Abstract
Rationale
Genetic mutations in a number of putative glycosyltransferases lead to the loss of glycosylation of dystroglycan and loss of its laminin binding activity in genetic forms of human muscular dystrophy. Human patients and glycosylation defective myd mice develop cardiomyopathy with loss of dystroglycan matrix receptor function in both striated and smooth muscle.
Objective
To determine the functional role of dystroglycan in cardiac muscle and smooth muscle in the development of cardiomyopathy in muscular dystrophies.
Methods and results
Using Cre/lox mediated gene targeting, we show here that loss of dystroglycan function in ventricular cardiac myocytes is sufficient to induce a progressive cardiomyopathy in mice characterized by focal cardiac fibrosis, increase in cardiac mass, and dilatation ultimately leading to heart failure. In contrast, disruption of dystroglycan in smooth muscle is not sufficient to induce cardiomyopathy. The specific loss of dystroglycan function in cardiac myocytes causes the accumulation of large, clustered patches of myocytes with membrane damage, which increase in number in response to exercise induced cardiac stress, while exercised mice with normal dystroglycan expression accumulate membrane damage limited to individual myocytes.
Conclusions
Our findings suggest dystroglycan function as an extracellular matrix receptor in cardiac myocytes plays a primary role in limiting myocardial damage from spreading to neighboring cardiac myocytes, and loss of dystroglycan matrix receptor function in cardiac muscle cells is likely important in the development of cardiomyopathy in glycosylation-deficient muscular dystrophies.
Keywords: Heart failure, cardiomyopathy, adhesion molecules, muscular dystrophy
Introduction
Clinically defined cardiovascular disease occurs in more than 70-90% of muscular dystrophy patients with defects in the dystrophin glycoprotein complex (DGC), and heart failure is the second leading cause of death in these patients 1. The DGC contains a key transmembrane glycoprotein, dystroglycan, which is believed to link the intracellular protein dystrophin to the extracellular matrix. Dystroglycan is composed of α and β subunits. β-dystroglycan binds intracellularly to dystrophin, which binds the actin cytoskeleton and binds extracellularly to α-dystroglycan (αDG). The heavily glycosylated αDG binds tightly to extracellular matrix proteins, such as laminin, with high affinity. In muscle cells, the DGC also contains a sarcoglycan-sarcospan complex composed of sarcoglycan proteins (alpha, beta, gamma and delta in striated muscle) and sarcospan. Whereas mutations in dystrophin and each of the four sarcoglycans can cause Duchenne/Becker and limb girdle muscular dystrophies respectively, no mutations in dystroglycan have been identified as a cause of human disease.
The distribution of dystroglycan and the DGC in both cardiac and vascular smooth muscle, have raised questions about whether the cardiomyopathy present in muscular dystrophy is primary to cardiac muscle dysfunction, or might be a consequence of changes in vascular function. Sarcoglycan-deficient mice and hamsters show coronary vasospasm 2-4, and coronary dysfunction has been suggested to be the primary cause of cardiomyopathy 2, 3. However, it is possible coronary vasospasm or peripheral vasoconstriction could be secondary to the release of factors by diseased cardiac myocytes or skeletal muscle cells 5, 6. In support of this hypothesis, transgenic overexpression of δ-sarcoglycan in cardiac myocytes of the δ-sarcoglycan knockout mouse appears to slow or prevent histological evidence of cardiomyopathy 7. The fact that mutations in δ-sarcoglycan can cause dilated cardiomyopathy without skeletal muscle disease 8, and the presence of an alternative sarcoglycan complex in smooth muscle9 suggests sarcoglycans may have a unique function in the cardiovascular system, acting through a mechanism that is distinct from the function of dystroglycan and dystrophin glycoprotein complex function.
While dystroglycan mutations have not been linked to human disease, mutation in genes including POMGnT-1, POMT1, POMT1, FKTN, LARGE and FKRP can cause a broad spectrum of disease severity ranging from severe congenital muscular dystrophy, such as Walker Warburg Syndrome, to mild LGMD, such as LGMD2I. the proteins encoded by these genes all have homology to glycosyltransferases and mutations in these genes lead to a convergent loss of glycosylation of α-DG and a loss of dystroglycan function as a receptor for its known extracellular matrix ligands 10. Myodystrophy mice (myd), due to mutations in LARGE, demonstrate hypoglycosylation of α-DG and abnormal dystroglycan-ligand binding in skeletal muscle, which is biochemically similar to that of human muscular dystrophy patients with glycosylation deficient muscular dystrophy 10, 11. LARGE is believed to be a “master” regulator of the glycosylation pathway necessary for dystroglycan glycosylation and function 12. Studies have shown that myd mice develop histological evidence of cardiomyopathy and a loss of glycosylated dystroglycan in the heart, similar to the cardiomyopathy and glycosylation deficiency observed in human patients 13, 14. The molecular mechanisms that lead to cardiomyopathy in these disorders are unknown.
In the present study, we show that glycosylation deficient myd mice have impaired dystroglycan glycosylation in smooth muscle, including coronary blood vessels, and a quantitatively comparable loss of high affinity laminin binding activity in both cardiac and smooth muscle. In order to address the significance of loss of dystroglycan function with respect to the cardiomyopathy in glycosylation deficient muscular dystrophies, we used cre-Lox technology to disrupt the dystroglycan gene in the cardiovascular system in a tissue specific manner. Our results indicate that gene targeted loss of dystroglycan function specifically in cardiac myocytes is sufficient to cause cardiomyopathy in mice. Moreover, in actively contracting myocardium and after increased cardiac stress induced by exercise, dystroglycan appears to play an important role in limiting cardiac myocyte membrane damage to individual cells, and loss of its function as an extracellular matrix receptor in mice, results in expansion of myocardial damage to nearest neighboring cells.
Methods (additional details in the supplementary data)
Mice
B6C3Fe-a/a-LARGEmyd mice were obtained from Jackson Laboratories. Mice expressing cre recombinase under control of the myosin light chain 2v regulatory region (MLC2vcre)15 or using the smooth muscle myosin heavy chain promoter16 were kind gifts from from Dr. Kenneth Chien and Dr. Gary Owens respectively. Mice deficient for DAG1 were generated by mating male mice heterozygous for the cre transgene and homozyogous for the DAG1 floxed allele, with females that were homozyogous for the DAG1 floxed allele17. The mice from these crosses were born in the predicted Mendelian ratios (see Supplemental Methods). All comparisons were made on age and sex matched littermate mice.
Dystroglycan glycosylation, DGC protein expression and laminin binding activity
Dystroglycan glycosylation, protein expression, immunolocalization and laminin binding activity were analyzed in WGA enriched glycoprotein preparations as previously described 10.
In vivo echocardiography
Mice were analyzed by in vivo echocardiography while conscious as previously described18
In vivo assessment of myocardial membrane damage
Five hours before a 1 hour graded treadmill exercise session, mice (<5 mos of age) were given an i.p. injection of 0.1 mg/g Evans blue dye in saline. Eighteen hours post injection, with or without exercise, hearts were removed, and 4 cryosections >250-300 microns apart were obtained. Immunostaining with anti-laminin 1 antibody (Sigma, 1:1000) was used to show cell boundaries and identify cells with dye uptake.
Results
Glycosylation deficient muscular dystrophy mice show loss of dystroglycan glycosylation in both cardiac and smooth muscle
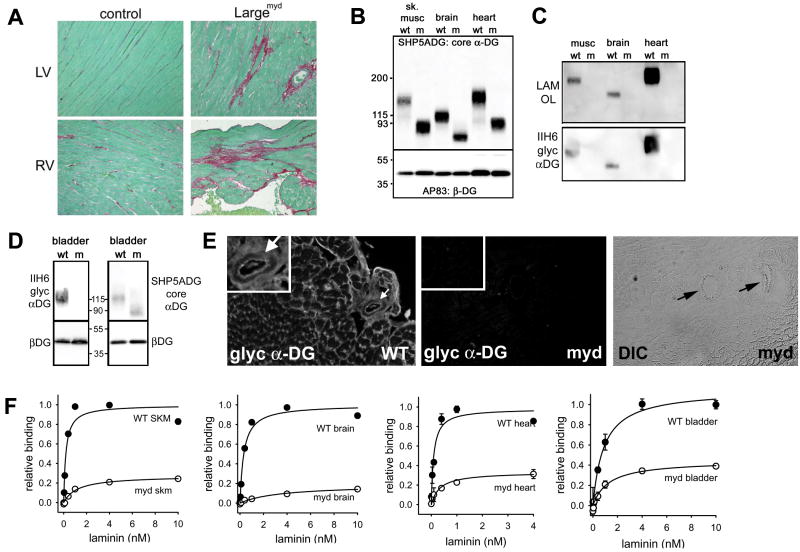
Hearts from myd mice at 10 months of age displayed evidence of significant but focal interstitial myocardial collagen deposition, consistent with cardiac remodeling that occurs following myocardial damage (Figure 1A). To address how the loss of LARGE activity affects dystroglycan glycosylation in myd mice across tissues, the apparent molecular weight shifts of α-DG protein from tissues of wild-type and myd mice were compared using an antibody against the core α-DG protein. In addition to skeletal muscle and brain as previously reported 10, both cardiac muscle and smooth muscle α-DG from myd mice (using bladder as a source of tissue composed primarily of smooth muscle), were also markedly reduced in molecular weight compared to their littermate WT controls (Figure 1B, 1D). In contrast, other tissues such as kidney, lung, testes, spleen and liver did not show as large a shift in molecular weight of α-DG, although more subtle shifts were still observed in the absence of LARGE activity (Online Figure 1). By comparing the amounts of IIH6 reactive α-DG to the amount of α-DG recognized by the core α-DG antibody, levels of the highly glycosylated form of α-DG (recognized by IIH6) were high in WT skeletal muscle, brain, heart, smooth muscle and kidney, but barely detectable in lung, testes, spleen and liver (Figures 1B, 1C, Online Figure 1). The reactivity of IIH6 antibody was tightly correlated with laminin binding activity of α-DG detected by laminin overlay assay (Figure 1C, Online Figure 1). In each adult tissue where the IIH6-reactive, laminin binding form of α-DG was highly expressed in WT mice, the IIH6 reactive α-DG and laminin binding activity by overlay assay was undetectable in samples from myd mice. Loss of α-DG glycosylation did not appear to markedly impact the expression levels of the dystroglycan peptide in these tissues. Staining of tissues from myd mice, including the myocardium, demonstrate that the glycosylated form of α-DG is lost from all tested vascular smooth muscle including coronary blood vessels (Figure 1E). This loss of dystroglycan glycosylation results in a significant loss of total high-affinity laminin binding activity in skeletal, cardiac and smooth muscle, and brain (Figure 1F). Together these results demonstrate that LARGE activity is necessary for the type of dystroglycan glycosylation that is required for its function as an extracellular matrix receptor in: striated muscle (including the heart), smooth muscle, nervous system, and to a lesser extent the kidney.
Figure 1. Distribution of dystroglycan glycosylation deficiency and laminin binding activity in the cardiovascular system and other tissues of glycosylation deficient myd mice.
A) Myocardium of 10 month old myd mice shows focal areas of collagen deposition. Collagen was stained with Sirius Red and counterstained with Fast Green. LV=left ventricle. RV=right ventricle B) Assessment of α-DG protein expression and molecular weight in tissues of myd mice using an antibody that recognizes the core α-DG protein. C) The fully glycosylated α-DG (recognized by IIH6) and laminin binding glycoform (determined by laminin overlay assay) of α-DG are easily detected in muscle, heart and, brain. D) Glycosylation of α-DG is also markedly affected in smooth muscle (from bladder) of myd mice as indicated by a loss of IIH6 staining and a reduction in molecular weight. E) IIH6 immunostaining in the myocardium of myd mice reveals a loss of staining at the plasma membrane in cardiac myocytes and a loss of staining in coronary blood vessels (arrows). A DIC image of the myd panel demonstrates two coronary vessels are in the field of view. F) High affinity laminin binding activity is markedly reduced in heart and smooth muscle of myd mice (p<0.05 at 1-10 nM laminin points). Data were normalized to the peak binding activity in littermate WT animals to allow comparisons of total activity and relative affinity. All data shown are means +/- SD (n=3) and in cases where the error bars are smaller than the data points the SD was less than 0.04. The biochemical data in panels B-F were performed in mice age 14-20 weeks.
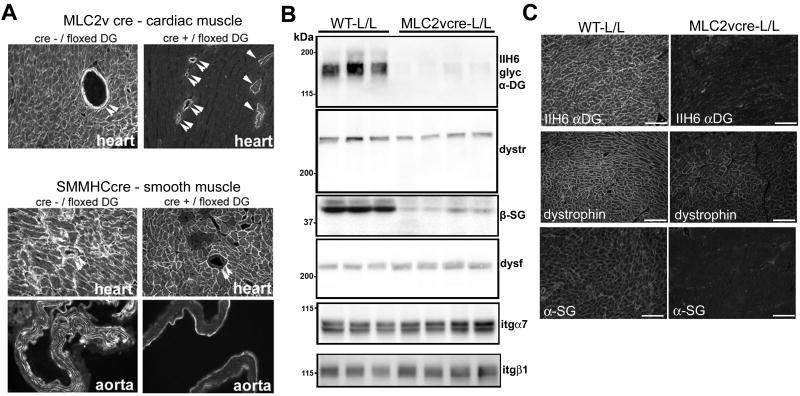
Genetic disruption of the dystroglycan gene in the cardiac myocytes or smooth muscle leads to a tissue specific loss of dystroglycan expression
To determine the significance that the loss of dystroglycan function in cardiac or vascular smooth muscle has for the development of cardiomyopathy, mice bearing an allele of the dystroglycan gene (DAG1) flanked by loxP sites (L/L) were crossed with mice that express cre-recombinase in either cardiac ventricular muscle (MLC2vcre-L/L), or in all smooth muscle (SMMHCcre-L/L). In MLC2vcre-L/L mice, there was loss of dystroglycan expression in nearly all cardiac ventricular myocytes while coronary vascular smooth muscle expression of dystroglycan was maintained (Figure 2A). A small percentage of cardiac myocytes (21 out of 2350 myocytes in random fields from 3 animals) showed residual dystroglycan expression (Figure 2A). Conversely, in the SMMHCcre-L/L mice showed complete loss of dystroglycan staining in vascular smooth muscle in coronary vessels, the aorta (although largely collagen-based tunica externa remained positive), and bladder smooth muscle (Figure 2A, Online Figure 2). Whereas nearly all ventricular myocytes in the SMMHCcre-L/L mice exhibited normal dystroglycan expression, a small subset of ventricular myocytes in this mouse was targeted by this promoter (Figure 2A). These targeted myocytes were observed in sample sections of 2 of 4 SMMHCcre-L/L hearts examined, but only 18 and 27 myocytes were disrupted in an entire cross section of the heart in those samples. This is likely due to the short-lived, developmental expression of the smooth muscle myosin heavy chain gene in a subset of myocytes that has been characterized previously 16.
Figure 2. Targeted tissue-specific deletion of dystroglycan gene expression in cardiac and smooth muscle.
A) Immunostaining with an antibody against beta-dystroglycan Double arrows indicate smooth muscle in coronary vessels. Single arrows indicate cardiac myocytes expressing residual beta-DG. In MLC2vcre- L/L mice, the field shown was specifically chosen to show the residual positive cells while most fields were negative for dystroglycan expression. In SMMHCcre- L/L mice dystroglycan expression was not detected in smooth muscle. A very small portion of cardiac myocytes also showed gene targeting, most likely due to the transient expression of smooth muscle markers in cardiac myocytes during development, although most fields analyzed showed normal dystroglycan expression in all cardiac myocytes B) Analysis of dystroglycan, dystrophin (dystr), dysferlin (dysf), sarcoglycan (β-SG) and integrin (itg) protein expression in MLC2vcre-L/L ventricular myocardium compared to littermate L/L (WT) mice. Each lane represents ventricular muscle from a different animal. C) Immunofluorescence localization of dystrophin is retained at the plasma membrane of cardiac myocytes in MLC2vcre-L/L mice despite loss of dystroglycan expression, while alpha-sarcoglycan expression is reduced. Samples in A-C were from mice age 14-20 weeks.
In order to determine how loss of dystroglycan protein expression impacted the expression of the DGC in cardiac muscle, DGC protein expression in ventricles of MLC2vcre-L/L mice was examined (Figure 2B). Dystroglycan expression was reduced 93.8 +/-1.5 % (mean +/-SEM, n=4, p<0.05). Somewhat surprisingly, the loss of dystroglycan protein expression in cardiac myocytes by gene targeting failed to cause a corresponding marked reduction in dystrophin expression, and the expressed dystrophin was retained at the sarcolemma (Figure 2C), even though dystroglycan is a direct binding partner of dystrophin in the DGC. In contrast, sarcoglycan expression levels were reduced to a much greater extent than the dystrophin expression levels, although not lost completely in MLC2vcre -L/L mice. These results suggest that dystrophin may interact with other proteins in cardiac muscle cells to confer dystrophin protein stability. There was also a slight increase in dysferlin expression (37% increase, p<0.05), but no change in alpha7 or beta1 integrin expression (p>0.05), as measured by densitometry, in MLC2vcre-L/L animals. In SMMHCcre-L/L mice, immunofluorescence staining also revealed a reduction of β-sarcoglycan immunofluorescence staining in vascular smooth muscle and bladder smooth muscle accompanying loss of dystroglycan expression in SMMHCcre-L/L mice (Online Figure 2).
Tissue specific loss of dystroglycan expression in cardiac myocytes is sufficient to cause a cardiomyopathic phenotype in mice
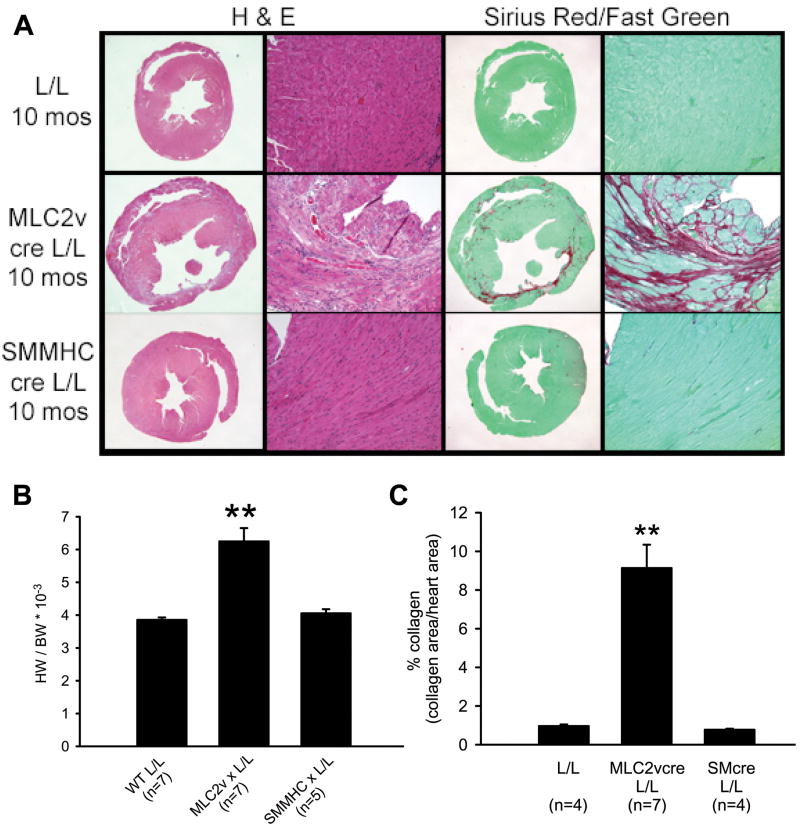
Ten month old MLC2vcre L/L mice showed marked cardiac pathology including myocardial cell degeneration marked by eosinophilic myocytes, infiltration of mononuclear cells in a subset of cases with severe pathology, and significant focal collagen deposition (Figure 3A). All hearts from MLC2v-L/L mice examined appeared enlarged in cross section, and a subset showed focal thinning of the left ventricular wall. Consistent with these histological observations, MLC2vcre-L/L mice showed a 65% increase in heart weight to body weight ratios compared to L/L mice (Figure 3B), and a significant increase in cardiac fibrosis with nearly 9% of the myocardium being replaced with focal fibrotic tissue in 10 month old mice (Figure 3C). Despite the focal nature of the fibrosis, microfil perfusion experiments did not reveal evidence of coronary vasospasms (Online Figure 3). SMMHCcre-L/L mice at 10 months of age failed to show alterations in cardiac mass, or any pathology by histological staining (Figure 3).
Figure 3. Mice with cardiac myocyte restricted deletion of dystroglycan gene show morphological evidence of a cardiomyopathic phenotype.
A) Morphological analysis of hearts from littermate control mice (L/L), MLC2vcre-L/L mice, and SMMHCcre-L/L mice at 10 months of age. Formalin fixed heart sections were stained with hematoxylin and eosin (left two panels) to assess gross histology and with sirius red (right two panels) for collagen deposition. MLC2vcre-L/L mice show evidence of increased myocardial damage, ventricular wall thinning, enlarged hearts and collagen deposition whereas SMMHCcre-L/L mice were indistinguishable from L/L mice. B) Heart weight (HW) to body weight (BW) ratios at 10 months age revealed a significant increase in cardiac mass in MLC2v cre- L/L animals. BW was unchanged in the three groups (46.4+/-1.8g WT; 47.4+/-2.5g MLC2vcre-L/L; 47.2+/-1.1g SMMHCcre-L/L, p>0.05) C) Collagen deposition was quantified using image analysis of Sirius red stained sections and revealed a significant increase in fibrotic area in MLC2vcre-L/L animals. ** p<0.001 by ANOVA and a Bonferroni's post hoc test compared to both L/L and SMMHCcre-L/L mice. Data shown are mean +/- SEM.
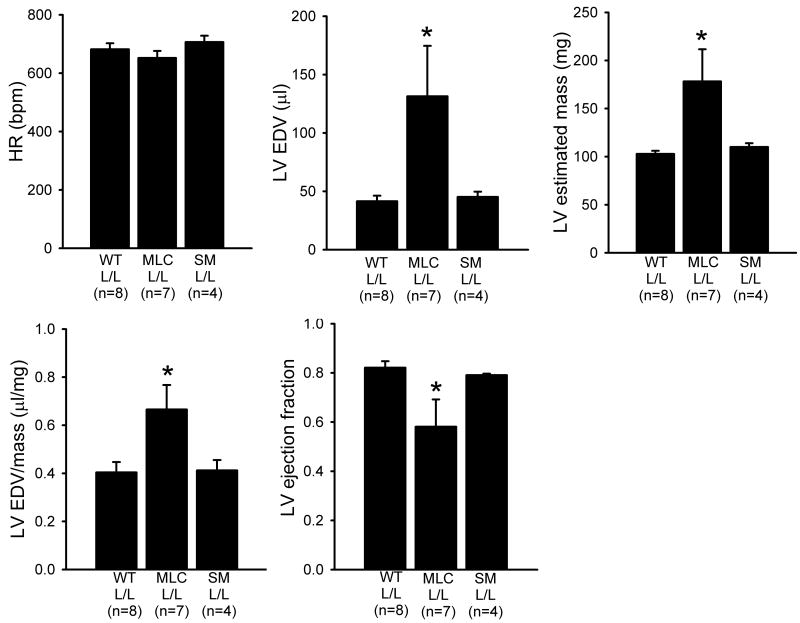
Consistent with the morphological findings, murine echocardiography showed the cardiac mass and end diastolic volume were significantly increased in MLC2vcre-L/L mice (Figure 4). The ratio of the end diastolic volume to mass was also increased, confirming these mice experienced pathological chamber dilation as opposed to a mere increase in overall heart size. MLC2vcre-L/L mice also showed a significant impairment of systolic function evidenced by a reduction in the left ventricular ejection fraction. In contrast, the loss of dystroglycan function in smooth muscle had no measureable impact on cardiac function as measured by echocardiography. Together, these echocardiography findings support the conclusion that the loss of dystroglycan function in cardiac myocytes is necessary and sufficient to cause a functional dilated cardiomyopathy.
Figure 4. Mice with cardiac restricted deletion of the dystroglycan gene show evidence for a cardiomyopathic phenotype by echocardiography.
Echocardiography was performed on 10 month old L/L littermate mice, MLC2vcre- L/L, and SMMHcre-L/L mice. MLC2vcre- L/L mice displayed a significant increase in end diastolic volume (EDV) that exceeded the measured increase in cardiac mass, and a decrease in ejection fraction that suggested an overall functional dilated cardiomyopathic phenotype. Data shown are means +/- SEM. * p<0.05 by ANOVA and a Bonferroni's post hoc test compared to both L/L and SMMHCcre-L/L mice.
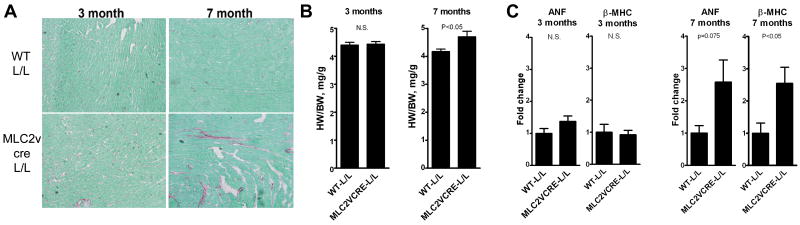
Examining mice at several ages indicated that the first detectable signs of cardiomyopathy by histology and pathological markers in MLC2vcre-L/L mice occurs around seven moths of age. MLC2vcre-L/L mice at 3 months of age appear histologically and morphologically normal (Figure 5). MLC2vcre-L/L mice at 7 months begin to show small focal areas of fibrosis and a small increase in HW/BW ratio. Furthermore, MLC2vcre-L/L mice at 7 months of age show significant activation of β–myosin heavy chain (MHC) gene expression, which as important molecular marker of cardiomyopathic remodeling associated with both cardiac hypertrophy and fibrosis 19, but mice at 3 months of age showed no activation of β–MHC or ANF (Figure 5). This data suggests that cardiomyopathy develops progressively in adult animals due to cumulative effects of lifetime loss of dystroglycan in cardiac myocytes, rather than a congenital defect in young animals.
Figure 5. Pathological evidence of cardiomyopathy in mice with cardiac restricted deletion of the dystroglycan is progressive and appears around 7 months of age.
A) Picrosirius red staining (red, collagen; green, tissue counterstain) of myocardium from representative 3 month-old and 7 month-old mice. B) HW/BW ratios at 3 month and 7 months of age. BW was not different between the two groups at 3 mos (24.3+/-1.1g WT, 24.52+/-0.9g MLC2vCRE-L/L, p>0.05) or 7 months (34.7+/-1.1g WT, 33.19 +/- 1.0g MLC2vcre, p>0.05). C) Expression of cardiomyopathic markers atrial natriuretic factor and beta myosin heavy chain by quantitative RT-PCR in 3 month and 7 month old hearts. Data are mean +/- SEM for B and C with n=5-7
Dystroglycan expression in cardiac myocytes is necessary for limiting stress-induced cardiac myocyte damage from spreading to nearest neighbor cells
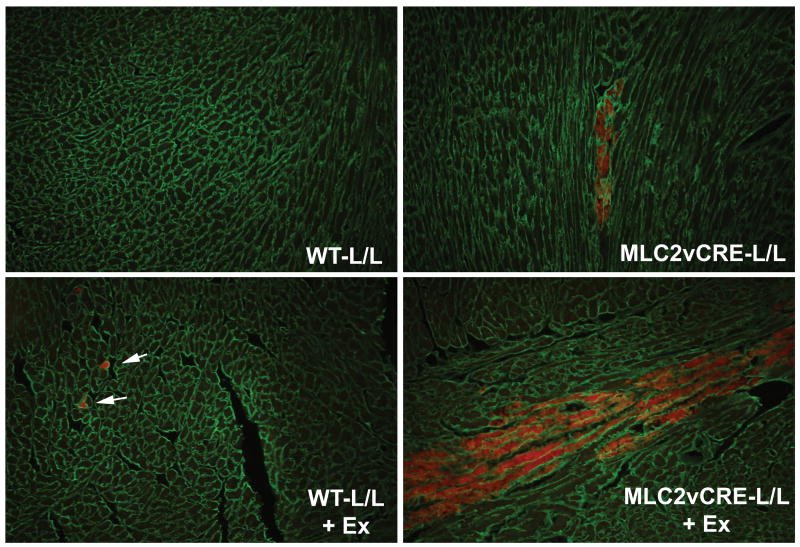
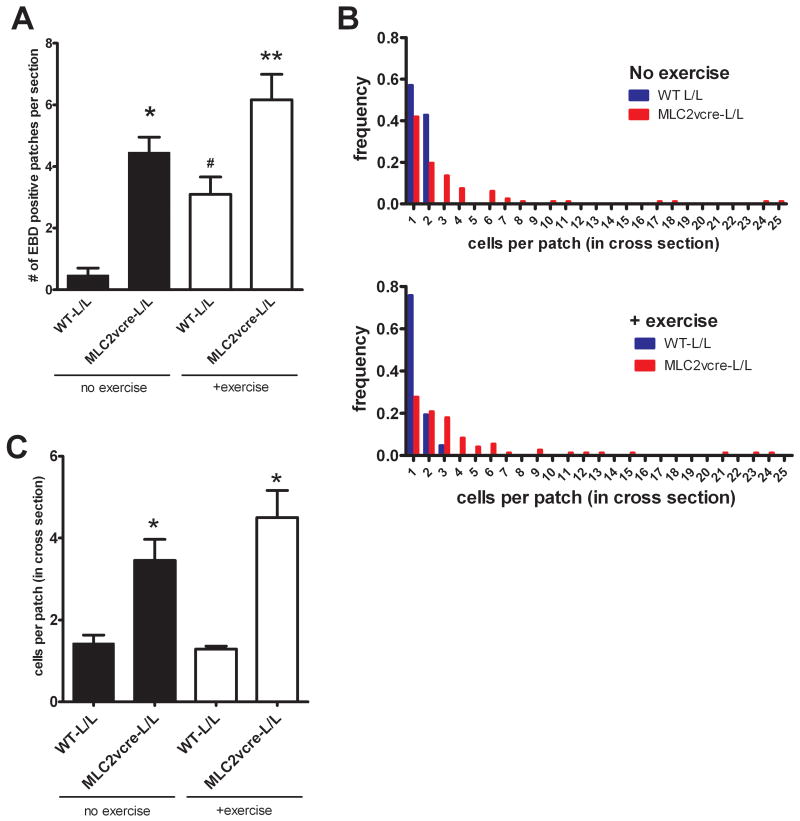
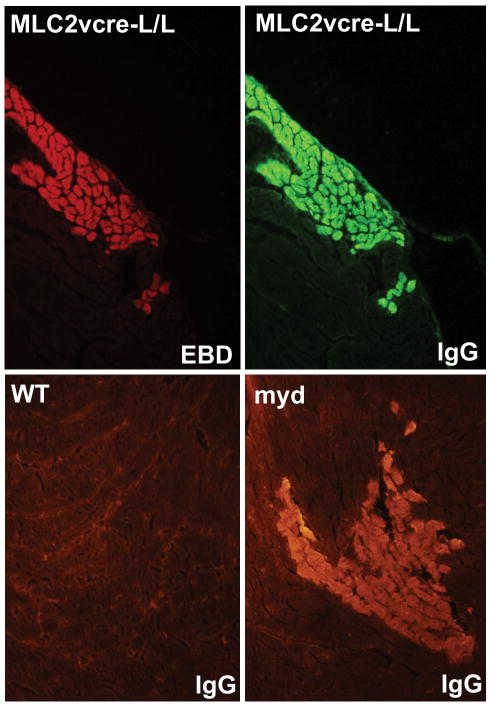
Because loss of dystroglycan expression in cardiac myocytes and loss of dystroglycan laminin binding activity in myd mice is sufficient to cause a progressive cardiomyopathy characterized by focal fibrosis, we hypothesized that dystroglycan's function as an extracellular matrix receptor in cardiac myocytes might play a primary role in preventing myocardial damage in young animals that accumulates with age leading to cardiomyopathic remodeling. To test this hypothesis, young WT and MLC2vcre-L/L mice (<5 months old, prior to detectable fibrosis or cardiac histopathology) were examined for sarcolemma damage in cardiac myocytes, by Evan's blue dye (EBD) uptake in sedentary and acutely exercised animals. In WT, unexercised animals, very few EBD positive cells were observed, with most sections devoid of any dye uptake. Interestingly, in WT animals, EBD positive myocytes were consistently observed after exercise, but the sarcolemma damage was almost always limited to single isolated myocytes. In contrast, MLC2vcre × L/L animals had a markedly different pattern of EBD uptake both in exercised and unexercised mice, where EBD uptake seemed to occur in large groups of neighboring cells, or cell “patches”(Figure 6). We also noted the patches of membrane damage in MLC2vcre-L/L mice appeared to be limited to cardiac myocytes that were organized in similar orientations of their longitudinal, or mechanical axis (Figure 6). To examine this curious pattern of dye uptake in more detail, the total number of patches of damaged cells as small as one cell, and the size of those patches were quantified (Figure 7). These results showed that acute exercise was able to produce a significant number of EBD positive cells in WT animals, but these patches were typically observed as one isolated cardiac myocyte (Figure 7). In contrast, loss of dystroglycan function resulted in slightly greater numbers of patches both in sedentary and exercised animals, but more significantly a dramatic increase in the size of those patches up to patch sizes that exceeded 25 contacting cells in cross sections of myocardium from MLC2vcre-L/L mice (Figure 7). Note that the measurements of patch size were sampled in cross sections suggesting these patches likely contain a significantly larger number of cells in three dimensions. Also, the greater number of patches observed in MLC2vcre-L/L animals could be in part due to the increased size of the patches increasing likelihood of observing a patch in a random section. The mean patch size in each genotype was not statistically different in control and exercised animals, indicating the size of the patch was not influenced by exercise stress. Taken together these results suggest that cardiac membrane damage and uptake of EBD can occur even in WT mice in response to increased activity, but the primary role of dystroglycan in cardiac myocytes is to protect against the expansion of that initial myocyte damage to neighboring cardiac myocytes. Myocytes that take up EBD, also take up large proteins from the extracellular space including immunoglobulin (Figure 8). By marking membrane damage with immunoglobulin, Figure 8 shows that sedentary myd mice also have large patches of neighboring myocytes with membrane damage similar to that observed in sedentary MLC2vcre-L/L animals. Therefore, loss of dystroglycan's function as an extracellular matrix receptor in cardiac myocytes appears critical to preventing expansion of initial myocyte membrane damage to neighboring cardiac myocytes.
Figure 6. Acute myocyte damage in WT and MLC2vcre-L/L in response to exercise stress.
Representative images showing EBD uptake (red) in sedentary and exercised (+ex) WT and MLC2vcre-L/L mice. A pan-laminin antibody was used to outline the cell boundaries to score EBD within individual myocytes and fibrosis was not observed in hearts where these sections were taken. Patches of myocytes in MLC2vcre-L/L mice consisted of a number of contacting neighboring cells with similar orientation of their longitudinal axis. Note there was no significant difference in the staining with laminin suggesting an intact basal lamina.
Figure 7. Loss of dystroglycan function in cardiac myocytes causes expansion of myocardial damage to neighboring myocytes.
The scoring of EBD patches is described in detail in the methods A) The number of EBD positive patches per section was significantly increased in MLC2vcre × L/L mice compared to WT animals and was significantly increased by exercise in both groups. Data are mean +/- SEM, *p<0.01 vs WT-L/L no ex, , #p<0.01 vs WTL/L, ** p<0.05 vs all three other groups. B) Frequency distribution of the size of EBD positive patches in cross section of WT and MLC2vcre-L/L mice, with and without exercise. C) Summary of the numbers of EBD positive cells per patch in all patches scored. Data are mean +/- SEM (n=64-87), except WT-L/L no exercise where only 8 total patches were found. *p<0.01 vs WT-L/L +ex.
Figure 8. Myd mice show focal patches of cardiac myocyte membrane damage.
The upper panels show cardiac myocytes that show EBD uptake, also show uptake of large extracellular proteins such as immunoglobulin. In the lower panels, sedentary myd mice show patches of membrane damage, marked by immunoglobulin staining, similar to that observed in MLC2vcre-L/L animals.
Discussion
The mechanistic role of dystroglycan function in muscular dystrophy and cardiomyopathy, and the exact function of dystroglycan in striated muscle and smooth muscle is still debated. While cytoskeletal to matrix linkages through dystroglycan and other receptors have been proposed to be critical for muscle cell survival 20, the association of the dystrophin glycoprotein complex with signaling molecules such as kinase cascades and nitric oxide synthase suggest several possible significant roles in striated and vascular smooth muscle21, 22. Our results indicate that the loss of dystroglycan function in cardiac myocytes results in increased myocyte membrane damage in response to increased activity and is sufficient to cause a progressive dilated cardiomyopathy, and loss of dystroglycan function in smooth muscle does not cause a detectable cardiac phenotype.
Despite the uniform genetic deletion of dystroglycan function in myocytes (Figure 2 and 7), the membrane damage observed in MLC2vcre-L/L mice is non-uniform and occurs in distinct focal patches of adjacent myocytes, rather than just randomly membrane damaged myocytes throughout the myocardium. Patchy areas of cell membrane damage were also observed in myd mice in the presence of uniform loss of dystroglycan laminin binding function (Figure 1 and 8). Previous studies in sarcoglycan deficient animals suggested the cardiomyopathy was correlated with coronary vasospasm and loss of sarcoglycan expression in smooth muscle 2, 3. However, we did not observe evidence coronary vasopasm by microfil perfusion in MLC2vcrexL/L mice, and dystroglycan and DGC expression in smooth muscle is normal in MLC2vcre-L/L mice. Furthermore, no patches of myocardial damage or fibrosis were observed in SMMHCcre-L/L mice. We also demonstrate that exercise can induce surprisingly significant numbers of EBD positive cells in WT mice compared to unexcercised WT mice, but the damage is always limited to one or two cells. Taken together, these data suggest that the interaction of dystroglycan with the extracellular matrix is required for stabilizing neighboring cells against sarcolemma injury, and preventing expansion of membrane damage to neighboring cells in response to an individual cell becoming damaged. Because significant focal patches of membrane damage are observed in younger ML2vcrexL/L mice even in the absence of exercise, the accumulation of focal myocardial damage even under sedentary conditions is likely playing a primary role in the development of dystroglycan-deficient cardiomyopathy and the development of focal myocardial fibrosis. The relatively slow progression of the cardiac disease in MLC2vcre-L/L mice may be due to many cells in the patches with membrane damage undergoing successful plasma membrane repair resulting in fewer cells with permanent damage.
In the working myocardium, cardiac myocytes are attached to neighboring cells by interacting laterally with matrix that separates adjacent cells, and longitudinally through intercalated disks that mechanically and electrically connect neighboring cells. If isolated membrane damage in a single cell results in transient or permanent contractile failure, the surrounding myocytes may experience increased mechanical load. In the absence of stabilizing lateral interactions of those myocytes with extracellular matrix, this may result in inappropriate stretch or strain on neighboring myocytes that leads to damage of the sarcolemma. The localization of dystroglycan to costameres in striated muscle, places dystroglycan at an important location for stabilizing myocytes, the sarcolemma, and their sarcomeres against mechanical activity or stretch. Dystrophin deficient skeletal muscle has been shown to be particularly sensitive to the damaging effects of lengthening contractions 23. Other matrix receptors in muscle such as integrins, may also provide a parallel pathway toward in stabilizing cardiac myocytes. The combined loss of dystrophin and integrin in myocytes results in a more severe histological phenotype then the loss of either gene alone, especially under conditions of stress such as isoproterenol infusion 24. It is possible other mechanisms may be involved, including increased sensitivity of dystroglycan deficient myocytes to circulating factors released by myocytes with membrane damage. We noted the patches of membrane damage also appeared to be limited to myocytes that were organized in similar orientations of their longitudinal axis (Figure 6) suggesting the damage was related to orientation of the mechanical strain or stretch, and not simple diffusion of molecules to neighboring cells.
A qualitative comparison of the histopathological cardiac phenotypes of MLC2vcre-L/L mice (Figure 3), the phenotypes of myd mice (Figure 1 and Online Figure 4) and previous studies in dystrophin deficient mdx mice, suggests that MLC2vcre-L/L mice may have a more severe cardiac phenotype than both myd and mdx mice. This could be due to the fact that myd mice retain dystroglycan protein expression and preserve some of the intracellular functions of the DGC21, 22 Alternatively, the lack of skeletal muscle disease in MLC2v cre mice, may result in increased locomotor activity and added stress on the heart as compared to other dystrophic mouse models. Dystrophic mdx mice fatigue very quickly 22 and myd mice have observable reduced mobility and hindlimb paralysis due to severe dystrophy and peripheral nerve dysfunction. Rescue of skeletal muscle disease in mdx mice by a skeletal muscle-specific mini-dystrophin transgene has been shown to lead to increased voluntary activity, and a worse cardiac phenotype, possibly due to increased stress placed on these hearts 25. Therefore, there may be a paradoxical inverse relationship between cardiac disease severity in dystrophic models, and the good health of skeletal muscle. This would fit well with our observation that acute exercise appears to increase the number of patches of cell membrane damage in MLC2vcre mice. While myd mice are a genetic model of the relatively rare congenital muscular dystrophy MDC1D, the loss of dystroglycan glycosylation and the function of dystroglycan as an extracellular matrix receptor is so far biochemically identical to many forms of glycosylation deficient muscular dystrophy including the relatively common LGMD2I 10. Our results suggest that if a specific genetic disruption of this glycosylation pathway affects dystroglycan matrix receptor function in cardiac myocytes, this will likely lead to increased susceptibility to activity-induced myocardial damage and cardiovascular disease in human patients.
Supplementary Material
Acknowledgments
We would like to thank Sally Prouty for technical assistance and the University of Iowa Hybridoma Facility for preparing the antibody IIH6. We thank the Nathan Shock Center In Vivo Functionality Core and Center for Integrative Genomics at the University Michigan for support. We would like to thank members of the Michele laboratory and Campbell laboratory for critical comments on the manuscript.
Sources of Funding: This work is funded by a grant in aid from the American Heart Association and NIH R01-HL080388 to DEM, an American Heart Association post-doctoral fellowship to ZK, and NIH RR017369 to RMW. This work was supported in part by the Senator Paul D. Wellstone Muscular Dystrophy Cooperative Research Center grant 1 U54 NS053672. KPC is an investigator of the Howard Hughes Medical Institute.
Non-Standard Abbreviations and Acronyms
- DGC
dystrophin-glycoprotein complex
- DG
dystroglycan
- myd
myodystrophy
- LGMD
limb girdle muscular dystrophy
- MLC2v
myosin light chain 2v
- SMMHC
smooth muscle myosin heavy chain
- HW/BW
heart weight/body weight
- EBD
Evan's blue dye
Footnotes
Disclosures: None
References
- 1.Finsterer J, Stollberger C. The heart in human dystrophinopathies. Cardiology. 2003;99(1):1–19. doi: 10.1159/000068446. [DOI] [PubMed] [Google Scholar]
- 2.Coral-Vazquez R, Cohn RD, Moore SA, Hill JA, Weiss RM, Davisson RL, Straub V, Barresi R, Bansal D, Hrstka RF, Williamson R, Campbell KP. Disruption of the sarcoglycan-sarcospan complex in vascular smooth muscle: a novel mechanism for cardiomyopathy and muscular dystrophy. Cell. 1999;98(4):465–474. doi: 10.1016/s0092-8674(00)81975-3. [DOI] [PubMed] [Google Scholar]
- 3.Durbeej M, Cohn RD, Hrstka RF, Moore SA, Allamand V, Davidson BL, Williamson RA, Campbell KP. Disruption of the beta-sarcoglycan gene reveals pathogenetic complexity of limb-girdle muscular dystrophy type 2E. Mol Cell. 2000;5(1):141–151. doi: 10.1016/s1097-2765(00)80410-4. [DOI] [PubMed] [Google Scholar]
- 4.Nigro V, Okazaki Y, Belsito A, Piluso G, Matsuda Y, Politano L, Nigro G, Ventura C, Abbondanza C, Molinari AM, Acampora D, Nishimura M, Hayashizaki Y, Puca GA. Identification of the Syrian hamster cardiomyopathy gene. Hum Mol Genet. 1997;6(4):601–607. doi: 10.1093/hmg/6.4.601. [DOI] [PubMed] [Google Scholar]
- 5.Thomas GD, Sander M, Lau KS, Huang PL, Stull JT, Victor RG. Impaired metabolic modulation of alpha-adrenergic vasoconstriction in dystrophin-deficient skeletal muscle. Proc Natl Acad Sci U S A. 1998;95(25):15090–15095. doi: 10.1073/pnas.95.25.15090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heydemann A, Huber JM, Kakkar R, Wheeler MT, McNally EM. Functional nitric oxide synthase mislocalization in cardiomyopathy. J Mol Cell Cardiol. 2004;36(2):213–223. doi: 10.1016/j.yjmcc.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 7.Wheeler MT, Allikian MJ, Heydemann A, Hadhazy M, Zarnegar S, McNally EM. Smooth muscle cell-extrinsic vascular spasm arises from cardiomyocyte degeneration in sarcoglycan-deficient cardiomyopathy. J Clin Invest. 2004;113(5):668–675. doi: 10.1172/JCI20410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsubata S, Bowles KR, Vatta M, Zintz C, Titus J, Muhonen L, Bowles NE, Towbin JA. Mutations in the human delta-sarcoglycan gene in familial and sporadic dilated cardiomyopathy. Journal of Clinical Investigation. 2000;106(5):655–662. doi: 10.1172/JCI9224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Straub V, Ettinger AJ, Durbeej M, Venzke DP, Cutshall S, Sanes JR, Campbell KP. epsilon-sarcoglycan replaces alpha-sarcoglycan in smooth muscle to form a unique dystrophin-glycoprotein complex. J Biol Chem. 1999;274(39):27989–27996. doi: 10.1074/jbc.274.39.27989. [DOI] [PubMed] [Google Scholar]
- 10.Michele DE, Barresi R, Kanagawa M, Saito F, Cohn RD, Satz JS, Dollar J, Nishino I, Kelley RI, Somer H, Straub V, Mathews KD, Moore SA, Campbell KP. Post-translational disruption of dystroglycan-ligand interactions in congenital muscular dystrophies. Nature. 2002;418(6896):417–422. doi: 10.1038/nature00837. [DOI] [PubMed] [Google Scholar]
- 11.Grewal PK, Holzfeind PJ, Bittner RE, Hewitt JE. Mutant glycosyltransferase and altered glycosylation of alpha-dystroglycan in the myodystrophy mouse. Nat Genet. 2001;28(2):151–154. doi: 10.1038/88865. [DOI] [PubMed] [Google Scholar]
- 12.Barresi R, Michele DE, Kanagawa M, Harper HA, Dovico SA, Satz JS, Moore SA, Dumanski JP, Schachter H, Cohn RD, Nishino I, Campbell KP. LARGE functionally bypasses alpha-dystroglycan glycosylation defects in congenital muscular dystrophies. Nature Medicine. 2004;10(7):696–703. doi: 10.1038/nm1059. [DOI] [PubMed] [Google Scholar]
- 13.Holzfeind PJ, Grewal PK, Reitsamer HA, Kechvar J, Lassmann H, Hoeger H, Hewitt JE, Bittner RE. Skeletal, cardiac and tongue muscle pathology, defective retinal transmission, and neuronal migration defects in the Large(myd) mouse defines a natural model for glycosylation-deficient muscle - eye - brain disorders. Hum Mol Genet. 2002;11(21):2673–2687. doi: 10.1093/hmg/11.21.2673. [DOI] [PubMed] [Google Scholar]
- 14.Hayashi YK, Ogawa M, Tagawa K, Noguchi S, Ishihara T, Nonaka I, Arahata K. Selective deficiency of alpha-dystroglycan in Fukuyama-type congenital muscular dystrophy. Neurology. 2001;57(1):115–121. doi: 10.1212/wnl.57.1.115. [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Kubalak SW, Chien KR. Ventricular muscle-restricted targeting of the RXRalpha gene reveals a non-cell-autonomous requirement in cardiac chamber morphogenesis. Development. 1998;125(10):1943–1949. doi: 10.1242/dev.125.10.1943. [DOI] [PubMed] [Google Scholar]
- 16.Regan CP, Manabe I, Owens GK. Development of a smooth muscle-targeted cre recombinase mouse reveals novel insights regarding smooth muscle myosin heavy chain promoter regulation. Circ Res. 2000;87(5):363–369. doi: 10.1161/01.res.87.5.363. [DOI] [PubMed] [Google Scholar]
- 17.Moore SA, Saito F, Chen J, Michele DE, Henry MD, Messing A, Cohn RD, Ross-Barta SE, Westra S, Williamson RA, Hoshi T, Campbell KP. Deletion of brain dystroglycan recapitulates aspects of congenital muscular dystrophy. Nature. 2002;418(6896):422–425. doi: 10.1038/nature00838. [DOI] [PubMed] [Google Scholar]
- 18.Weiss RM, Ohashi M, Miller JD, Young SG, Heistad DD. Calcific aortic valve stenosis in old hypercholesterolemic mice. Circulation. 2006;114(19):2065–2069. doi: 10.1161/CIRCULATIONAHA.106.634139. [DOI] [PubMed] [Google Scholar]
- 19.Pandya K, Kim HS, Smithies O. Fibrosis, not cell size, delineates beta-myosin heavy chain reexpression during cardiac hypertrophy and normal aging in vivo. Proc Natl Acad Sci U S A. 2006;103(45):16864–16869. doi: 10.1073/pnas.0607700103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Towbin JA, Bowles NE. Molecular genetics of left ventricular dysfunction. Curr Mol Med. 2001;1(1):81–90. doi: 10.2174/1566524013364077. [DOI] [PubMed] [Google Scholar]
- 21.Higginson JR, Winder SJ. Dystroglycan: a multifunctional adaptor protein. Biochem Soc Trans. 2005;33(Pt 6):1254–1255. doi: 10.1042/BST0331254. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi YM, Rader EP, Crawford RW, Iyengar NK, Thedens DR, Faulkner JA, Parikh SV, Weiss RM, Chamberlain JS, Moore SA, Campbell KP. Sarcolemma-localized nNOS is required to maintain activity after mild exercise. Nature. 2008;456(7221):511–515. doi: 10.1038/nature07414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dellorusso C, Crawford RW, Chamberlain JS, Brooks SV. Tibialis anterior muscles in mdx mice are highly susceptible to contraction-induced injury. Journal of Muscle Research & Cell Motility. 2001;22(5):467–475. doi: 10.1023/a:1014587918367. [DOI] [PubMed] [Google Scholar]
- 24.Elsherif L, Huang MS, Shai SY, Yang Y, Li RY, Chun J, Mekany MA, Chu AL, Kaufman SJ, Ross RS. Combined deficiency of dystrophin and beta1 integrin in the cardiac myocyte causes myocardial dysfunction, fibrosis and calcification. Circ Res. 2008;102(9):1109–1117. doi: 10.1161/CIRCRESAHA.108.173153. [DOI] [PubMed] [Google Scholar]
- 25.Townsend D, Yasuda S, Li S, Chamberlain JS, Metzger JM. Emergent dilated cardiomyopathy caused by targeted repair of dystrophic skeletal muscle. Mol Ther. 2008;16(5):832–835. doi: 10.1038/mt.2008.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.