Summary
Methylphenidate is a frequently prescribed stimulant for the treatment of attention deficit hyperactivity disorder (ADHD). An important assumption in the animal models that have been employed to study methylphenidate’s effects on the brain and behavior is that bioavailability of methylphenidate in the animal models reflects that in human subjects. From this perspective, the dose and route of administration of methylphenidate assume critical importance because both these factors likely influence rate of uptake, plasma and brain concentrations of the drug. In the present study, plasma and brain concentrations of D- and L-methylphenidate and D- and L-ritalinic acid were measured in 2-month old mice (equivalent to young adulthood in humans) following a single oral administration of a racemic mixture. Our data show that oral administration of 0.75 mg/kg dose produced within 15 min, plasma levels of D-methylphenidate that correspond to the clinically effective plasma levels in human subjects (estimated to be 6-10 ng/ml). Brain concentrations of D- and L-methylphenidate tended to exceed their plasma concentrations, while the plasma concentrations of D- and L-ritalinic acid exceeded their brain concentrations. A single oral administration at 0.75 mg/kg dose increased dopamine content of the frontal cortex within 1 hr, without producing statistically significant changes in serotonin or noradrenaline contents. Striatal monoamine levels remained unaltered. These data highlight disparities between plasma and brain concentrations of methylphenidate and its metabolites following oral administration and illustrate brain region- and monoamine-specific changes produced by the low oral dose of methylphenidate.
Keywords: Methylphenidate, ritalinic acid, dopamine, striatum, frontal cortex
Introduction
Methylphenidate is one of the most frequently prescribed medications for the treatment of attention deficit hyperactivity disorder (ADHD). Its prescription usage has been rising along with increasing public awareness of the morbidity and disability associated with this disorder, and the critical importance of successful diagnosis and treatment (Olfson et al., 2002; Zuvekas et al., 2006). At the same time, diversion of methylphenidate for use as a recreational street-drug has also been on the rise (Klein-Schwartz, 2002; McCabe et al., 2004; Teter et al., 2003) causing serious and persisting concerns about the potential addictive properties of this compound (Biederman et al., 1997; Biederman et al., 1998; Klein-Schwartz, 2002; Robbins, 2002; Volkow, 2006).
Since these safety concerns cannot be adequately studied in humans, animal models have been employed to examine the mechanisms of action of methylphenidate and its addictive potential (Andersen et al., 2002; Augustyniak et al., 2006; Bolanos et al., 2003; Brandon et al., 2001; Carlezon et al., 2003; Davids et al., 2003; Randall and Hannigan, 1999; Russell et al., 2005; Sagvolden et al., 2005; Thanos et al., 2007). However, a drawback associated with some earlier animal studies is that methylphenidate was administered parenterally and at 2-10 times the therapeutic dose used in humans. Such administration can cause rapid rise in plasma and brain methylphenidate levels, which can reach peaks 5-10 times those seen in patients taking therapeutic doses of methylphenidate orally as ADHD treatment (Gerasimov and Volkova, 1991; Kuczenski and Segal, 2002, 2005; Patrick and Markowitz, 1997).
Although D-methylphenidate is thought to be the active ingredient of the compound, a racemic mixture of methylphenidate containing both the D and L enantiomers is used most frequently in the treatment of ADHD (Ding et al., 1997; Ding et al., 2004; Schweri et al., 1985). Yet, the plasma and brain distributions of each enantiomer following oral administration of the racemic mixture are not fully characterized in the animal models. Since, we do not know if L-methylphenidate has therapeutic or adverse influences or if it modifies the actions of D-methylphenidate (Ding et al., 2004), understanding bioavailability of both the enantiomers is of high clinical and scientific importance. Equally significantly, the bioavailability of methylphenidate metabolites, D- and L-ritalinic acid following oral methylphenidate administration is also not well characterized in animal models.
To address these issues in an animal model, we analyzed plasma and brain levels of D- and L-methylphenidate and their metabolites D- and L-ritalinic acid at 15, 30, 60 and 120 min after a single oral administration of racemic methylphenidate at 0.75, 2.0 or 5.0 mg/kg doses to 2-month old mice. We report that 0.75 mg/kg oral dose produces within 15 min of administration, plasma levels of D-methylphenidate that are comparable to those seen in ADHD patients taking oral therapeutic doses of methylphenidate. Moreover, this dose produces significant increases (∼ 6-fold) in dopamine concentration in the frontal cortex in 1 hr. Neither serotonin nor noradrenaline showed statistically significant changes in the frontal cortex within 1 hr at this dose. Interestingly, no statistically significant changes were seen in the concentrations of any of the monoamines in the striatum within 1 hr.
Materials and Methods
Animals
We used 2-month old male CD1 mice purchased from Charles River Laboratories (Wilmington, MA). The mice were maintained in the institutional animal facility on a 12 hr light-dark cycle for approximately 10 days prior to the experimentation. The experiments were approved by the animal care and use committee of the Massachusetts General Hospital and conformed to NIH guidelines for care and use of laboratory animals.
Methylphenidate administration and sample collection
Mice were administered an aqueous solution of methylphenidate (racemic mixture, Sigma-Aldrich, St. Louis, MO) or saline by oral gavage using a feeding needle (Fisher Scientific, Pittsburgh, PA). Mice were not anesthetized during the gavage procedure. We did not withhold food prior to the gavage. Methylphenidate was administered at 0.75, 2.0 or 5.0 mg/kg dose. Following 15, 30, 60 or 120 minutes of the gavage, the mice were decapitated and trunk blood was collected in 1 ml lithium-heparin tubes (Fischer Scientific, Pittsburgh, PA) and mixed 3-4 times and stored on ice. The heparinized samples were centrifuged at 3,000 rpm for 20 min at 4°C. Plasma (100-300μl) was aspirated from the centrifuged specimen using a transfer pipette. The entire procedure, from decapitation to plasma collection, was completed within 40 minutes. Plasma samples were stored at -80°C.
Brain was dissected from the skull and the two hemispheres were separated by a midline cut and each hemisphere was snap frozen in liquid nitrogen. One hemisphere from each brain was used for analysis of methylphenidate and ritalinic acid levels and the other was used for analysis of neurotransmitter and metabolite content.
For each dose and each survival time, we used 10 mice. Only male mice were used. Plasma and brain samples were shipped on dry ice to the Human Toxicology Research Center, University of Utah, Salt Lake City, Utah for analysis of methylphenidate and ritalinic acid levels.
Analysis of methylphenidate and ritalinic acid concentrations
Each serum sample was extracted into a combination of acetonitrile and methyl t-butyl ether. Each extract was concentrated and injected into a chiral liquid chromatography column and a tandem mass spectrometer that employed electrospray ionization and selected-reaction monitoring. The calibration standards contained each of the enantiomers of ritalinic acid and methylphenidate with concentrations ranging from 0.5 ng/mL to 100 ng/mL. Quality control samples were also prepared (1.0, 10 and 75 ng/mL) and analyzed.
The brain tissues were moved from the -80°C freezer to an ice bucket to keep the samples cold while the tissue specimens were weighed. The tissue was homogenized with a 0.1 M phosphate buffer containing 1% sodium fluoride. The volume of buffer was 9-fold the weight of each tissue specimen. For example, 0.2 grams of tissue would be mixed with 1.8 milliliters of buffer.
Each homogenized tissue sample was extracted into 400 μL of acetonitrile and 50 μL of methyl t-butyl ether. Each extract was then concentrated and injected into the chiral liquid chromatography column and the tandem mass spectrometer. The calibration standards contained each of the enantiomers of ritalinic acid and methylphenidate with concentrations ranging from 1.0 to 100 ng/g.
Analysis of monoamines and their metabolites in samples of the striatum and frontal cortex
We analyzed monoamine neurotransmitter and metabolite concentrations in the striatum and frontal cortex of mice receiving 0.75 mg/kg methylphenidate at 15 min, 30 min and 60 min intervals. Baseline measurements were obtained from mice receiving saline instead of methylphenidate. The brains snap-frozen in liquid nitrogen were sectioned on a cryostat to the level of the anterior forceps, where a 1.0 mm coronal slab was cut. The striatum and frontal cortex were visually identified and tissue punches were obtained from each region bilaterally using a biopsy punch. We used 5 mice for each of the 3 survival periods. Samples from the right and left sides for each region from each brain were pooled for further analysis. The tissue punches were weighed and homogenized by ultrasonification in PE buffer (150 mM phosphoric acid; 0.2 mM ethylenediamine tetra acetic acid [EDTA]). Crude homogenates were centrifuged at 14,000 rpm for 20 minutes and the supernatant was filtered through a 0.22 μm cellulose acetate membrane.
In each tissue sample, we analyzed dopamine (DA) and its metabolites 3,4 dihydroxyphenylacetic acid (DOPAC) and 3-methoxy-4-hydroxyphenyl acetic acid (homovanillic acid, HVA); 5-hydroxytryptamine (serotonin, 5-HT) and its metabolite 5-hydroxyindole-3-acetic acid (5-HIAA) and noradrenaline (DL-Norepinephrine hydrochloride, NE). Quantification of monoamines was performed isocratically at room temperature by HPLC through a model MD-150 analytical column (C18 3.2 mm × 150 mm; 3 μm particle size; 120 Å pore size) at a flow rate of 0.6 ml/min with electrochemical detection (model 5600A Coulochem II electrochemical detector; model 5011 analytical cell with palladium reference electrode; model 5020 guard cell; ESA, Chelmsford MA) at the following potentials: guard cell +250 mV; pre-oxidation -100 mV; detector +240 mV. The mobile phase consisted of 90 mM NaH2PO4 (monohydrate); 50 mM citric acid (monohydrate); 1.7 mM 1-octanesulfonic acid; 50 μM EDTA; and 10% (vol/vol) acetonitrile; pH=3.00 (phosphoric acid); filtered through a 0.22 μm cellulose acetate membrane, and degassed by ultrasonification. Under these conditions the retention time for monoamines was 3-11 minutes and the limit of detection was below 1.5 fmol. Monoamine levels were quantified by external standard curve calibration using CoulArray software (v.1.12; ESA). Monoamine standards (1.0 μM, 0.5 μM and 0.1 μM) were run at the beginning and end, and after every eighth sample for re-calibration during each experiment.
For the baseline group and each survival period, we used brains from 5 male mice.
Results
We compared plasma and brain levels of D- and L-methylphenidate as well as D- and L-ritalinic acid in mice receiving the 3 different oral doses of a racemic mixture of methylphenidate. Our principal goal was to establish the oral dose that produced a plasma D-methylphenidate level of ∼6-10 ng/ml, which is the plasma concentration achieved in ADHD patients (Kuczenski and Segal, 2005; Patrick and Markowitz, 1997; Sprague and Sleator, 1977; Swanson et al., 1999; Swanson and Volkow, 2002). We also examined the impact of this “therapeutic dose” on monoamine concentrations in the striatum and frontal cortex.
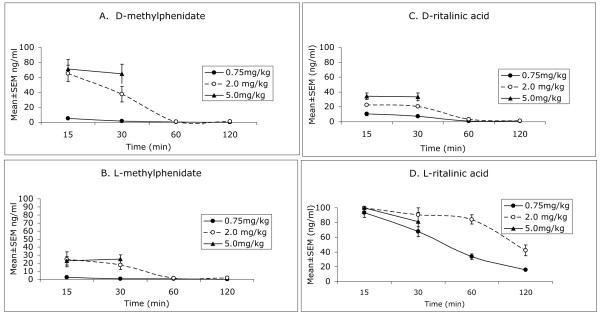
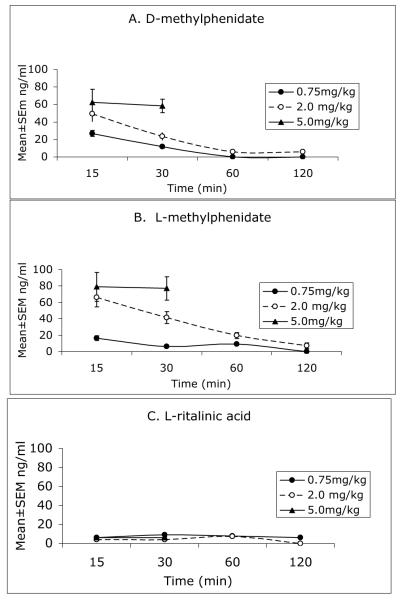
Concentrations (Mean±SEM) of D- and L-methylphenidate as well as D- and L-ritalinic acid in the plasma at the different doses and survival times are shown in Figure 1. Brain concentrations of the same compounds are shown in Figure 2. Our data suggest that an oral dose of 0.75 mg/kg methylphenidate can produce plasma levels of D-methylphenidate in the 6-10 ng/ml range within 15 min of administration. The plasma levels of D-methylphenidate produced by the 5 mg/kg dose reached nearly 8 times the estimated clinical levels by 15 min and remained at this high level at 30 min (Fig. 1). We did not consider this dose of methylphenidate to be therapeutically relevant and therefore discontinued its analysis beyond the 30 min interval.
Figure 1.
Plsama concentrations of D-methylphenidate (A), L-methylphenidate (B), D-ritalinic acid (C) and L-ritalinic acid (D) measured at 15, 30, 60 and 120 min following a single oral administration of racemic methylphenidate at 0.75, 2.0 or 5.0 mg/kg dose to 2-month old mice. Only the 15 and 30 min time points were used for the 5 mg/kg dose.
Figure 2.
Brain concentrations of D-methylphenidate (A), L-methylphenidate (B) and L-ritalinic acid (C) measured at 15, 30, 60 and 120 min following a single oral administration of racemic methylphenidate at 0.75, 2.0 or 5.0 mg/kg to 2-month old mice. Only the 15 and 30 min time points were used for the 5 mg/kg dose. D-ritalinic acid could not be detected in the brain at any dose or time point.
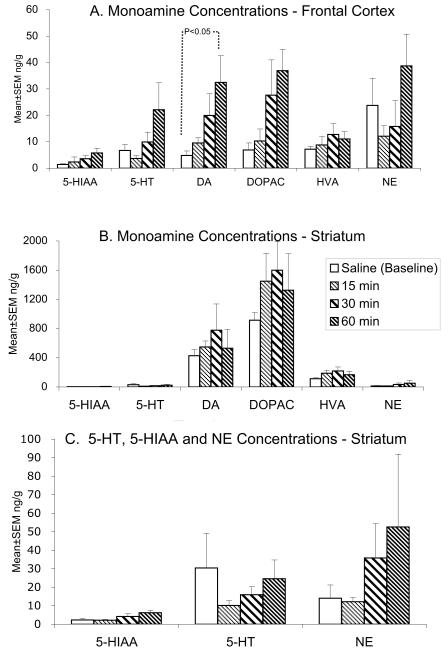
We analyzed the concentrations of dopamine, 5-HT and norepinephrine in the striatum and frontal cortex at 15, 30 and 60 minutes after administration of 0.75 mg/kg methylphenidate. We also measured dopamine metabolites HVA and DOPAC and 5-HT metabolite 5-HIAA in these 2 brain regions. We compared the measurements to baseline measurements obtained from mice that were administered saline instead of methylphenidate. The data are shown in Figure 3.
Figure 3.
Tissue concentrations of dopamine (DA), serotonin (5-HT), noradrenaline (NE) and the metabolites 3,4 dihydroxyphenylacetic acid (DOPAC), 3-methoxy-4-hydroxyphenyl acetic acid (homovanillic acid, HVA) and 5-hydroxyindole-3-acetic acid (5-HIAA) were measured in the frontal cortex (A) and striatum (B) by HPLC-EC in 2-month old mice at 15, 30 and 60 min following a single oral administration of a racemic mixture of methylphenidate at a dose of 0.75mg/kg. Data from age-matched controls receiving a single dose of saline were used as baseline measurements. There was a significant increase (p<0.05) in dopamine content of the frontal cortex at 60 min following methylphenidate administration compared to the baseline level (A). None of the other measurements showed statistically significant differences from the baseline data. Since the concentrations of DA, DOPAC and HVA are several fold higher than the concentrations of 5-HT, NE and 5-HIAA in the striatum, data for the latter 3 chemicals are shown separately in C. Differences among the groups were compared by using ANOVA and Tukey’s Multiple Comparisons Test.
We found that the only statistically significant difference that occurred was in the concentration of dopamine in the frontal cortex (Fig. 3A; ANOVA; F=3.32; p<0.05). This difference was due to a ∼6-fold increase in dopamine concentration at 60 minutes following the methylphenidate administration compared to the concentration in the saline-administered controls (Fig. 3A; Tukey’s Multiple Comparison Test, p<0.05).
Discussion
We analyzed plasma and brain concentrations of D- and L-methylphenidate and D- and L-ritalinic acid following oral administration of a racemic mixture of methylphenidate. Our data show different brain and plasma profiles for each of the compounds (Figs. 1, 2 and 4), suggesting differential enzymatic breakdown in the gastrointestinal tract or plasma, differential permeability of the blood-brain barrier and/or different rates of clearance from the brain (Patrick et al., 1984; Thai et al., 1999). We found that serum concentration of ∼6-10 ng/ml of D-methylphenidate, equivalent to the serum concentration of this compound in ADHD patients (Kuczenski and Segal, 2005; Patrick and Markowitz, 1997; Sprague and Sleator, 1977; Swanson et al., 1999; Swanson and Volkow, 2002), was achieved in 15 minutes at the oral dose of 0.75 mg/kg. In addition, brain concentrations of monoamines in response to 0.75mg/kg oral dose of methylphenidate showed selectivity in terms of the monoamine species affected and the brain region affected. Thus, dopamine content was significantly elevated at 60 min (but not earlier) in the frontal cortex and not in the striatum. Neither serotonin nor norepinephrine showed statistically significant changes in either brain region.
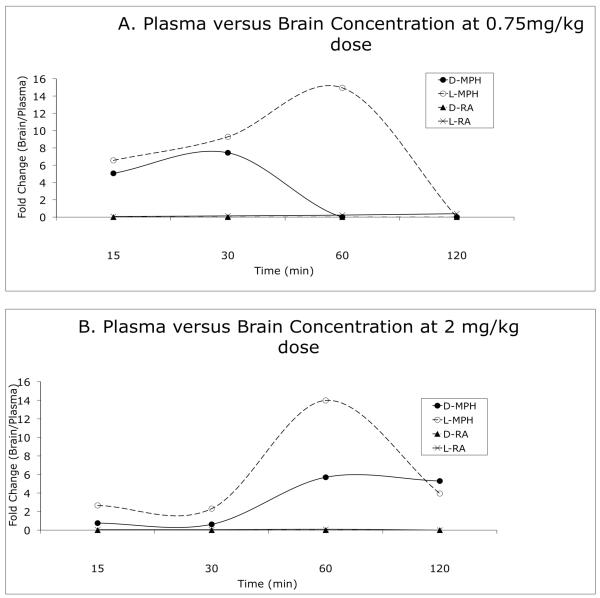
Figure 4.
Comparison of the concentrations of D- and L-methylphenidate and D- and L-ritalinic acid in the plasma and brain. A and B show quantitative relationship (fold-increase) between plasma and brain concentrations (brain concentration ÷ plasma concentration) of the 4 compounds following 0.75 mg/kg (A) or 2 mg/kg (B) oral dose. Brain concentrations of D- and L-methylphenidate differ from the plasma concentrations (generally higher) as a function of dose and time. Brain concentrations of L-ritalinic acid are only about a tenth of its plasma concentration. D-ritalinic acid is not present in the brain.
Interestingly, the 0.75 m/kg oral dose that was found to be therapeutically relevant is comparable to the 0.25 - 1.0 mg/kg oral dose administered clinically (Sprague and Sleator, 1977; Swanson et al., 1999; Swanson and Volkow, 2002). The therapeutic efficacy of this dose may result from the fact that at 0.25 mg/kg oral dose, methylphenidate can block 50% of the dopamine transporter in the human brain. This level of transporter occupancy corresponds to serum concentration of 6 ng/ml D-methylphenidate (Volkow et al., 1998). Furthermore, the maximum blockade of the dopamine transporter (∼ 80% occupancy) occurs at a serum methylphenidate concentrations of 8-10ng/ml (Swanson and Volkow, 2002). Thus, the therapeutic relevance of the 0.75 mg/kg oral dose appears to be valid based on the oral dose used in the clinic, plasma levels obtained in patients and occupancy level of the dopamine transporter.
A number of studies have examined the pharmacokinetics and bioavailability of methylphenidate in animal models. In rats, 10 mg/kg oral administration of methylphenidate produced 15-100 ng/ml plasma concentration of the racemic compound within 30 minutes (Wargin et al., 1983) whereas 1 mg/kg oral administration produced a plasma concentration of 20 ng/ml (Patrick et al., 1984). In another study, an oral dose of 1 mg/kg methylphenidate produced plasma levels of 9.3 ng/ml within 15 min (Kuczenski and Segal, 2002). An earlier study reported ∼2 ng/ml plasma levels of methylphenidate following 0.5 mg/kg oral dose, 36 ng/kg following 2 mg/kg dose and 62 ng/ml following 3.5 mg/kg dose (Aoyama et al., 1990). Oral administration of 0.75 mg/kg was predicted to yield peak plasma concentrations between 2 and 9 ng/ml, representing lower limits of the clinical range, whereas a dose of 3 mg/kg was predicted to produce peak plasma concentrations between 30 and 60 ng/ml, corresponding to upper limits of the clinical range (Kuczenski and Segal, 2002). Our data support these predictions.
Preclinical models of methylphenidate bioavailability are often based on plasma concentrations of the drug rather than brain concentrations because brain concentrations of D-methylphenidate are difficult to measure accurately in human subjects. We found that the brain concentrations of D-methylphenidate and L-methylphenidate were 4- and 5-fold higher than the plasma concentrations, respectively, in 15 min of administration of the drug at 0.75 mg/kg dose (Fig. 4A). At 30 min, the brain concentrations were 6-8 fold higher than plasma concentrations for both these compounds. In fact, L-methylphenidate concentrations in the brain were 13-fold higher than the plasma concentrations at 60 min. At the 2 mg/kg dose (Fig. 4B), the plasma and brain concentrations of D-methylphenidate were similar at 15 and 30 min. However at 60 and 120 min, the brain concentrations of this compound were nearly 5-fold higher than the plasma concentrations. The brain concentrations of L-ritalinic acid are only a tenth of the concentrations in the plasma and D-ritalinic acid was not detectable in the brain (Fig. 4).
Both dose and elapsed time appear to modulate the disparities between brain and plasma concentrations of methylphenidate. At the 0.75 mg/kg dose, brain concentrations of D-methylphenidate were greater than plasma concentrations at 15 and 30 min and resembled the plasma concentrations at 60 and 120 min (Fig. 4A). In contrast, at the 2mg/kg dose, the brain concentrations of this compound resembled plasma concentrations at 15 and 30 min and are higher at 60 and 120 min (Fig. 4B).
Previous studies examining the pharmacokinetics of methylphenidate in rodent models also showed that brain concentrations of the compound were consistently higher than plasma concentrations (Patrick et al., 1984; Thai et al., 1999) and that similar to our results, maximal plasma and brain concentrations of D-methylphenidate were attained within 10 min of administration (Thai et al., 1999). A positron emission tomography study using orally administered radiolabeled D- and L-methylphenidate also found higher levels of L-methylphenidate in the brains of baboons and rats (Ding et al., 2004). Our finding of 4-8 fold difference between brain and plasma concentration of D-methylphenidate at 15 and 30 min following 0.75mg/kg methylphenidate administration tend to agree with findings from earlier studies using intraperitoneal (37μM/kg) or oral (1 mg/kg) administration (Kotaki et al., 1988; Patrick et al., 1984; Thai et al., 1999). Interestingly, we did not see a difference between plasma and brain levels of D-methylphenidate at 15 or 30 min following higher oral doses, namely 2 or 5 mg/kg. The principal mechanism by which methylphenidate accumulates in brain is passive diffusion with a saturable component of transport (facilitated transport). Following the higher doses of 2 and 5 mg/kg, passive diffusion and/or transport protein may be equilibrated or saturated by the higher plasma concentrations inhibiting passage of the drug into the brain. This may not be the only explanation for the lack of dose-dependent increase in brain concentrations of the drug, as lipophilicity and tissue affinity also determine tissue distributions.
Another interesting finding in the present study is that the concentrations of D- and L-methylphenidate in the brain were similar (Fig. 2). Although L-methylphenidate is thought to be pharmacologically inactive (Ding et al., 1997), studies in 6-hydroxydopamine lesioned rat models suggest that it may interfere with the effects of D-methylphenidate on locomotor function (Davids et al., 2002). Therefore, the high levels of L-methylphenidate in the brain shown here may warrant a closer examination of its potential impact on brain function by direct action or modulation of the activity of D-methylphenidate.
Our data on methylphenidate-induced changes in tissue dopamine content reveal significant insights into methylphenidate’s central actions. We found that at the 0.75 mg/kg dose, dopamine content of the frontal cortex was significantly increased but not that of the striatum (Fig. 3). The increase occurred at 60 min, and not earlier (Fig. 3A). Neither serotonin nor noradrenaline showed significant changes in either brain region (Fig. 3). The metabolites of dopamine or serotonin did not show significant changes (Fig. 3).
Our findings in the frontal cortex are unlikely to be artifacts of our technique because if they were, both the frontal cortex and striatum could be expected to show increases or decreases in tissue dopamine content. We found an increase only in the frontal cortex. We suggest that our findings highlight a regional difference in the actions of low dose methylphenidate on the dopaminergic system that could have significant implications for understanding methylphenidate’s therapeutic potential. Dopaminergic neurons in the ventral tegmentum that project to the frontal and cingulate cortices have either a dramatically reduced number of dopamine-synthesis modulating autoreceptors or they lack the receptors completely (Wolf and Roth, 1990). Additional differences between meso-preforntal and meso-striatal dopaminergic neurons also have been reported [reviewed in (Wolf and Roth, 1990)]. For example, dopamine turnover is faster in the frontal cortex than in the striatum. Frontal cortical neurons that lack autoreceptors show poor responses in terms of dopamine release and turnover following acute administration of dopaminergic drugs whereas regions that possess autoreceptors exhibit marked response. Frontal cortical neurons do possess release-modulating autoreceptors that influence dopamine synthesis. The lack of synthesis-modulating autorteceptors coupled with the expression of the release-modulating autoreceptors, creates a unique environment for dopamine turnover in the frontal cortex compared to the striatum.
It is possible that the initial reduction of tissue levels of dopamine due to methylphenidate-induced inhibition of the transporter triggers delayed dopamine synthesis in the frontal cortex. It might explain the lack of change in dopamine level until 60 min following the oral administration of methylphenidate. Precedence for this exists in the literature: Amphetamine can increase the synthesis of dopamine as well as increase extracellular dopamine levels (Sulzer et al., 2005). It is plausible that methylphenidate, like amphetamine, can increase dopamine synthesis in the frontal cortex in a region-specific manner, notwithstanding the differences between the two compounds in their mechanisms of action.
Another important consideration is the effects of very low doses of methylphenidate. Methylphenidate’s effects are highly dose-dependent (Volkow et al., 2002). The low dose may produce regional differences in dopamine synthesis, release or turnover (Berridge et al., 2006) that contribute to our finding of increased tissue levels of dopamine in the frontal cortex. Precedence for this also exists: Low oral doses of methylphenidate do not stimulate dopamine release in the nucleus accumbens (Gerasimov et al., 2000; Kuczenski and Segal, 2002) but trigger noradrenaline release in the hippocampus (Kuczenski and Segal, 2002).
Low versus high doses of methylphenidate produce different effects on locomotor sensitization (Kuczenski and Segal, 2001, 2002), expression of immediate early genes such as c-Fos (Chase et al., 2005), firing rate of midbrain dopamine neurons (Brandon et al., 2003) and in conditioned place preference paradigms (Meririnne et al., 2001). Although our study does not directly characterize the way in which low doses of methylphenidate increase tissue levels of dopamine in the frontal cortex, the fact that it does reflects interactions between low oral doses of methylphenidate and the unique mechanisms of dopamine release, turnover or synthesis in this brain region.
Interestingly, the increases in frontal cortical dopamine content occurred 60 min after the oral methylphenidate dose, when the brain concentration of D-methylphenidate had declined to virtually undetectable levels (Fig. 2). In earlier studies in rats, dopamine release in the striatum or nucleus accumbens measured by in vivo microdialysis showed significant increases within 20 min of intravenous, intraperitoneal or oral administration of methylphenidate (Aoyama et al., 1996; Gerasimov et al., 2000; Kuczenski and Segal, 2002). However, these rapid increases occurred only with significantly higher oral doses of methylphenidate (5 mg/kg) than the dose used in the present study (Gerasimov et al., 2000; Kuczenski and Segal, 2002). It is important to note that the kinetics of methylphenidate-induced dopamine release in the frontal cortex are not well characterized at low or high doses.
We point out that our data on the brain content of monoamines and their metabolites reflect total tissue content - intracellular and extracellular. The therapeutic effects of methylphenidate are linked to increased dopamine release. Measurement of total dopamine content may not accurately reflect released dopamine. Therefore, our methodology cannot determine whether 0.75 mg/kg oral methylphenidate produces ‘therapeutic’ increases in the dopamine content of the frontal cortex.
In summary, our data show that 0.75 mg/kg oral administration of a racemic mixture of methylphenidate produces serum levels of D-methylphenidate that are equivalent to those seen in human subjects administered the same compound at approximately the same oral dose for treatment of ADHD. The plasma levels of D-methylphenidate are not accurate predictors of brain levels of the same compound. A single oral administration of methylphenidate at 0.75 mg/kg dose increases dopamine content of the frontal cortex within 1 hr, without producing statistically significant changes in serotonin or noradrenaline levels. Striatal monoamine levels do not show significant changes at this dose. These region-specific and monoamine-specific effects may reflect interactions between low oral doses of methylphenidate and dopamine signaling mechanisms unique to the frontal cortex and may underlie behavioral and molecular changes produced by low doses of methylphenidate.
Acknowledgements
Analysis of plasma and brain methylphenidate concentrations were performed by Drs. Rodger Foltz and Shen-Nan Lin of the Human Toxicology Research Center at the University of Utah, Salt Lake City, UT under an NIH-NIDA sponsored program. We thank Dr. Michael Schwarzschild, Massachusetts General Hospital, Boston, MA for giving us access to the HPLC facility in his laboratory. This study was supported by PHS grant RO1DA020796. Additional partial support was received from The Pediatric Psychopharmacology Philanthropic Fund, Massachusetts General Hospital, Boston, MA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen SL, Arvanitogiannis A, Pliakas AM, LeBlanc C, Carlezon WA., Jr. Altered responsiveness to cocaine in rats exposed to methylphenidate during development. Nat Neurosci. 2002;5:13–14. doi: 10.1038/nn777. [DOI] [PubMed] [Google Scholar]
- Aoyama T, Kotaki H, Honda Y, Nakagawa F. Kinetic analysis of enantiomers of threo-methylphenidate and its metabolite in two healthy subjects after oral administration as determined by a gas chromatographic-mass spectrometric method. J Pharm Sci. 1990;79:465–469. doi: 10.1002/jps.2600790602. [DOI] [PubMed] [Google Scholar]
- Aoyama T, Kotaki H, Sawada Y, Iga T. Pharmacokinetics and pharmacodynamics of methylphenidate enantiomers in rats. Psychopharmacology (Berl) 1996;127:117–122. doi: 10.1007/BF02805984. [DOI] [PubMed] [Google Scholar]
- Augustyniak PN, Kourrich S, Rezazadeh SM, Stewart J, Arvanitogiannis A. Differential behavioral and neurochemical effects of cocaine after early exposure to methylphenidate in an animal model of attention deficit hyperactivity disorder. Behav Brain Res. 2006;167:379–382. doi: 10.1016/j.bbr.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AF, Kelley AE, Schmeichel B, Hamilton C, Spencer RC. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatry. 2006;60:1111–1120. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Biederman J, Wilens T, Mick E, Faraone SV, Weber W, Curtis S, Thornell A, Pfister K, Jetton JG, Soriano J. Is ADHD a risk factor for psychoactive substance use disorders? Findings from a four-year prospective follow-up study. J Am Acad Child Adolesc Psychiatry. 1997;36:21–29. doi: 10.1097/00004583-199701000-00013. [DOI] [PubMed] [Google Scholar]
- Biederman J, Wilens TE, Mick E, Faraone SV, Spencer T. Does attention-deficit hyperactivity disorder impact the developmental course of drug and alcohol abuse and dependence? Biol Psychiatry. 1998;44:269–273. doi: 10.1016/s0006-3223(97)00406-x. [DOI] [PubMed] [Google Scholar]
- Bolanos CA, Barrot M, Berton O, Wallace-Black D, Nestler EJ. Methylphenidate treatment during pre- and periadolescence alters behavioral responses to emotional stimuli at adulthood. Biol Psychiatry. 2003;54:1317–1329. doi: 10.1016/s0006-3223(03)00570-5. [DOI] [PubMed] [Google Scholar]
- Brandon CL, Marinelli M, Baker LK, White FJ. Enhanced reactivity and vulnerability to cocaine following methylphenidate treatment in adolescent rats. Neuropsychopharmacology. 2001;25:651–661. doi: 10.1016/S0893-133X(01)00281-0. [DOI] [PubMed] [Google Scholar]
- Brandon CL, Marinelli M, White FJ. Adolescent exposure to methylphenidate alters the activity of rat midbrain dopamine neurons. Biol Psychiatry. 2003;54:1338–1344. doi: 10.1016/s0006-3223(03)00787-x. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr., Mague SD, Andersen SL. Enduring behavioral effects of early exposure to methylphenidate in rats. Biol Psychiatry. 2003;54:1330–1337. doi: 10.1016/j.biopsych.2003.08.020. [DOI] [PubMed] [Google Scholar]
- Chase TD, Carrey N, Brown RE, Wilkinson M. Methylphenidate differentially regulates c-fos and fosB expression in the developing rat striatum. Brain Res Dev Brain Res. 2005;157:181–191. doi: 10.1016/j.devbrainres.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Davids E, Zhang K, Tarazi FI, Baldessarini RJ. Stereoselective effects of methylphenidate on motor hyperactivity in juvenile rats induced by neonatal 6-hydroxydopamine lesioning. Psychopharmacology (Berl) 2002;160:92–98. doi: 10.1007/s00213-001-0962-5. [DOI] [PubMed] [Google Scholar]
- Davids E, Zhang K, Tarazi FI, Baldessarini RJ. Animal models of attention-deficit hyperactivity disorder. Brain Res Brain Res Rev. 2003;42:1–21. doi: 10.1016/s0165-0173(02)00274-6. [DOI] [PubMed] [Google Scholar]
- Ding YS, Fowler JS, Volkow ND, Dewey SL, Wang GJ, Logan J, Gatley SJ, Pappas N. Chiral drugs: comparison of the pharmacokinetics of [11C]d-threo and L-threo-methylphenidate in the human and baboon brain. Psychopharmacology (Berl) 1997;131:71–78. doi: 10.1007/s002130050267. [DOI] [PubMed] [Google Scholar]
- Ding YS, Gatley SJ, Thanos PK, Shea C, Garza V, Xu Y, Carter P, King P, Warner D, Taintor NB, Park DJ, Pyatt B, Fowler JS, Volkow ND. Brain kinetics of methylphenidate (Ritalin) enantiomers after oral administration. Synapse. 2004;53:168–175. doi: 10.1002/syn.20046. [DOI] [PubMed] [Google Scholar]
- Gerasimov AA, Volkova AM. Treatment of patients with lumbar osteochondrosis by the method of intra-tissular electric stimulation. Ortop Travmatol Protez. 1991:13–17. [PubMed] [Google Scholar]
- Gerasimov MR, Franceschi M, Volkow ND, Gifford A, Gatley SJ, Marsteller D, Molina PE, Dewey SL. Comparison between Intraperitoneal and Oral Methylphenidate Administration: A Microdialysis and Locomotor Activity Study. J Pharmacol Exp Ther. 2000;295:51–57. [PubMed] [Google Scholar]
- Klein-Schwartz W. Abuse and toxicity of methylphenidate. Curr Opin Pediatr. 2002;14:219–223. doi: 10.1097/00008480-200204000-00013. [DOI] [PubMed] [Google Scholar]
- Kotaki H, Aoyama T, Nakazato F, Saitoh Y, Nakagawa F. Interactions in tissue distribution between methylphenidate and pemoline. II. Effects of methylphenidate or its metabolite on plasma and tissue concentrations of pemoline in the rat. Chem Pharm Bull (Tokyo) 1988;36:4560–4566. doi: 10.1248/cpb.36.4560. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Locomotor effects of acute and repeated threshold doses of amphetamine and methylphenidate: relative roles of dopamine and norepinephrine. J Pharmacol Exp Ther. 2001;296:876–883. [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Exposure of adolescent rats to oral methylphenidate: preferential effects on extracellular norepinephrine and absence of sensitization and cross-sensitization to methamphetamine. J Neurosci. 2002;22:7264–7271. doi: 10.1523/JNEUROSCI.22-16-07264.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Stimulant actions in rodents: implications for attention-deficit/hyperactivity disorder treatment and potential substance abuse. Biol Psychiatry. 2005;57:1391–1396. doi: 10.1016/j.biopsych.2004.12.036. [DOI] [PubMed] [Google Scholar]
- McCabe SE, Teter CJ, Boyd CJ, Guthrie SK. Prevalence and correlates of illicit methylphenidate use among 8th, 10th, and 12th grade students in the United States, 2001. J Adolesc Health. 2004;35:501–504. doi: 10.1016/j.jadohealth.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Meririnne E, Kankaanpaa A, Seppala T. Rewarding properties of methylphenidate: sensitization by prior exposure to the drug and effects of dopamine D1- and D2-receptor antagonists. J Pharmacol Exp Ther. 2001;298:539–550. [PubMed] [Google Scholar]
- Olfson M, Marcus SC, Weissman MM, Jensen PS. National trends in the use of psychotropic medications by children. J Am Acad Child Adolesc Psychiatry. 2002;41:514–521. doi: 10.1097/00004583-200205000-00008. [DOI] [PubMed] [Google Scholar]
- Patrick KS, Ellington KR, Breese GR. Distribution of methylphenidate and p-hydroxymethylphenidate in rats. J Pharmacol Exp Ther. 1984;231:61–65. [PubMed] [Google Scholar]
- Patrick KS, Markowitz JS. Pharmacology of methylphenidate, amphetamine enantiomers and pemoline in attention-deficit hyperactivity disorder. Human Psychopharmacol. 1997;12:527–546. [Google Scholar]
- Randall S, Hannigan JH. In utero alcohol and postnatal methylphenidate: locomotion and dopamine receptors. Neurotoxicol Teratol. 1999;21:587–593. doi: 10.1016/s0892-0362(99)00017-3. [DOI] [PubMed] [Google Scholar]
- Robbins TW. ADHD and addiction. Nat Med. 2002;8:24–25. doi: 10.1038/nm0102-24. [DOI] [PubMed] [Google Scholar]
- Russell VA, Sagvolden T, Johansen EB. Animal models of attention-deficit hyperactivity disorder. Behav Brain Funct. 2005;1:9. doi: 10.1186/1744-9081-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagvolden T, Russell VA, Aase H, Johansen EB, Farshbaf M. Rodent models of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1239–1247. doi: 10.1016/j.biopsych.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Schweri MM, Skolnick P, Rafferty MF, Rice KC, Janowsky AJ, Paul SM. [3H]Threo-(+/-)-methylphenidate binding to 3,4-dihydroxyphenylethylamine uptake sites in corpus striatum: correlation with the stimulant properties of ritalinic acid esters. J Neurochem. 1985;45:1062–1070. doi: 10.1111/j.1471-4159.1985.tb05524.x. [DOI] [PubMed] [Google Scholar]
- Sprague RL, Sleator EK. Methylphenidate in hyperkinetic children: differences in dose effects on learning and social behavior. Science. 1977;198:1274–1276. doi: 10.1126/science.337493. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol. 2005;75:406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Swanson J, Gupta S, Guinta D, Flynn D, Agler D, Lerner M, Williams L, Shoulson I, Wigal S. Acute tolerance to methylphenidate in the treatment of attention deficit hyperactivity disorder in children. Clin Pharmacol Ther. 1999;66:295–305. doi: 10.1016/S0009-9236(99)70038-X. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Volkow ND. Pharmacokinetic and pharmacodynamic properties of stimulants: implications for the design of new treatments for ADHD. Behav Brain Res. 2002;130:73–78. doi: 10.1016/s0166-4328(01)00433-8. [DOI] [PubMed] [Google Scholar]
- Teter CJ, McCabe SE, Boyd CJ, Guthrie SK. Illicit methylphenidate use in an undergraduate student sample: prevalence and risk factors. Pharmacotherapy. 2003;23:609–617. doi: 10.1592/phco.23.5.609.34187. [DOI] [PubMed] [Google Scholar]
- Thai DL, Yurasits LN, Rudolph GR, Perel JM. Comparative pharmacokinetics and tissue distribution of the d-enantiomers of para-substituted methylphenidate analogs. Drug Metab Dispos. 1999;27:645–650. [PubMed] [Google Scholar]
- Thanos PK, Michaelides M, Benveniste H, Wang GJ, Volkow ND. Effects of chronic oral methylphenidate on cocaine self-administration and striatal dopamine D2 receptors in rodents. Pharmacol Biochem Behav. 2007;87:426–433. doi: 10.1016/j.pbb.2007.05.020. [DOI] [PubMed] [Google Scholar]
- Volkow ND. Stimulant medications: how to minimize their reinforcing effects? Am J Psychiatry. 2006;163:359–361. doi: 10.1176/appi.ajp.163.3.359. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang G, Ding Y, Gatley SJ. Mechanism of action of methylphenidate: insights from PET imaging studies. J Atten Disord. 2002;6(Suppl 1):S31–43. doi: 10.1177/070674370200601s05. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Gatley SJ, Logan J, Ding YS, Hitzemann R, Pappas N. Dopamine transporter occupancies in the human brain induced by therapeutic doses of oral methylphenidate. Am J Psychiatry. 1998;155:1325–1331. doi: 10.1176/ajp.155.10.1325. [DOI] [PubMed] [Google Scholar]
- Wargin W, Patrick K, Kilts C, Gualtieri CT, Ellington K, Mueller RA, Kraemer G, Breese GR. Pharmacokinetics of methylphenidate in man, rat and monkey. J Pharmacol Exp Ther. 1983;226:382–386. [PubMed] [Google Scholar]
- Wolf ME, Roth RH. Autoreceptor regulation of dopamine synthesis. Ann N Y Acad Sci. 1990;604:323–343. doi: 10.1111/j.1749-6632.1990.tb32003.x. [DOI] [PubMed] [Google Scholar]
- Zuvekas SH, Vitiello B, Norquist GS. Recent trends in stimulant medication use among U.S. children. Am J Psychiatry. 2006;163:579–585. doi: 10.1176/ajp.2006.163.4.579. [DOI] [PubMed] [Google Scholar]