Abstract
Pharmacological activation of group II metabotropic glutamate (mGlu2 and mGlu3) receptors inhibits reward-seeking behavior and/or rewarding efficacy induced by drugs (cocaine, nicotine) or natural rewards (food, sucrose). In the present study, we investigated whether elevation of brain N-acetylaspartatylglutamate (NAAG), an endogenous group II mGlu receptor agonist, by the NAAG peptidase inhibitor 2-PMPA attenuates cocaine's rewarding effects, as assessed by intravenous cocaine self-administration and intracranial electrical brain-stimulation reward (BSR) in rats. Systemic administration of 2-PMPA (10, 30, 100 mg/kg, i.p.) or intranasal administration of NAAG (100, 300 μg/10 μl/nostril) significantly inhibited intravenous cocaine self-administration under progressive-ratio (PR), but not under fixed-ratio 2 (FR2), reinforcement conditions. In addition, 2-PMPA (1, 10, 30 mg/kg, i.p) or NAAG (50, 100 μg/10 μl/nostril) significantly inhibited cocaine-enhanced BSR, but not basal BSR. Pretreatment with LY341495 (1 mg/kg, i.p.), a selective mGlu2/3 receptor antagonist, prevented the inhibitory effects produced by 2-PMPA or NAAG in both the self-administration and BSR paradigms. In vivo microdialysis demonstrated that 2-PMPA (10, 30, 100 mg/kg) dose-dependently attenuated cocaine-enhanced extracellular dopamine (DA) in the nucleus accumbens (NAc). 2-PMPA alone inhibited basal NAc DA release, an effect that was prevented by LY341495. These findings suggest that systemic administration of 2-PMPA or intranasal administration of NAAG inhibits cocaine's rewarding efficacy and cocaine-enhanced NAc DA - likely by activation of presynaptic mGlu2/3 receptors in the NAc. These data suggest a potential utility for 2-PMPA or NAAG in the treatment of cocaine addiction.
Keywords: 2-PMPA, NAAG, LY341495, cocaine, DA, mGlu2, mGlu3, self-administration, brain reward
Cocaine-induced enhancement of extracellular dopamine (DA) and glutamate in the nucleus accumbens (NAc) is postulated to play an important role in drug reward and relapse (Wise, 2006; Kalivas, 2004; Stewart, 2008). Since group II metabotropic glutamate (mGlu2/3) receptors presynaptically inhibit DA and glutamate release (Hu et al., 1999; Xi et al., 2002a), it has been suggested that mGlu2/3 receptor agonists may be effective for the treatment of cocaine addiction (Xi et al., 2002a, 2002b). This is supported by recent findings that systemic or intracranial administration of the mGlu2/3 receptor agonist LY379268 significantly inhibits cocaine self-administration, incubation of cocaine craving, and cocaine- or cocaine-associated cue-induced reinstatement of drug-seeking behavior (Baptista et al., 2004; Adewale et al., 2006; Peters and Kalivas, 2006; Lu et al., 2007). In addition, mGlu2/3 receptor agonists, at doses that inhibit cocaine reward or relapse, also inhibit food seeking or incubation of sucrose craving (Peters and Kalivas, 2006; Liechti and Markou, 2007; Uejima et al., 2007). This effect appears to be mediated predominantly by activation of the mGlu2 receptor subtype, because: 1) mGlu2, but not mGlu3, receptor deletion abolishes the pharmacological action of the mGlu2/3 agonists LY379268, LY354740 or LY404039 on brain c-Fos expression and phencyclindine- or amphetamine-induced hyperlocomotion (Linden et al., 2006; Fell et al., 2008; Woolley et al., 2008); 2) the allosteric mGlu2-selective positive modulator LY487379 (EC50 = 0.27 ± 0.01 μM) produces effects similar to the orthosteric mGlu2/3 agonist LY379268 in mouse models predictive of antipsychotic activity (Johnson et al., 2003; Galici et al., 2005); and 3) mGluR2 deletion produces enhanced locomotor, conditioned place preference, and NAc DA response to cocaine (Morishima et al., 2005). However, it remains unclear whether mGlu3 receptors are involved in cocaine's action or in the pharmacological actions of mGlu2/3 receptor agonists.
N-acetylaspartylglutamate (NAAG) is reported to be an endogenous mGluR3 agonist (Wroblewska et al., 1997; Neal et al., 2000). NAAG is co-released with glutamate or other neurotransmitters and prevents excessive neurotransmitter release by activating presynaptic mGlu3 receptors (Neale et al., 2000). NAAG is inactivated by the enzyme NAAG peptidase (also called N-acetylated-α-linked-acidic dipepetidase or glutamate carboxypeptidase II), which hydrolyzes NAAG to N-acetyl-aspartate and glutamate (Tsukamoto et al., 2007). Previous studies have shown that the selective NAAG peptidase inhibitor 2-(phosphonomethyl)pentanedioic acid (2-PMPA) significantly elevates brain NAAG levels (Slusher et al., 1999), and produces significant neuroprotective effects (Neale et al., 2005). In addition, 2-PMPA also significantly inhibits cocaine-induced behavioral sensitization (Shippenberg et al, 2000), cocaine-induced conditioned place preference (Slusher et al, 2001), and cocaine-kindled seizures (Witkin et al, 2002), suggesting possible involvement of the endogenous NAAG-mGlu3 signaling pathway in cocaine addiction. However, no studies have reported the effects of NAAG or 2-PMPA on intravenous cocaine self-administration or cocaine-enhanced electrical brain-stimulation reward (BSR).
Therefore, in the present study, we investigated the effects of systemic administration of 2-PMPA or intranasal administration of NAAG on intravenous cocaine self-administration and cocaine-enhanced BSR in the presence or absence of the selective mGlu2/3 receptor antagonist LY341495. We also observed the effects of 2-PMPA pretreatment on basal and cocaine-enhanced extracellular NAc DA by in vivo microdialysis.
Materials and Methods
Animals
Experimentally naïve male Long-Evans rats (Charles River Laboratories, Raleigh, NC, USA) weighing 250 to 300 g were used. They were housed individually in a climate-controlled room on a reversed light-dark cycle (lights on at 7:00 PM, lights off at 7:00 AM) with free access to food and water. The animal facility was fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. All experimental procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals of the U.S. National Academy of Sciences, and were approved by the Animal Care and Use Committee of the National Institute on Drug Abuse of the U.S. National Institutes of Health.
Experiment 1: Cocaine Self-Administration
Intravenous (i.v.) catheterization surgery and cocaine self-administration procedures were as previously described (Xi et al., 2006b). Briefly, i.v. catheterization was performed under sodium pentobarbital (60 mg/kg, i.p.) anesthesia with aseptic surgical technique. After 7 days of recovery from surgery, animals were placed into standard operant chambers from Med Associates Inc. (Saint Albans, VT, USA) for cocaine self-administration (0.5 mg/kg/infusion) under FR2 reinforcement. To avoid cocaine overdose, each animal was limited to 50 cocaine injections per 3 hr session.
Effects of 2-PMPA on cocaine self-administration under FR2 reinforcement
After stable cocaine-maintained responding was achieved (i.e., less than 10% variability in inter-response interval and less than 10% variability in active lever presses for at least 3 consecutive days), each rat randomly received 1 of 3 doses of 2-PMPA (10, 30, 100 mg/kg, i.p.) or vehicle (saline) 30 min prior to the test session. Animals then received an additional 5-7 days of cocaine self-administration until baseline response rate was re-established prior to testing the next dose of 2-PMPA or vehicle. The order of testing for the various doses of 2-PMPA or vehicle was counterbalanced according to a Latin square design.
Effects of systemic administration of 2-PMPA on cocaine self-administration under PR reinforcement
Additional groups of animals were used to observe the effects of 2-PMPA on PR cocaine self-administration. Initial cocaine self-administration under FR2 reinforcement schedules was identical to that outlined above. After stable cocaine self-administration under FR2 reinforcement was established, the subjects were switched to cocaine self-administration under a PR reinforcement schedule, during which the work requirement (lever presses) needed to receive a single i.v. cocaine infusion was progressively raised within each test session (see details in Richardson and Roberts, 1996; Xi et al., 2006a). The break-point was defined as the maximal work load (i.e., number of lever presses) completed for the last cocaine infusion prior to a 1 hr period during which no infusions were obtained by the animal. Animals were allowed to continue daily cocaine self-administration under PR reinforcement conditions until day-to-day variability in break-point fell within 1-2 ratio increments for 3 consecutive days. Once a stable break-point was established, subjects were assigned to 5 subgroups to determine the effects of 4 different doses of 2-PMPA (0, 10, 30, 100 mg/kg, i.p.) in the absence or presence of LY341495 (1 mg/kg, i.p.) on PR break-point for cocaine self-administration. Due to difficulty in re-achieving stable basal break-point levels after drug tests, we chose a between-subjects design to determine the effects of 2-PMPA on PR break-point.
Effects of intranasal administration of NAAG on cocaine self-administration under PR reinforcement
Additional groups of animals were used to observe the effects of NAAG on PR cocaine self-administration. The protocols for cocaine self-administration under FR2 and PR reinforcement schedules were identical to those outlined above. After stable cocaine self-administration under PR reinforcement was established, the subjects were divided into 4 subgroups to determine the effect of different doses of NAAG (0, 100, 300 μg/10 ml/side, intranasal, 30 min prior to cocaine self-administration testing) on PR break-point for cocaine self-administration in the absence (vehicle) or presence of LY341495 (1 mg/kg, i.p.). Intranasal drug administration was performed under inhalant isoflurane anesthesia using the Fluovac System (Harvard Apparatus, Hollinston, MA, USA). The vehicle or LY341495 was given 15 min prior to intra-nasal NAAG administration. An additional group of rats was used to determine the effects of LY341495 alone (1 mg/kg, i.p., 45 min prior to cocaine self-administration testing) on PR cocaine self-administration in the absence of NAAG.
Experiment 2: Intracranial electrical brain-stimulation reward
The general procedures for electrical BSR were as we have reported previously (Xi et al., 2006; Spiller et al., 2008). Briefly, rats were anesthetized under sodium pentobarbital (65 mg/kg i.p.), and unilateral monopolar stainless-steel stimulating electrodes (Plastics One, Roanoke, VA, USA) were surgically placed into the lateral hypothalamus (AP -2.56, ML ±1.9, and DV -8.6, according to the rat brain stereotaxic atlas of Paxinos and Watson, 1998). After 7 days of recovery from surgery, rats were allowed to self-train (autoshape) to lever-press for rewarding BSR. Following establishment of lever-pressing for BSR, animals were presented with a series of 16 different pulse frequencies, ranging from 141 to 25 Hz in descending order. Throughout the experiment, animals were run for 3 sessions per day. Since lever-pressing behavior was varied during the first session (the “warm up” session), but was stable during the second and third sessions, the data from the first session were discarded, and the data from the second and third sessions were designated as the baseline session data and test session data, respectively.
The BSR threshold (θ0) was defined as the minimum frequency at which the animal responded for rewarding stimulation. Ymax was defined as the maximal rate of response. The BSR threshold (θ0) and Ymax were mathematically derived for each baseline run and each test session run by analyzing each rate-frequency BSR function generated by a given animal over a given descending series of pulse frequencies using best-fit mathematical algorithms as reported previously (Xi et al., 2006; Spiller et al., 2008).
Effects of systemic administration of 2-PMPA on cocaine-enhanced BSR
Once stable baseline θ0 and Ymax values were achieved (<10% variation over 5 continuous days), the effects of cocaine, 2-PMPA and/or LY341495 on BSR were assessed. To determine basal drug effects, animals randomly received one injection of cocaine (1, 2, or 10 mg/kg, i.p.), 2-PMPA (1, 10, or 30 mg/kg, i.p.), LY341495 (1 mg/kg, i.p.), or vehicle (saline) on test day. To determine the effects of 2-PMPA or LY341495 on cocaine's actions, animals randomly received one injection of 2-PMPA (1, 20, or 30 mg/kg, i.p.) and/or LY341495 (1 mg/kg, i.p.) and/or vehicle administered 30 min prior to a cocaine injection (2 mg/kg, i.p.). After each test, animals received an additional 5-7 days of BSR re-stabilization until a new baseline θ0 was established. The order of testing for various doses of drugs was counterbalanced according to a Latin square design. The effect of 2-PMPA on cocaine-enhanced BSR was evaluated by comparing cocaine-induced alterations in θ0 value in the presence or absence of LY341495.
Effects of intranasal administration of NAAG on cocaine-enhanced BSR
To determine whether 2-PMPA's action is mediated by NAAG, we directly observed the effects of intranasal administration of NAAG on basal and cocaine-enhanced BSR. NAAG was given by intranasal administration because systemic administration (i.p.) of NAAG had no effect on any behavioral (locomotion, self-administration, BSR) or neurochemical (NAc DA, glutamate) measurements observed in our pilot preliminary studies, possibly due to its poor bioavailability and/or poor blood-brain barrier penetration capability.
The effects of intranasal administration of NAAG on basal or cocaine-enhanced BSR were assessed in additional groups of rats after stable baseline θ0 and Ymax values were achieved. On test days to determine basal drug effects, animals randomly received one injection of NAAG (50 or 100 μg/10 μl/side, intra-nasal) or vehicle (saline). On test days to determine the effects of NAAG or LY341495 on cocaine's actions, animals randomly received one injection of NAAG (50 or 100 μg/10 μl/side, intra-nasal, 30 min prior to cocaine injection) and LY341495 (1 mg/kg, i.p.) or vehicle administered 15 min prior to a NAAG injection. The effect of NAAG on cocaine-enhanced BSR was evaluated by comparing cocaine-induced alterations in θ0 value in the presence or absence of LY341495.
Effects of intranasal administration of glutamate on cocaine-enhanced BSR
To determine whether NAAG-induced inhibition of cocaine-enhanced BSR is mediated by glutamate contamination of NAAG as recently reported (Chopra et al., 2009; Fricker et al., 2009), we further observed the effects of intranasal administration of the same doses of glutamate (50, 100 mg/10 ml/side) as NAAG on basal and cocaine-enhanced BSR in a separate group (n=11) of rats. The protocol for intranasal glutamate administration was identical to that for intranasal NAAG administration described above.
Experiment 3: In vivo microdialysis
In vivo microdialysis procedures were as reported previously (Xi et al., 2006). Briefly, rats were anesthetized with sodium pentobarbital, and guide cannulae (20 gauge, Plastics One, Roanoke, VA) were surgically implanted into the NAc (AP+1.7 mm, ML±2.0 mm, DV-4.0 mm, 6° from vertical), according to the rat brain atlas of Paxinos and Watson (1998). The guide cannulae were fixed to the skull with 4 stainless steel jeweler screws (Small Parts Inc., Miami Lakes, FL, USA) and dental acrylic. After 7-14 days of recovery from surgery, in vivo microdialysis began. Dialysis probes were inserted into the NAc 12 hr before the onset of microdialysis to minimize damage-induced neurotransmitter release. Microdialysis samples were collected every 20 min into 10 μl 0.5 M perchloric acid to prevent DA degradation. After collection, samples were frozen at -80°C. Dialysate DA was measured using high pressure liquid chromatography (HPLC) with electrochemical detection as reported previously (Xi et al., 2006). DA values were quantified with external DA standard curves (0.1-1.0 nM). The limits of detection for DA were 0.01-10 nM.
After microdialysis experiments were completed, rats were anesthetized with a high dose of pentobarbital (>100mg/kg i.p.) and perfused transcardially with 0.9% saline followed by 10% formalin. Brains were removed and placed in 10% formalin for histological verification of microdialysis probe locations in rat brain.
Drugs
Cocaine HCl (Sigma Chemical Co., Saint Louis, MO, USA) was dissolved in physiological saline. 2-PMPA [2-(phosphonomethyl)pentanedioic acid] was provided by Guilford Pharmaceuticals Inc. (Baltimore, MD, USA). N-acetylaspartatylglutamate (NAAG), glutamate and LY341495 were purchased from Tocris Bioscience (Ellisville, MO, USA). 2-PMPA was dissolved in saline for systemic (i.p.) administration or dissolved in artificial cerebrospinal fluid (aCSF) for intracranial microdialysis. NAAG and 2-PMPA were dissolved in saline for i.p. or intranasal administration.
Data analyses
All data are presented as means (± S.E.M.). One-way analysis of variance (ANOVA) and/or two-way ANOVA for repeated measurements were used to analyze the effects of test drugs on cocaine-induced behavioral and neurochemical changes. Individual group comparisons were carried out using the Student-Newman-Keuls method.
Results
2-PMPA has no effect on cocaine self-administration under FR2 reinforcement
Figure 1A illustrates representative cocaine self-administration patterns before and after 2-PMPA administration. Figure 1B shows the total number of cocaine infusions within a 3-hr session of cocaine self-administration. Pretreatment with 2-PMPA (10, 30, 100 mg/kg, i.p.) did not significantly alter i.v. cocaine self-administration (F3,18=0.92, p=NS).
Figure 1.
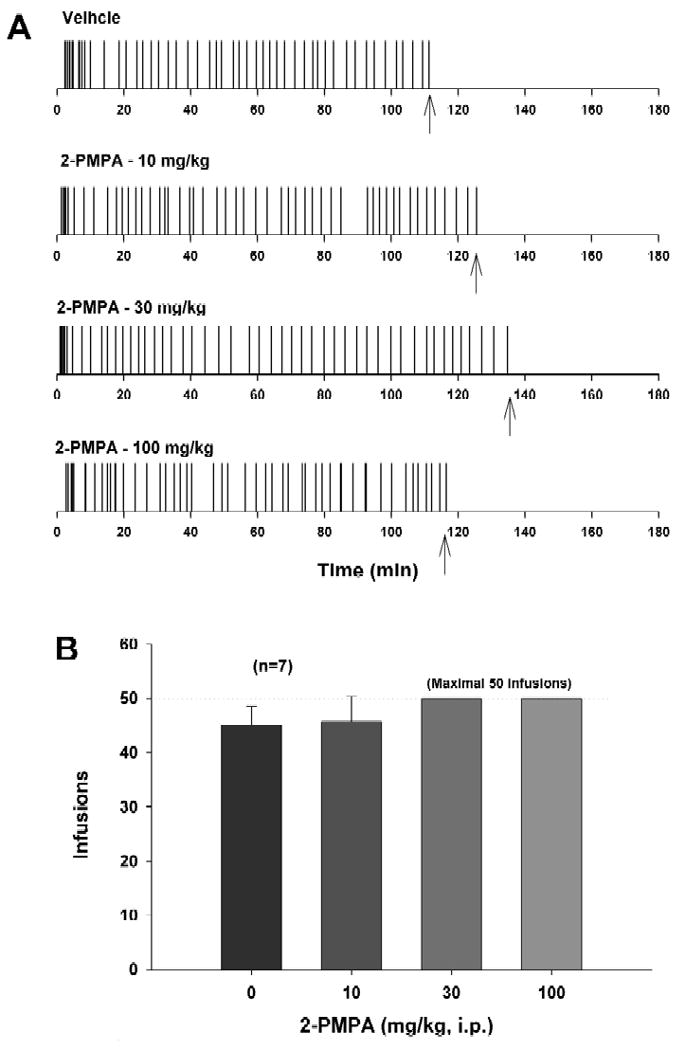
Effects of 2-PMPA on cocaine self-administration under FR2 reinforcement. Panel A shows representative cocaine self-administration records illustrating that systemic administration of 2-PMPA (10, 30, 100 mg/kg, i.p.) failed to alter the pattern of cocaine self-administration. Each vertical line represents a cocaine infusion (0.5 mg/kg/infusion). The arrows (↑) indicate the last cocaine infusion. Panel B shows the total numbers of cocaine infusions during 3 hr session of cocaine self-administration.
2-PMPA inhibits cocaine self-administration under PR reinforcement
Figures 2A and 2B illustrate representative records of cocaine self-administration under PR reinforcement, indicating that 100 mg/kg 2-PMPA significantly lowered the break-point from 145 after vehicle (Fig. 2A) to 25 after 2-PMPA administration (Fig. 2B). Figure 2C illustrates the % changes in PR break-point after each dose of 2-PMPA administration. One-way ANOVA revealed a statistically significant reduction in break-point after 2-PMPA administration (F4,35=9.23, p<0.001). Individual group comparisons revealed a statistically significant reduction in break-point after 10 mg/kg (q=5.05, p<0.01), 30 mg/kg (q=7.79, p<0.001) or 100 mg/kg (q=5.76, p=0.01), when compared with the vehicle treatment group. Pretreatment with the selective mGlu2/3 receptor antagonist LY341495 (1 mg/kg, i.p.) prevented 100 mg/kg 2-PMPA-induced reduction in break-point levels for cocaine self-administration (q= 3.58, p<0.05, compared to 100 mg/kg 2-PMPA treatment group). LY341495 alone (1 mg/kg) had no significant effect on PR cocaine self-administration (data not shown).
Figure 2.
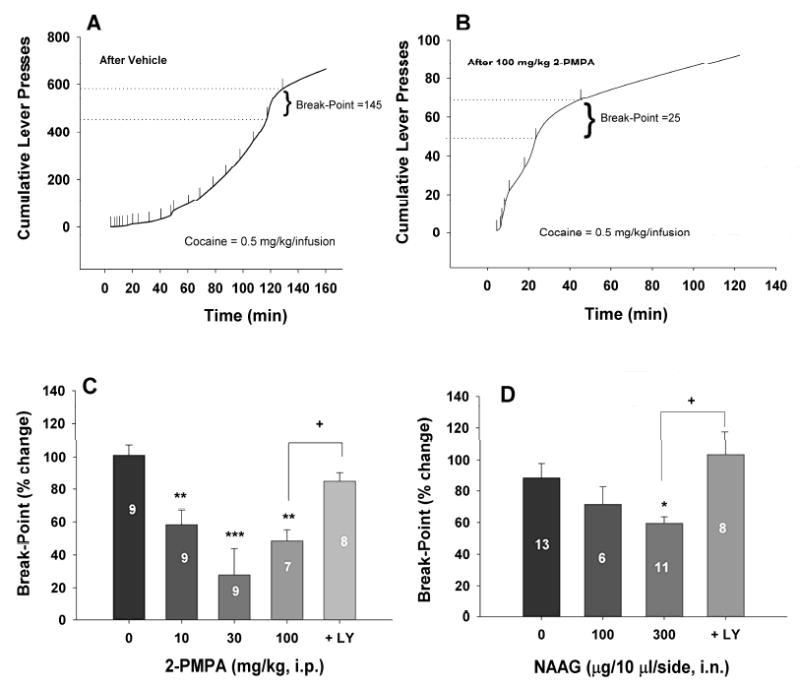
Effects of 2-PMPA or NAAG on cocaine self-administration under PR reinforcement. Panels A and B show representative records of an individual animal illustrating a reduction in the PR break-point for cocaine self-administration from 145 after vehicle to 25 after 100 mg/kg 2-PMPA. Each vertical line indicates a cocaine infusion (0.5 mg/kg/infusion). The break-point was defined as the highest completed work requirement (lever-presses) to receive the last cocaine infusion. Panel C depicts the percent changes in break-point for cocaine self-administration after each dose of 2-PMPA administration. Panel D depicts the percent changes in break-point for cocaine self-administration after each dose of NAAG administration. *p<0.05, **p<0.01, ***p<0.001, compared to the vehicle (0 mg/kg 2-PMPA or NAAG) control group. +p<0.05, compared to the 2-PMPA (100 mg/kg, i.p.) or NAAG (300 μg/side, i.n.) treatment group.
Intranasal NAAG inhibits cocaine self-administration under PR reinforcement
Figure 2D illustrates the % changes in PR break-point after each dose of intranasal NAAG administration. One-way ANOVA revealed a statistically significant reduction in break-point after 2-PMPA administration (F3,34=4.26, p<0.05). Individual group comparisons revealed a statistically significant reduction in break-point after 300 μg/side (q=3.48, p<0.05), but not 100 μg/side (q=1.69, p=NS) intranasal NAAG, when compared with the vehicle treatment group. Pretreatment with LY341495 (1 mg/kg, i.p.) attenuated 300 μg/side NAAG-induced reduction in break-point levels for cocaine self-administration (q=4.70, p<0.05, compared to 300 μg/side NAAG treatment group).
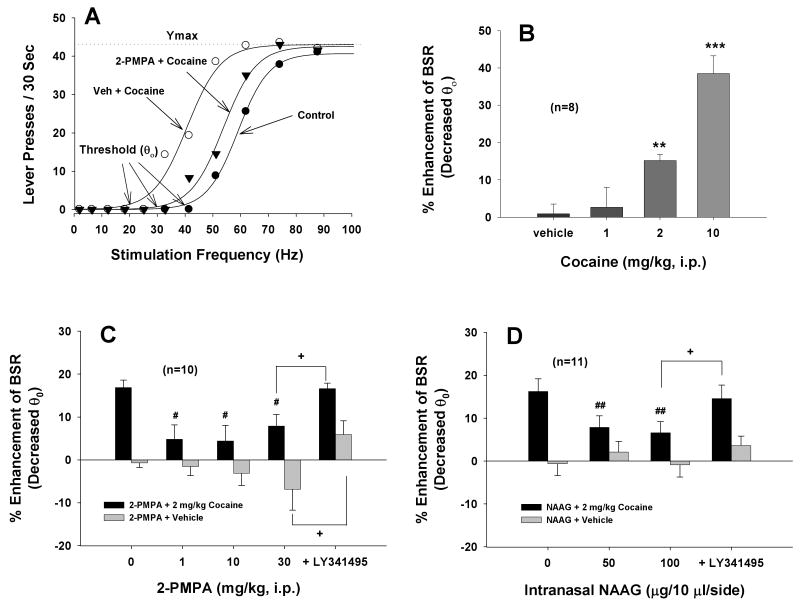
2-PMPA inhibits cocaine enhanced BSR
Figure 3A illustrates representative rate-frequency function curves for BSR, indicating BSR threshold (θ0, Hz) and Ymax (maximal lever presses/30 sec), and representative effects of cocaine and 2-PMPA on BSR. Cocaine, at 2 mg/kg, produced a significant enhancement of BSR, as indicated by the leftward shift in the rate-frequency function curve, reflecting lowered BSR threshold (θ0) values. This cocaine-enhanced BSR was substantially attenuated by 2-PMPA (10 mg/kg, i.p., 30 min prior to cocaine administration). Figure 3B illustrates the mean dose effects of cocaine. One-way ANOVA for repeated measures over cocaine dose revealed a statistically significant treatment main effect (F3,21=19.90, p<0.001). Individual group comparisons revealed significantly enhanced BSR after 2 mg/kg (q=3.25, p<0.05) or 10 mg/kg (q=9.47, p<0.001), but not 1 mg/kg (q=0.013, p=NS) cocaine. Figure 3C shows the mean effects of 2-PMPA pretreatment on basal and cocaine-enhanced BSR. Two-way ANOVA for repeated measures over 2-PMPA dose revealed a statistically significant treatment (cocaine vs. vehicle) main effect (F1,9=34.56, p<0.001) and time (2-PMPA dose) main effect (F4,36=6.37, p<0.001), but no significant treatment × time interaction (F4,36=1.50, p=NS). Individual group comparisons revealed that 2 mg/kg cocaine-enhanced BSR was significantly attenuated after 1 mg/kg (q=3.32, p<0.05), 10 mg/kg (q=3.97, p<0.05) or 30 mg/kg (q=3.98, p<0.05) 2-PMPA. Pretreatment with LY341495 prevented 2-PMPA (30 mg/kg)-induced reduction of cocaine-enhanced BSR (q=5.44, p<0.01, compared to 30 mg/kg 2-PMPA treatment group). In addition, neither cocaine nor 2-PMPA altered Ymax levels, at all doses tested (data not shown).
Figure 3.
Effects of 2-PMPA or NAAG on basal and cocaine-enhanced brain-stimulation reward (BSR). Panel A shows representative rate-frequency function curves for BSR, indicating that cocaine (2 mg/kg, i.p.) shifted the rate-frequency function curve to the left, lowering the BSR threshold θ0 value (i.e., enhancing BSR), without a change in Ymax level. Pretreatment with 2-PMPA (10 mg/kg, i.p.) significantly attenuated the cocaine-enhanced BSR without changing the Ymax level. Panel B shows mean dose effects of cocaine on BSR. Panels C shows mean dose effects of 2-PMPA (1-30 mg/kg, i.p.) on basal and cocaine-enhanced BSR. Panel D shows mean dose effects of intranasal NAAG on basal and cocaine-enhanced BSR. Pretreatment with LY341495 (1 mg/kg, i.p.) blocked the inhibitory effects of 2-PMPA (30 mg/kg, i.p.) or NAAG (100 μg/side, i.n.) on cocaine-enhanced BSR. 2-PMPA or NAAG alone did not alter BSR. *p<0.05, **p<0.01, ***p<0.001, compared with the vehicle control group (Panel B). #p<0.05, ##p<0.01, compared to the cocaine (0 mg/kg 2-PMPA or NAAG) control group (Panels C and D). +p<0.05, compared to the 2-PMPA (30 mg/kg, i.p.) or NAAG (100 μg/side, i.n.) treatment group.
Intranasal NAAG inhibits cocaine enhanced BSR
Fig. 3D shows the effects of intranasal administration of NAAG on basal and cocaine-enhanced BSR. Two-way ANOVA for repeated measures revealed a statistically significant treatment (cocaine vs. vehicle) main effect (F1,10=20.74, p<0.001), and time main effect (F3,30=3.60, p<0.05) and treatment × time interaction (F3,30=3.19, p<0.05). Individual group comparisons indicate that 2 mg/kg cocaine-enhanced BSR was significantly attenuated after 50 μg/side (q=4.37, p<0.01) or 100 μg/side (q=5.04, p<0.01) intranasal NAAG administration. Pretreatment with LY341495 (1 mg/kg, i.p.) blocked NAAG (100 μg/side)-induced reduction of cocaine-enhanced BSR (q=4.04, p<0.05, compared to 100 μg/side NAAG treatment group). Neither the NAAG alone nor the combination of NAAG and cocaine altered Ymax levels, at all doses tested (data not shown).
Intranasal glutamate fails to alter cocaine-enhanced BSR
It has been suggested that the in vitro electrophysiological effects of NAAG on Xenopus oocytes or rat hippocampal neurons expressing rat or human mGlu2 or mGlu3 receptors may be mediated by glutamate contamination of NAAG rather than by NAAG itself (Chopra et al., 2009; Fricker et al., 2009). To test this hypothesis, we compared the effects of intranasal administration of the same doses of NAAG (Fig. 3D) and glutamate on basal and cocaine-enhanced BSR. Figure 4 shows that intranasal microinjections of glutamate (50-100 mg/10 ml/side) altered neither basal BSR nor cocaine-enhanced BSR. Two-way ANOVA for repeated measures on the data shown in Fig. 4 revealed a statistically significant difference only in the cocaine vs. vehicle treatment main effect (F1,15=44.65, p<0.001), but not in the glutamate vs. vehicle treatment main effect (F2,30=0.59, p=NS) nor in the cocaine × glutamate interaction (F2,30=0.24, p=NS).
Figure 4.
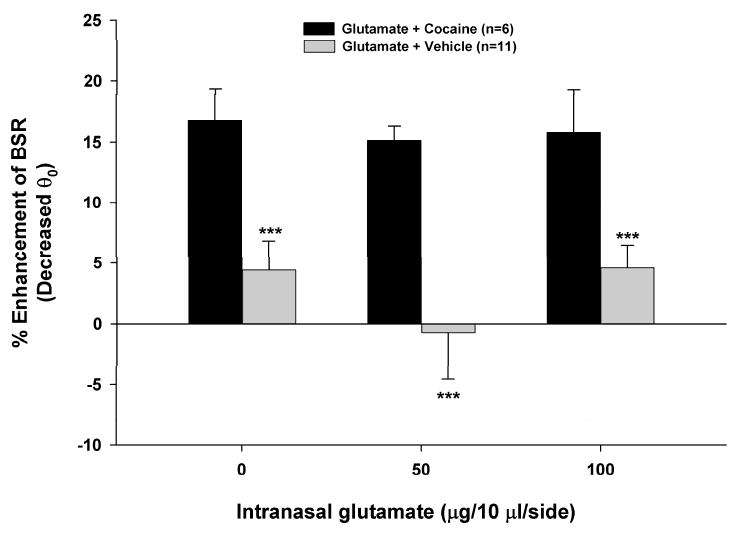
Effects of intranasal administration of glutamate on basal orcocaine-enhanced BSR. Systemic administration of cocaine (2 mg/kg, i.p.) produced a significant enhancement in BSR. Intranasal administration of glutamate altered neither basal nor cocaine-enhanced BSR. ***p<0.001, compared to vehicle.
2-PMPA inhibits basal and cocaine-enhanced NAc DA
Figure 5A illustrates that systemic administration of 2-PMPA (10, 30, 100 mg/kg) produced a dose-dependent reduction in extracellular DA in the NAc. Two-way ANOVA for repeated measurements over time revealed a statistically significant 2-PMPA treatment main effect (F4,25=6.77, p<0.001) and a significant treatment × time interaction (F72,447=1.58, p<0.01), but no time main effect (F18,72=1.56, p=NS). Individual overall group comparisons revealed a significant reduction in NAc DA after 30 mg/kg (q=3.88, p<0.05) or 100 mg/kg (q=4.81, p<0.01), but not 10 mg/kg (q=0.88, p=NS) 2-PMPA. Pretreatment with LY341495 (1 mg/kg) blocked the 2-PMPA (100 mg/kg)-induced reduction in NAc DA (q=4.93, p<0.01, compared to 100 mg/kg 2-PMPA treatment group).
Figure 5.
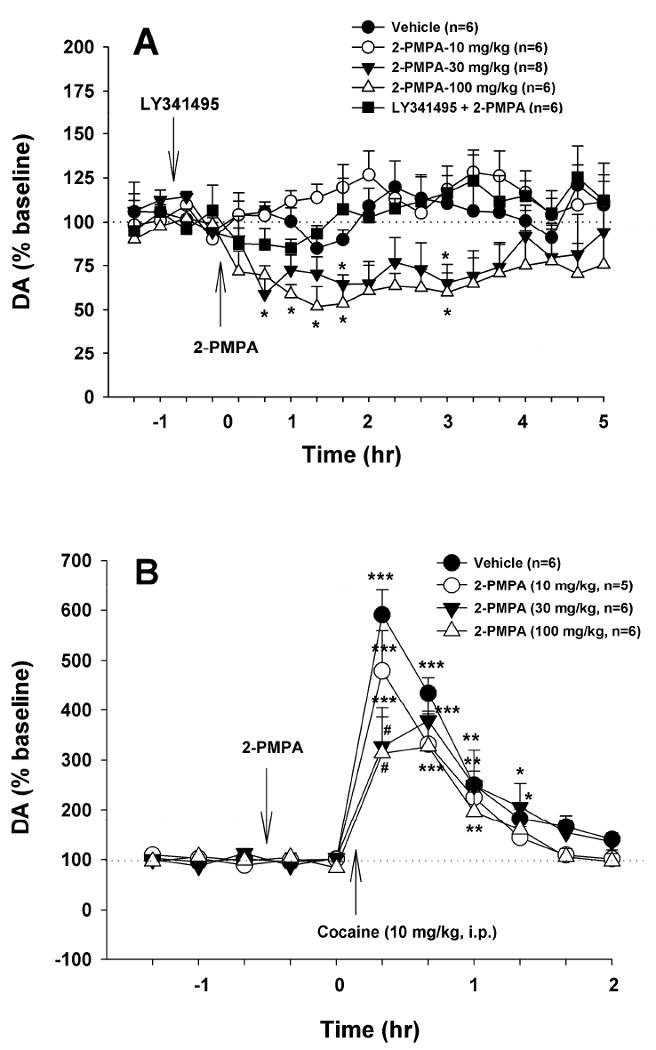
Effects of 2-PMPA on basal and cocaine-enhanced extracellular DA in the NAc. Systemic administration of 2-PMPA (10, 30, 100 mg/kg, i.p.) alone dose-dependently lowered extracellular DA (Panel A). The effect produced by 100 mg/kg 2-PMPA was blocked by LY341495 (1 mg/kg, i.p.). Pretreatment with 2-PMPA (10, 30, 100 mg/kg, i.p., 40 min prior to cocaine) dose-dependently attenuated cocaine-enhanced NAc DA (Panel B).*p<0.05, **p<0.01, compared to baseline (before cocaine) in each treatment group. #p<0.05, compared to the vehicle treatment group at the same time point (i.e., 20 min after cocaine injection).
Figure 5B illustrates that pretreatment with 2-PMPA (10, 30, 100 mg/kg, i.p., 40 min prior to cocaine) dose-dependently inhibited cocaine-enhanced extracellular DA in the NAc. Two-way ANOVA with repeated measures over time did not reveal a statistically significant 2-PMPA treatment main effect (F3,19=1.51, p=NS), but revealed a significant time main effect (F7,133=73.64, p<0.001) and treatment × time interaction (F21,133=2.65, p<0.001). Individual group comparisons revealed a significant reduction in cocaine-enhanced NAc DA at 20 min (i.e., the first sample after cocaine) after 30 mg/kg (q=7.27, p<0.001) or 100 mg/kg (q=7.63, p<0.001) 2-PMPA, but not at any other time point or dose.
Histology
Figure 6 depicts microdialysis probe locations, demonstrating that the microdialysis probe active membranes were located within the NAc core and shell (left panels). There were no apparent differences in microdialysis probe placement across the different experimental groups.
Figure 6.
Schematic reconstructions of positions of intracranial microdialysis probes in rat brain, indicating that active microdialysis membranes tended to span the length of the core and shell compartments of the NAc.
Discussion
The major findings of the present study include: 1) systemic administration of 2-PMPA or intranasal administration of NAAG inhibited cocaine self-administration under PR (but not FR2) reinforcement and cocaine-enhanced BSR. Neither 2-PMPA nor NAAG alone altered BSR itself; 2) pretreatment with LY341495, a selective mGlu2/3 receptor antagonist, attenuated the action of 2-PMPA or NAAG on cocaine self-administration and cocaine-enhanced BSR; 3) 2-PMPA produced a dose-dependent reduction in both basal and cocaine-enhanced extracellular DA in the NAc; and 4) LY341495 pretreatment blocked the 2-PMPA-induced reduction in NAc DA. These data suggest that NAAG or the NAAG peptidase inhibitor 2-PMPA attenuates cocaine's rewarding efficacy, likely by attenuating cocaine-induced increases in NAc DA via presynaptic group II mGlu receptors.
Intravenous drug self-administration is one of the most commonly used animal models to study drug reward and relapse (O'Brien and Gardner, 2005). In the present study, we found that systemic administration of 2-PMPA or intranasal administration of NAAG significantly inhibited cocaine self-administration under PR, but not FR2, reinforcement. There are several possibilities for the ineffectiveness of 2-PMPA on FR cocaine self-administration. First, the FR2 reinforcement schedule demands less work and provides a much higher cumulative cocaine dose payoff than does the PR reinforcement schedule. Thus, the stronger rewarding effects produced by the higher cumulative cocaine dose may overcome 2-PMPA's antagonism of cocaine's effect. Second, animals may compensate for 2-PMPA's action by increasing their drug intake or their self-administration rate under FR2 conditions. We consider this possibility to be unlikely, as we did not observe such a compensatory increase in cocaine intake (Fig. 1A). Third, it is possible that PR cocaine self-administration is more sensitive to changes in reinforcement efficacy than FR reinforcement. This is congruent with the view that FR reinforcement measures the fact of drug reinforcement, while PR reinforcement measures the degree of reinforcing efficacy (Arnold and Roberts, 1997; Wise and Gardner, 2004). The present finding that 2-PMPA or NAAG inhibited cocaine self-administration under PR but not FR reinforcement again contrasts with previous studies demonstrating that the mGlu2/3 receptor agonist LY379268 inhibits self-administration of cocaine, nicotine or alcohol under either FR (FR1-FR5) reinforcement in rats (Baptista et al., 2004; Bäckström and Hyytiä, 2005; Liechti et al., 2007) or under second-order reinforcement in non-human primates (Adewale et al., 2006).
Electrical BSR is a reliable and sensitive animal model for assessing the reward-relevant properties of addictive drugs (Wise and Gardner, 2004). Here, we found that cocaine significantly decreased BSR thresholds, indicative of summation between the reward induced by the electrical brain stimulation and that produced by cocaine. Strikingly, this cocaine-enhanced BSR was significantly attenuated by 2-PMPA or NAAG. In contrast, 2-PMPA or NAAG itself produced neither reward-like nor aversive-like effects (Figs. 3C, 3D). This contrasts with a previous study demonstrating that the mGlu2/3 agonist LY379268 significantly inhibits BSR itself (Liechti and Markou, 2007).
We note that higher doses of 2-PMPA (10-100 mg/kg) are required to inhibit PR cocaine self-administration than those (1-30 mg/kg) required to inhibit cocaine-enhanced BSR. This could be related to the fact that the cumulative doses (0.5 mg/kg/infusion × 10-20 infusions = 5-10 mg/kg) of cocaine under PR cocaine self-administration conditions were higher than the single dose of cocaine (2 mg/kg) used in the BSR study. In addition, sensitization to the reinforcing effects of cocaine observed under PR reinforcement (Morgan and Roberts, 2004; Morgan et al., 2006) may also overcome the antagonism produced by 2-PMPA. Also, we note that systemic administration of 2-PMPA did not produce a typical pharmacological dose-response relationship. That is, higher doses of 2-PMPA appeared to be less effective than lower doses in attenuating the actions of cocaine in both the self-administration and BSR paradigms. The mechanisms for the loss of the pharmacological action at high doses are unclear. First, it is likely that a high dose of 2-PMPA may produce non-selective binding to other functional proteins. Slushner et al (1999) reported that 2-PMPA was inactive in over 100 different receptor, transporter, ion channel and enzyme assays, but only tested 2-PMPA at 10 μM. Second, a high level of brain NAAG may act on other receptor signaling systems, which action may attenuate the pharmacological action of NAAG on mGlu2/3 receptors.
NAAG was given intranasally, not by intraperitoneally (i.p.), in the present study because i.p. injections of NAAG had no effect in our pilot preliminary study. This could be related to the compound's poor bioavailability or difficulty in penetrating blood-brain barrier. Recent research indicates that a wide variety of therapeutic compounds such as peptides and proteins can be delivered intranasally (Illum, 2002, 2003; Costantino et al., 2007). This route offers numerous benefits for drug delivery, including a large surface area for delivery, rapid drug onset, no first-pass metabolism, and direct drug delivery into the CNS. Three pathways may underlie drug delivery from nose to brain: 1) the transcellular pathway, i.e., the drug is transferred by receptor-mediated endocytosis or fluid phase endocytosis; 2) the paracellular pathway, i.e., through the tight junction or open clefts in the membrane; and 3) intracellular axonal transport from olfactory nerve terminals to the olfactory bulb (Illum, 2003). The present finding that intranasal NAAG significantly inhibits cocaine's rewarding efficacy suggests that the olfactory tubercle and/or other forebrain structures nearby are the possible loci of the action produced by NAAG or 2-PMPA.
Mesolimbic DA has long been thought to play a critical role in mediating drug reward (Wise, 2006). Although there is controversy as to whether BSR itself stimulates NAc DA release (Fiorino et al., 1993; Nakahara et al., 1992; Philips et al., 1989; Hernández and Shizgal, 2009; but see Miliaressis et al., 1991; Kruk et al., 1998), systemic administration of cocaine significantly elevates extracellular NAc DA by blocking DA reuptake (Giros et al., 1996; Carboni et al., 2001) and increasing DA release (Venton et al., 2006; Aragona et al., 2008). In the present study, we found that 2-PMPA itself produced a dose-dependent reduction in extracellular DA, while pretreatment with 2-PMPA also dose-dependently inhibited cocaine-enhanced NAc DA, suggesting that a DA-dependent mechanism may underlie 2-PMPA's action. Thus, a straightforward explanation for the present findings is that 2-PMPA elevates brain NAAG levels, which subsequently activates presynaptic mGlu2/3 receptors, attenuating cocaine-enhanced NAc DA and cocaine reinforced self-administration and BSR. This is supported by the finding that systemic administration of 2-PMPA selectively elevates brain NAAG levels (Slusher et al., 1999; Nagel et al., 2006). An early study indicates that NAAG is a selective mGlu3 agonist as assessed in HEK293 cells expressing mGlu3/mGlu1a chimeric receptors (EC50 = 65 ± 20 μM) (Wroblewska et al., 1997). In contrast, at 1 mM, NAAG has no effect on intracellular Ca++ levels or phosphoinositol hydrolysis in HEK 293 or CHO cells expressing mGlu1a, mGluR2, mGlu2/mGlu1a, mGlu4, mGlu5 or mGlu6 receptors (Wroblewska et al., 1997). However, additional studies suggest that NAAG may also bind to mGlu2 receptors with similar affinity in CHO cells expressing rat mGlu2 receptors [Ki = 134 ± 55 μM in inhibition of [3H]-DCG IV binding or EC50 = 68 ± 0.3 μM in stimulation of GTPγ35S binding] (Cartmell et al., 1998). These data suggest that NAAG may be an endogenous mGlu2/3 receptor agonist with relatively higher selectivity on mGlu3 over mGlu2 receptors, in contrast to the mGlu2/3 agonist LY379268 that has relatively higher selectivity for mGlu2 (EC50=2.69 nM) over mGlu3 (EC50=4.48 nM) receptors (Collado et al., 2002). This is further supported by the finding that pretreatment with the group II mGlu receptor antagonist LY341495 significantly blocked 2-PMPA- or NAAG-induced inhibition of cocaine self-administration, cocaine-enhanced BSR or NAc DA release. Here, we used the group II-selective mGlu receptor antagonist LY341495, because it has relatively higher selectivity on mGlu3 over mGlu2 receptors with the Ki/IC50 values for human mGlu2 (21 nM), mGu3 (14 nM), mGlu8 (173 nM), mGlu7a (990 nM), mGlu1a (7800 nM), mGluR5a (8200 nM) and mGlu4a (22000 nM) (Kingston et al., 1998), and that highly selective mGlu3 receptor antagonists are currently either unavailable or problematic. The NAAG analog β-NAAG has been reported to be a selective mGluR3 antagonist, preventing NAAG-induced long-term depression in hippocampus or NAAG-induced reduction of cGMP formation (Lea et al., 2001; Poschel et al., 2005; Wroblewska et al., 2006). However, β-NAAG (Ki = 0.70 μM) is a substantially less potent competitive NAAG peptidase inhibitor (Serval et al., 1990), compared to 2-PMPA (Ki = 0.2 nM) (Jackson et al., 1996). Unpublished data from our laboratory show that microinjection of β-NAAG into the NAc produced an inhibitory effect on cocaine-induced relapse similar to that of 2-PMPA. This is consistent with a previous report that both NAAG and β-NAAG produce similar neuroprotective effects against injury induced by either NMDA or hypoxia in primary spinal cord cultures (Yourick et al., 2003). In addition, we note recent reports that the electrophysiological actions of NAAG may be mediated by glutamate in unpurified NAAG, suggesting that NAAG may be not a selective mGluR3 agonist (Fricker et al., 2009; Chopra et al., 2009). However, this view is not supported by our findings in vivo that intranasal administration of the same doses of NAAG (Fig. 3D), but not glutamate (Fig. 4), inhibited cocaine-enhanced BSR in rats.
Compared to mGlu2/3 receptor agonists (such as LY379268), endogenous neuropeptide NAAG or the NAAG peptidase inhibitor 2-PMPA have several unique pharmacological properties: 1) LY379268 inhibits basal locomotion and amphetamine-induced hyperactivity (Bäckström and Hyytiä, 2005; Galici et al., 2005), while 2-PMPA, at very high doses in rats (500 mg/kg, i.v.), caused no overt behavioral changes such as sedation, stereotypy, ataxia or tremor (Slushner et al., 1999); 2) LY379268, at the doses that inhibit cocaine reward or relapse, inhibits natural (food/sucrose) reward or reward-seeking behavior (Peters and Kalivas, 2006; Liechti et al., 2007; Lu et al., 2007; Uejima et al., 2007), while 2-PMPA has no effect on food-induced CPP (Slushner et al., 2001); 3) LY379268 inhibits BSR itself, suggesting aversive-like effects (Liechti and Markou, 2007), while 2-PMPA or NAAG does not (present study); 4) the mGlu2/3 receptor agonist LY354740 significantly impairs learning and memory functions (Spinelli et al., 2005), while 2-PMPA does not (Slushner et al., 1999). In addition, repeated administration of LY379267 produces rapid tolerance in attenuation of locomotion, amphetamine- or phencyclidine-induced hyperactivity, or nicotine self-administration (Cartmell et al., 2000; Galici et al., 2005; Liechti et al., 2007). These data suggest that NAAG or 2-PMPA may be superior to the mGlu2/3 agonist LY379269 as potential medications for the treatment of cocaine addiction. The reasons for such differences are unclear. They could relate to their potency, relative selectivity for mGlu2 or mGlu3 receptors, blood-brain barrier penetration ability or other pharmacokinetic properties. More studies are required to address these issues.
In conclusion, the present study demonstrates that NAAG or 2-PMPA produces a significant inhibitory effect on cocaine's rewarding efficacy as assessed by cocaine self-administration and electrical BSR. The underlying mechanisms may relate to an increase in brain NAAG level, which subsequently activates presynaptic mGlu2/3 receptors, producing a reduction in cocaine-enhanced NAc DA and cocaine reward. The present findings support the potential use of 2-PMPA or other NAAG peptidase inhibitors in the treatment of cocaine addiction.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health, Department of Health and Human Services.
Footnotes
Disclosure/Conflict of Interest: All authors hereby declare that, except for income received from their respective primary employers, no financial support or compensation has been received from any individual or corporate entity over the past three years for research or professional services. There are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adewale AS, Platt DM, Spealman RD. Pharmacological stimulation of group II metabotropic glutamate receptors reduces cocaine self-administration and cocaine-induced reinstatement of drug seeking in squirrel monkeys. J Pharmacol Exp Ther. 2006;318:922–931. doi: 10.1124/jpet.106.105387. [DOI] [PubMed] [Google Scholar]
- Aragona BJ, Cleaveland NA, Stuber GD, Day JJ, Carelli RM, Wightman RM. Preferential enhancement of dopamine transmission within the nucleus accumbens shell by cocaine is attributable to a direct increase in phasic dopamine release events. J Neurosci. 2008;28:8821–8831. doi: 10.1523/JNEUROSCI.2225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold JM, Roberts DCS. A critique of fixed ratio and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacol Biochem Behav. 1997;57:441–447. doi: 10.1016/s0091-3057(96)00445-5. [DOI] [PubMed] [Google Scholar]
- Bäckström P, Hyytiä P. Suppression of alcohol self-administration and cue-induced reinstatement of alcohol seeking by the mGlu2/3 receptor agonist LY379268 and the mGlu8 receptor agonist (S)-3,4-DCPG. Eur J Pharmacol. 2005;528:110–118. doi: 10.1016/j.ejphar.2005.10.051. [DOI] [PubMed] [Google Scholar]
- Baptista MA, Martin-Fardon R, Weiss F. Preferential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on conditioned reinstatement versus primary reinforcement: comparison between cocaine and a potent conventional reinforcer. J Neurosci. 2004;24:4723–4727. doi: 10.1523/JNEUROSCI.0176-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carboni E, Spielewoy C, Vacca C, Nosten-Bertrand M, Giros B, Di Chiara G. Cocaine and amphetamine increase extracellular dopamine in the nucleus accumbens of mice lacking the dopamine transporter gene. J Neurosci. 2001;21:RC141, 1–4. doi: 10.1523/JNEUROSCI.21-09-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartmell J, Monn JA, Schoepp DD. Tolerance to the motor impairment, but not to the reversal of PCP-induced motor activities by oral administration of the mGlu2/3 receptor agonist, LY379268. Naunyn Schmiedebergs Arch Pharmacol. 2000;361:39–46. doi: 10.1007/s002109900151. [DOI] [PubMed] [Google Scholar]
- Chopra M, Yao Y, Blake TJ, Hampson DR, Johnson EC. The neuroactive peptide N-acetylaspartylglutamate (NAAG) is not an agonist at the mGluR3 subtype of metabotropic glutamate receptor. J Pharmacol Exp Ther. 2009 doi: 10.1124/jpet.109.152553. Epub ahead of print, 23 Apr 09. [DOI] [PubMed] [Google Scholar]
- Collado I, Pedregal C, Mazón A, Espinosa JF, Blanco-Urgoiti J, Schoepp DD, Wright RA, Johnson BG, Kingston AE. (2S,1′S,2′S,3′R)-2-(2′-carboxy-3′-methylcyclopropyl) glycine is a potent and selective metabotropic group 2 receptor agonist with anxiolytic properties. J Med Chem. 2002;45:3619–3629. doi: 10.1021/jm0110486. [DOI] [PubMed] [Google Scholar]
- Costantino HR, Illum L, Brandt G, Johnson PH, Quay SC. Intranasal delivery: physicochemical and therapeutic aspects. Int J Pharm. 2007;337:1–24. doi: 10.1016/j.ijpharm.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Coyle JT. The nagging question of the function of N-acetylaspartylglutamate. Neurobiol Dis. 1997;4:231–238. doi: 10.1006/nbdi.1997.0153. [DOI] [PubMed] [Google Scholar]
- Fell MJ, Svensson KA, Johnson BG, Schoepp DD. Evidence for the role of metabotropic glutamate (mGlu)2 not mGlu3 receptors in the preclinical antipsychotic pharmacology of the mGlu2/3 receptor agonist (-)-(1R,4S,5S,6S)-4-amino-2-sulfonylbicyclo[3.1.0]hexane-4,6-dicarboxylic acid ( LY404039) J Pharmacol Exp Ther. 2008;326:209–217. doi: 10.1124/jpet.108.136861. [DOI] [PubMed] [Google Scholar]
- Fiorino DF, Coury A, Fibiger HC, Phillips AG. Electrical stimulation of reward sites in the ventral tegmental area increases dopamine transmission in the nucleus accumbens of the rat. Behav Brain Res. 1993;55:131–141. doi: 10.1016/0166-4328(93)90109-4. [DOI] [PubMed] [Google Scholar]
- Fricker AC, Selina Mok MH, de la Flor R, Shah AJ, Woolley M, Dawson LA, Kew JN. Effects of N-acetylaspartylglutamate (NAAG) at group II mGluRs and NMDAR. Neuropharmacology. 2009 doi: 10.1016/j.neuropharm.2009.03.002. Epub ahead of print 12 Mar 09. [DOI] [PubMed] [Google Scholar]
- Fuhrman S, Palkovits M, Cassidy M, Neale JH. The regional distribution of N-acetylaspartylglutamate (NAAG) and peptidase activity against NAAG in the rat nervous system. J Neurochem. 1994;62:275–281. doi: 10.1046/j.1471-4159.1994.62010275.x. [DOI] [PubMed] [Google Scholar]
- Galici R, Echemendia NG, Rodriguez AL, Conn PJ. A selective allosteric potentiator of metabotropic glutamate (mGlu) 2 receptors has effects similar to an orthosteric mGlu2/3 receptor agonist in mouse models predictive of antipsychotic activity. J Pharmacol Exp Ther. 2005;315:1181–1187. doi: 10.1124/jpet.105.091074. [DOI] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Hernández G, Shizgal P. Dynamic changes in dopamine tone during self-stimulation of the ventral tegmental area in rats. Behav Brain Res. 2009;198:91–97. doi: 10.1016/j.bbr.2008.10.017. [DOI] [PubMed] [Google Scholar]
- Hu G, Duffy P, Swanson C, Ghasemzadeh MB, Kalivas PW. The regulation of dopamine transmission by metabotropic glutamate receptors. J Pharmacol Exp Ther. 1999;289:412–416. [PubMed] [Google Scholar]
- Illum L. Nasal drug delivery: new developments and strategies. Drug Discov Today. 2002;7:1184–1189. doi: 10.1016/s1359-6446(02)02529-1. [DOI] [PubMed] [Google Scholar]
- Illum L. Nasal drug delivery--possibilities, problems and solutions. J Control Release. 2003;87:187–198. doi: 10.1016/s0168-3659(02)00363-2. [DOI] [PubMed] [Google Scholar]
- Jackson PF, Cole DC, Slusher BS, Stetz SL, Ross LE, Donzanti BA, Trainor DA. Design, synthesis, and biological activity of a potent inhibitor of the neuropeptidase N-acetylated-α-linked acidic dipeptidase. J Med Chem. 1996;39:619–622. doi: 10.1021/jm950801q. [DOI] [PubMed] [Google Scholar]
- Johnson MP, Baez M, Jagdmann GE, Jr, Britton TC, Large TH, Callagaro DO, Tizzano JP, Monn JA, Schoepp DD. Discovery of allosteric potentiators for the metabotropic glutamate 2 receptor: synthesis and subtype selectivity of N-(4-(2-methoxyphenoxy)phenyl)-N-(2,2,2-trifluoroethylsulfonyl)pyrid-3-ylmethylamine. J Med Chem. 2003;46:3189–3192. doi: 10.1021/jm034015u. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Glutamate systems in cocaine addiction. Curr Opin Pharmacol. 2004;4:23–29. doi: 10.1016/j.coph.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Kingston AE, Ornstein PL, Wright RA, Johnson BG, Mayne NG, Burnett JP, Belagaje R, Wu S, Schoepp DD. LY341495 is a nanomolar potent and selective antagonist of group II metabotropic glutamate receptors. Neuropharmacology. 1998;37:1–12. doi: 10.1016/s0028-3908(97)00191-3. [DOI] [PubMed] [Google Scholar]
- Kruk ZL, Cheeta S, Milla J, Muscat R, Williams JE, Willner P. Real time measurement of stimulated dopamine release in the conscious rat using fast cyclic voltammetry: dopamine release is not observed during intracranial self stimulation. J Neurosci Methods. 1998;79:9–19. doi: 10.1016/s0165-0270(97)00156-8. [DOI] [PubMed] [Google Scholar]
- Lea PM, 4th, Wroblewska B, Sarvey JM, Neale JH. β-NAAG rescues LTP from blockade by NAAG in rat dentate gyrus via the type 3 metabotropic glutamate receptor. J Neurophysiol. 2001;85:1097–1106. doi: 10.1152/jn.2001.85.3.1097. [DOI] [PubMed] [Google Scholar]
- Liechti ME, Lhuillier L, Kaupmann K, Markou A. Metabotropic glutamate 2/3 receptors in the ventral tegmental area and the nucleus accumbens shell are involved in behaviors relating to nicotine dependence. J Neurosci. 2007;27:9077–9085. doi: 10.1523/JNEUROSCI.1766-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechti ME, Markou A. Metabotropic glutamate 2/3 receptor activation induced reward deficits but did not aggravate brain reward deficits associated with spontaneous nicotine withdrawal in rats. Biochem Pharmacol. 2007;74:1299–1307. doi: 10.1016/j.bcp.2007.05.020. [DOI] [PubMed] [Google Scholar]
- Linden AM, Baez M, Bergeron M, Schoepp DD. Effects of mGlu2 or mGlu3 receptor deletions on mGlu2/3 receptor agonist ( LY354740)-induced brain c-Fos expression: specific roles for mGlu2 in the amygdala and subcortical nuclei, and mGlu3 in the hippocampus. Neuropharmacology. 2006;51:213–228. doi: 10.1016/j.neuropharm.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Lu L, Uejima JL, Gray SM, Bossert JM, Shaham Y. Systemic and central amygdala injections of the mGluR2/3 agonist LY379268 attenuate the expression of incubation of cocaine craving. Biol Psychiatry. 2007;61:591–598. doi: 10.1016/j.biopsych.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Miliaressis E, Emond C, Merali Z. Re-evaluation of the role of dopamine in intracranial self-stimulation using in vivo microdialysis. Behav Brain Res. 1991;46:43–48. doi: 10.1016/s0166-4328(05)80095-6. [DOI] [PubMed] [Google Scholar]
- Morgan D, Liu Y, Roberts DCS. Rapid and persistent sensitization to the reinforcing effects of cocaine. Neuropsychopharmacology. 2006;31:121–128. doi: 10.1038/sj.npp.1300773. [DOI] [PubMed] [Google Scholar]
- Morgan D, Roberts DCS. Sensitization to the reinforcing effects of cocaine following binge-abstinent self-administration. Neurosci Biobehav Rev. 2004;27:803–812. doi: 10.1016/j.neubiorev.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Morishima Y, Miyakawa T, Furuyashiki T, Tanaka Y, Mizuma H, Nakanishi S. Enhanced cocaine responsiveness and impaired motor coordination in metabotropic glutamate receptor subtype 2 knockout mice. Proc Natl Acad Sci USA. 2005;102:4170–4175. doi: 10.1073/pnas.0500914102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel J, Belozertseva I, Greco S, Kashkin V, Malyshkin A, Jirgensons A, Shekunova E, Eilbacher B, Bespalov A, Danysz W. Effects of NAAG peptidase inhibitor 2-PMPA in model chronic pain - relation to brain concentration. Neuropharmacology. 2006;51:1163–1171. doi: 10.1016/j.neuropharm.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Nakahara D, Fuchikami K, Ozaki N, Iwasaki T, Nagatsu T. Differential effect of self-stimulation on dopamine release and metabolism in the rat medial frontal cortex, nucleus accumbens and striatum studied by in vivo microdialysis. Brain Res. 1992;574:164–170. doi: 10.1016/0006-8993(92)90813-o. [DOI] [PubMed] [Google Scholar]
- Neale JH, Bzdega T, Wroblewska B. N-Acetylaspartylglutamate: the most abundant peptide neurotransmitter in the mammalian central nervous system. J Neurochem. 2000;75:443–452. doi: 10.1046/j.1471-4159.2000.0750443.x. [DOI] [PubMed] [Google Scholar]
- Neale JH, Olszewski RT, Gehl LM, Wroblewska B, Bzdega T. The neurotransmitter N-acetylaspartylglutamate in models of pain, ALS, diabetic neuropathy. CNS injury and schizophrenia. Trends Pharmacol Sci. 2005;26:477–484. doi: 10.1016/j.tips.2005.07.004. [DOI] [PubMed] [Google Scholar]
- O'Brien CP, Gardner EL. Critical assessment of how to study addiction and its treatment: human and non-human animal models. Pharmacol Ther. 2005;108:18–58. doi: 10.1016/j.pharmthera.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. fourth. Academic Press; San Diego, CA: 1998. [Google Scholar]
- Peters J, Kalivas PW. The group II metabotropic glutamate receptor agonist, LY379268, inhibits both cocaine- and food-seeking behavior in rats. Psychopharmacology. 2006;186:143–149. doi: 10.1007/s00213-006-0372-9. [DOI] [PubMed] [Google Scholar]
- Phillips AG, Coury A, Fiorino D, LePiane FG, Brown E, Fibiger HC. Self-stimulation of the ventral tegmental area enhances dopamine release in the nucleus accumbens: a microdialysis study. Ann NY Acad Sci. 1992;654:199–206. doi: 10.1111/j.1749-6632.1992.tb25968.x. [DOI] [PubMed] [Google Scholar]
- Pöschel B, Wroblewska B, Heinemann U, Manahan-Vaughan D. The metabotropic glutamate receptor mGluR3 is critically required for hippocampal long-term depression and modulates long-term potentiation in the dentate gyrus of freely moving rats. Cereb Cortex. 2005;15:1414–1423. doi: 10.1093/cercor/bhi022. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DCS. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Serval V, Barbeito L, Pittaluga A, Cheramy A, Lavielle S, Glowinski J. Competitive inhibition of N-acetylated-α-linked acidic dipeptidase activity by N-acetyl-l-aspartyl-β-linked l-glutamate. J Neurochem. 1990;55:39–46. doi: 10.1111/j.1471-4159.1990.tb08818.x. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Rea W, Slusher BS. Modulation of behavioral sensitization to cocaine by NAALADase inhibition. Synapse. 2000;38:161–166. doi: 10.1002/1098-2396(200011)38:2<161::AID-SYN7>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Slusher BS, Thomas A, Paul M, Schad CA, Ashby CR., Jr Expression and acquisition of the conditioned place preference response to cocaine in rats is blocked by selective inhibitors of the enzyme N-acetylated-α-linked-acidic dipeptidase (NAALADASE) Synapse. 2001;41:22–28. doi: 10.1002/syn.1056. [DOI] [PubMed] [Google Scholar]
- Slusher BS, Vornov JJ, Thomas AG, Hurn PD, Harukuni I, Bhardwaj A, Traystman RJ, Robinson MB, Britton P, Lu XC, Tortella FC, Wozniak KM, Yudkoff M, Potter BM, Jackson PF. Selective inhibition of NAALADase, which converts NAAG to glutamate, reduces ischemic brain injury. Nat Med. 1999;5:1396–1402. doi: 10.1038/70971. [DOI] [PubMed] [Google Scholar]
- Spiller K, Xi ZX, Peng XQ, Newman AH, Ashby CR, Jr, Heidbreder C, Gaál J, Gardner EL. The selective dopamine D3 receptor antagonists SB-277011A and NGB 2904 and the putative partial D3 receptor agonist BP-897 attenuate methamphetamine-enhanced brain stimulation reward in rats. Psychopharmacology. 2008;196:533–542. doi: 10.1007/s00213-007-0986-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinelli S, Ballard T, Gatti-McArthur S, Richards GJ, Kapps M, Woltering T, Wichmann J, Stadler H, Feldon J, Pryce CR. Effects of the mGluR2/3 agonist LY354740 on computerized tasks of attention and working memory in marmoset monkeys. Psychopharmacology. 2005;179:292–302. doi: 10.1007/s00213-004-2126-x. [DOI] [PubMed] [Google Scholar]
- Stewart J. Psychological and neural mechanisms of relapse. Philos Trans R Soc Lond B Biol Sci. 2008;363:3147–3158. doi: 10.1098/rstb.2008.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto T, Wozniak KM, Slusher BS. Progress in the discovery and development of glutamate carboxypeptidase II inhibitors. Drug Discov Today. 2007;12:767–776. doi: 10.1016/j.drudis.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Uejima JL, Bossert JM, Poles GC, Lu L. Systemic and central amygdala injections of the mGluR2/3 agonist LY379268 attenuate the expression of incubation of sucrose craving in rats. Behav Brain Res. 2007;181:292–296. doi: 10.1016/j.bbr.2007.04.019. [DOI] [PubMed] [Google Scholar]
- Venton BJ, Seipel AT, Phillips PE, Wetsel WC, Gitler D, Greengard P, Augustine GJ, Wightman RM. Cocaine increases dopamine release by mobilization of a synapsin-dependent reserve pool. J Neurosci. 2006;26:3206–3209. doi: 10.1523/JNEUROSCI.4901-04.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbrook GL, Mayer ML, Namboodiri MA, Neale JH. High concentrations of N-acetylaspartylglutamate (NAAG) selectively activate NMDA receptors on mouse spinal cord neurons in cell culture. J Neurosci. 1986;6:3385–3392. doi: 10.1523/JNEUROSCI.06-11-03385.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Role of brain dopamine in food reward and reinforcement. Philos Trans R Soc Lond B Biol Sci. 2006;361:1149–1158. doi: 10.1098/rstb.2006.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Gardner EL. Animal models of addiction. In: Charney DS, Nestler EJ, editors. Neurobiology of Mental Illness. second. Oxford Univ. Press; London: 2004. pp. 683–697. [Google Scholar]
- Witkin JM, Gasior M, Schad C, Zapata A, Shippenberg T, Hartman T, Slusher BS. NAALADase (GCP II) inhibition prevents cocaine-kindled seizures. Neuropharmacology. 2002;43:348–356. doi: 10.1016/s0028-3908(02)00124-7. [DOI] [PubMed] [Google Scholar]
- Woolley ML, Pemberton DJ, Bate S, Corti C, Jones DNC. The mGlu2 but not the mGlu3 receptor mediates the actions of the mGluR2/3 agonist, LY379268, in mouse models predictive of antipsychotic activity. Psychopharmacology. 2008;196:431–440. doi: 10.1007/s00213-007-0974-x. [DOI] [PubMed] [Google Scholar]
- Wroblewska B, Wegorzewska IN, Bzdega T, Olszewski RT, Neale JH. Differential negative coupling of type 3 metabotropic glutamate receptor to cyclic GMP levels in neurons and astrocytes. J Neurochem. 2006;96:1071–1077. doi: 10.1111/j.1471-4159.2005.03569.x. [DOI] [PubMed] [Google Scholar]
- Wroblewska B, Wroblewski JT, Pshenichkin S, Surin A, Sullivan SE, Neale JH. NAAG selectively activates mGluR3 receptors in transfected cells. J Neurochem. 1997;69:174–181. doi: 10.1046/j.1471-4159.1997.69010174.x. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Baker DA, Shen H, Carson DS, Kalivas PW. Group II metabotropic glutamate receptors modulate extracellular glutamate in the nucleus accumbens. J Pharmacol Exp Ther. 2002a;300:162–171. doi: 10.1124/jpet.300.1.162. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Newman AH, Gilbert JG, Pak AC, Peng XQ, Ashby CR, Jr, Gitajn L, Gardner EL. The novel dopamine D3 receptor antagonist NGB 2904 inhibits cocaine's rewarding effects and cocaine-induced reinstatement of drug-seeking behavior in rats. Neuropsychopharmacology. 2006;31:1393–1405. doi: 10.1038/sj.npp.1300912. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Ramamoorthy S, Baker DA, Shen H, Samuvel DJ, Kalivas PW. Modulation of group II metabotropic glutamate receptor signaling by chronic cocaine. J Pharmacol Exp Ther. 2002b;303:608–615. doi: 10.1124/jpet.102.039735. [DOI] [PubMed] [Google Scholar]
- Yourick DL, Koenig ML, Durden AV, Long JB. N-acetylaspartylglutamate and β-NAAG protect against injury induced by NMDA and hypoxia in primary spinal cord cultures. Brain Res. 2003;991:56–64. doi: 10.1016/s0006-8993(03)03533-9. [DOI] [PubMed] [Google Scholar]