Abstract
Serotonin (5-HT) is involved in many developmental processes and influences behaviors including anxiety, aggression, and cognition. Disruption of the serotonergic system has been implicated in human disorders including autism, depression, schizophrenia, and ADHD. Although pharmacological, neurotoxin, and dietary manipulation of 5-HT and tryptophan hydroxylase has added to our understanding of the serotonergic system, the results are complicated by multiple factors. A newly identified ETS domain transcription factor, Pet-1, has direct control of major aspects of 5-HT neuronal development. Pet-1 is the only known factor that is restricted in the brain to 5-HT neurons during development and adulthood and exerts dominant control over 5-HT neuronal phenotype. Disruption of Pet-1 produces an ∼80% loss of 5-HT neurons and content and results in increased aggression in male Pet-1-/- mice (Hendricks et al., 2003). We hypothesized that Pet-1-/- mice would also exhibit changes in anxiety and cognition. Pet-1-/- mice were hypoactive which may have affected the observed lack of anxious behavior in the elevated zero maze and light-dark test. Pet-1-/- mice, however, were more defensive during marble burying and showed acoustic startle hyper-reactivity. No deficits in spatial, egocentric, or novel object recognition learning were found in Pet-1-/- mice. These findings were unexpected given that 5-HT depleting drugs given to adult or developing animals result in learning deficits (Mazer et al., 1997;Morford et al., 2002;Vorhees et al., 2007). Lack of differences may be the result of compensatory mechanisms in reaction to a constitutive knockout of Pet-1 or 5-HT may not be as important in learning and memory as previously suspected.
Keywords: Serotonin (5-HT), Morris water maze (MWM), locomotor activity, startle, marble burying
The serotonergic system is involved in many physiological and behavioral processes including cognition, anxiety, and aggression (Whitaker-Azmitia et al., 1996). Serotonin (5-hydroxytryptamine, 5-HT) is one of the first neurotransmitters to appear during development and aids in organizing the brain (Lauder and Bloom, 1974;Lauder and Krebs, 1978;Lauder et al., 1981).
In humans, decreased cognitive ability in children and adults has been linked to decreases in 5-HT. Cognitive deficits associated with schizophrenia and autism have been suggested to be associated with perinatal insults to the 5-HT system (Murray et al., 1992;Scott and Deneris, 2005;Torrey et al., 1994;Whitaker-Azmitia, 2005). In animal studies, early developmental exposure to drugs that decrease 5-HT also result in long-term learning and memory deficits (Mazer et al., 1997;Morford et al., 2002;Williams et al., 2002;Williams et al., 2003b).
Most of the evidence for the role of 5-HT in brain development and function comes from pharmacological agents that inhibit 5-HT biosynthesis, destroy 5-HT containing neurons, or block 5-HT reuptake. For example, maternal administration (prenatal exposure) of the tryptophan hydroxylase inhibitor, p-chlorophenylalanine (PCPA), has been shown in the offspring to delay the onset of neuronal differentiation in the raphe nuclei and presumptive serotonergic targets, and alter postnatal expression of serotonergic receptors (Lauder and Krebs, 1978;Lauder et al., 1985). PCPA-induced 5-HT depletions during the neonatal period have been shown to result in decreased learning and memory ability (Mazer et al., 1997). Both p-chloroamphetamine (PCA) and 5, 7 dihydroxytryptamine (5,7-DHT), 5-HT neurotoxins, produce decreases in dendritic spine density and length in granule cells when administered to developing rats (Yan et al., 1997). Although pharmacological manipulations of 5-HT have been informative, drugs also produce peripheral effects, secondary central effects, and variable responses even at their intended targets (Chang et al., 1979;Choi et al., 2004;Knuth and Etgen, 2004;Stokes et al., 2000). Constitutive gene-targeted deletions of several 5-HT receptors are also known to produce changes in learning ability, aggression, and anxiety, although these mutations are not cell-type specific (reviewed by (Sodhi and Sanders-Bush, 2004)).
Pet-1 (plasmacytoma-expressed transcript 1) is an ETS domain transcription factor that is restricted in the brain to 5-HT expressing neurons and is suspected to play a key role in the development and differentiation of serotonergic progenitors, as well as maintain the expression of tryptophan hydroxylase (TPH) and other genes required for serotonergic function in adulthood (Cheng et al., 2003;Ding et al., 2003;Hendricks et al., 1999;Hendricks et al., 2003;Pfaar et al., 2002). In the absence of Pet-1, there is an 80% reduction of both the total number of serotonergic neurons in the brain and a 70-80% decrease in the level of 5-HT in target regions, such as the hippocampus, striatum, and cortex (Hendricks et al., 2003). Although the remaining serotonergic neurons do not appear to require Pet-1 for differentiation, observed TPH and serotonin transporter (SERT) deficiencies in Pet-1-/- mice supports the idea that it is a critical protein controlling transcription of these genes (Hendricks et al., 2003). Furthermore, the genetic deletion of Pet-1 and subsequent reductions of 5-HT neuronal differentiation have been shown to increase anxiety-like behavior and aggression in male Pet-1 nulls compared to male wild type mice without affecting motor behavior (Hendricks et al., 2003). Our interest was primarily to test the role of 5-HT in cognition. In order to more thoroughly phenotype these mice we also included tests of anxiety, depression, locomotion, and sensorimotor gating. Because previous experiments have focused on males (Hendricks et al., 2003), we evaluated both males and females to obtain a more comprehensive assessment.
Experimental procedures
Animals
Pet-1-/- founder mice (129sv × C57BL/6J genetic background) were obtained from Case Western Reserve University (Cleveland, OH) as previously described by (Hendricks et al., 2003). Once acclimated, the received Pet-1-/- mice were backcrossed 3 times to wild type (WT) C57BL/6J mice (Jackson Laboratories, Bar Harbor, Maine). Pet-1+/- mice were then mated to each other. Offspring from these heterozygous pairings were used as breeders and their offspring were used as subjects in the present experiment. Wild-type (WT) and Pet-1-/- males and females, up to a maximum of 2 pups per litter of the same sex and genotype were used from a total of 31 litters. All mice were maintained on a 14 h light/10 h dark cycle. Ears were notched for identification and tails were clipped between P21-28 for genotyping. Mice were genotyped according to a previous protocol (Hendricks et al., 2003). Mice were housed separately after weaning on postnatal day (P) 28 because of high-level aggression (both combative and sexual). Behavioral testing began between P60-75 and was conducted in four separate cohorts in which both sex and genotype were equally represented in order to control for possible seasonal fluctuations in behavioral responses. Breeding and testing were done at Cincinnati Children's Research Foundation (CCRF) so that no shipping stress occurred. All procedures were approved by the institutional Laboratory Animal Care and Use Committee. Behavior was assessed during the light portion of the light/dark cycle and food and water were available ad libitum. Tests intended to determine anxiety-related responses (elevated zero maze, spontaneous locomotor activity, marble burying, light-dark box exploration) were assessed before tests of learning and memory or acoustic startle (File et al., 2004;Hogg, 1996).
Elevated zero maze
The first test administered was the elevated zero maze (Shepherd et al., 1994) with minor modification (Williams et al., 2003a;Moseley et al., 2007). Animals were brought to a room adjacent to the test room and allowed 1 h to acclimate before being tested. The test room was dimly lit based on preliminary experiments showing that this induced the proper conditions for mice to explore the test environment. The experimenter exited the room immediately after placing the mouse in one of the closed quadrants of the apparatus. Sessions were video recorded and later scored for time in open, number of head dips, number of open arm entries, and latency to first enter an open quadrant (ODLog, Macropod Software).
Spontaneous locomotor activity
At least 1 h following completion of the elevated zero maze, locomotor activity was assessed in infrared photocell activity chambers (41 × 41 cm; Accuscan Electronics, Columbus, OH) for 1 h (Moseley et al., 2007). Total horizontal, peripheral, and central distances were analyzed in 5 min intervals.
Marble Burying
Immediately after spontaneous locomotor activity assessment, animals were moved to an adjacent room and tested in a defensive marble burying task adapted from a previous procedure (Williams et al., 2007). Fifteen blue marbles (1.5 cm in diameter) were arranged in 5 rows of 3 using a template that spaced the marbles 4.5 cm apart, 4.5 cm from the long edge and 3.5 cm from the short edge of a 16 × 27 cm mouse cage containing 5 cm of wood chip bedding. Animals remained in these cages with a tall filter-top lid for 20 min. Latency to begin active digging and the number of marbles at least 2/3 buried at the end of the session were recorded. New cages and bedding were used for each animal and marbles were cleaned with 70% ethanol between animals.
Light-Dark test
In the light dark-chamber, assessment of boundary crossings and time spent in each compartment may be used to assess anxiolytic activity or screen for new 5-HT reuptake inhibitors (Crawley and Goodwin, 1980). On the day after spontaneous locomotor assessment, the locomotor chambers were fitted with a dark enclosure (1/2 the size of the chamber) placed against one wall and mice were placed in the corner of the open (light) side facing the wall. Latency to enter the dark, number of transitions from light to dark, and the total time spent in each side of the chamber were recorded for 10 min. The room was illuminated with overhead fluorescent lights. The chambers were cleaned with a 70% ethanol solution.
Novel object recognition
Testing began 1-3 days following the light-dark test as described previously (Brunskill et al., 2005) with minor modification. Briefly, a gray circular PVC arena (60 cm in diameter, 41 cm high) was used. Mice were habituated to the empty arena for 10 min/day for 4 days. On day 5, testing consisted of two phases. Phase-1 was familiarization which consisted of mice being placed in the test arena in the presence of two identical objects and the cumulative time exploring either object was recorded until a combined total of 30 s elapsed. Phase-2 was retention (1 h after completion of familiarization) with one object being an identical copy of the familiarization object and the other a never before seen novel object, and mice had to accumulate 30 s attending to either object. ODLog (Macropod Software) was used to score this task by the experimenter in real-time. Any animal that failed to acquire 30 s of attending to the objects during either phase within 10 min was discontinued from the test.
Morris water maze (MWM)
Spatial learning and memory were assessed in the MWM using procedures described previously (Vorhees and Williams, 2006). Testing began 1-4 days following novel object recognition and was performed in a 122 cm circular tank (Moseley et al., 2007). Animals were first tested for proximal cue learning in the cued version of the task. This consisted of 6 trials on day-1 with the start position (west) and platform (east) in fixed positions. This was done to train the animals to the task requirements (i.e., swimming, moving away from the perimeter, and climbing on the platform to escape). On the next 5 days, 2 trials per day were administered in which both the platform and start positions were randomly moved. On all cued trials, curtains were drawn around the maze to obscure distal cues. The platform (10 cm diameter) was submerged 1 cm below water level and had a spherical orange marker positioned 7 cm above the surface of the water on a brass rod. Latency to reach the cued platform was recorded using a stop watch and all trials had a maximum time limit of 1 min. Following the cued learning, animals were tested in 3 phases of the hidden platform version of the MWM: acquisition, reversal, and shift (Moseley et al., 2007). Briefly, animals received 4 trials per day (maximum of 1 min) for 6 days followed by a 30 s probe trial on day 7 with the platform removed. For all platform trials (cued and hidden) mice either stayed on the platform or were placed on the platform for 15 s. Each phase used a platform of a different size (10, 7, and 5 cm diameter, respectively). During hidden platform trials, a video camera and tracking software were used to map the animals' performance (AnyMaze, Stoelting Company,Wood Dale, IL). On platform trials, latency, cumulative distance, path length, and speed were analyzed. For probe trials, platform site crossovers, speed, and percent distance and time in the target quadrant were assessed.
Cincinnati water maze (CWM)
The CWM test was used to assess egocentric learning ability and began 1-3 days following the MWM. The maze, as described elsewhere (Vorhees, 1987), was originally designed and scaled for rats. The maze consists of a series of nine black Ts that branch from a central circuitous channel. For mice, the maze was scaled-down such that the width of the Ts and channel were ∼50% narrower (8 cm) than for rats (15 cm), with walls 25 cm high filled half-way with water. The maze was fabricated of high density 0.6 cm black polyethylene. Water was drained and refilled daily and allowed to equilibrate overnight to room temperature (21 ± 1 °C). Testing was conducted in complete darkness using infrared light emitters and a camera placed above the maze and connected to a closed circuit TV monitor located in an adjacent room where the experiment watched and recorded performance. Testing under infrared light was designed to eliminate extramaze cues and prevent the animal from using distal cues for a spatial strategy to navigate the maze. The animals were started facing the wall from the point originally described as position-B (Vorhees, 1987) and were allowed a maximum of 5 min per trial to find the escape platform with a minimum 15 min intertrial interval (ITI) if an animal failed to locate the goal. Animals were given two daily trials for 10 days. Errors, latency to escape, and number of start returns were recorded. An error was defined as a head and shoulder entry into the dead-end portion of any T. Perseverations within a given T were counted as individual errors. Occasionally an animal did not find the escape after completing all trials and sometimes stopped exploring. This lack of exploration left them with relatively few errors compared to animals that were escaping the maze in less than 5 min. To adjust for this, error scores for these animals are assigned a value of 1 more than the score of the worst performing animal in the experiment.
Acoustic startle/Prepulse Inhibition (PPI)
Prepulse inhibition (PPI) of the acoustic startle response (ASR) tests sensorimotor gating and was assessed 1-7 days following completion of the CWM using a previously described procedure (Brunskill et al., 2005). Briefly, each mouse was placed inside a sound-attenuating test chamber (SR Lab apparatus, San Diego Instruments, San Diego, CA) inside an inner cylindrical acrylic holder with sliding doors at each end and mounted on a platform. The platform had a piezoelectric force transducer mounted beneath it that was sensitive to the animal's movements. Mice were placed in the cylindrical test chamber for a 5 min acclimation period followed by a 4 × 4 Latin square sequence of trials that were of four types. The trial types were: zero stimulus, startle stimulus alone, 70 dB prepulse + startle stimulus, or 76 dB prepulse + startle stimulus. The Latin square was repeated three times for a total of 48 trials. The ITI was 8 s and the interstimulus interval was 70 ms from prepulse onset to startle signal onset. The startle signal was 115 dB (SPL) and lasted for 0.2 s.
Tail Suspension
The tail suspension test is known to be affected by acute 5-HT reductions or synaptic release and reduced immobility is commonly used to evaluate the effects of antidepressants in rodents (Crowley et al., 2004;O'Leary et al., 2007). Animals were moved to the testing room at least 1 h prior to testing. The tail suspension apparatus consisted of a clear acrylic plate mounted 15 cm above the table on plastic legs. A 1.3 cm hole fitted with a rubber grommet had an aperature large enough to accommodate the width of a mouse tail. Mice were suspended with their tails pulled all the way through the hole to prevent grabbing of their tails. Mice were disqualified if they used their claws to hold onto the grommet. Animals were scored for 6 min for time spent immobile (absence of struggling).
Forced Swim
Animals were moved to the testing room at least 1 h prior to testing 4-8 days after the tail suspension test. Mice were placed in a clear plastic chamber 19 cm in diameter and 50 cm tall. Water depth was 20 cm and immobility was scored for 6 min. Mice were considered immobile if they were not actively moving.
Locomotor Activity with Methamphetamine Challenge
Activity levels following a subcutaneous injection of the indirect dopamine agonist, (+)-methamphetamine (MA) (1 mg/kg), were assessed 6-10 days following forced swim testing. Mice were placed in the chambers for 30 min to re-habituate them and were then briefly removed, injected s.c. with MA and returned to the chambers for an additional 120 min.
Statistical Analysis
Data were analyzed using mixed linear ANOVA models (SAS Proc Mixed, SAS Institute 9.1, Cary, NC). The covariance matrix for each data set was checked using best fit statistics. In most cases the best fit was to the autoregressive-1 (AR(1)) covariance structure. Proc Mixed calculates Kenward-Rogers adjusted degrees of freedom that do not match those obtained from general linear model ANOVAs and can be fractional. Measures taken repetitively on the same animal, such as trial, day, or test interval, were repeated measure factors. Significant interactions were analyzed using simple-effect slice ANOVAs at each level of the repeated measure factor. Only genotype main effects and interaction F-ratios with genotype are shown for clarity of data presentation. Some data are presented only by genotype when sex did not influence the results. Significance was set at p ≤ 0.05 and trends at p< 0.10. Data are presented as least square (LS) mean ± LS SEM.
Results
Elevated Zero maze
There was a main effect of genotype for time in open (F(1,45)=8.49, p < 0.006) (Fig. 1A) and latency to enter an open quadrant (F(1,45)=7.97, p < 0.007) (Fig. 1 B). No main effect of Sex or Genotype × Sex interaction was present therefore, data are averaged across sex. Pet-1-/- mice (n = 14 males, 12 females) spent more time in the open and took longer to enter the first open quadrant than WT mice (n = 9 males, 14 females). No significant effects were observed for the number of open quadrant entries (Fig. 1C).
Figure 1.
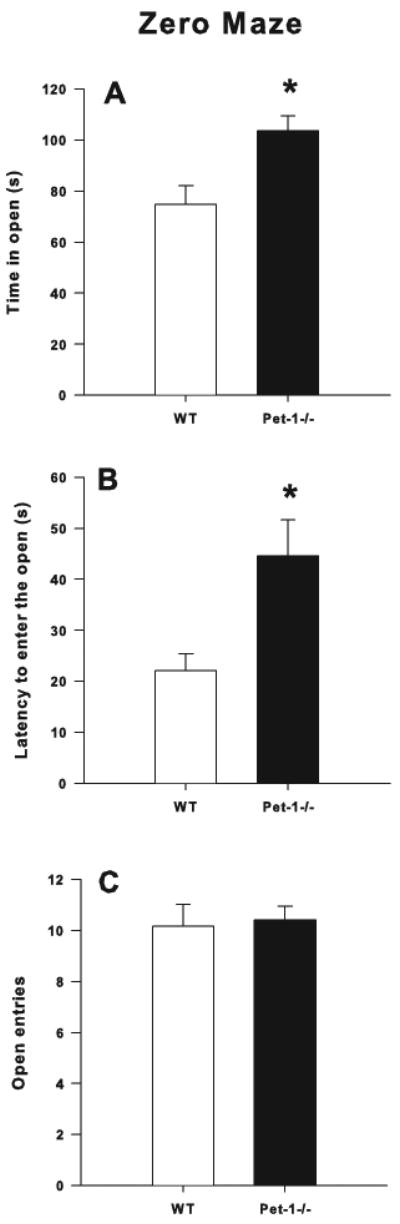
Elevated Zero Maze: A) Time in open (s), B) Latency to enter the open (s), C) Open quadrant entries during a 5 min test session. LS mean ± LS-SEM with males and females combined. *p < 0.05 vs. WT.
Spontaneous Locomotor Activity
For distance, there was a main effect of Genotype (F(1,70.1) = 6.73, p < 0.02) and a trend for a Genotype × Interval interaction (F(11,659) = 1.70, p < 0.07) (Fig. 2). For center and peripheral distance there was also an effect of Genotype (F(1,70) = 12.73, p < 0.001 and F(1,70.1) = 3.87, p < 0.05, respectively) and a Genotype × Interval interaction (F(11,770) = 2.73, p < 0.002 and F(11,658) = 3.00, p < 0.001, respectively). Pet-1-/- mice (n = 18 males, 20 females) traveled less distance overall with comparable reductions in the center and periphery (not shown) compared to WT mice (n = 18 males, 18 females).
Figure 2.
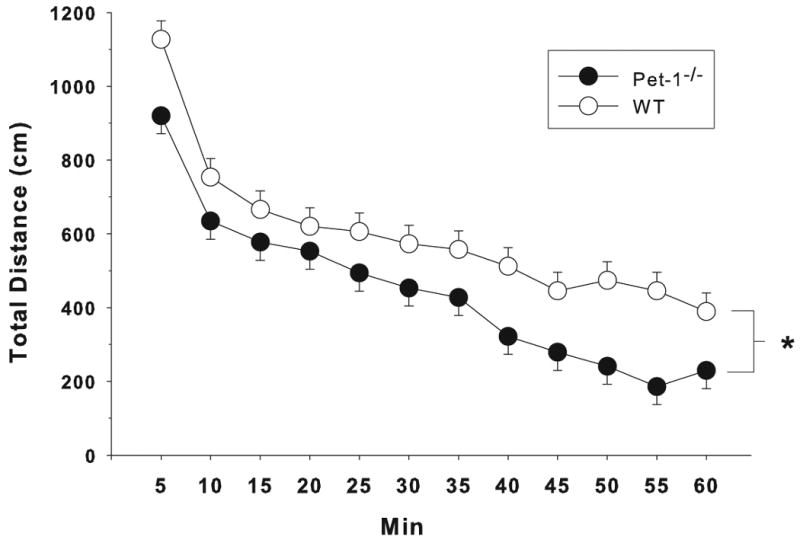
Locomotor Activity: Mice were tested for 1 h during the light phase of the light/dark cycle. Total distance (cm) as a function of genotype in 5 min intervals. Data are LS mean ± LS-SEM with males and females combined. *p < 0.05, vs. WT.
Marble Burying
There was a trend (F(1,76)= 3.71, p < 0.06) for shorter latency to begin burying in Pet-1-/- compared to WT mice (n= 20/genotype/sex) (Fig. 3A). For number of marbles at least 2/3 buried there was a main effect of Genotype (F(1,76)=25.34, p < 0.0001) in which Pet-1-/- buried more marbles than WT mice (Fig. 3B).
Figure 3.
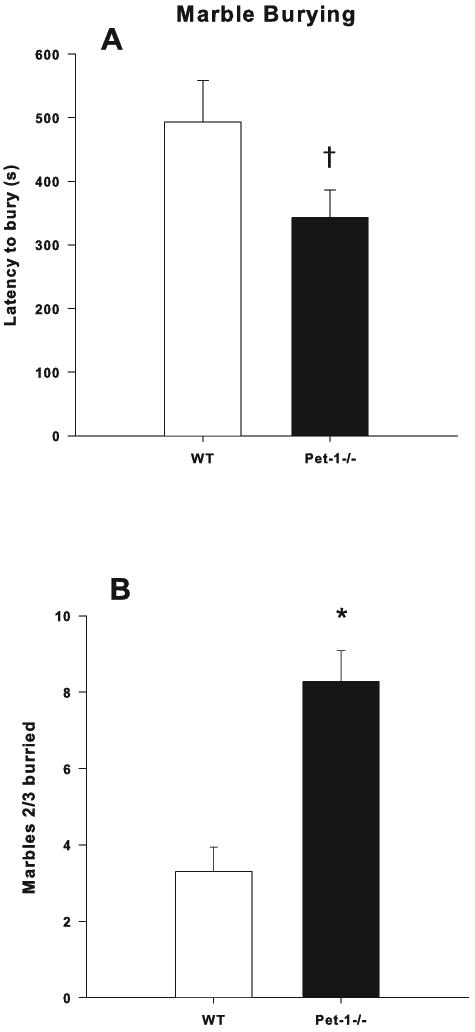
Marble Burying: A) Latency to start burying (s); B) Marbles at least 2/3rd buried. Mice were tested for 20 min. Data are LS mean ± LS-SEM with males and females combined. *p < 0.05, †p < 0.10 vs. WT.
Light-Dark Test
There was an effect of Genotype (F(1,55) = 4.36, p < 0.04) for latency to enter the dark in which Pet-1-/- mice (n = 13 males, 17 females) took longer to move from the light side to the dark side compared to WT mice (n = 17 males, 15 females; Fig. 4A). There were no differences in the number of light-side entries or time spent in the dark (Fig. 4B-C).
Figure 4.
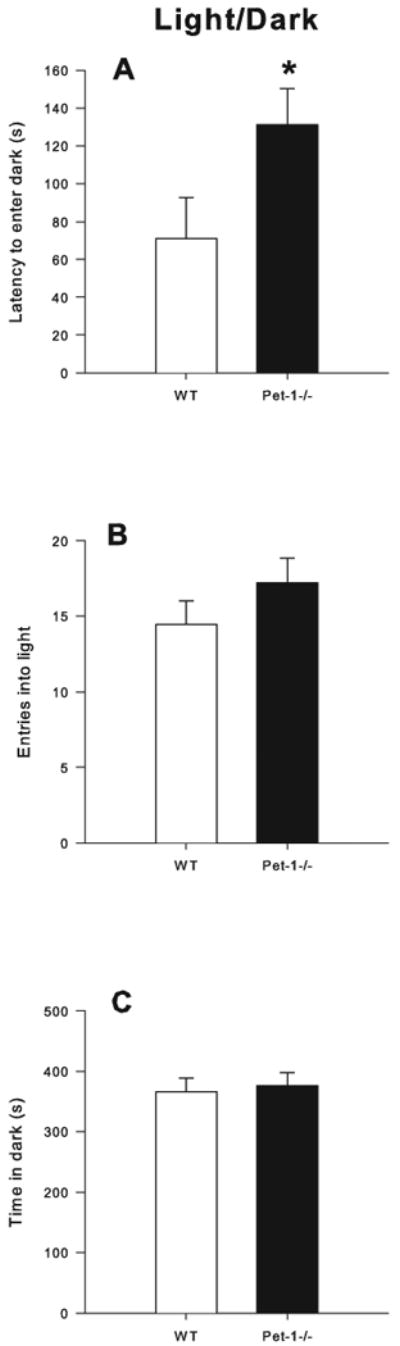
Light-Dark Box Exploration: A) Latency to enter the dark (s); B) Number of entries into the light; C) Time in dark (s). Animals were tested for 10 min. Data are LS mean ± LS-SEM with males and females combined. *p < 0.05 vs. WT.
Novel Object Recognition
For time spent with the novel object there were no differences between Pet-1-/- (20.31 ± 0.5 s; n = 16 males, 20 females) and WT mice (20.29 ± 0.62 s; n = 16 males, 14 females) (not shown).
Morris Water maze
During cued platform trials, latency to find the platform was not affected by Genotype or Sex (not shown; n = 20 male Pet-1-/-, 20 male wild type, 20 female Pet-1-/-, and 19 female WT mice).
During hidden platform trials, for the acquisition phase, there was a Genotype × Sex × Day interaction for path length (F(5,287) = 2.31, p < 0.04) and cumulative distance (F(5,283) = 2.20, p < 0.05). There were no differences between males (Fig. 5A), but in females, path length (Fig. 5B) and cumulative distance for Pet-1-/- mice were increased on day-1 and decreased on day-2 compared to female WT mice. Path lengths are plotted for each phase since this measure has been suggested to be less subject to performance effects than latency. Initial heading error showed a significant genotype × day interaction (F(5,290) = 2.97, p < 0.01). Pet-1-/- mice had a larger initial heading error on days 3 and 6 of testing (not shown). There was a trend for Genotype to affect latency (F(1,70.1) = 3.87, p < 0.05) such that Pet-1-/- mice had decreased latency to find the hidden platform compared to WT mice, even though speed did not differ between Pet-1-/- and WT mice (not shown).
Figure 5.
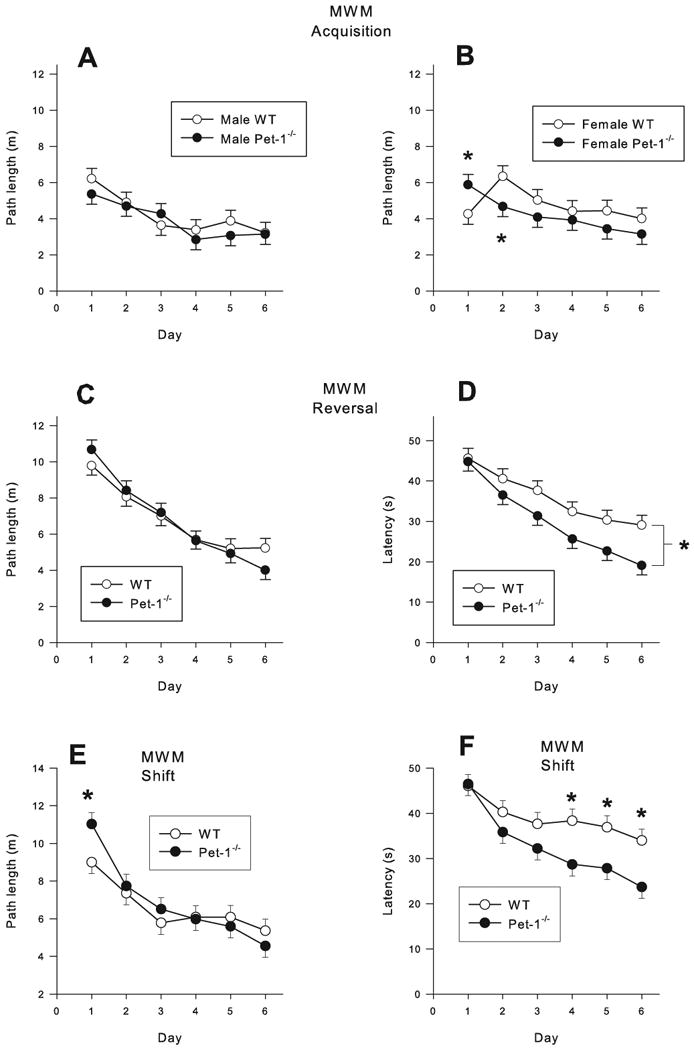
Morris water maze (MWM) hidden platform trials: A) acquisition path length males; B) acquisition path length females; C) reversal path length; D) reversal latency; E) shift path length; F) shift latency. Data are LS mean ± LS-SEM (path length was averaged across 4 trials/day). *p < 0.05 vs. same sex WT controls.
On the probe trial, average distance to the target, crossovers, percent time and distance in the target quadrant, heading error, and speed were not affected by Genotype or Sex (not shown).
During reversal with the 7 cm platform, there was a main effect of Genotype for latency (F(1,76.3) = 5.01, p < 0.03). Pet-1-/- mice had shorter latencies compared to WT mice (Fig. 5D). Path length (Fig. 5C), cumulative distance, initial heading error, and swim speed showed no significant effects (not shown).
On the reversal probe trial, there was a main effect of Genotype on crossovers (F(1,75) = 6.19, p < 0.02) and speed (F(1,75) = 4.26, p < 0.04). Pet-1-/- mice had more crossovers and swam faster than WT mice (not shown). No significant effects were seen on average distance or percent time or distance in the target quadrant.
During shift with the 5 cm platform, there was a main effect of Genotype for latency (F(1,75) = 4.95, p < 0.03) (Fig. 5F) and a Genotype × Day interaction (F(5,375) = 3.08, p < 0.01); the latter also being seen for path length (F(5,375) = 2.93, p < 0.01) (Fig. 5E). On days 4, 5, and 6, Pet-1-/- mice had decreased latencies compared to WT mice and conversely on day-1 Pet-1-/- mice had increased path lengths compared to WT mice. There was a main effect of Genotype (F(1,75.7) = 6.23, p < 0.01) and a Genotype × Sex × Day interaction (F(5,295) = 2.23, p < 0.05) for initial heading error. On day-2 male Pet-1-/- mice had an increased initial heading error (Fig. 6A) and on days 1, 2, and 5, female Pet-1-/- mice had an increased initial heading error (Fig. 6B) compared to WT same-sex controls. Speed was not affected.
Figure 6.
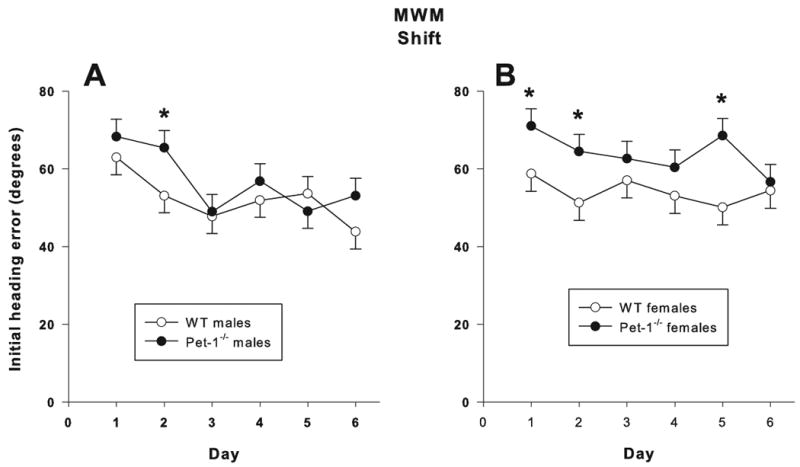
Initial heading error (degrees) during the Shift phase of the Morris water maze (MWM) hidden platform trials: A) males B) females. Data are LS mean ± LS-SEM (initial heading error was averaged across 4 trials/day). *p < 0.05 vs. same sex WT controls.
During the shift probe trial, there was a significant effect of genotype for speed (F(1,75) = 5.53, p < 0.02). Pet-1-/- mice swam faster than WT mice. Crossover, average distance, and percent time and distance in the target quadrant were not significantly affected by Genotype or Sex (not shown).
Cincinnati water maze
For latency (F(1,77.1) = 5.5, p < 0.02) and errors (F(1,77.1) = 3.97 p < 0.05) there was a Genotype × Sex interaction (n = 20 male Pet-1-/-, 20 male wild type, 20 female Pet-1-/-, and 19 female wild type mice). Pet-1-/- males had had shorter latencies (Fig. 7A) and committed fewer errors (Fig. 7C) compared to WT male mice. No differences were observed in females for latency (Fig. 7B) or errors (Fig. 7D). For start returns, there was a main effect of Genotype (F(1,77) = 13.54, p < 0.0004) and a Genotype × Day interaction (F(9,571) = 2.68, p < 0.005). On days 2 through 5, Pet-1-/- mice had more start returns than WT controls (Fig 7E).
Figure 7.
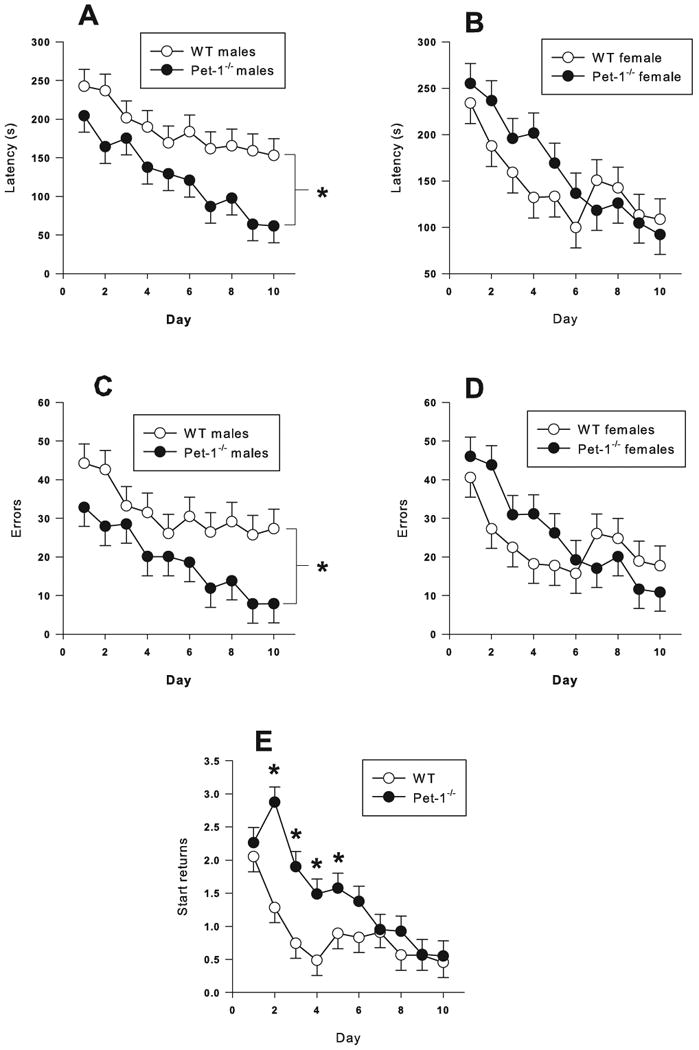
Cincinnati water maze (CWM): A) Latency (s) males; B) Latency (s) females; C) Errors males; D) Errors females; E) Start returns males and females combined. Data are LS mean ± LS-SEM averaged across trials (2 trials/day). *p < 0.05 vs. same sex WT for panels A, B, C, D; *p < 0.05 vs. WT with sexes combined.
Acoustic startle/PPI
There was a Genotype × Prepulse interaction on startle amplitude (Vmax, F(2,150) = 5.99, p < 0.003). Slice effect analysis showed there was only a significant effect when no prepulse was presented. Pet-1-/- mice (251.14 ± 21.15 mV) (n = 20 male, 20 female) had increased startle compared to WT mice (144.73 ± 21.15 mV) (n = 20 male, 19 female).
Tail Suspension
No immobility time differences were found (s); Pet-1-/- male (n=20, 183.3 ± 15.233) and female (n=20, 178.2 ± 14.804); WT male (n=20, 196.68 ± 14.044) and female (n=19, 174.83 ± 15.7015). Many mice grabbed their hind legs during testing despite our attempt to prevent this behavior. In order to determine if this affected the results, a separate analysis was performed in which time spent engaged in limb holding was subtracted from immobility time. No significant effects of genotype were obtained.
Forced Swim test
No effects of genotype were found. For Pet-1-/- mice, immobility times (s) were: male (94.9 ± 18.2) (n = 12) and female (99.5 ± 18.2) (n = 12) and for WT they were male (145.0 ± 22.3) (n = 8) and female (98.0 ± 18.2) (n = 12).
Methamphetamine challenge
Following methamphetamine challenge, there was a significant effect of Genotype (F(1,71.6) = 4.22, p < 0.04), Genotype × Interval (F(23, 1478) = 1.7, p < 0.02), and Genotype × Sex × Interval (F(23,1478) = 1.74, p < 0.02) by analysis of covariance with the last 10 min of the pre-challenge re-habituation period used as the covariate to ensure that post-challenge effects were not contaminated by baseline activity levels. All groups showed a significant methamphetamine-induced hyperactivity response, however, Pet-1-/- male mice (n = 18) showed an exaggerated increase and traveled more distance than WT male mice (n = 18) during intervals 3-16 (Fig. 8A). Female mice (n = 20 Pet-1-/-, 18 WT) did not differ from each other (Fig. 8B).
Figure 8.
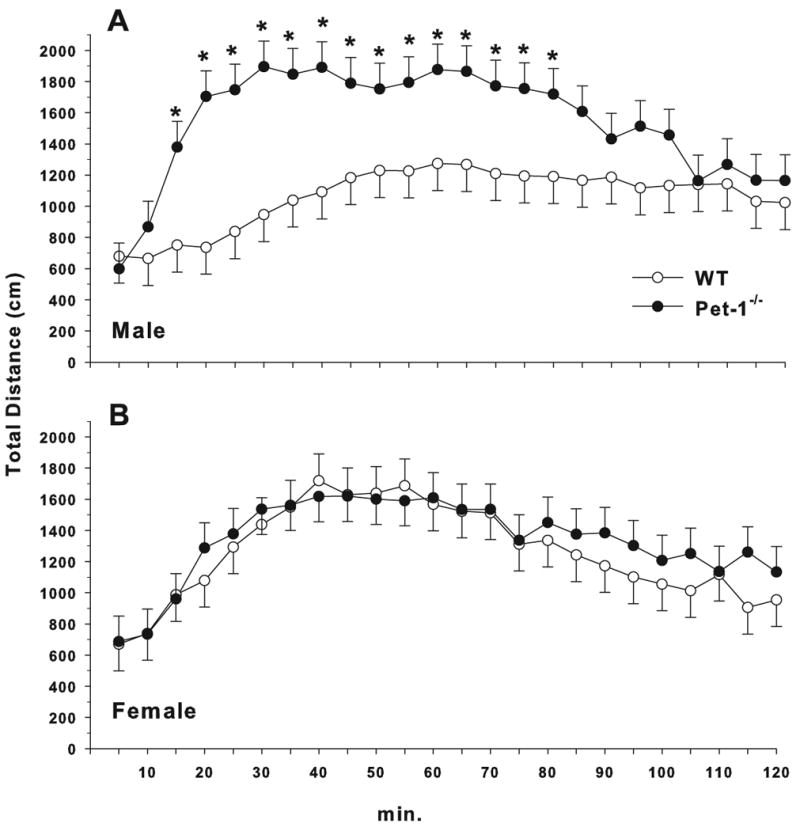
Locomotor activity with methamphetamine challenge: Total distance (cm) for: A) males; B) females. Methamphetamine dose was 1 mg/kg given s.c. Data are LS mean ± LS-SEM shown in 5 min intervals. *p < 0.05 vs. same sex WT controls.
Discussion
Pharmacological, lesion, and neurotoxin experiments suggest that 5-HT is involved in cognition. Accordingly, we postulated that Pet-1-/- mice, with 80% fewer 5-HT-expressing neurons and 70-80% reductions in 5-HT levels that originate from early in development onward, would exhibit cognitive deficiencies. The results, however, reveal that the expected cognitive deficits were not observed in the 5-HT depleted, Pet-1-/- mice. The lack of 5-HT throughout development did not negatively impact spatial learning in the MWM, egocentric learning in the CWM, or recognition memory in a novel object recognition task. Emotional behaviors including anxiety in the elevated zero maze and light-dark box exploration task and depression-related behaviors in the forced swim and tail suspension tests were not increased in this genetically-induced model of decreased 5-HT functioning, although the behavior of Pet-1-/- mice in the marble burying task suggested increased defensive anxiety and/or aggression. This is not to say that the alterations of the serotonergic system were without effect. For example, Pet-1-/- mice did exhibit hypoactivity in spontaneous locomotor activity, increased acoustic startle amplitude, and an increased sensitivity in locomotor responsiveness to a methamphetamine challenge. It may be that the lack of cognitive phenotypic changes in these mice was attributable to compensatory mechanisms that prevented the emergence of a ‘5-HT cognitive syndrome’. Alternatively, perhaps 5-HT is not as vital to cognitive and emotional functioning as reported, however, this interpretation is difficult to reconcile with the extensive accumulated evidence that acute reductions of 5-HT affect these functions or that increasing 5-HT in organisms with endogenous reductions tend to normalize these functions. It may be that the acuity of the 5-HT change is critical to the induction of cognitive effects. If so, the Pet-1 mouse is not a suitable model in which to test this notion.
Much of the work assessing the developmental role of 5-HT on adult cognition involves disrupting 5-HT function during critical periods. In these previous experiments, initially the 5-HT system develops normally until pharmacological or neurotoxic manipulation inhibits or ablates some or most serotonergic cellular function. In disease states, such as schizophrenia, autism, depression, and anxiety disorders, there is evidence that endogenous 5-HT is dysregulated, however while the dysregulation may be chronic it is not as severe or as early in development as that induced by the Pet-1 deletion (Murray et al., 1992;Scott and Deneris, 2005;Torrey et al., 1994;Whitaker-Azmitia, 2005). We have shown that P11-20 MDMA exposure in rats produces approximately a 50% decrease in 5-HT content in the hippocampus and neostriatum during dosing (P11 through P21) (Schaefer et al., 2006;Schaefer et al., 2008;Williams et al., 2005). This results in adult learning deficits in the MWM and CWM that are long lasting (Skelton et al., 2006;Vorhees et al., 2004;Vorhees et al., 2007;Williams et al., 2003b). Fenfluramine produces more dramatic decreases in 5-HT content when administered neonatally and produces more impaired learning in the MWM than developmental MDMA exposure (Morford et al., 2002;Schaefer et al., 2006). Others have shown that P10-20 exposure to the tryptophan hydroxylase inhibitor p-chlorophenylalanine produces spatial learning deficits in the eight-arm radial maze and that these animals fail to extinguish learned behaviors as adults (Mazer et al., 1997). Given such findings, it is surprising that Pet-1-/- mice do not exhibit learning deficits since they are lacking 70-80% of the normal level of 5-HT throughout development and in adulthood. While there is no direct evidence to support the idea of developmental compensation to explain the absence of cognitive deficits in Pet-1-/- animals, it is difficult to explain these data by other means. Alternatively, it may be that previously mentioned learning changes following developmental pharmacologic manipulations of 5-HT may have been dependent on changes induced by these drugs to other neurotransmitter systems, hypothalamic-pituitary-adrenal axis, or other factors.
In terms of assessing spatial learning in the MWM, path length has been suggested to be a more accurate index of spatial ability than latency because it is less affected by swimming ability or speed. It is noteworthy, therefore, that Pet-1-/- mice performed similarly in the cued phase of MWM suggesting no differences in swimming ability or motivation to escape from water. There were minor differences in path lengths between Pet-1-/- mice and WT controls (i.e., females in acquisition on day-1 and -2; males and females on shift day-1) although these differences occurred early in the phases in which they were seen and were not maintained on later test days. These data demonstrate that no profound differences in spatial learning ability exist in Pet-1-/- compared to WT mice. There were some differences during the most spatially demanding shift phase of MWM testing. Pet-1-/- mice exhibited increased path lengths on day 1 and larger initial heading errors in females on days 1, 2, and 5 and in Pet-1-/- males on day 2. These changes suggest that Pet-1-/- mice may have a deficit in spatial orientation that is not reflected in the principal indices of spatial learning. We surmise that the Pet-1-/- mice may leave the tank wall more off-course than WT controls, but correct their initial heading error quickly enough to not affect their performance for the trial as a whole.
The CWM is a task that requires egocentric learning and reliance on self-movement cues of the animal to determine their position within an environment (Etienne and Jeffery, 2004). This is one of the first experiments to show that mice will perform this task and successfully use path-finding ability in the absence of distal cues. This task may be useful for assessing genetic manipulations in mice in which disruptions of egocentric substrates are suspected, such as presubiculum head-direction cells, entorhinal cortex grid and border cells (Solstad et al., 2008), and subsets of hippocampal place cell (Fuhs and Touretzky, 2006;McNaughton et al., 2006;Rondi-Reig et al., 2006;Sargolini et al., 2006;Whishaw et al., 1997;Witter and Moser, 2006) that together constitute egocentric circuitry. We showed that male Pet-1-/- mice learn this task more rapidly (shorter latencies and fewer errors) than male WT controls whereas female Pet-1-/- mice perform the task similarly to WT females. It is conceivable that Pet-1-/- mice swam faster than controls which may result in more frequent chance encounters with the escape platform and therefore decreased latencies on later trials, however, the decreases in errors argue against this interpretation.
The serotonergic system is the target for numerous pharmacological agents including those for depression, anxiety, and affective disorders. This suggests that the 5-HT system is involved in the etiology of these behaviors, however, we do not show Pet-1-/- mice to have depressive-like symptoms or exhibit an anxiety phenotype. The elevated zero maze and light-dark box exploration tasks assess conflict between the drive to explore a novel environment versus neophobia. Pet-1-/- mice of both sexes, remained in the closed areas of the elevated zero maze longer than WT mice, which suggests increased anxiety, but this may also be the result of decreased activity levels since once the Pet-1-/- mice emerged from a closed quadrant they spent more time in the open arm than WT mice without a difference in the number of zone crossings suggesting, contrary to the longer latencies to leave the enclosed areas, that these mice were less anxious. The light-dark box exploration results also suggest that they may be less anxious since Pet-1-/- mice remained in the open side longer than controls. Here again, whether these results reflect hypoactivity or reduced anxiety is unclear based on the present data.
It was previously reported that Pet-1-/- males do not spend time in the open arms of the elevated plus maze suggesting that they may be more anxious (Hendricks et al., 2003). The apparent discrepancy between these data and the current results may be the product of testing differences. Firstly, the mice previously tested in the elevated plus maze experienced a variety of tests prior to anxiety assessment including the resident-intruder assay in which Pet-1-/- mice were more aggressive. The stress of this test may have played a role in their subsequent response to the elevated plus maze. Secondly, when tested in the elevated plus maze, standard lighting was used previously whereas in the present experiment animals were tested in the elevated zero maze under low-level lighting. Thirdly, like the elevated zero maze, the elevated plus maze results may have been influenced by the hypoactivity of the Pet-1-/- mice. Accordingly, a number of factors may explain the differences between this and the previous experiment in regard to anxiety differences.
We also included a different measure of anxiety, marble burying, in which the Pet-1-/- mice buried more marbles than the WT mice. While the marble burying task is regarded as a test of anxiety or depressive-like behavior (Kobayashi et al., 2008;Li et al., 2006), the previous observation that Pet-1-/- males are highly aggressive in the resident-intruder assay (short attack latencies;(Hendricks et al., 2003)) suggests that the increase in marbles buried and the tendency towards decreased latencies to begin to bury may be related to this aggressive phenotype rather than to anxiety (Sluyter et al., 1996).
Male Pet-1-/- mice appear to be more affected by the MA challenge than female Pet-1-/- mice even though their activity levels during the spontaneous locomotor assessment and immediately after methamphetamine injection (at the 5 min interval) are similar (Fig 7). 5-HT is thought to support the development of DA neurons in the substantia nigra (Lauder and Krebs, 1978;Lauder, 1993) and the 5-HT depletions during development in Pet-1-/- animals may play a role in the observed hyper-responsiveness to the indirect DA agonist methamphetamine. Why this pattern did not emerge in females is unclear.
Other reports of genetic manipulations of 5-HT content (constitutive mutants) have hinted at a lack of 5-HT involvement in emotional behavior. Tryptophan hydroxylase (TPH) 1 and 2 double knockouts do not exhibit any overt phenotypes even with nearly complete 5-HT depletion. For instance, no differences were observed in locomotor activity, acoustic startle, or tail suspension (cognitive function was not assessed) in TPH1/2 double knockouts (Savelieva et al., 2008). This group did show TPH1/2 double knockouts to bury more marbles in the defensive marble burying task just as we observed in Pet-1-/- mice, so on this assay good agreement was obtained. However, whereas the TPH double knockouts showed no change in acoustic startle, the Pet-1-/- mice showed augmented startle reactivity. This indicates a significant difference between complete and partial 5-HT ablation. It may be that partial ablation induces a response akin to neurotoxin-induced receptor supersensitivity to acoustic stimulation. Further experiments will be needed to test this hypothesis.
In conclusion, it has been shown that the incipient 5-HT neurons in Pet-1-/- mice do not undergo apoptosis, but rather their development is arrested (Krueger and Deneris, 2008). The remaining 20% serotonergic functioning in Pet-1-/- mice may be sufficient for development and execution of the behaviors assessed and unaffected by removal of Pet-1 in this experiment or other neurotransmitter systems may respond by aiding in compensatory development and reorganization of the brain under these circumstances. Specifically, the cholinergic system, which when lesioned or antagonized in addition to 5-HT depletion has been reported to potentiate learning deficits (Beiko et al., 1997;Harder et al., 1996), may in Pet-1-/- mice compensate for the loss of 5-HT functionality. It is possible that a conditional mutant in which Pet-1 and or 5-HT depletion can be deleted at different times during development may give better insight into the involvement of neonatal 5-HT disruption and subsequent impact on emotional and cognitive behaviors.
Acknowledgments
Portions of these data were presented at the annual International Behavioral Neuroscience Society meeting in St. Thomas, US Virgin Islands, 2008 and the Society for Neuroscience meeting in Washington, D.C. Supported by National Institutes of Health grants DA014269 (MTW) and DA006733 (CVV), and training grant ES007051 (TLS). The authors would like to thank Dr. Evan S. Deneris for supplying Pet-1-/- founder mice.
Abbreviations
- 5-HT
Serotonin
- MWM
Morris water maze
- CWM
Cincinnati water maze
- PCPA
p-chlorophenylalanine
- PCA
p-chloroamphetamine
- 5,7-DHT
5, 7 dihydroxytryptamine
- Pet-1
plasmacytoma-expressed transcript 1
- TPH
tryptophan hydroxylase
- SERT
serotonin transporter
- WT
wild-type
- P
postnatal day
- PPI
prepulse inhibition
- MA
methamphetamine
Footnotes
Section Editor: Dr. Joan I. Morrell
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Beiko J, Candusso L, Cain DP. The effect of nonspatial water maze pretraining in rats subjected to serotonin depletion and muscarinic receptor antagonism: a detailed behavioural assessment of spatial performance. Behav Brain Res. 1997;88:201–211. doi: 10.1016/s0166-4328(97)02298-5. [DOI] [PubMed] [Google Scholar]
- Brunskill EW, Ehrman LA, Williams MT, Klanke J, Hammer D, Schaefer TL, Sah R, Dorn GW, Potter SS, Vorhees CV. Abnormal neurodevelopment, neurosignaling and behaviour in Npas3-deficient mice. Eur J Neurosci. 2005;22:1265–1276. doi: 10.1111/j.1460-9568.2005.04291.x. [DOI] [PubMed] [Google Scholar]
- Chang N, Kaufman S, Milstien S. The mechanism of the irreversible inhibition ofrat liver phenylalanine hydroxylase due to treatment with p-chlorophenylalanine. The lack of effect on turnover of phenylalanine hydroxylase. J Biol Chem. 1979;254:2665–2668. [PubMed] [Google Scholar]
- Cheng L, Chen CL, Luo P, Tan M, Qiu M, Johnson R, Ma Q. Lmx1b, Pet-1, and Nkx2.2 coordinately specify serotonergic neurotransmitter phenotype. J Neurosci. 2003;23:9961–9967. doi: 10.1523/JNEUROSCI.23-31-09961.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S, Jonak E, Fernstrom JD. Serotonin reuptake inhibitors do not prevent 5,7-dihydroxytryptamine-induced depletion of serotonin in rat brain. Brain Res. 2004;1007:19–28. doi: 10.1016/j.brainres.2003.12.044. [DOI] [PubMed] [Google Scholar]
- Crawley J, Goodwin FK. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav. 1980;13:167–170. doi: 10.1016/0091-3057(80)90067-2. [DOI] [PubMed] [Google Scholar]
- Crowley JJ, Jones MD, O'Leary OF, Lucki I. Automated tests for measuring the effects of antidepressants in mice. Pharmacol Biochem Behav. 2004;78:269–274. doi: 10.1016/j.pbb.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Ding YQ, Marklund U, Yuan W, Yin J, Wegman L, Ericson J, Deneris E, Johnson RL, Chen ZF. Lmx1b is essential for the development of serotonergic neurons. Nat Neurosci. 2003;6:933–938. doi: 10.1038/nn1104. [DOI] [PubMed] [Google Scholar]
- Etienne AS, Jeffery KJ. Path integration in mammals. Hippocampus. 2004;14:180–192. doi: 10.1002/hipo.10173. [DOI] [PubMed] [Google Scholar]
- File SE, Lippa AS, Beer B, Lippa MT. Animal tests of anxiety. Curr Protoc Neurosci Chapter 8:Unit. 2004 doi: 10.1002/0471142301.ns0803s26. [DOI] [PubMed] [Google Scholar]
- Fuhs MC, Touretzky DS. A spin glass model of path integration in rat medial entorhinal cortex. J Neurosci. 2006;26:4266–4276. doi: 10.1523/JNEUROSCI.4353-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder JA, Kelly ME, Cheng CH, Costall B. Combined pCPA and muscarinic antagonist treatment produces a deficit in rat water maze acquisition. Pharmacol Biochem Behav. 1996;55:61–65. doi: 10.1016/0091-3057(96)00049-4. [DOI] [PubMed] [Google Scholar]
- Hendricks T, Francis N, Fyodorov D, Deneris ES. The ETS domain factor Pet-1 is an early and precise marker of central serotonin neurons and interacts with a conserved element in serotonergic genes. J Neurosci. 1999;19:10348–10356. doi: 10.1523/JNEUROSCI.19-23-10348.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks TJ, Fyodorov DV, Wegman LJ, Lelutiu NB, Pehek EA, Yamamoto B, Silver J, Weeber EJ, Sweatt JD, Deneris ES. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron. 2003;37:233–247. doi: 10.1016/s0896-6273(02)01167-4. [DOI] [PubMed] [Google Scholar]
- Hogg S. A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol Biochem Behav. 1996;54:21–30. doi: 10.1016/0091-3057(95)02126-4. [DOI] [PubMed] [Google Scholar]
- Knuth ED, Etgen AM. Neural and hormonal consequences of neonatal 5,7-dihydroxytryptamine may not be associated with serotonin depletion. Brain Res Dev Brain Res. 2004;151:203–208. doi: 10.1016/j.devbrainres.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Hayashi E, Shimamura M, Kinoshita M, Murphy NP. Neurochemical responses to antidepressants in the prefrontal cortex of mice and their efficacy in preclinical models of anxiety-like and depression-like behavior: a comparative and correlational study. Psychopharmacology (Berl) 2008;197:567–580. doi: 10.1007/s00213-008-1070-6. [DOI] [PubMed] [Google Scholar]
- Krueger KC, Deneris ES. Serotonergic transcription of human FEV reveals direct GATA factor interactions and fate of Pet-1-deficient serotonin neuron precursors. J Neurosci. 2008;28:12748–12758. doi: 10.1523/JNEUROSCI.4349-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauder JM. Neurotransmitters as growth regulatory signals: role of receptors and second messengers. Trends Neurosci. 1993;16:233–240. doi: 10.1016/0166-2236(93)90162-f. [DOI] [PubMed] [Google Scholar]
- Lauder JM, Bloom FE. Ontogeny of monoamine neurons in the locus coeruleus, Raphe nuclei and substantia nigra of the rat. I. Cell differentiation. J Comp Neurol. 1974;155:469–481. doi: 10.1002/cne.901550407. [DOI] [PubMed] [Google Scholar]
- Lauder JM, Krebs H. Serotonin as a differentiation signal in early neurogenesis. Dev Neurosci. 1978;1:15–30. doi: 10.1159/000112549. [DOI] [PubMed] [Google Scholar]
- Lauder JM, Towle AC, Patrick K, Henderson P, Krebs H. Decreased serotonin content of embryonic raphe neurons following maternal administration of p-chlorophenylalanine: a quantitative immunocytochemical study. Brain Res. 1985;352:107–114. doi: 10.1016/0165-3806(85)90092-6. [DOI] [PubMed] [Google Scholar]
- Lauder JM, Wallace JA, Krebs H. Roles for serotonin in neuroembryogenesis. Adv Exp Med Biol. 1981;133:477–506. doi: 10.1007/978-1-4684-3860-4_28. [DOI] [PubMed] [Google Scholar]
- Li X, Morrow D, Witkin JM. Decreases in nestlet shredding of mice by serotonin uptake inhibitors: comparison with marble burying. Life Sci. 2006;78:1933–1939. doi: 10.1016/j.lfs.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Mazer C, Muneyyirci J, Taheny K, Raio N, Borella A, Whitaker-Azmitia P. Serotonin depletion during synaptogenesis leads to decreased synaptic density and learning deficits in the adult rat: a possible model of neurodevelopmental disorders with cognitive deficits. Brain Res. 1997;760:68–73. doi: 10.1016/s0006-8993(97)00297-7. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Battaglia FP, Jensen O, Moser EI, Moser MB. Path integration and the neural basis of the ‘cognitive map’. Nat Rev Neurosci. 2006;7:663–678. doi: 10.1038/nrn1932. [DOI] [PubMed] [Google Scholar]
- Morford LL, Inman-Wood SL, Gudelsky GA, Williams MT, Vorhees CV. Impaired spatial and sequential learning in rats treated neonatally with D-fenfluramine. Eur J Neurosci. 2002;16:491–500. doi: 10.1046/j.1460-9568.2002.02100.x. [DOI] [PubMed] [Google Scholar]
- Moseley AE, Williams MT, Schaefer TL, Bohanan CS, Neumann JC, Behbehani MM, Vorhees CV, Lingrel JB. Deficiency in Na,K-ATPase alpha isoform genes alters spatial learning, motor activity, and anxiety in mice. J Neurosci. 2007;27:616–626. doi: 10.1523/JNEUROSCI.4464-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray RM, Jones P, O'Callaghan E, Takei N, Sham P. Genes, viruses and neurodevelopmental schizophrenia. J Psychiatr Res. 1992;26:225–235. doi: 10.1016/0022-3956(92)90029-n. [DOI] [PubMed] [Google Scholar]
- O'Leary OF, Bechtholt AJ, Crowley JJ, Hill TE, Page ME, Lucki I. Depletion of serotonin and catecholamines block the acute behavioral response to different classes of antidepressant drugs in the mouse tail suspension test. Psychopharmacology (Berl) 2007;192:357–371. doi: 10.1007/s00213-007-0728-9. [DOI] [PubMed] [Google Scholar]
- Pfaar H, von Holst A, Vogt Weisenhorn DM, Brodski C, Guimera J, Wurst W. mPet-1, a mouse ETS-domain transcription factor, is expressed in central serotonergic neurons. Dev Genes Evol. 2002;212:43–46. doi: 10.1007/s00427-001-0208-x. [DOI] [PubMed] [Google Scholar]
- Rondi-Reig L, Petit GH, Tobin C, Tonegawa S, Mariani J, Berthoz A. Impaired sequential egocentric and allocentric memories in forebrain-specific-NMDA receptor knock-out mice during a new task dissociating strategies of navigation. J Neurosci. 2006;26:4071–4081. doi: 10.1523/JNEUROSCI.3408-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargolini F, Fyhn M, Hafting T, McNaughton BL, Witter MP, Moser MB, Moser EI. Conjunctive representation of position, direction, and velocity in entorhinal cortex. Science. 2006;312:758–762. doi: 10.1126/science.1125572. [DOI] [PubMed] [Google Scholar]
- Savelieva KV, Zhao S, Pogorelov VM, Rajan I, Yang Q, Cullinan E, Lanthorn TH. Genetic disruption of both tryptophan hydroxylase genes dramatically reduces serotonin and affects behavior in models sensitive to antidepressants. PLoS ONE. 2008;3:e3301. doi: 10.1371/journal.pone.0003301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer TL, Ehrman LA, Gudelsky GA, Vorhees CV, Williams MT. Comparison of monoamine and corticosterone levels 24 h following (+)methamphetamine, (+/-)3,4-methylenedioxymethamphetamine, cocaine, (+)fenfluramine or (+/-)methylphenidate administration in the neonatal rat. J Neurochem. 2006;98:1369–1378. doi: 10.1111/j.1471-4159.2006.04034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer TL, Skelton MR, Herring NR, Gudelsky GA, Vorhees CV, Williams MT. Short- and long-term effects of (+)-methamphetamine and (+/-)-3,4-methylenedioxymethamphetamine on monoamine and corticosterone levels in the neonatal rat following multiple days of treatment. J Neurochem. 2008;104:1674–1685. doi: 10.1111/j.1471-4159.2007.05112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott MM, Deneris ES. Making and breaking serotonin neurons and autism. Int J Dev Neurosci. 2005;23:277–285. doi: 10.1016/j.ijdevneu.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Shepherd JK, Grewal SS, Fletcher A, Bill DJ, Dourish CT. Behavioural and pharmacological characterisation of the elevated “zero-maze” as an animal model of anxiety. Psychopharmacology (Berl) 1994;116:56–64. doi: 10.1007/BF02244871. [DOI] [PubMed] [Google Scholar]
- Skelton MR, Williams MT, Vorhees CV. Treatment with MDMA from P11-20 disrupts spatial learning and path integration learning in adolescent rats but only spatial learning in older rats. Psychopharmacology (Berl) 2006 doi: 10.1007/s00213-006-0563-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluyter F, Korte SM, Bohus B, Van Oortmerssen GA. Behavioral stress response of genetically selected aggressive and nonaggressive wild house mice in the shock-probe/defensive burying test. Pharmacol Biochem Behav. 1996;54:113–116. doi: 10.1016/0091-3057(95)02164-7. [DOI] [PubMed] [Google Scholar]
- Sodhi MS, Sanders-Bush E. Serotonin and brain development. Int Rev Neurobiol. 2004;59:111–174. doi: 10.1016/S0074-7742(04)59006-2. [DOI] [PubMed] [Google Scholar]
- Solstad T, Boccara CN, Kropff E, Moser MB, Moser EI. Representation of geometric borders in the entorhinal cortex. Science. 2008;322:1865–1868. doi: 10.1126/science.1166466. [DOI] [PubMed] [Google Scholar]
- Stokes AH, Xu Y, Daunais JA, Tamir H, Gershon MD, Butkerait P, Kayser B, Altman J, Beck W, Vrana KE. p-ethynylphenylalanine: a potent inhibitor of tryptophan hydroxylase. J Neurochem. 2000;74:2067–2073. doi: 10.1046/j.1471-4159.2000.0742067.x. [DOI] [PubMed] [Google Scholar]
- Torrey EF, Taylor EH, Bracha HS, Bowler AE, McNeil TF, Rawlings RR, Quinn PO, Bigelow LB, Rickler K, Sjostrom K. Prenatal origin of schizophrenia in a subgroup of discordant monozygotic twins. Schizophr Bull. 1994;20:423–432. doi: 10.1093/schbul/20.3.423. [DOI] [PubMed] [Google Scholar]
- Vorhees CV. Maze learning in rats: a comparison of performance in two water mazes in progeny prenatally exposed to different doses of phenytoin. Neurotoxicol Teratol. 1987;9:235–241. doi: 10.1016/0892-0362(87)90008-0. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Reed TM, Skelton MR, Williams MT. Exposure to 3,4-methylenedioxymethamphetamine (MDMA) on postnatal days 11-20 induces reference but not working memory deficits in the Morris water maze in rats: implications of prior learning. Int J Dev Neurosci. 2004;22:247–259. doi: 10.1016/j.ijdevneu.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Schaefer TL, Williams MT. Developmental effects of +/-3,4-methylenedioxymethamphetamine on spatial versus path integration learning: Effects of dose distribution. Synapse. 2007;61:488–499. doi: 10.1002/syn.20379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nature Protocols. 2006:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whishaw IQ, McKenna JE, Maaswinkel H. Hippocampal lesions and path integration. Curr Opin Neurobiol. 1997;7:228–234. doi: 10.1016/s0959-4388(97)80011-6. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM. Behavioral and cellular consequences of increasing serotonergic activity during brain development: a role in autism? Int J Dev Neurosci. 2005;23:75–83. doi: 10.1016/j.ijdevneu.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM, Druse M, Walker P, Lauder JM. Serotonin as a developmental signal. Behav Brain Res. 1996;73:19–29. doi: 10.1016/0166-4328(96)00071-x. [DOI] [PubMed] [Google Scholar]
- Williams MT, Herring NR, Schaefer TL, Skelton MR, Campbell NG, Lipton JW, McCrea AE, Vorhees CV. Alterations in body temperature, corticosterone, and behavior following the administration of 5-methoxy-diisopropyltryptamine (‘foxy’) to adult rats: a new drug of abuse. Neuropsychopharmacology. 2007;32:1404–1420. doi: 10.1038/sj.npp.1301232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MT, Moran MS, Vorhees CV. Refining the critical period for methamphetamine-induced spatial deficits in the Morris water maze. Psychopharmacology (Berl) 2003a;168:329–338. doi: 10.1007/s00213-003-1433-y. [DOI] [PubMed] [Google Scholar]
- Williams MT, Morford LL, Wood SL, Rock SL, McCrea AE, Fukumura M, Wallace TL, Broening HW, Moran MS, Vorhees CV. Developmental 3,4-methylenedioxymethamphetamine (MDMA) impairs sequential and spatial but not cued learning independent of growth, litter effects or injection stress. Brain Res. 2003b;968:89–101. doi: 10.1016/s0006-8993(02)04278-6. [DOI] [PubMed] [Google Scholar]
- Williams MT, Schaefer TL, Ehrman LA, Able JA, Gudelsky GA, Sah R, Vorhees CV. 3,4-Methylenedioxymethamphetamine administration on postnatal day 11 in rats increases pituitary-adrenal output and reduces striatal and hippocampal serotonin without altering SERT activity. Brain Res. 2005;1039:97–107. doi: 10.1016/j.brainres.2005.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MT, Vorhees CV, Boon F, Saber AJ, Cain DP. Methamphetamine exposure from postnatal day 11 to 20 causes impairments in both behavioral strategies and spatial learning in adult rats. Brain Res. 2002;958:312–321. doi: 10.1016/s0006-8993(02)03620-x. [DOI] [PubMed] [Google Scholar]
- Witter MP, Moser EI. Spatial representation and the architecture of the entorhinal cortex. Trends Neurosci. 2006;29:671–678. doi: 10.1016/j.tins.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Yan W, Wilson CC, Haring JH. Effects of neonatal serotonin depletion on the development of rat dentate granule cells. Brain Res Dev Brain Res. 1997;98:177–184. doi: 10.1016/s0165-3806(96)00176-9. [DOI] [PubMed] [Google Scholar]


