Abstract
Background & Aims
Cirrhotics undergoing transjugular intrahepatic portosystemic shunt (TIPS) for refractory ascites or recurrent variceal bleeding are at risk for decompensation and death. This study examined whether a new model for end-stage liver disease (MELDNa), which incorporates serum sodium, is a better predictor of death or transplant after TIPS than the original MELD.
Methods
148 consecutive patients undergoing non-emergent TIPS for refractory ascites or recurrent variceal bleeding from 1997 to 2006 at a single center were evaluated retrospectively. Cox model analysis was performed with death or transplant within 6 months as the end point. The models were compared using the Harrell’s C index. Recursive partitioning determined the optimal MELDNa cut-off to maximize the risk-benefit ratio of TIPS.
Results
The predictive ability of MELDNa was superior to MELD, particularly in patients with low MELD scores. The C indices (95% CI) for MELDNa and MELD were 0.65 (0.55, 0.71) and 0.58 (0.51, 0.67) using a cut-off score of 18, and 0.72 (0.60, 0.85) and 0.62 (0.49, 0.74) using a cut-off score of 15. Using a MELDNa > 15, 22% of patients were reclassified to a higher risk with an event rate of 44% compared to 10% when the score was ≤ 15.
Conclusions
MELDNa performed better than MELD in predicting death or transplant after TIPS, especially in patients with low MELD scores. In cirrhotics undergoing non-emergent TIPS, a MELD score ≤ 18 can provide a false positive prognosis; a MELDNa score ≤ 15 provides a more accurate risk prediction.
Refractory ascites and variceal bleeding represent manifestations of decompensated cirrhosis with a 2-year mortality rate of up to 50% or higher 1–4. Transjugular intrahepatic portosystemic shunts (TIPS) is a treatment option for these patients, but has associated risks including death 5. Appropriate patient selection for TIPS is therefore critical for optimizing patient outcomes.
Several models have been developed to predict outcomes in patients undergoing TIPS 6–13. The Model for End-Stage Liver Disease (MELD), originally developed in a cohort of cirrhotic patients undergoing non-emergent TIPS, is a commonly applied risk prediction tool as it effectively predicts short-term mortality 8, 10, 12. Current evidence suggests that patients with a MELD score above 18 are at a higher risk of death after TIPS compared to patients with a MELD score of less than 18 8, 11, 12.
MELD does not include serum sodium, a well-described predictor of mortality in end stage liver disease 14–21. Kim et al developed and validated a new model, MELDNa, which incorporates serum sodium into MELD 20. Compared with MELD, MELDNa improved prediction of short-term mortality in a large cohort of patients awaiting liver transplant, particularly in those with low MELD scores. In this study, we examined whether MELDNa was superior to MELD in predicting death or transplant in patients undergoing elective TIPS, the majority of whom have low MELD scores.
Methods
Patient Selection
Consecutive adult patients ≥ 18 years who underwent TIPS at the University of California, San Francisco from 1997 to 2006 were evaluated retrospectively by chart review. Inclusion criteria were non-emergent TIPS for indications of refractory ascites (not responsive to or intolerant of high dose diuretics, and/or requiring large volume paracentesis every 2 weeks), symptomatic hepatohydrothorax (effusion causing shortness of breath and requiring at least 2 thoracenteses), symptomatic umbilical hernia (including ulceration and rupture), or recurrent variceal bleeding. We defined recurrent variceal bleeding as a history of rebleeding after initial endoscopic therapy but with stable hematocrit and absence of blood transfusions or signs of re-bleeding for at least 48 hours prior to non-emergent TIPS. Patients were excluded if they had any of the following: active GI bleeding requiring emergent TIPS, active infection, hepatocellular carcinoma or other cancers, ascites (but not refractory), missing clinical information or laboratory measurements within 7 days of TIPS, or previous surgical shunt, TIPS, or liver transplant.
An Interventional Radiologist performed TIPS following standard techniques. Self-expandable 10mm-Wallstents (Boston Scientific, Natick, MA) were used prior to 2003. Thereafter, 10-mm Viatorr stent-grafts (W.L. Gore and Associates, Inc., Flagstaff, AZ) were routinely inserted. To evaluate for shunt complications, an ultrasound was routinely obtained within 24 hours after TIPS placement and at one month and six months thereafter. Abnormal ultrasound results were followed up with a hepatic venogram. Stent occlusion occurred in 23 patients (16%); 5 of these occurred within 6 months of TIPS placement. There was no identifiable era effect in study outcomes before and after covered stents were routinely performed.
Patients were followed until transplant or death. Patients alive at the end of the study period or who were lost to follow-up were censored at the last date of clinical contact. We administratively censored our data at 6 months based on the convention that MELD and MELDNa are most often used to predict short-term mortality as well as to mitigate the impact of competing risks of TIPS-related complications. The UCSF Institutional Review Board approved the study protocol.
Data collection
Patient age, etiology of liver disease, presence of histologic or radiologic cirrhosis, indication for and date of TIPS, and date of outcomes of death or transplant were retrieved from medical records. Death status was cross-referenced with the Social Security Death Index. Laboratory values for serum sodium, total bilirubin, creatinine, and the International Normalized Ratio (INR) were recorded within 24 hours prior to TIPS placement for 142 patients; the remaining patients had data within the prior 7 days. We performed calculations based on previously published formulas for MELD 22 [MELD=11.2 × ln(INR) + 3.78 × ln(total bilirubin) + 9.57 × ln(creatinine) + 6.43] and MELDNa 20 [MELDNa = (MELD) − Na −[0.025 × MELD × (140-Na)] + 140 with a cap of Na between 125 and 140].
Statistical Analysis
Chi square and Student’s t-test were used to compare dichotomous and continuous variables. Cox models generated hazard ratios for the composite end point of death or transplant within 6 months using MELD and MELDNa as continuous predictors. The assumption of proportional hazards was tested by the Schoenfeld statistical test. The Harrell’s C index 23 was employed to compare the predictive ability of MELD versus MELDNa models. The 95% confidence intervals for the C indices were created using bootstrap techniques. To identify the MELDNa cutoff that maximized the difference in probability of death or transplant between 2 groups, we compared log rank tests for Kaplan Meier survival curves stratified by various MELDNa cutoffs, and confirmed these findings by fitting a tree-structured survival model using recursive partitioning in R version 2.8.1. Statistical analysis was otherwise performed using Stata version 10.0 [College Station, TX].
Results
Patient Characteristics
Of the 258 consecutive adult patients who underwent TIPS at our institution from 1997 to 2006, 148 patients met inclusion criteria for non-emergent TIPS: 108 (73%) for refractory ascites, hepatohydrothorax, or umbilical hernia, and 40 (27%) for recurrent variceal bleeding. Patient characteristics stratified by indication are shown in Table 1. Patients with ascites and bleeding had statistically significant differences in age, MELD, MELDNa, serum sodium, and creatinine (p < 0.05). There were 109 (74%) patients with a MELD score of ≤ 18 and 74 (50%) patients with a MELD score of ≤ 15.
Table 1.
Patient Characteristics
| Ascites (n=108) | Bleeding (n=40) | |
|---|---|---|
| Age, median (IQR) | 54 (49,62) | 50 (44,57) |
| Male, (n, %) | 69 (64) | 22 (55) |
| Liver Disease, (n, %) | ||
| HCV | 31 (29) | 11 (28) |
| ALD | 25 (23) | 8 (20) |
| HCV + ALD | 24 (20) | 10 (25) |
| Other cirrhotic | 28 (26) | 11 (28) |
| MELD, median (IQR) | 16 (12,19) | 12 (10,16) |
| MELDNa, median (IQR) | 20 (16,23) | 14 (13,19) |
| Sodium, median (IQR) | 134 (131,137) | 136 (134,138) |
| Creatinine, median (IQR) | 1.3 (1.0, 1.6) | 0.8 (0.6,1.0) |
| INR, median (IQR) | 1.4 (1.2, 1.6) | 1.3 (1.2, 1.5) |
| Bilirubin, median (IQR) | 1.9 (1.3, 2.7) | 1.8 (1.1, 3.8) |
IQR, interquartile range. n, number.
Patient Outcomes
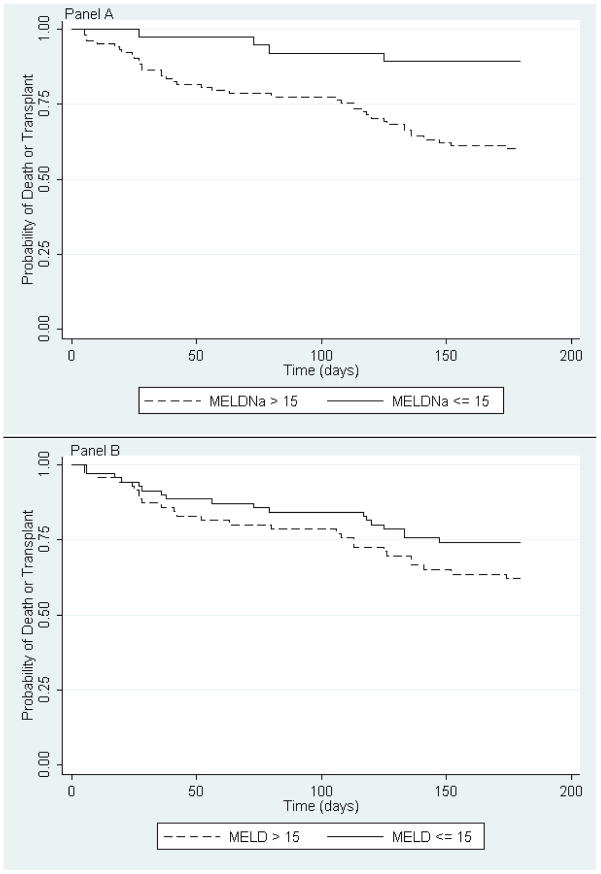
During the study period, 61 patients died and 43 patients underwent transplant. The median follow-up time (interquartile range) was 10.6 months (3.4, 29.1). By 6 months, 22 patients died and 22 patients underwent transplant; the 6-month event rate (95% CI) was 32% (25, 40). To determine the optimal cut-off score that distinguishes between events and non-events, we compared log rank tests of dichotomized Kaplan Meier curves at various MELDNa scores and performed recursive partitioning. Both methods identified a MELDNa score of 15 as the optimal cut-off (p=.0015) [Figure 1].
Figure 1.
Probability of Death or Transplant within 6 months in patients with a MELDNa score of > 15 (dashed line) as compared to ≤ 15 (solid line) [Panel A, log rank test p value = 0.0015] and with a MELD score of > 15 (dashed line) as compared to ≤ 15 (solid line) [Panel B, log rank test p value = 0.0131].
Univariate analysis in the entire cohort revealed that age, serum sodium, MELD, MELDNa, and indication of refractory ascites as compared to recurrent variceal bleeding were significant predictors of death or transplant within 6 months [Table 2]. Refractory ascites was no longer a significant predictor of outcomes in the multivariate Cox models that accounted for either MELD or MELDNa. There were no clinically or statistically significant interactions between ascites and the following predictors: serum sodium, MELD, and MELDNa (p>0.05). An interaction between serum sodium and MELD was present (p=0.02).
Table 2.
Univariate Analysis of Predictors of Events within 6 months
| HR | 95% CI | P value | |
|---|---|---|---|
| Age* | 1.05 | 1.02,1.09 | 0.001 |
| Ascites§ | 2.66 | 1.12,6.30 | 0.026 |
| Sodium¶ | 0.94 | 0.89,0.99 | 0.017 |
| MELD | 1.08 | 1.01,1.14 | 0.015 |
| MELDNa | 1.09 | 1.04,1.16 | 0.001 |
Per year.
Reference indication of bleeding
Per mEq/dl.
Risk Prediction and Reclassification
MELDNa improved upon MELD for prediction of death or transplant within 6 months in the entire cohort, as well as in patients with low MELD scores [Table 3]. Low MELD score cutoffs, defined as MELD ≤ 18 and MELD ≤ 15, were chosen a priori based on previous reports 8, 11 and on the cut-point determined by our recursive partitioning analysis. In the entire cohort, the Harrell’s C index (95% CI) for MELDNa was 0.64 (0.59, 0.74) and for MELD was 0.60 (0.53, 0.70), for an overall improvement in event discrimination with MELDNa of 4% (CI 0.008, 0.089). In low MELD score patients, the improved performance of MELDNa over MELD was even more pronounced. In patients with scores ≤ 18 as compared to those with a score of > 18, the C indices (95% CI) for MELDNa and MELD were 0.65 (0.55, 0.71) and 0.58 (0.51, 0.67). In patients with scores ≤ 15 as compared to those with a score of > 15, the C indices (95% CI) for MELDNa and MELD were 0.72 (0.60, 0.85) and 0.62 (0.49, 0.74). MELDNa improves discrimination of events over MELD in both low MELD groups by 12% (CI 0.01, 0.21).
Table 3.
Hazard Ratio of Death or Transplant within 6 months Comparing MELD and MELDNa models
| MELD | MELDNa | |
|---|---|---|
|
All patients n=148 | ||
| Hazard Ratio | 1.08 (1.01,1.14) | 1.09 (1.04, 1.16) |
| p value | 0.015 | 0.001 |
| C index | 0.60 (0.53,0.70) | 0.64 (0.59,0.74) |
|
| ||
|
MELD ≤ 18 n=109 | ||
| Hazard Ratio | 1.10 (0.97,1.25) | 1.13 (1.04,1.23) |
| p value | 0.135 | 0.004 |
| C index | 0.58 (0.51, 0.67) | 0.65 (0.55,0.71) |
|
| ||
|
MELD ≤ 15 n=74 | ||
| Hazard Ratio | 1.20 (0.95, 1.51) | 1.28 (1.11, 1.47) |
| p value | 0.121 | 0.001 |
| C index | 0.62 (0.49, 0.74) | 0.72 (0.60, 0.85) |
We next examined reclassification of a patient’s risk of death or transplant based on MELD and MELDNa cut-offs of 18 and 15 [Table 4]. Patients with discordant MELD and MELDNa scores are of the most interest as they were reclassified to a higher risk of death or transplant using their MELDNa score [Group 1]. For example, in the reclassified group, a patient’s MELDNa score would be > 18 but his MELD score would be ≤ 18. Reclassified patients represented 25% of cohort (37 patients) using a cut-off of 18, and 22% of the cohort (32 patients) using a cut-off of 15. The remaining patients had concordant MELD and MELDNa scores and their risk was not reclassified [Groups 2 and 3].
Table 4.
Cumulative Event Rate of Death or Transplant within 6 months comparing groups with MELD and MELDNa cut-off scores of 18 and 15
| Cut-off Score 18 | Cut-off Score 15 | |||||
|---|---|---|---|---|---|---|
| Group | 1 | 2 | 3 | 1 | 2 | 3 |
| MELD | ≤ 18 | > 18 | ≤ 18 | ≤ 15 | > 15 | ≤ 15 |
| MELDNa | > 18 | > 18 | ≤ 18 | > 15 | > 15 | ≤ 15 |
| Events | 14 | 15 | 15 | 14 | 26 | 4 |
| Number at risk (n,%) | 37 (25) | 39 (26) | 72 (49) | 32 (22) | 74 (50) | 42 (28) |
| Event Rate (%, CI*) | 38 (22,54) | 39 (23,54) | 21 (12,30) | 44 (27,61) | 35 (24,46) | 10 (1,18) |
CI, 95% confidence interval. Number at risk (n) and percent (%) of total cohort.
Using MELD instead of MELDNa would result in an underestimation of the risk of events in the reclassified group as shown by comparing the cumulative event rate of death or transplant for each group [Table 4]. Patients with concordant MELD and MELDNa scores below 18 and 15 [Group 3] have the lowest event rates of 21% (p value 0.057 vs. reclassified group) and 9.5% (p<0.001 vs. reclassified group) respectively. However, in patients who are reclassified due to their MELDNa score being higher than their MELD score [Group 1], their event rate is higher. In this scenario, the event rate for the reclassified patients [Group 1] is similar to those patients with concordant MELD or MELDNa scores above 18 and 15 [Group 2]: 38% vs. 39% for a MELD cut-off 18, and 44% vs. 35% for a MELD cut-off of 15.
Discussion
MELDNa improves discrimination of the risk of death or transplant within 6 months as compared to MELD in patients undergoing non-emergent TIPS for refractory ascites or recurrent variceal bleeding. The clinical implication of this finding is most evident in patients with low MELD scores who are reclassified to a higher risk based on their MELDNa score. We determined that a MELDNa score of 15 best optimizes discrimination between events and non-events, with less than 10% experiencing death or transplant with MELDNa ≤ 15 as compared to 44% with MELDNa > 15.
Several studies have shown that serum sodium, in addition to MELD, captures additional risk of death in cirrhotic patients 14–20. In patients undergoing elective TIPS, investigators have similarly demonstrated that serum sodium is an independent predictor of outcomes 13, 24. Our study provides further support of these observations and suggests that for every 1 mEq/dl decrease in serum sodium there is a 6% increase in the risk of death or transplant within 6 months after TIPS. The important statistical interaction of MELD and serum sodium that has been documented here and in other studies resulted in the development of the MELDNa model 16, 18–20. Our results support the expansion of the application of the MELDNa model to cirrhotic patients undergoing elective TIPS.
MELDNa is particularly effective at predicting risk in patients with low MELD scores, and results in the reclassification of some patients to a higher risk of death or transplant 19, 20. Patients undergoing elective TIPS typically have low MELD scores. The median MELD score in our cohort was 15, which is similar to other studies 8, 11–13. We demonstrated that low MELD score patients continue to have an increased risk of death or transplant after TIPS, and that the MELDNa model more effectively captures this risk, resulting in the reclassification of up to 25% of patients.
To evaluate the effectiveness of MELDNa and MELD based-models, we employed the Harrell’s C index 23. This index compares the expected to observed outcomes generated by Cox proportional models and is analogous to the receiver operative curve statistic, or C statistic, that is derived from logistic regression models. A higher C index indicates improved model performance. The C indices for MELD in patients with scores of ≤ 18 and ≤ 15 were 0.58 (0.51, 0.67) and 0.62 (0.49, 0.74), suggesting that this model performs inadequately in patients with low scores. In comparison, the C indices for MELDNa in patients with scores of ≤ 18 and ≤ 15 were 0.65 (0.55, 0.71) and 0.72 (0.60, 0.85). Clinically, the application of MELDNa in those with low MELD scores improves discrimination of risk of death or transplant by over 12%. As expected, because our population of patients undergoing non-emergent TIPS does not represent the entire spectrum of MELD scores (less than 10% of patients have a MELD > 22), the C indices in our analysis are lower than those reported in previous studies that evaluated MELD and MELDNa in patients awaiting liver transplant where the full range of scores is represented 20, 25.
Our findings are subject to some limitations. The size of the cohort and the relatively small number of outcomes within 6 months tempers our ability to validate the MELDNa score of 15 we generated as the optimal cut-off for discrimination of events versus non-events. Application of this score in a larger cohort would strengthen this conclusion by providing a continuous estimation of risk rather than a dichotomous cut-point and could allow for testing in shorter post-TIPS time intervals. Our cohort consisted mainly of patients undergoing non-emergent TIPS for the indication of refractory ascites, in contrast to other studies that included emergent variceal bleeding or where the majority of patients underwent elective TIPS for prevention of variceal re-bleeding 6–8, 10. We excluded patients with emergent variceal bleeding due to their higher risk of death in the early post-TIPS period as compared with patients undergoing TIPS for non-emergent indications 7, 9, 26. Advances in endoscopic and pharmacologic management of variceal bleeding likely contribute to the decreased number of patients undergoing TIPS for prevention of variceal rebleeding in our cohort 5, 27. To confirm that MELDNa performs well independent of indication for TIPS, we conducted a sensitivity analysis restricting the results to ascitic patients alone and found very similar hazards ratios, p values, and C indices [data not shown]. This would be expected since ascites as compared to bleeding was no longer a significant predictor of outcomes in multivariate models accounting for MELD or MELDNa. These findings suggest that the MELDNa model is applicable for both non-emergent indications.
Given ongoing controversy regarding the optimal management strategy for refractory ascites 24, 28–32 and the continued improvements in endoscopic and pharmacologic management of variceal bleeding 2, 33, our results establishing that MELDNa is a superior risk prediction tool compared to MELD can be helpful to clinicians who must determine the risk-benefit ratio of elective TIPS for individual patients. Furthermore, this analysis suggests that a MELD score of ≤18 is falsely reassuring, particularly in patients with concurrent hyponatremia. A cut-off MELDNa score of 15 may become the new benchmark for categorizing risk of elective TIPS in the future, though a validation study is necessary before widespread application is adopted.
Acknowledgments
This work was funded in part by grants from National Institute of Diabetes and Digestive and Kidney Diseases (DK060414) (JG) (DK076565) (SWB), from the National Center for Research Resources (KL2 RR024130) (SWB), from Agency for Healthcare Research and Quality (DK076565) (SWB), from the ASGE Endoscopic Research Award (MS), and P30 DK026743 (UCSF Liver Center). Amy J. Markowitz, JD provided editorial assistance with funding under grant DK076565.
Abbreviations
- ALD
alcoholic liver disease
- HCV
hepatitis C virus
- INR
International Normalized Ratio
- MELD
Model for End Stage Liver Disease
- MELDNa
Model for End Stage Liver Disease-Na
- TIPS
Transjugular Intrahepatic Portosystemic Shunting
Footnotes
Author Responsibilities: Study design (JG, RK, JI, SWB); Acquisition of data (JG, RK, SWB); analysis and interpretation of data (JG, MS, SS, RK, JI, SWB); drafting of manuscript (JG, SWB); critical revision of manuscript (JG, MS, SS, JI, SWB); statistical analysis (JG, MS, SS, JI, SWB); study supervision (JI, SWB)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gines P, Cardenas A, Arroyo V, Rodes J. Management of cirrhosis and ascites. N Engl J Med. 2004;350:1646–54. doi: 10.1056/NEJMra035021. [DOI] [PubMed] [Google Scholar]
- 2.Chalasani N, Kahi C, Francois F, Pinto A, Marathe A, Bini EJ, Pandya P, Sitaraman S, Shen J. Improved patient survival after acute variceal bleeding: a multicenter, cohort study. Am J Gastroenterol. 2003;98:653–9. doi: 10.1111/j.1572-0241.2003.07294.x. [DOI] [PubMed] [Google Scholar]
- 3.D’Amico G, De Franchis R. Upper digestive bleeding in cirrhosis. Post-therapeutic outcome and prognostic indicators. Hepatology. 2003;38:599–612. doi: 10.1053/jhep.2003.50385. [DOI] [PubMed] [Google Scholar]
- 4.Thomopoulos K, Theocharis G, Mimidis K, Lampropoulou-Karatza C, Alexandridis E, Nikolopoulou V. Improved survival of patients presenting with acute variceal bleeding. Prognostic indicators of short- and long-term mortality. Dig Liver Dis. 2006;38:899–904. doi: 10.1016/j.dld.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Boyer TD, Haskal ZJ. The role of transjugular intrahepatic portosystemic shunt in the management of portal hypertension. Hepatology. 2005;41:386–400. doi: 10.1002/hep.20559. [DOI] [PubMed] [Google Scholar]
- 6.Jalan R, Elton RA, Redhead DN, Finlayson ND, Hayes PC. Analysis of prognostic variables in the prediction of mortality, shunt failure, variceal rebleeding and encephalopathy following the transjugular intrahepatic portosystemic stent-shunt for variceal haemorrhage. J Hepatol. 1995;23:123–8. doi: 10.1016/0168-8278(95)80325-4. [DOI] [PubMed] [Google Scholar]
- 7.Chalasani N, Clark WS, Martin LG, Kamean J, Khan MA, Patel NH, Boyer TD. Determinants of mortality in patients with advanced cirrhosis after transjugular intrahepatic portosystemic shunting. Gastroenterology. 2000;118:138–44. doi: 10.1016/s0016-5085(00)70422-7. [DOI] [PubMed] [Google Scholar]
- 8.Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864–71. doi: 10.1053/he.2000.5852. [DOI] [PubMed] [Google Scholar]
- 9.Brensing KA, Raab P, Textor J, Gorich J, Schiedermaier P, Strunk H, Paar D, Schepke M, Sudhop T, Spengler U, Schild H, Sauerbruch T. Prospective evaluation of a clinical score for 60-day mortality after transjugular intrahepatic portosystemic stent-shunt: Bonn TIPSS early mortality analysis. Eur J Gastroenterol Hepatol. 2002;14:723–31. doi: 10.1097/00042737-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Schepke M, Roth F, Fimmers R, Brensing KA, Sudhop T, Schild HH, Sauerbruch T. Comparison of MELD, Child-Pugh, and Emory model for the prediction of survival in patients undergoing transjugular intrahepatic portosystemic shunting. Am J Gastroenterol. 2003;98:1167–74. doi: 10.1111/j.1572-0241.2003.07515.x. [DOI] [PubMed] [Google Scholar]
- 11.Angermayr B, Cejna M, Karnel F, Gschwantler M, Koenig F, Pidlich J, Mendel H, Pichler L, Wichlas M, Kreil A, Schmid M, Ferlitsch A, Lipinski E, Brunner H, Lammer J, Ferenci P, Gangl A, Peck-Radosavljevic M. Child-Pugh versus MELD score in predicting survival in patients undergoing transjugular intrahepatic portosystemic shunt. Gut. 2003;52:879–85. doi: 10.1136/gut.52.6.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferral H, Gamboa P, Postoak DW, Albernaz VS, Young CR, Speeg KV, McMahan CA. Survival after elective transjugular intrahepatic portosystemic shunt creation: prediction with model for end-stage liver disease score. Radiology. 2004;231:231–6. doi: 10.1148/radiol.2311030967. [DOI] [PubMed] [Google Scholar]
- 13.Luca A, Angermayr B, Bertolini G, Koenig F, Vizzini G, Ploner M, Peck-Radosavljevic M, Gridelli B, Bosch J. An integrated MELD model including serum sodium and age improves the prediction of early mortality in patients with cirrhosis. Liver Transpl. 2007;13:1174–80. doi: 10.1002/lt.21197. [DOI] [PubMed] [Google Scholar]
- 14.Arroyo V, Rodes J, Gutierrez-Lizarraga MA, Revert L. Prognostic value of spontaneous hyponatremia in cirrhosis with ascites. Am J Dig Dis. 1976;21:249–56. doi: 10.1007/BF01095898. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez-Esparrach G, Sanchez-Fueyo A, Gines P, Uriz J, Quinto L, Ventura PJ, Cardenas A, Guevara M, Sort P, Jimenez W, Bataller R, Arroyo V, Rodes J. A prognostic model for predicting survival in cirrhosis with ascites. J Hepatol. 2001;34:46–52. doi: 10.1016/s0168-8278(00)00011-8. [DOI] [PubMed] [Google Scholar]
- 16.Heuman DM, Abou-Assi SG, Habib A, Williams LM, Stravitz RT, Sanyal AJ, Fisher RA, Mihas AA. Persistent ascites and low serum sodium identify patients with cirrhosis and low MELD scores who are at high risk for early death. Hepatology. 2004;40:802–10. doi: 10.1002/hep.20405. [DOI] [PubMed] [Google Scholar]
- 17.Ruf AE, Kremers WK, Chavez LL, Descalzi VI, Podesta LG, Villamil FG. Addition of serum sodium into the MELD score predicts waiting list mortality better than MELD alone. Liver Transpl. 2005;11:336–43. doi: 10.1002/lt.20329. [DOI] [PubMed] [Google Scholar]
- 18.Biggins SW, Rodriguez HJ, Bacchetti P, Bass NM, Roberts JP, Terrault NA. Serum sodium predicts mortality in patients listed for liver transplantation. Hepatology. 2005;41:32–9. doi: 10.1002/hep.20517. [DOI] [PubMed] [Google Scholar]
- 19.Biggins SW, Kim WR, Terrault NA, Saab S, Balan V, Schiano T, Benson J, Therneau T, Kremers W, Wiesner R, Kamath P, Klintmalm G. Evidence-based incorporation of serum sodium concentration into MELD. Gastroenterology. 2006;130:1652–60. doi: 10.1053/j.gastro.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 20.Kim WR, Biggins SW, Kremers WK, Wiesner RH, Kamath PS, Benson JT, Edwards E, Therneau TM. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359:1018–26. doi: 10.1056/NEJMoa0801209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mathur S, Gane EJ, McCall JL, Plank LD. Serum sodium and hydration status predict transplant-free survival independent of MELD score in patients with cirrhosis. J Gastroenterol Hepatol. 2008;23:239–43. doi: 10.1111/j.1440-1746.2007.04891.x. [DOI] [PubMed] [Google Scholar]
- 22.Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D’Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–70. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 23.Harrell FE, Jr, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. Jama. 1982;247:2543–6. [PubMed] [Google Scholar]
- 24.Salerno F, Camma C, Enea M, Rossle M, Wong F. Transjugular intrahepatic portosystemic shunt for refractory ascites: a meta-analysis of individual patient data. Gastroenterology. 2007;133:825–34. doi: 10.1053/j.gastro.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 25.Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P, Kremers W, Lake J, Howard T, Merion RM, Wolfe RA, Krom R. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91–6. doi: 10.1053/gast.2003.50016. [DOI] [PubMed] [Google Scholar]
- 26.Patch D, Nikolopoulou V, McCormick A, Dick R, Armonis A, Wannamethee G, Burroughs A. Factors related to early mortality after transjugular intrahepatic portosystemic shunt for failed endoscopic therapy in acute variceal bleeding. J Hepatol. 1998;28:454–60. doi: 10.1016/s0168-8278(98)80320-6. [DOI] [PubMed] [Google Scholar]
- 27.Escorsell A, Banares R, Garcia-Pagan JC, Gilabert R, Moitinho E, Piqueras B, Bru C, Echenagusia A, Granados A, Bosch J. TIPS versus drug therapy in preventing variceal rebleeding in advanced cirrhosis: a randomized controlled trial. Hepatology. 2002;35:385–92. doi: 10.1053/jhep.2002.30418. [DOI] [PubMed] [Google Scholar]
- 28.Ochs A, Rossle M, Haag K, Hauenstein KH, Deibert P, Siegerstetter V, Huonker M, Langer M, Blum HE. The transjugular intrahepatic portosystemic stent-shunt procedure for refractory ascites. N Engl J Med. 1995;332:1192–7. doi: 10.1056/NEJM199505043321803. [DOI] [PubMed] [Google Scholar]
- 29.Rossle M, Ochs A, Gulberg V, Siegerstetter V, Holl J, Deibert P, Olschewski M, Reiser M, Gerbes AL. A comparison of paracentesis and transjugular intrahepatic portosystemic shunting in patients with ascites. N Engl J Med. 2000;342:1701–7. doi: 10.1056/NEJM200006083422303. [DOI] [PubMed] [Google Scholar]
- 30.Gines P, Uriz J, Calahorra B, Garcia-Tsao G, Kamath PS, Del Arbol LR, Planas R, Bosch J, Arroyo V, Rodes J. Transjugular intrahepatic portosystemic shunting versus paracentesis plus albumin for refractory ascites in cirrhosis. Gastroenterology. 2002;123:1839–47. doi: 10.1053/gast.2002.37073. [DOI] [PubMed] [Google Scholar]
- 31.Sanyal AJ, Genning C, Reddy KR, Wong F, Kowdley KV, Benner K, McCashland T. The North American Study for the Treatment of Refractory Ascites. Gastroenterology. 2003;124:634–41. doi: 10.1053/gast.2003.50088. [DOI] [PubMed] [Google Scholar]
- 32.Albillos A, Banares R, Gonzalez M, Catalina MV, Molinero LM. A meta-analysis of transjugular intrahepatic portosystemic shunt versus paracentesis for refractory ascites. J Hepatol. 2005;43:990–6. doi: 10.1016/j.jhep.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Garcia-Tsao G, Sanyal AJ, Grace ND, Carey WD. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Am J Gastroenterol. 2007;102:2086–102. doi: 10.1111/j.1572-0241.2007.01481.x. [DOI] [PubMed] [Google Scholar]