Abstract
Recent data suggest that opioids can activate immune-like cells of the central nervous system (glia). This opioid-induced glial activation is associated with decreased analgesia, owing to the release of proinflammatory mediators. Here we examine in rats whether the putative microglial inhibitor, minocycline, may affect morphine-induced respiratory depression and/or morphine-induced reward (conditioned place preference). Systemic co-administration of minocycline significantly attenuated morphine-induced reductions in tidal volume, minute volume, inspiratory force and expiratory force, but did not affect morphine-induced reductions in respiratory rate. Minocycline attenuation of respiratory depression was also paralleled with significant attenuation by minocycline of morphine-induced reductions in blood oxygen saturation. Minocycline also attenuated morphine conditioned place preference. Minocycline did not simply reduce all actions of morphine, as morphine analgesia was significantly potentiated by minocycline co-administration. Lastly, morphine dose-dependently increased cyclooxygenase-1 gene expression in a rat microglial cell line, an effect that was dose-dependently blocked by minocycline. Together, these data support that morphine can directly activate microglia in a minocycline-suppressible manner and suggest a pivotal role for minocycline-sensitive processes in the mechanisms of morphine-induced respiration depression, reward, and pain modulation.
Keywords: rats, opioid, conditioned place preference, microglia, respiratory depression, blood oxygen saturation
Introduction
Within the past six years, opioids have been recognized to activate the immune cells of the central nervous system, microglia and astrocytes, and that this glial activation acts as an opponent process, compromising the ability of opioids to suppress pain (Hutchinson et al., 2007; Watkins et al., 2007). Administration of inhibitors of glial activation (Hutchinson et al., 2007; Raghavendra et al., 2002; Raghavendra et al., 2003b; Raghavendra et al., 2004) including minocycline (Cui et al., 2008; Song and Zhao, 2001), inhibitors of neuroexcitatory products of glia such as proinflammatory cytokines (Hutchinson et al., 2007; Johnston et al., 2004; Shavit et al., 2005), and genetic disruption of proinflammatory cytokine signaling (Shavit et al., 2005) have each been shown to enhance the analgesic efficacy of acute and chronic morphine. Conversely, manipulations that would cause glial activation compromise the ability of morphine to suppress pain (Johnston and Westbrook, 2005; Raghavendra et al., 2003b).
The results from animal models summarized above suggest that suppressing glial activation and/or its downstream consequences may be useful in increasing the efficacy of opioids in the treatment of pain. However, a valid concern is that the wanted positive actions of opioids (analgesia) and the negative unwanted side effects (respiratory depression, dependence, etc) may also be potentiated. If this were true, a glially-based strategy to potentiate morphine analgesia would be of limited benefit as the therapeutic window would not have been widened, just shifted.
As a first investigation into this novel arena of potential glial involvement in other morphine-induced effects, we explore whether minocycline, a putative microglial inhibitor, may influence morphine-induced respiratory depression and/or morphine-induced reward in animal models. Respiratory depression was chosen due to its major clinical relevance and impact on morbidity and mortality. Reward was chosen for study given that it is associated with drug seeking/drug craving aspects of drug abuse (Hutchinson et al., 2007), and conditioned place preference was used to measure reward as it is a well established and thoroughly characterized measure of the motivational aspects of drugs including morphine (Tzschentke, 2007). If minocycline, acting as a glial attenuator in these animal models, were to enhance either opioid respiratory depression or reward, this would prove a major difficulty for the pharmacological targeting of glial activation as a treatment of pain.
In the following series of studies, systemic delivery of minocycline in combination with systemic morphine was chosen for study. This choice was made for several reasons. First, microglia are widely considered to be more reactive to inflammatory stimuli than astrocytes, leading them to generally be the first glial cell activated (Watkins et al., 2007). Second, preventing microglial activation with minocycline has been observed, in turn, to prevent astrocyte activation (Ryu et al., 2004; Ryu and McLarnon, 2006), again suggestive of the primary importance of microglia in initiating activation of proinflammation within the CNS. Third, minocycline is blood-brain barrier permeable (Raghavendra et al., 2003a), so is appropriate for systemic administration unlike many other glial modulatory candidates (Watkins and Maier, 2003). Finally, minocycline has previously been shown to be effective in reducing morphine-induced microglial activation as reflected by a suppression of microglial morphine-induced p38 MAP kinase activation in vivo (Cui et al., 2008). Given these considerations, the following series of studies explore the effects of systemic minocycline on unwanted side effects of systemically administered morphine.
Methods and Materials
Subjects
Pathogen-free adult male Sprague–Dawley rats (300–375 g; Harlan Labs, Madison, WI, USA) were used in all experiments (n=5–6 rats per group for Experiments 1 and 3; 12–14 rats per group for Experiment 2). Rats were housed in temperature (23±3 °C) and light (12 h:12 h light:dark cycle; lights on at 0700) controlled rooms with standard rodent chow and water available ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Colorado at Boulder.
Drugs
Morphine sulfate was kindly gifted by Mallinckrodt, Inc. (St. Louis, MO, USA). Minocycline was purchased from Sigma (St. Louis, MO, USA). Where applicable, drugs were prepared and are reported as free base concentrations. Minocycline was administered by gavage (5 ml/kg) whereas morphine was administered subcutaneously (s.c.; 1 ml/kg). Minocycline was administered via gavage to mimic the clinically employed route of delivery and to avoid peritoneal irritation by this drug. The vehicle for morphine was sterile endotoxin-free isotonic saline (Abbott Laboratories, North Chicago, IL, USA) and the vehicle for minocycline was sterile endotoxin-free water. Vehicles were administered equivolume to the drugs under test.
Quantification of morphine induced respiratory depression
Respiratory rate (breaths per minute) and tidal volume (ml per breath) were measured using full body plethysmography (DBL plethysmograph box type 855; Validyne type DP 45-14 differential pressure transducer). Rats were habituated to the chamber for 20 min, with the last 5 min serving as the pre-morphine baseline measure the day prior to test day. On test day, animals were administered 0 (vehicle), 25 or 50 mg/kg minocycline by gavage followed 30 min later by 0 (vehicle) or 10 mg/kg morphine s.c. to provide a reliably quantifiable respiratory depression allowing statistically reliable reductions to be observed. Immediately following this second injection, rats were returned to the full body plethysmography apparatus and respiration measured for 30 min. From these measures, minute volume (ml per minute), inspiratory force (ml per sec), and expiratory force (ml per sec) were calculated using the HSE-HA-BDAS software (Hugo Sachs Elektronik, March-Hugstetten, Germany).
A parallel series of rats were tested for changes in blood oxygen saturation using pulseoxymetry (PulseOx; Oximax N-600 with Maxfast adhesive forehead reflective sensor held to each rat’s chest; Nellcor, Boulder, CO). At least 24 hr prior to test day rats were briefly anesthetized (isoflurane) and had their chest hair removed (Nair). The same dosing protocol was employed for PulseOx as for the full body plethysmography experiment. On test day, four baseline PulseOx readings were recorded prior to the gavage, following which the subcutaneous drug administration was given and PulseOx measures recorded every 2 min for 30 min. As the Nellcor equipment was calibrated for human use, rather than being calibrated for rat anatomy, relative changes are reported only, as full quantitative analysis is not possible given equipment constraints. Hence, data are simply expressed as a percent of baseline.
Morphine conditioned place preference
The Plexiglas place preference apparatus measured 72 (l) × 30 (w) × 30 (h) cm and was composed of two distinct conditioning environments and a neutral area in between. Each conditioning environment measured 30 × 30 × 30 cm. The floor of one environment consisted of metal bars 5 mm in diameter spaced 1.5 cm apart, the floor of the second environment was a black anodized aluminum perforated plate with 5 mm holes evenly spaced and staggered across the surface. The neutral area measured 12 × 30 × 30 cm, and had black sanded Plexiglas as a floor. During the conditioning phase, black Plexiglas partitions matching the respective environment they enclosed were inserted to restrict the rats to their designated conditioning environment.
The activity of each rat was monitored via Logitech Quickcam Pro 5000 webcams (Fremont, CA, USA) mounted 1.0 m above the center of the conditioned place preference apparatus. The camera relayed the information of the rat’s location to a computer running AnyMaze (Stoelting Co, Wood Dale, IL, USA) and recorded the time a subject spent within each of the three compartments.
An unbiased conditioned place preference procedure was used for all experiments. Prior to experimentation, all rats were extensively handled. On day 1 of testing, between 1 and 3 h after lights on, all rats were individually placed in the conditioned place preference apparatus and allowed to freely explore the entire apparatus for 20 min. This day served as a baseline to assess individual biases to either environment. Any rat that spent less than 4 min (20% of total time) in either environment was eliminated from the study. On the evening of day 1, rats were randomly assigned to their treatment groups and received a loading dose of minocycline (50 mg/kg in water given by gavage in a dose volume of 5 ml/kg; to provide microglial attenuation and shorten the time to achieve a steady state level of drug) or vehicle. On day 2 and thereafter rats received (matched to the prior evening’s drug) either a dose of minocycline (25 mg/kg in water given by gavage in a dose volume of 5 ml/kg) or vehicle twice daily. These gavages occurred at 120 min and 15 h after lights on, on days 2 through 5. As in the unbiased conditioned place preference procedure, conditioning assignments were counterbalanced such that half of the rats in each treatment group where assigned to be conditioned in the environment they originally preferred on day 1, and half were conditioned in the environment they did not prefer. There were four treatment groups: minocycline + morphine, minocycline + saline, vehicle + morphine and vehicle + saline. The minocycline + saline group was included to assess the effect of systemic minocycline, so “drug” in this group refers to conditioning with saline. The conditioning phase consisted of four pairings, two with drug (morphine/saline) and two with saline. Rats were conditioned once a day, 30 min following gavage dosing (and 150 min after lights on), with drug (7.5 mg/kg s.c. morphine/equivolume saline) or equivolume saline. The presentation of drug or equivolume saline alternated across the 4 days of conditioning.. Following injection of all rats, subjects were immediately placed into the appropriate conditioning environment for 45 min. Identical to day 1, on day 6 rats were placed in the conditioning apparatus in a drug-free state and allowed to explore the entire apparatus for 20 min. The time spent in each environment was measured and conditioning was calculated as a difference of the time spent in the drug-paired environment before and after the conditioning phase. Therefore, a positive score would reflect a preference for the environment.
Quantification of morphine analgesia
Rats received three 60 min habituations to the test environment before behavioral testing. Latencies for behavioral response to heat stimuli applied to the plantar surface of each hind-paw and tail were assessed using a modified Hargreaves test (Hargreaves et al., 1988). All testing was conducted blind with respect to group assignment. Briefly, baseline withdrawal values were calculated from an average of 2 consecutive withdrawal latencies of the tail and the left and the right hind-paws, measured at 15-min intervals. Latencies at baseline ranged from 3 to 4 s, and a cut-off time of 10 s was imposed to avoid tissue damage. The order of paw and tail testing varied randomly. After baseline assessment, rats received 0 (vehicle), 25 or 50 mg/kg minocycline by gavage followed 30 min later by 0 (vehicle) or 4 mg/kg morphine s.c. This morphine dose was chosen to produce reliable but submaximal analgesia, allowing for either potentiation or inhibition of analgesia to be observed. Nociceptive assessments for acute administration experiments were then made at 0 (immediately following the second drug delivery) 5 and every 10 min thereafter through 125 min.
Rat microglial cell culture and mRNA quantification
To test the acute effects of morphine and minocycline on microglial cell function, a rat microglial cell line (Highly Aggressive and Proliferative In culture; HAPI cells; n=3 replicates per condition) was used (kindly provided by Dr. P. Cheepsunthorn) (Cheepsunthorn et al., 2001). Cyclooxygenase-1 (COX-1) mRNA was chosen for study based on: (a) its robust upregulation in these cells by morphine in pilot studies, (b) its upregulation in brain in response to chronic morphine (Tumbaga et al., 1999), and (c) its involvement in morphine dependence (Capasso and Sorrentino, 1997).
HAPI cells were grown at 37°C (5% CO2) in 10 cm dishes in normal supplement selection media (DMEM media supplemented with 10% fetal bovine serum; Penicillin 10,000U/ml; Streptomycin 10 mg/ml, Normocine, and 200 nM L-Glutamin). The cells were then plated for 24 hr in 96 well plates at 8 × 106 cells/well with the same media. After 24 hr, supernatants were removed and replaced with 180 μl artificial cerebrospinal fluid to model in vivo conditions within the CNS (i.e. low protein). Drugs under test were then added in 20 μl and incubated for 4 hr (final well DMSO concentration 2%). At this time supernatants were removed and 100 μl of Trizol reagent (Invitrogen) was added to each well and plates frozen at −80°C until later analysis. Samples were the centrifuged (12,000 × g) at 4 °C. After chloroform and isopropyl alcohol isolation steps, samples were vortexed, incubated, and centrifuged (12,000 × g) at 4°C. Nucleic acid precipitates were washed twice in 75% ethanol and centrifuged (7500 × g) at 4°C. UV spectrophotometry was used to assess purity and concentration. Samples were DNase treated (DNA-free kit; Ambion), followed by requantitation before cDNA synthesis. Amplification of cDNA was performed using the QuantiTect SYBR Green PCR Kit (Qiagen) in iCycler iQ 96 well PCR plates (Bio-Rad) on a MyiQ Single Color Real-Time PCR Detection System (Bio-Rad). The reaction mixture (26 μl) was composed of 1× QuantiTect SYBR Green PCR Master Mix (containing the fluorescent dye SYBR Green I, 2.5 mM MgCl2, dNTP mix, and HotStart Taq DNA polymerase), 10 nM fluorescein, 500 nM each of forward and reverse primers, 25 ng cDNA and nuclease-free H2O. The reaction conditions were an initial 15 min at 95 °C, followed by 40 cycles of 15 s at 94 °C, 30 s at 55–60 °C, and 30 s at 72 °C. Melt curve analyses were conducted to assess uniformity of product formation, primer-dimer formation, and amplification of non-specific products. Linearity and efficiency of PCR amplification were assessed using standard curves generated by increasing amounts of cDNA. SYBR Green 1 fluorescence (PCR product formation) was monitored in real time using the MyiQ Single Color Real-Time PCR Detection System (Bio-Rad). Threshold for detection of PCR product was set in the log–linear phase of amplification and the threshold cycle (CT, the number of cycles to reach threshold of detection) was determined for each reaction. The levels of the COX1 mRNAs were quantified, using blinded procedures, relative to the level of the housekeeping gene glyceraldehyde-3-phosphate-dehydrogenase (GAPDH) using the comparative CT (ΔCT) method. Primers for each gene examined were the same as those previously reported (Ledeboer et al., 2005). Expression of the housekeeping gene was not significantly altered by experimental treatment.
Statistics
Respiratory variables obtained from the full body plesthymography recordings were smoothed using a 20 point moving average. Area under the curve for each of the variables was then obtained and a one-way analysis of variance with Bonferroni posthoc analysis was conducted. PulseOx data were smoothed using a 4 point moving average. The analgesic responses were calculated as the percent of maximal possible effect (%MPE) using the following test latency - baseline latency equation (Carmody, 1995) . A cut off of 10 sec was cut off - baseline latency used to avoid tissue damage. Baseline latencies for the Hargreaves tests ranged from 2–3 sec. Conditioned place preference data were expressed as the difference in time spent in the drug-paired environment before and after conditioning. Statistical significance was assessed using repeated measures ANOVA with Bonferroni post hoc test was used when comparing the analgesic responses of morphine versus vehicle controls and for all area under the curve analyses of respiratory function. A 2-way ANOVA was used for the conditioned place preference data and for the microglial cell mRNA results. Statistical comparisons are indicated on the figures for clarity.
Results
Experiment 1. Minocycline suppresses respiratory depression induced by morphine
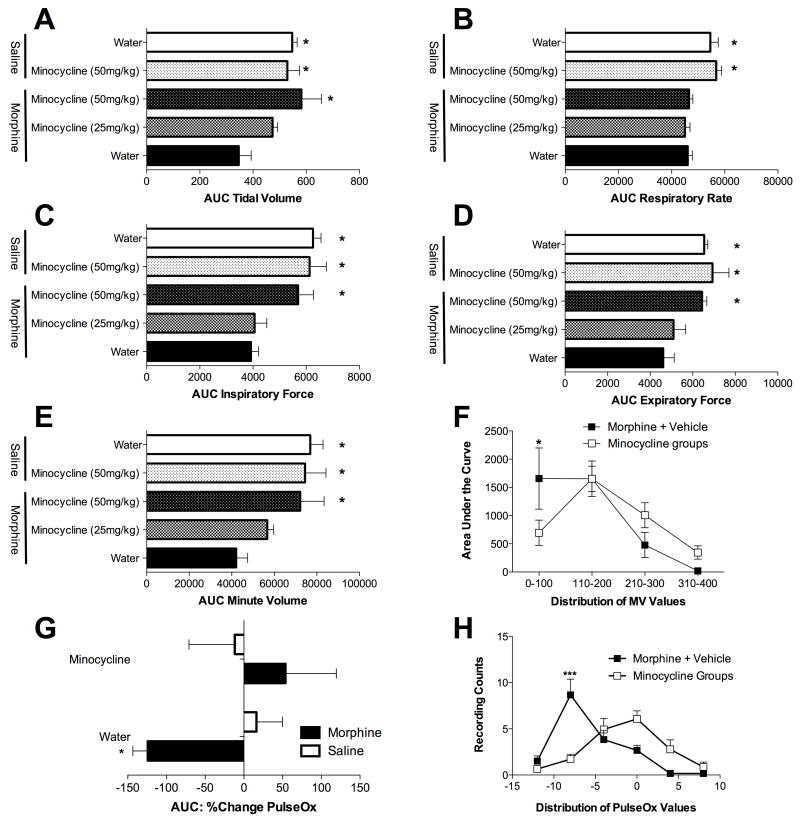
Before morphine treatment, no differences were observed in any index of respiration across groups. Subcutaneous administration of morphine (10 mg/kg) produced respiratory suppression, as comparison of pre- and post-drug measures revealed significant decreases in respiration rate tidal volume (Figure 1A and 2A), respiratory rate (Figure 1B and 2B), minute volume (Figure 1E and 2E), inspiratory force (Figure 1C and 2C), and expiratory force (Figure 1D and 2D). In addition, when the distribution of minute volume values were analyzed (Figure 1F), it is clear that morphine selectively enhanced very low minute volume values, a finding supportive of respiratory depression. Whilst comparison of pre- and post-minocycline (50 mg/kg) values did not indicate that minocycline affected respiratory rate alone (Figure 1B and 2B), it did attenuate other measures of morphine-induced respiratory depression, in a dose-dependent manner. Specifically, the highest minocycline dose attenuated morphine-induced suppression of tidal volume, minute volume, inspiratory force and expiratory force (Figures 1 and 2).
Figure 1. Minocycline attenuates morphine induced respiratory depression without effecting respiratory rate.
Morphine induced respiratory depression and its attenuation by minocycline were quantified using full body plesthysmography allowing the quantification of tidal volume (A), respiratory rate (B), minute volume (E), inspiratory force (C) and expiratory force (D). The data are displayed as area under the curve (AUC) for each parameter. (F) Analysis of the distribution of minute volume (MV) values reveals that morphine significantly increases very low minute volume values, consistent with significant respiratory depression. As distributions for minocycline+morphine and minocycline+vehicle groups were superimposable, these groups were pooled for simplicity of data presentation in this figure. (G) Morphine induced decreases in blood oxygen saturation (PulseOx), an effect significantly attenuated by minocycline. (H) Analysis of the distribution of PulseOx values reveals that morphine significantly increases low PulseOx values, consistent with significant respiratory depression which is attenuated by minocycline. A one-way ANOVA with Bonferroni posthoc test comparing morphine + water with each other data group. * = P < 0.05
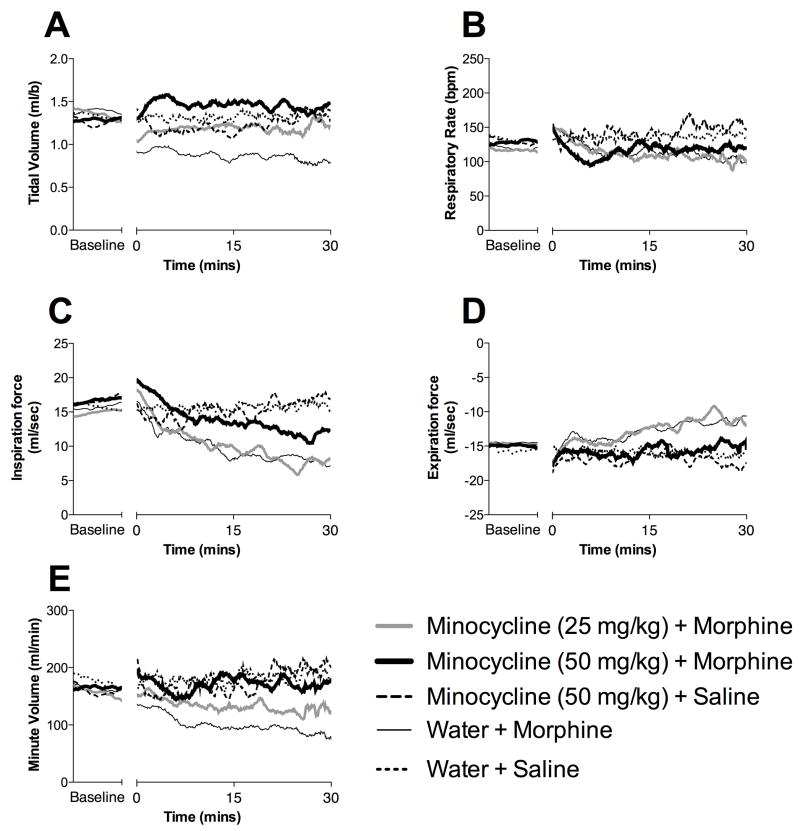
Figure 2. Minocycline attenuates morphine induced respiratory depression without effecting respiratory rate.
Morphine induced respiratory depression and its attenuation by minocycline were quantified using full body plesthysmography allowing the quantification of tidal volume (A), respiratory rate (B), minute volume (E), inspiratory force (C) and expiratory force (D).). Minocycline (50 mg/kg) + morphine (
 ), minocycline (25 mg/kg) + morphine (
), minocycline (25 mg/kg) + morphine (
 ), water + morphine (—), minocycline (50 mg/kg) + saline (
), water + morphine (—), minocycline (50 mg/kg) + saline (
 ), water + saline (
), water + saline (
 ). The mean data for each group are displayed as the time course of respiratory changes at baseline and following morphine administration (10 data point moving average to smooth data). A one-way ANOVA with Bonferroni posthoc test comparing morphine + water with each other data group. * = P < 0.05
). The mean data for each group are displayed as the time course of respiratory changes at baseline and following morphine administration (10 data point moving average to smooth data). A one-way ANOVA with Bonferroni posthoc test comparing morphine + water with each other data group. * = P < 0.05
Blood oxygen saturation (PulseOx) was also measured in a parallel experiment to the mechanical respiratory depression measure. Morphine alone (10 mg/kg) produced a significant reduction in PulseOx values compared to vehicle treated controls. Minocycline pretreatment as above significantly attenuated the morphine-induced decrease in PulseOx (Figure 1G) and caused a normalization of the distribution of the PulseOx values (Figure 1H).
Experiment 2. Minocycline attenuates conditioned place preference induced by morphine
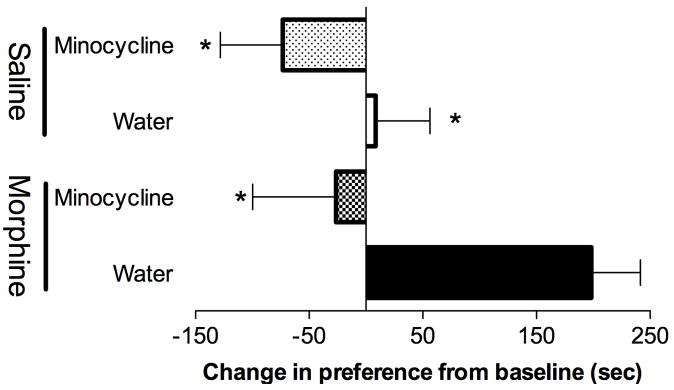
Here, we assessed whether minocycline could attenuate a very different morphine-induced side effect, that being reward. Vehicle + morphine rats displayed significant conditioning to the morphine paired environment, demonstrating that the conditioned place preference design with 4 conditionings (2 pairings with morphine, 2 pairings with vehicle) across four days created a significant preference for the morphine-paired environment (Figure 3). Minocycline treatment significantly attenuated morphine conditioned place preference when compared to morphine + vehicle.
Figure 3. Minocycline attenuates morphine conditioned place preference.
Morphine (7.5 mg/kg) produces significant conditioned place preference (morphine + water (vehicle): black fill). Minocycline (50 mg/kg per day; gavage) significantly attenuated this morphine induced conditioned place preference (morphine + minocycline: checkerboard fill). In the absence of morphine, neither water (saline + vehicle; white fill) nor minocycline (saline + minocycline: dotted fill) significantly altered place preference. A 2-way ANOVA with Bonferroni was conducted. * = P < 0.05 compared to morphine + water (vehicle).
Experiment 3. Minocycline enhances analgesia induced by morphine
While the results above appear to indicate that systemic minocycline decreases both systemic morphine-induce respiratory depression and systemic morphine-induced conditioned place preference, an alternative explanation could potentially be that minocycline somehow modified morphine pharmacokinetics by decreasing the access of morphine to the central nervous system (CNS). As we have reported that intrathecal minocycline potentiates the analgesic effect of intrathecal morphine (Hutchinson et al., 2007), here we tested whether the analgesic effects of systemic morphine would be potentiated or inhibited by systemic minocycline. If analgesia were indeed inhibited under this condition, it would suggest that minocycline decreases morphine levels in CNS. However, if morphine analgesia were potentiated, it would provide not only novel data regarding microglial regulation of opioid actions, but would also argue against a pharmacokinetic confound.
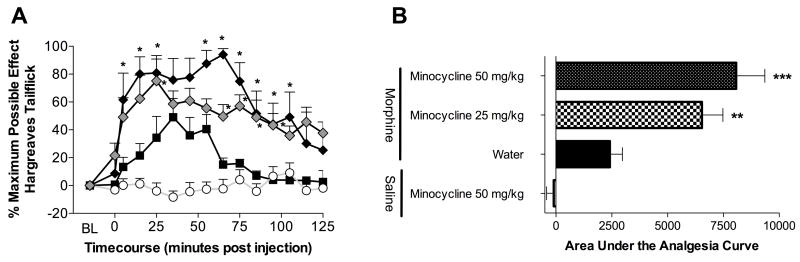
Prior to drug, no differences in baseline response latencies to radiant heat were observed across groups. As pre- and post-drug results were consistent for both hindpaws and tail, only data from the tailflick test are included here for simplicity. The 4 mg/kg morphine dose in the absence of minocycline produced reliable but submaximal analgesia as expected (Figure 4A). While in the absence of morphine, 50 mg/kg minocycline had no effect on escape latencies, both 25 and 50 mg/kg minocycline significantly potentiated the onset, peak magnitude, and duration of systemic morphine analgesia (Figure 4).
Figure 4. Minocycline potentiates systemic morphine analgesia.
Minocycline (25 mg/kg
 and 50 mg/kg ◆ gavages) significantly potentiated morphine (4 mg/kg, ■) analgesia, whilst saline treatment had no effect (○). Minocycline potentiated the onset, peak and duration of analgesia (A) as well as the total area under the analgesia curve (B). A one-way ANOVA with Bonferroni posthoc test comparing morphine + water with each other data group. ** = P < 0.01; *** = P < 0.001.
and 50 mg/kg ◆ gavages) significantly potentiated morphine (4 mg/kg, ■) analgesia, whilst saline treatment had no effect (○). Minocycline potentiated the onset, peak and duration of analgesia (A) as well as the total area under the analgesia curve (B). A one-way ANOVA with Bonferroni posthoc test comparing morphine + water with each other data group. ** = P < 0.01; *** = P < 0.001.
Experiment 4. Minocycline suppresses morphine-induced upregulation of COX-1 mRNA in rat microglial cells in vitro
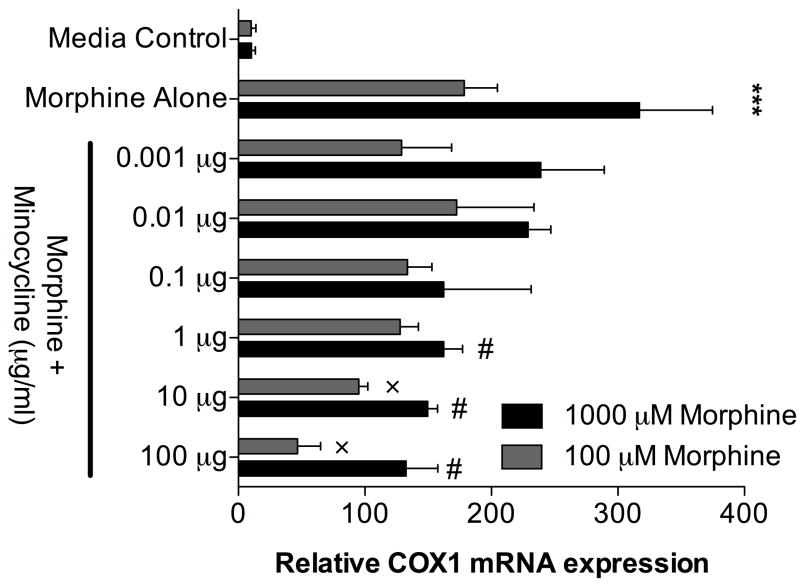
Acute 4 hr in vitro incubation of HAPI cells in artificial CSF with morphine dose dependently induces a significant increase in COX-1 mRNA (Figure 5). Minocycline dose dependently reverses this proinflammatory COX-1 activation of HAPI cells in vitro (Figure 5). Thus the present COX-1 mRNA data extend the prior report by Cui et al. (2008) that minocycline reduces morphine-induced p38 MAP kinase activation in microglia in vivo.
Figure 5. Morphine enhances COX-1 mRNA expression in a rat microglial cell line, an effect blocked by minocycline.
HAPI microglia cells response in a proinflammatory fashion in vitro to acute (4 hr) morphine exposure under CNS conditions by significantly up regulating COX-1 mRNA expression. Minocycline dose dependently attenuates this proinflammatory activation by significantly attenuating COX-1 mRNA upregulation. n=3 replicates per condition. *** P < 0.001 media controls vs, morphine alone treatments; # P < 0.05 1000 μM morphine alone vs. morphine+minocycline; × P < 0.05 100 μM morphine alone vs morphine+minocycline.
Discussion
These studies provide the first pharmacological evidence that minocycline-sensitive processes contribute to clinically relevant effects of opioids, namely respiratory depression and reward. Here we demonstrate that systemic minocycline, a blood brain barrier permeable microglial inhibitor with no known opioid receptor activity (Festoff et al., 2006), suppressed morphine-induced respiratory depression, as measured by tidal volume, minute volume, inspiratory force, expiratory force and blood oxygen saturations. In contrast, at the doses tested, minocycline produced no effect on morphine-induced suppression of respiratory rate. In addition, morphine-induced conditioned place preference also was attenuated by minocycline. These same doses of minocycline potentiated analgesia produced by systemic morphine, both providing new evidence that minocycline-sensitive processes can enhance the analgesic efficacy of systemically administered opioids and suggesting that the minocycline-induced decrease in morphine-induced respiratory depression and conditioned place preference is unlikely to be accounted for by decreases in morphine penetrance of CNS tissues or increased morphine clearance. The lack of effect of minocycline on morphine-induced reduction in respiratory rate would support this conclusion as well, as higher concentrations of morphine are required to suppress respiration rate relative to tidal volume (White and Irvine, 1999). Thus if less morphine were to reach CNS centers, an attenuated decrease respiration rate in minocycline treated animals would have been observed rather than the changes in tidal volume that were recorded. Whether the same or altered effects would be seen in the presence of ongoing persistent pain is, at present, unknown. Use of pain-free rats in the present study was designed to avoid potential confounds from superimposing morphine on ongoing glial activation induced in persistent pain models. Lastly, in vitro study of a rat microglial cell line provided clear evidence that morphine does activate pure microglia (as measured by elevated COX-1 mRNA), an effect dose-dependently suppressed by minocycline.
Numerous prior studies document that microglia become activated in response to opioids. One question is how such activation occurs. Both direct binding to opioid receptors (Chao et al., 1997), activation via non-classical opioid receptors (Hutchinson et al., 2007), and indirect activation via opioid-induced release of neuron-to-microglial signals (Johnston et al., 2004) have been described. Under conditions of higher doses of morphine in vivo, hypoxia becomes a fourth option as respiratory depression caused by morphine can induce a hypoxic state (Modalen et al., 2006). Hypoxia is a potent stimulus for microglial activation and minocycline markedly inhibits hypoxia-induced production of inflammatory mediators such as nitric oxide, interleukin (IL)-1 and tumor necrosis factor (Suk, 2004). How hypoxia may influence microglial activation when it occurs simultaneously with the direct and indirect influences noted above has not been explored. Similarly, as noted previously, exploring how microglia become activated in response to opioids under conditions of ongoing persistent pain may provide a rich avenue for a deeper understanding of opioid-glial interactions.
The rapidity of effects observed in the present study are also notable as they suggest a rapid activation of glia by morphine. This observation is consistent with prior literature. For example, Shavit et al. observed that analgesia was reliably potentiated in IL-1 receptor antagonist (IL-1ra) overexpressing mice by 30 min after systemic morphine (Shavit et al., 2005). Similarly, they observed potentiation of systemic morphine by 30 min in mice with disrupted IL-1 receptor signaling (IL-1 receptor knock out; IL-1 receptor accessory protein knock out) (Shavit et al., 2005). We have recently extended these findings by documenting that analgesia induced by intrathecal morphine is reliably enhanced by intrathecal IL-1 receptor antagonist within 10 min (Hutchinson et al., 2008). Very rapid (within 10 min) enhancement of morphine analgesia is also observed in response to intrathecal blockade of the effects of tumor necrosis factor and IL-6 as well (Hutchinson et al., 2008). Whether morphine directly activates microglia within 30 min is as yet unknown.
An additional question is why glia suppress the actions of opioids for analgesia, yet appear, based on the effects of minocycline to enhance both reward and respiratory depression. While speculative, perhaps a parsimonious view is not that opioid-induced glial activation opposes opioid analgesia per se, but rather that glially-induced proinflammatory cytokines enhance pain responsivity. Thus opioid-induced pain enhancement counter-regulates, or opposes, the pain suppressive effects of opioids. The sum of the analgesic actions of opioids combined with the glially-induced proinflammatory cytokine response would therefore behavioral present and be interpreted as a suppression of analgesia. This interpretation would allow for consistent opioid-induced effects on glia; namely, enhancement of pain responsivity, drug reward, and respiratory depression. Whether it is glial proinflammatory cytokines, alterations in glutamate, or other glial products that enhance drug reward and respiratory depression is at yet unknown.
The mechanisms via which opioid-induced glial activation produces these behavioral outcomes remain to be fully characterized. Opposition of opioid analgesia by activated glia may be attributed to several candidate mechanisms, such as interleukin-1 phosphorylation of NMDA receptors (Viviani et al., 2003), or increased glutamate tone due to activated glial down regulation of glutamate transporters (Tilleux and Hermans, 2007). Similar glutamate (Nakagawa et al., 2005; Nakagawa and Satoh, 2004) and proinflammatory (Narita et al., 2006) theories have also been posited for reward.
The conclusions of the present studies rest on the use of minocycline. Minocycline is a tetracycline derivative that exhibits selectivity for microglia. It exhibits anti-inflammatory effects that are independent of its antimicrobial actions (Ledeboer et al., 2005). Minocycline can inhibit: microglial activation, activation of p38 MAP kinase, activation of interleukin-1 converting enzyme which cleaves pro-interleukin-1 into its active form (interleukin-1), tumor necrosis factor release, nitric oxide production, and mRNA expression of interleukin-1, tumor necrosis factor, interleukin-1 converting enzyme, and tumor necrosis factor converting enzyme (Ledeboer et al., 2005; Tikka et al., 2001; Yrjanheikki et al., 1998). While the literature by-and-large supports that minocycline inhibits microglial activation independent of direct effects on astrocytes and neurons (Tikka and Koistinaho, 2001), neuroprotective effects of minocycline on neuronal cultures exposed to toxic levels of nitric oxide have been reported (Lin et al., 2001). As minocycline’s specificity and mechanisms of action are not fully characterized, further studies with other glial modulators will be needed before the effects of minocycline in the present study can be conclusively attributed to actions on glia.
In line with known actions of minocycline on microglia, minocycline inhibits enhanced pain in diverse animal models in which the manipulations are known to activate microglia and the outcomes involve mediation by proinflammatory cytokines (Ledeboer et al., 2005; Raghavendra et al., 2003a; Raghavendra et al., 2003b). However, concerns have been raised regarding potential clinical relevance of this compound, given that it prevents yet fails to reverse pain in most (Ledeboer et al., 2005; Raghavendra et al., 2003a), but not all (Hains and Waxman, 2006), animal models of pain enhancement studied to date. Given this, it will be important in future to define whether minocycline will still be effective in enhancing morphine analgesia and suppressing respiratory depression and reward under conditions where glia are already perseveratively activated. If it were found that minocycline again fails to be effective under such conditions, other blood brain barrier permeable glial modulators, such as propentofylline (Sweitzer et al., 2001) and AV411 (ibudilast) (Ledeboer et al., 2007), may prove efficacious as both of these agents reverse as well as prevent glial activation.
It has not previously been tested whether glia contribute to respiratory depression. Respiratory depression is one of the most frequently observed adverse effects of clinically administered morphine, with a higher incidence of respiratory depression observed in opioid-naive patients (Momeni et al., 2006). Fear of respiratory depression leads to under-medication with opioids, resulting in unnecessary suffering (Smith, 2007). Regarding the potential for glial involvement in this adverse action of morphine, there are suggestions in the literature that glia may play such a role, arising from studies of sudden infant death syndrome (SIDS). While clearly speculative, given that these cases are in immature systems rather than adult, it remains that autopsy of SID cases reveal activated microglia and astrocytes in brainstem respiratory centers, compared to controls, suggestive that such glial activation may be related to suppressed neural respiratory control (Becker and Takashima, 1985; Biondo et al., 2004; Storm et al., 1994). Elevated IL-6 was also detected in cerebrospinal fluid of SID cases (Vege et al., 2005). Several studies have reported associations between SIDS and high-producing interleukin-6 polymorphisms (Dashash et al., 2006; Moscovis et al., 2006) and low-producing interleukin-10 polymorphisms (Summers et al., 2000). Such evidence led to the suggestion that proinflammatory cytokines may cause respiratory depression (Vege et al., 2005).
Given that minocycline affected tidal volume but not respiratory rate, it is likely that minocycline acts, as anticipated, at central sites rather than at peripheral chemoreceptors that provide inputs to central respiratory centers. An action at these sensory cells or afferent fibers would not allow the separation of effects observed with minocycline, as differential regulation of respiratory rate and tidal volume occurs within the brain (Bianchi et al., 1995).
There is precedence for separating effects on tidal volume from respiratory rate. For example, activation of GABA receptors by intracerebroventricular injection of GABA or muscimol or systemic administration of amino-oxyacetic acid, which increases endogenous GABA levels, depresses tidal volume without affecting respiratory rate (Bianchi et al., 1995). Local application of acetylcholine to the ventral medullary surface selectively increases tidal volume, with no effect on respiratory rate (Bianchi et al., 1995). Regarding opioid regulation of respiration, neurons in the dorsal respiratory group (ventrolateral division of the nucleus tractus solitarius) are thought to create opioid-induced reduction in tidal volume whereas neurons of the pontine respiratory group (nucleus parabrachialis medialis and Kolliker-Fuse nucleus) and pre-Botzinger complex reduce respiratory frequency in response to opioids (Bianchi et al., 1995; Horner, 2006). As opioid-induced glial activation shows regional heterogeneity (Lewis et al., 2006), exploring site-specific effects of opioids on glial activation, and of proinflammatory cytokines (or other products of activated glia) on respiration, may prove fruitful.
It is also intriguing to speculate that a glial contribution to respiratory depression may contribute to the relatively slow and incomplete tolerance that develops for this negative side effect, relative to other effects of opioids such as analgesia (White and Irvine, 1999). Glia have been reported to become increasingly activated in response to repeated opioids, leading to increased production and release of proinflammatory cytokines (Johnston et al., 2004). Increased glial activation in response to repeated opioids has been implicated in the development of analgesic tolerance as proinflammatory cytokines oppose analgesia (Johnston et al., 2004; Raghavendra et al., 2004). In the case of respiratory depression, if it is true that glia contribute to reduced respiration then increasing glial activation with repeated opioid administration may oppose development of tolerance in this case.
The ability of minocycline to also attenuate morphine conditioned place preference is intriguing as it appears to implicate opioid-induced microglial activation in the rewarding actions of morphine. However, as the amount of conditioned place preference generated is affected by the animal’s ability to learn and remember as well as by the rewarding effects of the drug, one issue is whether minocycline’s disruption of conditioned place preference was due to effects on reward versus on learning and memory. Currently available evidence suggests that, if anything, minocycline enhances learning and memory rather than disrupting it (Choi et al., 2007; Fan et al., 2007; Liu et al., 2007; Mizoguchi et al., 2007). Hence, the most likely conclusion from the conditioned place preference data reported here is that minocycline disrupted morphine-induced drug reward.
The conditioned place preference results support previous data from Narita et al. (2006) who demonstrated that microinjection of activated astrocyte-derived soluble factors amplifies the rewarding effect of opioids when injected into in the nucleus accumbens and cingulate cortex. That glia may generally regulate the rewarding effects of drugs of abuse is supported by the observation that another glial modulator, propentofylline, suppresses methamphetamine-induced conditioned place preference (Narita et al., 2006). These data suggest that a major change in the traditional notion of purely neuronally mediated reward pathways is warranted.
In summary, minocycline, a putative microglial attenuator, reduces morphine-induced respiratory depression and, conditioned place preference, while potentiating morphine analgesia. Our data implicate opioid-induced glial activation in respiration, reward and nociception. Further research is required to determine if attenuating glial activation may be a clinically viable method for improving the safety and efficacy of opioids.
Acknowledgments
International Association for the Study of Pain International Collaborative grant, American Australian Association Merck Company Foundation Fellowship, National Health and Medical Research Council CJ Martin Fellowship (ID 465423) and NIH Grants DA015642, DA017670 and DE017782. The authors thank Dr. Jack L. Feldman (UCLA) for helpful discussions. Thanks also to Harvard Apparatus and Nellcor for the loan of the plesthysmography and PulseOx equipment, respectively.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Becker LE, Takashima S. Chronic hypoventilation and development of brain stem gliosis. Neuropediatrics. 1985;16:19–23. doi: 10.1055/s-2008-1052538. [DOI] [PubMed] [Google Scholar]
- Bianchi AL, Denavit-Saubie M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiol Rev. 1995;75:1–45. doi: 10.1152/physrev.1995.75.1.1. [DOI] [PubMed] [Google Scholar]
- Biondo B, Magagnin S, Bruni B, Cazzullo A, Tosi D, Matturri L. Glial and neuronal alterations in the nucleus tractus solitarii of sudden infant death syndrome victims. Acta Neuropathol (Berl) 2004;108:309–318. doi: 10.1007/s00401-004-0895-2. [DOI] [PubMed] [Google Scholar]
- Capasso A, Sorrentino L. Arachidonic acid and its metabolites are involved in the expression of morphine dependence in guinea-pig isolated ileum. European journal of pharmacology. 1997;330:199–204. doi: 10.1016/s0014-2999(97)00177-5. [DOI] [PubMed] [Google Scholar]
- Carmody J. Avoiding fallacies in nociceptive measurements. Pain. 1995;63:136. doi: 10.1016/0304-3959(95)90018-7. [DOI] [PubMed] [Google Scholar]
- Chao CC, Hu S, Shark KB, Sheng WS, Gekker G, Peterson PK. Activation of mu opioid receptors inhibits microglial cell chemotaxis. J Pharmacol Exp Ther. 1997;281:998–1004. [PubMed] [Google Scholar]
- Cheepsunthorn P, Radov L, Menzies S, Reid J, Connor JR. Characterization of a novel brain-derived microglial cell line isolated from neonatal rat brain. Glia. 2001;35:53–62. doi: 10.1002/glia.1070. [DOI] [PubMed] [Google Scholar]
- Choi Y, Kim HS, Shin KY, Kim EM, Kim M, Kim HS, Park CH, Jeong YH, Yoo J, Lee JP, Chang KA, Kim S, Suh YH. Minocycline attenuates neuronal cell death and improves cognitive impairment in Alzheimer’s disease models. Neuropsychopharmacology. 2007;32:2393–2404. doi: 10.1038/sj.npp.1301377. [DOI] [PubMed] [Google Scholar]
- Cui Y, Liao XX, Liu W, Guo RX, Wu ZZ, Zhao CM, Chen PX, Feng JQ. A novel role of minocycline: attenuating morphine antinociceptive tolerance by inhibition of p38 MAPK in the activated spinal microglia. Brain, behavior, and immunity. 2008;22:114–123. doi: 10.1016/j.bbi.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Dashash M, Pravica V, Hutchinson IV, Barson AJ, Drucker DB. Association of sudden infant death syndrome with VEGF and IL-6 gene polymorphisms. Hum Immunol. 2006;67:627–633. doi: 10.1016/j.humimm.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Fan R, Xu F, Previti ML, Davis J, Grande AM, Robinson JK, Van Nostrand WE. Minocycline reduces microglial activation and improves behavioral deficits in a transgenic model of cerebral microvascular amyloid. J Neurosci. 2007;27:3057–3063. doi: 10.1523/JNEUROSCI.4371-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains BC, Waxman SG. Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. J Neurosci. 2006;26:4308–4317. doi: 10.1523/JNEUROSCI.0003-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Horner RL. Morphine-induced acetylcholine release at the hypoglossal motor nucleus: implications for opioid-induced respiratory suppression. Sleep. 2006;30:551–552. doi: 10.1093/sleep/30.5.551. [DOI] [PubMed] [Google Scholar]
- Hutchinson MR, Bland ST, Johnson KW, Rice KC, Maier SF, Watkins LR. Opioid-induced glial activation: mechanisms of activation and implications for opioid analgesia, dependence and reward. The Scientific World JOURNAL. 2007;7:98–111. doi: 10.1100/tsw.2007.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Coats BD, Lewis SS, Zhang Y, Sprunger DB, Rezvani N, Baker EM, Jekich BM, Wieseler JL, Somogyi AA, Martin D, Poole S, Judd CM, Maier SF, Watkins LR. Proinflammatory cytokines oppose opioid-induced acute and chronic analgesia. Brain, behavior, and immunity. 2008 doi: 10.1016/j.bbi.2008.05.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston IN, Milligan ED, Wieseler-Frank J, Frank MG, Zapata V, Campisi J, Langer S, Martin D, Green P, Fleshner M, Leinwand L, Maier SF, Watkins LR. A role for proinflammatory cytokines and fractalkine in analgesia, tolerance, and subsequent pain facilitation induced by chronic intrathecal morphine. J Neurosci. 2004;24:7353–7365. doi: 10.1523/JNEUROSCI.1850-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston IN, Westbrook RF. Inhibition of morphine analgesia by LPS: role of opioid and NMDA receptors and spinal glia. Behavioural brain research. 2005;156:75–83. doi: 10.1016/j.bbr.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Ledeboer A, Hutchinson MR, Watkins LR, Johnson KW. Ibudilast (AV-411): a new class therapeutic candidate for neuropathic pain and opioid withdrawal syndromes. Expert Opinion on Investigational Drugs. 2007;16:935–950. doi: 10.1517/13543784.16.7.935. [DOI] [PubMed] [Google Scholar]
- Ledeboer A, Sloane EM, Milligan ED, Frank MG, Mahony JH, Maier SF, Watkins LR. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain. 2005;115:71–83. doi: 10.1016/j.pain.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Lewis SS, Hutchinson MR, Coats BD, Brzeski AL, Maier SF, Watkins LR, Johnson KW. AV411, a blood brain barrier permeable glial activation inhibitor, reduces morphine withdrawal behaviors in rats. Proc Soc Neurosci 2006 [Google Scholar]
- Lin S, Zhang Y, Dodel R, Farlow MR, Paul SM, Du Y. Minocycline blocks nitric oxide-induced neurotoxicity by inhibition p38 MAP kinase in rat cerebellar granule neurons. Neurosci Lett. 2001;315:61–64. doi: 10.1016/s0304-3940(01)02324-2. [DOI] [PubMed] [Google Scholar]
- Liu Z, Fan Y, Won SJ, Neumann M, Hu D, Zhou L, Weinstein PR, Liu J. Chronic treatment with minocycline preserves adult new neurons and reduces functional impairment after focal cerebral ischemia. Stroke. 2007;38:146–152. doi: 10.1161/01.STR.0000251791.64910.cd. [DOI] [PubMed] [Google Scholar]
- Mizoguchi H, Takuma K, Fukakusa A, Ito Y, Nakatani A, Ibi D, Kim HC, Yamada K. Improvement by minocycline of methamphetamine-induced impairment of recognition memory in mice. Psychopharmacology (Berl) 2007 doi: 10.1007/s00213-007-0955-0. [DOI] [PubMed] [Google Scholar]
- Modalen AO, Quiding H, Frey J, Westman L, Lindahl S. A novel molecule with peripheral opioids properties: the effects on hypercarbic and hypoxic ventilation at steady-state compared with morphine and placebo. Anesth Analg. 2006;102:104–109. doi: 10.1213/01.ANE.0000184254.85567.80. [DOI] [PubMed] [Google Scholar]
- Momeni M, Crucitti M, De Kock M. Patient-controlled analgesia in the management of postoperative pain. Drugs. 2006;66:2321–2337. doi: 10.2165/00003495-200666180-00005. [DOI] [PubMed] [Google Scholar]
- Moscovis SM, Gordon AE, Al Madani OM, Gleeson M, Scott RJ, Roberts-Thomson J, Hall ST, Weir DM, Busuttil A, Blackwell CC. IL6 G-174C associated with sudden infant death syndrome in a Caucasian Australian cohort. Hum Immunol. 2006;67:819–825. doi: 10.1016/j.humimm.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Fujio M, Ozawa T, Minami M, Satoh M. Effect of MS-153, a glutamate transporter activator, on the conditioned rewarding effects of morphine, methamphetamine and cocaine in mice. Behavioural brain research. 2005;156:233–239. doi: 10.1016/j.bbr.2004.05.029. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Satoh M. Involvement of glial glutamate transporters in morphine dependence. Annals of the New York Academy of Sciences. 2004;1025:383–388. doi: 10.1196/annals.1307.047. [DOI] [PubMed] [Google Scholar]
- Narita M, Miyatake M, Narita M, Shibasaki M, Shindo K, Nakamura A, Kuzumaki N, Nagumo Y, Suzuki T. Direct evidence of astrocytic modulation in the development of rewarding effects induced by drugs of abuse. Neuropsychopharmacology. 2006;31:2476–2488. doi: 10.1038/sj.npp.1301007. [DOI] [PubMed] [Google Scholar]
- Raghavendra V, Rutkowski MD, DeLeo JA. The role of spinal neuroimmune activation in morphine tolerance/hyperalgesia in neuropathic and sham-operated rats. J Neurosci. 2002;22:9980–9989. doi: 10.1523/JNEUROSCI.22-22-09980.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendra V, Tanga F, DeLeo JA. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J Pharmacol Exp Ther. 2003a;306:624–630. doi: 10.1124/jpet.103.052407. [DOI] [PubMed] [Google Scholar]
- Raghavendra V, Tanga F, Rutkowski MD, DeLeo JA. Anti-hyperalgesic and morphine-sparing actions of propentofylline following peripheral nerve injury in rats: mechanistic implications of spinal glia and proinflammatory cytokines. Pain. 2003b;104:655–664. doi: 10.1016/S0304-3959(03)00138-6. [DOI] [PubMed] [Google Scholar]
- Raghavendra V, Tanga FY, DeLeo JA. Attenuation of morphine tolerance, withdrawal-induced hyperalgesia, and associated spinal inflammatory immune responses by propentofylline in rats. Neuropsychopharmacology. 2004;29:327–334. doi: 10.1038/sj.npp.1300315. [DOI] [PubMed] [Google Scholar]
- Ryu JK, Franciosi S, Sattayaprasert P, Kim SU, McLarnon JG. Minocycline inhibits neuronal death and glial activation induced by beta-amyloid peptide in rat hippocampus. Glia. 2004;48:85–90. doi: 10.1002/glia.20051. [DOI] [PubMed] [Google Scholar]
- Ryu JK, McLarnon JG. Minocycline or iNOS inhibition block 3-nitrotyrosine increases and blood-brain barrier leakiness in amyloid beta-peptide-injected rat hippocampus. Exp Neurol. 2006;198:552–557. doi: 10.1016/j.expneurol.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Shavit Y, Wolf G, Goshen I, Livshits D, Yirmiya R. Interleukin-1 antagonizes morphine analgesia and underlies morphine tolerance. Pain. 2005;115:50–59. doi: 10.1016/j.pain.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Smith LH. Opioid safety: is your patient at risk for respiratory depression? Clinical journal of oncology nursing. 2007;11:293–296. doi: 10.1188/07.CJON.293-296. [DOI] [PubMed] [Google Scholar]
- Song P, Zhao ZQ. The involvement of glial cells in the development of morphine tolerance. Neurosci Res. 2001;39:281–286. doi: 10.1016/s0168-0102(00)00226-1. [DOI] [PubMed] [Google Scholar]
- Storm H, Rognum TO, Saugstad OD, Skullerud K, Reichelt KL. Beta-endorphin immunoreactivity in spinal fluid and hypoxanthine in vitreous humour related to brain stem gliosis in sudden infant death victims. Eur J Pediatr. 1994;153:675–681. doi: 10.1007/BF02190691. [DOI] [PubMed] [Google Scholar]
- Suk K. Minocycline suppresses hypoxic activation of rodent microglia in culture. Neurosci Lett. 2004;366:167–171. doi: 10.1016/j.neulet.2004.05.038. [DOI] [PubMed] [Google Scholar]
- Summers AM, Summers CW, Drucker DB, Hajeer AH, Barson A, Hutchinson IV. Association of IL-10 genotype with sudden infant death syndrome. Hum Immunol. 2000;61:1270–1273. doi: 10.1016/s0198-8859(00)00183-x. [DOI] [PubMed] [Google Scholar]
- Sweitzer SM, Schubert P, DeLeo JA. Propentofylline, a glial modulating agent, exhibits antiallodynic properties in a rat model of neuropathic pain. J Pharmacol Exp Ther. 2001;297:1210–1217. [PubMed] [Google Scholar]
- Tikka T, Fiebich BL, Goldsteins G, Keinanen R, Koistinaho J. Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. J Neurosci. 2001;21:2580–2588. doi: 10.1523/JNEUROSCI.21-08-02580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikka TM, Koistinaho JE. Minocycline provides neuroprotection against N-methyl-D-aspartate neurotoxicity by inhibiting microglia. J Immunol. 2001;166:7527–7533. doi: 10.4049/jimmunol.166.12.7527. [DOI] [PubMed] [Google Scholar]
- Tilleux S, Hermans E. Neuroinflammation and regulation of glial glutamate uptake in neurological disorders. Journal of neuroscience research. 2007;85:2059–2070. doi: 10.1002/jnr.21325. [DOI] [PubMed] [Google Scholar]
- Tumbaga P, Beharry K, Akmal Y, Federico C, Modanlou HD. Biochemical changes in prostanoids and cerebral expression of cyclooxygenase (COX)-1 and COX-2 during morphine sulfate infusion in the newborn piglet. Prostaglandins & other lipid mediators. 1999;58:273–284. doi: 10.1016/s0090-6980(99)00042-8. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Vege A, Rognum TO, Scott H, Aasen AO, Saugstad OD. SIDS cases have increased levels of interleukin-6 in cerebropsinal fluid. Neurology. 2005;65:1958–1960. doi: 10.1111/j.1651-2227.1995.tb13608.x. [DOI] [PubMed] [Google Scholar]
- Viviani B, Bartesaghi S, Gardoni F, Vezzani A, Behrens MM, Bartfai T, Binaglia M, Corsini E, Di Luca M, Galli CL, Marinovich M. Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J Neurosci. 2003;23:8692–8700. doi: 10.1523/JNEUROSCI.23-25-08692.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Ledeboer A, Wieseler-Frank J, Milligan ED, Maier SF. Glia as the “bad guys”: Implications for improving clinical pain control and the clinical utility of opioids. Brain, behavior, and immunity. 2007;21:131–146. doi: 10.1016/j.bbi.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Maier SF. Glia: a novel drug discovery target for clinical pain. Nat Rev Drug Discov. 2003;2:973–985. doi: 10.1038/nrd1251. [DOI] [PubMed] [Google Scholar]
- White JM, Irvine RJ. Mechanisms of fatal opioid overdose. Addiction. 1999;94:961–972. [PubMed] [Google Scholar]
- Yrjanheikki J, Keinanen R, Pellikka M, Hokfelt T, Koistinaho J. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci U S A. 1998;95:15769–15774. doi: 10.1073/pnas.95.26.15769. [DOI] [PMC free article] [PubMed] [Google Scholar]