Abstract
TLRs have been implicated in promoting osteoclast-mediated bone resorption associated with inflammatory conditions. TLRs also activate homeostatic mechanisms that suppress osteoclastogenesis and can limit the extent of pathologic bone erosion associated with infection and inflammation. We investigated mechanisms by which TLRs suppress osteoclastogenesis. In human cell culture models, TLR ligands suppressed osteoclastogenesis by inhibiting expression of receptor activator of NF-κB (RANK), thereby making precursor cells refractory to the effects of RANKL. Similar but less robust inhibition of RANK expression was observed in murine cells. LPS suppressed generation of osteoclast precursors in mice in vivo, and adsorption of LPS onto bone surfaces resulted in diminished bone resorption. Mechanisms that inhibited RANK expression were down-regulation of RANK transcription, and inhibition of M-CSF signaling that is required for RANK expression. TLRs inhibited M-CSF signaling by rapidly down-regulating cell surface expression of the M-CSF receptor c-Fms by a matrix metalloprotease- and MAPK-dependent mechanism. Additionally, TLRs cooperated with IFN-γ to inhibit expression of RANK and of the CSF1R gene that encodes c-Fms, and to synergistically inhibit osteoclastogenesis. Our findings identify a new mechanism of homeostatic regulation of osteoclastogenesis that targets RANK expression and limits bone resorption during infection and inflammation.
Osteoclasts are multinucleated giant cells that differentiate from hematopoietic cells of the myeloid lineage. Mature osteoclasts effectively resorb bone and thus are directly responsible for physiological bone resorption and pathological bone destruction (1). Receptor activator of NF-κB ligand (RANKL)3 and M-CSF (also termed CSF-1) are essential molecules for differentiation of osteoclasts, and these osteoclastogenic molecules are abundantly expressed in inflammatory conditions such as rheumatoid arthritis and periodontitis (2, 3). M-CSF supports survival and proliferation of myeloid progenitors and promotes generation of osteoclast precursors that express the RANK receptor for RANKL, and thus are competent to differentiate into osteoclasts in response to RANKL stimulation. M-CSF effects are mediated by a cell surface receptor with intrinsic tyrosine kinase activity, CSF-1R (also termed c-Fms), which acts as a potent stimulator of RANK expression (4). Osteoprotegerin is soluble decoy receptor for RANKL that functions as a potent inhibitor of osteoclastogenesis. Under physiological conditions of bone homeostasis, the ratio of RANKL to osteoprotegerin is a key determinant of the rate of osteoclastogenesis and bone resorption.
Under inflammatory conditions, various activators and products of innate and acquired immunity also positively or negatively regulate osteoclastogenesis and bone resorption. Several inflammatory molecules, such as TLR ligands, TNF-α, IL-1β, IL-6, IL-17, and prostaglandins promote osteoclastogenesis indirectly by increasing expression of RANKL and M-CSF by stromal cells and T cells, and also by acting directly on osteoclast precursors to synergize with RANKL in driving osteoclastogenesis (5, 6). In contrast to stimulation of osteoclastogenesis, negative regulation of osteoclastogenesis under inflammatory conditions is less well investigated. Recently, it has become apparent that various activators or products of the immune system (TLR ligands, GM-CSF, IFN-α/β, IFN-γ, IL-4, and IL-10) can inhibit osteoclastogenesis by acting directly on osteoclasts or osteoclast precursors (5). IFN-α/β, IFN-γ, and IL-4 work by inhibiting RANK signaling, GM-CSF by suppressing differentiation of osteoclast precursors, and IL-10 by suppressing induction of NFATc1 (7–11). In general, these inhibitors are most effective at early stages of osteoclastogenesis, such as suppression of the generation of osteoclast precursors (OCPs), and lose their effectiveness (or even promote osteoclastogenesis) at later stages, after stimulation with RANKL. The extent of bone resorption in inflammatory diseases is determined by the balance between osteoclastogenic factors and the relative potency of these feedback and homeostatic molecules (5). During acute infection or in chronic inflammatory diseases such as in rheumatoid arthritis, pro-osteoclastogenic factors often predominate, leading to increased osteoclast formation and pathologic bone resorption.
TLRs are the best characterized “pattern recognition receptors” that recognize conserved microbial molecules and mediate immune and inflammatory cellular responses to infection and microbial products (12). TLRs are also activated by endogenous factors generated during cell death, inflammation, and tissue damage, such as fragments of the extracellular matrix. TLRs are potent inducers of inflammation and thus can promote bone resorption. TLR ligands can activate osteoblasts and stromal cells directly to express RANKL (1). Additionally, TLRs are the most potent inducers of inflammatory cytokines such as TNF-α and IL-1 that then act to increase RANKL expression on stromal cells, and synergize with RANK signals to drive osteoclastogenesis. Furthermore, TLR activation of committed or mature osteoclasts promotes their survival and possibly enhances their function (13–16). Consistent with these proresorptive functions, TLRs have been implicated in osteolysis associated with acute and chronic exposure to microbial products, such as acute infection, periodontitis, and in models where TLR ligands are injected over bone, such as the calvarial resorption model (5, 6, 17–19). The overall paradigm in the field currently is that on balance TLRs drive bone resorption and contribute to pathologic osteolysis.
In the immune system, TLRs engage potent feedback inhibitory and homeostatic mechanisms to limit the extent of inflammation and thus avoid the toxicity and tissue damage associated with excessive inflammation (20, 21). Similarly, it is becoming increasingly appreciated that TLRs also induce homeostatic mechanisms to limit osteoclastogenesis and thus limit the amount of bone resorption that is associated with infection and inflammation. Direct stimulation of various TLRs on osteoclast precursors inhibits RANKL-mediated osteoclastogenesis (22–24), but little is known about underlying mechanisms. The major currently appreciated inhibitory mechanism, which has been described in murine systems, is TLR-mediated induction of IFN-β (1, 25) that inhibits osteoclastogenesis by inhibiting RANKL-induced expression of Fos protein (9). We investigated mechanisms by which TLR ligands suppress osteoclastogenesis in primary human cells, because of previously described differences between the regulation of osteoclastogenesis and bone resorption in humans and mice (26), and because we wished to focus on mechanisms relevant for human inflammatory diseases. We found that TLR-induced IFN-β played a minimal role in inhibition of human osteoclastogenesis. Instead, TLR ligands suppressed human osteoclastogenesis by inhibiting expression of RANK, thereby making precursor cells refractory to the effects of RANKL. Similar inhibition of RANK expression was observed in murine cells, and TLRs attenuated osteoclastogenesis in an in vivo murine model of bone resorption. Mechanisms that inhibited RANK expression were down-regulation of RANK transcription and inhibition of M-CSF signaling that is required for RANK expression. M-CSF signaling was inhibited secondary to rapid down-regulation of cell surface c-Fms expression. TLRs cooperated with IFN-γ to suppress CSF1R gene expression with more delayed kinetics, and thus TLRs and IFN-γ synergized to suppress osteoclastogenesis. These findings reveal complex regulation of RANK expression and yield insights into mechanisms that limit pathologic bone resorption associated with infection and inflammation.
Materials and Methods
Cell culture
PBMCs were obtained from blood leukocyte preparations purchased from the New York Blood Center by density gradient centrifugation with Ficoll (Invitrogen) using a protocol approved by the Hospital for Special Surgery Institutional Review Board. Monocytes were obtained from peripheral blood, using anti-CD14 magnetic beads, as recommended by the manufacturer (Miltenyi Biotec). Monocytes were cultured for 1–2 days in α-MEM medium (Invitrogen) supplemented with 10% FBS (HyClone) with M-CSF (20 ng/ml) in the presence or absence of TLR ligands or IFN-γ. Monocyte-derived OCPs obtained after 2 days of culture with M-CSF were used unless otherwise noted in figure legends, and purity of monocytes was >97%, as verified by flow cytometric analysis.
Reagents
Trichostatin A was from Sigma-Aldrich. GF109203X, SB203580, MG-132, and actinomycin D were purchased from Calbiochem. Pam3CysSer(Lys)4 was purchased from EMC Microcollections. Ultra-pure LPS (Escherichia coli 11: B4) was purchased from Invivogen. Recombinant human IFN-γ was from R&D Systems, and human M-CSF and soluble RANKL were from Pepro-Tech. Anti-human IFN-α/β receptor chain 2 Ab was from PBL Biomedical Laboratories. MTT assays were performed using an MTT assay kit (Roche Diagnostics), according to the manufacturer's instructions.
Osteoclast differentiation
Human CD14+ cells were incubated with 20 ng/ml M-CSF for 2 days to generate osteoclast precursors. For osteoclastogenesis assays, cells were added to 96-well plates at a seeding density of 6 × 104 cells per well. Osteoclast precursors were further incubated with 20 ng/ml M-CSF and 40 ng/ml human soluble RANKL for an additional 6 days in α-MEM supplemented with 10% FBS. Cytokines were replenished every 3 days. On day 8, cells were fixed and stained for tartrate-resistant acid phosphatase (TRAP) using an acid phosphatase leukocyte diagnostic kit (Sigma-Aldrich) as recommended by the manufacturer. Multinucleated (greater than three nuclei) TRAP-positive osteoclasts were counted in triplicate wells. Bone marrow cells were flushed from femurs of mice. For detection of actin ring formation, cells were fixed and permeabilized with 0.1% Triton X-100 and incubated with FITC-phalloidin in a humidified chamber for 45 min at 37°C. After rinsing in PBS, cells were imaged using a Zeiss Axioplan microscope with an attached Leica DC 200 digital camera. Mouse bone marrow cells were cultured in α-MEM supplemented with 10% FBS with 50 ng/ml soluble RANKL and 50 ng/ml M-CSF for 4 days.
Murine osteoclastogenesis
Bone marrow cells were cultured on petri dishes (Midwest Scientific) with murine M-CSF (20 ng/ml; PeproTech) after lysis of RBCs using ACK lysis buffer (Cambrex). Then, the nonadherent cell population was recovered and further cultured with murine M-CSF for an additional 1 day. We defined this cell population as mouse OCPs. For murine osteoclastogenesis assays, we plated 2 × 104 OCPs per well in a 96-well plate and added M-CSF and RANKL for an additional 6 days, with exchange of fresh media every 3 days. To analyze the in vivo effects of TLR ligands on osteoclastogenesis, LPS (0.5 μg per mouse) was injected i.v. into C57BL/6 mice, and 2 days later, bone marrow cells were prepared.
In vivo bone resorption
For in vivo bone resorption assays, 4-mm-diameter calvarial discs of bone were excised from euthanized CBAF-B6 mice. After devitalization by freeze-thawing, discs were left untreated or treated with LPS (1 mg/ml). Discs were then implanted subcutaneously into the backs of recipient mice and the calvariae were harvested at 3, 6, and 8 wk and fixed with 4% paraformaldehyde. Bone disc implants were photomicrographed at a × 12.6 total magnification (all images included a disc and a standard of known length) followed by demineralization with 14% EDTA in PBS for 2 days. The tissues were processed and embedded in paraffin and 5-μm sections were prepared for histological analyses. These studies were approved by the Institutional Animal Care and Use Committee at Beth Israel Deaconess Medical Center.
Immunoblotting
Whole cell extracts were prepared by lysis in buffer containing 20 mM HEPES (pH 7.0), 300 mM NaCl, 10 mM KCl, 1 mM MgCl2, 0.1% Triton X-100, 0.5 mM DTT, 20% glycerol, and 1× proteinase inhibitor cocktail (Roche). The cell membrane-permeable protease inhibitor Pefablock (1 mM) was added immediately before harvesting cells. The protein concentration of extracts was quantitated using the Bradford assay (Bio-Rad). For immunoblotting, 10 μg of cell lysates was fractionated on 7.5% polyacrylamide gels using SDS-PAGE, transferred to polyvinylidene difluoride membranes (Millipore), incubated with specific Abs, and ECL was used for detection. TNFR-associated factor 6 (TRAF6) and p38 Abs were from Santa Cruz Biotechnology, and STAT3 Ab was from BD Transduction Laboratories. Triggering receptor expressed on myeloid cells 2 (TREM-2) and M-CSF receptor Abs were from R&D Systems, and RANK Ab was from Alexis Biochemicals.
Gene expression analysis
For real-time PCR, DNA-free RNA was obtained using the RNeasy Mini Kit from Qiagen with DNase treatment, and 1 μg of total RNA was reverse transcribed using a First Strand cDNA Synthesis kit (Fermentas). Real-time PCR was performed in triplicate using the iCycler iQ thermal cycler and detection system (Bio-Rad Laboratories) following the manufacturer's protocols. Expression of the tested gene was normalized relative to levels of GAPDH. For primary transcript analysis, relative amounts of primary transcripts were measured by real-time PCR using primer pairs that amplify exon-intron junctions or intronic sequences.
Chromatin immunoprecipitation (ChIP)
ChIP was performed using the ChIP Assay Kit (Upstate Biotechnology) following the manufacturer's instructions. Briefly, after stimulation, 107 human primary macrophages were fixed by adding formaldehyde directly to the medium to a final concentration of 1%. Cells were harvested, washed, and lysed. Chromatin was sheared by sonication (6 × 20 s) to lengths of ~500 bp. Sheared chromatin was precleared and then immunoprecipitated with specific Ab or Ig control. Immune complexes were subsequently collected and washed and DNA crosslinking was reversed by heating at 65°C for 4 h. Following proteinase K digestion, DNA was recovered by phenol/chloroform extraction and ethanol precipitation. The Abs used for ChIP were polyclonal Ab against RNA polymerase II (Santa Cruz Biotechnology) and control rabbit IgG (Santa Cruz Biotechnology). The primers used to amplify the human RANK promoter are: sense, GCTGGGGGACGCCTCAA; anti-sense, GCCAGCAGCCACTATCTCTTTTTC.
Flow cytometry
Staining for cell surface expression of c-Fms was performed using monoclonal anti-human c-Fms (R&D Systems). A FACScan flow cytometer with CellQuest software (BD Biosciences) were used.
Results
TLR stimulation inhibits human osteoclastogenesis
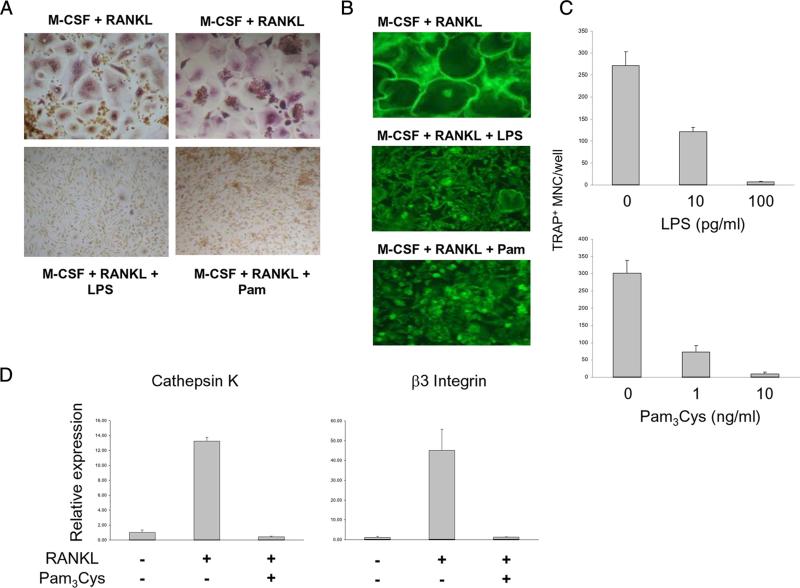
We tested the effects of the TLR2 agonist Pam3CysSer(Lys)4 (Pam3Cys) and the TLR4 agonist LPS on human osteoclastogenesis in a standard, validated culture system (27). In this system primary human monocytes are initially cultured for 1–2 days with M-CSF to induce RANK expression (thereby generating RANKL-responsive OCPs) and are subsequently treated with RANKL to induce formation of multinucleated TRAP+ osteoclast-like cells that are capable of resorbing calcified matrix (Osteologic) and dentin, which we confirmed in our system (data not shown). Control cells cultured with M-CSF and RANKL efficiently differentiated into multinucleated (more than three nuclei per cell) TRAP+ giant cells that formed large actin rings that represent a marker of the later stages of osteoclast differentiation (Fig. 1, A and B). Addition of TLR ligands strongly inhibited RANKL-induced osteoclastogenesis, as evidenced by diminished formation of TRAP+ multinucleated cells (Fig. 1A) and of cells exhibiting actin rings (Fig. 1B). Osteoclastogenesis was inhibited by TLR ligands in a dose-dependent manner, and LPS strongly inhibited osteoclast differentiation even at low concentrations (10 pg/ml) (Fig. 1C). TLR ligands most effectively inhibited osteoclastogenesis when added before or together with RANKL; however, in contrast to murine systems, TLR agonists still partially inhibited human osteoclastogenesis even when added several days after stimulation with RANKL (supplemental Fig. S1).4 There is minimal proliferation in this human osteoclastogenesis system that utilizes monocyte-derived OCPs and a dose-response experiment showed that even high concentrations of LPS minimally affected OCP viability (supplemental Fig. S2). This suggests that TLR agonists did not suppress osteoclastogenesis by inducing apoptosis but may suppress osteoclast differentiation. This notion was supported by evidence that Pam3Cys inhibited RANKL-induced expression of genes that serve as markers of osteoclast differentiation, such as cathepsin K and β3 integrin (Fig. 1D). In contrast to mouse OCPs, TRAP is expressed by human OCPs generated using M-CSF alone and expression is further increased by RANKL stimulation; TLR ligands suppressed TRAP expression (Fig. 2D), but mononuclear cells in cultures treated with TLR ligands plus RANKL still expressed low levels of TRAP positivity (Fig. 1A). These results show that TLR agonists strongly inhibit human RANKL-induced osteoclastogenesis and act directly on monocytes and myeloid precursor cells.
FIGURE 1.
TLR stimulation inhibits human osteoclastogenesis. A, Human monocytes were cultured with M-CSF (20 ng/ml) for 2 days, 6 × 104 cells were plated in each well of 96-well plate, and then RANKL (40 ng/ml) was added for an additional 6 days. TLR ligands (LPS 100 pg/ml, Pam3Cys 10 ng/ml) were added with RANKL. Cells were stained for TRAP expression. B, Cells were stained using FITC-phalloidin to detect actin ring formation. C, Cultures were performed in triplicate and TRAP-positive multinucleated (more than three nuclei per cell) cells were counted as osteoclasts. D, Human monocytes were cultured with M-CSF (20 ng/ml) for 2 days, and then RANKL (40 ng/ml) was added with or without Pam3Cys for 3 days. RANKL-inducible gene expression was analyzed by real-time PCR. mRNA levels were normalized relative to the expression of GAPDH. Data are shown as means ± SD of triplicate determinants and are representative of more than three experiments.
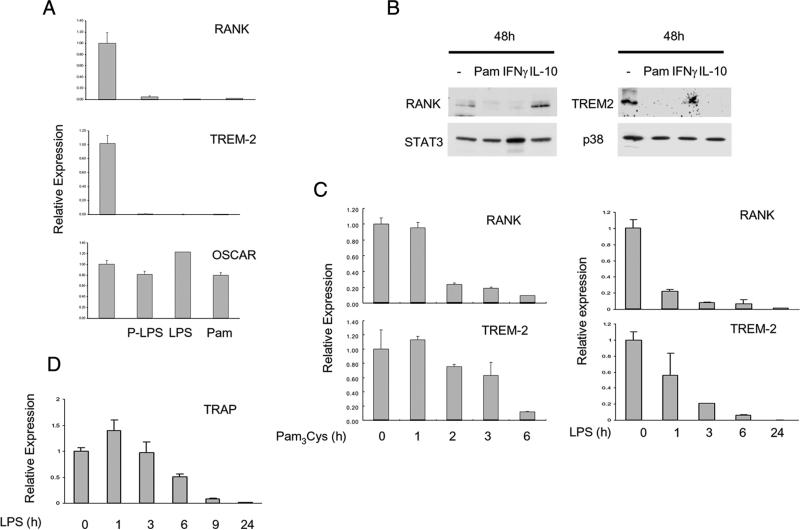
FIGURE 2.
TLR ligands suppress RANK and TREM-2 expression in human osteoclast precursors. A, Human monocytes were cultured with 20 ng/ml M-CSF in the presence or absence of TLR ligands (LPS 100 ng/ml, Pam3Cys 100 ng/ml, Porphyromonas gingivalis LPS (P-LPS) 100 ng/ml) for 2 days, and mRNA was isolated and analyzed using real-time PCR. B, Whole cell lyates from osteoclast precursors were subjected to SDS-PAGE and immunoblotted with RANK, TREM-2, STAT3, and p38 Abs. C and D, Human monocytes were cultured with 20 ng/ml M-CSF for 2 days, and then Pam3Cys or LPS was added for the indicated times. mRNA levels were measured using real-time PCR and were normalized relative to the expression of GAPDH. Data are shown as means ± SD of triplicate determinants and are representative of more than three experiments.
TLR ligands suppress RANK and TREM-2 expression
Stimulation of TLR2 with Pam3Cys does not induce type I IFN (IFN-α/β) production (28), which we confirmed in our system; additionally, blockade of the type I IFN receptor IFNAR did not detectably inhibit the effect of TLRs on osteoclastogenesis in the human system (supplemental Fig. S3). Thus, we investigated mechanisms by which TLR ligands inhibit human osteoclastogenesis that are distinct from the induction and autocrine action of IFNs previously described using murine cells (1, 9, 25). We examined expression of RANK and also of the key costimulatory molecule TREM-2 that is important for RANKL-induced human osteoclastogenesis (29–32). Addition of TLR2 and TLR4 ligands to human OCPs resulted in a striking decrease in RANK and TREM-2 mRNA (>90% decrease in many donors, n > 10), whereas control osteoclast-associated receptor (OSCAR) mRNA was not suppressed (Fig. 2A). RANK and TREM-2 protein expression was suppressed in parallel (Fig. 2B). RANK and TREM-2 mRNA decreased in a time- and dose-dependent manner after TLR stimulation (Fig. 2C and data not shown). Similar inhibitory effects were observed when TLR ligands were added at early or later time points in culture (data not shown). Additionally, TRAP mRNA that is expressed in human OCPs decreased in a time-dependent manner after TLR stimulation (Fig. 2D). Thus, TLR ligands potently suppress RANK and TREM-2 expression in human OCPs, suggesting that TLRs inhibit human osteoclastogenesis in part by making OCPs refractory to stimulation with RANKL and costimulation via TREM-2.
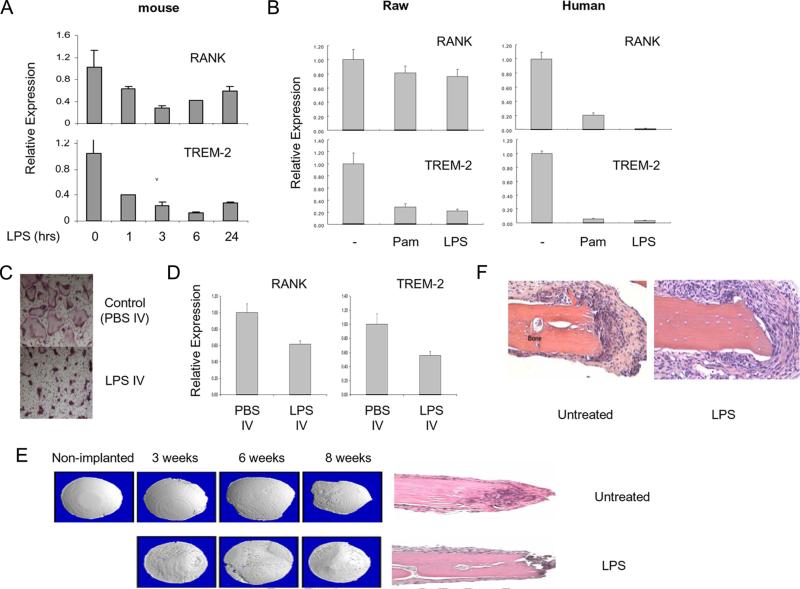
We wanted to determine whether TLR ligands similarly inhibited RANK and TREM-2 expression in murine cells. In primary mouse bone marrow-derived OCPs, TLR ligands inhibited RANK and TREM-2 expression (Fig. 3A), but inhibition was less effective than in human OCPs (Fig. 2C) and appeared to partially reverse with time (Fig. 3A). In RAW 264.7 cells, a murine cell line with osteoclastogenic potential, TLR ligands minimally inhibited RANK expression and inhibited TREM-2 expression less effectively than in human cells (Fig. 3B). Thus, the mechanism of inhibition of osteoclastogenesis by down-regulation of RANK expression appears to be operative in murine cells, but is less effective than in human cells; TREM-2 appears to have a minimal role in mouse osteoclastogenesis (30). Inhibition of osteoclastogenesis and RANK expression by TLRs was apparent after injection of mice with LPS followed by ex vivo analysis of RANK expression in parallel with suppression of formation of osteoclast precursors (Fig. 3, C and D). To further test the inhibitory effects of LPS on osteoclastogenesis and osteoclast-mediated bone resorbing activity, we utilized a calvarial disc implantation model that is associated with induction of inflammation and allows the analysis of the direct effects of LPS that has been adsorbed onto bone surfaces on the generation of myeloid lineage osteoclasts at the bone-inflammatory cell interface (33). Calvaria discs induced a mononuclear cell inflammatory reaction, and there was evidence of localized bone resorption that increased in a time-dependent manner, and multinucleated TRAP+ cells were present on the bone surfaces (Fig. 3, E and F, and data not shown). Calvaria pretreated with LPS induced a more extensive inflammatory reaction; however, there was minimal evidence of bone resorption (Fig. 3F) and multinucleated osteoclast-like cells were not detected (data not shown). These results suggest that homeostatic inhibition of oseoclastogenesis counterbalances the inflammatory effects of TLRs to attenuate the extent of bone resorption in an inflammatory setting.
FIGURE 3.
TLR ligands down-regulate murine RANK and TREM-2 expression and suppress bone resorption in vivo. A, BMDMs from C57BL/6 mice and human monocytes were cultured for 2 days in the presence of M-CSF (20 ng/ml) with or without LPS (100 ng/ml) and mRNA was measured using real-time PCR. B, Human monocytes cultured with M-CSF for 2 days and RAW 264.7 murine macrophage cell line were treated with or without LPS (100 ng/ml) or Pam3Cys (100 ng/ml) for 6 h. mRNA levels were measured using real-time PCR and were normalized relative to the expression of GAPDH. Data are shown as means ± SD of triplicate determinants and are representative of more than three experiments. C and D, Bone marrow cells were prepared from C57BL/6 mice 2 days after i.v. injection of LPS (0.5 μg/mice) or PBS. Harvested cells were cultured with M-CSF (50 ng/ml) and RANKL (50 ng/ml) for 4 days and stained for TRAP (C) or used to prepare RNA (D). mRNA levels were measured using real-time PCR and were normalized relative to the expression of GAPDH. Data are representative of more than three experiments. E and F, Implanted calvarial discs that had been coated with or without LPS were harvested and analyzed as described in Materials and Methods. Results are representative of two independent experiments.
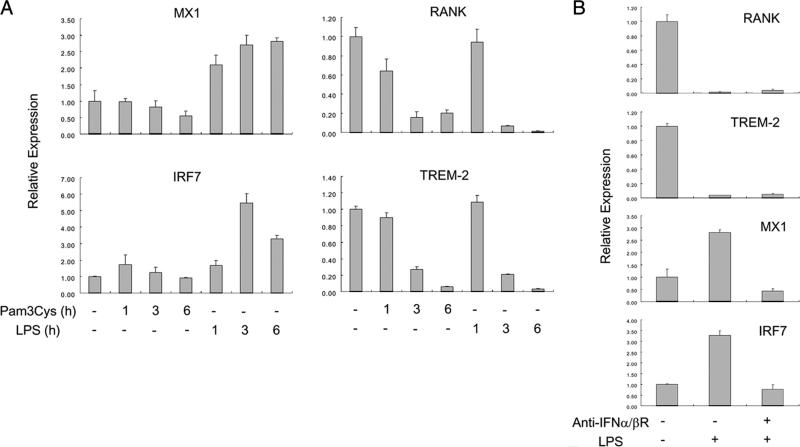
Inhibition of RANK and TREM-2 expression by LPS is not mediated by IFN-β production
Because of the established importance of autocrine IFN-β in TLR-mediated inhibition of osteoclastogenesis in murine systems (1, 9, 25), we experimentally addressed the possibility that TLR-induced type I IFN expression may be involved in down-regulation of RANK and TREM-2 expression in human OCPs. As expected, the TLR2 ligand Pam3Cys did not induce expression of IFN-inducible genes MX1 and IRF7, a sensitive measure of endogenous type I IFN production, but strongly suppressed RANK and TREM2 expression in the same experiment (Fig. 4A). LPS did induce expression of MX1 and IRF7, as expected (Fig. 4), and this induction was abolished by the addition of a type I IFN receptor blocking Ab (Fig. 4B), demonstrating the effectiveness of type I IFN blockade. However, LPS-induced inhibition of RANK and TREM2 expression was not detectably affected by blockade of the type I IFN receptor (Fig. 4B), which correlated with a lack of effect of type I IFN receptor blockade on LPS-induced suppression of osteoclastogenesis (supplemental Fig. S3). LPS induced relatively low amounts of IFN in these cultures, and addition of high concentrations of exogenous type I IFN suppressed human osteoclastogenesis but did not suppress RANK expression (data not shown). Collectively, these results indicate that TLR2 and TLR4 activation suppresses RANK and TREM2 expression independently of type I IFNs, and suggest that distinct IFN-dependent and IFN-independent mechanisms mediate TLR-induced inhibition of osteoclastogenesis and can function in parallel.
FIGURE 4.
TLR-induced inhibition of RANK and TREM-2 expression is not mediated by IFN-β. A, Human monocytes were cultured with 20 ng/ml M-CSF for 1 day, treated with Pam3Cys (100 ng/ml) or LPS (100 ng/ml) for indicated times, and mRNA was measured using real-time PCR. B, Human monocytes were cultured with 20 ng/ml M-CSF for 1 day and then treated with Pam3Cys (100 ng/ml) in the presence or absence of blocking IFN-α/β receptor Ab for 6 h. mRNA levels were measured using real-time PCR and were normalized relative to the expression of GAPDH. Data are shown as means ± SD of triplicate determinants and are representative of more than three experiments.
TLRs accelerate TREM-2 mRNA decay and inhibit RANK and TREM2 transcription
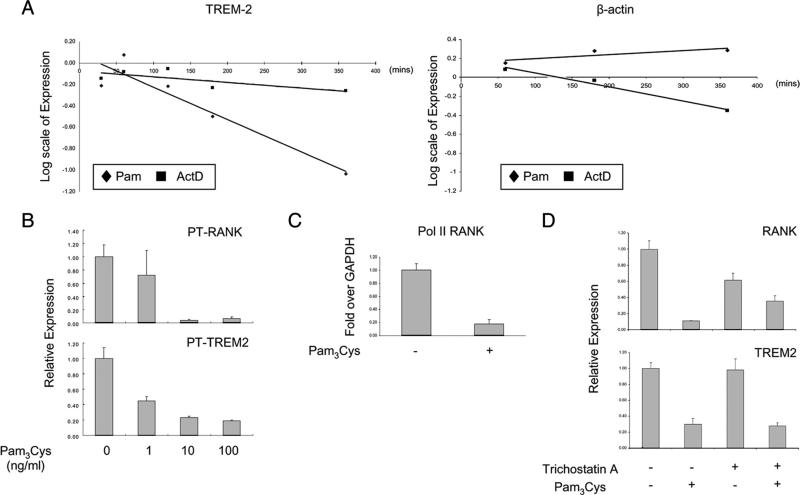
TLR ligands could inhibit RANK and TREM-2 mRNA expression by inhibiting transcription, by destabilizing mRNA and accelerating its degradation, or by a combination of these two mechanisms. The effects of TLR ligands on mRNA stability were assessed. Human monocytes were cultured with M-CSF to generate RANK+TREM-2+ OCPs, actinomycin D was added to inhibit transcription, and mRNA levels were followed over time. In the absence of de novo transcription, TREM-2 mRNA was quite stable (Fig. 5A), with a calculated half-life of 11.3 h. Addition of TLR ligands resulted in markedly accelerated decay of TREM-2 mRNA (Fig. 5A), with a drop in half-life to 4 h. TLR ligands did not accelerate decay of β-actin mRNA, which served as a negative control (Fig. 5A). These results indicate that TLR ligation generates a signal that destabilizes TREM-2 mRNA.
FIGURE 5.
TLR stimulation accelerates TREM-2 mRNA decay and inhibits RANK and TREM2 transcription. A, Human monocytes were cultured with M-CSF for 2 days, and then actinomycin D or Pam3Cys was added and mRNA levels were followed over time using real-time PCR. Data are representative of more than three experiments. B, Human monocytes were cultured with 20 ng/ml M-CSF with or without the indicated doses of Pam3Cys for 1 day. Primary transcript levels of indicated genes were measured using real-time PCR and were normalized relative to the expression of GAPDH. C, Human monocytes were cultured with 20 ng/ml M-CSF with or without Pam3Cys (100 ng/ml) for 1 day, and RNA polymerase II (Pol II) occupancy of the RANK promoter was analyzed by ChIP using real-time PCR. Data are shown as means ± SD of triplicate determinants and are representative of more than three experiments. D, Human monocytes were cultured with 20 ng/ml M-CSF for 2 days and then treated with trichostatin A (50 nM) 30 min before stimulation with Pam3Cys (100 ng/ml) for 3 h. mRNA levels were measured using real-time PCR and were normalized relative to the expression of GAPDH. Data are shown as means ± SD of triplicate determinants and are representative of more than three experiments.
We used primary transcript analysis (34) to determine the effects of TLR ligands on transcription of the RANK and TREM2 genes. Expression of primary transcripts reflects rates of transcription, rather than the balance between transcription and mRNA decay that determines levels of steady-state mRNA, and primary transcript analysis has gained broad acceptance as a method to measure transcription rates. The addition of Pam3Cys had a striking inhibitory effect on RANK and TREM2 transcription, with stronger suppression of RANK transcription (Fig. 5B); similar results were obtained with LPS (data not shown). Consistent with inhibition of transcription, Pam3Cys resulted in diminished RNA polymerase II occupancy at the endogenous RANK promoter, as assessed by ChIP assays (Fig. 5C). Additionally, the inhibitory effects of Pam3Cys on RANK (but not TREM2) transcription were partially attenuated by the histone deacetylase inhibitor trichostatin A (Fig. 5D, lanes 3 and 4), further supporting a role for transcriptional mechanisms that modulate histone acetylation at the RANK locus in the regulation of RANK expression. Collectively, the results establish that TLRs inhibit RANK transcription.
TLRs target c-Fms and inhibit M-CSF-induced RANK expression
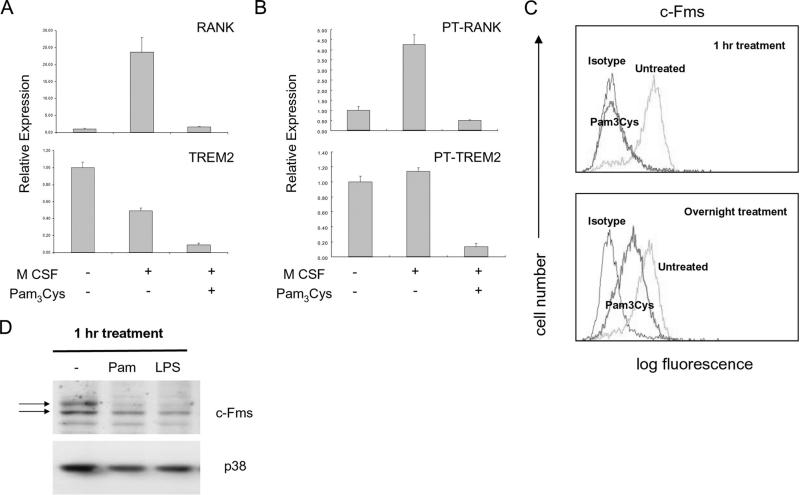
RANK expression is dependent on M-CSF, which binds to and signals via c-Fms/CSF-1R (4). We considered the possibility that TLRs suppressed RANK expression by interrupting M-CSF-mediated signaling. To study M-CSF-induced responses, we cultured monocytes overnight in the absence of M-CSF to maintain low basal RANK expression, and then acutely stimulated cells with M-CSF. M-CSF stimulation dramatically induced accumulation of RANK mRNA, and this induction was essentially completely blocked by Pam3Cys (Fig. 6A) and by LPS (data not shown). Thus, TLRs inhibited M-CSF-mediated induction of RANK expression. This inhibition occurred at the level of transcription, as M-CSF induction of RANK primary transcripts was inhibited by TLRs (Fig. 6B). These results suggest that TLRs inhibit RANK expression by inhibiting M-CSF responses.
FIGURE 6.
TLRs inhibit M-CSF-induced RANK expression by down-regulating cell surface c-Fms. A and B, Human monocytes were cultured overnight in the absence of M-CSF and then were stimulated with M-CSF with or without Pam3Cys (100 ng/ml) for 6 h. mRNA (A) or primary transcripts (B) were measured using real-time PCR. Data are representative of more than three experiments. C, Human monocytes were stimulated with Pam3Cys for 1 h or overnight and cell surface c-Fms levels were assessed by flow cytometry. D, Human monocytes were stimulated with Pam3Cys and whole cell lyates were analyzed by immunobloting. Upper arrow marks mature cell surface c-Fms; lower arrow marks immature intracellular form. Data are representative of more than three experiments.
To investigate the basis for TLR-mediated inhibition of M-CSF responses, we examined the effect of TLR ligands on the expression of c-Fms. Cell surface c-Fms expression in OCPs was down-regulated within 15 min after Pam3Cys stimulation (data not shown) and was essentially completely absent by 1 h after Pam3Cys stimulation (Fig. 6C, upper panel). Similar results were obtained when LPS was used (data not shown). This TLR-mediated inhibition of cell surface c-Fms expression was sustained, although cells typically recovered up to 10–30% of baseline c-Fms expression after overnight incubation with Pam3Cys or LPS (Fig. 6C, bottom panel, and data not shown). Down-regulation of c-Fms protein expression by TLRs was confirmed using immunoblotting (Fig. 6D). Of note, TLR stimulation rapidly down-regulated the mature cell surface form of c-Fms (Fig. 6D, upper band), but did not affect expression of the immature intracellular form (Fig. 6D, lower band). These results suggest that TLR ligands inhibit human osteoclastogenesis by down-regulating cell surface c-Fms expression on OCPs and thereby inhibit M-CSF/c-Fms-dependent expression of RANK.
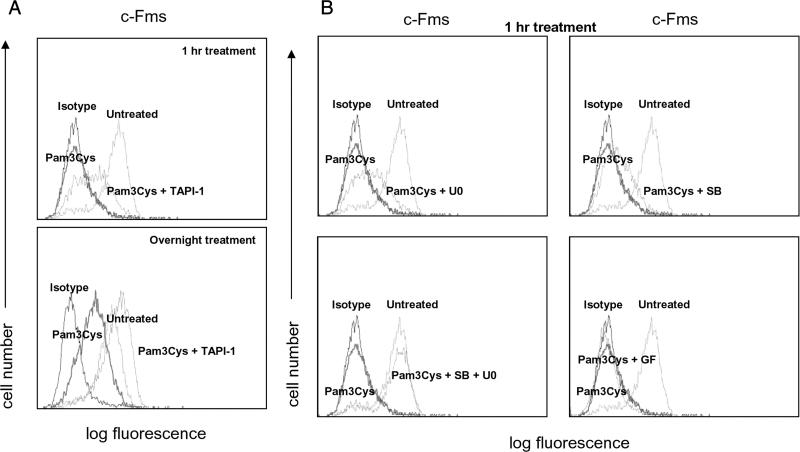
c-Fms cell surface expression can be rapidly down-regulated by proteolytic cleavage and shedding of the extracellular domain that is mediated by cell surface ADAM metalloproteases or by endocytosis (35–37). A role for metalloproteinases (MMPs) in c-Fms shedding was tested using the MMP inhibitor TAPI-1. TAPI-1 partially reversed the nearly complete down-regulation of c-Fms expression induced by a 1 h treatment with Pam3Cys (Fig. 7A, upper panel); the reversal of inhibition was in the range of 30–50% in a total of nine experiments. Inhibition of MMPs with TAPI-1 completely reversed the down-regulation of c-Fms cell surface expression induced by overnight treatment of Pam3Cys (Fig. 7A, lower panel). Thus, MMP-mediated proteolysis contributes to the rapid down-regulation of c-Fms observed after 1 h of TLR stimulation and is the major mechanism of down-regulation at later time points.
FIGURE 7.
TLR-mediated down-regulation of cell surface c-Fms is dependent on MMPs and ERK and p38 MAPKs. A, Human monocytes were cultured overnight in the absence of M-CSF and the stimulated for the indicated times with Pam3Cys with or without the MMP inhibitor TAPI-1. B, Cells were cultured as in A and then GF109203X (1 μM), U0126 (40 μM), SB203580 (10 μM), or SP600125 (10 μM) were added 1 h before adding Pam3Cys. Cell surface c-Fms levels were assessed by flow cytometry. Data are representative of more than three experiments.
The major cell surface sheddases in hematopoietic cells are ADAM10 and ADAM17 (also termed TACE); ADAM17 is activated by TLRs and the ERK MAPKs (38). Therefore, we examined the effects of inhibiting ERK activation on TLR-induced down-regulation of c-Fms expression. The MEK inhibitor U0126 (which blocks activation of downstream ERKs) partially reversed down-regulation of c-Fms expression when cells were treated with Pam3Cys for 1 h (Fig. 7B, upper left panel). The p38 inhibitor SB203580 when used alone had minimal effect on c-Fms expression (Fig. 7B, upper right panel). However, combined inhibition of ERKs and p38 essentially completely abrogated TLR2-induced down-regulation of cell surface c-Fms expression (Fig. 7B, lower left panel). In contrast, inhibitors of JNK, PKC, or proteosomes had no detectable effect on Pam3Cys-induced down-regulation of M-CSF receptor (Fig. 7 and data not shown). Collectively, the results suggest that TLRs induce proteolytic cleavage of c-Fms that contributes to receptor down-regulation, and that TLR-induced down-regulation of c-Fms cell surface expression is dependent on ERK and p38 MAPKs.
IFN-γ synergizes with TLRs to inhibit c-Fms and RANK expression and osteoclastogenesis
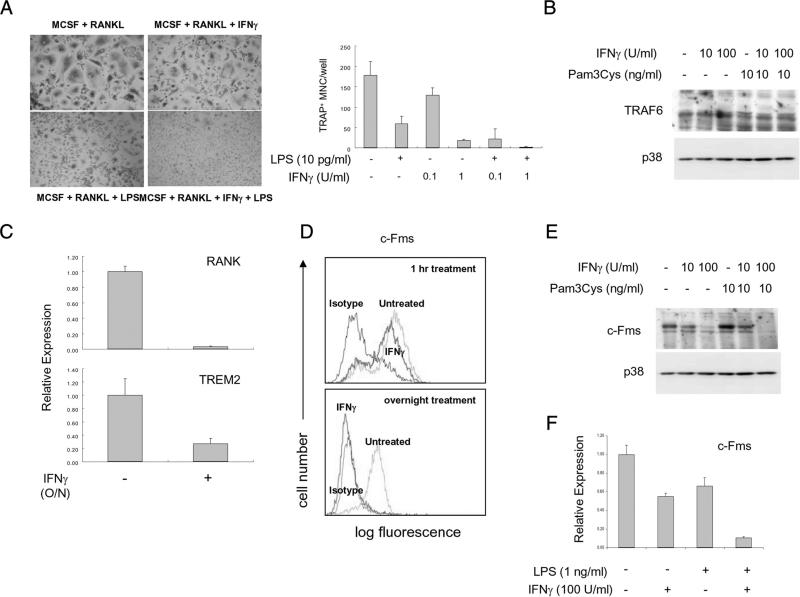
Pretreatment with IFN-γ sensitizes macrophages to exhibit enhanced responses to the activating effects of TLRs (39). We examined whether IFN-γ would similarly augment inhibitory effects of TLRs, such as suppression of osteoclastogenesis. IFN-γ has been previously described to inhibit osteoclastogenesis via down-regulation of TRAF6 in murine cells (10), and it is possible that IFN-γ and TLRs synergistically inhibit osteoclastogenesis using complementary mechanisms. As expected, IFN-γ inhibited human osteoclastogenesis (Fig. 8A); additionally, low concentrations of IFN-γ synergized with low concentrations of LPS to suppress osteoclastogenesis (Fig. 8A). However, IFN-γ, either when used alone or in combination with Pam3Cys, did not affect TRAF6 expression in human OCPs (Fig. 8B), and thus we investigated alternative mechanisms by which IFN-γ suppresses human osteoclastogenesis. Surprisingly, IFN-γ inhibited expression of RANK and TREM2 in human OCPs, with more effective and near complete inhibition of RANK expression by high concentrations of IFN-γ (Fig. 8C). Thus, both IFN-γ and TLRs suppress human osteoclastogenesis by targeting RANK expression for inhibition.
FIGURE 8.
IFN-γ synergizes with TLR ligands to suppress RANK and c-Fms expression. A, Human monocytes were cultured with M-CSF (20 ng/ml) for 2 days, and then RANKL (40 ng/ml) was added for 6 days. LPS and IFN-γ were added at the same time as RANKL. Data are shown as means ± SD of triplicate determinants and are representative of more than three experiments. B, Human monocytes were cultured with or without IFN-γ or Pam3Cys overnight. Whole cell lyates were subjected to SDS-PAGE and immunoblotted with either TRAF6 or p38 Abs. Data are representative of more than three experiments. C, Human monocytes were cultured with 20 ng/ml M-CSF in the presence or absence of IFN-γ (100 U/ml) for 1 day. mRNA was measured using real-time PCR. D, Human monocytes were cultured overnight with M-CSF. IFN-γ was added either at initiation of cultures (labeled overnight treatment) or for 1 h to cells that had been cultured overnight with M-CSF (1 h treatment). Cell surface M-CSF receptor levels were assessed by flow cytometry. Data are representative of more than three experiments. E, Whole cell lyates from B were analyzed by immunoblotting with c-Fms Abs. Data are representative of more than three experiments. F, Human monocytes were cultured with or without IFN-γ or Pam3Cys overnight. mRNA levels were measured using real-time PCR and were normalized relative to the expression of GAPDH. Data are shown as means ± SD of triplicate determinants and are representative of more than three experiments.
We then tested whether IFN-γ suppressed RANK expression by inhibiting cellular responses to M-CSF that are required for RANK expression. In contrast to rapid down-regulation of cell surface c-Fms by TLR ligands (Fig. 6C), a 1-h treatment with IFN-γ had minimal effect on cell surface c-Fms expression (Fig. 8D). However, overnight incubation with IFN-γ essentially completely suppressed cell surface and total cellular c-Fms protein expression (Fig. 8, D and E). Overnight IFN-γ treatment also suppressed c-Fms mRNA levels, and this suppression was potentiated by LPS (Fig. 8F). Collectively, the results reveal a complex interplay between IFN-γ and TLRs that results in sustained suppression of c-Fms expression over an extended time frame. The results show that IFN-γ and TLRs synergize to inhibit human osteoclastogenesis by triggering complementary mechanisms that work in tandem to suppress expression of c-Fms and its downstream target RANK.
Discussion
The differentiation and function of osteoclasts are regulated to maintain bone mass and to limit the extent of pathological bone resorption in the setting of inflammation or infection. Mechanisms that limit bone resorption during inflammation and infection are not well understood. In this study we found that microbial products that activate host defense and inflammation at the same time divert differentiation away from the osteoclast lineage by suppressing formation of RANK-responsive precursor cells. The mechanism of inhibition was down-regulation of RANK transcription mediated in part by interruption of M-CSF-induced signals required for RANK expression. Our findings identify a new mechanism of homeostatic regulation of osteoclastogenesis during infection and acute inflammation. In chronic inflammatory diseases, such as rheumatoid arthritis and periodontitis, the rate of progression of bone resorption will be determined by the balance between factors that promote osteoclastogenesis and homeostatic regulation mediated by mechanisms such as those that we have described.
Mechanisms that promote osteoclastogenesis have been extensively studied but less is known about negative regulation, especially in the setting of inflammation. Inhibition of osteoclastogenesis by potent proinflammatory factors such as IFN-γ and TLR ligands has been previously shown to occur by suppression of RANK signaling in mouse OCPs. The molecular mechanisms of inhibition of RANK signaling are IFN-γ-induced degradation of TRAF6 and TLR-induced production of IFN-β, which suppresses RANK-mediated induction of Fos protein (1, 9, 10). Liu et al. recently showed that TLR stimulation inhibited RANKL-induced NFATc1 expression by decreased activation of JNK (40). We have shown that TLRs also inhibit a more proximal step in osteoclastogenesis, the generation of RANK+ osteoclast precursors. This inhibition is mediated by down-regulation of c-Fms and suppression of RANK transcription and both of these mechanisms cooperate to effectively suppress osteoclastogenesis. Additionally, because TLR ligands still partially inhibited osteoclastogenesis when added after RANKL, more downstream signals are also inhibited by TLRs. It is likely that these distinct TLR-induced homeostatic mechanisms work in tandem, although our data suggest a more prominent role for inhibition of RANK expression in human cells relative to murine cells. We propose that down-regulation of RANK expression is one mechanism by which TLRs regulate myeloid cell fate decisions and “reroute” precursor cells from becoming osteoclasts toward becoming inflammatory macrophages.
RANK expression in OCPs is dependent on M-CSF-induced signals that are transmitted via its receptor termed CSF-1R or c-Fms (4). TLR stimulation inhibited M-CSF signaling at a proximal step by very rapidly (within 1 h) down-regulating cell surface c-Fms expression. This rapidly acting inhibitory mechanism involved, at least in part, proteolytic shedding of cell surface c-Fms and completely but temporarily abolished cell surface c-Fms expression, thus making cells unresponsive to M-CSF immediately after encountering microbial products and engagement of TLR receptors. This inhibitory mechanism is also likely induced by endogenous TLR2 and TLR4 ligands such as ECM fragments or necrotic cells, and thus can be engaged under conditions of tissue damage and associated sterile inflammation. Shedding of c-Fms has been previously described in other systems (35–37), but our findings are the first to provide a biological function for c-Fms shedding and to link it to regulation of RANK expression and osteoclastogenesis.
Although rapidly induced down-regulation of cell surface c-Fms by TLRs was very effective, it was not sustained, as c-Fms expression partially recovered when cells were cultured overnight after TLR stimulation. The early phase of c-Fms down-regulation was dependent on ERK and p38 MAPKs, and the partial (~30%) recovery in c-Fms expression over time is likely secondary to the resolution of acute MAPK signaling and synthesis and maturation of new c-Fms proteins. The sustained decrease in c-Fms expression that was observed at later time points after TLR stimulation was mediated by ongoing shedding, as it was reversed by inhibition of MMPs (Fig. 7A). Additionally, TLRs induced a modest and delayed decrease in c-Fms mRNA (Fig. 8F) and accordingly decreased de novo synthesis of c-Fms protein (Fig. 8E). Inhibition of CSF1R mRNA expression was markedly potentiated by IFN-γ, which also synergized with TLR ligands in suppressing osteoclastogenesis. These findings suggest that myeloid precursor responses to M-CSF are coordinately regulated by inflammatory stimuli, and full inhibition requires cooperation between microbial products present at the onset of infection (such as TLR ligands) and products of innate and acquired immunity that would be produced later during the immune response (such as IFN-γ). Thus, a maturing productive immune response can potently suppress M-CSF responses by several mechanisms, thereby limiting osteoclast differentiation and promoting host defense by shunting myeloid precursors away from the pathway of osteoclast differentiation.
In our system TLR-treated myeloid precursors exhibited decreased RANK expression and remained refractory to RANKL stimulation over prolonged periods of up to 14 days of culture even in the absence of exogenous or endogenous IFNs. As c-Fms was expressed at ~30% of control levels beginning 1 day after TLR stimulation, these results suggest that, in addition to down-regulation of M-CSF responsiveness, TLRs inhibit RANK expression and osteoclastogenesis via additional mechanisms. One such inhibitory mechanism is inhibition of transcription at the RANK locus, which can occur by modification of chromatin or induction of transcriptional repressors. A role for such more direct inhibition of RANK transcription is suggested by evidence showing strongly diminished RANK transcription and occupancy of the RANK promoter by RNA polymerase II, even at later points in culture when c-Fms was partially expressed (Fig. 5, B and C), and by increased RANK expression after treatment with a histone deacetylase inhibitor (Fig. 5D). Another inhibitory mechanism is TLR-induced production of autocrine-acting IFN-β (1), although IFN-β appeared to play a minimal role in experiments to date in the human system. Instead, TLR stimulation strongly down-regulated expression of TREM-2, a receptor that provides a necessary costimulatory signal for RANK in human OCPs. TREM-2 has been reported to play an important role in human osteoclastogenesis in patients with TREM-2 loss-of-function mutations that manifest as Nasu-Hakola disease (29, 31, 32), and we have recently confirmed a role for TREM-2 in human osteoclastogenesis using RNA interference (41). Thus, coordinate down-regulation of RANK and TREM-2 expression helps explain the potent down-regulation of human osteoclastogenesis by TLRs.
Supplementary Material
Acknowledgments
We thank A. Yarilina and X. Hu for critical review of the manuscript.
Footnotes
This work was supported by grants from the National Institutes of Health (to L.B.I.), the Arthritis Foundation (to K.-H.P.-M.), and by a grant of the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare and Family Affairs, Republic of Korea (A084224 to J.-D.J.).
Abbreviations used in this paper: RANK, receptor activator of NF-κB; OCP, osteoclast precursor; ChIP, chromatin immunoprecipitation; MMP, metalloproteinase; TRAF6, TNFR-associated factor 6; TRAP, tartrate-resistant acid phosphatase; TREM-2, triggering receptor expressed on myeloid cells 2.
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Lorenzo J, Horowitz M, Choi Y. Osteoimmunology: interactions of the bone and immune system. Endocr. Rev. 2008;29:403–440. doi: 10.1210/er.2007-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartold PM, Marshall RI, Haynes DR. Periodontitis and rheumatoid arthritis: a review. J. Periodontol. 2005;76:2066–2074. doi: 10.1902/jop.2005.76.11-S.2066. [DOI] [PubMed] [Google Scholar]
- 3.McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat. Rev. Immunol. 2007;7:429–442. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- 4.Arai F, Miyamoto T, Ohneda O, Inada T, Sudo T, Brasel K, Miyata T, Anderson DM, Suda T. Commitment and differentiation of osteoclast precursor cells by the sequential expression of c-Fms and receptor activator of nuclear factor kappaB (RANK) receptors. J. Exp. Med. 1999;190:1741–1754. doi: 10.1084/jem.190.12.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat. Rev. Immunol. 2007;7:292–304. doi: 10.1038/nri2062. [DOI] [PubMed] [Google Scholar]
- 6.Teitelbaum SL. Osteoclasts; culprits in inflammatory osteolysis. Arthritis Res. Ther. 2006;8:201. doi: 10.1186/ar1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans KE, Fox SW. Interleukin-10 inhibits osteoclastogenesis by reducing NFATc1 expression and preventing its translocation to the nucleus. BMC Cell Biol. 2007;8:4. doi: 10.1186/1471-2121-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lari R, Fleetwood AJ, Kitchener PD, Cook AD, Pavasovic D, Hertzog PJ, Hamilton JA. Macrophage lineage phenotypes and osteoclastogenesis: complexity in the control by GM-CSF and TGF-β. Bone. 2007;40:323–336. doi: 10.1016/j.bone.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Takayanagi H, Kim S, Matsuo K, Suzuki H, Suzuki T, Sato K, Yokochi T, Oda H, Nakamura K, Ida N, Wagner EF, Taniguchi T. RANKL maintains bone homeostasis through c-Fos-dependent induction of interferon-β. Nature. 2002;416:744–749. doi: 10.1038/416744a. [DOI] [PubMed] [Google Scholar]
- 10.Takayanagi H, Ogasawara K, Hida S, Chiba T, Murata S, Sato K, Takaoka A, Yokochi T, Oda H, Tanaka K, Nakamura K, Taniguchi T. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-γ. Nature. 2000;408:600–605. doi: 10.1038/35046102. [DOI] [PubMed] [Google Scholar]
- 11.Wei S, Wang MW, Teitelbaum SL, Ross FP. Interleukin-4 reversibly inhibits osteoclastogenesis via inhibition of NF-κB and mitogen-activated protein kinase signaling. J. Biol. Chem. 2002;277:6622–6630. doi: 10.1074/jbc.M104957200. [DOI] [PubMed] [Google Scholar]
- 12.Kawai T, Akira S. TLR signaling. Semin. Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Itoh K, Udagawa N, Kobayashi K, Suda K, Li X, Takami M, Okahashi N, Nishihara T, Takahashi N. Lipopolysaccharide promotes the survival of osteoclasts via Toll-like receptor 4, but cytokine production of osteoclasts in response to lipopolysaccharide is different from that of macrophages. J. Immunol. 2003;170:3688–3695. doi: 10.4049/jimmunol.170.7.3688. [DOI] [PubMed] [Google Scholar]
- 14.Kikuchi T, Matsuguchi T, Tsuboi N, Mitani A, Tanaka S, Matsuoka M, Yamamoto G, Hishikawa T, Noguchi T, Yoshikai Y. Gene expression of osteoclast differentiation factor is induced by lipopolysaccharide in mouse osteoblasts via Toll-like receptors. J. Immunol. 2001;166:3574–3579. doi: 10.4049/jimmunol.166.5.3574. [DOI] [PubMed] [Google Scholar]
- 15.Mochizuki A, Takami M, Kawawa T, Suzumoto R, Sasaki T, Shiba A, Tsukasaki H, Zhao B, Yasuhara R, Suzawa T, et al. Identification and characterization of the precursors committed to osteoclasts induced by TNF-related activation-induced cytokine/receptor activator of NF-κB ligand. J. Immunol. 2006;177:4360–4368. doi: 10.4049/jimmunol.177.7.4360. [DOI] [PubMed] [Google Scholar]
- 16.Sato N, Takahashi N, Suda K, Nakamura M, Yamaki M, Ninomiya T, Kobayashi Y, Takada H, Shibata K, Yamamoto M, et al. MyD88 but not TRIF is essential for osteoclastogenesis induced by lipopolysaccharide, diacyl lipopeptide, and IL-1α. J. Exp. Med. 2004;200:601–611. doi: 10.1084/jem.20040689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han X, Kawai T, Taubman MA. Interference with immune-cell-mediated bone resorption in periodontal disease. Periodontol. 2000. 2007;45:76–94. doi: 10.1111/j.1600-0757.2007.00215.x. [DOI] [PubMed] [Google Scholar]
- 18.Walsh NC, Crotti TN, Goldring SR, Gravallese EM. Rheumatic diseases: the effects of inflammation on bone. Immunol. Rev. 2005;208:228–251. doi: 10.1111/j.0105-2896.2005.00338.x. [DOI] [PubMed] [Google Scholar]
- 19.Chiang CY, Kyritsis G, Graves DT, Amar S. Interleukin-1 and tumor necrosis factor activities partially account for calvarial bone resorption induced by local injection of lipopolysaccharide. Infect. Immun. 1999;67:4231–4236. doi: 10.1128/iai.67.8.4231-4236.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 21.Liew FY, Xu D, Brint EK, O'Neill LA. Negative regulation of Toll-like receptor-mediated immune responses. Nat. Rev. Immunol. 2005;5:446–458. doi: 10.1038/nri1630. [DOI] [PubMed] [Google Scholar]
- 22.Takami M, Kim N, Rho J, Choi Y. Stimulation by Toll-like receptors inhibits osteoclast differentiation. J. Immunol. 2002;169:1516–1523. doi: 10.4049/jimmunol.169.3.1516. [DOI] [PubMed] [Google Scholar]
- 23.Zou W, Bar-Shavit Z. Dual modulation of osteoclast differentiation by lipopolysaccharide. J. Bone Miner. Res. 2002;17:1211–1218. doi: 10.1359/jbmr.2002.17.7.1211. [DOI] [PubMed] [Google Scholar]
- 24.Zou W, Schwartz H, Endres S, Hartmann G, Bar-Shavit Z. CpG oligonucleotides: novel regulators of osteoclast differentiation. FASEB J. 2002;16:274–282. doi: 10.1096/fj.01-0586com. [DOI] [PubMed] [Google Scholar]
- 25.Ha H, Lee JH, Kim HN, Kwak HB, Kim HM, Lee SE, Rhee JH, Kim HH, Lee ZH. Stimulation by TLR5 modulates osteoclast differentiation through STAT1/IFN-β. J. Immunol. 2008;180:1382–1389. doi: 10.4049/jimmunol.180.3.1382. [DOI] [PubMed] [Google Scholar]
- 26.Takayanagi H. Mechanistic insight into osteoclast differentiation in osteoimmunology. J. Mol. Med. 2005;83:170–179. doi: 10.1007/s00109-004-0612-6. [DOI] [PubMed] [Google Scholar]
- 27.Sorensen MG, Henriksen K, Schaller S, Henriksen DB, Nielsen FC, Dziegiel MH, Karsdal MA. Characterization of osteoclasts derived from CD14+ monocytes isolated from peripheral blood. J. Bone Miner. Metab. 2007;25:36–45. doi: 10.1007/s00774-006-0725-9. [DOI] [PubMed] [Google Scholar]
- 28.Toshchakov V, Jones BW, Perera PY, Thomas K, Cody MJ, Zhang S, Williams BR, Major J, Hamilton TA, Fenton MJ, Vogel SN. TLR4, but not TLR2, mediates IFN-β-induced STAT1α/β-dependent gene expression in macrophages. Nat. Immunol. 2002;3:392–398. doi: 10.1038/ni774. [DOI] [PubMed] [Google Scholar]
- 29.Cella M, Buonsanti C, Strader C, Kondo T, Salmaggi A, Colonna M. Impaired differentiation of osteoclasts in TREM-2-deficient individuals. J. Exp. Med. 2003;198:645–651. doi: 10.1084/jem.20022220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Colonna M, Turnbull I, Klesney-Tait J. The enigmatic function of TREM-2 in osteoclastogenesis. Adv. Exp. Med. Biol. 2007;602:97–105. doi: 10.1007/978-0-387-72009-8_13. [DOI] [PubMed] [Google Scholar]
- 31.Paloneva J, Kestila M, Wu J, Salminen A, Bohling T, Ruotsalainen V, Hakola P, Bakker AB, Phillips JH, Pekkarinen P, et al. Loss-of-function mutations in TYROBP (DAP12) result in a presenile dementia with bone cysts. Nat. Genet. 2000;25:357–361. doi: 10.1038/77153. [DOI] [PubMed] [Google Scholar]
- 32.Paloneva J, Manninen T, Christman G, Hovanes K, Mandelin J, Adolfsson R, Bianchin M, Bird T, Miranda R, Salmaggi A, et al. Mutations in two genes encoding different subunits of a receptor signaling complex result in an identical disease phenotype. Am. J. Hum. Genet. 2002;71:656–662. doi: 10.1086/342259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldring SR, Roelke M, Glowacki J. Multinucleated cells elicited in response to implants of devitalized bone particles possess receptors for calcitonin. J. Bone Miner. Res. 1988;3:117–120. doi: 10.1002/jbmr.5650030118. [DOI] [PubMed] [Google Scholar]
- 34.Murray PJ. The primary mechanism of the IL-10-regulated antiinflammatory response is to selectively inhibit transcription. Proc. Natl. Acad. Sci. USA. 2005;102:8686–8691. doi: 10.1073/pnas.0500419102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baccarini M, Dello Sbarba P, Buscher D, Bartocci A, Stanley ER. IFN-γ/lipopolysaccharide activation of macrophages is associated with protein kinase C-dependent down-modulation of the colony-stimulating factor-1 receptor. J. Immunol. 1992;149:2656–2661. [PubMed] [Google Scholar]
- 36.Rettenmier CW, Roussel MF, Ashmun RA, Ralph P, Price K, Sherr CJ. Synthesis of membrane-bound colony-stimulating factor 1 (CSF-1) and downmodulation of CSF-1 receptors in NIH 3T3 cells transformed by cotransfection of the human CSF-1 and c-fms (CSF-1 receptor) genes. Mol. Cell Biol. 1987;7:2378–2387. doi: 10.1128/mcb.7.7.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rovida E, Paccagnini A, Del Rosso M, Peschon J, Dello Sbarba P. TNF-α-converting enzyme cleaves the macrophage colony-stimulating factor receptor in macrophages undergoing activation. J. Immunol. 2001;166:1583–1589. doi: 10.4049/jimmunol.166.3.1583. [DOI] [PubMed] [Google Scholar]
- 38.Blobel CP. ADAMs: key components in EGFR signalling and development. Nat. Rev. Mol. Cell Biol. 2005;6:32–43. doi: 10.1038/nrm1548. [DOI] [PubMed] [Google Scholar]
- 39.Hu X, Chen J, Wang L, Ivashkiv LB. Crosstalk among Jak-STAT, Toll-like receptor, and ITAM-dependent pathways in macrophage activation. J. Leukocyte Biol. 2007;82:237–243. doi: 10.1189/jlb.1206763. [DOI] [PubMed] [Google Scholar]
- 40.Liu J, Wang S, Zhang P, Said-Al-Naief N, Michalek SM, Feng X. Molecular mechanism of the bifunctional role of lipopolysaccharide in osteoclastogenesis. J. Biol. Chem. 2009;284:12512–12523. doi: 10.1074/jbc.M809789200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park-Min KH, Ji JD, Antoniv T, Reid AC, Silver RB, Humphrey MB, Nakamura M, Ivashkiv LB. IL-10 suppresses calcium-mediated costimulation of receptor activator NF-κB signaling during human osteoclast differentiation by inhibiting TREM-2 expression. J. Immunol. 2009;183:2444–2455. doi: 10.4049/jimmunol.0804165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.