Abstract
Background and Purpose
Alterations of neuro-angiogenic response play important roles in the development of aging-related neurodisorders, and affect gene-based therapies. We tested brain response to vascular endothelial growth factor (VEGF) in aged mice.
Methods
AAV-VEGF, an adeno-associated viral vector expressing VEGF, was injected into the brain of 3-, 12- and, 24-month old mice. AAV-LacZ-injected mice were used as control (n=6). Before euthanization at 6 weeks after vector injection, the mice were intraperitoneally injected with BrdU for 3 consecutive days. The vascular density and the number of neuroprogenitors were analyzed.
Results
Injection of AAV-VEGF increased the vascular density in the brain of 3-, 12-and 24-month old mice by 22±7% (AAV-VEGF: 320±15 per 10X field vs. AAV-LacZ: 263±8, p<0.05), 20%±8 (AAV-VEGF: 300±9 vs. AAV-LacZ: 250±11, p<0.05), and 7±16% (AAV-VEGF: 257±27 vs. AAV-LacZ: 236±13, p=0.283), respectively. There were more VEGF receptor positive neuroprogenitors in the subventricular zone of AAV-VEGF injected 3- (22±2) and 12-month old mice (21±5) than that of 24-month old mice (7±1). More BrdU positive endothelial cells and neuroprogenitors were detected around the injection site and SVZ of 3- (13±4) and 12-month old mice (14±5) than that of 24-month old mice (1±1). VEGF receptor 2 was upregulated in AAV-VEGF-injected brains of 3- and 12-month old mice, but not in 24-month old mice.
Conclusion
The angiogenic and neurogenic response to VEGF stimulation is attenuated in the aged mouse brain, which may be due to reduced VEGF receptor activity.
Keywords: aging, brain, angiogenesis, neurogenesis, VEGF
Introduction
Vascular endothelial growth factor (VEGF) is a promising candidate gene for the treatment of ischemic stroke because it mediates both angiogenesis and neurogenesis.1 VEGF exerts its mitogenic, neurogenic and angiogenic effects mainly through VEGF receptor-2 (VEGFR-2).2, 3
VEGF expression is increased during cerebral ischemia in both patients and experimental animals.4, 5 However, endogenous VEGF does not appear to be sufficient to completely protect the brain from ischemic injury. Interestingly, many in vivo preclinical studies have shown that exogenous administration of VEGF-induced angiogenic changes in the ischemic brain results in a reduction of ischemic injury and better functional outcomes.6, 7 We have shown that adeno-associated viral vector (AAV)-mediated VEGF gene transfer induces angiogenesis in the mouse brain and reduces ischemic brain injury caused by transient middle cerebral artery occlusion.8–10 Thus, overexpression of VEGF is a promising strategy for the treatment of ischemic brain injury.
There is accumulating evidence suggesting that aging affects both angiogenesis and vasculogenesis.11–13 Angiogenesis responsible for collateral development in limb ischemia is impaired with aging.14 Aging also affects vascularization during fracture repair.15 Reduced angiogenic activity in the elderly is mostly associated with a reduction in VEGF expression in response to injury, attributed to varying mechanisms such as low promoter activity14 or reduced upstream signaling, e.g. HIF1-α.15 In vitro studies have suggested that aged endothelial cells show impaired proliferation and migration in response to other important angiogenic-related signals, such as platelet-derived growth factor and fibroblast growth factor.16, 17
Stroke occurs mostly in the elderly population. It is important to know if brain responsiveness to VEGF is affected by advancing age. In this study, we chose AAV serotype 1 to mediate VEGF gene transfer to compare the angiogenic and neurogenic responses of the mouse brain in three age groups because AAV1 has been successfully used to deliver therapeutic genes into the brain.8 We used 3-, 12- and 24-month old mice to represent young, middle-aged and aged individuals. We found that angiogenic and neurogenic responses to VEGF stimulation were attenuated in the aged mouse brain.
Methods and Materials
Animals
All animal procedures were carried out according to a protocol approved by the Institutional Animal Care and Use Committee of the University of California, San Francisco. C57BL/6J mice weighing 30–35 g, aged 3, 12, and 24 months, were used.
AAV Vector Construction and Production
The AAV-VEGF and AAV-LacZ were constructed as previously described.18 AAV vectors were produced using the 3 plasmid co-transfection system,19 and purified by CsCl2 centrifugation. Viral titers were determined by dot blot analysis of DNA content and expressed as genome copies (gcs).
Injection of AAV Vectors into the Mouse Brain
Two μl of viral suspensions containing 2×109 gcs of viruses were injected into the caudate putamen as described previously.8
Histological Analysis
BrdU (Sigma), a thymidine analogue, was injected intraperitoneally (i.p.) twice daily, 100mg/kg, for 3 consecutive days before the animals were euthanized 6 weeks after vector injection. Mouse brains were perfusion-fixed with 4% paraformaldehyde, embedded in paraffin and sectioned (6μm in thickness). Capillary density was analyzed as described previously.8
For immunostaining of BrdU positive cells, sections were treated with 2 M HCl at 37°C for 30 min, and rinsed in 0.1 M boric acid (pH 8.5) at room temperature for 10 min before being treated with the following procedures. Sections were incubated in 0.3% H2O2 in methanol for 30 min to quench the endogenous peroxidase activity, and heated up to 95°C for 15 min in 10mM sodium citrate buffer, pH 6.0, for antigen retrieval. The immunohistochemical staining was done using the protocol of standard Elite Vectastain ABC Kit (Vector Laboratories) using primary antibodies listed in Table 1, including Doublecortin (DCX), VEGFR2, CD31, CD68, and BrdU.
Table 1.
List of primary of antibodies
| Antigen | Company | Host | Reactivity | Dilution |
|---|---|---|---|---|
| Lectin | Vector Lab | 1:200 | ||
| LacZ | AbCam | Rabbit | 1:500 | |
| BrdU | sigma | mouse | Mouse | 1:500 |
| CD68 | Serotec | Rat | Mouse | 1:75 |
| Doublecortin | Cell signaling | Rabbit | Mouse | 1:200 |
| VEGF | Santa Cruz | Rabbit | Mouse | 1:50 |
| VEGFR-2 | Cell signaling | Rabbit | Mouse | 1:250 |
BrdU-, VEGFR-2-, and DCX-immunopositive cells were counted blindly in the needle track and sub-ventricular zone (SVZ) under a microscope with 40X objective lens. The data were expressed as mean ± SD per section, and 3 sections were counted for each animal.
Western Blot Analysis
Proteins were isolated from the needle injection site, separated in 14% of Tris-Glycine gel, and electrotransferred onto a nitrocellulose membrane (Bio-Rad Laboratories). After blocking in 5% milk, the membrane was incubated overnight with rabbit anti-mouse VEGF antibody (1:200, Santa Cruz Biotechnology) at 4°C. After incubating with horseradish peroxidase conjugated anti-rabbit secondary antibody (Amersham, Buckinghamshire, UK) diluted at 1:10,000, and reacting with FEMO detection reagent (Pierce Biotechnology), the membrane was exposed to Kodak film.
Statistical Analysis
All data are presented as mean ± SD and were compared using one-way ANOVA followed by post hoc t test least significant differences (LSD). A P value of less than 5% was considered statistically significant.
Results
AAV-mediated Gene Transfer not Affected by Animal Age
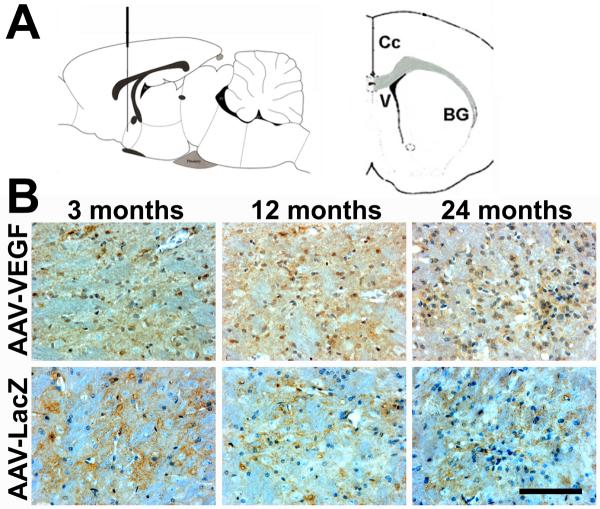
To examine the gene transduction efficiency mediated by AAV-LacZ or AAV-VEGF, LacZ and VEGF gene expressions were analyzed using immunostaining. We detected LacZ and VEGF expression around the injection sites in all the animals. Similar transgene expression among all the age groups suggests that age, per se, does not affect gene transduction efficiency (Figure 1).
Figure 1. VEGF and LacZ expression detected at the injection sites.
A. Drawings illustrate the position of the vector injection site, sagittal section (left) and coronal section (right). Cc= Corpus callosum; V= ventricle; and BG= basal ganglia. B. Photomicrographs show VEGF and LacZ positive cells (brown, indicated by arrows) in AAV-VEGF (VEGF) or AAV-LacZ (LacZ)-injected brains. The nuclei were counterstained with hematoxylin. Scale bars=100μm. 3M, 12M, and 24M represent 3-, 12-, and 24-month old mice, respectively.
Attenuated Angiogenic Response to VEGF Overexpression in the Brain of Aged Mice
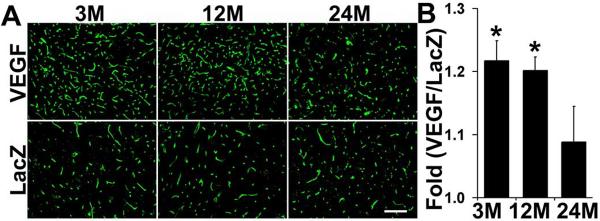
To analyze if AAV-VEGF induces a similar degree of angiogenesis in the brain of mice in the different age groups, capillaries were quantified on lectin-stained sections. As shown in Fig. 2, AAV-VEGF induced angiogenesis in the brain of all age groups. However, the induction level was different among the groups. Injection of AAV-VEGF increased capillary density (count per 10X field) by 22±7% (AAV-VEGF: 320 ± 15 vs. AAV-LacZ: 263 ± 8, p<0.05) and 20±8 % (AAV-VEGF 300 ± 9 vs. AAV-LacZ 250 ± 11, p<0.05) in the brain of 3- and 12-month old mice, respectively. There was a trend toward a minimal increase (7±16 %) in capillary density in the brain of AAV-VEGF-treated 24-month old mice (AAV-VEGF: 257 ± 27 vs. AAV-LacZ: 236±13, p=0.283).
Figure 2. Vascular density in the AAV-VEGF-injected brain.
A. Photomicrographs show lectin-stained microvessels in the brain of 3-, 12- and 24-month old mice 6 weeks after AAV vector injection. B. Bar graph shows the fold increase of capillaries in the AAV-VEGF-injected brain vs. AAV-LacZ-injected brain. There were more capillaries in the brain of 3- and 12-month old mice than 24-month old mice (Asterisk, p<0.05). VEGF: number of capillaries in the AAV-VEGF-injected brain; LacZ: number of capillaries in the AAV-LacZ-injected brain. Scale bar = 100μm.
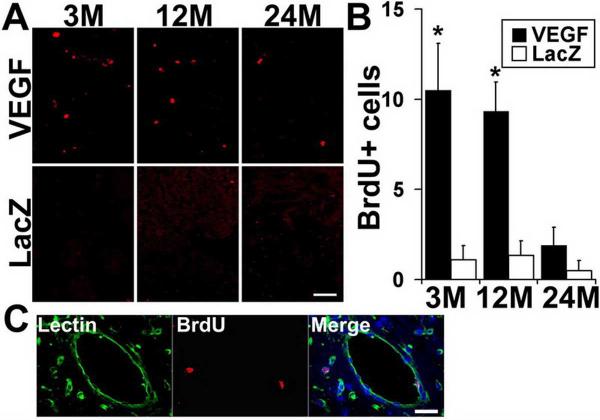
In addition, BrdU positive cells were detected in the vascular walls around AAVVEGF injection sites of 3- and 12-month old mice. In contrast, however, minimal BrdU positive cells were observed in the brain of 24-month old mice (Fig 3). Few BrdU positive cells were detected in the brain of AAV-LacZ vector injected mice regardless of age. Double-labeling with anti-BrdU antibody and lectin showed that the BrdU positive cells at the AAV-VEGF injection sites were endothelial cells, indicating that active angiogenesis continued 6 weeks after AAV-VEGF injection (Fig 3).
Figure 3. BrdU positive endothelial cells in the vector-injected brain.
A. More BrdU positive cells (red) were detected around injection sites of 3- and 12-month old mice than in 24-month old mice after AAV-VEGF injection. Few BrdU-positive cells were detected in AAV-LacZ-injected brains. B. Bar graph shows quantification of BrdU positive cells (per section; asterisk, p<0.05). C. Double labeling shows that BrdU positive cells (red) were colocalized with Lectin-positive vessels (green). Scale bars for A = 50 μm, for C = 25 μm.
Attenuated Neurogenic Response to VEGF Stimulation in the Brain of 24-Month Old Mice
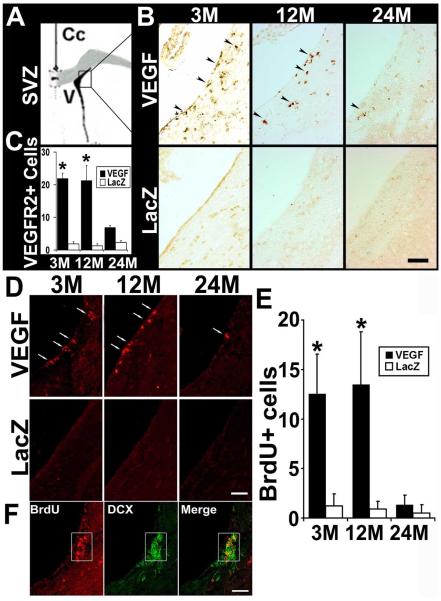
To investigate whether injection of AAV-VEGF to the basal ganglia stimulates neuroprogenitor cell (NPC) proliferation in the SVZ, we used VEGFR-2 and doublecortin (DCX) as markers and quantified the number of NPCs in this region 6 weeks after the vector injection. We detected an increased number of VEGFR-2 positive NPCs in the SVZ of AAV-VEGF-injected mice compared to AAV-LacZ-injected mice (3-month old mice: AAV-VEGF - 22.0 ± 1.5 per 40X fields vs. AAV-LacZ - 1.9 ± 0.7; 12-month old mice: AAV-VEGF - 21.3 ± 4.6 vs. AAV-LacZ - 1.3 ± 0.6; 24-month-old mice: AAV-VEGF - 7.0 ± 0.6 vs. AAV-LacZ - 2.3 ± 0.6, p<0.05) (Fig 4). Compared to 3- and 12-month old mice, 24-month old mice had fewer VEGFR-2 positive NPCs in the SVZ after receiving AAV-VEGF injection (p<0.05).
Figure 4. VEGFR-2 positive NPCs in SVZ.
A. Schematic drawing shows the SVZ region of the mouse brain. B. Photomicrographs show VEGFR-2 positive cells in the SVZ regions. The AAV-VEGF-injected brain had more VEGFR-2 positive cells in the SVZ than in the AAV-LacZ-injected brain. There were more VEGFR-2-positive cells (arrow) in the SVZ of 3- and 12-month old mice than in 24-month old mice after receiving AAV-VEGF injection (per section; asterisk, p<0.05). C. Bar graph shows the numbers of VEGFR-2 positive cells per 40X objective field in the different groups. D. Photomicrographs show BrdU positive cells (red) in the SVZ. E. Bar graph shows the quantification of BrdU positive cells in the SVZ (per section, asterisk, p<0.05). VEGF: AAV-VEGF injected brains; LacZ: AAV-LacZ injected brain. F. Double-label with BrdU (red) and DCX (green) shows most of the BrdU positive cells in SVZ were DCX positive NPCs. Scale bars = 50μm.
Injection of AAV-VEGF increased BrdU positive cells in the SVZ of 3- (AAV-VEGF: 12.6 ± 4.0 per 40X fields vs. AAV-LacZ: 1.2 ± 1.2) and 12-month old mice (AAV-VEGF: 13.5 ± 5.3 vs AAV-LacZ: 0.9 ± 0.8, p<0.05). Overexpression of VEGF did not increase BrdU positive cells significantly in the SVZ of 24-month old mice (AAV-VEGF: 1.3 ± 1.0, vs AAV-LacZ: 0.5 ± 0.8, p>0.05). Double-labeling with antibodies specific to DCX and BrdU showed that many DCX positive NPCs were BrdU positive (Fig 4), suggesting that VEGF overexpression stimulated NPC proliferation. Few DCX and BrdU double-positive cells were detected in the SVZ of 24-month mice compared to that of 3-and 12-month old mice after AAV-VEGF injection (Fig 4).
VEGF Overexpression Did Not Induce VEGFR-2 Expression in 24-Month Old Mice
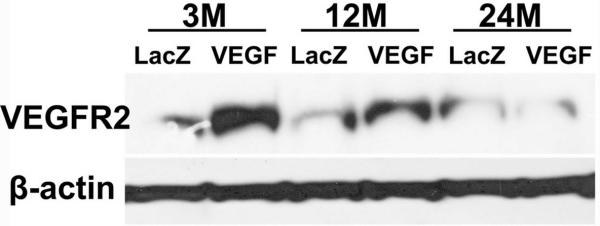
To study whether attenuated angiogenesis and neurogenesis in the aged brain in response to VEGF stimulation are due to the decrease of VEGF receptor functions, we performed Western blot analysis. We found that VEGFR-2 expression was increased 6 weeks after AAV-VEGF injection in the brain of 3- and 12-month old mice. However, this increase was attenuated in the brain of 24-month old mice (Fig 5). The expression of VEGFR-1 was the same in the 3 groups with or without AAV-VEGF injection (data not shown). Thus, overexpression of VEGF in the brain of old mice did not upregulate VEGFR-2 expression.
Figure 5. VEGFR-2 expression in the vector-injected brain.
Representative Western blot image shows VEGFR-2 expression was not increased in the brain of 24-month old mice treated with AAV-VEGF. LacZ: AAV-LacZ-injected brain: VEGF: AAV-VEGF-injected brain. Expression of β-actin was used as internal control.
We further analyzed the number of VEGFR-2 positive endothelial cells after VEGF stimulation. We found more VEGFR-2 positive endothelial cell in the AAV-VEGF-injected brain of 3- and 12 month-old mice, although the increase did not reach statistical significance with the sample size we used (3-month old mice: AAV-VEGF – 40 ± 8 per 40X field vs AAV-LacZ – 35 ± 6; 12-month old mice: AAV-VEGF – 45 ± 10 vs AAV-LacZ – 35 ± 8). Injection of AAV-VEGF vector did not increase the VEGFR-2 positive endothelial cells in the brain of 24-month old mice (AAV-VEGF – 23 ± 4 vs AAV-LacZ – 30 ± 3) (See Supplemental Figure I, available online at…). The brain of 3- and 12-month old mice with or without AAV-VEGF injection had significantly more VEGFR-2 positive endothelial cells than the brain of 24-month old mice (3 vs 24 month old mice: p=0.03; 12 vs 24 month-old mice: p=0.01). Thus, the endothelial cells in the 24-month old mouse brain expressed less VEGFR-2 before and after VEGF stimulation.
Discussion
In the present study, we demonstrated that: 1) AAV1-mediated overexpression of VEGF in the normal adult mouse brain induced angiogenesis and neurogenesis; 2) the angiogenic and neurogenic responses to VEGF stimulation in the brain of 24-month old mice were attenuated; and 3) overexpression of VEGF upregulated VEGFR-2 expression in the brain of 3- and 12-month old mice, but not in 24-month old mice. Although advanced age does not preclude augmentation of collateral vessel development in the ischemic limb in response to exogenous angiogenic cytokines14, we found that AAV-mediated VEGF gene transfer resulted in less angiogenesis and neurogenesis in the normal brain of aged mice. Our results also suggest that changes in VEGF receptor expression or function may contribute to the reduced responses of the aged brain to VEGF stimulation.
Angiogenesis and neurogenesis are prominent features of neurological disease, either as pathophysiological factors or as responses to injury.1 One common thread that connects angiogenesis, neurogenesis and pathogenesis is VEGF, which has been identified on the basis of its vascular effects, but recognized as an important signaling molecule in neural tissue as well. Recent insights into the role of VEGF in a variety of neurological disorders, including stroke and motor neuron disease, suggest that VEGF or its downstream effectors may be promising therapeutic targets for these diseases.
Recent studies indicate that VEGF can stimulate neurogenesis. VEGF increases the incorporation of BrdU into cells expressing immature neuronal markers both in mouse cortical cultures in vitro and in the SVZ and subgranular zone of the adult rat brain in vivo.20, 21 VEGFR-2 is implicated in each of these cases. Since neural stem cells from the hippocampus of adult rats express VEGF and its receptors,22 transient forebrain ischemia-induced cell proliferation and differentiation in the dentate gyrus may be mediated by enhanced VEGF receptor intracellular signaling pathways.23 All of these facts suggest that VEGF receptors in the early period of reperfusion may contribute to neurogenesis rather than angiogenesis in the hippocampal dentate gyrus.24
We found that expression of VEGFR-2 was upregulated after injection of AAV-VEGF into the mouse brain. There was no increase of VEGFR-2 expression in the brain of 24-month old mice after AAV-VEGF injection, suggesting that functional VEGFR-2 signaling may be reduced in the aged mouse brain. A recent study indicates that with advancing age, impairment of VEGFR-2/PI3-kinase signaling contributes to the reduction of flow-induced vasodilation in coronary arterioles.25 By ligating femoral artery unilaterally in mice, Qian et al26 found that ischemia led to an increase in the expression of VEGFR-2 in younger endothelial nitric oxide synthase (eNOS)-deficient mice; however, this increase in VEGFR-2 expression was absent in the older eNOS knock-out animals, which was followed by the severe disease phenotype. Taken together, reduction of VEGF expression or function may contribute to the reduced angiogenic and neurogenic responses of the older brain to VEGF stimulation.
This study has several limitations. We only tested one virus dose because our previous study showed that this dose effectively induced angiogenesis in the young mouse brain.8 It is possible that the aged brain requires a higher viral load to be effective. VEGFR-2 expression in the brain of 24-month old mice was not upregulated by our chosen dose of AAV-VEGF. The phosphorylation of VEGFR-2 and the activities of VEGFR-2 downstream genes were not studied. The roles of other factors, such as Caveolin-1,27 that influence VEGFR-2 functions were not analyzed. We have examined the effect of aging on the VEGF response in the normal mouse brain. The responses and regulation of receptors are likely different and one cannot simply infer that similar changes would take place during ischemia. Future studies will need to address these issues.
In summary, we have shown in this study that angiogenic and neurogenic responses to VEGF stimulation are reduced in the brain of aged mice. Our data also suggest that reduced VEGFR-2 quantity or function may be a potential mechanism for the diminished response of the aged brain to VEGF stimulation.
Supplementary Material
Acknowledgment
The authors thank Professor Feng Ling, Department of Neurosurgery, Xuanwu Hospital, Capital University of Medical Sciences, Beijing, China, for providing partial fellowship support to Peng Gao.
Sources of Funding These studies were supported by grants from the National Institutes of Health (R01 NS27713 to W.L.Y., and P01 NS44155 to W.L.Y. and H.S.), and from the American Heart Association (AHA SDG 0535018N to H.S.).
Footnotes
Disclosures None.
References
- 1.Greenberg DA, Jin K. From angiogenesis to neuropathology. Nature. 2005;438:954–959. doi: 10.1038/nature04481. [DOI] [PubMed] [Google Scholar]
- 2.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 3.Zachary I, Gliki G. Signaling transduction mechanisms mediating biological actions of the vascular endothelial growth factor family. Cardiovasc Res. 2001;49:568–581. doi: 10.1016/s0008-6363(00)00268-6. [DOI] [PubMed] [Google Scholar]
- 4.Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke. 1994;25:1794–1798. doi: 10.1161/01.str.25.9.1794. [DOI] [PubMed] [Google Scholar]
- 5.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 6.Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, Greenberg DA. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest. 2003;111:1843–1851. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang R, Wang L, Zhang L, Chen J, Zhu Z, Zhang Z, Chopp M. Nitric oxide enhances angiogenesis via the synthesis of vascular endothelial growth factor and cGMP after stroke in the rat. Circ Res. 2003;92:308–313. doi: 10.1161/01.res.0000056757.93432.8c. [DOI] [PubMed] [Google Scholar]
- 8.Shen F, Su H, Liu W, Kan YW, Young WL, Yang GY. Recombinant adeno-associated viral vector encoding human VEGF165 induces neomicrovessel formation in the adult mouse brain. Front Biosci. 2006;11:3190–3198. doi: 10.2741/2042. [DOI] [PubMed] [Google Scholar]
- 9.Shen F, Su H, Fan Y, Chen Y, Zhu Y, Liu W, Young WL, Yang GY. Adeno-associated viral vector-mediated hypoxia-inducible vascular endothelial growth factor gene expression attenuates ischemic brain injury after focal cerebral ischemia in mice. Stroke. 2006;37:2601–2606. doi: 10.1161/01.STR.0000240407.14765.e8. [DOI] [PubMed] [Google Scholar]
- 10.Shen F, Fan Y, Su H, Zhu Y, Chen Y, Liu W, Young WL, Yang GY. Adeno-associated viral vector-mediated hypoxia-regulated VEGF factor gene transfer promotes angiogenesis following focal cerebral ischemia in mice. Gene Ther. 2008;15:30–39. doi: 10.1038/sj.gt.3303048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Behonick DJ, Xing Z, Lieu S, Buckley JM, Lotz JC, Marcucio RS, Werb Z, Miclau T, Colnot C. Role of matrix metalloproteinase 13 in both endochondral and intramembranous ossification during skeletal regeneration. PLoS ONE. 2007;2:e1150. doi: 10.1371/journal.pone.0001150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kreisle RA, Stebler BA, Ershler WB. Effect of host age on tumor-associated angiogenesis in mice. J Natl Cancer Inst. 1990;82:44–47. doi: 10.1093/jnci/82.1.44. [DOI] [PubMed] [Google Scholar]
- 13.Reed MJ, Karres N, Eyman D, Cruz A, Brekken RA, Plymate S. The effects of aging on tumor growth and angiogenesis are tumor-cell dependent. Int J Cancer. 2007;120:753–760. doi: 10.1002/ijc.22351. [DOI] [PubMed] [Google Scholar]
- 14.Rivard A, Fabre JE, Silver M, Chen D, Murohara T, Kearney M, Magner M, Asahara T, Isner JM. Age-dependent impairment of angiogenesis. Circulation. 1999;99:111–120. doi: 10.1161/01.cir.99.1.111. [DOI] [PubMed] [Google Scholar]
- 15.Lu C, Hansen E, Sapozhnikova A, Hu D, Miclau T, Marcucio RS. Effect of age on vascularization during fracture repair. J Orthop Res. 2008;26:1384–1389. doi: 10.1002/jor.20667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phillips GD, Stone AM. PDGF-BB induced chemotaxis is impaired in aged capillary endothelial cells. Mech Ageing Dev. 1994;73:189–196. doi: 10.1016/0047-6374(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 17.Garfinkel S, Hu X, Prudovsky IA, McMahon GA, Kapnik EM, McDowell SD, Maciag T. FGF-1-dependent proliferative and migratory responses are impaired in senescent human umbilical vein endothelial cells and correlate with the inability to signal tyrosine phosphorylation of fibroblast growth factor receptor-1 substrates. J Cell Biol. 1996;134:783–791. doi: 10.1083/jcb.134.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Su H, Lu R, Kan YW. Adeno-associated viral vector-mediated vascular endothelial growth factor gene transfer induces neovascular formation in ischemic heart. Proc Natl Acad Sci U S A. 2000;97:13801–13806. doi: 10.1073/pnas.250488097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsushita T, Elliger S, Elliger C, Podsakoff G, Villarreal L, Kurtzman GJ, Iwaki Y, Colosi P. Adeno-associated virus vectors can be efficiently produced without helper virus. Gene Ther. 1998;5:938–945. doi: 10.1038/sj.gt.3300680. [DOI] [PubMed] [Google Scholar]
- 20.Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2002;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schanzer A, Wachs FP, Wilhelm D, Acker T, Cooper-Kuhn C, Beck H, Winkler J, Aigner L, Plate KH, Kuhn HG. Direct stimulation of adult neural stem cells in vitro and neurogenesis in vivo by vascular endothelial growth factor. Brain Pathol. 2004;14:237–248. doi: 10.1111/j.1750-3639.2004.tb00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maurer MH, Tripps WK, Feldmann RE, Jr., Kuschinsky W. Expression of vascular endothelial growth factor and its receptors in rat neural stem cells. Neurosci Lett. 2003;344:165–168. doi: 10.1016/s0304-3940(03)00407-5. [DOI] [PubMed] [Google Scholar]
- 23.Zhu Y, Sun Y, Xie L, Jin K, Sheibani N, Greenberg DA. Hypoxic induction of endoglin via mitogen-activated protein kinases in mouse brain microvascular endothelial cells. Stroke. 2003;34:2483–2488. doi: 10.1161/01.STR.0000088644.60368.ED. [DOI] [PubMed] [Google Scholar]
- 24.Kawai T, Takagi N, Mochizuki N, Besshoh S, Sakanishi K, Nakahara M, Takeo S. Inhibitor of vascular endothelial growth factor receptor tyrosine kinase attenuates cellular proliferation and differentiation to mature neurons in the hippocampal dentate gyrus after transient forebrain ischemia in the adult rat. Neuroscience. 2006;141:1209–1216. doi: 10.1016/j.neuroscience.2006.04.064. [DOI] [PubMed] [Google Scholar]
- 25.Leblanc AJ, Shipley RD, Kang LS, Muller-Delp JM. Aging impairs Flk-1 signaling and NO-mediated vasodilation in coronary arterioles. Am J Physiol Heart Circ Physiol. 2008;295:H2280–2288. doi: 10.1152/ajpheart.00541.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qian HS, de Resende MM, Beausejour C, Huw LY, Liu P, Rubanyi GM, Kauser K. Age-dependent acceleration of ischemic injury in endothelial nitric oxide synthase-deficient mice: potential role of impaired VEGF receptor 2 expression. J Cardiovasc Pharmacol. 2006;47:587–593. doi: 10.1097/01.fjc.0000211736.55583.5c. [DOI] [PubMed] [Google Scholar]
- 27.Fang K, Fu W, Beardsley AR, Sun X, Lisanti MP, Liu J. Overexpression of caveolin-1 inhibits endothelial cell proliferation by arresting the cell cycle at G0/G1 phase. Cell Cycle. 2007;6:199–204. doi: 10.4161/cc.6.2.3740. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.