather than to the planarity of the β-ionone ring. In DDM, the λmax of Meta II is at 382 nm, as observed for the all-trans retinal SB in a hydrophobic solvent, suggesting that the Schiff base nitrogen is not involved in strong hydrogen bonding interactions.53 One way to possibly reconcile the unusual chemical shifts of the retinal C5 carbon and the SB nitrogen with the 382 nm λmax of Meta II is to conclude that there are appreciable twists about the C6-C7 bond, which disrupts the conjugated π-system, and that polarization of the C=N bond results from the SB nitrogen being oriented toward a hydrophobic environment, while the C15H proton is oriented toward a very polar environment.
The 13C T1 relaxation data on the retinal chromophore clearly show that we have predominantly a 6-s-cis conformer in both rhodopsin and Meta II. However, these data do not allow us to differentiate between negatively or positively twisted 6-s-cis retinal enantiomers. Lau et al.68 have analyzed the conformation of retinal in rhodopsin based on molecular dynamic simulations of rhodopsin. Their study indicates that the polyene chain of the retinal is locked in a single, twisted conformation due to multiple steric interactions with the surrounding protein. However, they observe structural heterogeneity for the retinal β-ionone ring in agreement with deuterium NMR measurements.24 Their analysis yields a model of rhodopsin that can accommodate both negatively and positively twisted 6-s-cis retinal enantiomers; the deuterium NMR line shape of the C18 methyl group is best fit with a mixture of enantiomers where the negatively twisted conformer is more abundant. Recently, we described 2D dipolar-assisted rotational resonance (DARR) NMR experiments on the position of the C18 methyl group relative to Gly121 and to Tyr26828 that agree with this conclusion. With a negative twist, the C18 methyl group is predicted to be in close proximity to Gly121, while a positive twist places the C18 methyl group near Tyr268. We observed 13C…13C correlations between the C18 methyl group and Gly121 consistent with a ~4 Å distance, but failed to observe contacts between the C18 methyl group and Tyr268. We can estimate on the basis of the signal-to-noise ratios in our DARR NMR experiments that we would have been able to observe a C18…Tyr268 contact within ~5 Å if the population of the positively twisted 6-s-cis conformer were above 40%. In the section below, we discuss additional NMR experiments that support the conclusion that the β-ionone ring has predominantly a single conformation with a negatively twisted 6-s-cis bond.
Finally, it is known that retinal analogs in which the β-ionone ring is replaced with two ethyl groups are not able to fully activate rhodopsin.69 In membranes, rhodopsin activity is reduced to ~20% of wild type levels as assayed by GTPγS binding to transducin. As a result, steric interactions between the retinal β-ionone ring and helix H5 in Meta II are absent in retinal analogs lacking the ionone ring. Without these interactions, which may be responsible for the unusual C5 chemical shift and associated 6-s-cis ring conformation, changes in the structure or position of H5 that are necessary for receptor activation may not occur.
3.5. Ring conformation and assignment of the 13C16 and 13C17 chemical shifts
According to IUPAC nomenclature, the retinal C16 methyl group in Figure 1 is oriented into the plane of the page, whereas the C17 methyl group is oriented out of the plane of the page. In solution, the 13C16 and 13C17 resonances are at the same chemical shift of ~ 28.9 ppm in both 11-cis PSB and all-trans SB retinal model compounds.43 In rhodopsin, the 13C16 and 13C17 resonances are observed at 30.6 ppm and 26.1 ppm, respectively (Table 1). The difference in chemical shift between the retinal in solution and bound to the rhodopsin is attributed to the tight binding site within the receptor and the inability of different ring conformations to rapidly interconvert. Ring inversion can change the position of the methyl groups from an axial orientation to an equatorial orientation. In an axial orientation, the protons of the methyl group are in steric contact with the proton of the C3 carbon of the β-ionone ring. This interaction produces an upfield chemical shift of the methyl group22,43 (another example of a γ effect). Assuming that the difference in the C16, C17 chemical shifts are due to a γ effect, we assign the resonance at 26.1 ppm to the methyl group in the axial orientation. An open question has been whether the 26.1 ppm resonance corresponds to the C16 or the C17 methyl group.
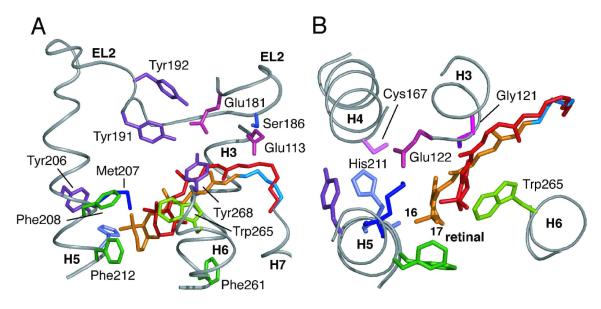
In order to determine which methyl group (C16 or C17) is at 26.1 ppm, we rely on recent 13C NMR studies that define the position of the β-ionone ring in rhodopsin and in Meta II on the basis of distance measurements between 13C groups on the retinal and amino acids lining the retinal binding site.28 For example, 2D DARR NMR spectra have been obtained using rhodopsin containing 13C-labels at the carbonyl carbons of Met207 and His211 on H5, and regenerated with retinal 13C-labeled at both the C16 and C17 methyl groups. The strongest crosspeak, corresponding to the shortest internuclear distance, is observed to the retinal methyl group resonating at 30.6 ppm. According to the rhodopsin crystal structures (pdb codes: 1U19 and 1GZM), the C16 methyl group is closer to the carbonyls of Met207 (4.9 Å) and His211 (4.9 Å) on H5 than the C17 methyl group, which is 7.0 Å away from the carbonyl of Met207 and 7.2 Å away from the carbonyl of His211.11 On this basis, we can assign the resonance at 26.1 ppm to the C17 methyl group in an axial orientation and the resonance at 30.6 ppm to C16 methyl group in an equatorial orientation.
On conversion to Meta II, the retinal moves toward H5 and is positioned between Met207/His211 and Phe208/Phe212. The C16 and C17 methyl group resonances are observed at 28.6 ppm and 33.4 ppm, respectively. These assignments are based on the following observations. First, a relatively strong DARR NMR crosspeak is observed between the methyl resonance at 28.6 ppm and both the carbonyl carbons of Met207 and His211 on H5.28 Second, 2D DARR NMR data show that the side chain of Met207 in Meta II is positioned between the β-ionone ring and Cys167 on H4. Figure 6B shows that these constraints are only consistent with assignment of the resonance at 28.6 ppm to the C16 methyl group.
Figure 6.
Two views of the retinal-binding site in Meta II highlighting the polar and aromatic residues interacting with the retinal chromophore (orange). The position of the retinal (red) in the dark state of rhodopsin (pdb code: 1U1911) is shown for comparison. (A) View showing the position of EL2 relative to the retinal chromophore. (B) View from the extracellular surface; EL2 is not shown for clarity. On the basis of the observed retinal chemical shifts and T1 relaxation measurements we conclude that the β-ionone ring has a 6-s-cis conformation about the C6-C7 single bond in rhodopsin and Meta II. The location of the retinal in Meta II has been defined by 2D DARR NMR measurements between the retinal chromophore and surrounding amino acids in the retinal binding site28. The Cε-methyl resonance of Met207 exhibits DARR NMR crosspeaks with His211, Cys167 and the retinal carbons (C6, C7, C16 and C18). These contacts allow us to position the β-ionone ring between Met207 and Phe208/Phe212 (Panel B) with the retinal C16 and C18 methyl groups on the H3/H4 side of the retinal binding site. The Met207-C18 contact suggests a negative twist for the C6-C7 bond.
The upfield shift in the 28.6 ppm resonance in Meta II is attributed to an axial orientation of the C16 methyl group and the γ-effect produced by interaction with the protons on C3 on the β-ionone ring. As a consequence, in the rhodopsin to Meta II transition the C16 methyl resonance changes from 30.6 ppm to 28.6 ppm, and the C17 methyl resonance changes from 26.1 ppm to 33.4 ppm. These assignments, in turn, indicate that there is an inversion in the ring conformation. Buss and coworkers70,71 have calculated that the energy barrier for ring inversion is between 5-6 kcal/mol. Moreover, these results show that in both rhodopsin and Meta II, the C16 and C17 chemical shifts are unique and that averaging by rotation of the ionone ring does not occur in the retinal binding site of the protein.
4. Conclusions
Crystal structures of the dark state of rhodopsin have provided a number of clues for how the 11-cis retinal PSB chromophore functions as an inverse agonist for activation.11,48,72 The lack of a corresponding crystal structure of the active Meta II intermediate has limited progress on understanding the mechanism by which the all-trans retinal SB functions as a full agonist for activation. The recent crystal structure of opsin at low pH,73,74 which contains several features of an activated GPCR, but lacks the covalently bound all-trans SB chromophore that likely holds the extracellular side of the receptor in its active conformation.28 Here, solid-state NMR measurements of the 13C chemical shifts and relaxation times of the all-trans SB chromophore in Meta II provide the most detailed description to date on the conformation and environment of a GPCR agonist. The observation that the retinal C18 methyl group has a short T1 relaxation time in both the dark-state of rhodopsin and the Meta II intermediate demonstrates that the C6-C7 single bond has a twisted 6-s-cis conformation, which does not change upon receptor activation. The 6-s-cis conformation contrasts with the planar s-trans conformation of the retinal chromophore in bacteriorhodopsin.17 The unusually high upfield chemical shift of the retinal 13C5 resonance may be associated with steric interactions of the β-ionone ring with helix H5 resulting in a significant twist of the C6-C7 bond. Steric interactions involving the β-ionone portion of the retinal are essential for rhodopsin activation.69 In this regard, the β-ionone ring appears to be well positioned to hold H5 in an active conformation. Recently, we have shown that retinal isomerization is coupled to the motion of EL2 and H5.29 Rotation of H5 is important for the insertion of Tyr223 into the region of the ionic lock between H3 and H6. 29,73,74
The comparison between △MII values for the retinal C5-C20 carbons relative to model compounds in polar and non-polar solvents suggests that the SB linkage of the all-trans retinal chromophore in Meta II is in a polar environment despite the fact the SB cannot be titrated. These results are in agreement with previous studies that the C20 methyl group rotates roughly 180° toward the extracellular surface 28 and EL2 is displaced slightly from the retinal binding site. 29 The displacement of EL2 allows water to enter on the extracellular side of binding site. In this regard, it is known that the Schiff base is more accessible to hydroxylamine in Meta II.5,6 The orientation of the C20 methyl group suggests that the C15H proton is directed toward Glu113 and the Schiff base nitrogen is oriented toward the hydrophobic receptor interior. We propose that polarization of the C=N bond, reflected in the NMR chemical shifts reported here, results from this orientation of the retinal SB in Meta II and that this facilitates Schiff base hydrolysis by dramatically increasing the partial positive charge on the C15 carbon in the presence of water.
Finally, it is possible to activate several opsin mutants above basal (or constitutive) levels in membranes by the addition of all-trans retinal as a diffusible ligand.75 However, the activity never reaches the full light-induced activity of Meta II. This observation supports the notion that there are specific retinal-protein contacts that form in the active site upon photoisomerization that might stabilize unique “agonist” structural features (i.e. structural changes on the extracellular side of the receptor are allosterically coupled to changes in the intracellular loops). In fact, the agonist binding pocket optimal for receptor activity is probably only achieved in the presence of the bound G protein, analogous to the “high affinity” agonist state in ligand-activated GPCRs.
Acknowledgements
This work was supported by a research grant to S.O.S. from the NIH (GM-41412), and NIH-NSF instrumentation grants (S10 RR13889 and DBI-9977553) and from US-Israel Binational Science Foundation to M.S. We gratefully acknowledge the W.M. Keck Foundation for support of the NMR facilities in the Center of Structural Biology at Stony Brook. MS acknowledges support from Kimmelman center for Biomolecular Structure and Assembly.
References
- (1).Sakmar TP. Curr. Opin. Cell Biol. 2002;14:189–195. doi: 10.1016/s0955-0674(02)00306-x. [DOI] [PubMed] [Google Scholar]
- (2).Okada T, Ernst OP, Palczewski K, Hofmann KP. Trends Biochem. Sci. 2001;26:318–324. doi: 10.1016/s0968-0004(01)01799-6. [DOI] [PubMed] [Google Scholar]
- (3).Cohen GB, Yang T, Robinson PR, Oprian DD. Biochemistry. 1993;32:6111–6115. doi: 10.1021/bi00074a024. [DOI] [PubMed] [Google Scholar]
- (4).Vogel R, Siebert FJ. Biol. Chem. 2001;276:38487–38493. doi: 10.1074/jbc.M105423200. [DOI] [PubMed] [Google Scholar]
- (5).Zhukovsky EA, Oprian DD. Science. 1989;246:928–930. doi: 10.1126/science.2573154. [DOI] [PubMed] [Google Scholar]
- (6).Sakmar TP, Franke RR, Khorana HG. Proc. Natl. Acad. Sci. U.S.A. 1989;86:8309–8313. doi: 10.1073/pnas.86.21.8309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Struts AV, Salgad GFJ, Tanaka K, Krane S, Nakanishi K, Brown MF. J. Mol. Biol. 2007;372:50–66. doi: 10.1016/j.jmb.2007.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Crocker E, Eilers M, Ahuja S, Hornak V, Hirshfeld A, Sheves M, Smith SO. J. Mol. Biol. 2006;357:163–172. doi: 10.1016/j.jmb.2005.12.046. [DOI] [PubMed] [Google Scholar]
- (9).Patel AB, Crocker E, Reeves PJ, Getmanova EV, Eilers M, Khorana HG, Smith SO. J. Mol. Biol. 2005;347:803–812. doi: 10.1016/j.jmb.2005.01.069. [DOI] [PubMed] [Google Scholar]
- (10).Verdegem PJE, Bovee-Geurts PHM, De Grip WJ, Lugtenburg J, de Groot HJM. Biochemistry. 1999;38:11316–11324. doi: 10.1021/bi983014e. [DOI] [PubMed] [Google Scholar]
- (11).Okada T, Sugihara M, Bondar AN, Elstner M, Entel P, Buss V. J. Mol. Biol. 2004;342:571–583. doi: 10.1016/j.jmb.2004.07.044. [DOI] [PubMed] [Google Scholar]
- (12).Sugihara M, Hufen J, Buss V. Biochemistry. 2006;45:801–810. doi: 10.1021/bi0515624. [DOI] [PubMed] [Google Scholar]
- (13).Schoenlein RW, Peteanu LA, Wang Q, Mathies RA, Shank CV. J. Phys. Chem. 1993;97:12087–12092. [Google Scholar]
- (14).Tsutsui K, Imai H, Shichida Y. Biochemistry. 2007;46:6437–6445. doi: 10.1021/bi7003763. [DOI] [PubMed] [Google Scholar]
- (15).Honig B, Dinur U, Nakanishi K, Baloghnair V, Gawinowicz MA, Arnaboldi M, Motto MG. J. Am. Chem. Soc. 1979;101:7084–7086. [Google Scholar]
- (16).Smith SO, Courtin J, de Groot H, Gebhard R, Lugtenburg J. Biochemistry. 1991;30:7409–7415. doi: 10.1021/bi00244a007. [DOI] [PubMed] [Google Scholar]
- (17).Harbison GS, Smith SO, Pardoen JA, Courtin JM, Lugtenburg J, Herzfeld J, Mathies RA, Griffin RG. Biochemistry. 1985;24:6955–6962. doi: 10.1021/bi00345a031. [DOI] [PubMed] [Google Scholar]
- (18).Smith SO, Palings I, Copie V, Raleigh DP, Courtin J, Pardoen JA, Lugtenburg J, Mathies RA, Griffin RG. Biochemistry. 1987;26:1606–1611. doi: 10.1021/bi00380a018. [DOI] [PubMed] [Google Scholar]
- (19).Gröbner G, Burnett IJ, Glaubitz C, Choi G, Mason AJ, Watts A. Nature. 2000;405:810–813. doi: 10.1038/35015604. [DOI] [PubMed] [Google Scholar]
- (20).Singh D, Hudson BS, Middleton C, Birge RR. Biochemistry. 2001;40:4201–4204. doi: 10.1021/bi001911o. [DOI] [PubMed] [Google Scholar]
- (21).Fujimoto Y, Ishihara J, Maki S, Fujioka N, Wang T, Furuta T, Fishkin N, Borhan B, Berova N, Nakanishi K. Chem.-Eur. J. 2001;7:4198–4204. doi: 10.1002/1521-3765(20011001)7:19<4198::aid-chem4198>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- (22).Spooner PJR, Sharples JM, Verhoeven MA, Lugtenburg J, Glaubitz C, Watts A. Biochemistry. 2002;41:7549–7555. doi: 10.1021/bi020007o. [DOI] [PubMed] [Google Scholar]
- (23).Spooner PJR, Sharples JM, Goodall SC, Seedorf H, Verhoeven MA, Lugtenburg J, Bovee-Geurts PHM, DeGrip WJ, Watts A. Biochemistry. 2003;42:13371–13378. doi: 10.1021/bi0354029. [DOI] [PubMed] [Google Scholar]
- (24).Salgado GFJ, Struts AV, Tanaka K, Fujioka N, Nakanishi K, Brown MF. Biochemistry. 2004;43:12819–12828. doi: 10.1021/bi0491191. [DOI] [PubMed] [Google Scholar]
- (25).Salom D, Lodowski DT, Stenkamp RE, Le Trong I, Golczak M, Jastrzebska B, Harris T, Ballesteros JA, Palczewski K. Proc. Natl. Acad. Sci. U.S.A. 2006;103:16123–16128. doi: 10.1073/pnas.0608022103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Luca S, White JF, Sohal AK, Filippov DV, van Boom JH, Grisshammer R, Baldus M. Proc. Natl. Acad. Sci. U.S.A. 2003;100:10706–10711. doi: 10.1073/pnas.1834523100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Ratnala VRP, Kiihne SR, Buda F, Leurs R, de Groot HJM, DeGrip WJ. J. Am. Chem. Soc. 2007;129:867–872. doi: 10.1021/ja0652262. [DOI] [PubMed] [Google Scholar]
- (28).Ahuja S, Crocker E, Eilers M, Hornak V, Hirshfeld A, Ziliox M, Syrett N, Reeves PJ, Khorana HG, Sheves M, Smith SO. J. Biol. Chem. 2009;284:10190–10201. doi: 10.1074/jbc.M805725200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Ahuja S, Hornak V, Yan ECY, Syrett N, Goncalves JA, Hirshfeld A, Ziliox M, Sakmar TP, Sheves M, Reeves PJ, Smith SO, Eilers M. Nat. Struct. Mol. Biol. 2009;16:168–175. doi: 10.1038/nsmb.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Reeves PJ, Kim JM, Khorana HG. Proc. Natl. Acad. Sci. U.S.A. 2002;99:13413–13418. doi: 10.1073/pnas.212519199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Reeves PJ, Thurmond RL, Khorana HG. Proc. Natl. Acad. Sci. U.S.A. 1996;93:11487–11492. doi: 10.1073/pnas.93.21.11487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Eilers M, Reeves PJ, Ying WW, Khorana HG, Smith SO. Proc. Natl. Acad. Sci. U.S.A. 1999;96:487–492. doi: 10.1073/pnas.96.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Lugtenburg J. Pure Appl. Chem. 1985;57:753–762. [Google Scholar]
- (34).Crocker E, Patel AB, Eilers M, Jayaraman S, Getmanova E, Reeves PJ, Ziliox M, Khorana HG, Sheves M, Smith SO. J. Biomol. NMR. 2004;29:11–20. doi: 10.1023/B:JNMR.0000019521.79321.3c. [DOI] [PubMed] [Google Scholar]
- (35).Metz G, Wu X, Smith SO. J. Magn. Reson. A. 1994;110:219–227. [Google Scholar]
- (36).Bennett AE, Rienstra CM, Auger M, Lakshmi KV, Griffin RG. J. Chem. Phys. 1995;103:6951–6958. [Google Scholar]
- (37).Fung BM, Khitrin AK, Ermolaev K. J. Magn. Reson. 2000;142:97–101. doi: 10.1006/jmre.1999.1896. [DOI] [PubMed] [Google Scholar]
- (38).Harris RK, Becker ED, De Menezes SMC, Granger P, Hoffman RE, Zilm KW. Solid State Nucl. Magn. Reson. 2008;33:41–56. doi: 10.1016/j.ssnmr.2008.02.004. [DOI] [PubMed] [Google Scholar]
- (39).Morcombe CR, Zilm KW. J. Magn. Reson. 2003;162:479–486. doi: 10.1016/s1090-7807(03)00082-x. [DOI] [PubMed] [Google Scholar]
- (40).Harbison GS, Herzfeld J, Griffin RG. Biochemistry. 1983;22:1–4. doi: 10.1021/bi00270a600. [DOI] [PubMed] [Google Scholar]
- (41).Wang JX, Balazs YS, Thompson LK. Biochemistry. 1997;36:1699–1703. doi: 10.1021/bi962578k. [DOI] [PubMed] [Google Scholar]
- (42).Hu JG, Sun BQ, Bizounok M, Hatcher ME, Lansing JC, Raap J, Verdegem PJE, Lugtenburg J, Griffin RG, Herzfeld J. Biochemistry. 1998;37:8088–8096. doi: 10.1021/bi973168e. [DOI] [PubMed] [Google Scholar]
- (43).Creemers AFL, Kiihne S, Bovee-Geurts PHM, DeGrip WJ, Lugtenburg J, de Groot HJM. Proc. Natl. Acad. Sci. U.S.A. 2002;99:9101–9106. doi: 10.1073/pnas.112677599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Kusnetzow AK, Altenbach C, Hubbell WL. Biochemistry. 2006;45:5538–5550. doi: 10.1021/bi060101v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Shriver JW, Mateescu GD, Abrahamson EW. Biochemistry. 1979;18:4785–4792. doi: 10.1021/bi00589a004. [DOI] [PubMed] [Google Scholar]
- (46).Smith SO, Palings I, Miley ME, Courtin J, de Groot H, Lugtenburg J, Mathies RA, Griffin RG. Biochemistry. 1990;29:8158–8164. doi: 10.1021/bi00487a025. [DOI] [PubMed] [Google Scholar]
- (47).Han M, DeDecker BS, Smith SO. Biophys. J. 1993;65:899–906. doi: 10.1016/S0006-3495(93)81117-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, Yamamoto M, Miyano M. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- (49).Lüdeke S, Beck R, Yan ECY, Sakmar TP, Siebert F, Vogel R. J. Mol. Biol. 2005;353:345–356. doi: 10.1016/j.jmb.2005.08.039. [DOI] [PubMed] [Google Scholar]
- (50).Yan ECY, Kazmi MA, De S, Chang BSW, Seibert C, Marin EP, Mathies RA, Sakmar TP. Biochemistry. 2002;41:3620–3627. doi: 10.1021/bi0160011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Hall KF, Vreven T, Frisch MJ, Bearpark MJ. J. Mol. Biol. 2008;383:106–121. doi: 10.1016/j.jmb.2008.08.007. [DOI] [PubMed] [Google Scholar]
- (52).Yan ECY, Kazmi MA, Ganim Z, Hou JM, Pan DH, Chang BSW, Sakmar TP, Mathies RA. Proc. Natl. Acad. Sci. U.S.A. 2003;100:9262–9267. doi: 10.1073/pnas.1531970100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Gat Y, Sheves M. Photochem. Photobiol. 1994;59:371–378. [Google Scholar]
- (54).Farrens DL, Khorana HG. J. Biol. Chem. 1995;270:5073–5076. doi: 10.1074/jbc.270.10.5073. [DOI] [PubMed] [Google Scholar]
- (55).Wald G, Brown PK. J. Gen. Physiol. 1953;37:189–200. doi: 10.1085/jgp.37.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Harbison GS, Smith SO, Pardoen JA, Winkel C, Lugtenburg J, Herzfeld J, Mathies R, Griffin RG. Proc. Natl. Acad. Sci. U.S.A. 1984;81:1706–1709. doi: 10.1073/pnas.81.6.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Bagley KA, Balogh-Nair V, Croteau AA, Dollinger G, Ebrey TG, Eisenstein L, Hong MK, Nakanishi K, Vittitow J. Biochemistry. 1985;24:6055–6071. doi: 10.1021/bi00343a006. [DOI] [PubMed] [Google Scholar]
- (58).Palings I, Pardoen JA, van den Berg E, Winkel C, Lugtenburg J, Mathies RA. Biochemistry. 1987;26:2544–2556. doi: 10.1021/bi00383a021. [DOI] [PubMed] [Google Scholar]
- (59).Okada T, Fujiyoshi Y, Silow M, Navarro J, Landau EM, Shichida Y. Proc. Natl. Acad. Sci. U.S.A. 2002;99:5982–5987. doi: 10.1073/pnas.082666399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Farrar MR, Lakshmi KV, Smith SO, Brown RS, Raap J, Lugtenburg J, Griffin RG, Herzfeld J. Biophys. J. 1993;65:310–315. doi: 10.1016/S0006-3495(93)81065-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Vogel R, Siebert F, Mathias G, Tavan P, Fan GB, Sheves M. Biochemistry. 2003;42:9863–9874. doi: 10.1021/bi034684+. [DOI] [PubMed] [Google Scholar]
- (62).Jäger F, Fahmy K, Sakmar TP, Siebert F. Biochemistry. 1994;33:10878–10882. doi: 10.1021/bi00202a005. [DOI] [PubMed] [Google Scholar]
- (63).Knierim B, Hofmann KP, Ernst OP, Hubbell WL. Proc. Natl. Acad. Sci. U.S.A. 2007;104:20290–20295. doi: 10.1073/pnas.0710393104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Vogel R, Mahalingam M, Luedke S, Huber T, Siebert F, Sakmar TP. J. Mol. Biol. 2008;380:648–655. doi: 10.1016/j.jmb.2008.05.022. [DOI] [PubMed] [Google Scholar]
- (65).Vogel R, Sakmar TP, Sheves M, Siebert F. Photochem. Photobiol. 2007;83:286–292. doi: 10.1562/2006-06-19-IR-937. [DOI] [PubMed] [Google Scholar]
- (66).Tsutsui K, Imai H, Shichida Y. Biochemistry. 2008;47:10829–10833. doi: 10.1021/bi801377v. [DOI] [PubMed] [Google Scholar]
- (67).Kusnetzow AK, Dukkipati A, Babu KR, Ramos L, Knox BE, Birge RR. Proc. Natl. Acad. Sci. U.S.A. 2004;101:941–946. doi: 10.1073/pnas.0305206101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Lau PW, Grossfield A, Feller SE, Pitman MC, Brown MF. J. Mol. Biol. 2007;372:906–917. doi: 10.1016/j.jmb.2007.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Vogel R, Siebert F, Lüdeke S, Hirshfeld A, Sheves M. Biochemistry. 2005;44:11684–11699. doi: 10.1021/bi0508587. [DOI] [PubMed] [Google Scholar]
- (70).Terstegen F, Buss V. Chem. Phys. 1997;225:163–171. [Google Scholar]
- (71).Terstegen F, Carter EA, Buss V. Int. J. Quantum Chem. 1999;75:141–145. [Google Scholar]
- (72).Li J, Edwards PC, Burghammer M, Villa C, Schertler GFX. J. Mol. Biol. 2004;343:1409–38. doi: 10.1016/j.jmb.2004.08.090. [DOI] [PubMed] [Google Scholar]
- (73).Scheerer P, Park JH, Hildebrand PW, Kim YJ, Krauss N, Choe HW, Hofmann KP, Ernst OP. Nature. 2008;455:497–502. doi: 10.1038/nature07330. [DOI] [PubMed] [Google Scholar]
- (74).Park JH, Scheerer P, Hofmann KP, Choe HW, Ernst OP. Nature. 2008;454:183–187. doi: 10.1038/nature07063. [DOI] [PubMed] [Google Scholar]
- (75).Han M, Smith SO, Sakmar TP. Biochemistry. 1998;37:8253–8261. doi: 10.1021/bi980147r. [DOI] [PubMed] [Google Scholar]
- (76).13C chemical shift differences between Meta II and the all-trans-N-retinylidene-n-butylimine model compound in a nonpolar solvent (CDCl3) are taken from Shriver et al. (1979).
- (77).13C chemical shift differences between Meta II and the all-trans-N-retinylidene-n-butylimine model compound in a nonpolar solvent (CDCl3) and polar solvent (CD3OD) were obtained as part of this study.