Abstract
Age-related changes in non-adrenergic, non-cholinergic (NANC) neurotransmission might contribute to differences in gastrointestinal motility. Our aim was to determine age-related changes in functional innervation with vasoactive intestinal polypeptide (VIP) and substance P (Sub P) in rat jejunum. We hypothesized that maturation causes changes in neurotransmission with these two neuropeptides. Longitudinal and circular jejunal muscle strips from young (3 months) and middle-aged (15 months) rats (total: 24 rats) were studied; the response to exogenous VIP and Sub P and the effect of their endogenous release from the enteric nervous system during electrical field stimulation (EFS) were evaluated. In longitudinal muscle, response to exogenous VIP and endogenously released VIP during EFS were increased in middle-aged rats, while the effect of endogenously released Sub P was decreased. In the circular muscle, the response to endogenously released VIP was increased in middle-aged rats, while the effects of exogenous VIP and endogenously released Sub P were unchanged. Response to exogenous Sub P was unaffected by maturation in both muscle layers. Spontaneous contractile activity was increased in the longitudinal and circular muscle of the older rats. In the jejunum of middle-aged rats, participation of VIP in functional NANC innervation was increased, while functional innervation with Sub P was decreased. These changes in the balance of inhibitory and excitatory neurotransmission occur during the year of maturation in rats and demonstrate an age-dependant plasticity of neuromuscular bowel function.
Keywords: Aging, enteric nervous system, maturation, motility, small intestine, substance P, vasoactive intestinal polypeptide
INTRODUCTION
Functional gastrointestinal disorders, such as abdominal pain, bloating, diarrhea, constipation, and irritable bowel syndrome, are more common in the elderly, conferring detrimental effects on the well-being of the person (Camilleri et al., 2008; Camilleri et al., 2000; Firth et al., 2002; Orr et al., 2002). Particularly with regard to the aging population of our society, age-related changes in gastrointestinal function are of increasing interest in the field of gastrointestinal research.
Age-related loss of neurons within the myenteric plexus of the enteric nervous system (ENS) was first reported in the rat by Santer and Baker (Santer et al., 1988) and was later confirmed in guinea pig (Gabella, 1989), mice (El-Salhy et al., 1999), and humans (de Souza et al., 1993). Morphologic studies carried out predominantly in rat demonstrated that nitrergic neurons are spared largely from this process, while about 60% of cholinergic neurons within the ENS are lost from the ileum of 24-month-old rats (Cowen et al., 2000; Phillips et al., 2003); cholinergic neurons which express certain specific, calcium-binding proteins are affected more severely (Phillips et al., 2007; Thrasivoulou et al., 2006). These findings appear to vary among anatomic regions, species, and different strains of rats (Phillips et al., 2007). In the myenteric plexus of Fischer 344 rats, neuronal loss begins at the age of 12 months, follows a linear course over time, and is organ-specific with an increasing severity from the proximal to the distal gut (Phillips et al., 2001). Although similar changes occur in the central nervous system, the extent of neurodegeneration of the ENS appears to be far more pronounced (Wade et al., 2004).
Reports about age-related changes in peptidergic innervation with VIP and Sub P are rare. One study showed that VIP- and Sub P-positive neurons are preserved in old rats (24 months), although their number decreased dramatically in senile rats (36 months) (Feher et al., 1987). A recent study confirmed the preservation of VIP- and Sub P-containing neurons in the ileum of 24-month-old rats (Johnson et al., 1998); however, animals in this study had been maintained on a calorie-restricted diet that is known to prevent age-related neuronal loss (Cowen et al., 2000). A study in human colon was unable to demonstrate age-related changes in the VIP concentration within the bowel wall (Koch et al., 1991). To the best of our knowledge, functional studies of age-related changes in functional neuromuscular transmission with VIP and Sub P in the rat jejunum have not been published. We believe that such studies are of particular importance in order to try to link the morphologic changes described above with any objective effects on motor activity than may affect function.
Therefore, the aim of our study was to determine age-related changes in functional innervation of jejunal longitudinal and circular smooth muscle in Lewis rats with the inhibitory neuropeptide VIP and the excitatory neuropeptide Sub P. We hypothesized that distinct, age-related changes in the response to exogenously applied neurotransmitters and their endogenous release during EFS occur during maturation. These changes might then provide clues to explaining gastrointestinal motility disorders in the elderly.
MATERIALS AND METHODS
Preparation of Animals
The study was approved by the Institutional Animal Care and Use Committee (IACUC) of the Mayo Foundation. Animal care and procedures followed the guidelines of the IACUC of the Mayo Foundation in accordance with the guidelines of the National Institutes of Health and the Public Health Service Policy of the Humane Use and Care of Laboratory Animals.
Experimental Groups
Healthy, male, Lewis rats (Harlan, Indianapolis, IN) were housed two rats per cage in a strictly maintained, 12-h light/dark room (6 am to 6 pm) at a room temperature of 20°C, humidity of 45-55%, and with free access to water and standard rat chow (5001 Rodent Diet; PMI Nutrition International LLC, Brentwood, MO) in all experiments. Young rats were 3 months of age and weighed 313 g (200-350 g) [median (range)], while “middle-aged” rats were 15 months of age and weighed 525 g (475-600 g). Longitudinal jejunal muscle was studied in six young and six middle-aged rats, while circular muscle was studied in a different set of six young and six middle-aged rats.
Recording of Contractile Activity
Under anesthesia induced by inhalation of isoflurane 2% (Abbott Laboratories, North Chicago, IL) and maintained by subsequent intraperitoneal sodium pentobarbital (30-50 mg•kg−1; AmproPharmacy, Arcadia, CA), a segment of jejunum harvested 10 cm distal to the ligament of Treitz was immersed in chilled, modified Krebs-Ringer's bicarbonate solution (concentrations in mmol L−1: NaCl 116.4, KCl 4.7, CaCl2 2.5, MgSO4 1.2, KH2PO4 1.2, NaHCO3 23.8, calcium disodium edetate 0.26, and glucose 11.1) pre-oxygenated with 95% oxygen/5% carbon dioxide (Puritan-Bennett Corp., Lenexa, Canada). The rats were killed by exsanguination under anesthesia. The jejunal segment was opened along its mesenteric border, and eight, full-thickness muscle strips (8 mm long and 2 mm wide) per rat were cut either in the direction of the longitudinal or the circular muscle layer. Full thickness muscle strips were studied specifically to preserve the transmural neuromuscular integrity. The strips were then suspended vertically in 10-ml tissue chambers filled with modified Krebs-Ringer's bicarbonate solution which was bubbled continuously with 95% oxygen/5% carbon dioxide and maintained at 37.5°C. The two ends of the muscle strip were at tached to a fixed hook and to a noncompliant force transducer (Kulite Semiconductors Products, Inc., Leonia, NJ) to measure isometric force.
Contractile activity was monitored online with a chart recorder (Grass 7D polygraph; Grass Instrument Co, Quincy, MA) and was saved digitally on a personal computer using dedicated software (MP-100A-CE and AcqKnowledge; Biopac Systems, Inc., Goleta, CA) to allow detailed computer analysis later on.
Experimental Design
After 90 min of equilibration with intervening washouts of the bath solution every 15 min, each muscle strip was stretched incrementally at 10-min intervals to its optimal length (L0) beyond which further stretching did not increase further the amplitude or frequency of spontaneous contractile activity (Murr et al., 1996). Thereafter, non-adrenergic, non-cholinergic (NANC) conditions were established by adding atropine (10−7 M), phentolamine (10−5 M), and propranolol (5×10−6 M) into the bath solution. All subsequent experiments were performed at L0 under NANC conditions starting with the recording of spontaneous contractile activity for 5 min as area under the contractile curve (g × 5 min mg−1 tissue) in all eight muscle strips per rat. A representative recording of spontaneous contractile activity is given in Figure 1A. Strips without spontaneous contractile activity were excluded from the study (<2% of strips). Each subsequent experimental condition was studied in at least two muscle strips per rat. The mean effect of neurotransmitters or EFS was then summarized by calculating the mean effect on contractile activity in these muscle strips from the raw data.
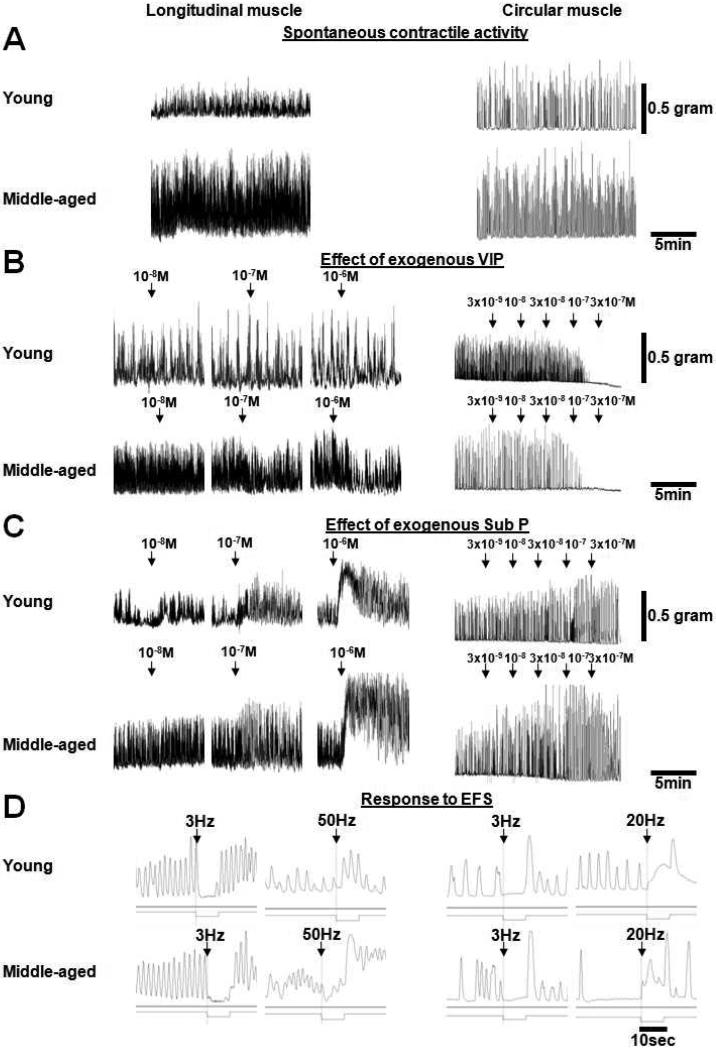
Figure 1.
Representative tracings show raw data from young and middle-aged rats in longitudinal and circular muscle for: A) Spontaneous contractile activity. B) The effect of exogenous VIP. C) The effect of exogenous Sub P. D) The response to EFS at different frequencies.
The response to increasing concentrations of VIP was studied in two strips per rat. When longitudinal muscle strips were studied, three increasing concentrations of VIP (10−8, 10−7, 10−6 M) were administered with washouts between each concentration, while circular muscle strips were exposed to stepwise, escalating, cumulative concentrations of VIP (3×10−9, 10−8, 3×10−8, 10−7, 3×10−7 M; Fig. 1B). Thereafter, the response to the greatest concentration of VIP (longitudinal muscle: 10−6 M; circular muscle: 3×10−7 M) was studied without and with the VIP antagonist ([D-p-Cl-Phe6,Leu17]-VIP; 10−6 M), the NO synthase inhibitor L-NG-nitro arginine (L-NNA; 10−4 M), and the combination of both antagonists. L-NNA was used to determine VIP effects related to a potential interaction between VIP and NO (Van Geldre et al., 2004).
In two other strips per rat, the effect of exogenous Sub P was studied. As for VIP, the longitudinal muscle was exposed to three, increasing concentrations of Sub P (10−8, 10−7, 10−6 M) with intervening washouts, while stepwise, escalating, cumulative concentrations of Sub P were administered when studying circular muscle (3×10−9, 10−8, 3×10−8, 10−7, 3×10−7 M; Fig. 1C). In addition based on other observations (Kasparek et al., 2007a; Kasparek et al., 2007b; Kasparek et al., 2008), muscle strips were precontracted with a submaximal concentration of Sub P (10−7 M), and the effect of a single application of VIP (longitudinal muscle: 10−6 M; circular muscle: 10−7 M) on stimulated contractile activity was studied to determine if the effect of VIP was altered by precontraction. A submaximal concentration of Sub P, instead of the maximal concentration of Sub P, was administered to stimulate phasic contractile activity instead of causing a nonphysiologic, tonic contraction.
The experimental designs for the dose-response experiments varied between muscle layers with single concentrations in longitudinal and cumulative concentrations in circular muscle. These experiments in young and mature rats in the longitudinal and circular muscle layer were done as separate experiments in different time periods using a slightly different study design.
Four muscle strips per rat were subjected to EFS with a constant voltage (longitudinal muscle: 10 V; circular muscle: 20 V), pulse width (longitudinal muscle: 0.5 msec; circular muscle: 4 msec), and duration of stimulation (10 sec for both muscle layers; Fig. 1D). Based on preliminary experiments, 3 and 6 Hz were used as inhibitory frequencies in both muscle layers, and 50 and 20 Hz were used as stimulatory frequencies in longitudinal and circular muscle, respectively. Direct effects of EFS on smooth muscle were excluded by preliminary experiments using tetrodotoxin. Between each EFS, 10 min were allowed for spontaneous activity to recover before the next EFS was applied. After each series of stimulations, the bath solution was exchanged. Two of the four strips were exposed to EFS without and with the VIP antagonist alone, L-NNA alone, and the combination of VIP antagonist and L-NNA. L-NNA was used to antagonize the profound inhibitory effect of endogenously released NO in order to unmask the more subtle effects of endogenously released VIP. The other two strips were studied without and with the Sub P antagonist ([D-Pro2,D-Trp7,9]-Sub P).
At the conclusion of each experiment, the muscle strips were blotted on filter paper and weighed to normalize motility data per mg tissue weight.
Data Analysis
Before studying drug or EFS responses, baseline contractile activity expressed as the area under the contractile curve (g × 5 min mg−1 tissue) was measured for 5 min under NANC conditions at L0 for each strip. Typically, drug effects on contractile activity were evaluated for the 5-min interval after administration and compared to baseline contractile activity during an equally long interval immediately before the drug was administered. When muscle strips were precontracted with Sub P for 2 min, the effect of VIP was measured over the next 5-min interval and compared to the last 90 sec (longitudinal muscle) or 60 sec (circular muscle) of precontraction directly before administration of VIP. Intervals differ between both muscle layers, because stable, stimulated contractile activity was established more rapidly in longitudinal than in circular muscle. Thus, drug responses are given as the percentage change from baseline contractile activity (defined as 0%), which represents the contractile activity 5 min, 90 sec, or 60 sec before drug administration. Positive values represent an increase and negative values a decrease in contractile activity.
The EFS response was determined only during the 10-sec period of stimulation; we did not evaluate the response to termination of EFS (the so-called “off contraction”). Contractile activity was expressed as the percentage change from baseline contractile activity for an equally long interval measured during the 20 sec (longitudinal muscle) or 40 sec (circular muscle) immediately before EFS. Because the frequency of contractions appeared to be less in the circular muscle, a greater duration of baseline interval was used for the circular muscle to obtain a stable and representative measurement of baseline contractile activity.
Data are summarized as mean±SEM. Analysis of variance (ANOVA) was used to examine the effect of different drugs or EFS on contractile activity. Repeated measures ANOVA was used for repeated measurements within the same rat and thus were treated as repeated factors (e.g. for concentration response to VIP, Sub P, or EFS response under different conditions). The two groups of rats were independent factors. When the overall main effect was statistically significant when analyzed by ANOVA, pairwise comparisons were performed post-hoc using paired Student's t-tests (for repeated factors) or two-sample t-tests (for independent group factors). Additional t-tests were used for single comparisons (e.g. versus baseline contractile activity). A Bonferroni correction was applied when evaluating the statistical significance of multiple t-tests. ANOVA on Ranks was used when data were not distributed normally.
Drugs
Atropine sulfate, phentolamine hydrochloride, DL-propranolol hydrochloride, L-NG-nitro arginine, Sub P, [D-Pro2,D-Trp7,9]-Sub P, VIP, and [D-p-Cl-Phe6,Leu17]-VIP were purchased from Sigma-Aldrich, St. Louis, MO.
RESULTS
Longitudinal Muscle
Spontaneous contractile activity
After equilibration, stretching, and establishing NANC conditions, the area under the contractile curve was measured during a 5-min interval and normalized by the tissue weight. The spontaneous contractile activity was less in young versus middle-aged rats (2.5±0.5 vs. 6.0±0.9 g × 5 min mg−1 tissue; p<0.01).
Response to exogenous VIP
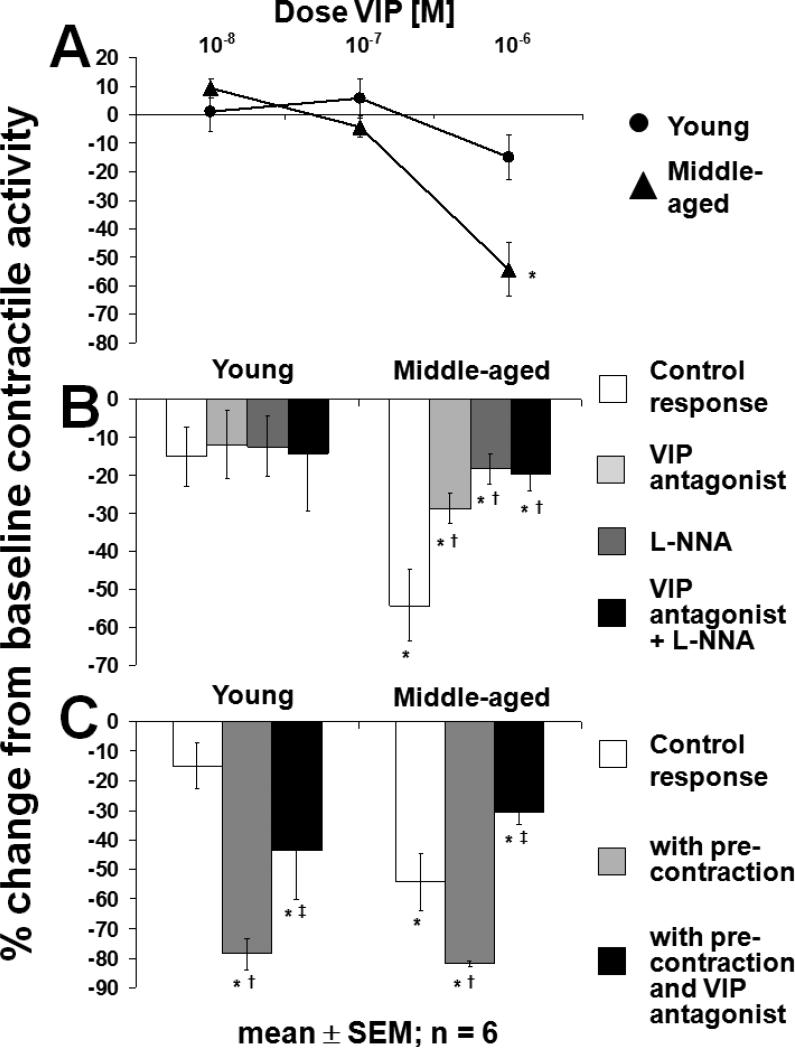
The inhibitory effect of VIP on spontaneous contractile activity was greater in middle-aged rats (p<0.05; ANOVA) in which VIP caused a concentration-dependent inhibition of contractile activity; in contrast, VIP had no measurable effect on spontaneous contractile activity in young rats (Fig. 2A). The inhibitory effect of VIP (10−6 M) in the middle-aged rats was prevented in part by the VIP-antagonist, L-NNA, and their combination (p<0.05; ANOVA); however, L-NNA and the VIP-antagonist did not have additive effects (Fig. 2B). Precontraction of muscle strips with a sub-maximal concentration of Sub P (10−7 M) increased the relative percent inhibitory effect of VIP (10−6 M) in both groups. The inhibitory effect of VIP (10−6 M) on stimulated contractile activity was prevented in part by the VIP antagonist in both groups (p<0.05; ANOVA; Fig. 2C).
Figure 2.
Longitudinal muscle of rat jejunum. A) Response to increasing concentrations of exogenous VIP. VIP caused a concentration-dependent inhibition of spontaneous contractile activity only in middle-aged rats; *p<0.05 for concentration-dependent inhibition of spontaneous contractile activity and vs. young rats (ANOVA). B) Effect of exogenous VIP (10−6 M) in presence of L-NNA (10−4 M) and VIP antagonist (10−6 M). The inhibitory effect of VIP (10−6 M) in middle-aged rats was prevented partially by the VIP antagonist (10−6 M), L-NNA (10−4 M), and the combination of both antagonists with no additional effect of the VIP antagonist and L-NNA; *p<0.01 compared to baseline contractile activity (t-test); †p<0.05 compared to control response (ANOVA). C) Response to VIP (10−6 M) after precontraction with a sub-maximal concentration of Sub P (10−7 M). The inhibitory effect of VIP (10−6 M) was increased after precontraction and was prevented in part by the VIP antagonist (10−6 M) in both groups; *p<0.01 compared to baseline contractile activity (t-test); †p<0.05 compared to control response without precontraction (ANOVA); ‡p<0.05 compared to response with precontraction without VIP antagonist (10−6 M; ANOVA). All experiments were carried out under NANC conditions.
Response to Exogenous Sub P
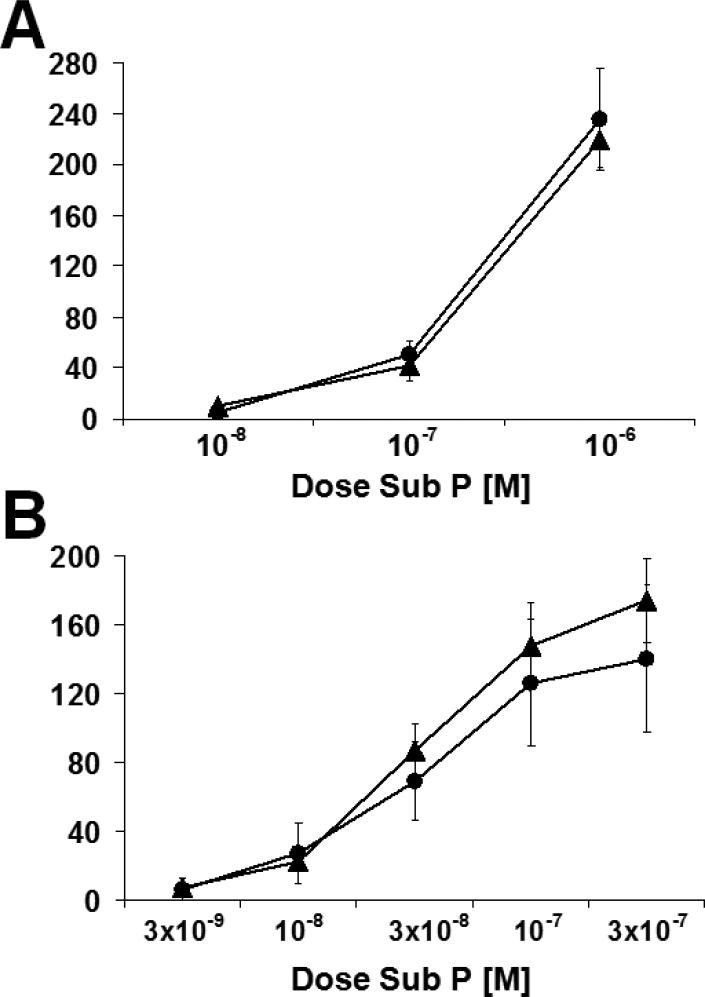
Sub P caused a concentration-dependent increase in spontaneous contractile activity which was comparable between young and middle-aged rats (p<0.05; ANOVA; Fig. 3A).
Figure 3.
Response to increasing concentrations of exogenous Sub P. A) Longitudinal muscle of rat jejunum. B) Circular muscle of rat jejunum. Sub P caused a concentration-dependent increase in spontaneous contractile activity in both groups in both muscle layers (p<0.05; ANOVA). Circles are young rats and triangles are middle-aged rats. All experiments were carried out under NANC conditions. Data is mean±SEM.
Response to EFS
EFS at 3 Hz under control conditions (NANC conditions) inhibited spontaneous contractile activity in both groups (p<0.05; Fig. 4A). Neither the VIP antagonist nor L-NNA alone altered the response to EFS at 3 Hz in either group. In contrast, the combination of the VIP antagonist and L-NNA increased the EFS response compared to all other conditions in middle-aged but not in young rats (p<0.05; ANOVA).
Figure 4.
Response of spontaneous contractile activity of longitudinal muscle of rat jejunum to EFS at A) 3 Hz, B) 6 Hz, and C) 50 Hz. The combination of VIP antagonist and L-NNA increased the response to EFS at 3 Hz in middle-aged but not in young rats. In contrast, at 6 Hz, L-NNA alone and in combination with the VIP antagonist increased the EFS response in both groups. Under control conditions, the EFS response at 50 Hz was greater in young compared to middle-aged rats. The Sub P antagonist decreased the EFS response at 50 Hz only in young rats; *p<0.05 compared to baseline contractile activity; †p<0.05 compared to control response in same group (ANOVA); #p<0.05 compared to response with VIP antagonist in same group (ANOVA); §p<0.05 compared to L-NNA in same group (ANOVA); ‡p<0.05 compared to young rats under same condition (t-test). All experiments were carried out under NANC conditions.
Under control conditions, EFS at 6 Hz inhibited spontaneous contractile activity to a similar extent in both groups (p<0.05; Fig. 4B). While the VIP antagonist alone had no effect on EFS-induced inhibition, L-NNA reversed the inhibitory EFS response compared to the control response in both groups (p<0.05; ANOVA). When the VIP antagonist and L-NNA were combined, the EFS response was increased compared to the response under control conditions and with the VIP antagonist alone in both groups (p<0.05; ANOVA). Overall, EFS responses at 6 Hz did not differ markedly between young and middle-aged rats (p=NS; t-test).
EFS at 50 Hz under control conditions caused a net excitatory response in young rats, while in middle-aged rats, contractile activity was inhibited (p<0.05; Fig. 4C). The Sub P antagonist decreased the EFS response at 50 Hz in young rats (p=0.04) and also in the middle-aged rats, but the effect in middle-aged rats was not significant (p=0.11)
Circular Muscle
Spontaneous contractile activity
As with longitudinal muscle, spontaneous contractile activity was also less in the circular muscle of the young versus the middle-aged rats (4.6±0.5 g × 5 vs. 7.5±1.3 g × 5 min mg−1; p=0.06).
Response to exogenous VIP
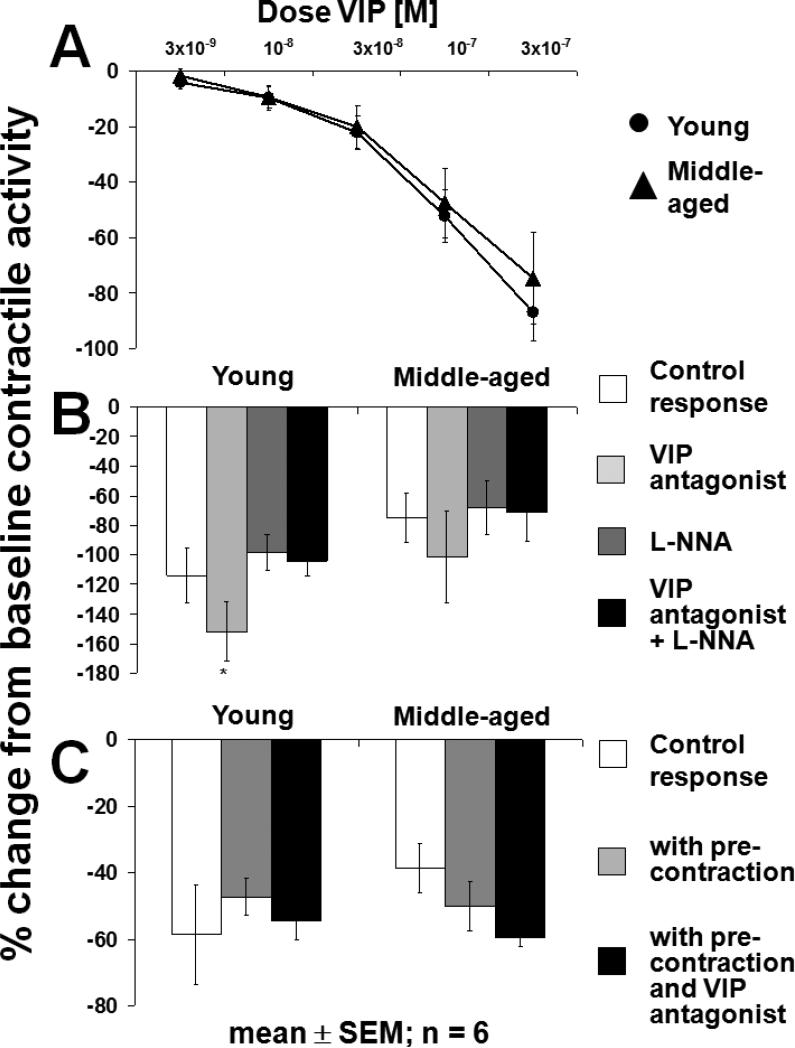
The profound inhibition of spontaneous contractile activity by exogenous VIP in the circular muscle was comparable between groups (p<0.05; Fig. 5A) but was more pronounced compared to the longitudinal muscle (see Fig. 2). The inhibitory effect of VIP (3×10−7 M; p<0.05 vs. baseline; ANOVA) was unaffected by the VIP antagonist, L-NNA, and their combination in both groups (p=NS; Fig. 5B); however, in young rats, the VIP-induced inhibition (3×10−7 M) was more pronounced in the presence of the VIP antagonist compared to the response with L-NNA (p<0.05; ANOVA). After precontraction of muscle strips with a sub-maximal concentration of Sub P (10−7 M; p<0.05 vs. baseline; ANOVA), the inhibitory response to VIP (10−7 M) was unchanged, and the VIP antagonist did not inhibit the effect of VIP (p=NS; Fig. 5C).
Figure 5.
Circular muscle of rat jejunum. A) Response to escalating, cumulative concentrations of exogenous VIP. VIP caused a concentration-dependent inhibition of spontaneous contractile activity in both groups (p<0.05; ANOVA). B) Effect of exogenous VIP in the presence of L-NNA (10−4 M) and VIP antagonist (10−6 M). The inhibitory effect of VIP was not blocked by the VIP antagonist, L-NNA, or the combination of both, was greater in presence of the VIP antagonist than in presence of L-NNA (*p<0.05; ANOVA). C) Response to VIP (10−7 M) after precontraction with a sub-maximal concentration of Sub P (10−7 M). The inhibitory effect of VIP on precontracted muscle was unaffected by the VIP antagonist (p=NS). All experiments were carried out under NANC conditions.
Response to exogenous Sub P
Sub P caused a comparable, concentration-dependent increase in contractile activity in young and middle-aged rats (p<0.05; ANOVA; Fig. 3B).
Response to EFS
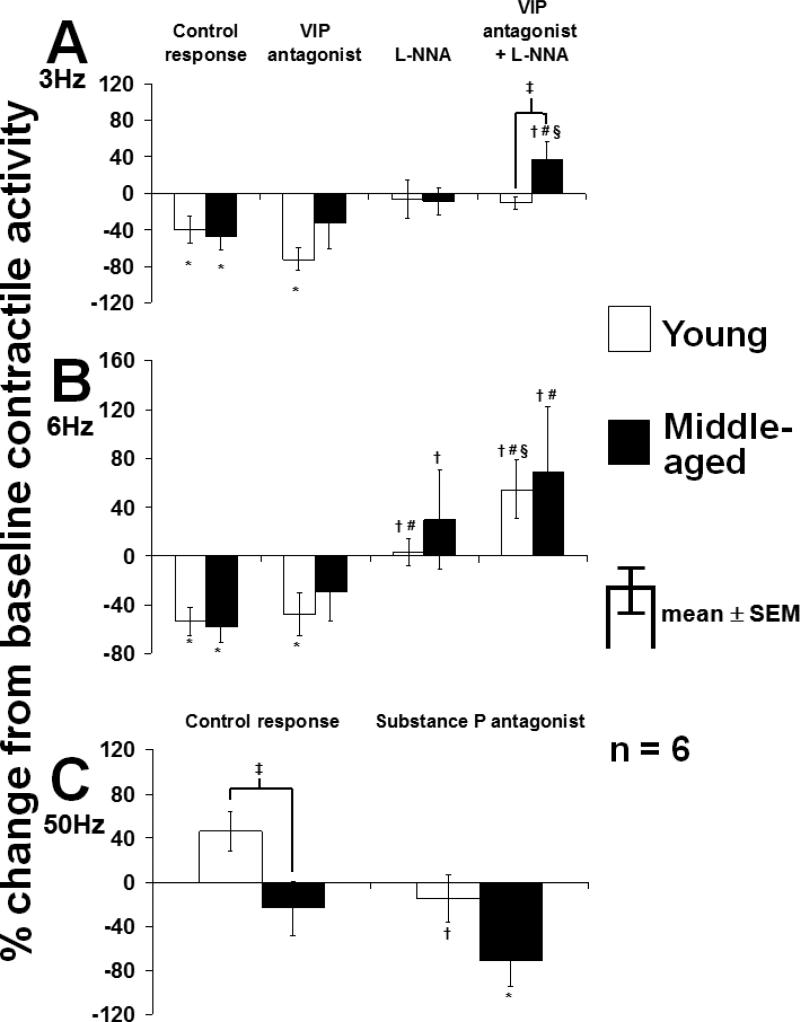
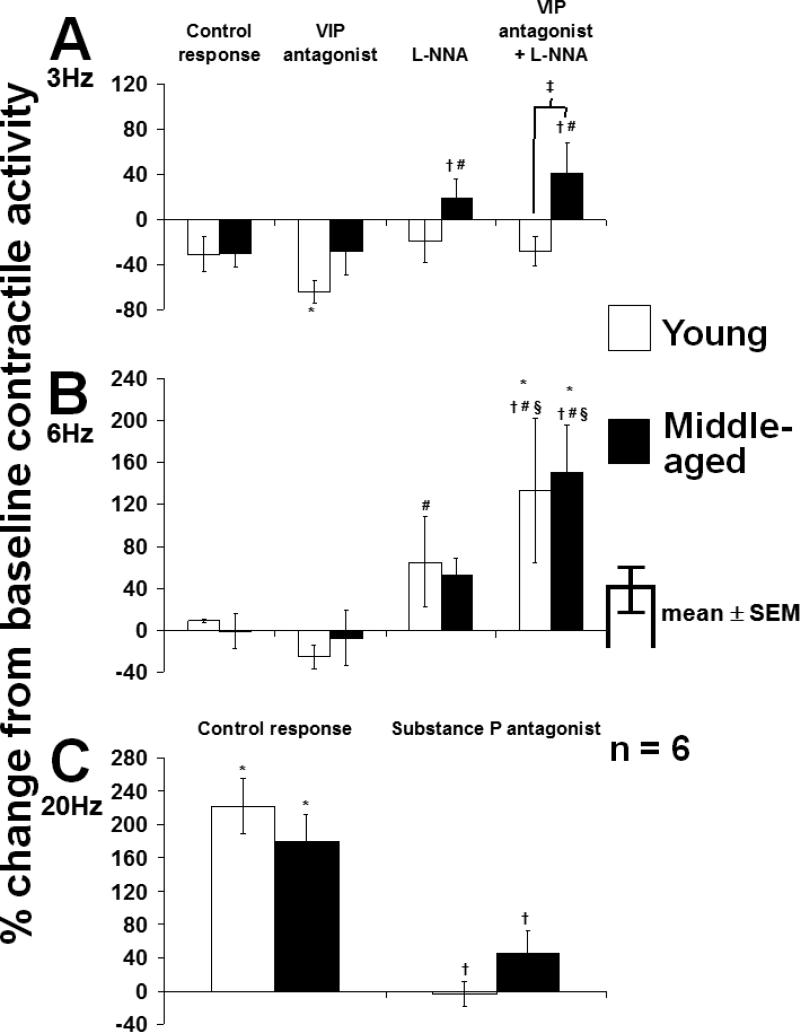
Under NANC conditions, EFS at 3 Hz tended to inhibit contractile activity (Fig. 6A). L-NNA alone and in combination with the VIP antagonist had no effect on the response to EFS in young rats but increased the EFS response compared to the control response and the response with the VIP antagonist alone in middle-aged rats (p<0.05; ANOVA). In contrast to longitudinal muscle, in circular muscle, EFS at 6 Hz did not alter contractile activity under control conditions (Fig. 6B). The VIP antagonist had no noticeable effect in either group; however, L-NNA converted the response to EFS at 6 Hz to a net contractile response, while the combination of L-NNA and the VIP antagonist caused a further increase in contractile activity in both groups. The excitatory response to EFS at 20 Hz was comparable between groups and was inhibited by the Sub P antagonist to a similar extent in both young and middle-aged rats (p<0.05; 6C).
Figure 6.
Response of spontaneous contractile activity of circular muscle of rat jejunum to EFS at A) 3 Hz, B) 6 Hz, and C) 20 Hz. The combination of VIP antagonist and L-NNA increased the response to EFS at 3 Hz in middle-aged but not in young rats. In contrast, at 6 Hz, the combination of VIP antagonist and L-NNA increased the EFS response in both groups. The Sub P antagonist prevented the excitatory EFS response at 20 Hz in both groups; *p<0.05 compared to baseline contractile activity; †p<0.05 compared to control response in same group (ANOVA); #p<0.05 compared to response with VIP antagonist in same group (ANOVA); §p<0.05 compared to response with L-NNA in same group (ANOVA); ‡p<0.05 compared to young rats under same condition (t-test). All experiments were carried out under NANC conditions.
DISCUSSION
The aim of our study was to explore age-related changes in neuromuscular responses mediated by the inhibitory neuropeptide VIP and the excitatory neuropeptide Sub P in the longitudinal and circular smooth muscle of rat jejunum. By studying the response to exogenously applied neurotransmitters, our aim was to delineate changes in the response of the two smooth muscle layers to their respective local release of neurotransmitters, while we studied the effect of endogenously released (by EFS) neurotransmitters to investigate changes in the neuronal release of neurotransmitters from the enteric nervous system as a consequence of maturation. Our study showed changes, not only between young and middle-aged rats, but also in the responses of longitudinal and circular muscle to VIP without and with a VIP antagonist and to inhibitory and excitatory frequencies of EFS as revealed by a VIP antagonist, L-NNA, and the combination of these two targeted agents.
NO and VIP appear to be the dominant, inhibitory, NANC neurotransmitters in the ENS. Moreover, there is an intense interaction between these enteric neurotransmitters, in that both neurotransmitters can influence the production, release, and effects of each other (Van Geldre et al., 2004). The role of NO and VIP in the control of contractile function, however, varies considerably among species, anatomic regions, and different muscle layers (Niioka et al., 1997; Okishio et al., 2000; Tomita et al., 2005). The age-related increase in the sensitivity to exogenous VIP in the longitudinal muscle (Kasparek et al., 2007b; Niioka et al., 1997; Okishio et al., 2000) accompanied by an increase in the VIP-mediated component of EFS-induced inhibition suggests an increased role of VIP in the NANC inhibition of contractile activity in the longitudinal jejunum of middle-aged rats. This finding is supported by the observation that, although nitrergic neurons appear to be spared largely from age-related neuronal loss (Phillips et al., 2003), expression of NO synthase and NO production (Takahashi et al., 2000), as well as the effects of endogenously released NO during EFS (Smits et al., 1996; Takeuchi et al., 1998), are decreased in the longitudinal muscle of jejunum and ileum in old rats. The overall magnitude of EFS-induced inhibition, however, was unchanged in the longitudinal muscle of jejunum and ileum in old rats in that study, implying that the muscle adapts with other inhibitory NANC neurotransmitters replacing the presumed decreased influence of NO (Takeuchi et al., 1998); indeed, VIP was identified as this other inhibitory NANC neurotransmitter in the longitudinal muscle of the rat colon (Takeuchi et al., 2000). In addition, findings from our studies at 3 Hz in circular muscle suggest, albeit indirectly, that not only the participation of VIP but also that of NO appears to be increased in middle-aged rats and thereby further extends this concept of age-related changes in functional NANC innervation.
Interestingly, these age-related changes in functional innervation with VIP in the longitudinal muscle of the jejunum were prevented when animals underwent complete extrinsic denervation by syngeneic, orthotopic small bowel transplantation 12 months previously (Kasparek et al., 2008). This observation raises the question about the role of the extrinsic nervous system in maturation of the ENS, a question which cannot be elucidated any further by the design of our study. Together, these findings highlight differences in the innervation with VIP and NO between anatomic regions, as well as potential variations in their interaction (Van Geldre et al., 2004). We did not find any obvious, age-related changes in the endogenous release of NO during EFS, but our study was not designed specifically to explore age-related changes in nitrergic neurotransmission. The differences between the EFS response at 3 Hz and 6 Hz demonstrates that the quantity and/or spectrum of neurotransmitters released during EFS depends on the frequency of EFS; however, we cannot draw any further conclusions on in vivo functional innervation with VIP and NO from this observation.
One drawback of our study is the variable effect of the VIP antagonist we used in the longitudinal and circular muscle layer. We used this antagonist because it demonstrated the ability to antagonize the effect of exogenous as well as endogenously released VIP in the longitudinal muscle layer (Kasparek et al., 2007b; Kasparek et al., 2008). Surprisingly, this VIP antagonist (10−6 M) was unable to block the effects of VIP in the circular muscle layer. The reason for this variable effect of the VIP antagonist between muscle layers remains unclear.
In contrast to VIP, the response to exogenous Sub P was not affected by maturation. The only study we could find evaluating age-related, functional changes in the innervation with Sub P was performed in the longitudinal muscle of guinea pig ileum; this study showed an increase in the excitatory response to exogenous Sub P in older animals which was paralleled, interestingly, by an age-dependent decrease in the innervation of the longitudinal muscle layer by excitatory motor neurons (Abalo et al., 2007). These results differ from our study but were performed in a different species and in a different anatomic region. The differences we showed between young and middle-aged rats in the EFS-response at 50 Hz under NANC conditions in the longitudinal muscle might reflect a decreased release of Sub P in middle-aged rats, which is supported by the finding that the Sub P antagonist inhibited the EFS response in young but not in middle-aged rats. A decrease in endogenous release of Sub P would not be too surprising, because cholinergic excitatory motor neurons are known to be affected more severely by age-related neuronal loss and because acetylcholine and Sub P are co-localized in these neurons and interact with one another (Brookes et al., 1991; Holzer et al., 1997; Phillips et al., 2003). Data about age-related changes in functional cholinergic innervation of gastrointestinal smooth muscle are limited and inconsistent. In rat colon, aging is associated with a decreased release of acetylcholine caused not only by the loss of cholinergic neurons but also by an impaired calcium influx via membrane calcium channels in cholinergic neurons (Roberts et al., 1994). These findings might explain the increased expression of muscarinic receptors in the jejunum of old rats, although no age-related changes in response to exogenous acetylcholine nor expression of acetylcholinesterase were found in this study (Tezuka et al., 2004). A limitation of our study is that we cannot exclude the possibility of a more pronounced release of inhibitory NANC neurotransmitters, such as VIP, NO, or other inhibitory neurotransmitters, in the response to EFS at 50 Hz in longitudinal muscle of middle-aged rats or the release and effect of other excitatory NANC neurotransmitters, such as neurokinin A or gamma amino butyric acid.
Based on the overall observation from our study that functional NANC innervation with VIP appeared to be increased in middle-aged rats, while innervation with Sub P was decreased when compared to young rats, we assume that the age-related increase in spontaneous contractile activity in the longitudinal and, although less pronounced, in the circular muscle of rat jejunum is the consequence of a change in the balance between excitatory and inhibitory innervation; whether this increase in spontaneous contractile activity is important as a compensatory or adaptive response that dominates over inhibitory NANC innervation is unknown. Most other studies have explored the response to EFS or exogenously applied drugs but have not evaluated spontaneous contractile activity. Only one study implied a similar phenomenon in the ileal longitudinal muscle of the guinea pig, but there was no specific analysis of changes in spontaneous contractile activity (Abalo et al., 2007). We cannot exclude an effect of age-related changes in muscle volume on our results; however, we attempted to control for this by normalizing the contractile data by the weight of the muscle strips.
Many of the previous studies exploring age-related changes in the gastrointestinal tract were performed in Fisher 344 rats (Phillips et al., 2003; Phillips et al., 2004; Phillips et al., 2001; Phillips et al., 2007) in which survival curves are established, and the course of these changes is best characterized. Although the median life span of Lewis rats seems to be comparable to those of other strains (Altun et al., 2007), objective data about age-related changes in the gastrointestinal tract do not exist for Lewis rats. We were interested particularly in studying Lewis rats, because inbred Lewis rats are used frequently for studies of gut function after the extrinsic denervation necessitated by syngeneic small bowel transplantation; indeed, age-related changes in functional neurotransmission might affect these results, especially in the long-term follow-up (Hirose et al., 1995; Kasparek et al., 2007a; Kasparek et al., 2007b; Tomita et al., 2005).
Age-related functional changes or cell loss in the gastrointestinal tract are not restricted to cells of the myenteric plexus but also affect extrinsic sympathetic and parasympathetic (vagal) innervation (Phillips et al., 2001; Phillips et al., 2007; Phillips et al., 2006), intrinsic primary afferent neurons (Wade et al., 2004), glia cells (Phillips et al., 2004), and maybe even interstitial cells of Cajal (Bernard et al., 2009; Hall, 2002). In addition, age-related changes in the thickness of the muscle layers, composition of contractile fibers in smooth muscle cells, mitochondrial function as well as diminished sarcoplasmatic release, and impaired resequestration of cytoplasmatic calcium with age have also been described (Hall, 2002; Lopes et al., 2006). Furthermore, age-related changes in downstream mechanisms have been reported for ion channel activity (Roberts et al., 1994) and for pathways involving tyrosine kinase and phosphokinase C (Bitar, 2003; Wiley, 2002). Although we cannot further elucidate the contribution of these factors in our results, we believe that our results may represent the summated effect of maturation on functional neurotransmission with VIP and Sub P in the longitudinal and circular muscle of rat jejunum.
In conclusion, we have demonstrated that age-related changes occur in functional, peptidergic neuromuscular transmission with VIP and Sub P. These changes are associated with an increased response to exogenous VIP and a decreased endogenous release of Sub P in the longitudinal muscle, while the effect of endogenously released VIP is increased in both muscle layers. These age-related changes may represent a relative change in the balance between inhibitory and excitatory innervation during the process of maturation of the gastrointestinal tract. Although these alterations demonstrated in middle-aged rats might reflect maturation or physiologic, age-related adaption or degeneration of the gastrointestinal tract, they might also participate in the pathophysiology of gastrointestinal motility disorders in the elderly.
ACKNOWLEDGEMENTS
We want to thank Deborah I. Frank for her expert assistance in the preparation of the manuscript.
Supported by grant DK 39337 from the National Institutes of Health, United States Public Health Services (M.G.S.) and grant KA 2329/1-1 from the Deutsche Forschungsgemeinschaft, Germany (M.S.K.).
ABBREVIATIONS USED IN THIS MANUSCRIPT
- ANOVA
analysis of variance
- EFS
electrical field stimulation
- ENS
enteric nervous system
- IACUC
Institutional Animal Care and Use Committee
- L0
optimal length
- L-NNA
L-NG-nitro arginine
- NANC
non-adrenergic, non-cholinergic
- NO
nitric oxide
- Sub P
substance P
- VIP
vasoactive intestinal polypeptide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests: the authors have no competing interests.
REFERENCES
- Abalo R, Vera G, Rivera AJ, Martin MI. Age-related changes in the gastrointestinal tract: a functional and immunohistochemical study in guinea-pig ileum. Life Sci. 2007;80:2436–2445. doi: 10.1016/j.lfs.2007.04.004. Epub 2007 Apr 2421. [DOI] [PubMed] [Google Scholar]
- Altun M, Bergman E, Edstrom E, Johnson H, Ulfhake B. Behavioral impairments of the aging rat. Physiol Behav. 2007;92:911–923. doi: 10.1016/j.physbeh.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Bernard CE, Gibbons SJ, Gomez-Pinilla PJ, Lurken MS, Schmalz PF, Roeder JL, Linden D, Cima RR, Dozois EJ, Larson DW, Camilleri M, Zinsmeister AR, Pozo MJ, Hicks GA, Farrugia G. Effect of age on the enteric nervous system of the human colon. Neurogastroenterol Motil. 2009;21:746–e746. doi: 10.1111/j.1365-2982.2008.01245.x. Epub 2009 Feb 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitar KN. Function of gastrointestinal smooth muscle: from signaling to contractile proteins. Am J Med. 2003;115:15S–23S. doi: 10.1016/s0002-9343(03)00189-x. [DOI] [PubMed] [Google Scholar]
- Brookes SJ, Steele PA, Costa M. Identification and immunohistochemistry of cholinergic and non-cholinergic circular muscle motor neurons in the guinea-pig small intestine. Neuroscience. 1991;42:863–878. doi: 10.1016/0306-4522(91)90050-x. [DOI] [PubMed] [Google Scholar]
- Camilleri M, Cowen T, Koch TR. Enteric neurodegeneration in ageing. Neurogastroenterol Motil. 2008;20:418–429. doi: 10.1111/j.1365-2982.2008.01134.x. [DOI] [PubMed] [Google Scholar]
- Camilleri M, Lee JS, Viramontes B, Bharucha AE, Tangalos EG. Insights into the pathophysiology and mechanisms of constipation, irritable bowel syndrome, and diverticulosis in older people. J Am Geriatr Soc. 2000;48:1142–1150. doi: 10.1111/j.1532-5415.2000.tb04793.x. [DOI] [PubMed] [Google Scholar]
- Cowen T, Johnson RJ, Soubeyre V, Santer RM. Restricted diet rescues rat enteric motor neurones from age related cell death. Gut. 2000;47:653–660. doi: 10.1136/gut.47.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza RR, Moratelli HB, Borges N, Liberti EA. Age-induced nerve cell loss in the myenteric plexus of the small intestine in man. Gerontology. 1993;39:183–188. doi: 10.1159/000213532. [DOI] [PubMed] [Google Scholar]
- El-Salhy M, Sandstrom O, Holmlund F. Age-induced changes in the enteric nervous system in the mouse. Mech Ageing Dev. 1999;107:93–103. doi: 10.1016/s0047-6374(98)00142-0. [DOI] [PubMed] [Google Scholar]
- Feher E, Penzes L. Density of substance P, vasoactive intestinal polypeptide and somatostatin-containing nerve fibers in the ageing small intestine of the rats. Gerontology. 1987;33:341–348. doi: 10.1159/000212901. [DOI] [PubMed] [Google Scholar]
- Firth M, Prather CM. Gastrointestinal motility problems in the elderly patient. Gastroenterology. 2002;122:1688–1700. doi: 10.1053/gast.2002.33566. [DOI] [PubMed] [Google Scholar]
- Gabella G. Fall in the number of myenteric neurons in aging guinea pigs. Gastroenterology. 1989;96:1487–1493. doi: 10.1016/0016-5085(89)90516-7. [DOI] [PubMed] [Google Scholar]
- Hall KE. Aging and neural control of the GI tract. II. Neural control of the aging gut: can an old dog learn new tricks? Am J Physiol Gastrointest Liver Physiol. 2002;283:G827–832. doi: 10.1152/ajpgi.00162.2002. [DOI] [PubMed] [Google Scholar]
- Hirose R, Taguchi T, Hirata Y, Yamada T, Nada O, Suita S. Immunohistochemical demonstration of enteric nervous distribution after syngeneic small bowel transplantation in rats. Surgery. 1995;117:560–569. doi: 10.1016/s0039-6060(05)80256-9. [DOI] [PubMed] [Google Scholar]
- Holzer P, Holzer-Petsche U. Tachykinins in the gut. Part I. Expression, release and motor function. Pharmacol Ther. 1997;73:173–217. doi: 10.1016/s0163-7258(96)00195-7. [DOI] [PubMed] [Google Scholar]
- Johnson RJ, Schemann M, Santer RM, Cowen T. The effects of age on the overall population and on sub-populations of myenteric neurons in the rat small intestine. J Anat. 1998;192:479–488. doi: 10.1046/j.1469-7580.1998.19240479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasparek MS, Fatima J, Iqbal CW, Duenes JA, Sarr MG. Long-term effects of extrinsic denervation on VIP and substance P innervation in circular muscle of rat jejunum. J Gastrointest Surg. 2007a;11:1339–1350. doi: 10.1007/s11605-007-0212-1. [DOI] [PubMed] [Google Scholar]
- Kasparek MS, Fatima J, Iqbal CW, Duenes JA, Sarr MG. Role of VIP and substance P in NANC innervation in the longitudinal smooth muscle of the rat jejunum - influence of extrinsic denervation. J Surg Res. 2007b;141:22–30. doi: 10.1016/j.jss.2007.01.021. [DOI] [PubMed] [Google Scholar]
- Kasparek MS, Fatima J, Iqbal CW, Duenes JA, Sarr MG. Effect of chronic, extrinsic denervation on functional NANC innervation with vasoactive intestinal polypeptide and substance P in longitudinal muscle of rat jejunum. Neurogastroenterol Motil. 2008;20:242–252. doi: 10.1111/j.1365-2982.2007.01021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch TR, Carney JA, Go VL, Szurszewski JH. Inhibitory neuropeptides and intrinsic inhibitory innervation of descending human colon. Dig Dis Sci. 1991;36:712–718. doi: 10.1007/BF01311226. [DOI] [PubMed] [Google Scholar]
- Lopes GS, Ferreira AT, Oshiro ME, Vladimirova I, Jurkiewicz NH, Jurkiewicz A, Smaili SS. Aging-related changes of intracellular Ca2+ stores and contractile response of intestinal smooth muscle. Exp Gerontol. 2006;41:55–62. doi: 10.1016/j.exger.2005.10.004. Epub 2005 Dec 2015. [DOI] [PubMed] [Google Scholar]
- Murr MM, Miller VM, Sarr MG. Contractile properties of enteric smooth muscle after small bowel transplantation in rats. Am J Surg. 1996;171:212–217. doi: 10.1016/S0002-9610(99)80102-0. discussion 217-218. [DOI] [PubMed] [Google Scholar]
- Niioka S, Takeuchi T, Kishi M, Ishii T, Nishio H, Takewaki T, Hata F. Nonadrenergic, noncholinergic relaxation in longitudinal muscle of rat jejunum. Jpn J Pharmacol. 1997;73:155–161. doi: 10.1254/jjp.73.155. [DOI] [PubMed] [Google Scholar]
- Okishio Y, Niioka S, Yamaji M, Yamazaki Y, Nishio H, Takeuchi T, Hata F. Mediators of nonadrenergic, noncholinergic relaxation in Sprague Dawley rat intestine: comparison with the mediators of other strains. J Vet Med Sci. 2000;62:821–828. doi: 10.1292/jvms.62.821. [DOI] [PubMed] [Google Scholar]
- Orr WC, Chen CL. Aging and neural control of the GI tract: IV. Clinical and physiological aspects of gastrointestinal motility and aging. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1226–1231. doi: 10.1152/ajpgi.00276.2002. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Kieffer EJ, Powley TL. Aging of the myenteric plexus: neuronal loss is specific to cholinergic neurons. Auton Neurosci. 2003;106:69–83. doi: 10.1016/S1566-0702(03)00072-9. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Kieffer EJ, Powley TL. Loss of glia and neurons in the myenteric plexus of the aged Fischer 344 rat. Anat Embryol (Berl) 2004;209:19–30. doi: 10.1007/s00429-004-0426-x. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Powley TL. As the gut ages: timetables for aging of innervation vary by organ in the Fischer 344 rat. J Comp Neurol. 2001;434:358–377. doi: 10.1002/cne.1182. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Powley TL. Innervation of the gastrointestinal tract: Patterns of aging. Auton Neurosci. 2007;136:1–19. doi: 10.1016/j.autneu.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RJ, Rhodes BS, Powley TL. Effects of age on sympathetic innervation of the myenteric plexus and gastrointestinal smooth muscle of Fischer 344 rats. Anat Embryol (Berl) 2006;211:673–683. doi: 10.1007/s00429-006-0123-z. Epub 2006 Sep 2021. [DOI] [PubMed] [Google Scholar]
- Roberts D, Gelperin D, Wiley JW. Evidence for age-associated reduction in acetylcholine release and smooth muscle response in the rat colon. Am J Physiol. 1994;267:G515–522. doi: 10.1152/ajpgi.1994.267.4.G515. [DOI] [PubMed] [Google Scholar]
- Santer RM, Baker DM, Conboy VB, Swift GL, Owen RG. Enteric neuron numbers and sizes in Auerbach's plexus in the small and large intestine of adult and aged rats. J Auton Nerv Syst. 1988;25:59–67. doi: 10.1016/0165-1838(88)90008-2. [DOI] [PubMed] [Google Scholar]
- Smits GJ, Lefebvre RA. Influence of age on cholinergic and inhibitory nonadrenergic noncholinergic responses in the rat ileum. Eur J Pharmacol. 1996;303:79–86. doi: 10.1016/0014-2999(96)00089-1. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Qoubaitary A, Owyang C, Wiley JW. Decreased expression of nitric oxide synthase in the colonic myenteric plexus of aged rats. Brain Res. 2000;883:15–21. doi: 10.1016/s0006-8993(00)02867-5. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Negoro T, Yamaji M, Yamazaki Y, Fujita A, Nishio H, Takewaki T, Takatsuji K, Hata F. Increase in participation of vasoactive intestinal peptide in relaxation of the distal colon of Wistar rats with age. Br J Pharmacol. 2000;131:942–948. doi: 10.1038/sj.bjp.0703643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi T, Niioka S, Yamaji M, Okishio Y, Ishii T, Nishio H, Takatsuji K, Hata F. Decrease in participation of nitric oxide in nonadrenergic, noncholinergic relaxation of rat intestine with age. Jpn J Pharmacol. 1998;78:293–302. doi: 10.1254/jjp.78.293. [DOI] [PubMed] [Google Scholar]
- Tezuka A, Ishihata A, Aita T, Katano Y. Aging-related alterations in the contractile responses to acetylcholine, muscarinic cholinoceptors and cholinesterase activities in jejunum and colon of the male Fischer 344 rats. Exp Gerontol. 2004;39:91–100. doi: 10.1016/j.exger.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Thrasivoulou C, Soubeyre V, Ridha H, Giuliani D, Giaroni C, Michael GJ, Saffrey MJ, Cowen T. Reactive oxygen species, dietary restriction and neurotrophic factors in age-related loss of myenteric neurons. Aging Cell. 2006;5:247–257. doi: 10.1111/j.1474-9726.2006.00214.x. [DOI] [PubMed] [Google Scholar]
- Tomita R, Fujisaki S, Park E, Ikeda T, Koshinaga T. Substance P and vasoactive intestinal peptide in rat small-bowel isografts. Am J Surg. 2005;189:63–70. doi: 10.1016/j.amjsurg.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Van Geldre LA, Lefebvre RA. Interaction of NO and VIP in gastrointestinal smooth muscle relaxation. Curr Pharm Des. 2004;10:2483–2497. doi: 10.2174/1381612043383890. [DOI] [PubMed] [Google Scholar]
- Wade PR, Cowen T. Neurodegeneration: a key factor in the ageing gut. Neurogastroenterol Motil. 2004;16:19–23. doi: 10.1111/j.1743-3150.2004.00469.x. [DOI] [PubMed] [Google Scholar]
- Wiley JW. Aging and neural control of the GI tract: III. Senescent enteric nervous system: lessons from extraintestinal sites and nonmammalian species. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1020–1026. doi: 10.1152/ajpgi.00224.2002. [DOI] [PubMed] [Google Scholar]