Abstract
Irritable bowel syndrome (IBS) is a functional gastrointestinal disorder affecting up to 3-15% of the general population in western countries. It is characterized by unexplained abdominal pain, discomfort, and bloating in association with altered bowel habits. The pathophysiology of IBS is multifactorial involving disturbances of the brain-gut-axis. The pathophysiology provides the rationale for pharmacotherapy: abnormal gastrointestinal motor functions, visceral hypersensitivity, psychosocial factors, autonomic dysfunction, and mucosal immune activation. Understanding the mechanisms, and their mediators or modulators including neurotransmitters and receptors have led to several therapeutic approaches including agents acting on the serotonin receptor or serotonin transporter system, antidepressants, novel selective anticholinergics, α-adrenergic agonists, opioid agents, cholecystokinin-antagonists, neurokinin-antagonists, somatostatin receptor agonists, corticotropin releasing factor antagonists, chloride-channel activators, guanylate-cyclase-c agonists, melatonin, atypical benzodiazepines, antibiotics, immune modulators and probiotics. The mechanisms and current evidence regarding efficacy of these agents are reviewed.
Keywords: pharmacology, pharmacodynamics, clinical trials, serotonergics, opioids
Introduction
Irritable bowel syndrome (IBS) is one of the most common functional gastrointestinal disorders (1,2) characterized by abdominal pain and discomfort in association with altered bowel habits; symptoms are not explained by structural abnormalities using current standard diagnostic tests (3). The pathophysiology of IBS is still not well understood, but is most likely multifactorial. Several factors such as motor and sensory dysfunction, neuroimmune mechanisms, psychological factors, and changes in the intraluminal milieu appear to play a role [Figure 1 (3,4)]. The increased release of serotonin in the circulation especially in the D-IBS group (5-7) and increased serine proteases (derived from mast cells) in stool of patients with IBS (8) provide evidence for the potential role of neurotransmitters or chemical mediators such as proteases in the disorder.
Figure 1.
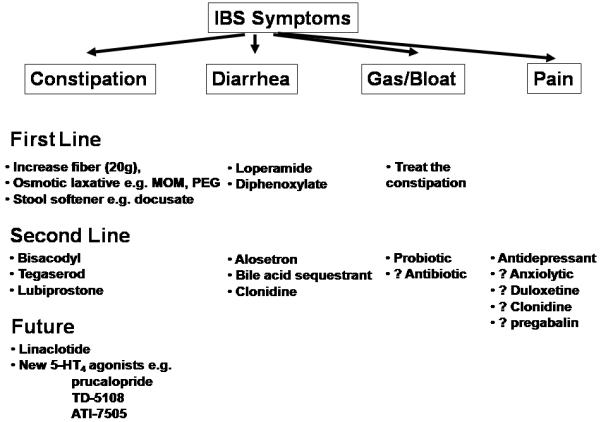
Current Management of IBS and Potential Role of Therapies in Pipeline for IBS
Changes in mucosal serotonin levels may affect sensory and motor functions possibly contributing to IBS symptoms (9). There is increasing evidence of mucosal dysfunction, immune activation or inflammation (10-12) and, possibly, alterations in colonic bacterial flora. Increased number of activated mast-cells in the proximity of colonic nerves in the lamina propria is associated with abdominal pain severity (13). Mucosal immune activation is associated with systemic evidence for a pro-inflammatory state (decreased IL10/IL12 ratios) in IBS patients (14) and changes in local defense mechanisms in the sigmoid and colonic mucosa in IBS (15). These provide novel promising targets for future therapies. Traditional IBS therapies (Figure 1) are mainly directed at the relief of individual symptoms, e.g. antidiarrheals for diarrhea, laxatives for constipation or smooth muscle relaxants for pain. They are often of limited efficacy in addressing the overall symptom complex.
This article reviews recent and novel approaches in IBS therapy (Table I, Figure 2) that have preclinical efficacy, Phase IIa studies of pharmacodynamics or proof of concept, and Phase IIb or phase III studies that show evidence of efficacy.
Table I. Drugs in Use or in Clinical Development for IBS.
| Target System | Receptor Activity | Compounds | Human Physiological Effects |
Potential or Approved Indication |
|---|---|---|---|---|
|
Serotonergic Receptor System |
5-HT4-agonists | Prucalopride, ATI-7505 TD-5108 |
Prokinetic, secretagogue | C-IBS FC |
| 5-HT3-antagonist | Alosetron, Cilansetron |
Decrease motility and secretion, increase compliance decrease pain |
D-IBS FD |
|
| 5-HT3-agonist | DDP-733 | Accelerates small bowel transit |
C-IBS FC |
|
| Antidepressants | SSRI SNRI |
e.g. Venlafaxine | Increase compliance, decrease tone, and sensation |
IBS FAP |
|
Cholinergic System |
Selective M3- antagonists |
Zamifenacin Darifenacin |
Reduce colonic motility Decrease Pain? |
D-IBS FD |
|
α Adrenergic System |
α2-agonist | Clonidine | Increases compliance, decreases tone, and sensation |
D-IBS FAP |
| Opioid System | Peripheral μ-opioid antagonists |
Alvimopan, Methylnaltrexone |
Prokinetic Increase laxation |
C-IBS FC, OIC |
| κ-opioid-agonist | Asimadoline | Decrease sensation | IBS, FAP | |
| Corticoids | CRH antagonist CRF1 antagonist |
αhCRH, Pexacerfont |
Reduce stimulation induced motility and sensitivity |
D-IBS FAP |
| Benzodiazepine | 2,3-benzodiazepine receptor |
Dexofisopam | Reduces stool frequency, Increases stool consistency |
D-IBS |
| Melatonin | Receptor ? | Melatonin | Decreases Pain | IBS, FAP |
| CCK | CCK antagonists | Loxiglumide Dexloxiglumide |
Accelerategastric emptying, Delay prox. colonic transit |
IBS |
| Somatostatin | Somatostatin- Receptor Agonist |
Octreotide | Slows down transit, decreases secretion |
D-IBS FD |
|
Neurokinin (NK) |
NK antagonists 1& 2 NK antagonist 3 |
Ezlopitant Nepadutant Talnetant |
Reduce visceral sensation (NK1) and motility (NK2) |
IBS FAP |
|
Chloride Channel |
Activator | Lubiprostone | Accelerates transit, increases secretion |
C-IBS FC |
|
Guanylate Cyclase C |
Agonist | Linaclotide | Decreases stool consistency, increases stool frequency |
C-IBS FC |
| Antibiotics | neomycin, rifaximin | overall symptom relief | C-IBS FC |
|
| Probiotics | Bacterial flora | VSL#3, Lactobacilli Bifidobacteriae |
Decrease bloating and pain, slow colon transit |
IBS Bloating Flatulence |
Figure 2.
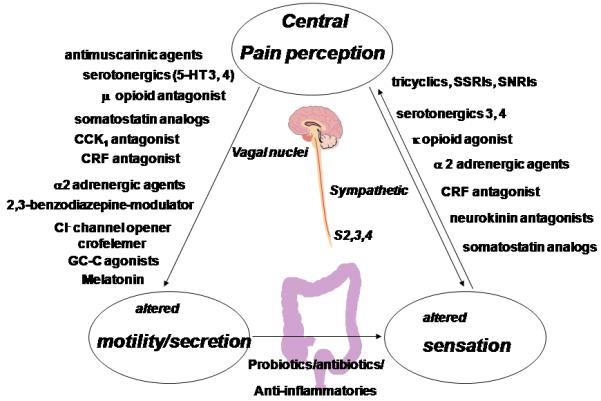
Current, Novel and Experimental Treatments for IBS
SEROTONERGIC SYSTEM
Serotonin (5-hydroxytryptamine) is an important neurotransmitter of the enteric nervous system [ENS (16,17)], the peristaltic reflex (chiefly 5-HT4 receptor), the brain-gut axis and vagal and visceral afferent cell bodies or pathways, chiefly 5-HT3 receptors (16-18). 5-HT plays a pivotal role in the modulation of multiple gut functions such as motility, sensation, blood flow and secretion (19). In humans, the highest level of specific binding has been found in the amygdalae, which are integral to the emotional responses to visceral stimulation.
Activity at 5-HT receptors is regulated by the 5-HT reuptake transporter protein [SERT (20)]. It is controversial whether there is reduced expression of SERT (mRNA or immunohistochemistry) in IBS (21,22).
5-HT3 antagonists and 5-HT4 agonists have demonstrated efficacy and effectiveness in the treatment of multiple or global symptoms of IBS. The prototype medications were alosetron and tegaserod respectively.
5-HT3-receptor antagonists retard colonic transit, reduce secretion, and increase colonic compliance in response to distension (23) and have central effects that contribute to their beneficial effects on sensation in IBS (24).
Alosetron and subsequently cilansetron were efficacious in several large, multicenter, randomized controlled clinical trials, reviewed in an updated meta-analysis (25). 5-HT3 antagonists more effective than the comparators in achieving global improvement in IBS symptoms (pooled relative risk, 1.60; 95% confidence interval [CI], 1.49-1.72; heterogeneity index, I2 = 0%) and relief of abdominal pain and discomfort (pooled relative risk, 1.30; 95% CI, 1.22-1.39; I2 = 22%). Benefit was apparent for both agents, in both genders. These agents were more likely to cause constipation (pooled relative risk, 4.28; 95% CI, 3.28-5.60, I2= 65%); 0.2% using 5-HT3 antagonists had possible ischemic colitis versus none in control groups. Cilansetron was never approved; alosetron is associated with clear benefits on quality of life (26), but warnings about potential lack of safety has resulted in only a minority of patients being prescribed the medication.
5HT3-receptor agonists
The 5-HT3 agonist, MKC-733, also named DDP733 and pumosetrag in the literature, stimulated fasting antroduodenal migrating motor complex activity and accelerated small intestinal transit (27). A preliminary report of a phase 2a study in IBS-C showed a significant overall benefit in overall clinical response rate (54%) in patients receiving 1.4 mg t.i.d. versus to a 15% for placebo (28).
5HT4-receptor agonists generally accelerate small bowel and colonic transit.
Tegaserod
Tegaserod is a selective partial agonist of the 5-HT4-receptor. Several trials in IBS and chronic constipation were summarized in a Cochrane meta-analysis (29). In IBS-C, tegaserod did not significantly improve the patients’ individual symptoms of abdominal pain and discomfort although bowel habit showed a statistically significant improvement with tegaserod 4 mg and there was a non-significant trend in this outcome in favor of tegaserod, 12 mg. In patients with chronic constipation, the RR of being a responder in terms of complete spontaneous bowel movements per week with tegaserod 12 mg was 1.54 (95% CI 1.35 to 1.75). The drug was essentially withdrawn because of possible, rare cardiovascular adverse effects.
Newer 5-HT4-receptor agonists
ATI-7505 is a novel, potent agonist of the 5-HT4 receptor and is chemically related to the 5-HT4-receptor agonist, cisapride. However, it has been chemically designed to eliminate cardiac liabilities (e.g., QT prolongation, tachycardia) and CYP450-dependent metabolism at therapeutically relevant concentrations. Pharmacodynamic data in healthy human volunteers (30) indicate prokinetic effects with acceleration of gastric emptying (20 mg tid) and colonic transit (10 mg tid; overall and ascending colon emptying), as well as inducing looser stool consistency. Thus, it appears to be promising for C-IBS. Extensive human safety data are not available. To date, there are no reports of adverse cardiac side effects.
Prucalopride is a 5-HT4-receptor agonist that has been extensively studied, including pharmacodynamics and large clinical trials in patients with severe chronic constipation (31-34). The medication has greater selectivity (∼150-fold) for 5-HT4-receptors than for hERG channel, and it is anticipated that this will be sufficient to prove safe from a cardiac arrhythmia perspective. Certainly, administration for approximately 1 year in more than 80 people with cardiovascular co-morbidity proved safe (35).
TD-5108 (or velusetrag) is a potent agonist at human 5-HT4 receptors with high intrinsic activity that displays preferential binding to the 5-HT4 receptor compared with other 5-HT receptor subtypes or hERG channel (36). In vivo studies have shown that TD-5108 increases colonic transit in guinea pigs, relaxes esophageal body musculature in rats, and increases contractility in multiple regions of the GI tract including the gastric antrum, duodenum, jejunum and proximal colon in conscious dogs with implanted strain gauges (37). In healthy volunteers, single dose, 30- and 50-mg, TD-5108 accelerated colonic and small bowel transit; with multiple doses, TD-5108 30-mg also accelerated colonic transit (38). A 400 patient, randomized, controlled trial demonstrated efficacy on bowel frequency and consistency in chronic constipation (39).
ANTIDEPRESSANTS
Many patients with IBS receive psychoactive agents for their co-morbid psychiatric illnesses, including anxiety, mood, and somatoform disorders, and for the potential effects on visceral sensation. Some SSRIs accelerate small bowel transit; others have effects on colorectal sensation, compliance or tone [e.g. citalopram, venlafaxine (40-42)].
There may also be racial or ethnic differences, as efficacy appears greatest in trials conducted in Iran (43,44). In intention-to-treat analysis, complete responses were significantly more common in the amitriptyline group. Efficacy of antidepressants in treatment of IBS has been appraised in a recent meta-analysis (45). At first evaluation, one might conclude that this class of drugs is extremely effective. The calculated relative risk of IBS symptoms persisting or remaining unimproved after treatment with antidepressant therapy versus placebo was 0.66 (95% CI 0.57 to 0.78), with the NNT with antidepressant therapy to prevent IBS symptoms persisting of 4 (95% CI 3 to 6). However, it is worth noting that very few (typically small single center) trials individually show significance, and the meta-analysis involves diverse medications and doses, study designs, endpoints, questionable generalizability, and small studies. The analysis included 13 studies comparing antidepressants (total n=432) to placebo (n=357) for IBS; 7 studies were in secondary, and 6 in tertiary care with none in primary or community cohorts. Moreover, there was marginal statistically significant heterogeneity detected between studies (I2 = 26.4%, P = 0.17) and funnel plot asymmetry (P = 0.02), suggestive of publication bias.
In the meta-analysis, only three papers are associated with clear efficacy and they involve small samples of patients: two in Iranian patients (43,44) and one in German patients (46). Intriguingly, the most efficacious treatment attributed to a tricyclic agent was reported with a trial of amitryptiline, 10 mg, that did not significantly affect abdominal pain, bloating or diarrhea, relative to placebo. Similarly, the trial of the SSRI, fluoxetine, (43) in 44 patients in Iran shows very large differences in patients with significant abdominal discomfort and bloating, but the proportion of placebo responders over 12 weeks was uncharacteristically low (<15% average), and it is unclear whether these results are generalizable.
ANTIMUSCARINICS/ANTICHOLINERGICS/SMOOTH MUSCLE RELAXANTS
Gastrointestinal effects of acetylcholine are mediated by nicotinic receptors in the myenteric plexus, and by muscarinic receptor subtypes M1-3, in the myenteric plexus, as well as the neuromuscular junction (47,48). Acetylcholine is the main excitatory neurotransmitter in the gastrointestinal (GI) tract. Nonselective anticholinergics or specific antimuscarinic agents reduce bowel motility and associated pain. Relaxant drugs act directly on the smooth muscle e.g. by blocking smooth muscle Ca 2+ channels. Several compounds (mebeverine, otilinium, pinaverium, and cimetropium), used in Europe, have never been approved in the U.S. Several meta-analyses suggest global improvement with this class of agents (49,50), with a mean odds ratio of 2.13 [95% CI: 1.77-2.58]. The low quality (Jadad Scores) of the individual clinical trials compromises the overall interpretation of efficacy of this drug class.
Newer anticholinergics specifically targeting the muscarinic type-3 receptor (M3) on smooth muscle decrease non-specific anticholinergic side effects (dry mouth or increased heart rate) and have promising gastrointestinal effects in animal models (51). Among M3-selective antagonists, darifenacin (used to treat overactive bladder) is associated with constipation (52), and zamifenacin (53) significantly reduced colonic motility without other anticholinergic effects in 36 IBS patients.
α-ADRENERGIC AGENTS
The adrenergic nervous system provides extrinsic tonic inhibitory control of non-sphincteric gut motility. The α2-adrenergic agents such as clonidine affect human colonic and rectal motor and sensory functions (54-56), increasing colonic compliance, reducing fasting tone without altering the colonic response to a meal or colonic transit, and significantly reducing sensation within a dose range of 0.1 to 0.3 mg bid. In a study of 44 patients with D-IBS (57), 67% of the patients treated with clonidine 0.1.mg twice daily compared to 46% in the placebo group achieved satisfactory relief, the primary endpoint. Clonidine also significantly improved bowel functions without altering gastrointestinal transit. Drowsiness, dizziness and dry mouth were observed at doses >0.1 mg bid. This suggests benefits of α2-adrenergic agents in IBS.
GABA-ergic AGENTS AND α2δ LIGAND
Gabapentin, a 3-alkylated analogue of γ-amino butyric acid (GABA), has been shown to reduce elements of central sensitization in human experimental hyperalgesia. Forty patients with D-IBS were randomized for 5-day treatment with gabapentin, 300 mg/day and then 600 mg/day, which reduced rectal sensory thresholds through attenuating rectal sensitivity to distension and enhancing rectal compliance (58).
Preclinical studies suggest that the α2δ ligand, pregabalin, reduces both visceral allodynia and hyperalgesia, but is inactive on basal sensitivity. The perception of rectal distension was tested in 26 hypersensitive IBS patients randomized to increasing doses of oral pregabalin for 3 weeks or placebo control. Pregabalin significantly increased the sensory thresholds from baseline for first sensation desire to defecate and pain compared with placebo; it also increased rectal compliance, an effect unrelated to the changes in sensitivity (59). Studies of GABA-ergic agents and α2δ ligands on clinical outcomes in IBS are not available.
OPIOID AGENTS
Three major opioid receptors, μ, δ, and κ, are distributed in the peripheral and central nervous systems. μ-agonists modulate visceral nociception and to slow down gastrointestinal transit resulting in constipation or central side effects (60-62). κ-opioid receptors are involved in visceral perception (63).
κ-opioid agonist
Asimadoline
The κ-opioid agonist, asimadoline (64), was shown to reduce sensation in response to distensions in the non-noxious range applied to the colon and to relax colonic and gastric tone during fasting. There were no significant effects on gastrointestinal or colonic transit or postprandial tone response to meal ingestion. Delvaux et al. (65) showed that this drug increased sensory thresholds in patients with IBS. On demand treatment of IBS pain with asimadoline was not significantly better than placebo (66); however, a recent trial using daily administration of asimadoline demonstrated efficacy in IBS-D and IBS-M patients (67).
Peripheral μ-opioid antagonists
Alvimopan
Alvimopan, a novel, peripherally-restricted μ is effective in the treatment of opioid-induced constipation and postoperative ileus (68-70). It reverses the peripheral effects of narcotics without influencing the pain relief desired by concomitant opioid administration. A pharmacodynamic study in healthy volunteers (71) confirmed that alvimopan normalized the colonic transit delay induced by co-administered codeine. Interestingly, alvimopan alone accelerated colonic transit, suggesting that the μ-opiate mechanisms participate in the physiological control of colonic transit (71).
Oral naltrexone does not accelerate colonic transit (72), but oral methylnaltrexone accelerated gut transit in opiate-naïve controls (73) and it reversed the prolongation of orocecal transit induced by long-term methadone (74). Trials to test the effects of peripheral μ-opioid antagonists such as alvimopan and methylnaltrexone in patients with constipation, C-IBS, and opiate-induced constipation are warranted.
CRF RECEPTOR ANTAGONISTS
Corticotropin releasing factor (CRF) is a key mediator of stress response in the brain gut axis (75). Two CRF receptor subtypes have been cloned: CRF1 and CRF2. In animals, stress activation of the CRF receptors alters gastrointestinal motility (76), effects that can be blocked with selective CRF1-receptor antagonists [antalarmin (77)] and the nonselective CRF-receptor antagonist α-helical CRF 9-41 [αhCRF (78)]. The increased colonic motility response to rectal electrical stimulation in IBS patients was significantly suppressed after αhCRF infusion (79). However, the CRF1 antagonist, pexacerafont, was not effective in normalizing colonic transit or bowel function in patients with diarrhea-predominant IBS (80). The role of CRF modulation requires further study.
2,3-BENZODIAZAPINE RECEPTOR MODULATOR
Dextofisopam (81) is the R-enantiomer of the nonsedating homophthalazine anxiolytic, tofisopam. It binds to 2,3-benzodiazepine receptors located in subcortical and hypothalamic regions. In animal models, dextofisopam reduced stimulation-induced colonic motility and visceral sensitivity (81). A 12-week, placebo-controlled, phase II study in patients with diarrhea-predominant or alternating-IBS showed more months with adequate relief in the dextofisopam compared to the placebo group (82). The effects were most prominent within the first 4 weeks of treatment, and also included an improvement of stool consistency and a reduction of stool frequency. The drug was well tolerated, with only 3% reporting constipation; however, 12% of the patients experienced a worsening of abdominal pain (versus 4% in the placebo group).
MELATONIN
Melatonin is a pineal gland neurohormone involved in the regulation of the sleep-wake cycle; it is also synthesized in the gastrointestinal tract, and may participate in the regulation of gastrointestinal motility and sensitivity (83), possibly by blocking nicotinic channels (84) or Ca2+ activated K+ channels (85). Two small studies of the effects of melatonin in IBS patients (86,87) reported improvement of abdominal pain and IBS symptom score or a reduction of rectal pain sensitivity. This appears to be a peripheral effect, since there was no alteration of sleep or anxiety or depression. The role of melatonin modulation in IBS requires further study.
CCK1 ANTAGONISTS
Cholecystokinin [CCK (88)], a neuropeptide released by endocrine cells within the duodenal and jejunal mucosa in response to a variety of nutrients, has been proposed as a mediator of IBS symptoms. Colonic responses to CCK in vivo and in vitro were increased in IBS patients (89). The effects of CCK are mediated by two distinct receptors, CCK1 and CCK2 (90,91), which are located predominantly in the periphery and the central nervous system respectively. The CCK1 (also called CCKA) receptors are present in gastrointestinal tract smooth muscles and vagal afferents. Blockade of CCK1 receptors in the gastrointestinal tract was proposed as an approach to stimulate gut motility and to change colonic transit time in patients with C-IBS.
Loxiglumide and Dexloxiglumide
Loxiglumide is a highly specific, competitive antagonist of the CCK1 receptor (92). Earlier pharmacodynamic (93) and clinical (94) studies suggested that loxiglumide might improve constipation. A twelve-week, phase II trial of 200 mg/day dexloxiglumide (95) in female IBS-C patients showed improvement in abdominal pain and discomfort compared to placebo, but two large phase III trials in IBS-C showed no efficacy for dexloxiglumide (96). The latter result was consistent with a pharmacodynamics study in C-IBS (97) which showed no significant effect on overall colonic transit or satisfactory relief of IBS. However, in a phase III randomized withdrawal study, dexloxiglumide demonstrated sustained relief of symptoms in female (not male) patients (98). Further studies with this mediation are awaited.
SOMATOSTATIN ANALOGS
The somatostatin analog, octreotide, activates predominantly somatostatin type 2 receptors, and reduces gastrointestinal secretion, retards gastrointestinal transit and has antinociceptive properties. Octreotide retards orocecal and small bowel transit time in patients with D-IBS (99,100) and normalizes visceral perception and discomfort thresholds in IBS patients without changing rectal compliance (101,102). There are no clinical trials with octreotide in IBS.
NEUROKININ-ANTAGONISTS
NK1-receptors play a role in nociception (103), whereas NK2 receptors have a greater influence on smooth muscle contractility (103-105) and of NK2-antagonists on gut motility (105). In a small pharmacodynamic study of IBS patients, the NK1-receptor antagonist, ezlozipant (106), reduced the emotional response to rectosigmoid distension but did not significantly decrease rectal sensitivity. In healthy controls, the NK2-receptor antagonist, nepadutant (107), reduced contraction frequency and amplitude on migrating motor complexes in the small intestine and effectively antagonized the motility-stimulating effects of infused NKA. The NK3 antagonist, talnetant, was tested in pharmacodynamics (108) and clinical trials (109), and proved ineffective.
CHLORIDE CHANNEL MODULATORS
Cl- Channel (ClC2) Opener
Intestinal Cl- secretion is critical for intestinal fluid and electrolyte transport. In the gastrointestinal tract, chloride channels type 2, ClC2, has been found in gastric parietal cells, small intestinal and colonic epithelia. Lubiprostone is a prostone that acts as a selective ClC2 activator and increases intestinal water secretion (110). In a pharmacodynamic study, lubiprostone was shown to accelerate small bowel and colonic transit time in healthy volunteers (111). In several clinical trials, lubiprostone, 24 μg b.i.d., had positive effects on stool consistency, frequency and straining, and was safe and effective for treating constipation (112-114). Interestingly, lubiprostone accelerated colonic transit without accelerating ascending colon emptying, suggesting it affects distal colonic function. Lubisprostone also reduced abdominal pain and improved bowel dysfunction in IBS-C (115,116). Side effects of lubiprostone include diarrhea and nausea that is usually mild and transient. In January 2006, the FDA approved lubiprostone.
Guanylate Cyclase-C Agonist
Linaclotide is a novel agonist of the human guanylate cyclase-c (GC-C), a transmembrane protein located in the gut epithelium. Activation of the GC-C induces secretion of fluid, sodium and bicarbonate in the intestinal lumen (117-120). In animal and early human safety studies, linaclotide has been observed to increase stool frequency, decrease stool consistency and decrease visceral pain (121). A phase IIA pilot, randomized, controlled study shows efficacy of this drug in treatment of chronic constipation (122). Therefore, the drug seems to be promising for the treatment of C-IBS and chronic constipation.
Crofelemer
Crofelemer reduces excess chloride ion secretion via the CFTR channel. Crofelemer does not affect gut motility and is not absorbed systemically to any significant level. It has been used in the past to treat diarrhea associated with intestinal secretion. In a dose-ranging study of crofelemer versus placebo b.i.d. for 12 weeks in 242 IBS-D patients (123), crofelemer did not produce improvement in the primary endpoint related to bowel function, urgency or adequate relief. On the other hand, female IBS-D patients exposed to the highest dose of 500 mg b.i.d. crofelemer had a higher proportion of pain and discomfort free days. These post-hoc observations suggest further studies are indicated to assess the visceral analgesic potential of crofelemer.
PROBIOTICS
Probiotics may have beneficial effects on altered colonic inflammation or barrier function that may be found in some IBS patients. The precise mechanism of action of probiotics is unclear and change of intraluminal milieu and modification of fermentation processes and gas production, inactivation of bile acids with decreased effect of endogenous bile acids on colonic fluid secretion and motility, and alteration of gastrointestinal motility may also contribute to the symptom improvement (124). A recent meta-analysis (125) identified 19 randomized, controlled trials (18 papers) in 1650 IBS patients. Trial quality was generally good. Among 10 randomized, controlled trials involving 918 patients providing outcomes as a dichotomous variable, probiotics were significantly better than placebo with an NNT = 4 (95% CI = 3 to 12.5); however, there was significant heterogeneity (I2 = 68%, p=0.001) and possible funnel plot asymmetry. Among 15 trials assessing 1351 patients that reported an improvement in IBS score as a continuous outcome, the standard mean difference was -0.34 (95% CI -0.60 to -0.07); however, there was statistically significant heterogeneity (I2 = 79%, p=0.001). These data suggest overall benefit for probiotics, but the precision of the NNT is uncertain. The safety profile of probiotics suggests they may be considered for the treatment of bloating and flatulence.
ANTIBIOTICS
The role of bacterial overgrowth of the small intestine (SIBO) in IBS is controversial. A placebo-controlled study (126) showed that antibiotic treatment with neomycin normalized the lactulose hydrogen breath test (LBT), and this was associated with significant reduction of IBS symptoms over the short term (∼1 week). More convincing evidence of the efficacy of antibiotics is provided by the randomized, controlled trials (127) and a preliminary report from Lembo et al. (128). Although there are many unanswered questions, regarding long-term effects, and frequency of retreatment, some patients may benefit from rifaximin treatment.
IMMUNE MODULATORS
Sodium Cromoglycate is a mast cell stabilizer; there is experimental evidence of increased mast cell infiltration and greater proximity of mast cells to intramural nerve endings. Proteases which may arise from mast cells have been associated with activation of visceral algesia in experimental studies. Sodium cromoglycate alone or in association with dietary exclusion of suspected allergens has been tested in trials conducted in IBS-D (129) and childhood IBS (130).
5-ASA Compounds or Budesonide
Given the efficacy of budesonide in microscopic or collagenous colitis (131-133) and the increasing evidence of immune activation in IBS, we anticipate more formal trials of these agents, especially in post-infectious IBS. In a recent meta-analysis, Chande et al. (133) report that, among 3 trials with budesonide for collagenous colitis, the odds ratio for inducing a response was 12.32 (95% CI 5.53-27.46), maintaining response was OR 8.82, and NNT of 2. Similar efficacy was observed for trials that included both collagenous and lymphocytic colitis (NNT of 3). Mesalazine and bismuth compounds were less effective for microscopic colitis. However, a small, pilot clinical trial in IBS suggests that mesalazine reduced total number of immune cells and mast cells and pain severity score, and increased global relief relative to placebo treatment (134). These interesting and provocative data suggest that 5-ASA compounds deserve further study.
Conclusion
The field of IBS therapeutics is vibrant, with greater understanding of neuroenteric mechanisms, effectors and transmitters in the brain-gut axis. These should provide opportunities for development of new therapeutic agents for IBS treatment. For almost all of the drug classes described here, rigorous phase III trials and assessment of drug safety are eagerly awaited.
Acknowledgements
The study was supported in part by grant RO1-DK54681 (MC) from National Institutes of Health. The excellent secretarial support of Mrs. Cindy Stanislav is gratefully acknowledged.
Abbreviations used
- IBS
irritable bowel syndrome
- IBS-C
irritable bowel syndrome with predominant constipation
- IBS-D
irritable bowel syndrome with predominant diarrhea
- FC
functional constipation
- FD
functional diarrhea
- FAP
functional abdominal pain
- OR
odds ratio
- CI
confidence interval
- SNRI
Serotonin and norepinephrine reuptake inhibitor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Drossman DA, Li Z, Andruzzi E, et al. U.S. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Dig Dis Sci. 1993;38(9):1569–80. doi: 10.1007/BF01303162. [DOI] [PubMed] [Google Scholar]
- 2.Cremonini F, Talley NJ. Irritable bowel syndrome: epidemiology, natural history, health care seeking and emerging risk factors. Gastroenterol Clin North Am. 2005;34(2):189–204. doi: 10.1016/j.gtc.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 3.Drossman DA, Camilleri M, Mayer EA, et al. AGA technical review on irritable bowel syndrome. Gastroenterology. 2002;123(6):2108–31. doi: 10.1053/gast.2002.37095. [DOI] [PubMed] [Google Scholar]
- 4.Camilleri M. Mechanisms in IBS: something old, something new, something borrowed. Neurogastroenterol Motil. 2005;17(3):311–6. doi: 10.1111/j.1365-2982.2004.00632.x. [DOI] [PubMed] [Google Scholar]
- 5.Dunlop SP, Coleman NS, Blackshaw E, et al. Abnormalities of 5-hydroxytryptamine metabolism in irritable bowel syndrome. Clin Gastroenterol Hepatol. 2005;3(4):349–57. doi: 10.1016/s1542-3565(04)00726-8. [DOI] [PubMed] [Google Scholar]
- 6.Bearcroft CP, Perrett D, Farthing MJ. Postprandial plasma 5-hydroxytryptamine in diarrhoea predominant irritable bowel syndrome: a pilot study. Gut. 1998;42(1):42–6. doi: 10.1136/gut.42.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Houghton LA, Atkinson W, Whitaker RP, et al. Increased platelet depleted plasma 5-hydroxytryptamine concentration following meal ingestion in symptomatic female subjects with diarrhoea predominant irritable bowel syndrome. Gut. 2003;52(5):663–70. doi: 10.1136/gut.52.5.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roka R, Rosztoczy A, Leveque M, et al. Fecal serine-protease activity: a pathophysiological marker in diarrhea-predominant irritable bowel syndrome. Clin Gastroenterol Hepatol. doi: 10.1016/j.cgh.2006.12.004. in press. [DOI] [PubMed] [Google Scholar]
- 9.Borman R. Serotonergic modulation and irritable bowel syndrome. Expert Opin Emerg Drugs. 2001;6(1):57–68. doi: 10.1517/14728214.6.1.57. [DOI] [PubMed] [Google Scholar]
- 10.Spiller RC. Postinfectious irritable bowel syndrome. Gastroenterology. 2003;124(6):1662–71. doi: 10.1016/s0016-5085(03)00324-x. [DOI] [PubMed] [Google Scholar]
- 11.Spiller RC, Jenkins D, Thornley JP, et al. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47(6):804–11. doi: 10.1136/gut.47.6.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spiller RC. Infection, immune function, and functional gut disorders. Clin Gastroenterol Hepatol. 2004;2(6):445–55. doi: 10.1016/s1542-3565(04)00159-4. [DOI] [PubMed] [Google Scholar]
- 13.Barbara G, Stanghellini V, De Giorgio R, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126(3):693–702. doi: 10.1053/j.gastro.2003.11.055. [DOI] [PubMed] [Google Scholar]
- 14.O’Mahony L, McCarthy J, Kelly P, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128(3):541–51. doi: 10.1053/j.gastro.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 15.Aerssens J, Camilleri M, Talloen W, et al. Alterations in mucosal immunity identified in the colon of patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 1008;6:194–205. doi: 10.1016/j.cgh.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gershon MD. Review article: roles played by 5-hydroxytryptamine in the physiology of the bowel. Aliment Pharmacol Ther. 1999;13(Suppl 2):15–30. [PubMed] [Google Scholar]
- 17.Gershon MD. Review article: serotonin receptors and transporters -- roles in normal and abnormal gastrointestinal motility. Aliment Pharmacol Ther. 2004;20(Suppl 7):3–14. doi: 10.1111/j.1365-2036.2004.02180.x. [DOI] [PubMed] [Google Scholar]
- 18.Hicks GA, Coldwell JR, Schindler M, et al. Excitation of rat colonic afferent fibres by 5-HT(3) receptors. J Physiol. 2002;544(Pt 3):861–9. doi: 10.1113/jphysiol.2002.025452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Camilleri M. Serotonin in the gastrointestinal tract. Curr Opin Endocrinol Diabetes Obes. 2009;16:53–9. doi: 10.1097/med.0b013e32831e9c8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gershon MD. Plasticity in serotonin control mechanisms in the gut. Curr Opin Pharmacol. 2003;3(6):600–7. doi: 10.1016/j.coph.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Coates MD, Mahoney CR, Linden DR, et al. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126(7):1657–64. doi: 10.1053/j.gastro.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 22.Andrews C, Camilleri M, Bharucha AE, et al. Serotonin-transporter (SERT) polymorphism genotype and SERT expression in mucosal biopsies of patients with irritable bowel syndrome. Gastroenterology. 2006;130:A24. abstract. [Google Scholar]
- 23.Gunput MD. Review article: clinical pharmacology of alosetron. Aliment Pharmacol Ther. 1999;13(Suppl 2):70–6. doi: 10.1046/j.1365-2036.1999.00009.x. [DOI] [PubMed] [Google Scholar]
- 24.Mayer EA, Berman S, Derbyshire SW, et al. The effect of the 5-HT3 receptor antagonist, alosetron, on brain responses to visceral stimulation in irritable bowel syndrome patients. Aliment Pharmacol Ther. 2002;16(7):1357–66. doi: 10.1046/j.1365-2036.2002.01287.x. [DOI] [PubMed] [Google Scholar]
- 25.Andresen V, Montori VM, Keller J, et al. Effects of 5-hydroxytryptamine (serotonin) type 3 antagonists on symptom relief and constipation in nonconstipated irritable bowel syndrome: a systematic review and meta-analysis of randomized controlled trials. Clin Gastroenterol Hepatol. 2008;6:545–55. doi: 10.1016/j.cgh.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El-Serag HB, Olden K, Bjorkman D. Health-related quality of life among persons with irritable bowel syndrome: a systematic review. Aliment Pharmacol Ther. 2002;16:1171–85. doi: 10.1046/j.1365-2036.2002.01290.x. [DOI] [PubMed] [Google Scholar]
- 27.Coleman NS, Marciani L, Blackshaw E, et al. Effect of a novel 5-HT3 receptor agonist MKC-733 on upper gastrointestinal motility in humans. Aliment Pharmacol Ther. 2003;18(10):1039–48. doi: 10.1046/j.1365-2036.2003.01797.x. [DOI] [PubMed] [Google Scholar]
- 28.Paterson WG, Ford D, Ganguli SC, et al. A novel, oral 5HT3 partial agonist, DDP733, improves overall response in patients with irritable bowel syndrome and constipation (IBS-C): a randomized, double-blind, placebo-controlled, parallel-group, dose-ranging study. Gastroenterology. 2008;134:A546–7. abstract. [Google Scholar]
- 29.Evans BW, Clark WK, Moore DJ, et al. Tegaserod for the treatment of irritable bowel syndrome and chronic constipation. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.CD003960.pub3. CD003960. [DOI] [PubMed] [Google Scholar]
- 30.Camilleri M, Vazquez-Roque MI, Burton D, et al. Pharmacodynamic effects of a novel prokinetic 5-HT receptor agonist, ATI-7505, in humans. Neurogastroenterol Motil. 2007;19:30–8. doi: 10.1111/j.1365-2982.2006.00865.x. [DOI] [PubMed] [Google Scholar]
- 31.Bouras EP, Camilleri M, Burton DD, et al. Prucalopride accelerates gastrointestinal and colonic transit in patients with constipation without a rectal evacuation disorder. Gastroenterology. 2001;120:354–60. doi: 10.1053/gast.2001.21166. [DOI] [PubMed] [Google Scholar]
- 32.Camilleri M, Kerstens R, Rykx A, et al. A placebo-controlled trial of prucalopride for severe chronic constipation. N Engl J Med. 2008;358:2344–54. doi: 10.1056/NEJMoa0800670. [DOI] [PubMed] [Google Scholar]
- 33.Tack J, Van Outryve M, Beyens G, et al. Prucalopride (Resolor(R) in the treatment of severe chronic constipation in patients dissatisfied with laxatives. Gut. 2008 Nov 5; doi: 10.1136/gut.2008.162404. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 34.Quigley EM, Vandeplassche L, Kerstens R, et al. Clinical trial: the efficacy, impact on quality of life, and safety and tolerability of prucalopride in severe chronic constipation--a 12-week, randomized, double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2009;29:315–28. doi: 10.1111/j.1365-2036.2008.03884.x. [DOI] [PubMed] [Google Scholar]
- 35.Camilleri M, Kerstens R, Beyens G, et al. A double-blind, placebo-controlled trial to evaluate the safety and tolerability of prucalopride oral solution in constipated elderly patients living in a nursing facility. Gastroenterology. 2009 in press. [Google Scholar]
- 36.Smith JA, Beattie DT, Marquess D, et al. The in vitro pharmacological profile of TD-5108, a selective 5-HT(4) receptor agonist with high intrinsic activity. Naunyn Schmiedebergs Arch Pharmacol. 2008;378:125–37. doi: 10.1007/s00210-008-0282-y. [DOI] [PubMed] [Google Scholar]
- 37.Beattie DT, Armstrong SR, Shaw JP, et al. The in vivo gastrointestinal activity of TD-5108, a selective 5-HT(4) receptor agonist with high intrinsic activity. Naunyn Schmiedebergs Arch Pharmacol. 2008;378:139–47. doi: 10.1007/s00210-008-0281-z. [DOI] [PubMed] [Google Scholar]
- 38.Camilleri M, Manini M, McKinzie S, et al. Dose-related effects of TD-5108, a selective 5-HT4 receptor agonist with high intrinsic activity, on gastrointestinal (GI) and colonic transit in healthy volunteers. Neurogastroenterol Motil. 2008;20(Suppl 2):6. abstract. [Google Scholar]
- 39.Goldberg MR, Li Y-P, Pitzer K, et al. TD-5108, a selective 5-HT4 agonist, is consistently better than placebo regardless of response definition in patients with chronic constipation. Gastroenterology. 2008;134:A-545. [Google Scholar]
- 40.Gorard DA, Libby GW, Farthing MJ. Influence of antidepressants on whole gut and orocaecal transit times in health and irritable bowel syndrome. Aliment Pharmacol Ther. 1994;8(2):159–66. doi: 10.1111/j.1365-2036.1994.tb00273.x. [DOI] [PubMed] [Google Scholar]
- 41.Tack J. A placebo controlled trial of buspirone, a fundus relaxing drug, in functional dyspepsia: effect on symptoms and gastric sensory and motor functions. Gastroenterology. 2000;116:G1423. [Google Scholar]
- 42.Chial HJ, Camilleri M, Ferber I, et al. Effects of venlafaxine, buspirone, and placebo on colonic sensorimotor functions in healthy humans. Clin Gastroenterol Hepatol. 2003;1(3):211–8. doi: 10.1053/jcgh.2003.50031. [DOI] [PubMed] [Google Scholar]
- 43.Vahedi H, Merat S, Rashidioon A, et al. Effect of fluoxetine in patients with pain and constipation-predominant irritable bowel syndrome: a double-blind randomized-controlled study. Aliment Pharmacol Ther. 2005;22:381–5. doi: 10.1111/j.1365-2036.2005.02566.x. [DOI] [PubMed] [Google Scholar]
- 44.Vahedi H, Merat S, Momtahen S, et al. Clinical trial: effect of amitriptyline in patients with diarrhea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2008;27:678–84. doi: 10.1111/j.1365-2036.2008.03633.x. [DOI] [PubMed] [Google Scholar]
- 45.Ford AC, Talley NJ, Schoenfeld PS, et al. Efficacy of antidepressants and psychological therapies in irritable bowel syndrome: systematic review and meta-analysis. Gut. 2008 Nov 10; doi: 10.1136/gut.2008.163162. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 46.Bergmann M, Heddergott A, Schlosser T. Die therapie des colon irritabile mit trimaprimin (Herphonal) — Eine kontrollierte studie. Z Klin Med. 1991;46:1621–8. [Google Scholar]
- 47.Goyal RK. Muscarinic receptor subtypes. Physiology and clinical implications. N Engl J Med. 1989;321(15):1022–9. doi: 10.1056/NEJM198910123211506. [DOI] [PubMed] [Google Scholar]
- 48.Eglen RM. Muscarinic receptors and gastrointestinal tract smooth muscle function. Life Sci. 2001;68(2223):2573–8. doi: 10.1016/s0024-3205(01)01054-2. [DOI] [PubMed] [Google Scholar]
- 49.Ford AC, Talley NJ, Spiegel BM, et al. Effect of fibre, antispasmodics, and peppermint oil in the treatment of irritable bowel syndrome: systematic review and meta-analysis. BMJ. 2008 Nov 13; doi: 10.1136/bmj.a2313. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Poynard T, Regimbeau C, Benhamou Y. Meta-analysis of smooth muscle relaxants in the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 2001;15(3):355–61. doi: 10.1046/j.1365-2036.2001.00937.x. [DOI] [PubMed] [Google Scholar]
- 51.Kobayashi S, Ikeda K, Suzuki M, et al. Effects of YM905, a novel muscarinic M3-receptor antagonist, on experimental models of bowel dysfunction in vivo. Jpn J Pharmacol. 2001;86(3):281–8. doi: 10.1254/jjp.86.281. [DOI] [PubMed] [Google Scholar]
- 52.Foote J, Glavind K, Kralidis G, et al. Treatment of overactive bladder in the older patient: pooled analysis of three phase III studies of darifenacin, an M3 selective receptor antagonist. Eur Urol. 2005;48(3):471–7. doi: 10.1016/j.eururo.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 53.Houghton LA, Rogers J, Whorwell PJ, et al. Zamifenacin (UK-76, 654) a potent gut M3 selective muscarinic antagonist, reduces colonic motor activity in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 1997;11(3):561–8. doi: 10.1046/j.1365-2036.1997.00189.x. [DOI] [PubMed] [Google Scholar]
- 54.Bharucha AE, Camilleri M, Zinsmeister AR, et al. Adrenergic modulation of human colonic motor and sensory function. Am J Physiol. 1997;273(5 Pt 1):G997–1006. doi: 10.1152/ajpgi.1997.273.5.G997. [DOI] [PubMed] [Google Scholar]
- 55.Malcolm A, Phillips SF, Camilleri M, et al. Pharmacological modulation of rectal tone alters perception of distention in humans. Am J Gastroenterol. 1997;92(11):2073–9. [PubMed] [Google Scholar]
- 56.Malcolm A, Camilleri M, Kost L, et al. Towards identifying optimal doses for alpha-2 adrenergic modulation of colonic and rectal motor and sensory function. Aliment Pharmacol Ther. 2000;14(6):783–93. doi: 10.1046/j.1365-2036.2000.00757.x. [DOI] [PubMed] [Google Scholar]
- 57.Camilleri M, Kim DY, McKinzie S, et al. A randomized, controlled exploratory study of clonidine in diarrhea-predominant irritable bowel syndrome. Clin Gastroenterol Hepatol. 2003;1(2):111–21. doi: 10.1053/cgh.2003.50019. [DOI] [PubMed] [Google Scholar]
- 58.Lee KJ, Kim JH, Cho SW. Gabapentin reduces rectal mechanosensitivity and increases rectal compliance in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2005;22:981–8. doi: 10.1111/j.1365-2036.2005.02685.x. [DOI] [PubMed] [Google Scholar]
- 59.Houghton LA, Fell C, Whorwell PJ, et al. Effect of a second-generation alpha2delta ligand (pregabalin) on visceral sensation in hypersensitive patients with irritable bowel syndrome. Gut. 2007;56:1218–25. doi: 10.1136/gut.2006.110858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Borody TJ, Quigley EM, Phillips SF, et al. Effects of morphine and atropine on motility and transit in the human ileum. Gastroenterology. 1985;89(3):562–70. doi: 10.1016/0016-5085(85)90452-4. [DOI] [PubMed] [Google Scholar]
- 61.Steadman CJ, Phillips SF, Camilleri M, et al. Control of muscle tone in the human colon. Gut. 1992;33(4):541–6. doi: 10.1136/gut.33.4.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lembo T, Naliboff BD, Matin K, et al. Irritable bowel syndrome patients show altered sensitivity to exogenous opioids. Pain. 2000;87(2):137–47. doi: 10.1016/S0304-3959(00)00282-7. [DOI] [PubMed] [Google Scholar]
- 63.Delvaux M, Louvel D, Lagier E, et al. The kappa agonist fedotozine relieves hypersensitivity to colonic distention in patients with irritable bowel syndrome. Gastroenterology. 1999;116(1):38–45. doi: 10.1016/s0016-5085(99)70226-x. [DOI] [PubMed] [Google Scholar]
- 64.Delgado-Aros S, Chial HJ, Cremonini F, et al. Effects of asimadoline, a kappa-opioid agonist, on satiation and postprandial symptoms in health. Aliment Pharmacol Ther. 2003;18(5):507–14. doi: 10.1046/j.1365-2036.2003.01670.x. [DOI] [PubMed] [Google Scholar]
- 65.Delvaux M, Beck A, Jacob J, et al. Effect of asimadoline, a kappa opioid agonist, on pain induced by colonic distension in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2004;20(2):237–46. doi: 10.1111/j.1365-2036.2004.01922.x. [DOI] [PubMed] [Google Scholar]
- 66.Szarka LA, Camilleri M, Burton D, et al. Efficacy of on-demand asimadoline, a peripheral kappa-opioid agonist, in females with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2007;5:1268–75. doi: 10.1016/j.cgh.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mangel AW, Bornstein JD, Hamm LR, et al. Clinical trial: asimadoline in the treatment of patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2008;28:239–49. doi: 10.1111/j.1365-2036.2008.03730.x. [DOI] [PubMed] [Google Scholar]
- 68.Wolff BG, Michelassi F, Gerkin TM, et al. Alvimopan, a novel, peripherally acting mu opioid antagonist: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial of major abdominal surgery and postoperative ileus. Ann Surg. 2004;240(4):728–34. doi: 10.1097/01.sla.0000141158.27977.66. discussion 734-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Taguchi A, Sharma N, Saleem RM, et al. Selective postoperative inhibition of gastrointestinal opioid receptors. N Engl J Med. 2001;345(13):935–40. doi: 10.1056/NEJMoa010564. [DOI] [PubMed] [Google Scholar]
- 70.Delaney CP, Weese JL, Hyman NH, et al. Phase III trial of alvimopan, a novel, peripherally acting, mu opioid antagonist, for postoperative ileus after major abdominal surgery. Dis Colon Rectum. 2005;48(6):1114–25. doi: 10.1007/s10350-005-0035-7. discussion 1125-6; author reply 1127-9. [DOI] [PubMed] [Google Scholar]
- 71.Gonenne J, Camilleri M, Ferber I, et al. Effect of alvimopan and codeine on gastrointestinal transit: a randomized controlled study. Clin Gastroenterol Hepatol. 2005;3:784–91. doi: 10.1016/s1542-3565(05)00434-9. [DOI] [PubMed] [Google Scholar]
- 72.Foxx-Orenstein AE, Camilleri M, Szarka LA, et al. Does co-administration of a non-selective opiate antagonist enhance acceleration of transit by a 5-HT4 agonist in constipation-predominant irritable bowel syndrome? A randomized controlled trial. Neurogastroenterol Motil. 2007;19:821–30. doi: 10.1111/j.1365-2982.2007.00944.x. [DOI] [PubMed] [Google Scholar]
- 73.Yuan CS, Doshan H, Charney MR, et al. Tolerability, gut effects, and pharmacokinetics of methylnaltrexone following repeated intravenous administration in humans. J Clin Pharmacol. 2005;45:538–46. doi: 10.1177/0091270004273491. [DOI] [PubMed] [Google Scholar]
- 74.Yuan CS, Foss JF, O’Connor M, et al. Methylnaltrexone for reversal of constipation due to chronic methadone use: a randomized controlled trial. JAMA. 2000;283:367–72. doi: 10.1001/jama.283.3.367. [DOI] [PubMed] [Google Scholar]
- 75.Owens MJ, Nemeroff CB. Physiology and pharmacology of corticotropin-releasing factor. Pharmacol Rev. 1991;43(4):425–73. [PubMed] [Google Scholar]
- 76.Tache Y, Monnikes H, Bonaz B, et al. Role of CRF in stress-related alterations of gastric and colonic motor function. Ann N Y Acad Sci. 1993;697:233–43. doi: 10.1111/j.1749-6632.1993.tb49936.x. [DOI] [PubMed] [Google Scholar]
- 77.Greenwood-Van Meerveld B, Johnson AC, Cochrane S, et al. Corticotropin-releasing factor 1 receptor-mediated mechanisms inhibit colonic hypersensitivity in rats. Neurogastroenterol Motil. 2005;17(3):415–22. doi: 10.1111/j.1365-2982.2005.00648.x. [DOI] [PubMed] [Google Scholar]
- 78.Monnikes H, Schmidt BG, Tache Y. Psychological stress-induced accelerated colonic transit in rats involves hypothalamic corticotropin-releasing factor. Gastroenterology. 1993;104(3):716–23. doi: 10.1016/0016-5085(93)91006-4. [DOI] [PubMed] [Google Scholar]
- 79.Sagami Y, Shimada Y, Tayama J, et al. Effect of a corticotropin releasing hormone receptor antagonist on colonic sensory and motor function in patients with irritable bowel syndrome. Gut. 2004;53(7):958–64. doi: 10.1136/gut.2003.018911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sweetser SR, Linker Nord SJ, Burton DD, et al. Effects of a novel corticotrophin releasing factor receptor-1 antagonist, BMS-562086, on gastrointestinal and colonic transit and bowel habits in patients with diarrhea-predominant irritable bowel syndrome (D-IBS) Gastroenterology. 2008;134:A-548. [Google Scholar]
- 81.Leventer SM, Kucharik RF, Keogh JC, et al. The potential of dextofisopam for treatment of irritable bowel syndrome and inflammatory bowel disease. Am J Gastroenterol. 2004;99:S 279. [Google Scholar]
- 82.Leventer SM, Raudibaugh K, Frissora CL, et al. Clinical trial: dextofisopam in the treatment of patients with diarrhoea-predominant or alternating irritable bowel syndrome. Aliment Pharmacol Ther. 2008;27:197–206. doi: 10.1111/j.1365-2036.2007.03566.x. [DOI] [PubMed] [Google Scholar]
- 83.Storr M, Koppitz P, Sibaev A, et al. Melatonin reduces non-adrenergic, non-cholinergic relaxant neurotransmission by inhibition of nitric oxide synthase activity in the gastrointestinal tract of rodents in vitro. J Pineal Res. 2002;33(2):101–8. doi: 10.1034/j.1600-079x.2002.02909.x. [DOI] [PubMed] [Google Scholar]
- 84.Barajas-Lopez C, Peres AL, Espinosa-Luna R, et al. Melatonin modulates cholinergic transmission by blocking nicotinic channels in the guinea-pig submucous plexus. Eur J Pharmacol. 1996;312(3):319–25. doi: 10.1016/0014-2999(96)00481-5. [DOI] [PubMed] [Google Scholar]
- 85.Storr M, Schusdziarra V, Allescher HD. Inhibition of small conductance K+ -channels attenuated melatonin-induced relaxation of serotonin-contracted rat gastric fundus. Can J Physiol Pharmacol. 2000;78(10):799–806. [PubMed] [Google Scholar]
- 86.Lu WZ, Gwee KA, Moochhalla S, et al. Melatonin improves bowel symptoms in female patients with irritable bowel syndrome: a double-blind placebo-controlled study. Aliment Pharmacol Ther. 2005;22(10):927–34. doi: 10.1111/j.1365-2036.2005.02673.x. [DOI] [PubMed] [Google Scholar]
- 87.Song GH, Leng PH, Gwee KA, et al. Melatonin improves abdominal pain in irritable bowel syndrome patients who have sleep disturbances: a randomised, double blind, placebo controlled study. Gut. 2005;54(10):1402–7. doi: 10.1136/gut.2004.062034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Walsh JH. Physiology of the gastrointestinal tract. 3rd ed. Raven; New York: 1994. Gastrointestinal hormones; pp. 49–67. [Google Scholar]
- 89.Chey WY, Jin HO, Lee MH, et al. Colonic motility abnormality in patients with irritable bowel syndrome exhibiting abdominal pain and diarrhea. Am J Gastroenterol. 2001;96(5):1499–506. doi: 10.1111/j.1572-0241.2001.03804.x. [DOI] [PubMed] [Google Scholar]
- 90.Wank SA. Cholecystokinin receptors. Am J Physiol. 1995;269(5 Pt 1):G628–46. doi: 10.1152/ajpgi.1995.269.5.G628. [DOI] [PubMed] [Google Scholar]
- 91.Fourmy D, Escrieut C, Archer E, et al. Structure of cholecystokinin receptor binding sites and mechanism of activation/inactivation by agonists/antagonists. Pharmacol Toxicol. 2002;91(6):313–20. doi: 10.1034/j.1600-0773.2002.910608.x. [DOI] [PubMed] [Google Scholar]
- 92.D’Amato M, Rovati LC. Cholecystokinin-A receptor antagonists: therapies for gastrointestinal disorders. Expert Opin Investig Drugs. 1997;6(7):819–36. doi: 10.1517/13543784.6.7.819. [DOI] [PubMed] [Google Scholar]
- 93.Meyer BM, Werth BA, Beglinger C, et al. Role of cholecystokinin in regulation of gastrointestinal motor functions. Lancet. 1989;2(8653):12–5. doi: 10.1016/s0140-6736(89)90255-9. [DOI] [PubMed] [Google Scholar]
- 94.Cann PA, Rovati LC, Smart HL, et al. Loxiglumide, a CCK-A antagonist, in irritable bowel syndrome. A pilot multicenter clinical study. Ann N Y Acad Sci. 1994;713:449–50. doi: 10.1111/j.1749-6632.1994.tb44123.x. [DOI] [PubMed] [Google Scholar]
- 95.D’Amato M, Whorwell PJ, Thompson DG. The efficacy and safety of the CCKA-receptor antagonist dexloxiglumide in IBS. Gut. 1999;45(Suppl V):A 258. [Google Scholar]
- 96.Forest to discontinue development in U.S. of dexloxiglumide for irritable bowel syndrome. 2004 October; Pharmabiz.com. Pharmabiz.com. http://www.pharmabiz.com/article/detnews.asp?articleid=18255§ionid=14.
- 97.Cremonini F, Camilleri M, McKinzie S, et al. Effect of CCK-1 antagonist, dexloxiglumide, in female patients with irritable bowel syndrome: a pharmacodynamic and pharmacogenomic study. Am J Gastroenterol. 2005;100(3):652–63. doi: 10.1111/j.1572-0241.2005.41081.x. [DOI] [PubMed] [Google Scholar]
- 98.Whorwell PJ, Pace F, D’Amato M, et al. A phase III, 6-month, double-blind, placebo-controlled, randomized withdrawal trial of the selective CCK-1 antagonist dexloxiglumide in constipation-predominant IBS: the Darwin study. Gastroenterology. 2008;134:A-157. abstract. [Google Scholar]
- 99.O’Donnell LJ, Watson AJ, Cameron D, et al. Effect of octreotide on mouth-to-caecum transit time in healthy subjects and in the irritable bowel syndrome. Aliment Pharmacol Ther. 1990;4(2):177–81. doi: 10.1111/j.1365-2036.1990.tb00463.x. [DOI] [PubMed] [Google Scholar]
- 100.von der Ohe MR, Camilleri M, Thomforde GM, et al. Differential regional effects of octreotide on human gastrointestinal motor function. Gut. 1995;36(5):743–8. doi: 10.1136/gut.36.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schwetz I, Naliboff B, Munakata J, et al. Anti-hyperalgesic effect of octreotide in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2004;19(1):123–31. doi: 10.1111/j.1365-2036.2004.01774.x. [DOI] [PubMed] [Google Scholar]
- 102.Bradette M, Delvaux M, Staumont G, et al. Octreotide increases thresholds of colonic visceral perception in IBS patients without modifying muscle tone. Dig Dis Sci. 1994;39(6):1171–8. doi: 10.1007/BF02093780. [DOI] [PubMed] [Google Scholar]
- 103.Holzer P, Holzer-Petsche U. Tachykinins in the gut. Part I. Expression, release and motor function. Pharmacol Ther. 1997;73(3):173–217. doi: 10.1016/s0163-7258(96)00195-7. [DOI] [PubMed] [Google Scholar]
- 104.Moriarty D, Selve N, Baird AW, et al. Potent NK1 antagonism by SR-140333 reduces rat colonic secretory response to immunocyte activation. Am J Physiol Cell Physiol. 2001;280(4):C852–8. doi: 10.1152/ajpcell.2001.280.4.C852. [DOI] [PubMed] [Google Scholar]
- 105.Onori L, Aggio A, Taddei G, et al. Contribution of NK(2) tachykinin receptors to propulsion in the rabbit distal colon. Am J Physiol Gastrointest Liver Physiol. 2000;278(1):G137–47. doi: 10.1152/ajpgi.2000.278.1.G137. [DOI] [PubMed] [Google Scholar]
- 106.Oh-Young L, Manakata J, Naliboff B. A double-blind, parallel group pilot study of the effects o CJ-11974 and placebo on perceptual and emotional responses to rectosigmoid distension in IBS patients. Gastroenterology. 2000;118:A-846. [Google Scholar]
- 107.Lordal M, Navalesi G, Theodorsson E, et al. A novel tachykinin NK2 receptor antagonist prevents motility-stimulating effects of neurokinin A in small intestine. Br J Pharmacol. 2001;134(1):215–23. doi: 10.1038/sj.bjp.0704217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Houghton LA, Cremonini F, Camilleri M, et al. Effect of the NK3 receptor antagonist, talnetant, on rectal sensory function and compliance in healthy humans. Neurogastroenterol Motil. 2007;19:732–43. doi: 10.1111/j.1365-2982.2007.00934.x. [DOI] [PubMed] [Google Scholar]
- 109.Dukes GE, Dewit OE, Sanger GJ, et al. Lack of effect of the NK3 receptor antagonist, talnetant SB223242 on symptoms of IBS: results of 2 randomized, double-blind, placebo-controlled, dose-ranging trials. Gastroenterology. 2007;132:A60. [Google Scholar]
- 110.Lubiprostone: RU 0211, SPI 0211. Drugs R D. 2005;6(4):245–8. doi: 10.2165/00126839-200506040-00009. no authors listed. [DOI] [PubMed] [Google Scholar]
- 111.Camilleri M, Bharucha AE, Ueno R, et al. Effect of a selective chloride channel activator, lubiprostone, on gastrointestinal transit, gastric sensory and motor functions in healthy volunteers. Am J Physiol. 2006;290:G942–7. doi: 10.1152/ajpgi.00264.2005. [DOI] [PubMed] [Google Scholar]
- 112.Johanson JF, Gargano AM, Holland PC, et al. Phase III, efficacy and safety of RU-0211 a novel chloride channel activator, for the treatment of constipation. Gastroenterology. 2003;124:A48. [Google Scholar]
- 113.Johanson JF, Gargano MA, Holland PC, et al. Phase III, randomized withdrawal study of RU-0211, a novel chloride channel activator for the treatment of constipation. Gastroenterology. 2004;126(4 Suppl 2):A-100. [Google Scholar]
- 114.Johanson JF, Gargano M, Patchen M. Efficacy and safety of a novel compound, RU-0211, for the treatment of constipation. Gastroenterology. 2002;122(4 Suppl 1):A-315. [Google Scholar]
- 115.Johanson JF, Drossman DA, Panas R, et al. Clinical trial: phase 2 study of lubiprostone for irritable bowel syndrome with constipation. Aliment Pharmacol Ther. 2008;27(8):685–96. doi: 10.1111/j.1365-2036.2008.03629.x. [DOI] [PubMed] [Google Scholar]
- 116.Drossman DA, Chey WD, Johanson JF, et al. Clinical trial: lubiprostone in patients with constipation-associated irritable bowel syndrome--results of two randomized, placebo-controlled studies. Aliment Pharmacol Ther. 2009;29:329–41. doi: 10.1111/j.1365-2036.2008.03881.x. [DOI] [PubMed] [Google Scholar]
- 117.Currie MG, Fok KF, Kato J, et al. Guanylin: an endogenous activator of intestinal guanylate cyclase. Proc Natl Acad Sci U S A. 1992;89(3):947–51. doi: 10.1073/pnas.89.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hamra FK, Forte LR, Eber SL, et al. Uroguanylin: structure and activity of a second endogenous peptide that stimulates intestinal guanylate cyclase. Proc Natl Acad Sci U S A. 1993;90(22):10464–8. doi: 10.1073/pnas.90.22.10464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Giannella RA. Escherichia coli heat-stable enterotoxins, guanylins, and their receptors: what are they and what do they do? J Lab Clin Med. 1995;125(2):173–81. [PubMed] [Google Scholar]
- 120.Forte LR. Guanylin regulatory peptides: structures, biological activities mediated by cyclic GMP and pathobiology. Regul Pept. 1999;81(13):25–39. doi: 10.1016/s0167-0115(99)00033-6. [DOI] [PubMed] [Google Scholar]
- 121.Currie MG, Kurtz C, Mahajan-Miklos S, et al. Effects of single dose administration of MD-1100 on safety, tolerability, exposure, and stool consistency in healthy subjects. Am J Gastroenterol. 2005;100:S-328. abstract. [Google Scholar]
- 122.Johnston JM, Kurtz CB, Drossman DA, et al. Pilot study on the effect of linaclotide in patients with chronic constipation. Am J Gastroenterol. 2009;104:125–32. doi: 10.1038/ajg.2008.59. [DOI] [PubMed] [Google Scholar]
- 123.Mangel AW, Chaturvedi P. Evalution of crofelemer in the treatment of diarrhea-predominant irritable bowel syndrome patients. Digestion. 2008;78(4):180–6. doi: 10.1159/000185719. [DOI] [PubMed] [Google Scholar]
- 124.Verdu EF, Collins SM. Irritable bowel syndrome and probiotics: from rationale to clinical use. Curr Opin Gastroenterol. 2005;21(6):697–701. doi: 10.1097/01.mog.0000182861.11669.4d. [DOI] [PubMed] [Google Scholar]
- 125.Moayyedi P, Ford AC, Talley NJ, et al. The efficacy of probiotics in the therapy of irritable bowel syndrome: a systematic review Gut online first. 2008 December 17; published on. [Google Scholar]
- 126.Pimentel M, Chow EJ, Lin HC. Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome. a double-blind, randomized, placebo-controlled study. Am J Gastroenterol. 2003;98(2):412–9. doi: 10.1111/j.1572-0241.2003.07234.x. [DOI] [PubMed] [Google Scholar]
- 127.Pimentel M, Park S, Mirocha J, et al. The effect of a nonabsorbed oral antibiotic (rifaximin) on the symptoms of the irritable bowel syndrome: a randomized trial. Ann Intern Med. 2006;145(8):557–63. doi: 10.7326/0003-4819-145-8-200610170-00004. [DOI] [PubMed] [Google Scholar]
- 128.Lembo A, Zakko SF, Ferreira NL, et al. Rifaximin for treatment of diarrhea-associated irritable bowel syndrome: short-term treatment leading to long-term sustained response. Gastroenterology. 2008;134:A-545. abstract. [Google Scholar]
- 129.Leri O, Tubili S, De Rosa FG, et al. Management of diarrhoeic type of irritable bowel syndrome with exclusion diet and disodium cromoglycate. Inflammo-pharmacology. 1997;5:153–8. doi: 10.1007/s10787-997-0024-7. [DOI] [PubMed] [Google Scholar]
- 130.Grazioli I, Melzi G, Balsamo V, et al. Food intolerance and irritable bowel syndrome of childhood: clinical efficacy of oral sodium cromoglycate and elimination diet. Minerva Pediatr. 1993;45:253–8. [PubMed] [Google Scholar]
- 131.Miehlke S, Madisch A, Bethke B, et al. Oral budesonide for maintenance treatment of collagenous colitis: a randomized, double-blind, placebo-controlled trial. Gastroenterology. 2008;135:1510–6. doi: 10.1053/j.gastro.2008.07.081. [DOI] [PubMed] [Google Scholar]
- 132.Bonderup OK, Hansen JB, Teglbjaerg PS, et al. Long-term budesonide treatment of collagenous colitis: a randomised, double-blind, placebo-controlled trial. Gut. 2009;58:68–72. doi: 10.1136/gut.2008.156513. [DOI] [PubMed] [Google Scholar]
- 133.Chande N, Macdonald JK, McDonald JW. Interventions for treating microscopic colitis: a Cochrane inflammatory bowel disease and functional bowel disorders review group systematic review of randomized trials. Am J Gastroenterol. 2009;104:235–41. doi: 10.1038/ajg.2008.16. [DOI] [PubMed] [Google Scholar]
- 134.Barbara G, Cremon C, Gargano L, et al. Mesalazine treatment for intestinal immune activation in patient with irritable bowel syndrome: a randomized controlled pilot trial. Gastroenterology. 2008;134:A-546. abstract. [Google Scholar]


