Abstract
Background
The primary cause of morbidity and mortality in patients with cystic fibrosis (CF) is progressive obstructive pulmonary disease due to chronic endobronchial infection, particularly with Pseudomonas aeruginosa (Pa). Risk factors for and clinical impact of early Pa infection in young CF patients are less well understood.
Purpose
The present studies are designed to evaluate risk factors and outcomes associated with early Pa acquisition, and the benefits and harms of four anti-pseudomonal treatment regimens in young CF patients initiated after the first Pa positive respiratory culture.
Methods
The Early Pseudomonas Infection Control (EPIC) program consists of two studies, a randomized multicenter trial in CF patients ages 1–12 years at first isolation of Pa from a respiratory culture, and a longitudinal cohort study enrolling Pa-negative patients. Using a factorial design, trial participants are assigned for 18 months to either anti-pseudomonal treatment on a scheduled quarterly basis (cycled therapy) or based on recovery of Pa from quarterly respiratory cultures (culture-based therapy). The study drugs include inhaled tobramycin (300 mg BID) for 28 days, combined with either oral ciprofloxacin (15–20 mg/kg BID) or oral placebo for 14 days. The primary endpoints of the trial are the time to pulmonary exacerbation requiring IV antibiotics or hospitalization for respiratory symptoms, and the proportion of patients with new Pa-positive respiratory cultures during the study. The broad goals of the observational study are to describe the risk factors and outcomes associated with early acquisition of Pa. 306 patients were randomized in the clinical trial and 1,787 were enrolled in the cohort study.
Conclusions
These companion studies will provide valuable epidemiological and microbiological information on early CF lung disease and Pa acquisition, and safety and clinical efficacy data on anti-pseudomonal treatment strategies for early Pa infections in the airways of young children with CF.
Keywords: Antimicrobials, Inhaled tobramycin, Ciprofloxacin, Pseudomonas aeruginosa, Mucoviscidosis, Clinical trial
INTRODUCTION
Study Rationale
Cystic fibrosis (CF), an autosomal recessive disease lacking a curative therapy, has a current median survival of over 36 years, and affects approximately 25,000–30,000 individuals in the United States and 70,000 people worldwide [1–3]. The primary cause of morbidity and mortality in patients with CF is progressive obstructive pulmonary disease associated with chronic Pseudomonas aeruginosa (Pa) endobronchial bacterial infection and an intense neutrophil-dominated host inflammatory response [4, 5]. Pa, a ubiquitous environmental bacterium, is the most important pathogen in CF lung disease. Pa infection can begin very early in life, and the prevalence of Pa in respiratory cultures increases with age, from 10–30% at ages 0–5 years to 80% at ages ≥18 years [1]. Unlike established Pa infection, features of early Pa infection, including susceptibility to antibiotics, non-mucoid phenotype, and low bacterial density, appear to provide a “window of opportunity” during which time anti-pseudomonal therapy may be effective in eradicating Pa [6–8]. Over time, the distinct microenvironment in the CF airways allows selection of Pa uniquely adapted for chronic, persistent infection. These organisms are mucoid, form biofilms, become increasingly antibiotic-resistant, are present at high density, and are virtually impossible to eradicate. Chronic Pa infection is clearly associated with poorer clinical outcomes among patients with CF [9–12]. The risk factors for and clinical impact of early Pa infection are even less understood, yet are of great import to clinicians caring for young patients with CF. Preliminary data suggest a favorable effect of aggressive treatment at first isolation of Pa from respiratory cultures, but data from large randomized trials are lacking [5, 12–14].
Thus, we designed a randomized trial in children with CF to investigate the benefits and harms of aggressive, early anti-pseudomonal interventions with the goal of delaying or preventing chronic Pa infection and its clinical consequences. Companion to this clinical trial is the first large, multicenter, longitudinal observational study of early lung disease in CF, which enrolled young Pa-negative patients and clinical trial participants. The goal of the observational study is to determine improved strategies for prevention and treatment of early Pa infection.
Study Overview
In order to assess the clinical and microbiologic efficacy of early anti-pseudomonal therapy, and more thoroughly address issues of safety and antimicrobial resistance, the Early Pseudomonas Infection Control multi-center clinical trial (EPIC-CT) and an observational study (EPIC-OBS) target children with CF younger than 12 years of age.
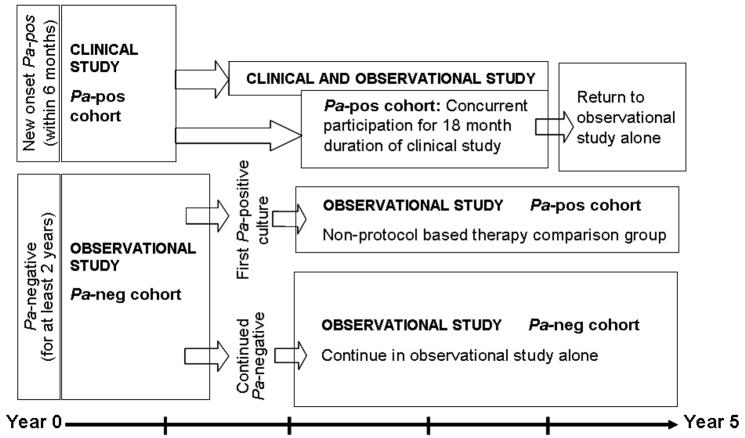
The EPIC-OBS serves both as a freestanding epidemiologic study of risk factors associated with early Pa airway infection, and as an adjunct to the EPIC-CT by providing pre-study data on risk factors potentially affecting response to the trial regimens and post-enrollment follow-up for the clinical trial participants for up to five years (Figure 1).
Figure 1.
EPIC study diagram and overlap between the clinical trial and the observational study.
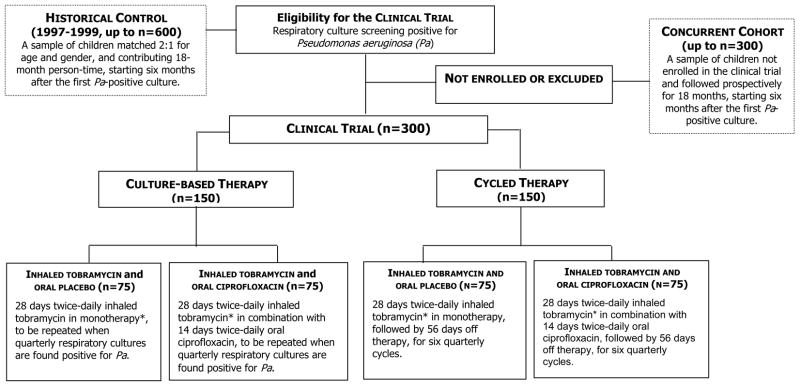
The EPIC-CT was designed to allow the randomized controlled evaluation of early intervention with inhaled and oral anti-pseudomonal therapy in young patients with CF at first isolation of Pa from respiratory cultures. Participants meeting the eligibility criteria were offered the opportunity to be enrolled in the clinical trial and initiate or continue simultaneous participation in the observational study. The clinical trial assigned children to two different antimicrobial treatment strategies: (1) Cycled antibiotic therapy, i.e., treatment provided in quarterly cycles regardless of findings from respiratory cultures obtained quarterly, and (2) Antibiotic therapy based upon cultures, i.e., treatment based on recovery of Pa from respiratory cultures obtained at scheduled quarterly intervals throughout the 18-month study period (Figure 2).
Figure 2.
Randomization assignment of participants enrolling in the clinical trial component of EPIC. *Patients with culture positive for Pa at the end of 28 days of inhaled therapy will receive a second 28-day treatment course for the first cycle only.
To summarize, the overall objectives of the clinical trial are to compare the clinical and microbiological efficacy and safety of cycled therapy versus culture-based therapy, initiated at the time of early Pa infection of the respiratory tract and given over an 18-month study period. Because a true placebo group receiving no anti-pseudomonal antibiotic therapy was not feasible at the time of study initiation in 2004, clinical trial participants will be also compared with two external controls, a concurrent cohort (derived from the EPIC Observational study) that will allow the evaluation of the generalizability of the clinical trial results, and a historical group that will allow the evaluation of low versus high intensity exposure to antipseudomonal antibiotics. This historical group, derived from an existing patient registry and covering a period ranging from 1997–1999, had significantly less exposure to inhaled tobramycin prior to the commercial product’s (TOBI) FDA approval in 1998. Children in the historical group will be matched 2:1 for age and gender to each EPIC-CT participant.
THE OBSERVATIONAL STUDY COMPONENT
Objectives and Aims of EPIC-OBS
The primary aim of EPIC-OBS is the identification of risk factors associated with early age at first isolation of Pa from respiratory cultures and with the early emergence of mucoid and antibiotic-resistant strains of Pa, in particular, modifiable exposures, such as environmental tobacco smoke, breastfeeding and daycare. The EPIC-OBS also aims to describe the longitudinal changes in clinical endpoints (e.g., lung function, growth, exacerbation frequency) associated with initial acquisition of Pa, as well as changes in clinical endpoints associated with emergence of mucoid Pa and antibiotic-resistant Pa. Among subjects not enrolled in the clinical trial, the effect of type and length of anti-pseudomonal therapy on clinical endpoints, subsequent Pa serology, Pa antimicrobial resistance, Pa genotype, and emergence of other pathogens will be examined. The longitudinal relationships between anti-pseudomonal serology, isolation of Pa from respiratory cultures, and evolving clinical signs and symptoms during early CF lung disease will be described. Clinical outcomes associated with isolation of Staphylococcus aureus (S. aureus) from respiratory cultures, and with emergence of methicillin-resistant S. aureus (MRSA) will also be evaluated. DNA samples extracted from whole blood are being collected and banked from participants and their parents of origin for a separate planned evaluation of genetic factors that may be associated with CF pathogenesis, disease progression, and clinical outcomes. Finally, Pa isolates and serum samples from observational participants are banked for future studies to enhance the understanding of microbiological aspects of early CF lung disease.
For subjects who enroll in the EPIC-CT, the observational study provides pre-enrollment data to allow examination of the association between risk factors prior to trial enrollment, particularly Pa serology and Pa phenotype (mucoid vs. non-mucoid), and response to trial regimens. Moreover, the observational study provides follow-up data on trial endpoints after completion of trial participation to allow assessment of the long-term effects of trial regimens (Figure 1). The observational study will also allow characterization of patients who declined enrollment in the clinical trial to estimate the presence of potential selection bias and to evaluate the effect of non-protocol based antimicrobial approaches on the clinical, microbiology, and serology endpoints of interest in the clinical trial.
Study Design of EPIC-OBS
The EPIC-OBS is an ongoing prospective, observational cohort study. The size of the original cohort was 1,787 participants at 59 CF centers. CF patients ≤12 years of age at the time of enrollment were eligible if they had no prior isolation of Pa from respiratory cultures, or if prior history of isolation of Pa from respiratory cultures, at least a two-year period with Pa negative cultures (documented by at least 1 culture annually) or were concurrently enrolled in the EPIC-CT. The study was approved by the Institutional Review Board at each participating site, and all participants or their surrogates provided written informed consent.
The EPIC-OBS involves prospective data and specimen collection (Table 1). Clinical care and monitoring is not affected by study participation. The Cystic Fibrosis Foundation (CFF) maintains a National Patient Registry containing a wide array of demographic, clinical, and actuarial data on all patients seen at CF care centers. Registry data entry is encounter-based and submitted via a secure web site (www.portcf.org). The EPIC-OBS study is made possible by the existence of this resource: data collection occurs at each encounter and via an annual family survey utilizing an augmented version of the CFF Registry containing the standard Registry data collection forms as well as additional, study-specific forms. The EPIC-OBS annual family survey is a parent/family questionnaire on potential risk factors for Pa acquisition, such as environmental tobacco smoke and daycare attendance. Study sites are also queried annually regarding issues such as infection control practices and use of standardized monitoring and treatment regimens in young CF patients.
Table 1.
EPIC-OBS Data and sample collection schedule
| Enrollment | Quarterly Encounter | Annually | Hospitalization or IV Antibiotic | |
|---|---|---|---|---|
| EPIC-OBS enrollment form | X | |||
| Registry clinical encounter form | X | |||
| EPIC-OBS clinical encounter forma | X | |||
| Registry year-end survey | X | |||
| EPIC-OBS year-end surveyb | X | X | ||
| Registry hospitalization/IV history | X | |||
| Results of respiratory culture (local site lab)c | X | |||
| Results of outpatient complete blood countd | X | |||
| Serum for serology and banking | X | |||
| Respiratory specimen sent to Core Microbiology Labe | X |
The EPIC-OBS clinical encounter form collects information about the following characteristics: use of oral and inhaled antibiotics since the previous visit, presence of crackles or wheezes on chest auscultation, cough frequency, cold symptoms, physical activity level, and activity limitations due to respiratory symptoms;
The EPIC-OBS year-end survey collects information about the following exposures: influenza and pneumococcal vaccines; environmental tobacco smoke; wood-burning stoves; hot tubs; swimming pools; attendance at social events with individuals who have CF; playing with other children who have CF; other household members with CF; daycare attendance; Synagis® prophylaxis; breast-feeding; mother’s education; and annual household income;
Culture results from routine clinical cultures performed at the local site laboratory are recorded in the Registry clinical encounter forms;
Result of outpatient complete blood counts are recorded on the EPIC-OBS clinical encounter form;
After the first isolation of Pa from a respiratory culture at the local site laboratory, annual respiratory specimens (OP swab or sputum) are shipped to and processed at the Cystic Fibrosis Foundation Therapeutics Development (TDN) Core Microbiology Laboratory. If possible, initial Pa isolates from the local site laboratory will also be shipped to the TDN Core Microbiology Laboratory.
EPIC-OBS participants have a serum sample collected annually for evaluation of Pa serology and for banking. After the first isolation of Pa from respiratory culture at the local site laboratory, an annual respiratory specimen is sent to the Coordinating Center Core Microbiology Laboratory for semi-quantitative culture, evaluation of Pa mucoidy and banking. If available, initial Pa isolates from the local site laboratory are also shipped to the Core Microbiology Laboratory for banking. A single whole blood sample is collected from participants and a whole blood sample or buccal swab is collected from parents of origin for DNA extraction and banking.
Sample Size and Power for EPIC-OBS
The sample size, number of sites for both the EPIC-OBS and EPIC-CT, and duration of enrollment were determined based on statistics from the 2000 CFF National Patient Registry [1] and questionnaire data from prospective sites participating in the EPIC studies. Combining the information from both sources indicated that at approximately 60 sites, a sample of about 2,000 total Pa negative patients age 12 and younger was available. The sample size justification was based on the primary EPIC-OBS aim, i.e., to define risk factors for early age at first isolation of Pa. We hypothesized that environmental tobacco smoke exposure and other modifiable exposures would have an effect on age at first isolation of Pa at least as great as that of other known risk factors evaluated in prior studies, such as aerosol use, mother’s education, and delta F508 homozygous status [15, 16]. The prevalence of smoking among parents of children with CF ranges from 15 to 40% nationally. The minimum detectable hazard ratios, varying the underlying prevalence of the risk factor from 0.2 to 0.5, and as a function of study power, ranged from 1.32 to 1.25 for a power of 0.8. These detectable hazard ratios are within the range of risk estimates reported in prior studies. All calculations were based on the log-rank statistic, assumed a two-sided .05 significance level and the occurrence of 650 events (conversions to Pa positive status during the study period) and uniform accrual of the study participants over the two year enrollment period.
Table 2 provides the projected number of patients converting to Pa-positive during the study period. The following assumptions were made to estimate duration of enrollment in EPIC-OBS at the participating sites: 1. An enrollment rate of 70% of eligible patients; 2. 15% annual rate of conversion to Pa-positive; 3. 40% of Pa positive patients would enroll in EPIC-CT; and 4. 10% of enrolled subjects would drop-out or be lost to follow-up by the end of the study. Based on these assumptions, enrollment into the EPIC-OBS was needed for the first two years of the study to achieve a sample of approximately 1,400 Pa negative patients, of whom 650 participants were expected to convert to Pa positive before completion of the observation period, from which approximately 300 were expected to enroll in the EPIC-CT. Enrollment rates exceeded expectations, and the observational study actually enrolled 1,787 rather than 1,400 participants.
Table 2.
Estimated annual number of eligible patients among 60 U.S. CF centers in 2000 based on the CFF National Patient Registry
| Age Group | No. previously Pa negative | No. with first Pa positive oropharyngeal culture | % Annual rate of first Pa positive oropharyngeal culture |
|---|---|---|---|
| 1–3 | 569 | 104 | 18.4 |
| 4–6 | 573 | 77 | 13.4 |
| 7–9 | 482 | 62 | 12.8 |
| 10–13 | 485 | 71 | 14.7 |
| Total | 2,109 | 314 | 14.9 |
THE CLINICAL TRIAL COMPONENT
Objectives of EPIC-CT
The primary objectives of the clinical trial are to investigate if an intensive quarterly anti-pseudomonal strategy (cycled therapy) reduces pulmonary exacerbations and the isolation of Pa from respiratory cultures, compared with a strategy of anti-pseudomonal administration solely based upon recovery of positive respiratory cultures collected quarterly (culture-based therapy). The specific primary aim is twofold and includes a clinical and a microbiological endpoint. The clinical endpoint is the time to first pulmonary exacerbation (Table 3) requiring intravenous antibiotics or hospital admission during the 18 month study period. The microbiological endpoint is the proportion of Pa-positive respiratory cultures among quarterly cultures obtained after randomization. Secondary independent clinical efficacy endpoints include: (1) time to pulmonary exacerbation not requiring intravenous antibiotic usage or hospitalization, (2) frequencies of pulmonary exacerbations, hospitalizations, and use of concomitant oral, inhaled, and intravenous antibiotics, (4) anthropometric measures (linear growth, weight gain), (5) pulmonary function tests including FVC, FEF25%–75%, and FEV1 (patients 4 years of age and older, able to reproducibly perform spirometry), and (6) total hospitalization days. Secondary microbiological endpoints include the microbiologic profile of Pa isolates from respiratory cultures as indicated by: (1) changes in antibiotic susceptibility patterns (minimal inhibitory concentrations of 12 antibiotics), (2) colony morphology, and (3) the presence of mucoid isolates from baseline to the end of the study. The emergence of intrinsically aminoglycoside- and ciprofloxacin-resistant non-pseudomonal organisms is also evaluated (e.g., B. cepacia, A. xylosoxidans, and S. maltophilia).
Table 3.
Definition of pulmonary exacerbation – minimal criteria for treatment with anti-pseudomonal antibiotics
| The presence of a pulmonary exacerbation is established by the following: One of the major criteria alone or two of the minor signs/symptoms and fulfillment of symptom duration. |
| Major Criteria: (One finding alone establishes the presence of a pulmonary exacerbation) |
|
| Minor Signs/Symptoms: (Two minor signs/symptoms are required with duration criteria in the absence of major criteria) |
|
| Signs/Symptom Duration: (Required with two minor signs/symptoms in absence of major criteria) |
|
Study participants are also followed with respect to the serologic response against selected Pa surface and secretory antigens and changes in inflammatory markers (white blood count with differential and C reactive protein).
The comparison of the safety profiles between the two groups include the emergence of organ toxicities detected by serial evaluation of articular/skeletal symptoms, renal function, hearing acuity, liver function, hematological profile, and adverse events.
Choice of Interventions in the EPIC-CT
In young patients in whom Pa is isolated for the first time, the goal of treatment is maintaining sustained eradication rather than controlling a chronic infection. The main considerations that were taken into account in the treatment selection process were the following: (1) a group receiving no active anti-pseudomonal antibiotics was not deemed feasible in consideration of current clinical practice, (2) 28-day therapy seemed appropriate based on previous literature concerning cycled inhaled tobramycin [5, 17], and (3) a quarterly cycle would allow sufficient time off antibiotics to limit exposure to antimicrobials.
The proposed regimens were discussed among a panel of experts from the CF community convened by the study principal investigators and the CFF to develop the optimal range of therapeutic approaches that would ensure adequate microbial coverage for all subjects and preserve clinical equipoise in the CF community.
A systematic review of the literature was prepared for the panel to assist in selection of the most effective and safest anti-pseudomonal agents for administration in this young age group. Aminoglycosides are the drug class with the largest amount of information available about sputum-antibiotic interactions in patients with CF. It was the general agreement that tobramycin for inhalation was a logical prototypical agent for trials of early intervention in CF, as its safety and efficacy in patients six years and older is the most thoroughly documented of all inhaled antibiotics. It is the only FDA approved inhaled antibiotic for the treatment of Pa infection in patients with CF. There have been several studies in children less than six years old demonstrating at least transient Pa eradication from upper and/or lower airways with inhaled tobramycin [5, 12, 18, 19].
The recommended dosage of preservative-free inhaled tobramycin for adults and children ≥6 years of age is 300 mg. This dose was demonstrated to attain sputum levels adequate to overcome potential sputum antagonism [17]. Inhaled tobramycin has also been shown to be safe and to achieve therapeutic concentrations in the lower airway of patients <6 years of age [20]. Dose adjustments for age or weight are not required.
Studies in patients chronically colonized with Pa support the use of inhaled tobramycin twice a day for cycles of 28 days receiving drug, followed by 1 month not receiving drug. In colonized patients, data suggested that the effect of a 28-day cycle persists at least 56 days in over half of the patients, indicating that a 28-day cycle followed by 56 days off therapy may be appropriate [5]. This discontinuous dosing has the theoretical advantages of minimizing emergence of resistance and of reducing drug exposure. Therefore, quarterly therapy was selected for this young population.
The consensus panel supported a combination of an inhaled antibiotic with an oral fluoroquinolone such as ciprofloxacin for initial eradication. There are several presumed advantages to this approach. First, oral fluoroquinolones are distributed systemically providing access to the sinuses and upper airway, a potential reservoir for re-infection [21]. Second, they are bactericidal agents which have a broad spectrum of antimicrobial activity with excellent in vitro activity against Pa strains from CF patients [22] and documented in vitro synergism with tobramycin [23]. Third, they are well tolerated and easily administered. Fourth, the risk of emergence of resistance can be minimized by short-term administration [24]. The pharmacokinetic profile of oral and intravenous ciprofloxacin was examined in 150 pediatric patients ages 0.3–17 years, including 28 children with CF. On average, the most frequently used dose of ciprofloxacin in children is twice daily 15–20 mg/kg/dose up to a maximum of 750 mg/dose, for a two-week course [25].
Study Design
This clinical trial is a multicenter, randomized study of young children with CF. Fifty seven clinical centers throughout the US participated and the enrollment goal of 300 participants was met in 2.5 years (Figure 2). Participating sites are listed in the Appendix. All participating centers obtained IRB approval from their respective institutions. After obtaining informed consent/assent, 306 participants have been equally randomized to one of two early anti-pseudomonal treatment algorithms (cycled or culture-based therapy groups). The duration of study participation for each subject is 18 months, during which time each participant receives up to six treatment cycles. In combination with inhaled tobramycin (Novartis Pharmaceutical Corp), patients were randomized to receive either oral ciprofloxacin or oral placebo (Bayer Healthcare AG).
Randomization was carried out by permuted blocks, and performed using a computer generated sequence. The randomization blocks did not account for clinical site since we assumed that the potential clustering effect of clinical site would be mitigated by the large number of sites (i.e., large number of clusters of small size).
At study enrollment, participants received an initial course of anti-pseudomonal antibiotic therapy consisting of 28 days of inhaled tobramycin with 14 days of oral ciprofloxacin or placebo. To promote initial eradication of Pa at the beginning of the study, participants randomized to any of the study groups could receive an additional 28-day course of inhaled tobramycin at the end of the first treatment cycle if their respiratory cultures sampled after three weeks of the first anti-pseudomonal cycle continued to be positive for Pa. They did not receive a second course of ciprofloxacin or placebo.
Following this initial antibiotic regimen, participants randomized to the cycled therapy group (n=153) received therapy administrated in quarterly cycles for five additional quarters, while participants randomized to the culture-based therapy group (n=153) received a course of therapy only when quarterly respiratory cultures, from either the central or the local laboratory, were found positive for Pa for the same study duration. The same anti-pseudomonal antibiotics, inhaled tobramycin twice-daily for 28 days and oral ciprofloxacin or placebo twice-daily for 14 days, were used in both groups. The inhaled tobramycin therapy was not blinded. The ciprofloxacin was administered as a pill to older children taking 250 mg or higher dose and placebo tablets were provided by Bayer. Younger children received ciprofloxacin suspension or taste masked placebo suspension provided by Bayer.
Overall, the study follows a factorial design and participants were allocated to the treatment regimens as displayed in Figure 2. Once patients were assigned to a treatment group, patients and treating physicians had to adhere to the assigned regimen for the 18-month study period. Except for the initial two study visits, patients were seen on a quarterly basis in conjunction with their routine clinic visits. Irrespective of randomization assignment, participants in each of these groups were allowed to receive necessary antibiotic therapy for treatment of a pulmonary exacerbation in addition to their assigned treatment regimen. An operational definition of pulmonary exacerbation was developed for the purpose of the study (Table 3). Participants presenting in a stable condition at the time of randomization were assigned to one of the study regimens immediately. Study participants presenting with new onset of a pulmonary exacerbation requiring IV antibiotics or hospital admission were treated at the discretion of the investigator and then randomized at the following quarter if they continued to meet the study eligibility criteria.
Blinding
Inhaled tobramycin was provided in an open label fashion, while oral ciprofloxacin was provided in a double-blinded fashion. To minimize potential bias due to the lack of blinding, we developed an objective and rigorous operational definition of pulmonary exacerbation (Table 3) and we verified all the hospitalization records to ensure that the reason of hospitalization was a pulmonary exacerbation. A patient diary was maintained as a corroborating mechanism to ensure the active reporting of any and all respiratory symptoms. A review committee is devoted to the quality control of this endpoint in a blinded fashion. Further, all of the secondary endpoints are objective measures. Additional measures to minimize bias included an extensive ongoing training of both physicians and research coordinators by quarterly newsletters, teleconferences and annual study meetings. Educational tools for families and primary care physicians who might also be involved in the care of the study participants have also been provided. All protocol deviations and violations have been evaluated on a case-by-case basis.
Choice of Study Population
Male and female subjects ≥1 year and ≤12 years of age with a diagnosis of CF with a documented new onset of oropharyngeal, sputum or lower respiratory tract culture positive for Pa within six months prior to study entry were eligible for participation in this study. For study purposes, first isolation of Pa was defined as the first lifetime documented respiratory culture positive for Pa or as a positive Pa culture after at least two-year absence of Pa growth (minimum of one documented negative Pa culture per year). For participants ages 12 to 15 months, at least one Pa positive respiratory culture since birth was required. Children below the age of 1 year were not considered for enrollment because oral ciprofloxacin could not be administered for safety concerns regarding potential arthropathy [26, 27]. Other eligibility criteria included: Diagnosis of CF [28, 29]; Clinically stable with no evidence of any significant respiratory symptoms at screening that would require administration of intravenous antipseudomonal antibiotics, oxygen supplementation and/or hospitalization; Signed informed consent by parent or legal guardian.
Patients were excluded from the study if they had a history of aminoglycoside hypersensitivity or adverse reaction to inhaled aminoglycoside, history of hypersensitivity or adverse event associated with ciprofloxacin or other fluoroquinolones, abnormal renal function (serum creatinine >1.5 times the upper limit of normal for age), clinically documented chronic hearing loss, serum transaminase levels at the screening visit >2 times the upper limit of normal range, administration of any investigational drug within 30 days prior to enrollment, chronic administration of loop diuretics, administration of theophylline or other methyl-xanthines within 30 days of the time of enrollment, administration of more than one course of intravenous anti-pseudomonal antibiotics (defined as at least 10 days of therapy) in the 2 years prior to baseline or more than one course (at least 28 continuous days of therapy) of inhaled anti-pseudomonal antibiotics within two years prior to study entry, and chronic macrolides use (more than 3-month duration) within 3 months of baseline; presence of a condition or abnormality that in the opinion of the Investigator would compromise the safety of the patient or the quality of the data. Intravenous or inhaled anti-pseudomonal antibiotics needed to be completed more than 30 days prior to baseline.
Study Subject Screening and Follow up Strategies
Potential study participants were screened at the participating sites whether or not they were previously enrolled in the observational study. Patients meeting the criteria for the EPIC-CT were enrolled and randomization assignment was made centrally via an interactive voice response system. Table 4 shows the content of the baseline encounter and subsequent quarterly study visits for the 18 month study duration. Patients had microbiology samples from oropharyngeal swab cultures at every quarterly visit. Anthropometric measures and nutritional assessment included length (children ≤18 months) or height, and weight. Spirometry data were collected in subjects 4 years of age or older, according to the guidelines stipulated in the 1994 American Thoracic Society Guidelines [30], with modified criteria for children [31].
Table 4.
EPIC-CT study visit schedule
| Base Visit | Visit 1 Week 3 | Visit 2 Week 10 | Visit 3 Week 22 | Visit 4 Week 34 | Visit 5 Week 46 | Visit 6 Week 58 | Visit 7 Week 70 | Visit for PE/AEL | |
|---|---|---|---|---|---|---|---|---|---|
| Informed consent | X | ||||||||
| Medical history reviewa | X | ||||||||
| Interim medical historya | X | X | X | X | X | X | X | X | |
| Concomitant medication review | X | X | X | X | X | X | X | X | X |
| Complete physical examb | X | X | X | X | X | X | X | X | |
| Height/Lengthc | X | X | X | X | X | X | X | X | |
| Weightc | X | X | X | X | X | X | X | X | |
| Vital signs | X | X | X | X | X | X | X | X | |
| Spirometryd | X | X | X | X | X | X | X | ||
| Oropharyngeal or | X | X | X | X | X | X | X | X | X |
| sputum culturee | |||||||||
| Serum creatinine | X | X | X | X | |||||
| AST, ALT, γGT | X | X | X | ||||||
| CBC with differential | X | X | X | X | |||||
| C-reactive protein | X | X | X | X | |||||
| Chest X-ray (PA & lateral)f | X | X | |||||||
| Audiologyg | X | X | X | ||||||
| Adverse event monitoring | X | X | X | X | X | X | X | X | X |
| Pa serology | X | X | X | X | |||||
| Additional plasma aliquot | X | X | X | X |
Neurological side effects based on medical history; if abnormalities are reported, patient is referred to the facility enrolling study subjects;
Complete physical exam, including standard articular/skeletal muscle exam;
Height/length and weight must be measured by same equipment throughout study period. Participants initiating study with length measurements must continue with length throughout study;
Spirometry in participants ≥4 years of age;
MICs at Week 0, Week 22, Week 46, and Week 70;
Chest x-ray must not be prior to 6 months prior to inclusion in the study;
Annual audiology at the sites is recommended. If abnormal results are found, a confirmatory audiology will be repeated four weeks later;
If needed, for pulmonary exacerbation (PE) or follow up of adverse events (AE), including musculo-skeletal, articular or neurological symptoms.
EPIC-CT participants, who were not previously enrolled in the observational study, had the option to enroll in EPIC-OBS prior to completion of the clinical trial in order to collect long-term clinical and safety follow-up data.
Due to potential for aminoglycoside ototoxicity, audiometry utilizing age appropriate testing [32, 33] with tympanometry to detect the presence of fluid in the middle ear was performed at study enrollment, at the end of the first year and at study completion. Abnormal hearing was defined as an auditory threshold ≥25 dB at any frequency (500–8000 Hz) in either ear. If abnormal results were found during the study period, confirmatory audiometry was repeated four weeks later. Children with abnormal audiologic findings had a tympanometry performed, and if both were abnormal, they were referred to the study investigator or medically-qualified sub-investigator for an ear examination. Audiometry was not collected on all participants because some sites did not have the capability to perform audiometric testing in this age group and some children were unable to comply with the testing procedure or had uninterpretable results due to pneumatic equalizing tube placement.
Blood samples were obtained for assessment of clinical status and included blood chemistry (creatinine, blood urea nitrogen, and liver function tests including hepatic transaminases [AST and ALT] and γGT, and C reactive protein), and a complete blood count (hemoglobin, hematocrit, red blood cell count, white blood cell count, and white blood cell differential count). After baseline, blood samples were obtained twice a year and at the end of the study. Blood sampling also included serum banking for Pa serology assays. For this purpose, the blood sample was centrifuged, the serum extracted, and the specimen stored at −70°C.
Chronic use of azithromycin was not permitted. All participants were encouraged to remain on the same medications throughout the entire study period, as medically feasible. Study participants maintained a diary while on the study and the information recorded in the patient diary was abstracted at every clinic visit. The diary collected data among others on treatment adherence and changes in concomitant medications.
Drug Distribution and Adherence to Treatment Regimen
Within seven days of the study visit participants were contacted to report the microbiology results and to review the treatment plan. If participants required study medication based on group assignment, the drug was prescribed at this time. Study drugs (tobramycin solution for inhalation, ciprofloxacin suspension or tablets, or a matched oral placebo) were distributed by a central pharmacy and mailed to the study participants’ domicile within 48 hours of prescription. At the first treatment cycle patients were also provided with a nebulizer (PARI-Proneb® Ultra compressors and PARI LC Plus® reusable nebulizers, PARI Respiratory Equipment Inc., Midlothian, VA). Participants were contacted within 48 hours of prescription of the study drugs to verify drug receipt, and were instructed to initiate treatment upon receipt of the study drugs. Subsequently, participants were contacted within 14 days of the clinic visit to identify occurrence of adverse events including a musculoskeletal assessment survey, and to discuss study medication adherence. All participants were again contacted within 6 weeks of the clinic visit to evaluate possible changes in health status.
Further monitoring for patient adherence to prescribed treatment regimen (cycled and culture-based treatment arms) consisted of parent recall at quarterly visits and review of parent diary. The diary collected data on daily drug consumption, any changes in patient health or new onset of symptoms, medication use, and encounters with other health-care providers. The diary was maintained to ensure, among other things, an active mechanism to capture the possible occurrence of new respiratory symptoms that could qualify as a study defined pulmonary exacerbation.
Microbiology Methods and Specimen Collection
Oropharyngeal specimens were obtained at all study visits. Specimens were collected with a cotton-tipped swab from the posterior oropharyngeal wall and tonsillar pillars. Participants were encouraged to cough prior to collection of the OP specimen. With the exception of the specimen obtained at the end of the first treatment cycle (which was processed at the site laboratory), all specimens were processed at the core microbiology laboratory at Children’s Hospital and Regional Medical Center in Seattle, WA. Oropharyngeal swab specimens were sent on wet ice by overnight express shipment to the core microbiology laboratory, and needed to be received and cultured within 2 calendar days of collection. Bacterial culture techniques were performed according to core microbiology laboratory standard procedures using a semi-quantitative bacterial culture technique [34, 35]. All organisms were identified using standard techniques, including standard biochemical testing and PCR techniques [36]. All Pa isolates were assessed for mucoid phenotype. Minimal inhibitory concentrations (MIC) for Pa of 12 antibiotics were determined using a semi-automated microbroth dilution (Sensititre, AccuMed, Westlake, OH), according to standard National Committee on Clinical Laboratory Standards methods. MICs for any Pa isolate were performed at baseline and every six months.
We conducted quality control procedures to ensure that the sample shipment and processing methods would yield accurate results. For this purpose we spiked 50 samples with inoculums of known micro-organism species (Pa, Stenotrophomonas maltophilia, Achromobacter xylosoxidans, and “no organism”) and density (103 to 105 CFU) that were sent blindly to the core microbiology laboratory. The specificity for Pa identification was excellent (100%), as there were no instances of Pa mis-identification. There was a single instance of the laboratory finding no isolates from a Pa positive sample, yielding a sensitivity for Pa isolation of 97%. To further evaluate measurement error due to sampling, manipulation, and transport, data were collected from the clinical site microbiology laboratory to evaluate concordance between results for those participants who had two simultaneous or sequential oropharyngeal cultures collected at the same study visit.
Sample Size and Power of EPIC-CT
By design, the primary study endpoint for which the study is powered is the clinical efficacy endpoint, time to first exacerbation requiring intravenous antibiotics and/or hospitalization, which will be compared between treatment groups using a hazard ratio as an estimate of the relative risk. The primary analysis will compare the more aggressive treatment group, cycled therapy, to the less aggressive treatment group, culture-based therapy to evaluate the relative reduction in pulmonary exacerbations achieved by the more aggressive therapy. To determine the statistical power and sample size for this clinical efficacy endpoint, we first obtained expected rates of exacerbation in this patient population.
To determine a reasonable relative risk size for which to power this clinical efficacy endpoint, we estimated the annual exacerbation event rate using data on over 40,000 person-years represented in the CFF National Patient Registry during 1985–2000 [37]. All patients born in 1985 or later and who were between the ages of 1 and 12 were classified into three mutually exclusive groups at each calendar year: (i) patients with no positive Pa culture since birth; (ii) patients who had the first positive culture during that year, and (iii) patients with at least one positive culture in previous years. Based on the percentages of patients experiencing at least one exacerbation related event during a calendar year, we estimated that the annual incidence rate of exacerbations was 0.17 in group (i), 0.36 in group (ii), and 0.37 in group (iii). These rates appeared to be consistent across age categories within each group. We assumed that an aggressive early antibiotic therapy could potentially reduce the risk of exacerbation by as much as 50% when comparing group (ii) to group (i). Based on the annual exacerbation incidence rates estimated in the registry, an 18-month study with 300 patients had 80% power to detect a relative risk of approximately 0.6 or lower, equivalent to detecting a 40% or greater reduction in risk of pulmonary exacerbations. The primary microbiologic analysis of the proportion of Pa positive cultures in the 18-month study period will also compare the cycled therapy arm to the culture-based therapy arm. For this objective, a sample size of 300 subjects provides 80% power to detect an odds ratio 0.6 or smaller for Pa-positive respiratory cultures between the two treatment strategies.
Factorial Aspects of Sample Size and Power
The design of this study makes it possible to perform separate evaluations of (1) the effect of two different tobramycin treatment strategies and (2) the effect of oral ciprofloxacin versus oral placebo, each based on comparing two groups of 150 study participants. The first evaluation would compare the cycled versus the culture-based group, and the second evaluation would compare the group assigned to receive oral ciprofloxacin (regardless of tobramycin regimen) with the group assigned to receive oral placebo. The secondary analysis will explore the main effect of ciprofloxacin by comparing all participants randomized to oral ciprofloxacin to all participants randomized to placebo. Since the sample size in each of these groups is the same as for the primary comparison, the study will have the same power for this analysis as the primary analysis. Assuming lack of interaction between cycled therapy and ciprofloxacin, the factorial structure of the study will allow performing two independent comparisons with adequate power, by spending only one degree of freedom on each comparison. The study would have reduced power for finding an effect if there is a negative interaction between cycled therapy and ciprofloxacin which is thought to be unlikely. However, we could also gain power if there was a positive synergistic effect between the cycled therapy and ciprofloxacin. Importantly, further exploratory analyses will be performed to investigate potential interactions between the tobramycin and ciprofloxacin regimens. These analyses will involve comparing smaller subgroups of participants in each of the four relevant subgroups (approximately 75 participants per group).
A modified intent-to-treat (ITT) population was defined as all randomized participants who received at least one dose of study drug.
Statistical analysis plan
By design, the primary study endpoint for which the study is powered is the clinical efficacy endpoint, time to first protocol-defined pulmonary exacerbation requiring intravenous antibiotics and/or hospitalization. The censored failure time will be taken as the diagnosis date of the pulmonary exacerbation, or the end of follow-up if no exacerbation meeting these criteria has occurred. End of follow-up is defined as the date of the last study visit completed by the participant. Time will be measured in number of days post Day 0, the first day study therapy was started. The null hypothesis is that there is no difference between the cycled therapy and culture-based therapy groups in terms of the time to first protocol-defined exacerbation requiring intravenous antibiotics and/or hospitalization.
A Kaplan-Meier plot will be used to graphically display estimates of the survivor function in terms of the proportion of participants who were exacerbation free over time for both the cycled and culture-based groups. Relative risk due to treatment will be estimated using a Cox proportional hazards regression model, with covariate adjustment for baseline age group (1–3, >3–6, and >6 years). The significance of the treatment group variable will be tested by the likelihood ratio test at a two-sided 0.05 level of significance. The relative risk due to treatment from this model and corresponding 95% confidence interval will be the primary measure of treatment effect. Secondary analyses will evaluate the interaction between the inhaled and oral treatment groups within the context of this model, and similar models will be used to investigate differences between treatment groups with respect to an important secondary endpoint, time to pulmonary exacerbation requiring any antibiotics (inhaled, oral, or IV) and/or hospitalization. The following covariates will be considered for adjustment in exploratory models for these endpoints: gender, enrollment season, Pa status at enrollment, and/or geographic region.
The microbiology endpoint is the proportion of Pa-positive respiratory cultures among the seven respiratory cultures taken during the 18 months of the follow-up. The respiratory culture results obtained from oropharyngeal cultures or expectorated sputum cultures obtained at weeks 3, 10, 22, 34, 46, 58, and 70 will be used in this analysis. The response will be binary (positive or negative culture). In the rare event that both an oropharyngeal culture and an expectorated sputum culture are available at a given visit and produce discordant results, a positive result will be used in the analysis for this visit. The ITT population will be used for this longitudinal analysis and missing data will not be included. A generalized estimating equation (GEE) model using a logit link will be used to model this data with an independence working correlation matrix. The significance of the treatment group variable will be tested by the Wald test using a two-sided 0.05 level of significance. The treatment associated odds ratio from this model and corresponding 95% confidence interval will be the primary measure of treatment effect. The estimated treatment effect will be interpreted as the marginal odds ratio of Pa-positive respiratory cultures during the 18 months.
Sensitivity analyses will be performed in order to evaluate the robustness of the primary microbiologic results to missing culture data, which by virtue of study design should be minimal. Specifically, we will perform three additional analyses for the primary microbiologic endpoint: (1) an analysis which imputes missing respiratory culture data using the Last Observation Carried Forward (LOCF) method, (2) an analysis which assumes all missing culture data is negative for Pa, and (3) an analysis which assumes all missing culture data is positive for Pa. A further analysis will investigate the sensitivity of the results to the augmentation of core laboratory results with available results from individual site microbiology laboratories. In some instances, participants were double-swabbed and one swab was sent to the core lab and one to the site lab. In instances for which the site lab result was positive for Pa and the core lab was negative, the final result will be treated as positive. Additional secondary endpoints including longitudinal changes in anthropometric measures and spirometry will be modeled using the GEE framework to test for differences in the 18-month change in each endpoint. Adverse events will be descriptively summarized by MEDRA system organ class and preferred term. Colony morphology will be summarized by the eradication and incidence patterns during the follow up period as compared to baseline for each of the following bacterial organisms: P. aeruginosa, A. xylosoxidan, B. cepacia, S. aureus and S. maltophilia. This analysis will primarily be descriptive, and the proportion of patients who have eradicated or newly acquired the organism by the end of the study will be summarized with corresponding 95% confidence intervals.
Adverse event monitoring
In the case of a serious adverse event (as defined by the FDA 21 CFR 312.32) the site investigator notifies the Coordinating Center within 24 hours of learning of the event.
Each adverse event is entered into the case report form and evaluated with respect to intensity, seriousness, causality (relationship to treatment) and actions taken. A summary of potential trends or unexpected events is provided to the Data Safety Monitoring Board for further review and evaluation.
Data and Safety Monitoring Board (DSMB)
Safety is monitored on an ongoing basis throughout the trial by a Data and Safety Monitoring Board (DSMB) appointed by the National Heart Lung and Blood Institute (NHLBI). The DSMB convenes at fixed times during the study in open and closed meetings at approximately 6 month intervals, including an annual in-person meeting. At each meeting, the DSMB reviews safety data, enrollment data, protocol violations, and overall study progress. Descriptions of serious adverse events are communicated to the DSMB Chair within 24 hours of their occurrence.
There are no pre-specified stopping rules for efficacy or futility. If the data from this study are unable to support the superiority of the cycled based treatment regimen to the culture based therapy in terms of both clinical and microbiologic efficacy, this would be an important result that could influence clinical care. In particular, there would be no supportive data to suggest that newly colonized CF patients be aggressively treated regardless of their culture positivity. If the two regimens are equal in terms of efficacy, the secondary endpoints regarding safety and microbiologic resistance become even more important for determining which regimen is superior and these must be evaluated for the entire duration of the study. The DSMB follows the safety (quarterly) and efficacy (semi-annually) endpoints on a regular basis and could stop the study at any time if they felt it was ethically necessary.
Study enrollment
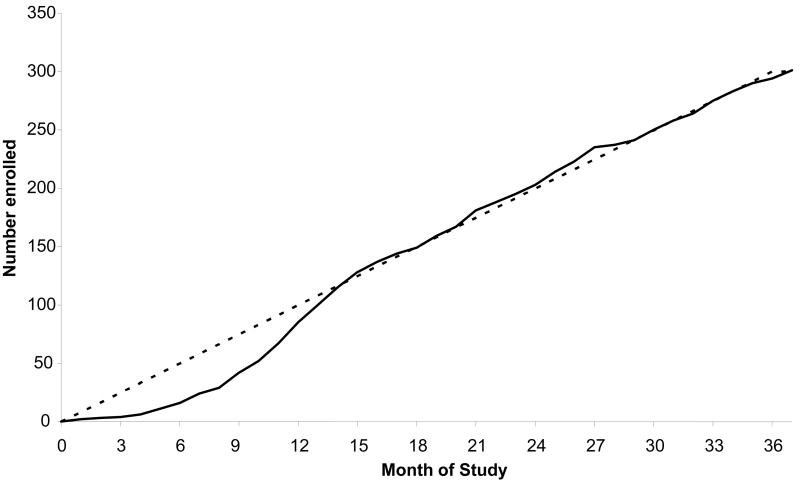
During the 2.5 years enrollment period, from December 20, 2004 to February 7, 2008, the actual accrual was excellent due to excellent participation by all sites, closely overlapping the projected figures (Figure 3). Clinical site performance is monitored on an ongoing basis and, if deficiencies are identified, these are addressed in a timely fashion.
Figure 3.
EPIC clinical trial enrollment by month. Solid line indicates actual enrollment, dotted line indicates projected enrollment.
DISCUSSION
The major objective of EPIC-CT is to help define a safe, effective and systematic approach to the treatment of first isolation of Pa from young CF patients. These young patients potentially have the most to gain from aggressive early intervention, but also the most to lose in terms of cumulative drug toxicities and acquisition of resistant pathogens. If the study demonstrates both a microbiologic effect and clinical efficacy of aggressive therapy without significant adverse events or high rates of acquisition of resistant organisms, then there would be a strong rationale for aggressive early intervention. If the two treatment approaches are not different then the use of aggressive cycled antibiotic therapy must be reassessed.
The major objectives of the EPIC-OBS study are 1) to provide a closely-monitored cohort of young children prior to potential EPIC-CT entry, 2) to provide long-term follow-up of the treatment regimens for the subset of randomized patients, and 3) to establish a large, multi-center, well-characterized cohort of young children with CF to obtain more generalizable data on the risk factors for and impact of Pa acquisition and infection. The ultimate goal is for this cohort to be closely monitored for at least 10 years with both study-specific data collection and ongoing use of the CF Patient Registry. We anticipate that this longitudinal study will be informative on both risks factors for and clinical outcomes of initial, chronic, and mucoid Pa infection, and will explore the impact of polymicrobial infections (interaction of S. aureus and Pa) in children with CF. The banked serum will provide a rich resource for the analyses of serologic markers of early and chronic Pa infection, and other potential biomarkers of early CF lung disease. The DNA bank from whole blood of EPIC-OBS participants will provide a unique resource for the evaluation of genetic modifiers of early CF disease.
This initiative represents the largest cohort and therapeutic study ever conducted in children with CF and provides a model of how to conduct a phase 3 trial in a population of young children. The project was made possible under the auspices of the Cystic Fibrosis Foundation and the National Heart, Lung and Blood Institute who joined forces to create a cooperative program, along with the support of industry who donated study drugs and supplies. The studies made use of existing resources both during the planning and implementation phase as provided by the linkage to the National CFF Registry. Similar to the design of the Women’s Health Initiative [38] that combined a cohort study and a clinical trial, the observational cohort study not only serves as a free-standing epidemiologic study of early CF lung disease, but also provides a pool of potential clinical trial participants and provides long term follow-up data on clinical trial participants. These features should provide an ideal setting for the interpretation of the clinical trial findings and their generalizability. A large number of sites were recruited to ensure timely enrollment and provide a broad geographic distribution and to ensure the inclusion of a representative sample of the population.
In the trial planning stage, a consensus process among experts in the CF field was used to ensure that the study treatment strategies were optimal in terms of administration schedule, dosing, safety, and efficacy in this young population. A site survey was also distributed to confirm that the proposed strategies were acceptable in the context of the local practices and in equipoise with current treatments in use at each participating institution. This process ensured that the proposed strategies would be well accepted by the CF community and facilitated successful enrollment throughout the study period.
In an effort to minimize the burden of a long term trial on participants and their families, we scheduled the clinical trial study visits to coincide with routine clinic visits and study drugs were shipped directly to the participant’s residence. Because of frequent microbiology sampling, the study also avoided the use of invasive sampling procedures and ensured that sampling occurred in conjunction with procedures performed (blood draws and oropharyngeal swabs) for routine care. Although Pa monitoring via oropharyngeal cultures is suboptimal, more invasive approaches were not feasible in a study of this size and in the age range of the study participants. Results of study oropharyngeal cultures were provided to the sites to allow continued clinical care.
This study did not examine patient/family reported outcomes due to the lack of availability of validated instruments for participants of ages 6 years or younger [39]. Future studies should consider collecting age-appropriate quality of life measures to evaluate the effect of long term interventions on treatment burden and health related quality of life.
The emphasis placed on engaging the clinical sites in the early phase of study development and designing the study to meet the needs of both the sites and the participant’s families likely contributed to the excellent enrollment rate that exceeded projections by 6 months. In addition, the attrition rate has remained at less than 10% easily meeting expectations.
In conclusion, this study will provide valuable clinical and microbiologic efficacy and safety data regarding the optimal use of antipseudomonal therapy in young children with CF, and the long-term follow-up of this unique cohort of children will supply important data on the effect of Pa infection on subsequent health status, and the linked serum and DNA banks will contribute valuable information on surrogate markers and genetic modifiers of early CF lung disease.
Acknowledgments
Financial support: The research for this article was supported in part by the Cystic Fibrosis Foundation grants number EPIC0K0 and OBSERV04K0, the National Heart Lung and Blood Institute (NHLBI) and National Institute for Digestive Disorders and Kidney (NIDDK) grant number U01-HL080310, and the National Center for Research Resources (NCRR) grant number ULI-RR2501401. Study drugs and devices were supplied free of charges by Novartis Pharmaceutical Corp. (inhaled tobramycin) and Bayer Healthcare AG (oral ciprofloxacin and oral placebo), compressors and nebulizers were provided by PARI Respiratory Equipment Inc.
The Sponsors had no role in designing the study, in the data collection, or in the writing of the manuscript. They have no access to the study data.
APPENDIX - EPIC INVESTIGATORS
Program Office: National Institute of Health
Susan Banks-Schlegel, PhD, National Heart, Lung and Blood Institute, Project Director; Gail Weinman, MD, National Heart, Lung and Blood Institute, Executive Secretary; Cystic Fibrosis Foundation Therapeutics: Robert J. Beall, Ph.D., Preston W. Campbell III, MD, Bruce C. Marshall, MD.
Data and Safety Monitoring Board
Lynne Quittell, MD (Chair) Columbia University; William Clarke, PhD, University of Iowa; Mary Jane Kennedy, PharmD, Kosair Charities Pediatric Clinical Research Unit, Louisville; Robert Nelson, MD, PhD, University of Pennsylvania; Kenneth N. Oliver, MD, MPH, National Institute of Allergy and Infectious Disease; Ronald Rubenstein, MD, PhD, Children’s Hospital of Philadelphia; O. Dale Williams, PhD, University of Alabama at Birmingham; Gail Weinman, MD, National Heart, Lung and Blood Institute; Susan Banks-Schlegel, PhD, National Heart, Lung and Blood Institute; Jungman Joo, PhD, National Heart, Lung and Blood Institute.
Coordinating Center
Study monitors: Amanda Bailey, Molly Andrina, Amy Feldman, Robin Hill, Tamara Potter, Deborah Chambers, Shirley Desmon; Data Management: Barbara Mathewson, MS, David Escobar, MPH; Biostatistical Unit: Umer Khan, MS, Kelli Joubran, MS; Microbiology Core Lab: Jane Burns, MD, Jenny Stapp, Anne Marie Buccatt, Griffith Adam; Medical Monitor: Christopher Goss, MD, MSc.
Clinical Centers
Albany Medical College, Albany, NY, Paul Comber, MD, PhD; All Children’s Hospital CF Center, St. Petersburg, FL, Magdalen Gondor, MD; Cardinal Glennon Children’s Hospital - St. Louis University, St. Louis, MO, Blakeslee E. Noyes, MD; Nationwide Children’s Hospital, Columbus, OH, Karen McCoy, MD; Children’s Hospital & Regional Medical Center, Seattle, WA, Ronald L. Gibson, MD, PhD (EPIC-CT), Margaret Rosenfeld, MD, MPH (EPIC-OBS); Denver Children’s Hospital, Denver, CO, Frank Accurso, MD and Jeffrey Wagener, MD; Children’s Hospital Medical Center of Akron, Akron, OH, Greg Omlor, MD; Children’s Hospital of Michigan, Detroit, MI, Debbie Toder, MD; Children’s Hospital of Pittsburgh, Pittsburgh, PA, David Orenstein, MD; Children’s Hospital of Wisconsin, Milwaukee, WI, William M. Gershan, MD; Children’s Hospital, Boston, Boston, MA, Terry Spencer, MD, Thomas Martin, MD, and David Waltz, MD; Children’s Hospitals & Clinics, Minneapolis, MN, John McNamara, MD; Children’s Medical Center of Dayton, Dayton, OH, Robert J. Fink, MD; Children’s Memorial Hospital, Chicago, IL, Adrienne Prestridge, MD; Children’s Mercy Hospital, Kansas City, MO, Philip Black, MD; Cook Children’s Medical Center, Ft. Worth, TX, Maynard Dyson, MD; Dartmouth-Hitchcock Medical Center, New Hampshire CF Center, Lebanon, NH, H. Worth Parker, MD and Dennis Stokes, MD; duPont Hospital for Children, A.I. duPont Inst. Med. Center, Wilmington, DE, Aaron Chidekel, MD; Emory University CF Center, Atlanta, GA, Michael Schecter, MD, Lawrence McKean, MD and Daniel Caplan, MD; Johns Hopkins University, Baltimore, MD, Peter Mogayzel, MD, PhD; LeBonheur Children’s Medical Center, Memphis, TN, Robert A. Schoumacher, MD; Maine Medical Center, Portland, ME, Anne Marie Cairns, DO; Massachusetts General Hospital, Boston, MA, Allen Lapey, MD, and Henry L. Dorkin, MD; Medical College of Georgia, Augusta, GA, Margaret F. Guill, MD; Miller Children’s Hospital, Memorial Miller Children’s Hosp, University of California, Irvine, Long Beach, CA, Felice Adler-Shohet, MD and Jay Lieberman, MD; Monmouth Medical Center, Long Branch, NJ, Robert L. Zanni, MD; Nemours Children’s Clinic, Jacksonville, FL, David Schaeffer, MD and Kathryn Blake, PharmD; New York Medical College, Westchester Medical Center, Valhalla, NY, Allen Dozor, MD and Nikhil Amin, MD; Northern California Kaiser CF Center, Kaiser Permenente Medical Center, Oakland, CA, Greg Shay, MD and Albin Leong, MD; Oregon Health Sciences University, Portland, OR, Michael Wall, MD; Penn State Milton S. Hershey Medical Center, Hershey, PA, Gavin Graff, MD; Rainbow Babies & Childrens Hospital, Cleveland, OH, Michael W. Konstan, MD; Rhode Island Hospital, Providence, RI, Mary Ann Passero (EPIC-OBS only); Riley Hospital, Indiana University, Indianapolis, IN, Michelle Howenstine, MD; Schneider Children’s Hospital, New Hyde Park, NY, Joan DeCelie-Germana, MD; Spectrum Health Hospitals - DeVos Children’s Butterworth Hospital, Grand Rapids, MI, Susan Millard, MD; St. Christopher’s Hospital for Children, Philadelphia, PA, Laurie Varlotta, MD; Stanford University, Packard Children’s Hosp., Palo Alto, CA, Richard Moss, MD; SUNY Upstate Medical University, Upstate Medical Center, Syracuse, NY, Ran D. Anbar, MD; Texas Children’s Hospital, Houston, TX, Peter Hiatt, MD; Tulane University School of Medicine, New Orleans, LA, Scott H. Davis, MD; University of Alabama at Birmingham, Birmingham, AL, Hector H. Gutierrez, MD; University of California, San Francisco, San Francisco, CA, Dennis Nielson, MD; University of Florida College of Medicine, Gainesville, FL, Terry Spencer, MD; University of Iowa, Iowa City, IA, Richard C. Ahrens, MD; University of Kentucky, Lexington, KY, Jamshed F. Kanga, MD; University of Mass Memorial Health Care, Worcester, MA, Brian P. O’Sullivan, MD; University of Michigan, Ann Arbor, MI, Samya Nasr, MD; University of Mississippi Medical Center, Jackson, MS, Alicia DePaula, MD and Fadel Ruiz, MD; University of Nebraska, Omaha, NE, John L. Colombo, MD; University of New Mexico, Albuquerque, NM, L. Francine Caffey, MD; University of North Carolina, Chapel Hill, Chapel Hill, NC, George Retsch-Bogart, MD; University of Rochester, Rochester, NY, Clement L. Ren, MD; University of Utah, Salt Lake City, UT, Barbara A. Chatfield, MD; University of Virginia, Charlottesville, VA, Deborah K. Froh, MD; University of Wisconsin Hospital and Clinics, Madison, WI, Michael Rock, MD; Vanderbilt University Medical Center, Nashville, TN, Elizabeth Perkett, MD and Christopher Harris, MD; Vermont Children’s Hospital at Fletcher Allen Health Care, Burlington, VT, Thomas Lahiri, MD; Washington University School of Medicine, St. Louis Children’s Hospital, St. Louis, MO, Thomas Ferkol, MD; Women & Children’s Hosp of Buffalo, Children’s Hospital of Buffalo, Buffalo, NY, Daniel Sheehan, MD; Children’s Hospital of Los Angeles, USC Medical School, Los Angeles, CA, Marlyn Woo, MD.
Footnotes
ClinicalTrial.gov numbers: NCT00676169 and NCT00097773.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cystic Fibrosis Foundation. Patient Registry 2004 Annual Data Report. Bethesda, Maryland: Cystic Fibrosis Foundation; 2005. [Google Scholar]
- 2.IACFA Newsletter. 1999;(56):8. [Google Scholar]
- 3.Kosorok MR, Wei WH, Farrell PM. The incidence of cystic fibrosis. Statistics in medicine. 1996;15:449–62. doi: 10.1002/(SICI)1097-0258(19960315)15:5<449::AID-SIM173>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 4.Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med. 2003;168:918–51. doi: 10.1164/rccm.200304-505SO. [DOI] [PubMed] [Google Scholar]
- 5.Gibson RL, Emerson J, McNamara S, Burns JL, Rosenfeld M, Yunker A, et al. Significant microbiological effect of inhaled tobramycin in young children with cystic fibrosis. Am J Respir Crit Care Med. 2003;167:841–9. doi: 10.1164/rccm.200208-855OC. [DOI] [PubMed] [Google Scholar]
- 6.Rosenfeld M, Gibson RL, McNamara S, Emerson J, Burns JL, Castile R, et al. Early pulmonary infection, inflammation, and clinical outcomes in infants with cystic fibrosis. Pediatric Pulmonology. 2001;32:356–66. doi: 10.1002/ppul.1144. [DOI] [PubMed] [Google Scholar]
- 7.Burns JL, Gibson RL, McNamara S, Yim D, Emerson J, Rosenfeld M, et al. Longitudinal assessment of Pseudomonas aeruginosa in young children with cystic fibrosis. Journal of Infectious Diseases. 2001;183:444–52. doi: 10.1086/318075. [DOI] [PubMed] [Google Scholar]
- 8.Dakin CJ, Numa AH, Wang H, Morton JR, Vertzyas CC, Henry RL. Inflammation, infection, and pulmonary function in infants and young children with cystic fibrosis. Am J Respir Crit Care Med. 2002;165:904–10. doi: 10.1164/ajrccm.165.7.2010139. [DOI] [PubMed] [Google Scholar]
- 9.Demko CA, Byard PJ, Davis PB. Gender differences in cystic fibrosis: Pseudomonas aeruginosa infection. J Clin Epidemiol. 1995;48:1041–9. doi: 10.1016/0895-4356(94)00230-n. [DOI] [PubMed] [Google Scholar]
- 10.Proesmans M, Balinska-Miskiewicz W, Dupont L, Bossuyt X, Verhaegen J, Hoiby N, et al. Evaluating the “Leeds criteria” for Pseudomonas aeruginosa infection in a cystic fibrosis centre. Eur Respir J. 2006;27:937–43. doi: 10.1183/09031936.06.00100805. [DOI] [PubMed] [Google Scholar]
- 11.Lee TW, Brownlee KG, Conway SP, Denton M, Littlewood JM. Evaluation of a new definition for chronic Pseudomonas aeruginosa infection in cystic fibrosis patients. J Cyst Fibros. 2003;2:29–34. doi: 10.1016/S1569-1993(02)00141-8. [DOI] [PubMed] [Google Scholar]
- 12.Valerius NH, Koch C, Hoiby N. Prevention of chronic Pseudomonas aeruginosa colonisation in cystic fibrosis by early treatment. Lancet. 1991;338(8769):725–6. doi: 10.1016/0140-6736(91)91446-2. [DOI] [PubMed] [Google Scholar]
- 13.Taccetti G, Campana S, Festini F, Mascherini M, Doring G. Early eradication therapy against Pseudomonas aeruginosa in cystic fibrosis patients. Eur Respir J. 2005;26:458–61. doi: 10.1183/09031936.05.00009605. [DOI] [PubMed] [Google Scholar]
- 14.Frederiksen B, Koch C, Hoiby N. Antibiotic treatment of initial colonization with Pseudomonas aeruginosa postpones chronic infection and prevents deterioration of pulmonary function in cystic fibrosis. Pediatric Pulmonology. 1997;23:330–5. doi: 10.1002/(sici)1099-0496(199705)23:5<330::aid-ppul4>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 15.Kosorok MR, Jalaluddin M, Farrell PM, Shen G, Colby CE, Laxova A, et al. Comprehensive analysis of risk factors for acquisition of Pseudomonas aeruginosa in young children with cystic fibrosis. Pediatr Pulmonol. 1998;26:81–8. doi: 10.1002/(sici)1099-0496(199808)26:2<81::aid-ppul2>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 16.Maselli JH, Sontag MK, Norris JM, MacKenzie T, Wagener JS, Accurso FJ. Risk factors for initial acquisition of Pseudomonas aeruginosa in children with cystic fibrosis identified by newborn screening. Pediatr Pulmonol. 2003;35:257–62. doi: 10.1002/ppul.10230. [DOI] [PubMed] [Google Scholar]
- 17.Ramsey BW, Pepe MS, Quan JM, Otto KL, Montgomery AB, Williams-Warren J, et al. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. Cystic Fibrosis Inhaled Tobramycin Study Group. N Engl J Med. 1999;340:23–30. doi: 10.1056/NEJM199901073400104. [DOI] [PubMed] [Google Scholar]
- 18.Ratjen F, Doring G, Nikolaizik WH. Effect of inhaled tobramycin on early Pseudomonas aeruginosa colonisation in patients with cystic fibrosis. Lancet. 2001;35:983–4. doi: 10.1016/S0140-6736(01)06124-4. [DOI] [PubMed] [Google Scholar]
- 19.Wiesemann HG, Steinkamp G, Ratjen F, Bauernfeind A, Przyklenk B, Doring G, et al. Placebo-controlled, double-blind, randomized study of aerosolized tobramycin for early treatment of Pseudomonas aeruginosa colonization in cystic fibrosis. Pediatr Pulmonol. 1998;25:88–92. doi: 10.1002/(sici)1099-0496(199802)25:2<88::aid-ppul3>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 20.Rosenfeld M, Gibson R, McNamara S, Emerson J, McCoyd KS, Shell R, et al. Serum and lower respiratory tract drug concentrations after tobramycin inhalation in young children with cystic fibrosis. J Pediatr. 2001;139:572–7. doi: 10.1067/mpd.2001.117785. [DOI] [PubMed] [Google Scholar]
- 21.Smith MJ, White LO, Bowyer H, Willis J, Hodson ME, Batten JC. Pharmacokinetics and sputum penetration of ciprofloxacin in patients with cystic fibrosis. Antimicrob Agents Chemother. 1986;30:614–6. doi: 10.1128/aac.30.4.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klinger JD, Aronoff SC. In-vitro activity of ciprofloxacin and other antibacterial agents against Pseudomonas aeruginosa and Pseudomonas cepacia from cystic fibrosis patients. J Antimicrob Chemother. 1985;15:679–84. doi: 10.1093/jac/15.6.679. [DOI] [PubMed] [Google Scholar]
- 23.Haller I. Comprehensive evaluation of ciprofloxacin-aminoglycoside combinations against Enterobacteriaceae and Pseudomonas aeruginosa strains. Antimicrob Agents Chemother. 1985;28:663–6. doi: 10.1128/aac.28.5.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ball P. Emergent resistance to ciprofloxacin amongst Pseudomonas aeruginosa and Staphylococcus aureus: clinical significance and therapeutic approaches. J Antimicrob Chemother. 1990;26 Suppl F:165–79. doi: 10.1093/jac/26.suppl_f.165. [DOI] [PubMed] [Google Scholar]
- 25.Bosso JA. Use of ciprofloxacin in cystic fibrosis patients. Am J Med. 1989;87:123S–7S. doi: 10.1016/0002-9343(89)90040-5. [DOI] [PubMed] [Google Scholar]
- 26.Burkhardt JE, Hill MA, Carlton WW, Kesterson JW. Histologic and histochemical changes in articular cartilages of immature beagle dogs dosed with difloxacin, a fluoroquinolone. Vet Pathol. 1990;27:162–70. doi: 10.1177/030098589002700303. [DOI] [PubMed] [Google Scholar]
- 27.Chysky V, Kapila K, Hullmann R, Arcieri G, Schacht P, Echols R. Safety of ciprofloxacin in children: worldwide clinical experience based on compassionate use. Emphasis on joint evaluation Infection. 1991;19:289–96. doi: 10.1007/BF01644970. [DOI] [PubMed] [Google Scholar]
- 28.Rosenstein BJ, Cutting GR. The diagnosis of cystic fibrosis: a consensus statement. Cystic Fibrosis Foundation Consensus Panel. J Pediatr. 1998;132:589–95. doi: 10.1016/s0022-3476(98)70344-0. [DOI] [PubMed] [Google Scholar]
- 29.De Boeck K, Wilschanski M, Castellani C, Taylor C, Cuppens H, Dodge J, et al. Cystic fibrosis: terminology and diagnostic algorithms. Thorax. 2006;61:627–35. doi: 10.1136/thx.2005.043539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152:1107–36. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 31.Beydon N, Davis SD, Lombardi E, Allen JL, Arets HG, Aurora P, et al. An official American Thoracic Society/European Respiratory Society statement: pulmonary function testing in preschool children. Am J Respir Crit Care Med. 2007;175:1304–45. doi: 10.1164/rccm.200605-642ST. [DOI] [PubMed] [Google Scholar]
- 32.Thompson G, Wilson WR. Clinical application of visual reinforcement audiometry. Sem Hearing. 1984;5:85–99. doi: 10.1044/jshd.4401.80. [DOI] [PubMed] [Google Scholar]
- 33.Gravel GS. Behavioral assessment of auditory function. Seminars in Hearing. 1989;10:216–28. [Google Scholar]
- 34.Burns JL, Emerson J, Stapp JR, Yim DL, Krzewinski J, Louden L, et al. Microbiology of sputum from patients at cystic fibrosis centers in the United States. Clin Infect Dis. 1998;27:158–63. doi: 10.1086/514631. [DOI] [PubMed] [Google Scholar]
- 35.Burns JL, Van Dalfsen JM, Shawar RM, Otto KL, Garber RL, Quan JM, et al. Effect of chronic intermittent administration of inhaled tobramycin on respiratory microbial flora in patients with cystic fibrosis. The Journal of infectious diseases. 1999;179:1190–6. doi: 10.1086/314727. [DOI] [PubMed] [Google Scholar]
- 36.Qin X, Emerson J, Stapp J, Stapp L, Abe P, Burns JL. Use of real-time PCR with multiple targets to identify Pseudomonas aeruginosa and other nonfermenting gram-negative bacilli from patients with cystic fibrosis. Journal of clinical microbiology. 2003;41:4312–7. doi: 10.1128/JCM.41.9.4312-4317.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cystic Fibrosis Foundation. Patient Registry 2002 Annual Data Report. Bethesda, Maryland: Cystic Fibrosis Foundation; 2003. [Google Scholar]
- 38.Design of the Women’s Health Initiative clinical trial and observational study. The Women’s Health Initiative Study Group. Controlled clinical trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 39.Modi AC, Quittner AL. Validation of a disease-specific measure of health-related quality of life for children with cystic fibrosis. Journal of pediatric psychology. 2003;28(8):535–45. doi: 10.1093/jpepsy/jsg044. [DOI] [PubMed] [Google Scholar]