Abstract
An enantioselective synthesis of the maoecrystal V (1) carbon skeleton is described. The key transformations include arylation of a 1,3-dicarbonyl compound with a triarylbismuth(V)dichloride species, oxidative dearomatization of a phenol, and a subsequent intramolecular Diels-Alder reaction.
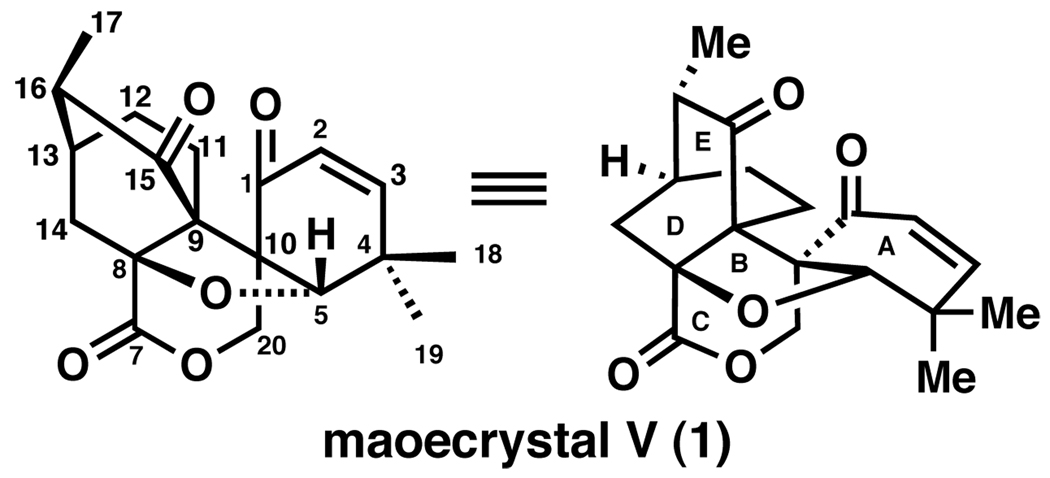
Maoecrystal V (1, Figure 1) was isolated and characterized in 2004 by Sun and coworkers from the Chinese medicinal herb Isodon Eriocalyx.1 It possesses an unusual architecture rendering it the most modified naturally occurring ent-kauranoid isolated to date.2 The structure was confirmed by X-ray crystallographic analysis to contain five highly congested rings and seven stereocenters, two of which are adjacent all carbon quaternary centers located in its interior. One of these all carbon quaternary centers is also the bridgehead carbon of the [2,2,2]-bicyclooctanone moiety. This letter reports an early approach to 1 pursued in our laboratory from November 2007 to May 2008.3
Figure 1.
Representations of maoecrystal V.
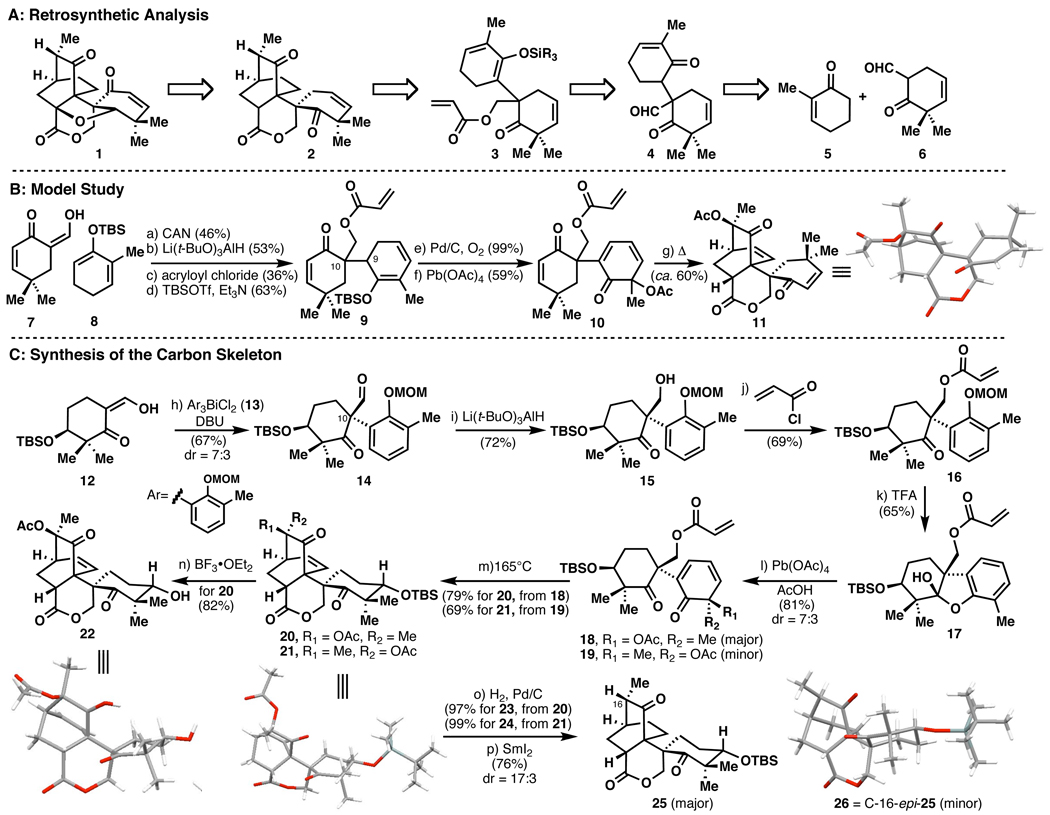
Our initial retrosynthesis, shown in Scheme 1A, began by disconnecting the tetrahydrofuran ring and simplifying the enone of the A ring to an olefin leading to 2. Next, an intramolecular Diels–Alder4 disconnection of the bicyclic ring system would generate a cyclohexadiene/acrylate conjugate 3. It was initially envisioned that silyl enol ether 3 could be used as the diene component in the Diels-Alder reaction. In order to construct this substrate via the corresponding enone 4, an oxidative coupling of enolates or related substrates was proposed (i.e. 5 + 6).5
Scheme 1.
Maoecrystal V: (A) Retrosynthetic Analysis, (B) Model Study, and (C) Synthesis of the Complete Carbon Skeletona
aDetails: reagents and conditions (a) CAN, NaHCO3, MeOH, 0°C, 46% , (b) Li(t-BuO)3AlH, THF, −78°C, 53%, (c) acryloyl chloride, Et3N, DMAP, DCM, 0°C, 36%, (d) TBSOTf, Et3N, DCM, 63%, (e) Pd/C, O2, PhMe, 110°C, 99%, (f) Pb(OAc)4, AcOH, 59%, (g) 165°C, o-DCB, ca. 60%, (h) Ar3BiCl2(13), DBU, PhMe, 67%, (i) Li(t-BuO)3AlH, THF, −78°C, 72%, (j) acryloyl chloride, DIEPA, DMAP, DCM, −78°C, 69%, (k) TFA, DCM, 0°C, 65%, (l) Pb(OAc)4, AcOH, 81%, dr = 3:7, (m) 165°C, o-DCB, BHT 79% for 20, 69% for 21 (n) BF3•OEt2, DCM, 82%, (o) H2, Pd/C, EtOAc, 97% for 23, 99% for 24 (p) SmI2, MeOH, THF, 0°C, 76%, dr = 17:3. CAN = cerium ammonium nitrate, DMAP = 4-dimethylaminopyridine, DCM = dichloromethane, TBSOTf = tert-butydimethylsilyl trifluoromethylsulfonate, o-DCB = ortho-dichlorobenzene, DIEPA = diisopropylethylamine, DBU = 1,8-diazobicylco[5.4.0]undec-7-ene, TFA = trifluoroacetic acid, BHT = 2,6-di-tert-butyl-p-cresol.
Initial studies (Scheme 1B) with a model system using β-ketoaldehyde 7 and enol ether 8 led to the generation of the C-10 quaternary center by treatment with cerium ammonium nitrate.6 Following reduction of the formyl group and esterification, silylation of the ketone under a variety of conditions led only to the undesired regioisomeric dienol ether 9 (presumably due to the sterically hindered α–proton at C-9). To circumvent this problem, the diene was further dehydrogenated (Pd/C, O2) and the resulting aromatic moiety was oxidized using a Wessely oxidation7 (Pb(OAc)4) to furnish cyclohexadienone 10 in 59% yield. A Diels–Alder reaction delivered polycycle 11 in ca. 60% yield (3 mg scale) upon heating to 165°C.8 X-ray crystallography confirmed the structure as that corresponding to the undesired regioisomeric outcome (based on the position of the geminal dimethyl group relative to the bicyclic portion of the molecule). Encouraged by the outcome of the intramolecular Diels-Alder, a revised route was devised to access the correct carbon framework found in 1 (Scheme 1C).
The sequence begins with β-ketoaldehyde 12, derived in three steps from a readily available symmetrical 1,3-diketone.9 Barton arylation10 of 12 with 139 led to 14 in 67% yield as a 7:3 readily separable mixture of diastereomers. In a similar fashion to the model study, reduction (to afford 15) and acylation led to 16. Removal of the MOM group with TFA and Wessely oxidation of the intermediate hemiketal 17 yielded an inconsequential mixture of diastereomeric acetates 18/19 in 53% yield over two steps.
Dienes 18 and 19 were heated in a microwave reactor in o-DCB to 165°C for 1.5 hours in the presence of BHT, to give the expected endo-cycloadducts in 79% and 69% yields. The stereochemistry of the cycloadducts was verified by X-ray crystallographic analysis.11 Once the structures had been determined, both adducts were elaborated further towards 1 by hydrogenation of the bicyclic olefin (H2, Pd/C). Next, the acetate group was excised using SmI2 to give an intermediate samarium enolate that was protonated by methanol to give the α-methyl ketone as a mixture of diastereomers in 76% yield (dr = 17:3 with 25 as major).12 An X-ray crystal structure of 26 (C-16-epi-25) was obtained from the minor diasteromer as shown in Scheme 1C, establishing that the major isomer posseses the proper orientation of the C-16 methyl group in 1.
Certain elements of the sequential Barton arylation/Wessely oxidation/Diels-Alder Strategy (i.e. 12→14, 17→18/19, 18/19→25) reported herein may well find use in an eventual synthesis of 1.13
Supplementary Material
Acknowledgment
We thank Dr. D.-H. Huang (TSRI) and Dr. L. Pasternack (TSRI) for NMR spectroscopic assistance, and Dr. G. Siuzdak (TSRI) for assistance with mass spectroscopy. Dr. Arnold Rheingold (UCSD) and Dr. Antonio DiPasquale (UCSD) are acknowledged for X-ray crystallographic assistance. Financial support for this work was provided by Bristol-Myers Squibb, the NIH (CA134785) and the German Research Foundation (DFG) (postdoctoral fellowship to N.S.)
Footnotes
Supporting Information Available: Detailed experimental procedures, copies of all spectral data, and full characterization. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Li S-H, Wang J, Niu X-M, Shen Y-H, Zhang H-J, Sun H-D, Li M-L, Tian Q-E, Lu Y, Cao P, Zheng Q-T. Org. Lett. 2004;6:4327–4330. doi: 10.1021/ol0481535. [DOI] [PubMed] [Google Scholar]
- 2.Sun H-D, Huang S-X, Han Q-B. Nat. Prod. Rep. 2006;23:673–698. doi: 10.1039/b604174d. [DOI] [PubMed] [Google Scholar]
- 3.During this period one of the corresponding authors of the preceding letter was employed as a postdoctoral associate (C.-C. Li) in this laboratory: Gong J, Lin G, Li C-C, Yang Z. Org. Lett. 2009 doi: 10.1021/ol9014392., DOI: 10.1021/ol9014392. This work was reported in an NIH proposal (received 8/11/2008 and reviewed on 10/9/2008 by the BCMB-B study section) and a final report to the DFG (submitted: 7/17/2009).
- 4.(a) Nicolaou KC, Snyder SA, Montagnon T, Vassilikogiannakis G. Angew. Chem., Int. Ed. 2002;41:1668–1698. doi: 10.1002/1521-3773(20020517)41:10<1668::aid-anie1668>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]; (b) Roush WR. In: Comprehensive Organic Synthesis. Trost BM, editor. Vol. 5. Oxford: Pergamon; 1991. pp. 513–550. [Google Scholar]
- 5.(a) Baran PS, DeMartino MP. Angew. Chem., Int. Ed. 2006;45:7083–7086. doi: 10.1002/anie.200603024. [DOI] [PubMed] [Google Scholar]; (b) DeMartino MP, Chen K, Baran PS. J. Am. Chem. Soc. 2008;130:11546–11560. doi: 10.1021/ja804159y. [DOI] [PubMed] [Google Scholar]
- 6.Nair V, Mathew J. J. Chem. Soc., Perkin Trans. 1995;1:187–188. [Google Scholar]
- 7.(a) Wessely F, Sinwel F. Monatsh. Chem. 1950;81:1055–1070. [Google Scholar]; (b) Barnes-Seeman D, Corey EJ. Org. Lett. 1999;1:1503–1504. doi: 10.1021/ol991070h. [DOI] [PubMed] [Google Scholar]; (c) Nicolaou KC, Simonsen KB, Vassilikogiannakis G, Baran PS, Vidali VP, Pitsinos EN, Couladouros EA. Angew. Chem., Int. Ed. 1999;38:3555–3559. doi: 10.1002/(sici)1521-3773(19991203)38:23<3555::aid-anie3555>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]; (d) Magdziak D, Meek SJ, Pettus TRR. Chem. Rev. 2004;104:1383–1429. doi: 10.1021/cr0306900. [DOI] [PubMed] [Google Scholar]
- 8.For recent examples using ortho-quinols as dienophiles see: Nicolaou KC, Toh Q-Y, Chen DY-K. J. Am. Chem. Soc. 2008;130:11292–11293. doi: 10.1021/ja804588r. Gao S-Y, Chittimalla SK, Chuang GJ, Liao C-C. J. Org. Chem. 2009;74:1632–1639. doi: 10.1021/jo802295p. Dory YL, Roy A-L, Soucy P, Deslongchamps P. Org. Lett. 2009;11:1197–1200. doi: 10.1021/ol8026768. Snyder SA, Kontes F. J. Am. Chem. Soc. 2009;131:1745–1752. doi: 10.1021/ja806865u..
- 9.See Supporting Information for details.
- 10.(a) Barton DHR, Lester DJ, Motherwell WB, Papoula MTB. J. Chem. Soc., Chem. Comm. 1980:246–247. [Google Scholar]; (b) Barton DHR, Blazejewski J-C, Charpiot B, Finet J-P, Motherwell WB, Papoula MTB, Stanforth SP. J. Chem. Soc., Perkin Trans. 1985;1:2667–2675. [Google Scholar]; (c) Barton DHR, Finet J-P, Giannotti C, Halley F. J. Chem. Soc., Perkin Trans. 1987;1:241–249. [Google Scholar]; (d) Combes S, Finet J-P. Tetrahedron. 1999;55:3377–3386. [Google Scholar]; (e) Fedorov AY, Finet J-P. J. Chem. Soc., Perkin Trans. 2000;1:3775–3778. [Google Scholar]; (f) Finet J-P, Fedorov AY. J. Organomet. Chem. 2006;691:2386–2393. [Google Scholar]
- 11.For adduct 21 a crystal was grown directly, whereas adduct 20 was crystallized after removal of the TBS group as alcohol 22.
- 12.Molander GA, Hahn G. J. Org. Chem. 1986;51:1135–1138. [Google Scholar]
- 13.While this paper was in review, two other groups reported progress towards 1: Peng F, Yu M, Danishefsky SJ. Tetrahedron Lett. 2009 doi: 10.1016/j.tetlet.2009.09.050. Nicolaou KC, Dong L, Deng L, Talbot AC, Chen DY-K. Chem. Commun. 2009 doi: 10.1039/b917045f. accepted..
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.