Abstract
Behavioral transitions characterize development. Young infant rats paradoxically prefer odors paired with shock but older pups learn aversions. This transition is amygdala- and corticosterone-dependent. Microarray and microdialysis studies here showed downregulated dopaminergic presynaptic function in the amygdala with preference learning. Corticosterone–injected 8-day-old pups and untreated 12-day-old pups learn aversions and had dopaminergic upregulation in the amygdala. Dopamine injection into the amygdala changed preferences to aversions; dopamine antagonism reinstated preference learning.
Transitional phases in development are associated with specific behaviors within specific social contexts. For example, infants of many altricial species, including birds, dogs and primates, learn attachments to their caregivers despite diverse environments that include both positive and noxious stimulation1, 2. These early conditioned preferences promote attachment to the mother and encourage infants to remain in proximity to the mother, regardless of the quality of care. This can be considered a prerequisite for both attachment, which consists of enhanced approach and attenuated avoidance and safety learning in that the learned cues, both positive and negative, signal the safety of the dam and nest2. However, with maturation, the infant transitions out of the specialized attachment learning. Since our understanding of mechanisms that control developmental transitions is limited, we used an attachment/fear transition model system to explore the amygdala neural changes underlying this transition.
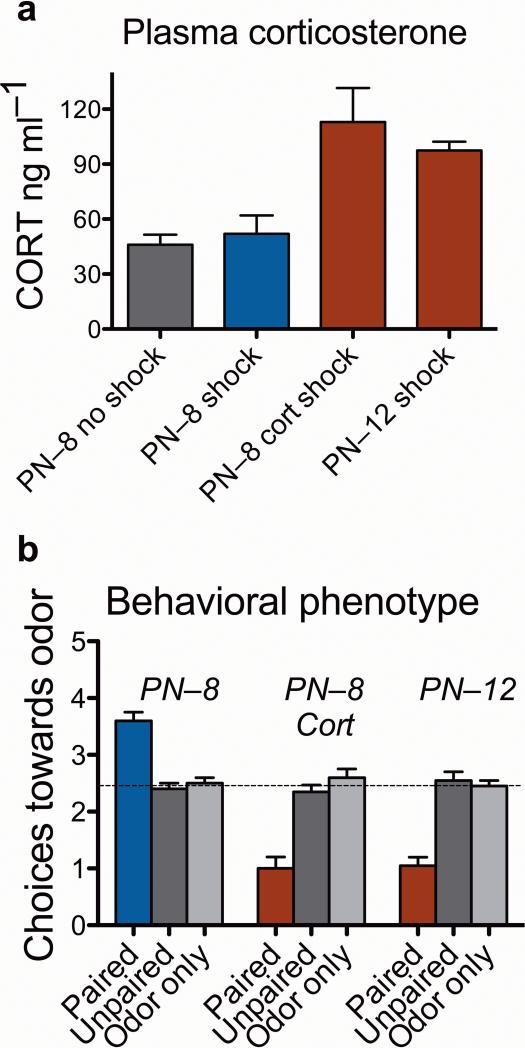
To understand mechanisms within the amygdala that mediate early preference learning and the corticosterone-induced switch to aversion learning, we used an olfactory odor-shock learning paradigm to condition rat pups to learn an odor preference at 8 days of age (postnatal day 8; PN–8) or an odor aversion at the same age when treated with corticosterone. We also tested 12-day-old animals (PN–12) that normally learn an aversion. Between these ages (at 10 days of age) pups transition from odor-shock preference learning to more adult-like amygdala dependent fear/odor-avoidance learning. There is an endogenous increase in shock-induced corticosterone that is essential for avoidance learning and induces the switch from odor preference to avoidance learning irrespective of age. The amygdala, which is rich in glucocorticoid receptors3, only participates in conditioning during aversion learning, again regardless of age but dependent on corticosterone levels4–6 (Fig. 1).
Figure 1.
Role of shock on corticosterone and behavior. (a) Pups at 8 days of age are within the stress hypo-responsive period15 when mild shock (0.5 mA) does not elevate mean plasma corticosterone levels. In contrast, injection of corticosterone in a saline vehicle (at 24 hours and 30 minutes before conditioning; 3.0 mg kg–1, i.p.) produces elevated plasma corticosterone levels following shock, equal about to that induced by mild shock at 12 days of age. (ANOVA: significant group effect; F3, 9 = 9.95, P < 0.005). (b) Infant odor-shock conditioning paradoxically causes an odor preference in 8–day–old pups, although similar conditioning in 12–day–old pups causes an odor aversion. This odor aversion can be learned in 8–day–old animals if injected with corticosterone prior to conditioning. Control groups (unpaired odor-shock and odor-only pups) failed to learn either an aversion or preference, regardless of the age or corticosterone treatment. The dotted line represents chance performance. [ANOVA: significant interaction between conditioning groups and drug treatment (F4,49 = 16.07, P < 0.0001)]. For all figures: bars represent means ± s.e.m.; blue indicates conditions under which preferences are learned and red indicates conditions under which aversions are learned.
For broad screening of possible phenotypic related changes within the amygdala we used microarrays (Affymetrix) to examine changes in gene expression. We determined significant differential gene expression using Ranked Products7 followed by confirmatory quantitative PCR analyses (Table 1; also Supplementary Methods; Supplementary Tables 1–3 online). One class of genes related to presynaptic dopamine function followed the behavioral phenotype: tyrosine hydroxylase (Th), the dopamine transporter (Slc6a3; DAT), dopa decarboxylase (Ddc), and the solute carrier 18a2 (Slc18a2, VAT2; which loads monoamines into vesicles). An aldehyde dehydrogenase (Aldh1a1), which is highly and specifically expressed in dopaminergic cells of the substantia nigra and ventral tegmental area8, also followed the phenotype. At PN–8, each of these dopaminergic components was significantly downregulated in the odor-shock paired group compared to the controls. Conversely, pups learning an odor aversion (PN–8 corticosterone paired and PN–12 paired) showed significant upregulation of a subset of these dopamine related genes. At 12 days of age, four dopamine-related genes were significantly upregulated (Th; Slc6a3; Slc18a2; Aldh1a1). Most strikingly, pretreatment of PN–8 pups with corticosterone resulted in upregulation of Th and Slc6a3, similar to the PN–12 pups. Dopamine receptors (Drd1a and Drd2) were regulated in a phenotypic direction but opposite to that of the presynaptic markers. Other genes for other transmitter systems were up- or down-regulated by learning but not in a phenotypically specific manner (Supplementary Tables 1–3).
Table 1.
Microarray and PCR results
| 8–DAY–OLD |
8–DAY–OLD CORT |
12–DAY–OLD |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | p-val | Array Fold | PCR fold | Direction | p-val | Array Fold | PCR fold | Direction | p-val | Array Fold | Direction |
| Aldh1a1 | .000 | –2.03 | –1.64 | DOWN | 0.584 | 1.30 | 2.21 | UP | 0.025 | 1.77 | UP |
| Ddc | .004 | –1.95 | DOWN | 0.782 | –1.23 | 0.846 | 1.18 | ||||
| Drd1a | .020 | 2.12 | UP | 0.212 | –1.72 | 0.213 | –1.40 | ||||
| Drd2 | .192 | 1.44 | UP | 0.417 | –1.29 | 0.408 | –1.33 | ||||
| Slc18a2 | .001 | –2.26 | DOWN | 0.564 | 1.24 | 0.070 | 1.80 | UP | |||
| Slc6a3 | .000 | –3.49 | –4.97 | DOWN | 0.052 | 2.14 | 2.27 | UP | 0.016 | 2.25 | UP |
| Th | 000 | –3.32 | –2.92 | DOWN | 0.003 | 1.89 | 1.82 | UP | 0.071 | 1.30 | UP |
Note. Comparisons are between pups for which odor was paired with shock compared to those for which odor and shock were unpaired. The p-value is corrected for multiple testing7. Fold change for the PCR data is by the delta-delta Ct method using GADPH as a control (see Supplementary methods). All animals were sacrificed immediately after conditioning. For PN–8 paired pups, all presynaptic markers were downregulated (blue) and both Drd1a and Drd2 were upregulated (red). Aspects of other neurotransmitters changed but not in concert with the phenotype (Supplementary Tables 1–3). At 12 days that pattern was reversed, although not all probesets were significant. Likewise, and consistent with the behavioral data, CORT changed the pattern of gene expression from that of a PN–8 pup to that of a PN–12 pup when conditioned (again not always significantly). The “n's” for the PN–12 (n = 4) and PN–8 CORT pups (n = 3) treated pups were smaller than those for the normal PN–8 pups (n = 7), which might account for the fewer significant effects. The University of Oklahoma IACUC committee approved all experiments. Data are on deposit in GEO (Accession code: GSE17651).
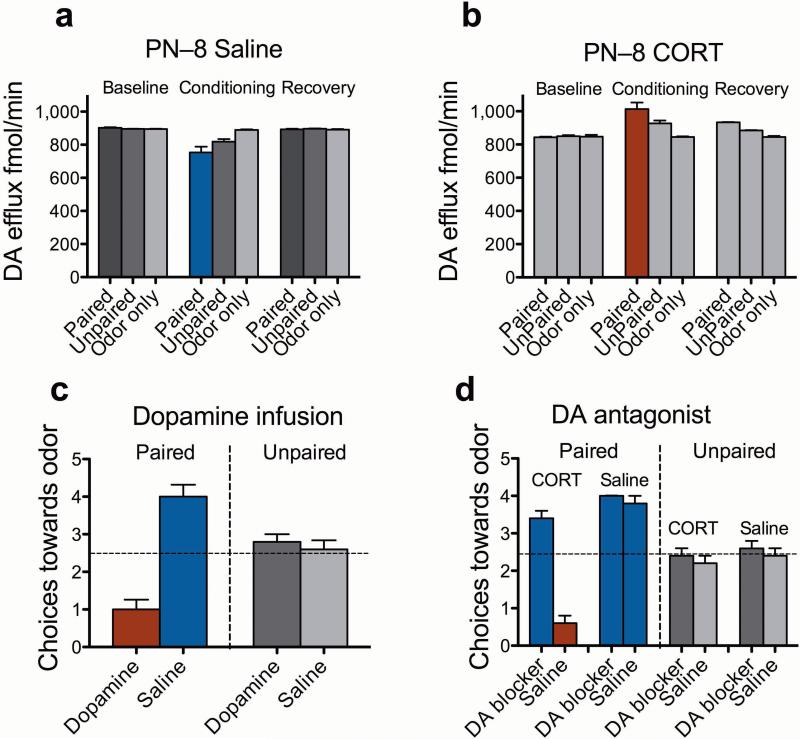
We then directly assessed amygdala dopamine efflux during odor-shock conditioning in 8–day–old pups using in vivo microdialysis with HPLC. Conditions that allow preference learning (without corticosterone) decreased dopamine efflux from the amygdala (Fig. 2a); conditions that favor aversion learning (with corticosterone) increased amygdala levels of dopamine (Fig. 2b). Pups receiving unpaired presentations showed less change in dopamine efflux than did paired pups (P > 0.05) but in the same direction (see Supplementary Figs. 1 and 2 online for metabolites). We did not see similar phenotypically consistent results with norepinephrine or serotonin efflux in the same samples (data not shown). These data are in concert with the adult literature on fear and safety learning and the role of dopamine within the amygdala9–11.
Figure 2.
Dopamine efflux and manipulation. (a) Measurements of extracellular dopamine efflux within the amygdala at PN–8 before (baseline), during (conditioning) and after (recovery) conditioning. Paired odor-shock treatment, which normally produces an odor preference, decreased amygdala dopamine, whereas in PN–8 animals that normally learn an aversion after injection with corticosterone showed increased amygdala dopamine [Data collapsed over 45 minute bins for presentation. significant interaction between conditioning groups × time; F18,135 = 88.81, P < 0.0001]; (b). PN–8 pups learning an aversion (with corticosterone) had increased amygdala levels of dopamine [significant interaction between conditioning groups × time; F18,135 = 71.87, P < 0.0001]. Post-hoc Fisher tests revealed that all Paired groups were significantly different from the control groups (P < 0.001); (c) At 8 days of age, dopamine infused (3–6 μg 2 μl–1; 0.1 μl min–1 beginning 5 minutes prior to conditioning) into the amygdala resulted in a learned odor aversion during an odor-shock conditioning. Saline infused animals of the same age learned the expected preference for the odor [condition × infusion interaction; F1,17 = 37.36, P < 0.0001]; (d) Blocking amygdala dopamine receptors with the receptor antagonist cis-Flupenthixol (20 μg 2 μl–1 as per dopamine) caused PN–8 pups receiving corticosterone to switch to an odor preference rather than the odor aversion seen with corticosterone injection during an odor-shock conditioning in the same aged rats [interaction between conditioning groups, drugs infusion and corticosterone/saline; F1,32 = 17.79, P < 0.0005].
The microdialysis experiments confirmed the microarray data and suggest that low levels of dopamine in the amygdala assist in preventing odor aversion learning in rat pups at young ages. To test this more directly, pups were implanted with bilateral amygdala cannulas at 6 days of age and returned to the nest (see Supplementary Figs. 3 and 4 online for probe placements) 5, 6. At 8 days of age, pups were infused with saline, dopamine or a dopamine receptor antagonist (cis-flupenthixol) during odor-shock conditioning and returned to the nest. The next day, a Y-maze test assessed odor preference/aversion learning. Infusions of dopamine into 8–day–old pups caused the age-typical odor preference learning to switch to aversion learning (Fig. 2c). In contrast, blocking amygdala dopamine receptors in pups treated with corticosterone resulted in preference learning even though those receptors were upregulated at this age (Table 1; Fig. 2d).
Infants are uniquely adapted to regulate transitions during development to insure that different age appropriate “safe” behavioral strategies are engaged. The experimental data and clinical experience suggest that attachment by the altricial infant to the caretaker has evolved to ensure that the infant forms a bond to that caregiver regardless of the quality of care received. Downregulation of the presynaptic elements of dopamine transmission within the amygdala may be one neural mechanism, at least in the rat, by which the amygdala is prevented from participating in avoidance/fear conditioning during attachment learning to the maternal odor. Importantly, the transition out of this specialized period of preference learning does not depend on the pups’ age (within limits) but rather on corticosterone levels normally regulated by the mother. Thus dopaminergic activity “flips” from inhibition to activation in a circuit that includes at least the amygdala, fully consistent with phenotype not age and is likely responsive to levels of stress. The amygdala and the ventral tegmental dopaminergic cells contain glucocorticoid receptors 3, 12 and corticosterone enhances the firing rates of dopaminergic cells 13. Rapid switches are crucial to survival for both adults and infants and the mechanisms required to react with age appropriate behaviors to similar stimuli in different contexts (e.g. stress) may predestine similar mechanisms in the adult. 14
Our results closely parallel the role of dopamine in amygdala-dependent fear conditioning in the adult, where increased activation of dopaminergic neurons within the amygdala modulates fear related behaviors9, 10. Moreover, there are parallels between dopaminergic mechanisms within the amygdala that mediate the preference learning shown here and safety learning in the adult10, 11: 1) dopamine function is decreased in the amygdala; 2) dopamine agonists decrease preference learning and abolish the safety response; and 3) dopamine antagonists abolish aversion learning and enhance the safety response. Thus, processes that mediate preference learning in the young pup, and which serve to keep the pup safely within the nest and to foster attachment to the dam, may also code safety in the adult.
Supplementary Material
Acknowledgments
This work was supported by grants NIH-NICHD-HD33402, NSF-IOB-0544406 and NIH-NIDCD-DC009910 to RMS and NIH-NIDA DA00325 and NIH-NIMH MH080603 to GAB. We thank Liat Levita and the anonymous reviewers for helpful criticisms.
REFERENCES
- 1.Maestripieri D, Lindell SG, Ayala A, Gold PW, Higley JD. Neurosci Biobehav Rev. 2005;29:51–57. doi: 10.1016/j.neubiorev.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan RM, Moriceau S, Raineki C, Roth TL. In: Cognitive Neuroscience IV. Gazzaniga M, editor. MIT Press; Cambridge, MA: 2009. pp. 889–904. [Google Scholar]
- 3.Meaney MJ, Sapolsky RM, McEwen BS. Brain Res. 1985;350:159–164. doi: 10.1016/0165-3806(85)90259-7. [DOI] [PubMed] [Google Scholar]
- 4.Sullivan RM, Landers M, Yeaman B, Wilson DA. Nature. 2000;407:38–39. doi: 10.1038/35024156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moriceau S, Sullivan RM. Nat Neurosci. 2006;9:1004–1006. doi: 10.1038/nn1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moriceau S, Wilson DA, Levine S, Sullivan RM. J Neurosci. 2006;26:6737–6748. doi: 10.1523/JNEUROSCI.0499-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breitling R, Armengaud P, Amtmann A, Herzyk P. FEBS Lett. 2004;573:83–92. doi: 10.1016/j.febslet.2004.07.055. [DOI] [PubMed] [Google Scholar]
- 8.Galter D, Buervenich S, Carmine A, Anvret M, Olson L. Neurobiol Dis. 2003;14:637–647. doi: 10.1016/j.nbd.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Rosenkranz JA, Grace AA. Nature. 2002;417:282–287. doi: 10.1038/417282a. [DOI] [PubMed] [Google Scholar]
- 10.Bissiere S, Humeau Y, Luthi A. Nat. Neurosci. 2003;6:587–592. doi: 10.1038/nn1058. [DOI] [PubMed] [Google Scholar]
- 11.Pollak DD, et al. Neuron. 2008;60:149–161. doi: 10.1016/j.neuron.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harfstrand A, et al. Proc Natl Acad Sci U S A. 1986;83:9779–9783. doi: 10.1073/pnas.83.24.9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Overton PG, Tong ZY, Brain PF, Clark D. Brain Res. 1996;737:146–154. doi: 10.1016/0006-8993(96)00722-6. [DOI] [PubMed] [Google Scholar]
- 14.Herry C, et al. Nature. 2008;454:600–606. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- 15.Levine S, Dent G, De Kloet ER. In: Encyclopedia of Stress. Fink G, editor. Academic Press; New York: 2000. pp. 518–526. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.