Abstract
Our laboratory has reported that mice that express a dominant negative form of transforming growth factor-β receptor restricted to T cells (dnTGFβRII) develop an inflammatory biliary ductular disease with raised serum levels of IL-12p40 and other proinflammatory cytokines and anti-mitochondrial autoantibodies (AMA), closely resembling human primary biliary cirrhosis (PBC). We have utilized this mouse model to address the potential mechanisms of immune modulation of the liver disease by creating two unique genetic strains, IL-12p40 KO-dnTGFβRII mice and IFN-γ KO-dnTGFβRII mice. The two colonies of genetically modified mice and, for purposes of controls the dnTGFβRII mice, were monitored for liver immunopathology, anti-mitochondrial autoantibodies, and intrahepatic cytokine production. Disease expressions in the IFN-γ KO-dnTGFβRII mice, including liver immunopathology, were similar to those of dnTGFβRII mice, whereas the IL-12p40 KO-dnTGFβRII mice had a dramatic reduction in histological autoimmune cholangitis and significant decreases in levels of intrahepatic proinflammatory cytokines, but similar levels of AMA compared to dnTGFβRII controls. In conclusion, these data indicate that in this mouse model of PBC, signaling via the IL-12p40 is an essential requirement for development of autoimmune cholangitis. The results of these studies will play an important role in identifying pathways and reagents that will selectively inhibit IL-12 signaling for the outlining of future therapeutic strategies for human PBC.
Keywords: dnTGFβRII mice, IL-12, Primary biliary cirrhosis, Anti-mitochondrial antibody
Primary biliary cirrhosis (PBC) is an autoimmune liver disease characterized by the presence of anti-mitochondrial antibodies (AMA) associated with non-suppurative destructive cholangitis in the interlobular bile ducts (1, 2). Several studies on human PBC have suggested that an autoimmune T cell response to the E-2 subunit of the mitochondrial enzyme complex PDC (PDC-E2) is a critical factor in the pathogenesis of PBC (3-6). We have recently reported that mice transgenic for directed expression of a dominant negative form of TGF-β receptor type II (dnTGFβRII), under the control of the CD4 promoter lacking the CD8 silencer, spontaneously develop an autoimmune biliary ductular disease, attributable to a dysregulated T-cell response, that histologically and serologically closely resembles human PBC (7). Moreover CD8 T cells isolated from dnTGFβRII mice upon adoptive transfer to Rag1 knockout (KO) mice induce a PBC-like cholangitis in recipient mice (8). However, the detailed mechanisms by which effector CD8+ T cells are recruited, and mediate biliary pathology in this mouse model remain unknown.
It is well established that cytokines produced by immune cells are major factors in the development of autoimmunity and, among these, IFN-γ and IL-12 have emerged as prototypic Th1 cytokines implicated in autoimmune inflammatory diseases (9-15). In the case of, IL-12, the functional form of the cytokine is a heterodimer (p70) comprised of two disulfide linked subunits, p35 and p40. IL-12p70 is secreted by dendritic cells (DC) and macrophages after activation of Toll-like receptors (TLR) by a variety of ligands which include especially ligands for TLR9. Such activation induces the generation of Th1 responses by stimulating the production of IFN-γ, TNF-α, and various other proinflammatory cytokines (16-18). IL-12 initiates its signaling by binding to its cognate IL-12 receptor expressed on NK cells and activated T cells (19, 20).
We report herein that the deletion of IL-12p40 in dnTGFβRII mice led to a marked diminution in the levels of proinflammatory Th1 cytokines in the liver of dnTGFβRII mice with accompanying reductions in cellular infiltrates in portal tracts associated with diminished bile duct damage. However the deletion of IFN-γ in dnTGFβRII mice had no significant effect on the immunopathology of autoimmune cholangitis. Thus our data indicate that signaling via the IL-12p40 pathway(s) is a major determinant of the autoimmune cholangitis that affects dnTGFβRII mice.
Materials and Methods
Mouse strains
C57Bl/6J (B6), B6.129S7-IFN-γtm1Ts (IFN-γ KO), and B6.129S1-Il12btm1Jm (IL-12p40KO) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Our colony of dnTGFβRII mice were bred onto a B6 strain background at the University of California at Davis animal facility (Davis, CA). To generate IL-12p40KO-dnTGFβRII mice, male dnTGFβRII mice were bred with female IL-12p40KO mice to obtain IL-12p40+/- dnTGFβRII mice, which were subsequently backcrossed with female IL-12p40KO mice to obtain IL-12p40KO- dnTGFβRII mice. The parental dnTGFβRII and the derived IL-12p40KO-dnTGFβRII mice at 3-4 weeks of age were genotyped to confirm the dnTGFβRII gene and IL-12p40KO in their genomic DNA (7). Similarly, IFN-γKO-dnTGFβRII mice were generated by backcrossing IFN-γKO mice to dnTGFβRII mice and the genotype confirmed. dnTGFβRII mice were fed sterile rodent Helicobacter Medicated Dosing System (three-drug combination) diets (Bio-Serv, Frenchtown, NJ), and maintained in individually ventilated cages under specific pathogen-free conditions. Sulfatrim (Hi-tech Pharmacal, Amityville, NY) was delivered through drinking water according to the manufacturer's instruction.
Serum immunoglobulins (Ig) and antimitochondrial antibodies
Levels of serum IgG, IgM, and IgA were determined using a murine IgG, IgM and IgA ELISA Quantitation kit (Bethyl laboratories, Montgomery, TX). Microtiter plates were coated with goat anti-mouse IgG, IgM, or IgA affinity-purified Ab and incubated overnight at 4°C. Plates were washed with PBS-T, and blocked with 200 μl of 50 mM Tris, 0.15 M NaCl, and 1% BSA (pH 8.0) for 30 min. Next the plates were washed with PBS-T, then 100 μl of test samples and standards were added to the wells and incubated for 1 hour at room temperature (RT), followed by PBS-T washes. 100 μl of a pre-determined optimum dilution of HRP-labeled goat anti-mouse IgG, M, or a Fc-specific mAb were added and incubated for 1 hour at RT. Plates were washed, developed with TMB substrate for 30 minutes, the reaction stopped with 2N H2SO4, and read at 450 nm with a microplate reader. Serum AMA was detected using an enzyme-linked immunosorbent assay (ELISA) based on recombinant murine PDC-E2 as described previously (21). Immunoreactivity was determined by measuring the optical density (O.D.) at 450 nm after incubation with 100 μl of TMB substrate (BD Biosciences, San Jose, CA) for 30 minutes.
Pathology of liver
Portions of freshly isolated liver were immediately fixed in 10% buffered formalin solution for 2 to 3 days. Paraffin-embedded tissues were cut into 4-μm slices and stained with hematoxylin (DakoCytomation, Carpinteria, CA) and eosin (American Master Tech Scientific, Lodi, CA) (HE). The scoring of pathological changes in liver was performed by a “blinded” pathologist.
Flow cytometry
PBS-perfused livers were passed through a 100-μm nylon cell strainer (BD Bioscience, Bedford, MA), and re-suspended in PBS/0.2% BSA. The hepatocytes were removed as pellets by centrifugation at 700 rpm for 1 minute and the residual cells collected. Splenic tissue was disrupted between 2 glass slides and suspended in PBS/0.2% BSA. Lymphocytes from the suspended residual liver and spleen cells were gradient-isolated using Accu-Paque (density: 1.086, Accurate Chemical & Scientific Corp., Westbury, NY). After centrifugation, cells at the interface were collected and washed with PBS/0.2% BSA, and the viability of cells confirmed by trypan blue dye exclusion. Mononuclear cells were enumerated using a hemocytometer. For flow cytometry, cell preparations were incubated with monoclonal antibody (mAb) 2.4G2 for FcR blocking (BioLegend, San Diego, CA), and then stained at 4°C with a combination of fluorochrome-conjugated mAbs which included PE-conjugated anti-NK1.1 (Pharmingen, San Diego, CA), PE-conjugated anti-CD62L (Biolegend), Per-CP-conjugated anti-CD8α (Biolegend), Per-CP conjugated anti-CD4 (Biolegend), Alexa Fluor 647 anti-TCR-β (Biolegend), APC conjugated NK1.1 (Biolegend), APC/Cy7 conjugated CD-4 (Biolegend), Alexa Fluor 750-TCRβ (eBiosciences), Fluorescein isothiocyanate (FITC)-conjugated anti-CD19 (eBiosciences, San Diego, CA), and FITC conjugated anti-CD44 (Biolegend). Multi-color flow analysis was performed using a FACScan flow cytometer (BD Immunocytometry System, San Jose, CA) upgraded by Cytec Development (Fremont, CA) to allow for a 5-color analysis. Acquired data were analyzed with CELLQUEST software (BD Biosciences). The numbers of CD4+, CD8+, CD19+ cells in liver and spleen were calculated based on flow cytometric analysis of stained cells with specific fluorochrome-conjugated Abs.
Liver cytokine and chemokine analysis
To extract total liver protein, 100-200 mg samples of frozen liver tissue were homogenized in TNE buffer (1% NP 40, 10 mM Tris-Hcl pH 7.5, 150 mM NaCl, 1 mM EDTA) containing a cocktail of protease and phosphatase inhibitors (Roche, Indianapolis, IN). The suspension was centrifuged at 12000 rpm for 20 min at 4 °C and the supernatant stored at -80 °C. Levels of IL-12p70, TNF-α, IFN-γ, MCP-1, IL-6, and IL-10 in the liver extracts were measured using the cytometric bead array (CBA) kit (Mouse Inflammation Cytokine Kit, BD Biosciences). Samples also were subject to flow cytometry using a FACScan flow cytometer and the data analyzed using appropriate BD CBA Software.
Levels of monokines induced by IFN-γ (MIG, CXCL9) and IFN-γ inducible protein (IP-10, CXCL10) in liver extracts were measured using the Quantikine mouse MIG/CXCL9 and mouse IP-10/CXCL10 kit (R&D systems, Minneapolis, MN), respectively. The protein concentration of liver samples was measured using the BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA), which included protein standards.
Statistical procedures
Statistical analysis was performed utilizing a two-tailed unpaired Mann-Whitney test, one-way ANOVA test followed by Bonferroni multiple comparisons test, Kruskal-Wallis test followed by Dunn's multiple comparisons test, and Fisher's exact test. P values less than 0.05 were considered statistically significant.
Results
Depletion of IFN-γ does not inhibit autoimmune biliary disease
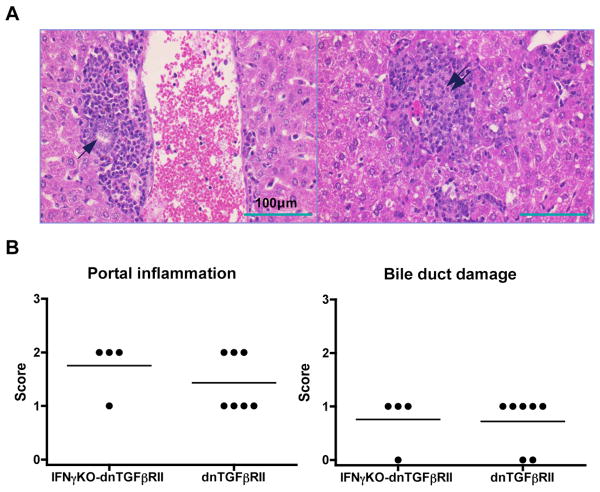
We have previously shown that the onset of autoimmune biliary ductular disease in dnTGFβRII mice is associated with a striking increase in the serum levels of the Th1 proinflammatory cytokine, IFN-γ (7). We therefore addressed the role of IFN-γ in this model by crossing IFN-γKO mice onto dnTGFβRII mice to generate the IFN-γKO-dnTGFβRII mice. Histological examination of the liver tissue sections from 6 month old mice demonstrated that IFN-γKO-dnTGFβRII mice had portal tract lymphocyte infiltrates and biliary ductular lesions equivalent to those in liver tissues from similarly aged wild-type dnTGFβRII mice (Fig. 1A and 1B). Thus lack of the Th1 cytokine IFN-γ was insufficient to influence the course of liver disease in dnTGFβRII mice.
Figure 1.
Histological evidence of cholangitis in the liver of IFN-γKO-dnTGFβRII mice. A. HE-stained liver sections of IFN-γKO-dnTGFβRII mice demonstrate lymphoid cell infiltration in portal tracts around bile ducts (arrow, left panel) and a damaged bile duct within the cell-infiltrated portal area (double arrow, right panel). B. Scoring of liver portal inflammation and bile duct damage in IFN-γKO-dnTGFβRII mice (left) compared to wild-type dnTGFβRII mice (right) was coded as follows: 0, no inflammation (or bile duct damage); 1, mild inflammation (or bile duct damage); 2, moderate inflammation (or bile duct damage); 3, severe inflammation (or bile duct damage). The scores were deemed to be equivalent.
IL-12p40KO-dnTGFβRII
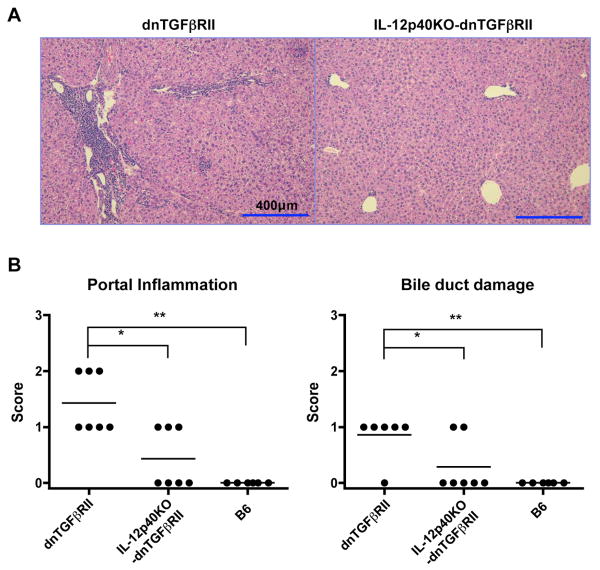
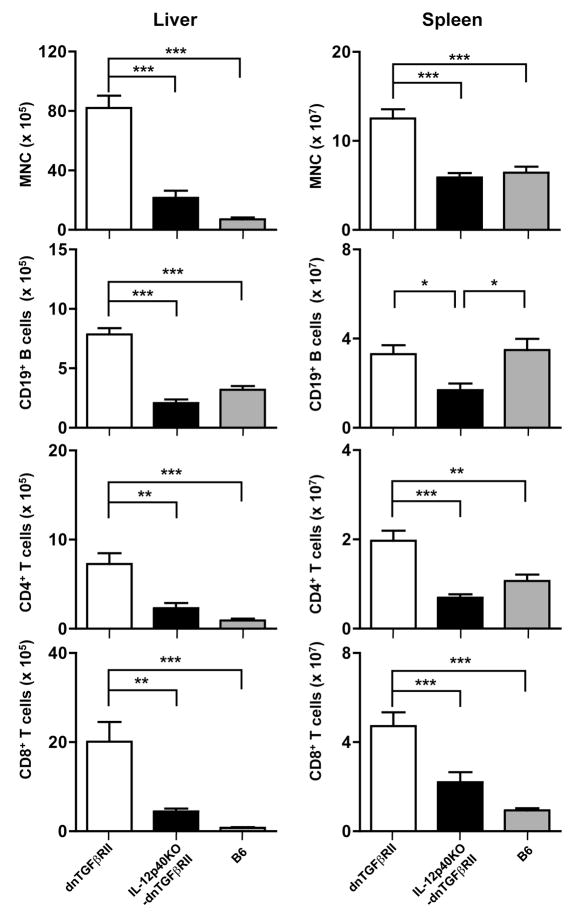
In contrast to the data on IFN-γ KO-dnTGFβRII mice and as illustrated in Fig. 2A, IL-12p40KO-dnTGFβRII mice had significantly fewer and smaller mononuclear cell (MNC) periductular infiltrates in hepatic portal tracts compared to dnTGFβRII mice. Indeed, of 7 IL-12p40KO-dnTGFβRII mice, 4 failed to show any detectable infiltrates (Fig. 2B), and 3 showed only minimal cellular infiltrates. In addition, IL-12p40KO mice had a marked reduction in levels of bile duct damage compared with the control dnTGFβRII mice. Analysis of the relatively depleted intrahepatic lymphoid cell populations within the liver and spleen of selected IL-12p40KO-dnTGFβRII mice corroborated reduced numbers of cellular infiltrates since, compared to dnTGFβRII mice, absolute numbers of MNCs were significantly reduced in livers and spleens of IL-12p40KO-dnTGFβRII mice (Fig. 3, dnTGFβRII liver: 81.9 ± 8.5 × 105, IL-12p40KO-dnTGFβRII liver: 21.6 ± 4.8 × 105, P<0.001; dnTGFβRII spleen: 12.5 ± 1.0 × 107, IL-12p40KO-dnTGFβRII spleen: 5.9 ± 0.5 × 107, P<0.001). Liver-infiltrating CD4+ T cells, CD8+ T cells, and CD19+ B cells were also reduced in the IL-12p40KO-dnTGFβRII mice (Fig. 3). Together, these data indicate that deficiency in IL-12p40 strongly protected dnTGFβRII mice from inflammatory portal lymphoid cell infiltration and bile duct damage.
Figure 2.
Protection from cholangitis in liver sections from IL-12p40KO-dnTGFβRII mice. A. HE-stained tissue sections of liver from dnTGFβRII mouse (left panel) demonstrate lymphoid infiltration in portal tract. In contrast, liver sections from IL-12p40KO-dnTGFβRII mice (right panel) demonstrate absence of lymphoid infiltration. B. Scoring of portal inflammation and bile duct damage in the liver of dnTGFβRII mice, IL-12p40KO-dnTGFβRII mice and normal B6 mice were coded as noted in Fig. 1B. * P <0.05, ** P <0.01, *** P <0.001 using Kruskal-Wallis test followed by Dunn's multiple comparisons test.
Figure 3.
Flow cytometric analysis of the number of total mononuclear cells (MNC), CD19+ B cells, CD4+ T cells and CD8+ T cells among liver and spleen cells from dnTGFβRII mice (n=7), IL-12p40KO-dnTGFβRII mice (n=7) and B6 mice (n=6). Data are shown as mean +/- S.D. *P<0.05, ** P <0.01, *** P <0.001 determined using the one-way ANOVA test followed by Bonferroni multiple comparisons test.
Serum levels of immunoglobulins and antimitochondrial antibodies
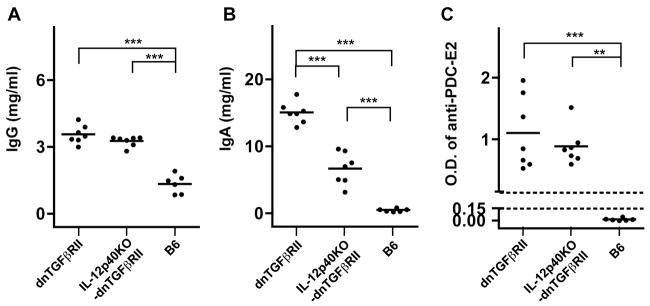
As shown in Fig. 4, while the levels of IgG were higher in the serum of the IL-12p40KO-dnTGFβRII mice compared to normal B6 mice, these levels were comparable with those of dnTGFβRII mice (Fig. 4A). On the other hand, the levels of IgA were significantly lower in IL-12p40KO-dnTGFβRII mice compared with those of dnTGFβRII mice, although were still higher than B6 mice (Fig. 4B). Serum reactivity for anti-PDC-E2 was positive in all IL-12p40KO-dnTGFβRII mice, with OD levels by ELISA comparable to those of dnTGFβRII mice (Fig. 4C), indicating that production of AMA, at least in this autoimmune cholangitis model, is not influenced to any detectable level by IL-12p40.
Figure 4.
Levels of immunoglobulins G and A, and anti-PDC-E2 antibody, in serum of dnTGFβRII mice, IL-12p40KO-dnTGFβRII mice, and normal B6 mice. Horizontal bars represent median values. An OD values of anti-PDC-E2 antibody > 3 standard deviations above the means of that for normal B6 mice was considered positive. ** P <0.01, ***P<0.001 determined by one-way ANOVA test followed by Bonferroni multiple comparisons test.
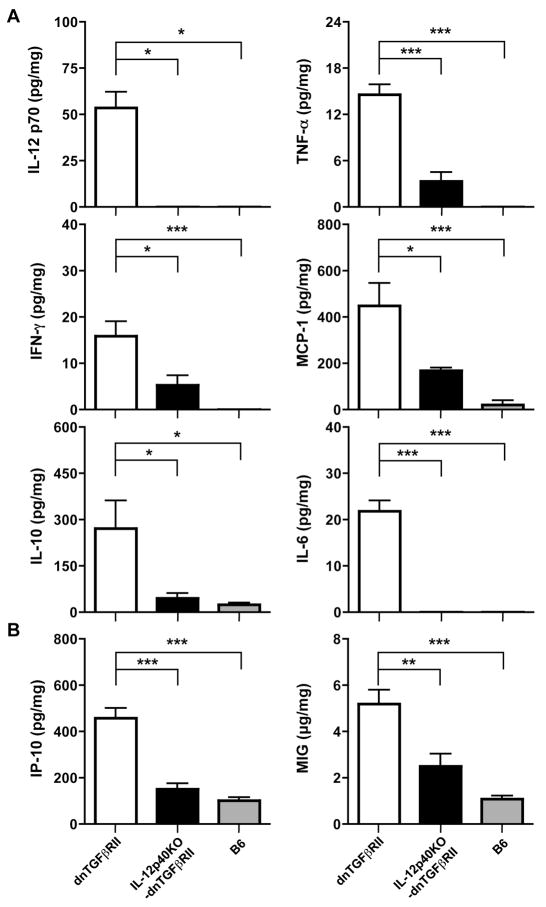
Effect of IL-12p40 deprivation on cytokine and chemokine production in the liver of IL-12p40KO-dnTGFβRII mice
Upregulation of the proinflammatory cytokines, IFN-γ, TNF-α, and IL-6 is associated with the expression of biliary disease in human PBC. Thus we analyzed the amount of proinflammatory cytokines and chemokine ligands in protein extracts prepared from liver tissues of 24-week old IL-12p40KO-dnTGFβRII mice; levels measured were normalized to the amount of total protein. As shown in Fig. 5, while the protein extracts from the liver of IL-12p40-dnTGFβRII mice were slightly elevated compared to B6 mice, they contained significantly lower levels of TNF-α, IFN-γ, IL-10 and IL-6, and CXCL2 (MCP-1) compared with levels in protein extracts from dnTGFβRII mice (Figure 5A). As expected, IL-12p70 was undetectable in the liver of IL-12p40KO-dnTGFβRII mice, indicating that knockout of IL-12p40 did abrogate IL-12p70 production. Since the chemokine ligands CXCL10 (IP-10) and CXCL9 (MIG) are potent chemoattractants for receptors on activated T cells, and promote the migration of these cells to foci of inflammation (22-25), levels were measured in protein extracts from liver tissue of these mice. As shown in Fig. 5B, such levels were significantly decreased in the liver of IL-12p40KO-dnTGFβRII mice compared to dnTGFβRII mice.
Figure 5.
Hepatic profiles of inflammatory cytokines and chemokines in whole protein extracts prepared from the liver of dnTGFβRII (n=7), IL-12p40KO-dnTGFβRII (n=7), and B6 (n=6) mice. Assays for levels of (A) IL-12p70, TNF-α, IFN-γ, MCP-1, IL-10, and IL-6, and (B) IP-10 (CXCL10) and MIG (CXCL9). Results are shown as mean levels +/- S.D. *P<0.05, ** P <0.01, *** P <0.001 determined using the one-way ANOVA test followed by Bonferroni multiple comparisons test.
Discussion
The aim of this study was to determine whether endogenous IL-12p40 and/or IFN-γ play a role in the development of inflammatory biliary disease in dnTGFβRII mice. We demonstrate herein that the deletion of IL-12 p40 gene by backcrossing IL-12p40KO mice to dnTGFβRII mice significantly protected offspring mice from developing autoimmune cholangitis as compared to control dnTGFβRII mice. Both infiltrating CD4 and CD8 T cells, and the production of proinflammatory cytokines in the liver were markedly decreased in IL-12p40KO-dnTGFβRII mice. Our data suggest that the development of autoimmune biliary disease in dnTGFβRII mice is dependent on signaling via the IL-12 pathway(s). Although IFN-γ is one of the proinflammatory Type 1 effector cytokines that could be a determinant of cholangitis, we found that deletion of the IFN-γ gene did not affect the liver immunopathology of dnTGFβRII mice.
IL-12 is a heterodimeric cytokine composed of a large (p40) and a small (p35) subunit. IL-23 (p40p19) has been recently described as a novel cytokine which shares the p40 subunit and several biological functions with IL-12 (26). It is important to note that lack of the p40 subunit of IL-12 results in the absence of both IL-12 and IL-23 (18, 26, 27). Although numerous studies suggest that IL-23 plays a critical role in autoimmune inflammation via triggering pathogenic Th-17 cells (28, 29), recent studies in EAE have demonstrated that IL-12- and IL-23-modulated T cells induce distinct effector populations that use disparate immunological pathways to achieve a similar pathological outcome (30). Given that TGF-β signaling is required by CD4+ T cells for the induction of pathogenic Th-17 cells, whereas in our dnTGFβRII mice, the TGF-β signaling has already been blocked and is incapable of Th-17 induction, the cholangitis in liver is most likely not due to the IL-23-mediated effector IL-17 pathway. Therefore, our findings suggest that signaling via the IL-12 pathway(s) is more important than IL-23 in the pathogenesis of autoimmune cholangitis in this model.
Although knocking out IL-12p40 strongly inhibited cholangitis in dnTGFβRII mice, these KO mice still had AMA in their serum, at levels comparable with those of control dnTGFβRII mice. This finding suggests that synthesis and upregulation of IL-12p40 is not a pre-requisite for the production of AMA by B cells in this model. Our recent data have also demonstrated that at least one subset of B cells has a “suppressor” effect on the inflammatory response in the development of cholangitis; a genetic deficiency of B cells led to the augmentation of not only the PBC-like liver disease but also the coexisting colitis in this model (31). These findings suggest that autoreactive B cells may not be a major factor in the induction of either cholangitis or colitis in the IL-12p40KO-dnTGFβRII mice. These data also highlight the independent feature of the altered immunopathology of IL-12p40 mice and AMA production. Future studies will focus on the possible role of B regulatory cells in modulating this disease. We also will study other combination of double genetic altered strains including, for example, IL-6 knockout in a dnTGFβRII mouse.
We also demonstrated that proinflammatory cytokine levels were not only increased in the serum but also in liver extracted protein of dnTGFβRII mice. The deletion of IL-12p40 causes a significant decrease in levels of these cytokines followed by protection from cholangitis, suggesting that the proinflammatory cytokines do participate in the pathogenesis of the PBC-like liver disease. However, deletion of IFN-γ in these dnTGFβRII mice did not confer protection from disease, indicating that IL-12 in this animal model acts ‘upstream’ in the development of PBC-like liver disease which can thus proceed in the absence of the IFN-γ cytokine. Our findings are in agreement with previous observations that IFN-γ is dispensable for the development of autoimmunity (32, 33). The basic question, however, remains the relationship between the immunopathology and the cholangitis in IL-12p40-triggering Th1 cells in the absence of IFN-γ. We suggest that further studies using other knockout combinations will focus on this problem.
Several studies have been conducted on the intracellular signaling pathways that are involved in translating dialog at the cell membrane into biological function following ligation of IL-12 receptor by the cognate IL-12 cytokine (34, 35). These studies demonstrate that there are marked differences in the amount of IL-12 that is synthesized by dendritic cells depending on the particular TLR pathway that is used for its induction (36-38). A more recent study appears to indicate that the kinetics of RelA activation and in particular serine536 phosphorylation of RelA plays a critical role in controlling the variables that regulate biological activity of IL-12 induction, with a prominent role for TLR9 in inducing high levels of IL-12 (39). Finally, we should also note the possibility that IL-12 may be integrally related to the pathogenic homing of autoreactive CD8 cells to liver. The results of these studies will play an important role in identifying pathways and reagents that will selectively inhibit IL-12 signaling for the outlining of future therapeutic strategies for human PBC.
Acknowledgments
Financial support: Financial support provided by National Institutes of Health grants DK39588 (M.E.G), DK077961 (M.E.G.).
Abbreviations in this paper
- PBC
primary biliary cirrhosis
- AMA
anti-mitochondrial antibody
- dnTGFβRII
dominant negative form of TGFβ receptor type II
- KO
knock out
- DC
dendritic cell
- MNC
mononuclear cell
- Ig
immunoglobulin
- AMA
anti-mitochondrial antibody
- PDC-E2
pyruvate dehydrogenase E2 complex
- RT
room temperature
- OD
optical density
- CBA
cytometric bead array
- ELISA
enzyme-linked immunosorbent assay
Contributor Information
Katsunori Yoshida, Email: yoshidkj@hotmail.co.jp.
Guo-Xiang Yang, Email: gxyang@ucdavis.edu.
Weici Zhang, Email: ddzhang@ucdavis.edu.
Masanobu Tsuda, Email: tsuda.masanobu@gmail.com.
Koichi Tsuneyama, Email: ktsune@med.u-toyama.ac.jp.
Yuki Moritoki, Email: ymoritoki@yahoo.co.jp.
Aftab A. Ansari, Email: pathaaa@emory.edu.
Kazuichi Okazaki, Email: okazaki@tnoc.kmu.ac.jp.
Zhe-Xiong Lian, Email: zxlian@ucdavis.edu.
Ross L. Coppel, Email: ross.coppel@med.monash.edu.au.
Ian R. Mackay, Email: Ian.MacKay@med.monash.edu.au.
M. Eric Gershwin, Email: megershwin@ucdavis.edu.
References
- 1.Gershwin ME, Ansari AA, Mackay IR, Nakanuma Y, Nishio A, Rowley MJ, et al. Primary biliary cirrhosis: an orchestrated immune response against epithelial cells. Immunol Rev. 2000;174:210–225. doi: 10.1034/j.1600-0528.2002.017402.x. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med. 2005;353:1261–1273. doi: 10.1056/NEJMra043898. [DOI] [PubMed] [Google Scholar]
- 3.Gershwin ME, Mackay IR, Sturgess A, Coppel RL. Identification and specificity of a cDNA encoding the 70 kd mitochondrial antigen recognized in primary biliary cirrhosis. J Immunol. 1987;138:3525–3531. [PubMed] [Google Scholar]
- 4.Kamihira T, Shimoda S, Harada K, Kawano A, Handa M, Baba E, et al. Distinct costimulation dependent and independent autoreactive T-cell clones in primary biliary cirrhosis. Gastroenterology. 2003;125:1379–1387. doi: 10.1016/j.gastro.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 5.Kita H, Matsumura S, He XS, Ansari AA, Lian ZX, Van de Water J, et al. Quantitative and functional analysis of PDC-E2-specific autoreactive cytotoxic T lymphocytes in primary biliary cirrhosis. J Clin Invest. 2002;109:1231–1240. doi: 10.1172/JCI14698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimoda S, Ishikawa F, Kamihira T, Komori A, Niiro H, Baba E, et al. Autoreactive T-cell responses in primary biliary cirrhosis are proinflammatory whereas those of controls are regulatory. Gastroenterology. 2006;131:606–618. doi: 10.1053/j.gastro.2006.05.056. [DOI] [PubMed] [Google Scholar]
- 7.Oertelt S, Lian ZX, Cheng CM, Chuang YH, Padgett KA, He XS, et al. Anti-mitochondrial antibodies and primary biliary cirrhosis in TGF-beta receptor II dominant-negative mice. J Immunol. 2006;177:1655–1660. doi: 10.4049/jimmunol.177.3.1655. [DOI] [PubMed] [Google Scholar]
- 8.Yang GX, Lian ZX, Chuang YH, Moritoki Y, Lan RY, Wakabayashi K, et al. Adoptive transfer of CD8+ T cells from dnTGFbetaRII mice induces autoimmune cholangitis into Rag1(-/-) mice. Hepatology. 2008;21:1974–1982. doi: 10.1002/hep.22226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dardalhon V, Korn T, Kuchroo VK, Anderson AC. Role of Th1 and Th17 cells in organ-specific autoimmunity. J Autoimmun. 2008;31:252–256. doi: 10.1016/j.jaut.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Field J, Alderuccio F, Hertzog P, Toh BH. GM-CSF-induced autoimmune gastritis in interferon alpha receptor deficient mice. J Autoimmun. 2008;31:274–280. doi: 10.1016/j.jaut.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Ingegnoli F, Fantini F, Favalli EG, Soldi A, Griffini S, Galbiati V, et al. Inflammatory and prothrombotic biomarkers in patients with rheumatoid arthritis: effects of tumor necrosis factor-alpha blockade. J Autoimmun. 2008;31:175–179. doi: 10.1016/j.jaut.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Lauwerys BR, Van Snick J, Houssiau FA. Serum IL-12 in systemic lupus erythematosus: absence of p70 heterodimers but presence of p40 monomers correlating with disease activity. Lupus. 2002;11:384–387. doi: 10.1191/0961203302lu213oa. [DOI] [PubMed] [Google Scholar]
- 13.Leung BP, McInnes IB, Esfandiari E, Wei XQ, Liew FY. Combined effects of IL-12 and IL-18 on the induction of collagen-induced arthritis. J Immunol. 2000;164:6495–6502. doi: 10.4049/jimmunol.164.12.6495. [DOI] [PubMed] [Google Scholar]
- 14.Monteleone G, Biancone L, Marasco R, Morrone G, Marasco O, Luzza F, et al. Interleukin 12 is expressed and actively released by Crohn's disease intestinal lamina propria mononuclear cells. Gastroenterology. 1997;112:1169–1178. doi: 10.1016/s0016-5085(97)70128-8. [DOI] [PubMed] [Google Scholar]
- 15.Yawalkar N, Karlen S, Hunger R, Brand CU, Braathen LR. Expression of interleukin-12 is increased in psoriatic skin. J Invest Dermatol. 1998;111:1053–1057. doi: 10.1046/j.1523-1747.1998.00446.x. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi M, Fitz L, Ryan M, Hewick RM, Clark SC, Chan S, et al. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989;170:827–845. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magram J, Connaughton SE, Warrier RR, Carvajal DM, Wu CY, Ferrante J, et al. IL-12-deficient mice are defective in IFN gamma production and type 1 cytokine responses. Immunity. 1996;4:471–481. doi: 10.1016/s1074-7613(00)80413-6. [DOI] [PubMed] [Google Scholar]
- 18.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 19.Langrish CL, McKenzie BS, Wilson NJ, de Waal Malefyt R, Kastelein RA, Cua DJ. IL-12 and IL-23: master regulators of innate and adaptive immunity. Immunol Rev. 2004;202:96–105. doi: 10.1111/j.0105-2896.2004.00214.x. [DOI] [PubMed] [Google Scholar]
- 20.Macatonia SE, Hosken NA, Litton M, Vieira P, Hsieh CS, Culpepper JA, et al. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J Immunol. 1995;154:5071–5079. [PubMed] [Google Scholar]
- 21.Moteki S, Leung PS, Dickson ER, Van Thiel DH, Galperin C, Buch T, et al. Epitope mapping and reactivity of autoantibodies to the E2 component of 2-oxoglutarate dehydrogenase complex in primary biliary cirrhosis using recombinant 2-oxoglutarate dehydrogenase complex. Hepatology. 1996;23:436–444. doi: 10.1002/hep.510230307. [DOI] [PubMed] [Google Scholar]
- 22.Liao F, Rabin RL, Yannelli JR, Koniaris LG, Vanguri P, Farber JM. Human Mig chemokine: biochemical and functional characterization. J Exp Med. 1995;182:1301–1314. doi: 10.1084/jem.182.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luster AD, Ravetch JV. Biochemical characterization of a gamma interferon-inducible cytokine (IP-10) J Exp Med. 1987;166:1084–1097. doi: 10.1084/jem.166.4.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Padovan E, Spagnoli GC, Ferrantini M, Heberer M. IFN-alpha2a induces IP-10/CXCL10 and MIG/CXCL9 production in monocyte-derived dendritic cells and enhances their capacity to attract and stimulate CD8+ effector T cells. J Leukoc Biol. 2002;71:669–676. [PubMed] [Google Scholar]
- 25.Romagnani P, Annunziato F, Lazzeri E, Cosmi L, Beltrame C, Lasagni L, et al. Interferon-inducible protein 10, monokine induced by interferon gamma, and interferon-inducible T-cell alpha chemoattractant are produced by thymic epithelial cells and attract T-cell receptor (TCR) alphabeta+ CD8+ single-positive T cells, TCRgammadelta+ T cells, and natural killer-type cells in human thymus. Blood. 2001;97:601–607. doi: 10.1182/blood.v97.3.601. [DOI] [PubMed] [Google Scholar]
- 26.Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 27.Gillessen S, Carvajal D, Ling P, Podlaski FJ, Stremlo DL, Familletti PC, et al. Mouse interleukin-12 (IL-12) p40 homodimer: a potent IL-12 antagonist. Eur J Immunol. 1995;25:200–206. doi: 10.1002/eji.1830250133. [DOI] [PubMed] [Google Scholar]
- 28.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 29.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kroenke MA, Carlson TJ, Andjelkovic AV, Segal BM. IL-12- and IL-23-modulated T cells induce distinct types of EAE based on histology, CNS chemokine profile, and response to cytokine inhibition. J Exp Med. 2008;205:1535–1541. doi: 10.1084/jem.20080159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wakabayashi K, Yoshida K, Leung PS, Moritoki Y, Yang GX, Tsuneyama K, et al. Induction of autoimmune cholangitis in non-obese diabetic (NOD). 1101 mice following a chemical xenobiotic immunization. Clin Exp Immunol. 2009;155:577–586. doi: 10.1111/j.1365-2249.2008.03837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eriksson U, Kurrer MO, Sebald W, Brombacher F, Kopf M. Dual role of the IL-12/IFN-gamma axis in the development of autoimmune myocarditis: induction by IL-12 and protection by IFN-gamma. J Immunol. 2001;167:5464–5469. doi: 10.4049/jimmunol.167.9.5464. [DOI] [PubMed] [Google Scholar]
- 33.Simpson SJ, Shah S, Comiskey M, de Jong YP, Wang B, Mizoguchi E, et al. T cell-mediated pathology in two models of experimental colitis depends predominantly on the interleukin 12/Signal transducer and activator of transcription (Stat)-4 pathway, but is not conditional on interferon gamma expression by T cells. J Exp Med. 1998;187:1225–1234. doi: 10.1084/jem.187.8.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bacon CM, McVicar DW, Ortaldo JR, Rees RC, O'Shea JJ, Johnston JA. Interleukin 12 (IL-12) induces tyrosine phosphorylation of JAK2 and TYK2: differential use of Janus family tyrosine kinases by IL-2 and IL-12. J Exp Med. 1995;181:399–404. doi: 10.1084/jem.181.1.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim J, Uyemura K, Van Dyke MK, Legaspi AJ, Rea TH, Shuai K, et al. A role for IL-12 receptor expression and signal transduction in host defense in leprosy. J Immunol. 2001;167:779–786. doi: 10.4049/jimmunol.167.2.779. [DOI] [PubMed] [Google Scholar]
- 36.Albrecht I, Tapmeier T, Zimmermann S, Frey M, Heeg K, Dalpke A. Toll-like receptors differentially induce nucleosome remodelling at the IL-12p40 promoter. EMBO Rep. 2004;5:172–177. doi: 10.1038/sj.embor.7400078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cowdery JS, Boerth NJ, Norian LA, Myung PS, Koretzky GA. Differential regulation of the IL-12 p40 promoter and of p40 secretion by CpG DNA and lipopolysaccharide. J Immunol. 1999;162:6770–6775. [PubMed] [Google Scholar]
- 38.Hermann C, Spreitzer I, Schroder NW, Morath S, Lehner MD, Fischer W, et al. Cytokine induction by purified lipoteichoic acids from various bacterial species--role of LBP, sCD14, CD14 and failure to induce IL-12 and subsequent IFN-gamma release. Eur J Immunol. 2002;32:541–551. doi: 10.1002/1521-4141(200202)32:2<541::AID-IMMU541>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 39.Bode KA, Schmitz F, Vargas L, Heeg K, Dalpke AH. Kinetic of RelA activation controls magnitude of TLR-mediated IL-12p40 induction. J Immunol. 2009;182:2176–2184. doi: 10.4049/jimmunol.0802560. [DOI] [PubMed] [Google Scholar]