Abstract
Familial hemiplegic migraine type 1 (FHM1), a severe migraine with aura variant, is caused by mutations in the CACNA1A gene. Mutant mice carrying the FHM1 R192Q mutation exhibit increased propensity for cortical spreading depression (CSD), a propagating wave of neuroglial depolarization implicated in migraine aura. The CSD phenotype is stronger in female R192Q mutants and diminishes after ovariectomy. Here, we show that orchiectomy reciprocally increases CSD susceptibility in R192Q mutant mice. Chronic testosterone replacement restores CSD susceptibility by an androgen receptor-dependent mechanism. Hence, androgens modulate genetically-enhanced CSD susceptibility and may provide a novel prophylactic target for migraine.
Keywords: cortical spreading depression, testosterone, flutamide
INTRODUCTION
Familial hemiplegic migraine (FHM) is an autosomal dominant subtype of migraine with aura associated with transient hemiparesis. Aura and headache features are otherwise identical to those in common forms of migraine1. FHM1 is caused by missense mutations in the CACNA1A gene, which encodes the pore-forming α1A-subunit of neuronal Cav2.1 voltage-gated Ca2+ channels (VGCC)2. When expressed in transfected cultured neurons, FHM1 mutations shift channel opening towards more negative membrane potentials and delay channel inactivation. Channels open with smaller depolarization and stay open longer, allowing more Ca2+ to enter presynaptic terminals3, 4. Increased action potential-evoked Ca2+ influx has been shown to enhance excitatory neurotransmission at pyramidal cell synapses of FHM1 mutant mice5. Accordingly, mutant mice carrying the FHM1 R192Q mutation show enhanced susceptibility to cortical spreading depression (CSD), the electrophysiological correlate of migraine aura, and a possible trigger of migraine headache mechanisms4, 6-8. CSD is characterized by an intense depolarization of neuronal and glial membranes propagating at a rate of approximately 3 mm/min. Evoked when extracellular K+ concentrations exceed a critical threshold, CSD is associated with massive K+ and glutamate efflux depolarizing adjacent neurons and glia and facilitate CSD spread.
Gonadal hormones are important modulators of migraine and cortical excitability9, 10. Incidence of common types of migraine both with or without aura is threefold higher in females (25%) than in males (8%)11. A female preponderance has also been described for familial (5:2) and sporadic (4.25:1) hemiplegic migraine1, 12.
Brennan et al. recently reported that KCl and electrical stimulation thresholds for CSD induction are both reduced by approximately 50% in wild type female mice compared to males13. We found a similar increase in CSD susceptibility in female FHM1 knockin mice compared to males; the sex difference was abrogated by ovariectomy and partly restored by estradiol replacement, suggesting that estrogens modulate CSD susceptibility7. Although the female preponderance of migraine has been largely attributed to ovarian sex steroids, anecdotal evidence suggests a role for testosterone and its synthetic derivatives in suppressing migraine in both men and women14-16. Here, we provide in vivo experimental evidence for androgenic suppression of CSD susceptibility, as a surrogate model for migraine aura. The data suggest that male and female gonadal hormones exert reciprocal effects on CSD susceptibility, and that androgens may contribute to the lower prevalence of FHM and common types of migraine in males.
METHODS
Experimental groups and the number of mice in each group are shown in Table 1 (n= 106). Adult (4−8 mo) or senescent (11−13 mo) male FHM1 knockin mice, homozygous for the R192Q mutation that was introduced in the mouse Cacna1a gene by a gene targeting approach4 were compared to wild type littermates and C57BL6/J mice. All experiments were carried out with the investigator blinded for the genotype, and confirmatory genotyping was done after the experiment.
Table 1.
Electrophysiological measures of CSD, and systemic physiological parameters.
| CSD |
Systemic physiology |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Age (mo) |
BW (g) |
Frequency (CSD/h) |
Speed (mm/min) |
Dur (sec) |
Amp (mV) |
BP (mmHg) |
pHa | paCO2 (mmHg) |
paO2 (mmHg) |
||
| WT | Naïve |
10 |
5±1 |
30±4 |
9±1 |
2.7±0.1 |
43±11 |
24±5 |
81±9 |
7.37±0.06 |
35±6 |
149±28 |
| Orx |
8 |
5±1 |
27±3 |
10±1 |
2.9±0.1 |
36±9 |
28±4 |
83±5 |
7.36±0.05 |
35±5 |
133±19 |
|
| Orx+TChronic |
4 |
4±0 |
27±1 |
9±1 |
2.8±0.2 |
41±7 |
23±1 |
86±1 |
7.40±0.03 |
31±3 |
142±18 |
|
| |
Aged |
5 |
11±0 |
37±3 |
9±1 |
2.7±0.0 |
23±9 |
24±4 |
87±5 |
7.43±0.02 |
31±3 |
157±19 |
| R192Q | Naïve |
19 |
5±1 |
27±3 |
12±1 |
3.8±0.2 |
37±10 |
23±5 |
84±5 |
7.38±0.05 |
32±6 |
133±22 |
| Orx |
16 |
5±1 |
27±2 |
17±2 |
4.1±0.1 |
32±8 |
25±7 |
86±5 |
7.37±0.05 |
32±4 |
140±20 |
|
| Orx+TChronic |
16 |
6±1 |
28±2 |
12±1 |
3.5±0.3 |
38±9 |
23±3 |
88±3 |
7.38±0.03 |
30±4 |
153±22 |
|
| Orx+TChronic+F25 |
7 |
8±0 |
30±6 |
12±0 |
3.7±0.2 |
32±6 |
18±2 |
85±7 |
7.42±0.04 |
33±5 |
161±18 |
|
| Orx+TChronic+F50 |
8 |
5±0 |
27±1 |
17±1 |
4.2±0.4 |
33±6 |
25±3 |
91±5 |
7.40±0.06 |
31±4 |
157±36 |
|
| Orx+TAcute |
6 |
7±0 |
28±2 |
16±2 |
4.3±0.3 |
33±10 |
25±4 |
90±5 |
7.40±0.03 |
31±3 |
151±37 |
|
| Aged | 7 | 13±2 | 37±4 | 12±1 | 3.6±0.3 | 42±12 | 27±3 | 80±6 | 7.37±0.05 | 34±5 | 146±19 | |
Values are mean ± standard deviation. CSD duration was measured at half amplitude.
The duration (Dur) and the amplitude (Amp) of only the first CSD are shown. Systemic physiological parameters were averaged over 1 hour recording duration.
BW: body weight; Orx: orchiectomized mice; TChronic: Testosterone 0.1 mg pellet for 21 days; TAcute: A single 1.2 mg dose of testosterone administered 1 hour prior to CSD testing; F25/50: Flutamide 25 or 50 mg/pellet for 21 days; BP: mean arterial blood pressure; pHa, paCO2, paO2: arterial blood gas values; WT, wild type; R192Q, homozygous R192Q knockin mice.
Experiments were conducted in accordance with the United States Public Health Service's Policy on Humane Care and Use of Laboratory Animals, and were approved by the institutional review committee. The femoral artery was catheterized for blood sampling and measurement of mean arterial pressure, and the trachea was intubated for mechanical ventilation under isoflurane anesthesia (2.5% induction, 1% maintenance, in 70% N2O/30% O2). Arterial blood gases and pH were measured every 20 min and maintained within normal limits by adjusting ventilation (Table 1). Mice were placed in a stereotaxic frame and burr holes were drilled at the coordinates described previously7. Two glass capillary microelectrodes were placed to record extracellular steady (DC) potential and electrocorticogram at a depth of 300 μm. After surgical preparation, the occipital cortex was allowed to recover for 20 minutes under saline irrigation. The frequency of CSDs evoked by epidural KCl (300 mM for 30 minutes) application was determined, as previously described7. The propagation speed, amplitude and duration of the first CSD were also measured.
Orchiectomy was performed under brief isoflurane anesthesia 3 weeks prior to CSD susceptibility testing. Subcutaneous testosterone pellets (0.1 mg/pellet, 21 day release; Innovative Research of America) were implanted into the dorsal neck and shoulder region on the day of orchiectomy. The pellets restore physiological circulating levels of testosterone for at least 21 days, but may cause an early peak in plasma levels during the first week after implantation. In order to test whether testosterone replacement exerts its effects on CSD via androgen receptors, a subgroup of orchiectomized testosterone-replaced mice also received pellets containing the androgen receptor antagonist, flutamide (25 or 50 mg/pellet, 21 day release; Innovative Research of America). In addition, acute effects of testosterone propionate (1.2 mg per mouse in 0.1 ml of β-cyclodextrin injected subcutaneously; Sigma) were tested one hour before electrophysiological recording in castrated mice. The effectiveness of orchiectomy, testosterone and flutamide treatments was confirmed by measuring the prostate and seminal vesicle weights after sacrifice.
Data were analyzed using SPSS (version 11.0). Using a general linear model of covariance analysis (ANACOVA), we tested for an effect of the independent variables genotype, age, orchiectomy, testosterone treatment (acute, chronic) and flutamide treatment (25mg, 50mg) on the dependent variables cortical SD frequency and propagation speed. Other electrophysiological measures of CSD and systemic physiological data were compared among groups using one-way ANOVA. Data are presented as mean ± standard deviation. P<0.05 was considered statistically significant.
RESULTS
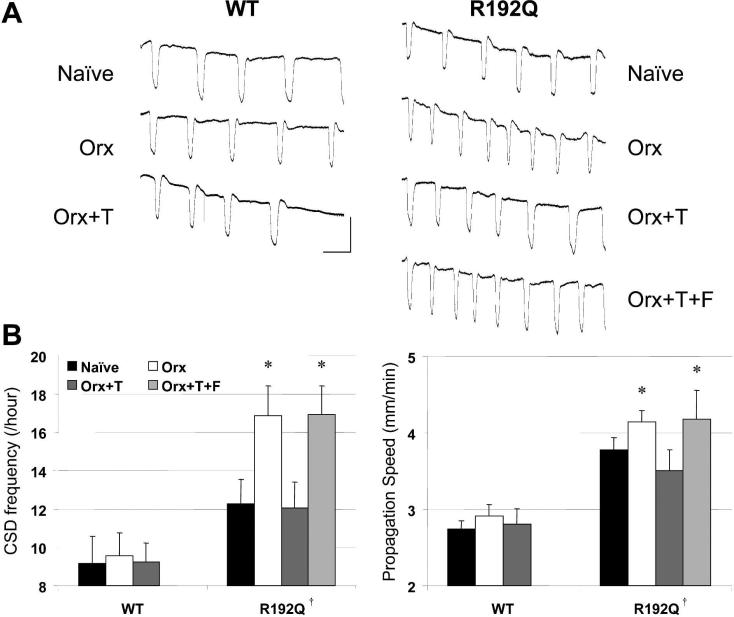
Continuous epidural KCl application evoked repetitive CSDs in all mice (Figure 1). Both the frequency and the propagation speed of CSDs were significantly higher in R192Q mutants compared to wild type, as reported previously7. Orchiectomy further increased CSD frequency (by 40%) and to a lesser extent the propagation speed in R192Q mutants, but not in wild type mice. Chronic testosterone replacement for 21 days completely prevented the orchiectomy-induced increase in CSD susceptibility in R192Q mutant mice (Figure 1, Table 1). In contrast, a single dose of testosterone propionate administered 1 hour before electrophysiological recordings had no effect (16±2 CSDs/h, 4.3±0.3 mm/min; p>0.05 vs. orchiectomized controls). The CSD suppression by chronic testosterone replacement was prevented by co-treatment with androgen receptor antagonist flutamide (50 mg pellet); a lower dose of flutamide was ineffective (25 mg pellet; data not shown). Aging (11−13 months) had no effect on CSD frequency and propagation speed in either wild type or R192Q mutant mice, consistent with the maintenance of plasma testosterone levels during aging in this wild type background strain 17. In wild type mice, gonadectomy or testosterone replacement did not significantly alter CSD susceptibility, suggesting that in our model androgens modulate CSD susceptibility only if the latter is genetically enhanced. The CSD duration and amplitude, and systemic physiological parameters did not significantly differ among groups (Table 1).
Figure 1. Androgenic modulation of CSD in R192Q mutant mice.
(A) Representative electrophysiological recordings from male wild type (WT) and homozygous R192Q mutant mice showing repetitive CSDs evoked by topical KCl application (300 mM) for 30 min. (B) Graphic representation of CSD frequency and propagation speed in WT and R192Q mutant mice. Naïve R192Q mutant mice developed higher frequency of CSDs compared to WT. Orchiectomy (Orx) further increased CSD frequency in the R192Q mutant, which was restored to the level of naïve R192Q mutants by chronic testosterone replacement (T). The androgen receptor blocker flutamide (F) completely abolished the effects of testosterone replacement. Vertical bar, 20 mV; horizontal bar, 4 min. Data are mean ± standard deviation. *, p<0.001 vs. naïve and Orx+T R192Q mutant. †, p<0.001 vs. WT. Numbers of mice for each group are shown in Table 1.
DISCUSSION
We showed that testosterone, acting via androgen receptors, suppresses genetically-enhanced CSD susceptibility. CSD suppression required chronic androgen replacement. We recently showed that estradiol augmented genetically-enhanced CSD susceptibility in FHM1 knockin mice7. To the extent that mice homozygous for the FHM1 allele represent the human condition, the data suggest that estrogen and androgen exert reciprocal effects on CSD susceptibility, providing a dual mechanism that may account for the female preponderance of migraine.
Observational studies suggest that methyl-testosterone and danazol, a synthetic testosterone derivative, may decrease attack frequency and severity in migraineurs14, 16 15, 18, 19. As androgens are known to downregulate estrogen receptor expression20 and danazol also inhibits ovarian sex hormone production, it is unclear whether the clinical effects of danazol are a direct result of androgen receptor activation or secondary to suppression of estrogen actions on excitability10, 21, 22. Complete cessation of migraine with aura attacks was reported in men treated with gonadotrophins for infertility, further implicating androgens secreted by the testes23. In a small cohort of male-to-female transsexuals, the prevalence of migraine with aura increased during anti-androgen combined with estrogen therapy to levels similar to that seen in females24.
Unlike estrogens, the influence of androgens on neuronal structure and function has not been studied in detail. There are data suggesting that androgens modulate both presynaptic and postsynaptic mechanisms. For example, orchiectomy enhances spontaneous acetylcholine release (i.e., increased frequency of miniature end-plate potentials) at the neuromuscular junction possibly related to altered expression and function of VGCCs (e.g., Cav2.2)25. Although a specific modulation of Cav2.1 channels has not been reported, similar mechanisms may be operational at the glutamatergic central synapses. Postsynaptic glutamate receptors, particularly the NMDA subtype, are critical for the propagation of CSD. The non-aromatizable androgen, 5-α-dihydrotestosterone (5αDHT), modulates NMDA responses in a complex manner in hippocampal slices from orchiectomized rats: despite larger NMDA-induced currents, irreversible depolarization and cell death at high NMDA concentrations were significantly inhibited by 5αDHT26. The latter effect required 5αDHT exposure times of 8 hours or more implicating transcriptional mechanisms.
There is a well established bidirectionally increased risk of comorbidity of migraine and epilepsy, suggesting shared underlying mechanisms27. Interestingly, there is a clinical association between androgen deficiency and epilepsy28. Consistent with this, androgens possess anticonvulsant activity in rodents by acutely enhancing GABAA receptor activity independent of androgen receptors29. However, we found that acute testosterone administration did not suppress CSD, and that suppression by chronic testosterone treatment was abolished by the androgen receptor blocker flutamide. Taken together with previous data suggesting that barbiturates do not significantly suppress CSD30, it is unlikely that GABAergic mechanisms play a significant role in androgenic CSD suppression.
In our study, orchiectomy and testosterone modulated CSD only in FHM1 mutant mice, and not in the wild type. The mechanisms of interaction between gonadal hormones and the mutant Cav2.1 channels are not known; however, the need for chronic treatment with testosterone implicates mechanisms linked to gene expression and, possibly, ultrastructural changes. Presynaptic, postsynaptic and astrocytic mechanisms may all be involved in the interaction between gonadal hormones and FHM1 mutations. The clear female preponderance in clinical migraine strongly suggests a reciprocal modulation of yet unidentified polygenetic migraine susceptibility factors by androgen and estrogen.
Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft (Ha5085/1-1, Haerter), National Institutes of Health (NS061505, Ayata; NS35611, Moskowitz), Netherlands Organization for Scientific Research (903-52-291 and Vici 918.56.602, Ferrari), EU “EUROHEAD” grant (LSHMCT-2004-504837; Ferrari, van den Maagdenberg), and the Centre for Medical Systems Biology (CMSB) in the framework of the Netherlands Genomics Initiative (NGI).
Footnotes
ALL REVISIONS IN THE MAIN TEXT ARE TRACKED.
Reviewer 2:
1. “The Introduction still contains the phrase “Here we provide the first in vivo...” which is still a dubious distinction and, more importantly, a distraction from developing the point that this manuscript addresses the role of androgen in suppressing CSD.”
We apologize for this oversight. The sentence is now revised according to the Reviewer's suggestion.
Bibliography
- 1.Thomsen LL, Eriksen MK, Roemer SF, et al. A population-based study of familial hemiplegic migraine suggests revised diagnostic criteria. Brain. 2002;125:1379–1391. doi: 10.1093/brain/awf132. [DOI] [PubMed] [Google Scholar]
- 2.Ophoff RA, Terwindt GM, Vergouwe MN, et al. Familial hemiplegic migraine and episodic ataxia type-2 are caused by mutations in the Ca2+ channel gene CACNL1A4. Cell. 1996;87:543–552. doi: 10.1016/s0092-8674(00)81373-2. [DOI] [PubMed] [Google Scholar]
- 3.Tottene A, Fellin T, Pagnutti S, et al. Familial hemiplegic migraine mutations increase Ca(2+) influx through single human CaV2.1 channels and decrease maximal CaV2.1 current density in neurons. Proc Natl Acad Sci U S A. 2002;99:13284–13289. doi: 10.1073/pnas.192242399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van den Maagdenberg AM, Pietrobon D, Pizzorusso T, et al. A Cacna1a knockin migraine mouse model with increased susceptibility to cortical spreading depression. Neuron. 2004;41:701–710. doi: 10.1016/s0896-6273(04)00085-6. [DOI] [PubMed] [Google Scholar]
- 5.Tottene A, Conti R, Fabbro A, et al. Enhanced excitatory transmission at cortical synapses as the basis for facilitated spreading depression in Ca(v)2.1 knockin migraine mice. Neuron. 2009;61:762–773. doi: 10.1016/j.neuron.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 6.Hadjikhani N, Sanchez Del Rio M, Wu O, et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci U S A. 2001;98:4687–4692. doi: 10.1073/pnas.071582498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eikermann-Haerter K, Dilekoz E, Kudo C, et al. Genetic and hormonal factors modulate spreading depression and transient hemiparesis in mouse models of familial hemiplegic migraine type 1. J Clin Invest. 2009;119:99–109. doi: 10.1172/JCI36059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolay H, Reuter U, Dunn AK, et al. Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nat Med. 2002;8:136–142. doi: 10.1038/nm0202-136. [DOI] [PubMed] [Google Scholar]
- 9.MacGregor EA. Oestrogen and attacks of migraine with and without aura. Lancet Neurol. 2004;3:354–361. doi: 10.1016/S1474-4422(04)00768-9. [DOI] [PubMed] [Google Scholar]
- 10.Woolley CS, Weiland NG, McEwen BS, Schwartzkroin PA. Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor-mediated synaptic input: correlation with dendritic spine density. J Neurosci. 1997;17:1848–1859. doi: 10.1523/JNEUROSCI.17-05-01848.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rasmussen BK, Jensen R, Schroll M, Olesen J. Epidemiology of headache in a general population--a prevalence study. J Clin Epidemiol. 1991;44:1147–1157. doi: 10.1016/0895-4356(91)90147-2. [DOI] [PubMed] [Google Scholar]
- 12.Thomsen LL, Kirchmann M, Bjornsson A, et al. The genetic spectrum of a population-based sample of familial hemiplegic migraine. Brain. 2007;130:346–356. doi: 10.1093/brain/awl334. [DOI] [PubMed] [Google Scholar]
- 13.Brennan KC, Romero Reyes M, Lopez Valdes HE, et al. Reduced threshold for cortical spreading depression in female mice. Ann Neurol. 2007;61:603–606. doi: 10.1002/ana.21138. [DOI] [PubMed] [Google Scholar]
- 14.Moehlig RC. Methyl testosterone for migraine of women; report of sixty cases. Journal - Michigan State Medical Society. 1955;54:577–579. passim. [PubMed] [Google Scholar]
- 15.Calton GJ, Burnett JW. Danazol and migraine. The New England journal of medicine. 1984;310:721–722. doi: 10.1056/nejm198403153101114. [DOI] [PubMed] [Google Scholar]
- 16.Jonsson B, Von Reis G, Sahlgren E. [Testosterone therapy of headache.]. Acta Psychiatr Neurol Scand Suppl. 1951;74:102–105. [PubMed] [Google Scholar]
- 17.Nelson JF, Latham KR, Finch CE. Plasma testosterone levels in C57BL/6J male mice: effects of age and disease. Acta Endocrinol (Copenh) 1975;80:744–752. doi: 10.1530/acta.0.0800744. [DOI] [PubMed] [Google Scholar]
- 18.Lichten EM, Bennett RS, Whitty AJ, Daoud Y. Efficacy of danazol in the control of hormonal migraine. The Journal of reproductive medicine. 1991;36:419–424. [PubMed] [Google Scholar]
- 19.Vincent FM. Migraine responsive to danazol. Neurology. 1985;35:618. doi: 10.1212/wnl.35.4.618. [DOI] [PubMed] [Google Scholar]
- 20.Thakur MK, Sharma PK. Transcription of estrogen receptor alpha and beta in mouse cerebral cortex: effect of age, sex, 17beta-estradiol and testosterone. Neurochemistry international. 2007;50:314–321. doi: 10.1016/j.neuint.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 21.Smith SS. Estrogen administration increases neuronal responses to excitatory amino acids as a long-term effect. Brain Res. 1989;503:354–357. doi: 10.1016/0006-8993(89)91691-0. [DOI] [PubMed] [Google Scholar]
- 22.Sato K, Matsuki N, Ohno Y, Nakazawa K. Estrogens inhibit l-glutamate uptake activity of astrocytes via membrane estrogen receptor alpha. J Neurochem. 2003;86:1498–1505. doi: 10.1046/j.1471-4159.2003.01953.x. [DOI] [PubMed] [Google Scholar]
- 23.Arango O, Bielsa O, Pascual-Calvet J, et al. [Disappearance of migraine crises in two patients with male infertility treated with human chorionic gonadotropin/human menopausal gonadotrophin]. Revista de neurologia. 1996;24:977–979. [PubMed] [Google Scholar]
- 24.Pringsheim T, Gooren L. Migraine prevalence in male to female transsexuals on hormone therapy. Neurology. 2004;63:593–594. doi: 10.1212/01.wnl.0000130338.62037.cc. [DOI] [PubMed] [Google Scholar]
- 25.Nudler SI, Pagani MR, Urbano FJ, et al. Testosterone modulates Ca(v2.2) calcium channels’ functional expression at rat levator ani neuromuscular junction. Neuroscience. 2005;134:817–826. doi: 10.1016/j.neuroscience.2005.03.061. [DOI] [PubMed] [Google Scholar]
- 26.Pouliot WA, Handa RJ, Beck SG. Androgen modulates N-methyl-D-aspartate-mediated depolarization in CA1 hippocampal pyramidal cells. Synapse (New York, N.Y. 1996;23:10–19. doi: 10.1002/(SICI)1098-2396(199605)23:1<10::AID-SYN2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 27.Haut SR, Bigal ME, Lipton RB. Chronic disorders with episodic manifestations: focus on epilepsy and migraine. Lancet Neurol. 2006;5:148–157. doi: 10.1016/S1474-4422(06)70348-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herzog AG, Seibel MM, Schomer DL, et al. Reproductive endocrine disorders in men with partial seizures of temporal lobe origin. Archives of neurology. 1986;43:347–350. doi: 10.1001/archneur.1986.00520040035015. [DOI] [PubMed] [Google Scholar]
- 29.Reddy DS. Testosterone modulation of seizure susceptibility is mediated by neurosteroids 3alpha-androstanediol and 17beta-estradiol. Neuroscience. 2004;129:195–207. doi: 10.1016/j.neuroscience.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 30.Kudo C, Nozari A, Moskowitz MA, Ayata C. The impact of anesthetics and hyperoxia on cortical spreading depression. Exp Neurol. 2008;212:201–206. doi: 10.1016/j.expneurol.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]