Abstract
Hepatocellular carcinoma (HCC) is a common human cancer with high mortality and currently there is no effective chemoprevention or systematic treatment. Recent evidence suggests that COX-2-derived PGE2 and Wnt/β-catenin signaling pathways are implicated in hepatocarcinogenesis. Here we report that ω-3 PUFAs, docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), inhibit HCC growth through simultaneously inhibition of COX-2 and β-catenin. DHA and EPA treatment resulted in a dose-dependent reduction of cell viability with cleavage of PARP, caspase-3 and caspase-9 in three human HCC cell lines (Hep3B, Huh-7, HepG2). In contrast, arachidonic acid (AA), a ω-6 PUFA, exhibited no significant effect. DHA and EPA treatment caused dephosphorylation and thus activation of GSK-3β, leading to β-catenin degradation in Hep3B cells. The GSK3-β inhibitor, LiCl, partially prevented DHA-induced β-catenin protein degradation and apoptosis. Additionally, DHA induced the formation of β-catenin/Axin/GSK-3β binding complex, which serves as a parallel mechanism for β-catenin degradation. Furthermore, DHA inhibited PGE2 signaling through downregulation of COX-2 and upregulation of the COX-2 antagonist, 15-hydroxyprostaglandin dehydrogenase (15-PGDH). Finally, the growth of HCC in vivo was significantly reduced when mouse HCCs (Hepa1–6) were inoculated into the Fat-1 transgenic mice which express a Caenorhabditis elegans desaturase converting ω-6 to ω-3 PUFAs endogenously. These findings provide important preclinical evidence and molecular insight for utilization of ω-3 PUFAs for the chemoprevention and treatment of human HCC.
Keywords: hepatocellular carcinoma, omega-3 polyunsaturated fatty acid, beta-catenin, cyclooxygenase-2, prostaglandin E2, 15-PGDH
INTRODUCTION
Hepatocellular carcinoma (HCC) is the fifth most common human cancer with high mortality and its incidence is rising worldwide. The overall survival of patients with HCC is dismal and currently no efficient secondary prevention or systemic treatments are available. HCC usually develops in the presence of continuous inflammation and hepatocyte regeneration in the setting of chronic hepatitis and cirrhosis (1). Increased cellular turnover and regeneration within the context of a noxious chronically inflamed environment cause accumulation of chromosomal damages, which eventually affect the structure and expression of oncogenes and tumor suppressor genes leading to carcinogenesis. Recent studies have shown that mediators of inflammation, such as prostaglandins (PGs), play an important role in hepatocarcinogenesis (2). For example, increased cyclooxygenase-2 (COX-2) expression has been found in human and animal HCCs and in dysplastic hepatocytes (3–9). Elevated levels of PGs, most notably PGE2, have also been detected in HCC (10). Overexpression of COX-2 or treatment with exogenous PGE2 increases human HCC cell growth and invasiveness (8, 11). The cyclooxygenase (COX) inhibitors, nonsteroidal anti-inflammatory drugs (NSAIDs), inhibit the proliferation and induce apoptosis in cultured HCC cells and in animal models of hepatocarcinogenesis (2), although these inhibitors are known to mediate effects through both COX-dependent and -independent mechanisms.
In addition to upregulation of COX-2, Wnt/β-catenin activation has also been implicated in various stages of hepatic tumorigenesis, including the dysplastic foci, hepatic adenoma, hepatoblastoma and HCC (12–16). Activation of the Wnt/β-catenin pathway occurs in approximately 30 to 40% of HCCs (17). Multiple mechanisms of β-catenin activation or stabilization have been reported in hepatic tumorigenesis, including mutations in the β-catenin gene (Ctnnb1), or components of its degradation machinery such as Axin and GSKβ inactivation (12–16). In mice, hepatic deletion of APC, another degradation component of β-catenin, leads to HCC (18). Recently, upregulation of a member of Wnt receptors, Frizzled-7, has been shown as another possible mechanism of β-catenin activation in HCC (19). In addition, Wnt/β-catenin also plays an important role in regulation of hepatocyte proliferation, survival, liver regeneration and in the maintenance and self-renewal of pluripotent stem cells and progenitor cells (12), hence, they may play a role in the maintenance of the cancer stem cell compartment. Indeed, β-catenin activation has been identified in oval cells (liver stem cells), which might be precursors of a subset of HCC (13). Thus, there appears to be multiple mechanisms of β-catenin activation leading to liver neoplasia. Although PGE2 has recently been shown to activate β-catenin in colon cancer cells (20, 21), it remains unknown whether the COX-2/PG and Wnt/β-catenin signaling pathways converge during hepatocarcinogenesis.
In contrast to the documented carcinogenic effect of the prostaglandins (PGE2 in particular) derived from arachidonic acid (an ω-6 PUFA), there is abundant experimental evidence that the ω-3 PUFAs rich in fish oil, such as docosahexaenoic acid (DHA) and eicosapentaenic acid (EPA), prevent carcinogenesis (22, 23). However, the molecular mechanisms for the anticancer actions of ω-3 PUFAs remain incompletely understood. This study was designed to investigate the effect and mechanism of ω-3 PUFAs in HCC cells. Our results show that DHA and EPA inhibited the growth of three human HCC cells (Hep3B, Huh-7, HepG2), in vitro. The growth of HCC in vivo was also significantly reduced when mouse HCC cells (Hepa1–6) were inoculated into the syngeneic Fat-1 transgenic mice which carry a Caenorhabditis elegans desaturase converting ω-6 to ω-3 PUFAs. Moreover, our data reveal that COX-2-derived PGE2 activates β-catenin signaling pathways in human HCC cells and that ω-3 PUFAs inhibit HCC growth by simultaneously blocking β-catenin and COX-2 signaling pathways. These findings provide important preclinical evidence and molecular framework for utilization of ω-3 PUFAs in the chemoprevention and treatment of HCC.
MATERIALS AND METHODS
Materials
α-MEM, DMEM, RPMI 1640, fetal bovine serum (FBS), glutamine, antibiotics, and Lipofectamine plus reagent were purchased from Life Technologies, Inc. (Rockville, MD). PGE2 was purchased from Calbiochem (San Diego, CA). The cell proliferation assay reagent WST-1 was purchased from Roche Molecular Biochemicals (Indianapolis, IN). The antibody for human COX-2, 15-hydroxyprostaglandin dehydrogenase (PGDH) were purchased from Cayman Chemical Company (Ann Arbor, MI). The antibodies against human Axin, β-catenin, PARP, caspase-3, caspase-9, and c-Met were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The antihuman β-actin monoclonal antibody was purchased from Sigma (St. Louis, MO). The horseradish peroxidase-linked streptavidin and chemiluminescence detection reagents were from Amersham Pharmacia Biotech, Inc. (Piscataway, NJ). The rabbit antibodies for phospho-Akt (Thr308), Akt, phospho-GSK-3β (Ser9), GSK-3β were purchased from Cell Signaling Technology (Beverly, MA). Mouse monoclonal anti GSK-3β was purchased from Transduction Laboratories and cytochrome c was purchased from BD Bioscience (Franklin Lakes, NJ). The Bio-Rad protein assay system was obtained from Bio-Rad Laboratories (Hercules, CA). The Tris-glycine gels were obtained from Invitrogen Life Technologies, Inc. (Carlsbad, CA). Hr. T. Hla at the University of Connecticut Health Center provided the COX-2 expression plasmid (containing full length of human COX-2 cDNA in sense orientation cloned in mammalian expression vector PCDNA3).
Cell culture
The human hepatocellular carcinoma cell lines (Hep3B, HepG2 and Huh7) were obtained from American Type Culture Collection (Manassas, VA) and cultured according to our previous described methods (8, 11, 24). Briefly, the cells were cultured in EMEM supplemented with 10%FBS, 2 mM L-glutamine, and penicillin/streptomycin. The cells were incubated at 37°C in a humidified CO2 incubator. The experiments were performed when cells reached ~80% confluence and conducted in serum-free medium (with serum deprivation for 24 hr before each experiment).
Cell growth assay
Cell growth was determined using the cell proliferation reagent WST-1, a tetrazolium salt that is cleaved by mitochondrial dehydrogenases in viable cells. Briefly, 100 µl of cell suspension (containing 0.5−2 × 104 cells) were plated in each well of 96-well plates. After 24 hr culture to allow reattachment, the cells then were treated with specific reagents such as DHA, EPA or Wnt3a-conditioned medium (Wnt3a-CM) for indicated time points. At the end of each treatment, the cell proliferation reagent WST-1 (10 µl) was added to each well, and the cells were incubated at 37°C for 0.5~5 hr. Absorbance at 450 nm was measured using an automatic ELISA plate reader.
Immunoprecipitations
Equal amount of cellular protein from the treated cells was incubated with 10 µl of rabbit antihuman Axin polyclonal antibody at 4°C for overnight, followed by addition of 20 µl Protein A/G PLUS agarose (Santa Cruz Biotechnology). The mixture was incubated for 2 hr and then washed three times with the cell lysis buffer [50 mM HEPES (pH 7.55), 1 mM EDTA, 1 mM DTT, and protease inhibitor cocktail tablets from Roche Diagnostics (Basel, Switzerland)]. The final pellets were dissolved in 20 µl 2X protein loading buffer, and the samples were subjected to SDS-PAGE and Western blot analysis using 1:1000 dilution mouse antihuman GSK-3β or β-catenin monoclonal antibodies and enhanced chemiluminescence Western blot detection system (Amersham Pharmacia Biotech, Inc.).
Transient transfection of COX-2 expression plasmid
Hep3B cells were exposed to the mixture of Lipofectamine plus reagents and COX-2 expression plasmid (full-lengh human COX-2 cDNA cloned in pcDNA3 vector), pcDNA3 control vector or TCF/LEF-Luc reporter plasmid for 4 hr. Following removal of the transfection mixtures, the cells were cultured in fresh serum-free medium with or without specific treatment as indicated in the text. The expression of COX-2 was verified by immunoblotting.
Luciferase reporter activity assay
The cultured cells were seeded at a concentration achieving 80% confluence in 12-well plates for eighteen hours before transfection. The cells were transiently transfected with 0.2 µg/per well translucent TCF/LEF-Luc reporter vector, which was designed to measure the β-catenin transcriptional activity of TCF/LEF responsive genes. After transfection, the cells were treated with specific reagents including DHA or Wnt3a conditioned medium in serum-free medium for 24 hr. The cell lysates were then obtained with 1X reporter lysis buffer (Promega). The luciferase activity was assayed in a Berthold AutoLumat LB953 Luminometer (Nashua, NH) by using the luciferase assay system from Promega. The relative luciferase activity was calculated after normalization of cellular proteins. All values are expressed as percentage of activity induction relative to control activity.
Immunoblotting
At the end of each indicated treatment, the cells were scraped off the plates and centrifuged, washed twice with cold phosphate-buffered saline (PBS) containing 0.5 mM PMSF and 10 µg/ml leupeptin and resuspended in 5-fold volume of hypotonic buffer consisting of 50 mM HEPES pH 7.55, 1 mM EDTA, 1 mM DTT and protease inhibitor cocktail tablets (Roche Diagnostics GmbH). After sonication, the whole cell lysate was collected by centrifugation at the speed of 15,000g at 4°C for 10 minutes to remove cell debris and stored in aliquots at −80°C until use. The protein concentration in the cell extracts were determined by the BioRad protein assay (Bio-Rad, CA). 30 µg of cellular protein was subjected to SDS-PAGE on 4–20% Tris-glycine gels for PARP, β-catenin, GSK-3β, phosphor-GSK-3β, Akt, phosphor-Akt, c-Met, Axin cytochrome c, caspase-3, caspase-9, COX-2, 15-PGDH or β-actin. The separated proteins were electrophoretically transferred onto the nitrocellulose membrane (BioRad, CA). Nonspecific binding was blocked with PBS-T (0.5% Tween 20 in PBS) containing 5% non-fat milk for 1 hr at room temperature. The membranes were then incubated overnight at 4°C with individual primary antibodies in PBS-T containing 1% non-fat milk at the dilutions specified by the manufactures. Following three washes with PBS-T, the membranes were then incubated with the horseradish peroxidase-conjugated secondary antibodies at 1:10,000 dilution in PBS-T containing 5% non-fat milk for 1 hour at room temperature. The membranes were then washed 3 times with PBS-T and the protein bands were visualized with the ECL Western blotting detection system.
Animals and tumorigenicity experiments
The Fat-1 transgenic mice (from Dr. J.X. Kang of Harvard University) (25). The animals were kept at 22°C under a 12-h light/dark cycle and fed standard mouse chow (Prolab® IsoPro® 5P75 RMH 3000) with water ad libitum. The fatty acid compositions in the Fat-1 transgenic mice and wild type control mice housed at our animal facility are shown in Supplementary Table 1. The mice were kept under specific pathogen-free conditions in standard cages, and used 8–10 weeks old male for this experiment. Each mouse was injected subcutaneously (s.c.) into the area overlying the right flank with 1.5 × 106 mouse hepatocellular carcinoma cells (Hepa1–6) suspended in 100 µl of serum-free medium. After inoculation, the animals were closely monitored for the development of subcutaneous tumor. The tumor size was measured with a caliper every 2 days. Upon sacrifice, the tumor volume was calculated according to the following formula: Tumor volume = L × W2 × 0.5. The animal experiments were carried out according to the protocol approved by the University of Pittsburgh Institutional Animal Care and Use Committee (#0201740B).
RESULTS
Immunohistochemical stains for COX-2 and β-catenin in human hepatocellular carcinoma tissues
Twenty paired human hepatocellular carcinomas and their matched nonneoplastic/nondysplastic liver tissues were analyzed by immunohistochemistry for the expression of COX-2 and β-catenin. Increased cytoplasmic staining for COX-2 and nuclear staining for β-catenin was observed in HCC cells when compared with the non-tumor liver tissue (Supplementary Figure 1). The average staining intensity for COX-2 in HCC is 2.10 ± 0.78, which is significantly higher than that in nontumor liver tissue (0.20 ± 0.09) (p<0.01, Student t test). Whereas COX-2 is expressed exclusively in the cytoplasm of both HCC cells and to a less degree in hepatocytes, the expression pattern of β-catenin between hepatocellular carcinoma cells and non-neoplastic hepatocytes are distinctly different. In nontumorous hepatocytes, β-catenin is expressed exclusively in the plasma membrane with no significant cytoplasmic staining and absence of nuclear staining in all 20 patients. In contrast, in hepatocellular carcinoma cells, nuclear staining for β-catenin was observed in 5/20 patients (25%), with focal cytoplasmic staining and decreased membrane staining, indicating β-catenin nuclear translocation and activation. Thus, COX-2 and β-catenin signaling pathways are active in a significant percentage of human HCCs.
ω-3 PUFAs induce hepatocellular carcinoma cell apoptosis
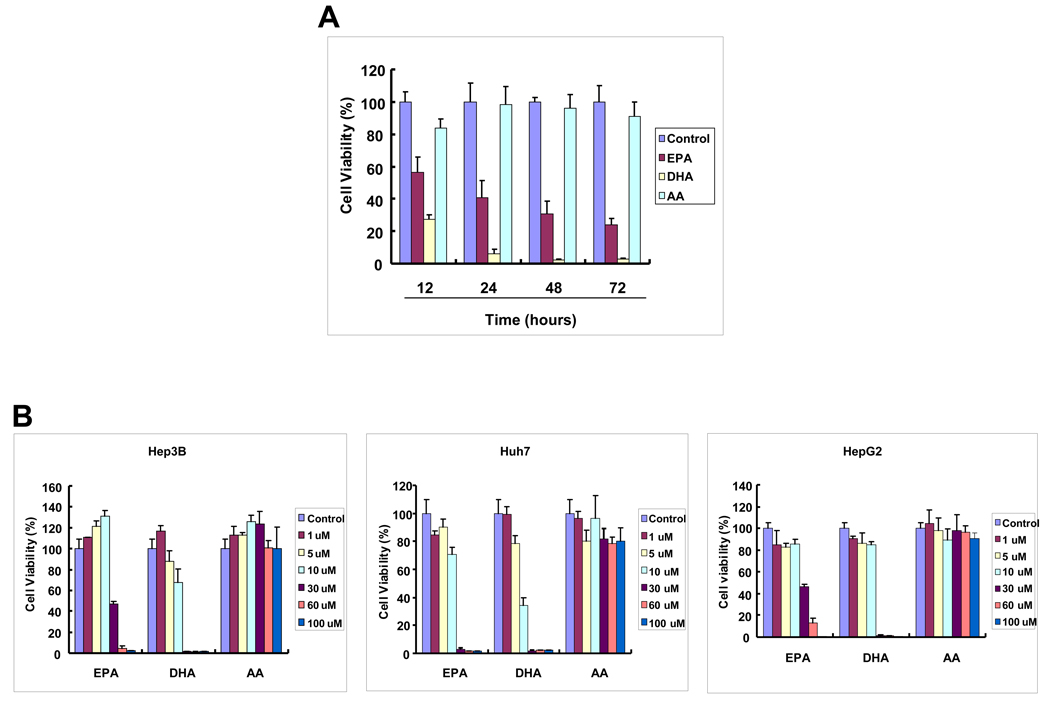
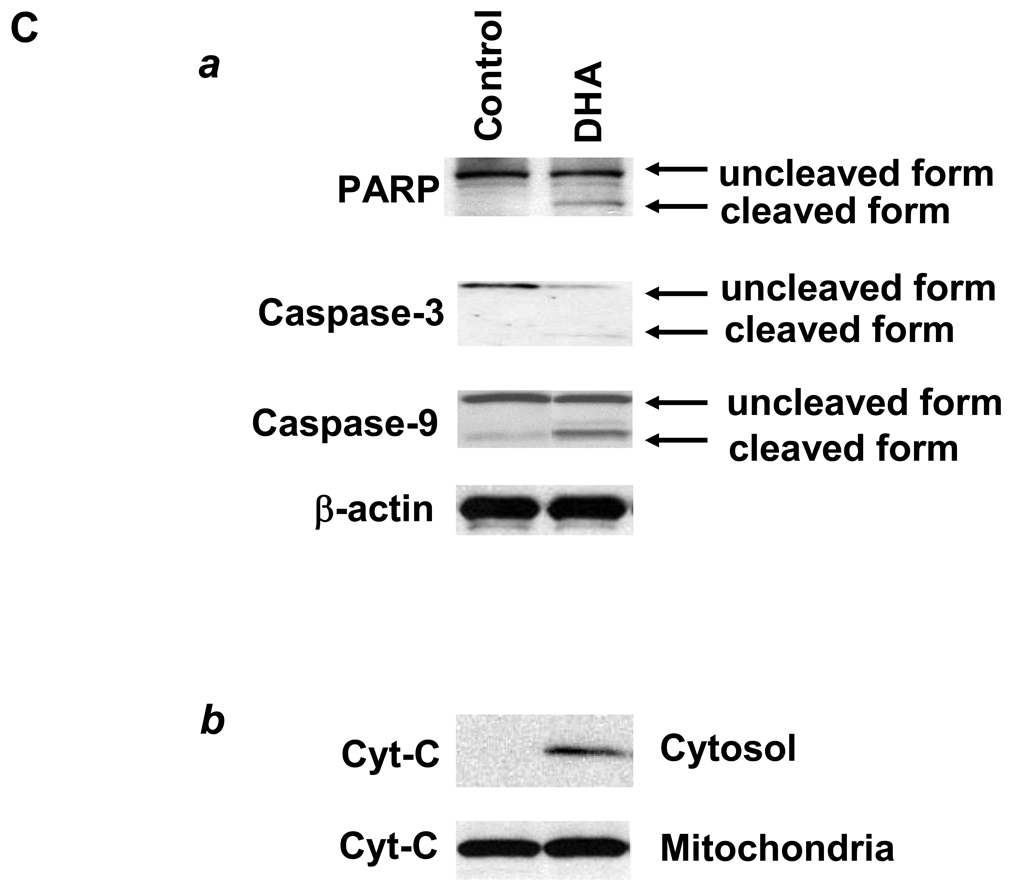
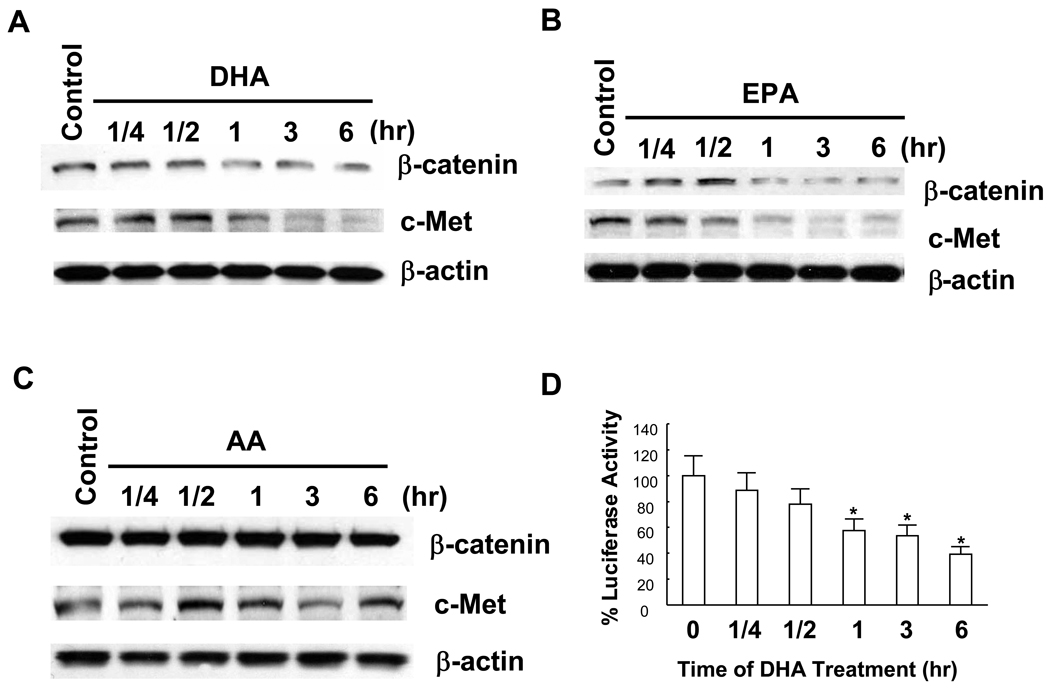
Human HCC cell lines were examined for their response to DHA, EPA and AA treatment. As shown in Figure 1A, treatment of Hep3B cells with two ω-3 PUFAs (30 µM), DHA and EPA, induced a time-dependent reduction of cell viability; in contrast AA, a ω-6 PUFA, had no significant effect. Treatment with 30 µM EPA for 12, 24, 48 and 72 hours induced approximately 45%, 60%, 70% and 75% reduction of viable cells, respectively. DHA appears to have more effect, with approximately 75% reduction of viable cells at 12 hours and more than 90% reduction at 24, 48 and 72 hours. The cells treated with DHA and EPA show morphological features of cell death, characterized by cell shrunken, round and detachment. In contrast, AA treatment did not significantly alter the cell morphology. The effect of DHA and EPA is dose-dependent in all three human HCC cell lines (Hep3B, Huh7 and HepG2 (Figure 1B). The observations that DHA induced the cleavage of PARP, caspase-3 and caspase-9, with concomitant release of cytochrome c from mitochondria to cytosol confirm the induction of apoptosis (Figure 1C). Taken together, these results document induction of apoptosis by ω-3 PUFAs in HCC cells. We have also tested the effect of DHA and EPA in primary cultures of liver parenchymal cells and these compounds were found to have no cytotoxic effect in primary cells (unpublished observations).
Figure 1. The effect of DHA, EPA and AA on the viability of hepatocellular carcinoma cells.
Equal numbers of human HCC cells (Hep3B, HepG2 and Huh-7) were seeded onto the 96-well plates (1 × 104 per well) and cultured in the medium supplemented with 10% fetal bovine serum for 24 hr. The cells were washed twice with PBS and maintained in serum-free medium for 24 hr before treatment with PUFAs. The cells were treated with DHA, EPA or AA at increasing concentrations (1–100 µM) for indicated time periods (12–72 hours). (A) The effect of DHA, EPA or AA on the viability of Hep3B cells (30 µM, 24 hours). The cell viability was determined using WST-1 assay. The data are presented as mean ± SD of six independent experiments. (B) Dose-dependent reduction of cell viability by DHA and EPA in Hep3B, Huh7 and HepG2. The cells were treated with 1–100 µM individual PUFAs for 24 hours. The cell growth was determined using WST-1 assay. The data are presented as mean ± SD of six independent experiments. (C) DHA induces the cleavage of caspase-3, caspase-9 and PARP and release of cytochrome c in Hep3B cells. (a) DHA induces the cleavage of caspase-3, caspase-9 and PARP. Hep3B cells were treated with DHA (30 µM) for 24 hr and the cell lysates were obtained for Western blot analysis using antibodies against PARP, caspase-3 and caspase-9. (b) DHA induces the release of cytochrome c in Hep3B cells. The levels of cytochrome c in the cytosolic and mitochondrial fractions were determined by western blotting analysis. EPA was not utilized in these experiments.
DHA decreases the level of β-catenin and inhibits TCF/LEF transcription activity in HCC cells
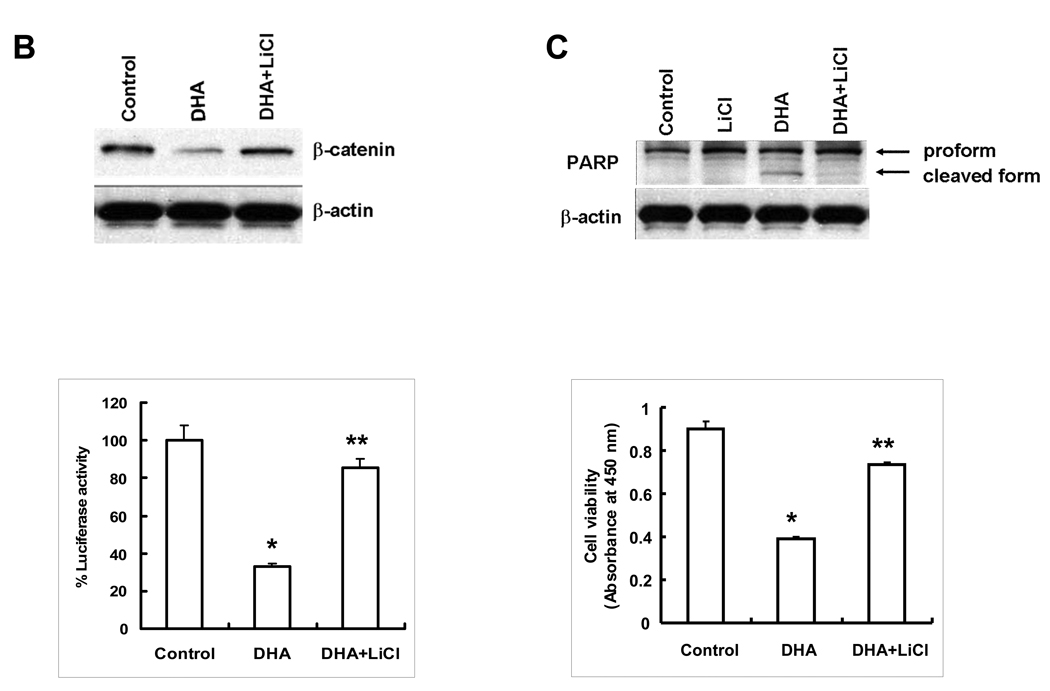
Further experiments were performed to assess the mechanisms by which ω-3 PUFAs induce HCC apoptosis. Since β-catenin activity is importantly involved in hepatocarcinogenesis, the potential effect of ω-3 PUFAs on β-catenin protein level and activity was examined. As shown in Figure 2A and 2B, treatment with DHA or EPA reduced the level of β-catenin protein; this effect was time-dependent (observed 1–6 hours after treatment). As c-Met is a β-catenin controlled downstream gene, the potential effect of DHA and EPA on c-Met protein expression was also examined. Indeed, DHA and EPA treatment also reduced the expression of c-Met. In contrast, treatment with AA did not alter β-catenin or c-Met level (Figure 2C).
Figure 2. ω-3 PUFAs reduce β-catenin protein level and TCF/LEF transcription activity in HCC cells.
(A–C) The effect of DHA and EPA on β-catenin protein. Hep3B cells were treated with 60 µM DHA, EPA or AA in serum-free medium for indicated time. The cell lysates were obtained for Western blot analysis using antibodies against β-catenin and c-Met as described in Materials and Methods. DHA and EPA decreased β-catenin and c-Met expression, whereas AA had no effect. (D) DHA treatment decrease TCF/LEF reporter activity. Hep3B cells were transiently tansfected with the pTCF/LEF-Luc reporter vector. After transfection the cells were cultured in serum-free medium with DHA (60 µM) for indicated time and the cell lysates were obtained for luciferase activity assay (n = 3, *p<0.01). The effect of EPA on TCF/LEF reporter vector was not examined.
Since β-catenin regulates gene expression via binding as a transcription factor in complex with the TCF/LEF transcription factor family to the promoter region of target genes, we further examined the effect of DHA on TCF/LEF reporter activity. The TCF/LEF transcription activity was assayed after transient transfection of a luciferase reporter construct under the control of TCF/LEF response element. As shown in Figure 2D, DHA treatment significantly inhibited the TCF/LEF reporter activity (approximately 5 fold, p<0.01). This result further confirms suppression of β-catenin activity by DHA.
ω-3 PUFAs induce β-catenin degradation through inhibition of GSK-3β phosphorylation
The level of β-catenin in cells is tightly controlled by its degradation complex composed of Axin, APC, GSK-3β and β-catenin, in which GSK-3β phosphorylates β-catenin and thus triggers its ubiquitination and subsequent proteosomal degradation. The activity of GSK-3β is regulated by its phosphorylation status, with GSK-3β phosphorylation at Ser-9 being functionally inactive. To determine whether ω-3 PUFAs might induce β-catenin degradation through inhibition of GSK-3β phosphorylation, we examined the phospho-Ser-9-GSK-3β and total GSK-3β protein levels in Hep3B cells treated with PUFAs. As shown in Figure 3A, DHA treatment reduced GSK-3β phosphorylation, whereas it had no effect on the protein level of total GSK-3β. Similarly, EPA treatment also decreased the level of phosphor-GSK-3β, whereas AA had no effect. Since the phosphorylation of GSK-3β is controlled by Akt, we also examined the potential effect of DHA on Akt phosphorylation. Our data showed that DHA had no effect on Akt phosphorylation (Figure 3A). Thus, DHA most likely inhibited GSK-3β phosphorylation through mechanism independent of Akt. Taken together, the above findings provide evidence for GSK-3β dephosphorylation (activation) in ω-3 PUFA-induced degradation of β-catenin in HCC cells.
Figure 3. ω-3 PUFAs inhibit GSK-3β phosphorylation in HCC cells.
(A) Hep3B cells were treated with 60 µM of DHA, EPA or AA in serum free medium for indicated time periods. The cell lysates were obtained for Western blot analysis using antibodies against p-GSK-3β and GSK-3β as well as p-Akt as described in Materials and Methods. DHA or EPA treatment resulted in p-GSK-3β dephosphorylation, whereas AA had no effect. (B) Inhibition of GSK-3β by LiCl prevents DHA-induced β-catenin degradation. (Upper Panel) Hep3B cells were pretreated with LiCl (20 mM) for 1 hr before DHA treatment (20 µM) for 24 hr; the cell lysates were obtained for Western blot analysis using the antibody against β-catenin. (Lower Panel) Inhibition of GSK-3β by LiCl prevents DHA-mediated reduction of TCF/LEF reporter activity. Hep3B cells transfected with the TCF/LEF reporter construct were treated with LiCl (20 mM) for 1 hr followed by DHA treatment (20 µM) for 24 hr. The cell lysates were obtained to determine the luciferase activity. The data are presented as mean ± SD of six independent experiments (*p<0.01 compared to control; **p<0.01 compared to DHA treatment). (C) LiCl prevents DHA-induced PARP cleavage and cell viability. (Upper Panel) Hep3B cells were pretreated with LiCl (20 mM) for 1 hr followed by DHA treatment (20 µM) for 24 hr. The cell lysates were obtained for Western blot analysis using the antibody against PARP. (Lower Panel) LiCl restores DHA-induced cell death. Hep3B cells pretreated with LiCl (20 mM) for 1 hr were incubated with DHA for 24 hr. The cell growth was determined using WST-1 assay. The data are presented as mean ± SD of six independent experiments (*p<0.01 compared to control; **p<0.01 compared to DHA treatment). EPA was not utilized for LiCl experiments.
The GSK-3β inhibitor, LiCl, prevents DHA-induced β-catenin degradation and cell death
To further determine the role of GSK-3β in DHA-induced β-catenin degradation, Hep3B cells were pretreated for 1 hr with LiCl prior to DHA treatment to determine the level of β-catenin protein, TCF/LEF reporter activity and cell growth. As shown in Figure 3B, inhibition of GSK-3β by LiCl prevented DHA-induced reduction of β-catenin protein and TCF/LEF reporter activity. Accordingly, LiCl pretreatment also prevented DHA-induced PARP and restored DHA-induced cell death (Figure 3C). These findings further support the role of GSK-3β activation (dephosphorylation) in DHA-induced β-catenin degradation in HCC cells.
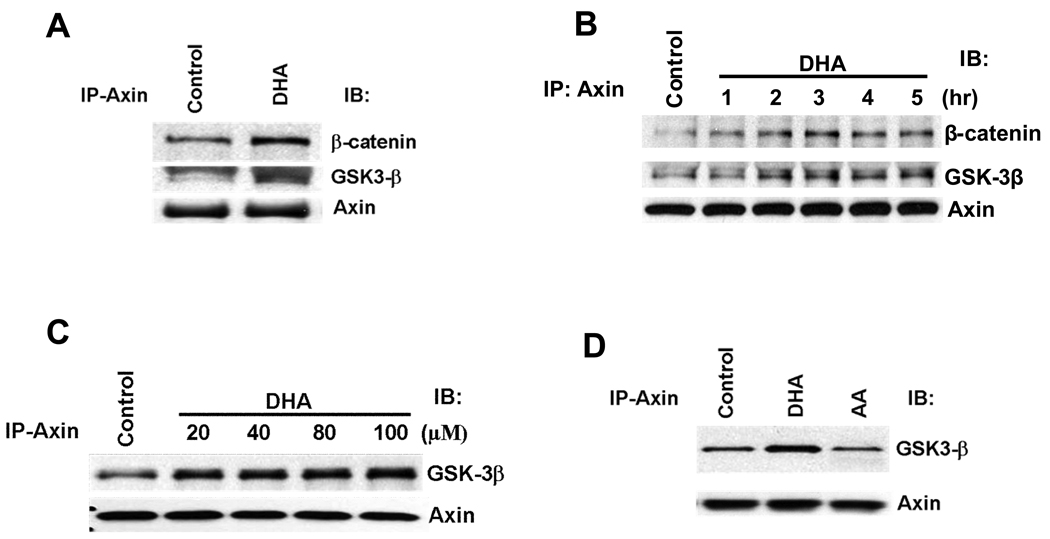
DHA induces the association of Axin with GSK-3β and β-catenin in HCC cells
The degradation of β-catenin strictly depends upon β-catenin phosphorylation, which occurs in a multiprotein complex containing Axin and GSK-3β and β-catenin. It is believed that in this complex assembled by Axin, GSK-3β phosphorylates the β-catenin primarily when it is bound to Axin. To determine whether DHA alters the assembly of the Axin/GSK-3β/β-catenin complex, immunoprecipitation and western blot experiments were performed to detect the Axin/GSK-3β/β-catenin binding complex. As shown in Figure 4A–C, treatment of Hep3B cells with DHA induced the association of Axin with GSK-3β as well as β-catenin. This effect was observed within 1 hour and persisted at 5 hours. In contrast, AA did not affect the association between Axin and GSK-3β (Figure 4D). These findings indicate that DHA induces the association of Axin with GSK-3β and β-catenin, thereby facilitating the formation of β-catenin destruction complex. Taken together, our data suggest that ω-3 PUFAs induce β-catenin degradation through dephosphorylation of GSK-3β and formation of β-catenin destruction complex in HCC cells.
Figure 4. DHA induces the formation of Axin/GSK-3β/β-catenin complex in HCC cells.
(A) The effect of DHA on the association of Axin with β-catenin and GSK-3β. Hep3B cells were treated with DHA (60 µM) for 1 hr. The cell lysates were immunoprecipitaed with anti-Axin antibody followed by immunoblotting with antibodies against GSK-3β, β-catenin, and Axin. (B) The time course effect of DHA. Hep3B cells were treated with DHA (60 µM) at different time points (1, 2, 3, 4, and 5 hours). The cell lysates were immunoprecipitaed with anti-Axin antibody followed by immunoblotting with antibodies against GSK-3β, β-catenin, and Axin. (C) The dose-dependent effect of DHA. Hep3B cells were treated with different concentration of DHA for 1hr. The cell lysates were immunoprecipitaed with anti-Axin antibody followed by immunoblotting with antibodies against GSK-3β and Axin. (D) AA did not alter Axin and GSK-3β association. Hep3B cells were treated with 60 µM AA, DHA or vehicle for 1 hr. The cell lysates were immunoprecipitaed with anti-Axin antibody followed by immunoblotting with antibodies against GSK-3β and Axin. EPA was not utilized in these experiments.
Activation of β-catenin by Wnt3a partially protects DHA-induced HCC cell death
Since Wnt3a is known to activate β-catenin signaling in cells, further experiments were carried out to determine whether Wnt3a might protect HCC cells from DHA-induced apoptosis. Indeed, treatment of Hep3B cells with Wnt3a conditioned medium partially prevented DHA-induced cell death (Supplementary Figure 2A). The effect of Wnt3a on β-catenin activation was confirmed by the observation that Wnt3a conditioned medium prevented DHA-induced reduction of TCF/LEF transcription activity (Supplementary Figure 2B). These results further demonstrate that DHA inhibits HCC growth at least in part through down-regulation of Wnt/β-catenin signaling pathway.
DHA inhibits COX-2 expression in HCC cells
We next examined whether DHA might also affect the expression of COX-2 in HCC cells. As shown in Supplementary Figure 3, DHA significantly inhibited the COX-2 promoter activity and COX-2 protein expression in HCC cells. These findings suggest that DHA inhibits the expression of COX-2 through suppression of gene transcription.
DHA induces 15-hydroxyprostaglandin dehydrogenase (15-PGDH) expression in HCC cells
15-PGDH catalyzes the rate-limiting step of prostaglandin catabolism and thus represents a physiological antagonist of COX-2 (26, 27). Recent emerging evidence suggests that elevated PGE2 in cancers may be the result of enhanced COX-2-mediated PGE2 synthesis as well as reduced 15-PGDH-mediated degradation of PGE2. Therefore, we sought to further determine whether DHA might affect 15-PGDH expression in HCC cells. As shown in Supplementary Figure 4, DHA treatment enhanced the expression of 15-PGDH in a dose-dependent manner in HCC cells (Hep3B, HepG2 and Huh7). These data are consistent with the observation that DHA and EPA inhibit PGE2 production in Hep3B cells (Supplementary Figure 5).
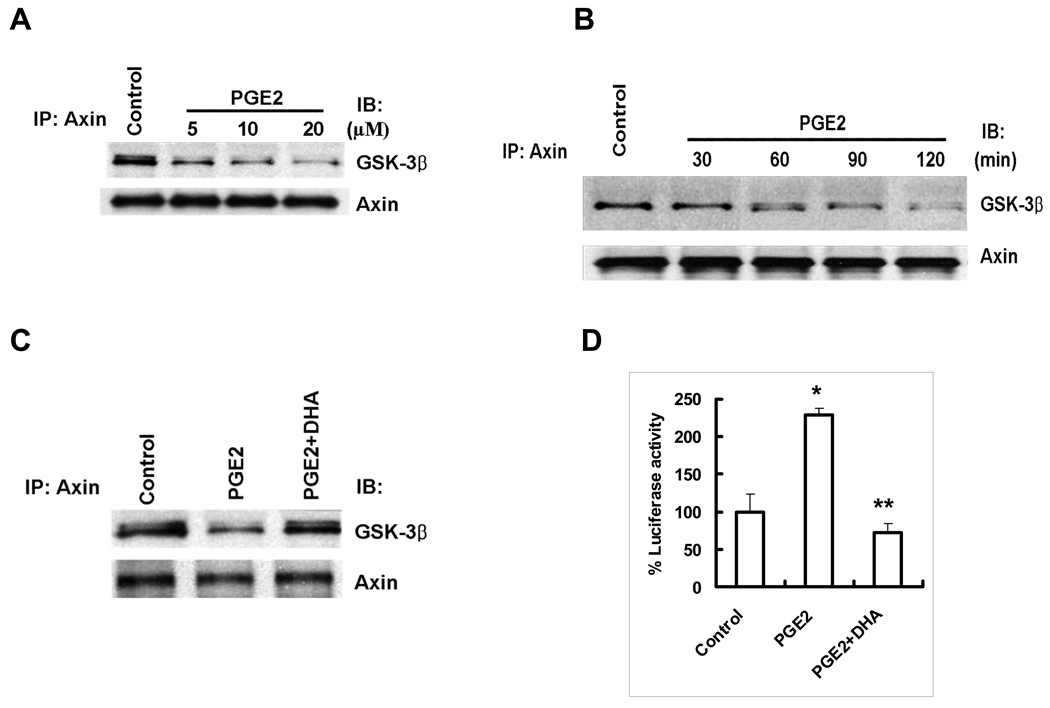
DHA prevents PGE2 induced β-catenin activation
Since DHA reduces PGE2 level through concomitant inhibition of COX-2 and induction of 15-PGDH, we postulate that DHA might also inhibit β-catenin through inhibition of PGE2. To evaluate this hypothesis, further experiments were performed to examine the direct effect of PGE2 on β-catenin activation and to determine whether DHA might prevent PGE2 effect. As shown in Figure 5, PGE2 treatment resulted in dissociation of Axin from GSK-3β and enhanced TCF/LEF reporter activity in Hep3B cells; these effects were significantly blocked by cotreatment with DHA. These findings suggest that suppression of PGE2 by DHA represents another mechanism for β-catenin degradation.
Figure 5. DHA prevents PGE2-induced dissociation of Axin/GSK-β complex in HCC cells.
(A) PGE2 induces dissociation of Axin from GSK-3β in Hep3B cells. The cells were treated with different concentration of PGE2 in the serum-free medium for 1 hr. The cell lysates were obtained for immunoprecipitation with Axin antibody followed by immunoblotting with antibodies against GSK-3β and Axin. (B) The time dependent effect of PGE2 on Axin/GSK-3β dissociation. Hep3B cells were treated with PGE2 (10 µM) for different time points in the serum-free medium. The cell lysates were obtained for immunoprecipitation with anti-Axin antibody followed by immunoblotting with antibodies against GSK-3β and Axin. (C) DHA prevents PGE2-induced Axin/GSK-3β dissociation. Hep3B cells were treated with PGE2 (10 µM) or vehicle in the absence or presence of DHA (60 µM) in serum-free medium for 1 hr. The cell lysates were obtained for immunoprecipitation with anti-Axin antibody followed by immunoblotting with antibodies against GSK-3β and Axin. (D) DHA inhibits PGE2-induced TCF/LEF reporter activity. Hep3B cells were transiently transfected with TCF/LEF-Luc reporter vector. After transfection the cells were treated with PGE2 with or without DHA in the serum-free medium for 24 hr. The cell lysates were obtained to determine the luciferase activity. The data are presented as mean ± SD of six independent experiments. DHA treatment significantly decreased TCF/LEF reporter activity (*p<0.01 compared to control; **p<0.01 compared to PGE2 treatment).
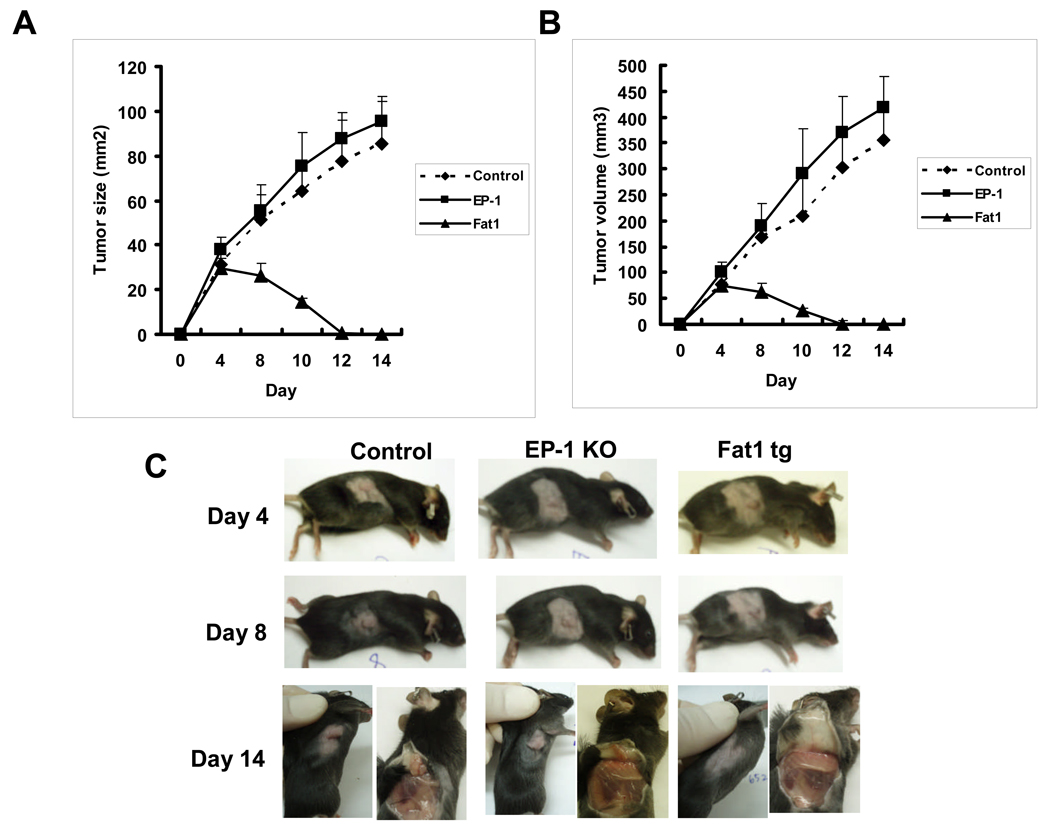
ω-3 PUFAs prevent HCC growth in vivo
After the in vitro effect of ω-3 PUFAs on HCC cell growth was documented, further experiments were carried out to evaluate the effect of ω-3 PUFAs on HCC growth in vivo. We implanted murine HCC cells (Hepa1-6) into the syngeneic Fat-1 transgenic and control mice (with C57BL/6 genetic background) and examined the growth of the inoculated tumor cells in these animals. The Fat-1 transgenic mice carry a Caenorhabditis elegans desaturase gene that adds a double bond into a saturated fatty-acid hydrocarbon chain and converts ω-6 to ω-3 polyunsaturated fatty acids, resulting in a significant increase in ω-3 PUFAs and reduction in ω-6 PUFAs in all the organs and tissues (25). The Hepa1–6 cell line was chosen because it was derived from hepatocellular carcinoma of C57BL/6 strain and can be grown to form tumors in mice with C57BL/6 genetic background. As shown in Figure 6, there is a marked difference in the tumor size and tumor volume between Fat-1 transgenic (n=10) and wild type mice (n=12). Over an observation period of 14 days, all wild type mice developed a palpable tumor by day 4, whereas only 5 of 10 Fat-1 transgenic mice developed a minor tumor palpable by day 4 day and the mass of all palpable tumor almost disappeared at 12 day. Mice with homozygous mutation for the prostaglandin receptor EP1 (in C57BL/6 background) was used as an additional control, which showed a similar degree of tumor growth as the wild type mice. These findings demonstrate that ω-3 PUFAs inhibit HCC growth, in vivo.
Figure 6. Tumorigenicity of Hepa1–6 mouse HCC cells in Fat-1 transgenic and wild type mice.
(A-B) Tumor size and tumor volume in the Fat-1 transgenic and wild type control mice. Hepa1–6 murine HCC cells were obtained from the American Type Culture Collection (ATCC) and cultured in DMEM containing 4 mmol/liter L-glutamine and 10% fetal bovine serum. 1.5 × 106 cells were injected subcutaneously (s.c.) into the right flank of each of Fat-1 transgenic and wild type mice (8-week-old male). Mice with homozygous mutation for the prostaglandin receptor EP1 (in C57BL/6 background) was used as an additional control. Tumor size was monitored at the indicated time with a caliper. Tumor volume was calculated on the basis of the following formula: Tumor volume = (length × width × width)/2. The values are mean ± SD of tumor size or volume of wild type group (n =12), EP1 knockout (n=10) or Fat-1 transgenic (n=10) mice. (C) Representative photographs showing tumor formation at three different time points after cell implantation.
The effect of ω-3 PUFAs on Hepa1–6 cell growth in vitro
Given the marked reduction of Hepa1–6 cell growth in the Fat-1 transgenic mice, we conducted subsequent experiments to evaluate ω3-PUFA actions in Hepa1–6 cell growth, in vitro. Both DHA and EPA significantly reduced the viability of cultured Hepa1–6 cells (Supplementary Figure 6). Treatment of Hepa1–6 with DHA led to reduction of β-catenin protein as well as TCF/LEF reporter activity (Supplementary Figure 7). In parallel, DHA also inhibited the expression of COX-2 and induced the expression of 15-PGDH in Hepa1–6 cells (Supplementary Figure 8A). Consistent with the latter observations, DHA treatment also inhibited the production of PGE2 in Hepa1–6 cells (Supplementary Figure 8B). Therefore, the effects of ω3-PUFAs in Hepa1–6 cells are similar to those in human HCC cells. These findings demonstrate that ω3-PUFAs inhibit β-catenin and COX-2 signaling in both murine and human HCC cells.
DISCUSSION
Both the COX-2/PGE2 and Wnt/β-catenin signaling pathways are active in human hepatocellular carcinomas. There is constitutively high expression and activation of COX-2 in human liver cancers and pre-cancerous inflammatory liver diseases; COX-2 activation enhance the production of PGs from AA that subsequently promote hepatic inflammation and neoplasia (2). In parallel, Wnt/β-catenin pathway is also activated in various stages of hepatic tumorigenesis (13–16, 28–31). Therefore, we postulate that therapies aimed at simultaneous disruption of the COX-2/PGE2 and Wnt/β-catenin pathways may produce effective chemopreventive and anti-tumorigenic effects. This study provides important experimental evidence and mechanisms for inhibition of both Wnt/β-catenin and COX-2/PGE2 signaling pathways by ω-3 PUFAs in HCC (Supplementary Figure 9).
Compelling epidemiological and experimental studies have indicated a relationship between PUFAs and the risk of cancer. For example, a high dietary intake of omega-6 polyunsaturated fatty acids, such as linoleic acid (18:2ω-6), is associated with a high risk for colon cancer, whereas high intake of omega-3 PUFAs from fish oils, such as DHA (22:6ω-3) and EPA (20:5ω-3), decreases it (22, 23). Experimental data have shown that the ω-6 fatty acids stimulate carcinogenesis, tumor growth and metastasis, whereas the ω-3 fatty acids exert suppressive effects. In this study, we utilized both in vitro and in vivo models to evaluate the effect of ω-3 PUFAs on HCC growth. Treatment with DHA and EPA induced a dose- and time-dependent growth inhibition and apoptosis in three human HCC cell lines. The induction of apoptosis is confirmed by cleavages of PARP, caspase-3 and caspase-9 and release of cytochrome c. To evaluate the anti-tumor effect of ω-3 PUFAs on HCC growth in vivo, we implanted murine hepatocellular carcinoma cells (Hepa1–6) into the syngeneic Fat-1 transgenic and control mice. The Fat-1 transgenic mice ubiquitously express a Caenorhabditis elegans desaturase, leading to significant increase in ω-3 PUFAs and reduction in ω-6 PUFAs in all the organs and tissues (25, 32). This model was selected because it provides a balanced ratio of ω-6 to ω-3 fatty acids in mouse tissues and eliminates the potential dietary variation associated with long-term feeding of PUFAs. A significant reduction of HCC tumor size and tumor volume was observed in the Fat-1 transgenic mice. These findings provide important in vivo evidence for inhibition of HCC by ω-3 PUFAs. The effect of dietary DHA and EPA on hepatocellular cancer growth remains to be further evaluated.
A prominent mechanism for the chemopreventive action of ω-3 PUFAs is their suppressive effect on the production of AA-derived prostanoids, particularly PGE2 (23, 33). This is important since PGE2 is implicated in multistages of tumorigenesis, including modulation of inflammation, cancer cell proliferation, differentiation, apoptosis, angiogenesis, metastasis and host immune response to cancer cells (2). Our data in this study show that ω-3 PUFAs inhibit COX-2 expression in HCC cells, which is consistent with recently reported downregulation of COX-2 by ω-3 PUFAs in colon cancer cells (34). Moreover, our findings provide novel evidence for induction of 15-PGDH, a rate-limiting key enzyme in prostaglandin catabolism, by ω-3 PUFAs in human cancer cells. The latter observation is noteworthy, since 15-PGDH is a prostaglandin-degrading enzyme that physiologically antagonizes COX-2 and suppresses tumor growth.
In addition to modulation of COX-2 and 15-PGDH by ω-3 PUFAs, our results reveal that degradation of β-catenin is a novel parallel mechanism for ω-3 PUFA-mediated anti-tumor effect. β-catenin is a key molecule in the canonical Wnt pathway that regulates multiple biological functions, including embryogenesis and tumorigenesis (35–38). In the absence of Wnt ligands, cytoplasmic β-catenin associates in a complex with GSK3β, Axin and APC, where it is phosphorylated and targeted for proteosomal degradation. Activation of Wnt signaling causes dissociation of the β-catenin degradation complex, leading to β-catenin accumulation in the cytoplasm and translocation into the cell nucleus. In the nucleus, β-catenin binds the transcription factor T-cell factor (TCF)/lymphoid enhancer factor (LEF) that induce transcription of important downstream target genes implicated in cell proliferation, differentiation, and apoptosis (35–38). Recent evidence has shown that PGE2 induces the cytoplasmic and nuclear accumulation of β-catenin in human colon cancer cells. Castellone and colleagues (20) reported that PGE2 activates its G protein-coupled receptor, EP2, resulting in direct association of the G protein alpha subunit with the regulator of G protein signaling (RGS) domain of axin; this causes release of GSK-3β from its complex with axin, thus leading to β-catenin accumulation. A separate study by Shao et al showed the involvement of cAMP/protein kinase A pathway in PGE2-induced β-catenin accumulation in colon cancer cells (21). The current study provides evidence that PGE2 induces dissociation of GSK-3β from Axin thus preventing β-catenin reduction in HCC cells.
Our data suggest that ω-3 PUFAs induce β-catenin degradation through three interrelated mechanisms. First, we show that DHA and EPA induce a rapid dephosphorylation of GSK-3β in HCC cells, suggesting that GSK-3β activation is involved in ω-3 PUFA-induced β-catenin degradation. This assertion is further supported by the observations that the GSK-3β inhibitor, LiCl, prevents DHA-induced reduction of β-catenin protein and transcription activity and restored DHA-induced cell death. Second, DHA treatment induces the association of Axin with GSK-3β forming β-catenin destruction complex. Third, ω-3 PUFAs suppress PGE2 signaling through concomitant inhibition of COX-2 and induction of 15-PGDH, thus preventing PGE2-induced β-catenin accumulation. The involvement of β-catenin degradation in ω-3 PUFA-induced inhibition of tumor growth is further supported by the observation that Wnt3a conditioned medium partially protects HCC cells from DHA-induced apoptosis.
In summary, this study provides encouraging preclinical evidence and important mechanism for utilization of ω-3 PUFAs in the chemoprevention and treatment of HCC, although the data should be interpreted with caution since the concentration of EPA and DHA used in the cultured cells is relatively high and most likely will not be achieved in vivo. Our findings have significant clinical implications, given that HCC is a common and highly malignant human cancer. It is conceivable that ω-3 PUFAs, either applied alone or in conjunction with current modalities, may represent an effective, nontoxic, and safe chemopreventive and therapeutic agent for patients with HCC or at high risks for development of this devastating tumor.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank Dr. J. X. Kang at the Harvard Medical School for providing the Fat-1 transgenic mice and thank Dr. Beverly Koller at the University of North Carolina and Dr. John McNeish at Pfizer Inc. for providing the EP1 knockout mice.
The work in the authors’ laboratories is supported in part by National Institutes of Health R01 grants CA134568, CA102325, CA106280 and DK077776 (to T.W.) and DK64207 (to Y.D.) and by KIEST and KOSEF through ISNRC (R13-2007-020-01000-0) (to KL).
ABBREVIATIONS
- AA
arachidonic acid
- COX-2
cyclooxygenase-2
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- GSK-3β
glycogen synthase kinase-3β
- HCC
hepatocellular carcinoma
- LEF
lymphoid enhancer factor
- PG
prostaglandin
- PGE2
prostaglandin E2
- 15-PGDH
15-hydroxyprostaglandin dehydrogenase
- PUFA
polyunsaturated fatty acids
- TCF
T cell factor
Footnotes
No conflicts of interest exist.
REFERENCES
- 1.Coleman WB. Mechanisms of human hepatocarcinogenesis. Curr Mol Med. 2003;3:573–588. doi: 10.2174/1566524033479546. [DOI] [PubMed] [Google Scholar]
- 2.Wu T. Cyclooxygenase-2 in hepatocellular carcinoma. Cancer Treat Rev. 2006;32:28–44. doi: 10.1016/j.ctrv.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Kondo M, Yamamoto H, Nagano H, et al. Increased expression of COX-2 in nontumor liver tissue is associated with shorter disease-free survival in patients with hepatocellular carcinoma. Clin Cancer Res. 1999;5:4005–4012. [PubMed] [Google Scholar]
- 4.Koga H, Sakisaka S, Ohishi M, et al. Expression of cyclooxygenase-2 in human hepatocellular carcinoma: relevance to tumor dedifferentiation. Hepatology. 1999;29:688–696. doi: 10.1002/hep.510290355. [DOI] [PubMed] [Google Scholar]
- 5.Shiota G, Okubo M, Noumi T, et al. Cyclooxygenase-2 expression in hepatocellular carcinoma. Hepatogastroenterology. 1999;46:407–412. [PubMed] [Google Scholar]
- 6.Bae SH, Jung ES, Park YM, Kim BS, Kim BK, Kim DG, Ryu WS. Expression of cyclooxygenase-2 (COX-2) in hepatocellular carcinoma and growth inhibition of hepatoma cell lines by a COX-2 inhibitor, NS-398. Clin Cancer Res. 2001;7:1410–1418. [PubMed] [Google Scholar]
- 7.Rahman MA, Dhar DK, Yamaguchi E, et al. Coexpression of inducible nitric oxide synthase and COX-2 in hepatocellular carcinoma and surrounding liver: possible involvement of COX-2 in the angiogenesis of hepatitis C virus-positive cases. Clin Cancer Res. 2001;7:1325–1332. [PubMed] [Google Scholar]
- 8.Leng J, Han C, Demetris AJ, Michalopoulos GK, Wu T. Cyclooxygenase-2 promotes hepatocellular carcinoma cell growth through Akt activation: evidence for Akt inhibition in celecoxib-induced apoptosis. Hepatology. 2003;38:756–768. doi: 10.1053/jhep.2003.50380. [DOI] [PubMed] [Google Scholar]
- 9.Cheng AS, Chan HL, To KF, Leung WK, Chan KK, Liew CT, Sung JJ. Cyclooxygenase-2 pathway correlates with vascular endothelial growth factor expression and tumor angiogenesis in hepatitis B virus-associated hepatocellular carcinoma. Int J Oncol. 2004;24:853–860. [PubMed] [Google Scholar]
- 10.Ikeda T, Tozuka S, Hasumura Y, Takeuchi J. Prostaglandin-E-producing hepatocellular carcinoma with hypercalcemia. Cancer. 1988;61:1813–1814. doi: 10.1002/1097-0142(19880501)61:9<1813::aid-cncr2820610915>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 11.Han C, Michalopoulos GK, Wu T. Prostaglandin E(2) receptor EP(1) transactivates EGFR/MET receptor tyrosine kinases and enhances invasiveness in human hepatocellular carcinoma cells. J Cell Physiol. 2006;207:261–270. doi: 10.1002/jcp.20560. [DOI] [PubMed] [Google Scholar]
- 12.Thompson MD, Monga SP. WNT/beta-catenin signaling in liver health and disease. Hepatology. 2007;45:1298–1305. doi: 10.1002/hep.21651. [DOI] [PubMed] [Google Scholar]
- 13.Monga SP. Hepatic adenomas: Presumed innocent until proven to be beta-catenin mutated. Hepatology. 2006;43:401–404. doi: 10.1002/hep.21110. [DOI] [PubMed] [Google Scholar]
- 14.Zucman-Rossi J, Jeannot E, Van Nhieu JT, et al. Genotype-phenotype correlation in hepatocellular adenoma: New classification and relationship with HCC. Hepatology. 2006;43:515–524. doi: 10.1002/hep.21068. [DOI] [PubMed] [Google Scholar]
- 15.Satoh S, Daigo Y, Furukawa Y, et al. AXIN1 mutations in hepatocellular carcinomas, and growth suppression in cancer cells by virus-mediated transfer of AXIN1. Nat Genet. 2000;24:245–250. doi: 10.1038/73448. [DOI] [PubMed] [Google Scholar]
- 16.Branda M, Wands JR. Signal transduction cascades and hepatitis B and C related hepatocellular carcinoma. Hepatology. 2006;43:891–902. doi: 10.1002/hep.21196. [DOI] [PubMed] [Google Scholar]
- 17.Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta. 2003;1653:1–24. doi: 10.1016/s0304-419x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 18.Colnot S, Decaens T, Niwa-Kawakita M, et al. Liver-targeted disruption of Apc in mice activates beta-catenin signaling and leads to hepatocellular carcinomas. Proc Natl Acad Sci U S A. 2004;101:17216–17221. doi: 10.1073/pnas.0404761101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merle P, de la Monte S, Kim M, et al. Functional consequences of frizzled-7 receptor overexpression in human hepatocellular carcinoma. Gastroenterology. 2004;127:1110–1122. doi: 10.1053/j.gastro.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 20.Castellone MD, Teramoto H, Williams BO, Druey KM, Gutkind JS. Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-beta-catenin signaling axis. Science. 2005;310:1504–1510. doi: 10.1126/science.1116221. [DOI] [PubMed] [Google Scholar]
- 21.Shao J, Jung C, Liu C, Sheng H. Prostaglandin E2 Stimulates the beta-catenin/T cell factor-dependent transcription in colon cancer. J Biol Chem. 2005;280:26565–26572. doi: 10.1074/jbc.M413056200. [DOI] [PubMed] [Google Scholar]
- 22.Hardman WE. (n-3) fatty acids and cancer therapy. J Nutr. 2004;134:3427S–3430S. doi: 10.1093/jn/134.12.3427S. [DOI] [PubMed] [Google Scholar]
- 23.Larsson SC, Kumlin M. Ingelman-Sundberg M, Wolk A. Dietary long-chain n-3 fatty acids for the prevention of cancer: a review of potential mechanisms. Am J Clin Nutr. 2004;79:935–945. doi: 10.1093/ajcn/79.6.935. [DOI] [PubMed] [Google Scholar]
- 24.Xu L, Han C, Lim K, Wu T. Cross-talk between peroxisome proliferator-activated receptor delta and cytosolic phospholipase A(2)alpha/cyclooxygenase-2/prostaglandin E(2) signaling pathways in human hepatocellular carcinoma cells. Cancer Res. 2006;66:11859–11868. doi: 10.1158/0008-5472.CAN-06-1445. [DOI] [PubMed] [Google Scholar]
- 25.Kang JX, Wang J, Wu L, Kang ZB. Transgenic mice: fat-1 mice convert n-6 to n-3 fatty acids. Nature. 2004;427:504. doi: 10.1038/427504a. [DOI] [PubMed] [Google Scholar]
- 26.Backlund MG, Mann JR, Holla VR, et al. 15-Hydroxyprostaglandin dehydrogenase is down-regulated in colorectal cancer. J Biol Chem. 2005;280:3217–3223. doi: 10.1074/jbc.M411221200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Myung SJ, Rerko RM, Yan M, et al. 15-Hydroxyprostaglandin dehydrogenase is an in vivo suppressor of colon tumorigenesis. Proc Natl Acad Sci U S A. 2006;103:12098–12102. doi: 10.1073/pnas.0603235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Torbenson M, Lee JH, Choti M, et al. Hepatic adenomas: analysis of sex steroid receptor status and the Wnt signaling pathway. Mod Pathol. 2002;15:189–196. doi: 10.1038/modpathol.3880514. [DOI] [PubMed] [Google Scholar]
- 29.Koch A, Denkhaus D, Albrecht S, Leuschner I, von Schweinitz D, Pietsch T. Childhood hepatoblastomas frequently carry a mutated degradation targeting box of the beta-catenin gene. Cancer Res. 1999;59:269–273. [PubMed] [Google Scholar]
- 30.Ogawa K, Yamada Y, Kishibe K, Ishizaki K, Tokusashi Y. Beta-catenin mutations are frequent in hepatocellular carcinomas but absent in adenomas induced by diethylnitrosamine in B6C3F1 mice. Cancer Res. 1999;59:1830–1833. [PubMed] [Google Scholar]
- 31.Ranganathan S, Tan X, Monga SP. beta-Catenin and met deregulation in childhood Hepatoblastomas. Pediatr Dev Pathol. 2005;8:435–447. doi: 10.1007/s10024-005-0028-5. [DOI] [PubMed] [Google Scholar]
- 32.Kang JX. From fat to fat-1: a tale of omega-3 fatty acids. J Membr Biol. 2005;206:165–172. doi: 10.1007/s00232-005-0790-3. [DOI] [PubMed] [Google Scholar]
- 33.Smith WL. Cyclooxygenases, peroxide tone and the allure of fish oil. Curr Opin Cell Biol. 2005;17:174–182. doi: 10.1016/j.ceb.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 34.Calviello G, Di Nicuolo F, Gragnoli S, et al. n-3 PUFAs reduce VEGF expression in human colon cancer cells modulating the COX-2/PGE2 induced ERK-1 and -2 and HIF-1alpha induction pathway. Carcinogenesis. 2004;25:2303–2310. doi: 10.1093/carcin/bgh265. [DOI] [PubMed] [Google Scholar]
- 35.Hoppler S, Kavanagh CL. Wnt signalling: variety at the core. J Cell Sci. 2007;120:385–393. doi: 10.1242/jcs.03363. [DOI] [PubMed] [Google Scholar]
- 36.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 37.Gordon MD, Nusse R. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem. 2006;281:22429–22433. doi: 10.1074/jbc.R600015200. [DOI] [PubMed] [Google Scholar]
- 38.Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.