Abstract
Anopheles mosquitoes have been shown to adapt to heavy metals in their natural habitats. In this study we explored the possibility of using Anopheles gambiae sensu stricto as bio-reporters for environmental heavy metal pollution through expressions of their metal responsive metallothionein and α-tubulin genes. The study was undertaken with third instar larvae after selection by cadmium, copper, or lead at LC30 through five successive generations. Expression levels were determined in the fifth generation by semi quantitative RT-PCR on the experimental and control populations. The data were analyzed using one-way ANOVA. The highest metallothionein (F3, 11= 4.574, P = 0.038) and α-tubulin (F3,11= 12.961, P = 0.002) responses were observed in cadmium-tolerant treatments. There was significantly higher expression of metallothionein in cadmium or copper treatments relative to the control (P = 0.012), and in cadmium than in lead treatments (P = 0.044). Expressions of α-tubulin were significantly higher in cadmium than in control treatments (P = 0.008). These results demonstrate capacity of An. gambiae s.s. to develop tolerance to increased levels of heavy metal challenge. The results also confirm the potential of heavy metal responsive genes in mosquitoes as possible bio-indicators of heavy metal environmental pollution. How the tolerance and expressions relate to An. gambiae s.s. fitness and vectorial capacity in the environment remains to be elucidated.
Keywords: Anopheles gambiae s.s, heavy metals, tolerance, pollution, α-tubulin, metallothionein
Introduction
There are currently seven recognized sibling species in the An. gambiae complex, including An. gambiae s.s. and An. arabiensis, the most prolific and widespread African malaria vectors in sub-Saharan Africa. Both species are highly anthropophilic and their larvae typically thrive in structurally simple, sunlit, temporary water collections devoid of vegetation (Gillies and de Meillon, 1968). Although malaria is generally associated with rural areas in Africa, studies have documented frequent occurrence of Anopheles larvae in urban environments (Robert et al., 2003; Hay et al., 2005; Donnelly et al., 2005). An important question is how these mosquitoes respond to different characteristics encountered in these environments. Absence of favorable mosquito larval habitats in urban environments has been associated with reduction in mosquito species diversity (Chinery, 1984; Trape and Zoulani, 1987; Coene, 1993; Coluzzi, 1993; Chinery, 1995). The species that succeed in these environments encounter a variety of larval habitats, some of which may be highly polluted with domestic and industrial sewage (Sattler et al., 2005; Awolola et al., 2007; Djouaka et al., 2007), and heavy metals (Mireji et al., 2008). Understanding the effects of environmental pollutants and responses of Anopheles mosquitoes is important for elucidating any adaptive changes that may take place and how these affect their population dynamics and vectorial capacity.
Tolerance to heavy metals in insects is associated with transcription of genes encoding for defense proteins such as metallothioneins, glutathione (Chin and Templeton, 1993; Coogan et al., 1994), α-tubulin and mucin (Rayms-Keller et al., 2000; Mattingly et al., 2001). Cysteine-rich, metal-binding metallothionein proteins have been implicated in regulating intracellular availability of some heavy metals (Klaassen et al., 1999). Recently, α-tubulin proteins, which are a component of the microtubules, have been linked to heavy metal tolerance (Mattingly et al., 2001). Expression levels of metallothionein or α-tubulin genes in aquatic organisms, such as Anopheles larvae, are useful indicators of evolving tolerance in the insect (Ayala and Coluzzi, 2005) and bio-reporters of environmental pollution by heavy metals (Reed et al., 2003).
In our previous study, we found evidence of global proteomic expressions in An. gambiae s.s. exposed to heavy metals (Mireji et al., 2006). This study explores the use of metallothionein and α-tubulin genes in An. gambiae s.s. as indicators of tolerance to some environmental heavy metal (copper, cadmium, or lead) pollutants.
Materials and Methods
Heavy Metals
Cadmium, copper and lead in the form of cadmium chloride (CdCl2, 99.99%), copper II nitrate hydrate (Cu (NO3)2. 25H2O, >99 %), and lead II nitrate (Pb(NO3)2, 99.5%) were obtained from Fisher Scientific, Fair Lawn, NJ, U.S.A., Sigma-Aldrich, Laborchemikalien, GMBH, Germany and Prolabo, Fontenay, France, respectively.
Test Insects
Anopheles gambiae s.s. (Mbita strain) larvae collected from water bodies at Mbita Point, Suba District, western Kenya (Seyoum et al., 2002), were maintained in an insectary at the International Center of Insect Physiology and Ecology (ICIPE), Nairobi, Kenya, at a density of 500 larvae per 3 liters of distilled water. To simulate natural conditions, the average temperature in the insectary was 29 ± 2°C (day) and 24 ± 2°C (night), with relative humidity (RH) ranging from 57 ± 4% (day) to 72 ± 5% (night). Larvae were fed Tetramin fish food daily. Adult mosquitoes were kept in standard 30×30×30 cm cages in an insectary at 27±2°C, 65–70% RH and a photoperiod of 12:12 h (L–D); 6% glucose solution was offered ad libitum. Three to four-day-old females were starved for 12 h, after which they were allowed to feed regularly on an anaesthetized mouse for approximately 10-minutes at 18:00 h. Engorged females were left in the cages until gravid.
Overall maintenance of the colony followed standard operating procedure for rearing Anopheles mosquitoes (Ford and Green 1972). Approval for feeding mosquitoes on mice was obtained from the Kenya National Ethical Review Board (protocol number KEMRI/RES/7/3/1); the protocol was reviewed and approved by the KEMRI Animal Care and Use Committee (ACUC).
Selection for heavy metal tolerance
Anopheles gambiae s.s. third-instar larvae were selected for heavy metal tolerance in the F1–F5 generations by exposing them to lethal concentrations (LC30) of cadmium, copper, or lead resulting in 30% mortality. The usual practice of selecting for 50% mortality (LC50) resulted in adult survival rates that were too low for analysis. In each of 3 replicates for each metal treatment, 300 larvae were selected from each generation and exposed to the test treatment for 24 h in 1,500 ml of water in polypropylene cylindrical pans (radius 10.5 cm and height 24.1 cm). The larvae were not fed during the exposure period. The susceptibility of An. gambiae s.s. to each metal in successive generations was monitored by determining the LC50 values, as indicated below. A control colony not exposed to metals was reared simultaneously in a separate room and handled in the same manner through all manipulations. Twenty-five LC30 survivors from the fifth generation selection were sampled from each treatment and replicate, and were frozen at −70°C for subsequent metallothionein and α-tubulin expression analyses.
Acute toxicity tests
Twenty-four hour toxicity range tests of cadmium, copper, or lead were conducted on each generation using third-instar An. gambiae s.s. larvae. Three replicates (n = 25 larvae per replicate) were exposed to five lead, cadmium, or copper concentrations within established toxicity response ranges (Finney, 1971), in 400 mL of distilled water in the polypropylene cylindrical pans. The concentrations were validated by direct quantitative determination of cadmium, copper or lead separately in each exposure concentration and replicate using a Buck Scientific 210VGP flame atomic absorption spectrophotometer (Buck Scientific, East Norwalk, Connecticut, USA). Quality control was achieved using certified reference sediment material for cadmium, copper, and lead (IAEA 433) from the International Atomic Energy Agency (Wyse et al., 2004). Larval mortalities were evaluated 24 h post exposure; lethal concentrations and; linear regressions were conducted using probit analysis (Finney, 1971). Changes in slope between generations indicated development of tolerance (Brown and Pal, 1971). The LC30 was used for selection, while LC50 was used to assess changes in tolerance.
RNA and DNA Extraction
Total RNA was isolated from each of the frozen (−70°C) larval samples using the guanidine isothiocyanate based protocol of the Total RNA Isolation System® (Promega, Madison, WI)‥ Integrity of extracted RNA was validated by electrophoresis in 1.0% agarose (Sigma – Aldrich Chemie, Gmbh) RNA denaturing gel in 1.4 % sodium phosphate with 1 µg/ml ethidium bromide staining for visualization. The yield and quality of RNA was determined spectroscopically (Sambrook et al., 1989). The genomic DNA was extracted from a pool of 25 third instar An. gambiae larvae by conventional phenol-chloroform DNA extraction method with RNAase (Sambrook et al. (1989). The RNA and DNA extracted were used for cDNA synthesis and for detection of gDNA contamination in each transcription product, respectively.
Reverse Transcriptions
Reverse transcriptions were conducted using TaqMan Reverse transcriptase reagents (Applied Biosystems, Foster City, CA). Oligo d(T)16 was used as primer in the first step of cDNA synthesis in the reaction mix. The mix consisted of 1X TaqMan RT buffer, 5.5 mM MgCl2, 1.5 µg total RNA, 2.5 µM oligo-dT, 0.4 U/µl RNAse inhibitor, 500 µM dNTPs, 125 U/µl Multiscribe Reverse Transcriptase and H20 in a total volume of 10 µl. This was incubated at 25°C for 10 min to maximize primer-RNA template binding, reverse-transcribed at 48°C for 30 min, and the reverse trancriptase inactivated at 95°C for 5 min. The cDNA generated was stored at −20°C.
PCR
a) Primer Selection
Metallothionein
Anopheles gambiae metallothionein gene (Genebank accession # AAX86006) was obtained from the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov) database using bioinformatic searches. In order to discriminate against possible genomic DNA in the cDNA, metallothionein primers were manually designed from the genomic sequence such that the 5' and the 3' primer pair spanned different exons.
α-Tubulin
A functional homologue of previously characterized heavy metal responsive Chironomus tentans α-tubulin gene (Genebank accession # AF272829) (Mattingly et al., 2001) in Anopheles gambiae was identified by BLAST analysis (Altschul et al., 1997) of the gene against the An. gambiae genome (Holt et al., 2002). The resultant BLAST results were screened for α-tubulin conserved domain through InterProScan (Mulder et al., 2003) analysis, following which the homologue (Genebank accession # XM_309723) was isolated. Primers for amplification of this homologue were designed in silico using primer3 software (Rozen and Skaletsky, 2000).
Ribosomal Protein S7
Anopheles gambiae Ribosomal Protein S7 (RP S7) gene was selected as an internal neutral/loading control (Salazar et al., 1993). The DNA sequence of the gene (Genebank accession # AAA03087) was obtained from the genomic database maintained by the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov). The primers were designed using similar procedure to those used for α-tubulin primers described above. The following primers were obtained: metallothionein 5’-ATGCCCTGCAA GTGCTGTGG-3’ and 5’-GAGCCGTACAACTCCATCCTG-3’, α-tubulin 5’-GGCGATCA TCAT CTACGTTGC-3’ and 5’-GCAGCCGCAGCAACCGCCGGAC-3’, and RP S7 5’-GTACGGCACCAGATTGGTCT-3’ and 5'-GTAGCTGCTGCAAAC TTCGG-3’
In all cases, it was ensured that the melting (Tm) and annealing temperatures, as determined by pDRAW32 software (ACACLONE Software version 1.0), of the respective forward and reverse primers generated were similar and that the expected amplification products did not overlap. Metallothionein, α-tubulin, and RP S7 were expected to yield amplification products of 130, 199 and 497 bp, respectively on cDNA amplification, with metallothionein yielding an additional product of 213 bp in the presence of gDNA contamination.
b) Standard reaction for metallothionein or α-tubulin genes
Standard reactions were conducted as described by Marone et al. (2002). Briefly, 1µl cDNA products were amplified with 1 unit of Taq polymerase (Promega, Madison, MO) in the buffer (provided by the manufacturer which contained no MgCl2) in the presence of the specific primers for metallothionein or α-tubulin genes together with RP S7 primers. A concentration of 5.5 mM MgCl2 (empirically determined) was used in each reaction, to provide optimum yield and specificity. Reactions were carried out in PTC 100 thermocycler. The first cycle included 2 minutes at 94°C, 45 seconds at 56°C, and 1 minute at 72°C. Subsequent cycles involved 1 minute at 94°C, 45 seconds at 56°C, and 1 minute at 72 °C for 22 and 25 cycles, respectively, for metallothionein and α-tubulin. Under these conditions it was confirmed that the cDNA were in the exponential phase of amplification, and that the two sets of primers used in each reaction were not competing with each other. A no-sample negative control was used. All the cDNA sampled were also screened for genomic DNA contamination via PCR using the metallothionein primers.
Documentation of expression products and analysis
The PCR products were loaded onto Ethidium Bromide 3 % agarose gels in a TBE buffer (Samrook et al., 1989). On every gel, a 50 bp DNA ladder molecular weight marker (Life Technologies, Rockville, MD) was run to confirm expected molecular weights of the amplification products. Band intensities were determined by ImageQuant TL 1D analysis software (Amersham Biosciences) and converted to nano gram (ng) quantities of cDNA by calibration against the 500 bp band of GeneRuler™ 50 bp DNA ladder (Fermatas, Hanover, MD). Expression of metallothionein and α-tubulin genes was normalized to that of RP S7 internal control.
Means and standard errors of the individual expressions were calculated. The cDNA expression data were further analyzed by one-way ANOVA with Dunnet's test (Dunnet, 1955) for comparison of treatment means versus control, and Tukey Student T-test post-hoc test was used for multiple mean comparisons. All statistical analyses were conducted using SPSS statistical software (SPSS Corporation, Chicago, Illinois Statistical Package version 11.5) and P values < 0.05 were considered significant.
Results
Changes in tolerance with metal-selection
Results of the changes in tolerance of An. gambiae s.s. larvae to heavy metal selection are summarized in Table 1. Cadmium was found to be the most toxic, followed by copper. The LC50 values for cadmium selection generally increased with successive generations, while those for cooper or lead tended to fluctuate. The LC50 distribution patterns indicate that the highest changes in tolerance associated with cadmium selections occurred in the 3rd and 4th generations while those of copper or lead occurred in the 1st and 2nd generations. The least change was observed in 4th and 5th generations in cadmium or lead treatments, and in 2nd and 3rd generations in copper treatment. Overall, there were approximately 17-, 12- and 4-fold increases in the LC50 values, following cadmium, copper and lead treatments, respectively. Linear regressions of data across generations gave slope values of 0.87 to 2.89, 0.56 to 1.10, and 0.69 to 1.77 for cadmium, copper and lead treatments, respectively.
Table 1.
Median toxicity (LC50) responses of An. gambiae s.s. 3rd instar larvae to cadmium, copper or lead treatments through five successive generations.
| Strain | Generation | LC50 (mg/l) | 95% CI | Slope (± SE) | χ2* |
|---|---|---|---|---|---|
| Cadmium | F1 | 0.61 | 0.57 –1.08 | 2.89 ± 0.47 | 0.94 |
| F2 | 3.12 | 2.14 – 20.18 | 0.87 ± 0.28 | 0.31 | |
| F3 | 5.86 | 3.31 – 8.31 | 1.42 ± 0.25 | 0.92 | |
| F4 | 10.32 | 7.38 –16.65 | 2.22 ± 0.49 | 0.25 | |
| F5 | 7.97 | 0.53 –14.88 | 1.00 ± 0.43 | 0.22 | |
| Copper | F1 | 4.326 | 2.84 – 16.83 | 0.78 ± 0.20 | 0.73 |
| F2 | 8.035 | 3.50 – 24.31 | 1.10 ± 0.23 | 1.30 | |
| F3 | 8.149 | 2.91 – 72.10 | 0.56 ± 0.23 | 0.32 | |
| F4 | 50.345 | 20.85 – 71.33 | 0.65 ± 0.22 | 0.38 | |
| F5 | 9.571 | 3.40 – 17.74 | 0.63 ± 0.20 | 0.62 | |
| Lead | F1 | 126.54 | 67.24 – 188.65 | 1.77 ± 0.32 | 0.93 |
| F2 | 543.03 | 241.63 – 666.54 | 0.86 ± 0.24 | 0.27 | |
| F3 | 205.49 | 52.86 – 487.59 | 0.69 ± 0.19 | 3.19 | |
| F4 | 470.05 | 436.01 – 561.28 | 1.41 ± 0.24 | 4.38 | |
| F5 | 302.68 | 217.07 – 441.98 | 1.51 ± 0.16 | 5.96 |
All Chi square values presented are significantly different at 0.05 levels of P and represent the goodness of fit of the regression lines in the probit analysis.
Expressions of metallothionein or α-tubulin in response to changes in tolerance
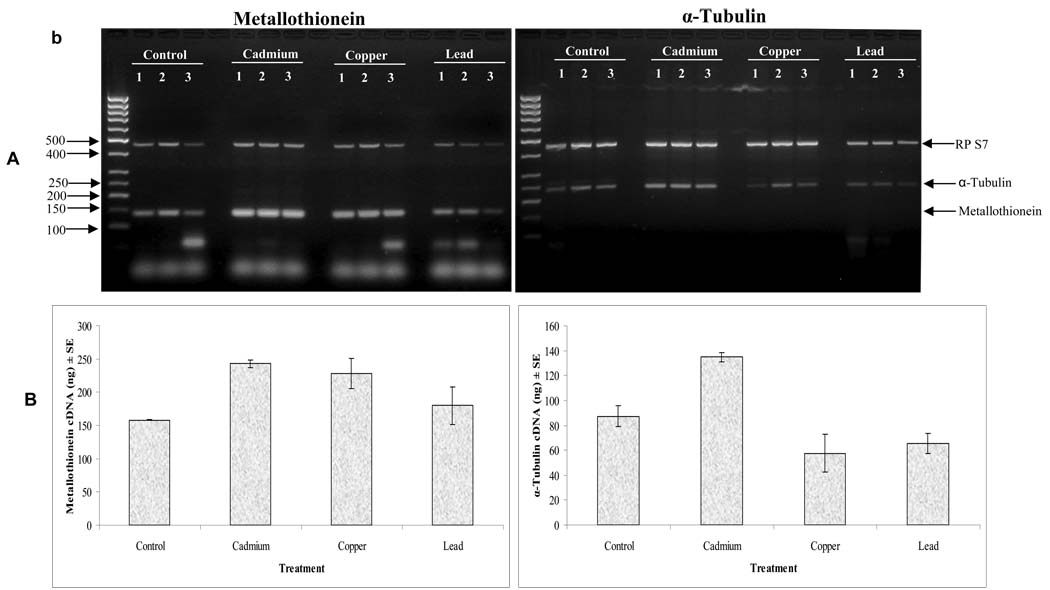
Results of the expressions of metallothionein or α-tubulin gene in An. gambiae s.s. larvae following five generational selections by cadmium, copper and lead are summarized in Fig 1. ANOVA revealed that the highest metallothionein (F3, 11= 4.574, P = 0.038) or α-tubulin (F3,11= 12.961, P = 0.002) responses were observed in cadmium treatment. Dunnett's test identified significant differences in the mean expressions of metallothionein for cadmium (95% confidence 8.64 to 160.69, P =0.031), but not for copper (95% confidence interval −5.69 to 146.36, P = 0.068) or lead (95% confidence interval −54.36 to 97.69, P = 0.749). Similar pattern of responses were obtained for α-tubulin (95% confidence intervals 8.31 to 87.03, P = 0.021 for cadmium, −69.03 to 9.69, P = 0.145 for copper, and −61.36 to 17.36, P = 0.318 for lead). Expression of each gene (for metallothionein and α-tubulin) was at least 1.5-fold higher in cadmium than in control treatments. The Tukey post hoc multiple comparisons of the means revealed significantly higher expression of metallothionein in cadmium (P = 0.012) and copper (P = 0.012) relative to the control treatments, and in cadmium than in lead (P = 0.044) treatments. Similarly, expression of α-tubulin was highest in cadmium treatment (P = 0.008), followed by that of copper and lead.
Fig. 1.
Induction of metallothionein or α-tubulin with RP S7 internal control in the fifth generation of An. gambiae s.s. third instar larvae treatment by heavy metals: Panel A shows products of multiplex PCR amplification cDNA from control and heavy metal tolerant populations. The products were fractionated by agarose gel electrophoresis. The larvae were subjected to five successive generational selections with separate exposures to cadmium, copper and lead of which each selection exposure lasted 24h. Panel B shows the mean expression quantities of metallothionein or α-tubulin (ng) when normalised with expressions of An. gambiae ribosomal protein S7 (RP S7). The expression quantities of metallothionein in the treatments were measured in independent triplicate; the means of the experiments are presented, and the bars represent the standard errors.
Discussion
This study demonstrates significant development of tolerance in An. gambiae s.s. to heavy metals within five generations. However, the exposed insects did not become heterozygous, which is normally associated with metal resistant genotypes indicated by successive drops in the slope of the regressions (Brown and Pal, 1971). Our results show the potential for mosquitoes to withstand relatively high levels of heavy metals, above those previously encountered in metal-polluted natural habitats (Mireji et al., 2008). This could influence their ability to proliferate in metal-polluted aquatic habitats (Sattler et al., 2005; Awolola et al., 2007; Djouaka et al., 2007). However, detailed follow up studies are needed to establish the relative level of mosquito fitness resulting from such heavy metal selection pressure and possible impact of this on their vectorial competence. Heavy metal pollution has been found to modulate immune response in some insect populations in nature (Sorvari et al., 2007).
The unexpected fluctuations in the levels of LC50 in copper and lead selections are similar to those observed in deltamenthrin-treated Anopheles minimus Theobald species A and may be relatively common in populations at early stages of selection pressure (Chareonviriyaphap et al., 2002). The difference in patterns of tolerance to different metal selections, reflected in the expression of metallothionein and α-tubulin genes, suggests that different underlying biological regulatory processes are operating for each metal. Differential expression of metallothionein by these metals has previously been documented (Kramer et al., 1996; Kaji et al., 1994, Hare, 1992). This could be related to differences in the interactions between the metals and metal-responsive transcription factor-1 (MTF-1), a key regulator of heavy metal homeostasis and detoxification in insects (Zhang et al., 2001). Interestingly, in the dipteran Chironomus tentans midge, it has been hypothesized that cadmium-induced α-tubulin is mediated by a calmodulin-dependent pathway (Mattingly et al., 2001) or by depolymerization of the microtubules (Perrino and Chou 1986), which are absent in copper or lead induced responses (Goldstein, 1993). If validated, it would be interesting to establish if the same tolerance mechanism also applies to Anopheles and other known arthropod pollution bio-reporters such as Mayflies (Ephemeroptera), Aedes aegypti (Rayms-Keller et al., 2000) and Culex quinquefasciatus (Sarkar et al., 2004). In addition, a detailed study of heavy metal homeostasis in the mosquito may help shed some light on the role and interactions between the metallothionein and α-tubulin genes and other molecular processes in countering the environmental heavy metal challenges.
In the past, the use of gene expression in Anopheles mosquitoes as indicators of heavy metal pollutants was impeded by previously held dogma that natural habitats of the mosquito are relatively devoid of such pollutants, a notion that has recently been disproved (Sattler et al., 2005;Awolola et al., 2007; Djouaka et al., 2007, Mireji et al., 2008). Moreover, several studies have now indicated that insect metallothionein expression is a potential biomarker for heavy metal pollution in the environment (Klerks and Weis, 1987; Hare, 1992, Roesijadi, 1994) and, indeed, it was used recently in the assessment of aquatic environmental levels of cadmium, copper and zinc through C. quinquefasciatus mosquito larvae (Sarkar et al., 2004). α-Tubulin has been also identified as a possible indicator of a heavy metal responsive gene in insect (Mattingly et al., 2001). Our study confirms that metallothionein and α-tubulin expressions in An. gambiae constitute promising candidate indicators of heavy metal pollution. However, a more exhaustive expression profiling of metal-selected populations of mosquitoes is needed to identify a global response pattern that can lay down a comprehensive groundwork for use in pollution monitoring.
Conclusion
Our results demonstrate a capacity of An. gambiae s.s. to develop tolerance to increased levels of heavy metal challenge under laboratory conditions. Our results also confirm the potential of heavy metal responsive genes in mosquitoes as possible bio-indicators of heavy metal environmental pollution. Application of metal responsive gene expressions in assessment of metal tolerance and related parameters in the wild Anopheles populations can be facilitated by further experiments. How the tolerance and expressions relate to An. gambiae s.s. fitness and vectorial capacity in the environment also remains to be elucidated.
Acknowledgements
We would like to thank Mr. Salim Mwatsahu of the Department of Chemistry, Kenyatta University, Nairobi, Kenya, for his technical assistance in Atomic absorption spectroscopy analysis of the samples and to Ms. Milkah Gitau (ICIPE, Nairobi) for her technical assistance with mosquito rearing. This study was funded by National Institutes of Health (NIH) grant NIH ICIDR U19 A145511 and NIH Fogarty ABC grant D43 TWO1142.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awolola TS, Oduola AO, Obansa JB, Chukwurar NJ, Unyimadu JP. Anopheles gambiae s.s. breeding in polluted water bodies in urban Lagos, southwestern Nigeria. J Vector Borne Dis. 2007;44:241–244. [PubMed] [Google Scholar]
- Ayala FJ, Coluzzi M. Chromosome speciation: humans, Drosophila, and mosquitoes. Proc Natl Acad Sci U S A. 2005;1:6535–6542. doi: 10.1073/pnas.0501847102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AWA, Pal R. Insecticide Resistance in Arthropods. World Health Organization; The 2nd WHO Monograph Ser. 38. 1971 [PubMed]
- Chareonviriyaphap T, Rongnoparut P, Juntarumporn P. Selection for pyrethroid resistance in a colony of Anopheles minimus species A, a malaria vector in Thailand. J. Vector Ecol. 2002;27:222–229. [PubMed] [Google Scholar]
- Chin TA, Templeton DM. Protective elevations of glutathione and metallothionein in cadmium-exposed mesangial cells. Toxicol. 1993;29:145–156. doi: 10.1016/0300-483x(93)90145-i. [DOI] [PubMed] [Google Scholar]
- Chinery WA. Effects of ecological changes on the malaria vectors Anopheles funestus and Anopheles gambiae complex of mosquitoes in Accra, Ghana. J. Trop. Med. Hyg. 1984;87:75–81. [PubMed] [Google Scholar]
- Chinery WA. Impact of rapid urbanization on mosquitoes and their disease transmission potential in Accra and Tema, Ghana. Afr. J. Med. Sci. 1995;24:179–188. [PubMed] [Google Scholar]
- Coene J. Malaria in urban and rural Kinshasa: The entomological input. Med. Vet. Entomol. 1993;7:127–137. doi: 10.1111/j.1365-2915.1993.tb00665.x. [DOI] [PubMed] [Google Scholar]
- Coluzzi M. Advances in the study of Afrotropical malaria vectors. Parasitologia. 1993;35:23–29. [PubMed] [Google Scholar]
- Coogan TP, Bare RM, Bjornson EJ, Waalkes MP. Enhanced metallothionein gene expression is associated with protection from cadmium-induced genotoxicity in cultured rat liver cells. J. Toxicol. Environ. Health. 1994;41:233–245. doi: 10.1080/15287399409531839. [DOI] [PubMed] [Google Scholar]
- Donnelly MJ, McCall PJ, Lengeler C, Bates I, D'lessandro U, Barnish G, Konradsen F, Klinkenberg E, Townson H, Trape JF, Hastings IH, Mutero C. Malaria and urbanization in sub-Saharan Africa. Malar. J. 2005;4:12. doi: 10.1186/1475-2875-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djouaka RF, Bakare AA, Bankole HS, Doannio JM, Coulibaly ON, Kossou H, Tamo M, Basene HI, Popoola OK, Akogbeto MC. Does the spillage of petroleum products in Anopheles breeding sites have an impact on the pyrethroid resistance? Malar J. 2007;6:159. doi: 10.1186/1475-2875-6-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunnet CW. A multiple comparison procedure for comparing several treatments with a control. J. Am. Stat. Assoc. 1955;50:1096–1121. [Google Scholar]
- Finney DJ. Probit Analysis. London: Cambridge University Press; 1971. [Google Scholar]
- Ford HR, Green E. Laboratory rearing of Anopheles albimanus. Mosquito News. 1972;32:509–513. [Google Scholar]
- Gillies MT, De Meillon B. The Anophelinae of Africa South of the Sahara. South African Institute for Medical Research. 1968;54:127–150. [Google Scholar]
- Goldstein GW. Evidence that lead acts as a calcium substitute in second messenger metabolism. Neurotoxicolog. 1993;14:97–101. [PubMed] [Google Scholar]
- Hare L. Aquatic insects and trace metals: Bio-availability, bio-accumulation and toxicity. Critical. Rev. Toxicol. 1992;22:327–369. doi: 10.3109/10408449209146312. [DOI] [PubMed] [Google Scholar]
- Hay SI, Guerra CA, Tatem AJ, Atkinson PM, Snow RW. Urbanization, malaria transmission and disease burden in Africa. Nature reviews. Microbiol. 2005;3:81–90. doi: 10.1038/nrmicro1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt RA, Subramanian GM, Halpern A, Sutton GG, Charlab R, Nusskern DR, Wincker P, Clark AG, et al. The genome sequence of the malaria mosquito Anopheles gambiae. Science. 2002;298:129–149. doi: 10.1126/science.1076181. [DOI] [PubMed] [Google Scholar]
- Kaji T, Suzuki M, Yamamoto C, Imaki Y, Mishima A, Fujiwara Y, Sakamoto M, Kozuka H. Induction of metallothionein synthesis by bismuth in cultured vascular endothelial cells. Res. Commun. Mol. Patho.l Pharmacol. 1994;86:25–35. [PubMed] [Google Scholar]
- Klaassen CD, Liu J, Choudhuri S. Metallothionein: An intracellular protein to protect against cadmium toxicity. Annu. Rev. Pharmacol. Toxicol. 1999;39:267–294. doi: 10.1146/annurev.pharmtox.39.1.267. [DOI] [PubMed] [Google Scholar]
- Klerks PL, Weis JS. Genetic adaptation to heavy metals in aquatic organisms: A review. Environ. Pollut. 1987;45:173–205. doi: 10.1016/0269-7491(87)90057-1. [DOI] [PubMed] [Google Scholar]
- Kramer KK, Liu J, Choudhuri S, Klaassen CD. Induction of metallothionein mRNA and protein in murine astrocyte cultures. Toxicol. Appl. Pharmacol. 1996;136:94–100. doi: 10.1006/taap.1996.0011. [DOI] [PubMed] [Google Scholar]
- Marone M, Mozzetti S, De Ritis D, Pierelli L, Scambia G. Semi-quantitative RT-PCR analysis to assess the expression levels of multiple transcripts from the same sample. Biol. Proced. Online. 2001;3:19–25. doi: 10.1251/bpo20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattingly KS, Beaty BJ, Mackie RS, McGaw M, Carlson JO, Rayms-Keller A. Molecular cloning and characterization of a metal responsive Chironomus tentans alpha-tubulin cDNA. Aquat. Toxicol. 2001;54:249–260. doi: 10.1016/s0166-445x(00)00181-8. [DOI] [PubMed] [Google Scholar]
- Mireji PO, Keating J, Kenya E, Mbogo C, Nyambaka H, Osir E, Githure J, Beier JC. Differential induction of proteins in Anopheles gambiae sensu stricto (Diptera: Culicidae) larvae in response to selection by heavy metals. Int. J. Trop. Ins. Sci. 2006;26:214–226. doi: 10.1017/S1742758406658955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mireji PO, Keating J, Hassanali A, Mbogo CM, Nyambaka H, Kahindi S, Beier JC. Heavy metals in mosquito larval habitats in urban Kisumu and Malindi, Kenya, and their impact. Ecotoxicol Environ Saf. 2008;70:147–153. doi: 10.1016/j.ecoenv.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder NJ, Apweiler R, Attwood TK, Bairoch A, Barrell D, Bateman A, Binns D, Biswas M, et al. The InterPro Database, 2003 brings increased coverage and new features. Nucl. Acids. Res. 2003;31:315–318. doi: 10.1093/nar/gkg046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrino BA, Chou IN. Role of calmodulin in cadmium-induced microtubule disassembly. Cell Biol Int Rep. 1986;10:565–73. doi: 10.1016/0309-1651(86)90031-7. [DOI] [PubMed] [Google Scholar]
- Rayms-Keller A, McGaw M, Oray C, Carlson JO, Beaty BJ. Molecular cloning and characterization of a metal responsive Aedes aegypti intestinal mucin cDNA. Insect Mol Biol. 2000;9:419–426. doi: 10.1046/j.1365-2583.2000.00202.x. [DOI] [PubMed] [Google Scholar]
- Reed DH, Lowe EH, Briscoe DA, Frankham R. Fitness and adaptation in a novel environment: Effect of inbreeding, prior environment, and lineage. Evolution. 2003;57:1822–1828. doi: 10.1111/j.0014-3820.2003.tb00589.x. [DOI] [PubMed] [Google Scholar]
- Robert V, Macintyre K, Keating J, Trape JF, Duchemin JB, Warren M, Beier JC. Malaria transmission in urban sub-Saharan Africa. Am J Trop Med Hyg. 2003;68:169–176. [PubMed] [Google Scholar]
- Roesijadi G. Metallothionein induction as a measure of response to metal exposure in aquatic animals. Environ. Health. Perspect. 1994;102:91–95. doi: 10.1289/ehp.94102s1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozen S, Skaletsky HJ. Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S, editors. Bioinformatics Methods and Protocols: Methods in Molecular Biology. Totowa, NJ: Humana Press; 2000. pp. 365–386. [DOI] [PubMed] [Google Scholar]
- Salazar CE, Mills-Hamm D, Kumar V, Collins FH. Sequence of a cDNA from the mosquito Anopheles gambiae encoding a homologue of human ribosomal protein S7. Nucleic. Acids. Res. 1993;21:4147. doi: 10.1093/nar/21.17.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: A laboratory manual. 2nd edn. New York: Cold Spring Harbour Laboratory Press; 1989. [Google Scholar]
- Sarkar S, Duttagupta AK, Mal TK. Effects of heavy metals on population growth and metallothionein gene expression in the mosquito Culex quinquefasciatus, from Calcutta, India. Environ Pollut. 2004;127:183–193. doi: 10.1016/j.envpol.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Sattler MA, Mtasiwa D, Kiama M, Premji Z, Tanner M, Killeen GF, Lengeler C. Habitat characterization and spatial distribution of Anopheles sp. mosquito larvae in Dar es Salaam (Tanzania) during an extended dry period. Malar J. 2005;14(4):4. doi: 10.1186/1475-2875-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyoum A, Pålsson K, Kung'a S, Kabiru EW, Lwande W, Killeen GF, Hassanali A, Knols BGJ. Traditional use of mosquito-repellent plants in western Kenya and their evaluation in semi-field experimental huts against Anopheles gambiae: ethnobotanical studies and application by thermal expulsion and direct burning. Trans. Royal Soc. Trop. Med. Hyg. 2002;96:225–231. doi: 10.1016/s0035-9203(02)90084-2. [DOI] [PubMed] [Google Scholar]
- Sorvari J, Rantala LM, Rantala MJ, Hakkarainen H, Eeva T. Heavy metal pollution disturbs immune response in wild ant populations. Environ.Pollut. 2006;145:324–328. doi: 10.1016/j.envpol.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Trape JF, Zoulani A. Malaria and urbanization in central Africa: the example of Brazzaville. Part II: Results of entomological surveys and epidemiological analysis. Trans. Royal. Soc. Trop. Med. Hyg. 1987;81:10–18. doi: 10.1016/0035-9203(87)90472-x. [DOI] [PubMed] [Google Scholar]
- Wyse EJ, Azemard S, de Mora SJ. Report on the world-wide intercomparison exercise for the determination of trace elements and methyl-mercury in marine sediment IAEA-433. IAEA/AL/147, IAEA/MEL/75, IAEA. 2004:113. [Google Scholar]
- Zhang B, Egli D, Georgiev O, Schaffner W. The Drosophila homolog of mammalian zinc finger factor MTF-1 activates transcription in response to heavy metals. Mol. Cell. Biol. 2001;21:4505–4514. doi: 10.1128/MCB.21.14.4505-4514.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]