Abstract
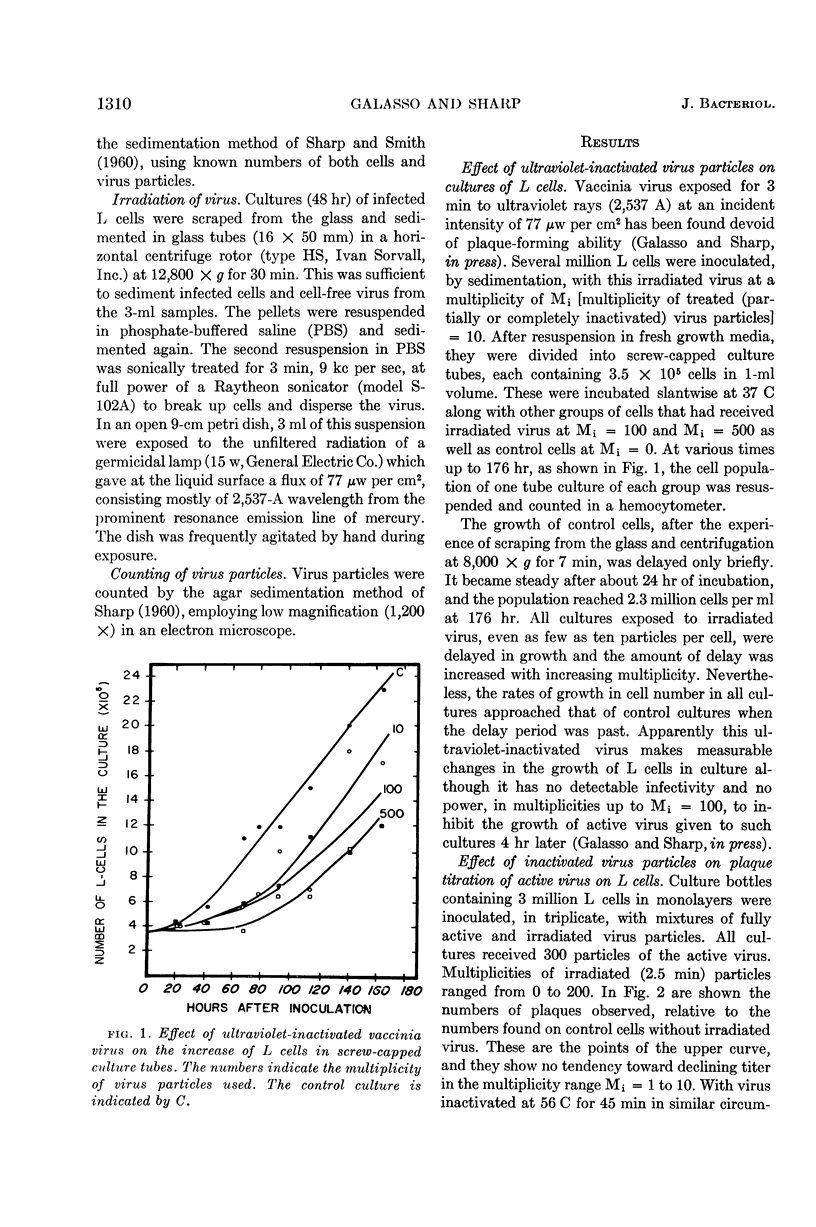
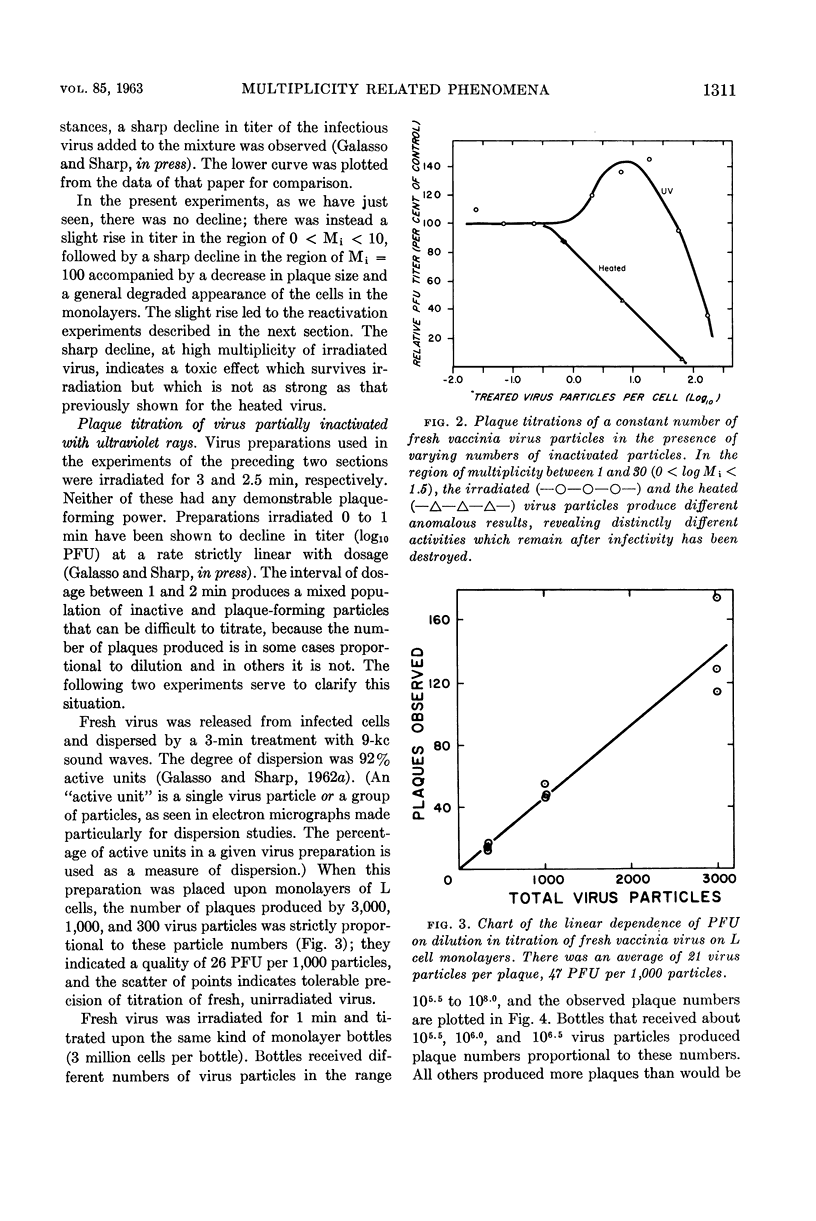
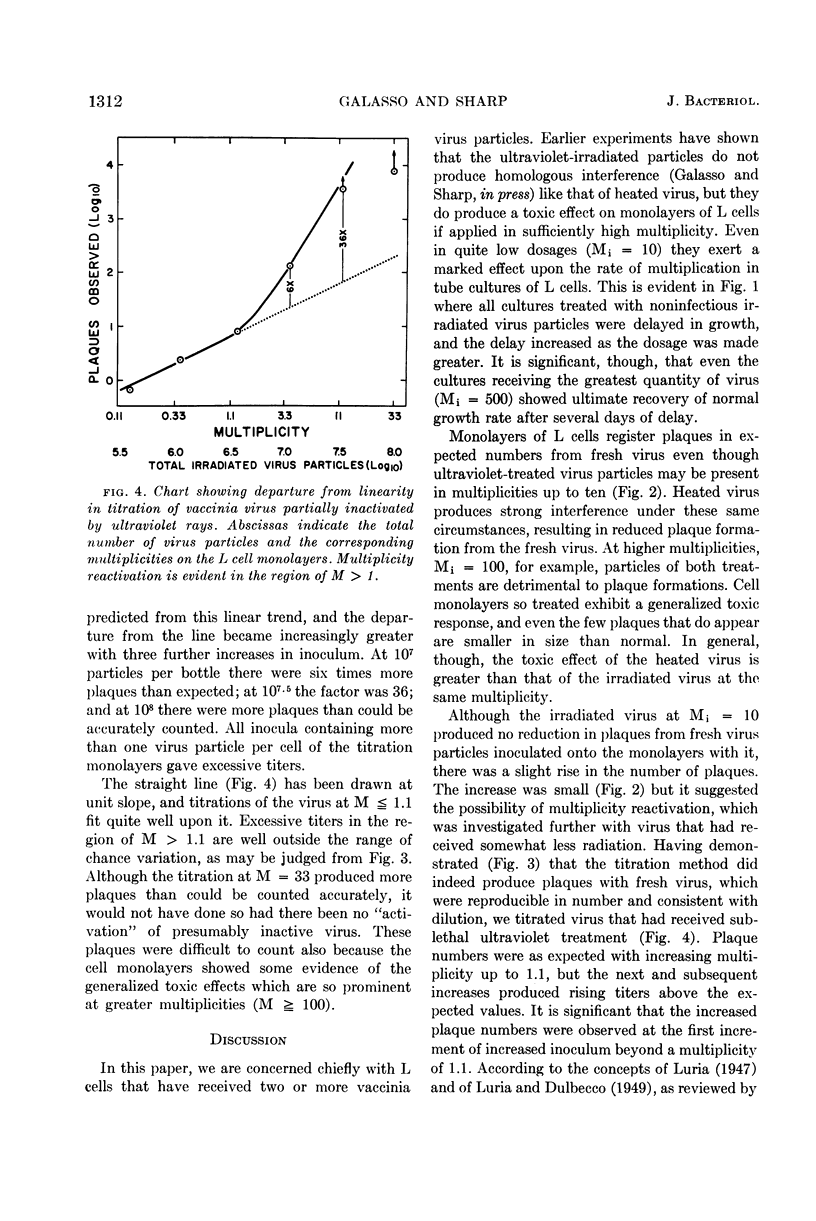
Galasso, G. J. (University of North Carolina, Chapel Hill) and D. G. Sharp. Homologous inhibition, toxicity, and multiplicity reactivation with ultraviolet-irradiated vaccinia virus. J. Bacteriol. 85:1309–1314. 1963.—Vaccinia virus whose plaque-forming capacity had been destroyed by ultraviolet rays (2,537 A) was shown to retard the growth of L cells in tube cultures. At input multiplicities (M) of 0 < M < 10, no interference was detected, but at M ≧ 100 the irradiated virus particles exerted a strong toxic effect on the L cells in monolayer cultures, affecting the plaque formation by active virus which was added. Multiplicity reactivation occurs in sublethally irradiated vaccinia, as shown by virus particle counts via electron microscopy and plaque counts. It is clearly demonstrated in this system because there is no complicating interference. It sets in at a total virus particle multiplicity of about one, even though the multiplicity of the original plaque-forming particles is much below one.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARRY R. D. The multiplication of influenza virus. I. The formation of incomplete virus. Virology. 1961 Aug;14:389–397. doi: 10.1016/0042-6822(61)90329-4. [DOI] [PubMed] [Google Scholar]
- BARRY R. D. The multiplication of influenza virus. II. Multiplicity reactivation of ultraviolet irradiated virus. Virology. 1961 Aug;14:398–405. doi: 10.1016/0042-6822(61)90330-0. [DOI] [PubMed] [Google Scholar]
- DRAKE J. W. Multiplicity reactivation of Newcastle disease virus. J Bacteriol. 1962 Aug;84:352–356. doi: 10.1128/jb.84.2.352-356.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUMBELL K. R., DOWNIE A. W., VALENTINE R. C. The ratio of the number of virus particles to infective titer of cowpox and vaccinia virus suspensions. Virology. 1957 Dec;4(3):467–482. doi: 10.1016/0042-6822(57)90080-6. [DOI] [PubMed] [Google Scholar]
- EASTERBROOK K. B. The multiplication of vaccinia virus in suspended KB cells. Virology. 1961 Dec;15:404–416. doi: 10.1016/0042-6822(61)90108-8. [DOI] [PubMed] [Google Scholar]
- GALASSO G. J., SHARP D. G. Heated vaccinia virus and plaque formation on L-cell monolayers. Virology. 1961 Nov;15:376–378. doi: 10.1016/0042-6822(61)90368-3. [DOI] [PubMed] [Google Scholar]
- GALASSO G. J., SHARP D. G. Inhibitory effect of heated vaccinia virus on growth of vaccinia virus in Earle's L cells. Proc Soc Exp Biol Med. 1961 Aug-Sep;107:957–960. doi: 10.3181/00379727-107-26808. [DOI] [PubMed] [Google Scholar]
- GALASSO G. J., SHARP D. G. Virus particle aggregation and the plaque-forming unit. J Immunol. 1962 Mar;88:339–347. [PubMed] [Google Scholar]
- ISAACS A. Particle counts and infectivity titrations for animal viruses. Adv Virus Res. 1957;4:111–158. doi: 10.1016/s0065-3527(08)60597-7. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K., HOLMES I. H., BRIGGS M. J. The reactivation of poxviruses. III. Properties of reactivable particles. Virology. 1960 May;11:202–218. doi: 10.1016/0042-6822(60)90062-3. [DOI] [PubMed] [Google Scholar]
- Luria S. E., Dulbecco R. Genetic Recombinations Leading to Production of Active Bacteriophage from Ultraviolet Inactivated Bacteriophage Particles. Genetics. 1949 Mar;34(2):93–125. doi: 10.1093/genetics/34.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luria S. E. Reactivation of Irradiated Bacteriophage by Transfer of Self-Reproducing Units. Proc Natl Acad Sci U S A. 1947 Sep;33(9):253–264. doi: 10.1073/pnas.33.9.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OVERMAN J. R., SHARP D. G. Ratios of vaccinia virus particles to virus infectious units; studies of ratio changes during growth and adaptation in eggs, guinea pigs, and rabbits. J Exp Med. 1959 Sep 1;110:461–480. doi: 10.1084/jem.110.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHARP D. G., SMITH K. O. Rapid adsorption of vaccinia virus on tissue culture cells by centrifugal force. Proc Soc Exp Biol Med. 1960 May;104:167–169. doi: 10.3181/00379727-104-25767. [DOI] [PubMed] [Google Scholar]


